Abstract
The architecture of the basement membranes is essential for proper function. This architecture is based on interactions among its components, which assemble in a complex network. Entactin-1 appears to be the mastermind of this assembling. In entactin-1-null transgenic mice, immunocytochemistry established the absence of entactin-1 in the glomerular basement membrane, and morphological thickening of this membrane was demonstrated. This prompted us to investigate the organization of other components of the glomerular basement membrane in the transgenic animals. The distribution of type IV collagen and laminin remained unchanged, whereas that of anionic charges was significantly altered. We also evaluated the impact of the absence of entactin-1 on cell relays by studying the α3- and the αv-integrins along the endothelial and epithelial glomerular cell plasma membranes. Only the density of αv was found to be increased. Finally, the filtration properties of the glomerular wall were evaluated by revealing endogenous albumin distribution across the basement membrane. This was altered in transgenic animals, suggesting changes in permselectivity properties. Entactin-1 appears to be an essential component in basement membranes because its absence appears to modify the molecular organization leading to alterations in functional properties.
Keywords: entactin-1, immunocytochemistry, glomerular basement membrane, collagen type IV, laminin, anionic charges, permselectivity
Basement membranes are thin layers of highly specialized extracellular matrix. Located in close contact with the plasma membrane of various cell types, they underlie sheets of endothelial and epithelial cells and surround muscle cells, adipocytes, peripheral neurons, and axons (Timpl and Dziadek 1986). They exert cell support functions, depending on their binding to cell receptors, and have filtration properties, particularly important in renal tissue (Bendayan et al. 1986; Timpl and Dziadek 1986). On binding to membrane receptors, they provide stimuli for cell differentiation, cell migration, and tissue development and repair (Paulsson 1988; Yurchenco and Schittny 1990; Timpl and Brown 1996). Basement membranes display particular molecular architecture through specific interactions among their components, mainly type IV collagen, laminin, entactin, and heparan sulfate proteoglycans (Chung and Durkin 1990; Yurchenco and Schittny 1990; Chung et al. 1993). Whereas type IV collagen creates a covalently stabilized framework by its COOH and NH2− terminals as well as by lateral associations, laminin self-assembles through terminal domain interactions, thus forming a second polymer network (Yurchenco and Schittny 1990). The heparan sulfate proteoglycan polyanionic proteins can bind weakly to laminin and type IV collagen and can also bind to themselves forming dimers and oligomers that together create a coherent basement membrane (Yurchenco and Schittny 1990).
Identified in 1977 (Chung et al. 1977; Carlin et al. 1981), entactin is also known as nidogen (Timpl et al. 1983). Molecular biology analysis by cDNA cloning has established that both terms refer to the same protein (Durkin et al. 1988; Mann et al. 1989). Entactin has recently been pointed out as an important component of the basement membranes (Chung and Durkin 1990). It has high affinity for laminin, being responsible for the molecular assembly of basement membranes (Chung and Durkin 1990; Aumailley et al. 1993; Chung et al. 1993; Mayer et al. 1998). It also connects the networks formed by type IV collagen and laminin to create the tridimensional network architecture particular to basement membranes (Chung and Durkin 1990; Yurchenco and Schittny 1990; Chung et al. 1993; Miosge et al. 1999). Entactin is also known for cell adhesion support (Chakravarti et al. 1990). In fact, two distinct molecular cell attachment sites have been identified (Dong et al. 1995). The one located in the second globular G2 domain of entactin binds a member of the β1 family of integrins receptors, probably the α3β1 (Dedhar et al. 1992; Dong et al. 1995; Wu et al. 1995), while the second, on the rigid stalk E domain within the RGD sequence, is recognized by the αvβ3-integrin (Dong et al. 1995; Yi et al. 1998). Moreover, this RGD sequence can also bind the leukocyte response integrin that stimulates neutrophil chemotaxis to sites of injury (Senior et al. 1992; Gresham et al. 1996). Recent findings have shown that entactin, through its G2 domain, constitutes a signal for stimulation of the Fc receptor-mediated phagocytosis via ligation of α3β1 (Gresham et al. 1996), therefore participating in acute inflammation responses. Furthermore, this protein binds fibrinogen (Wu and Chung 1991) and human trophoblasts (Yang et al. 1996), indicating potential roles in hemostasis and embryo implantation.
In this study we evaluated the importance of entactin-1 in the overall structural and functional properties of the glomerular basement membrane (GBM). Using renal tissues from entactin-1-null transgenic mice and applying cytochemical approaches, we evaluated the impact of the absence of entactin-1 on the molecular organization of other basement membrane components, on the distribution of anionic charges of the GBM, and on the expression by glomerular cells of extracellular matrix membrane receptors, the integrins. In view of the major roles played by the GBM in glomerular filtration, we also assessed the functional properties of the glomerular wall in renal tissues from entactin-1-null mice.
Materials and Methods
Animals
This study was performed on three groups of C57BL/6 mice. One group of three mice represents the wild-type or control group, +/+, a second group of three heterozygous mice (+/-) in which one of the two alleles for entactin-1 was inactivated, and a third group of four homozygous mice (-/-) in which the two alleles for entactin-1 were inactivated by homologous recombination (Dong et al. 2002). The absence of entactin-1 in the homozygous-null animals was demonstrated by Western blotting, immunostaining on fixed and frozen tissue sections, and by the absence of entactin-1 mRNA. All the animals were of the same age, between 7 and 8 months. Homozygous transgenic animals were all from the same litter.
Tissue Processing
After anesthesia, the kidneys were removed and cut into small samples for immunocytochemistry protocols. They were fixed by immersion with a periodate-lysine-paraformaldehyde 4% solution for 2 hr at room temperature (RT). Tissues were dehydrated in ethanol and embedded in Lowicryl K4M at −30C (Bendayan 1995). Thin sections were cut and mounted on Parlodion- and carbon-coated nickel grids and processed for immunocytochemistry.
Immunocytochemistry
Some grids were stained with uranyl acetate and lead citrate for electron microscopic examination, and others were subjected to immunocytochemistry. Antigenic sites for entactin-1, type IV collagen, laminin, integrins, and endogenous albumin were revealed using corresponding specific polyclonal and monoclonal antibodies [rabbit anti-entactin-1 (Chung and Durkin 1990); mouse anti-type IV collagen (MAB1430), Chemicon, Temecula, CA; mouse anti-laminin (clone Lam-1), ICN ImmunoBiologicals, Costa Mesa, CA; rabbit anti-integrins for α3 (AB1920) and αv (AB1930) subunits, both from Chemicon; rabbit anti-mouse serum albumin (Cappel, ICN Biomedical); and protein A-gold complexes (Bendayan 1995)]. The postembedding immunocytochemical labeling protocol was carried out as previously described (Bendayan 1995). Briefly, for the different antigens, the tissue thin sections were first incubated on a drop of 0.15 mol/liter glycine for 15 min and then on a drop of 1% ovalbumin in PBS, pH 7.3, for 20 min. The grids were then placed on a drop of 1% gelatin for an additional 15 min and transferred to a drop of one of the antibodies [anti-entactin-1 (1:100); anti-type IV collagen (1:10); anti-laminin (1:50); anti-integrins (1:100 for α3 and 1:50 for αv)] for an overnight incubation at 4C. After rinsing with PBS, the sections were incubated with 1% ovalbumin for 20 min and moved directly to a drop of the protein A-gold complex for 30 min. Protein A-gold was prepared with 10-nm gold particles according to protocols described previously (Bendayan 1995). The grids were then washed with PBS and distilled water before drying. Staining was performed with uranyl acetate before examination with a Philips 410 electron microscope. For endogenous albumin, the grids carrying the tissue thin sections were transferred directly from the PBS to a drop of the anti-mouse albumin polyclonal antibody (1:50) for a 90-min incubation at RT. The incubation step with 1% ovalbumin was omitted. After washing with PBS, they were incubated with the protein A-gold complex for 30 min. Specificity of each immunolabeling was demonstrated by control experiments: (a) incubation of the tissue sections with each antibody solution adsorbed with its corresponding antigen, followed by the protein A-gold complex, and (b) incubation with the protein A-gold complex alone, omitting the antibody step.
In addition, the distribution of the anionic charges through the GBM was revealed using the poly-l-lysine-gold (PLG) complex. Previous experiments have demonstrated the specificity of the PLG complex for heparan sulfate proteoglycans (Russo et al. 1993; Londoño et al. in press). The PLG complex was prepared according to Skutelsky and Roth (1986) as adapted by Russo et al. (1993) and applied on the tissue sections as described previously (Russo et al. 1993; Londoño et al. in press).
Data and Statistical Analysis
Morphometrical analysis of the labelings was performed using an image processing system (Videoplan 2; Carl Zeiss, Toronto, ONT, Canada). Labeling density for entactin-1 (gold particles/μm2) was evaluated for the glomerular and mesangial basement membranes. For the α3 and αv integrins, the density of labeling present over the cells' plasma membrane was evaluated in reference to the length of the membranes (gold particles/μm2). These measurements were made on 18 recorded fields at a final magnification of ×21,000 for each animal in each group. For type IV collagen, laminin, and albumin, as well as for the anionic sites, the exact location of the gold particles over the GBM was analyzed. The distribution of the labeling was carried out as reported in detail previously (Bendayan et al. 1986; Bendayan 1995). Briefly, the distance between each gold particle and the endothelial abluminal plasma membrane was first measured. Next, the thickness of the GBM at the same site and the distance between the endothelial abluminal plasma membrane and the podocyte basal plasma membrane was measured. Then, the ratio R = [distance (endothelium-gold particle)/distance (endothelium-epithelium)] was calculated and reported in histograms reflecting the distribution of the labeling over the GBM. These evaluations were carried out individually for each animal in each group. Over 900 measurements were recorded for each animal and for each protocol. Finally, the thickness of the GBM was measured according to methods previously described (Bendayan et al. 1986; Doucet et al. 2000). The distance between the endothelial abluminal plasma membrane and that of podocytes was directly measured by planimetry. Care was taken to evaluate fields cut at right angles. This was reflected by the clear presence of slit diaphragms between podocyte foot processes. Again, over 900 measurements were recorded for each animal. The quantitative data were statistically evaluated using the Mann-Whitney U-test or the Kruskal-Wallis test.
Results
Quantitative Immunocytochemistry for Entactin-1
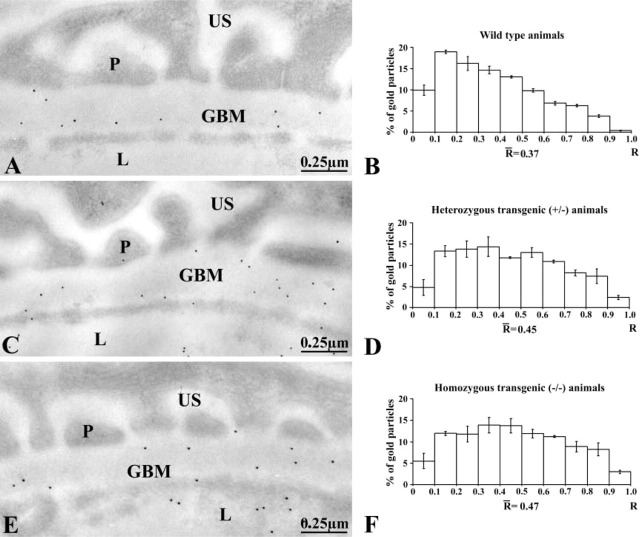
Our first concern was to demonstrate that entactin-1 was not expressed in the transgenic mice. To do so, we evaluated by quantitative immunocytochemistry the presence of entactin-1 antigenic sites in the GBM of renal tissues from the wild-type and the transgenic heterozygous +/- and homozygous -/- entactin-1-null animals. Immunolabeling for entactin-1 in renal tissues of the wild-type animals demonstrated strong labeling by gold particles throughout the entire thickness of the GBM, with a slight increase in intensity over the lamina lucida externa (Figure 1). Tissues from the homozygous -/- animals displayed practically no labeling, indicating absence of entactin-1 immunoreactivity, while tissues from the heterozygous +/- animals showed lower labeling than those of the wild-type animals, with no preferential distribution throughout the GBM (Figure 1). Morphometric analysis confirmed these observations (Table 1). In fact, tissues from the homozygous animals demonstrated labeling about nine times less intense than those of the wild-type and five times less than those of the heterozygous animals. For the mesangial region, the results were similar to those of the GBM (Table 1), with very low labeling in tissues of the entactin-1-null mice. Control experiments to evaluate the specificity of the entactin-1 immunolabeling resulted in very low levels of labeling in tissues of the wild-type animals (Table 1). Interestingly, in spite of the very low labeling registered for the tissues of the entactin-1-null homozygous animals, labeling was higher than that obtained in control experiments (Table 1). This may be due to very low levels of crossreactivity of the antibody with an isoform of entactin still present in the tissue, as suggested by Miosge et al. (2002).
Figure 1.
Entactin-1 immunolabeling. Glomerular wall of the wild-type +/+ (A), entactin-1-null heterozygous (+/-) (B), and homozygous (-/-) (C) animals. Tissues from wild-type mice demonstrate intense labeling in the glomerular basement membrane (GBM) compared to those of entactin-1-null mice +/- (B) and -/- (C). L, capillary lumen; P, podocytes; US, urinary space.
Table 1.
Entactin-1 immunolabeling: density values for the glomerular and mesangial basement membranes a
| Genotype | Control specificity ontissues of wild-type animals | ||||||
|---|---|---|---|---|---|---|---|
| Wild-type animals (+/+) | Heterozygous animals (+/-) | Homozygous animals | Antigen added in excess | Antibody step omitted | |||
| Glomerular basement membrane | 32.52 ± 0.63 | 18.03 ± 0.42 b | 3.89 ± 0.21 b , c | 2.12 ± 0.44 b | 1.63 ± 0.37 b | ||
| Mesangial basement membrane | 33.68 ± 0.64 | 20.45 ± 0.45 b | 4.71 ± 0.24 b , c | 2.16 ± 0.39 b | 1.32±0.17 b | ||
Number of gold particles per μm2. Mean values ± SEM.
Significantly different from the corresponding wild-type value (p<0.005).
Significantly different from the corresponding heterozygous value (p<0.005).
Morphological Examination
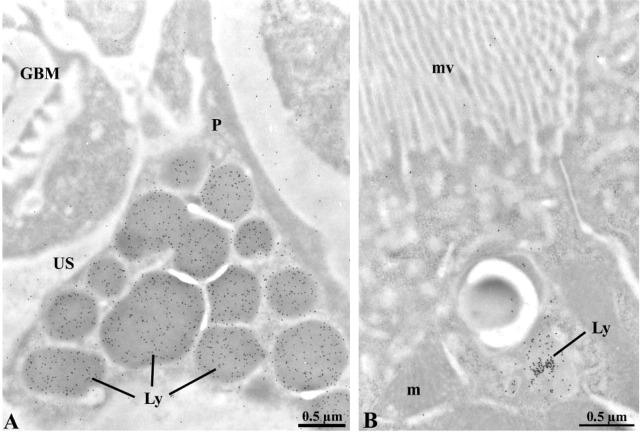
Once we confirmed that the renal basement membranes of entactin-1-null homozygous mice are devoid of this component, our next step consisted of evaluating changes in the appearance and structure of the basement membranes. The GBM in transgenic and in wild-type animals displayed its three characteristic regions: the lamina lucida interna on the subendothelial side, the lamina densa in the central part, and the lamina lucida externa on the epithelial side, with no major structural alterations (Figure 2). In the entactin-1-null homozygous and heterozygous animals, however, basement membrane thickenings and focal nodules were found (Figure 2). Albeit in fewer instances, the nodules were also detected in tissues of the wild-type animals. Morphometric evaluations of the GBM revealed small but significant thickening between tissues of the wild-type and the transgenic animals (Table 2). A significant 18% increase in GBM thickness was found for the transgenic homozygous mice compared to the wild-type, whereas only an 11% increase was found for the heterozygous transgenic mice. In addition, many lysosomes were present in the podocytes and tubule epithelial cells of the transgenic mice (see Figure 8).
Figure 2.
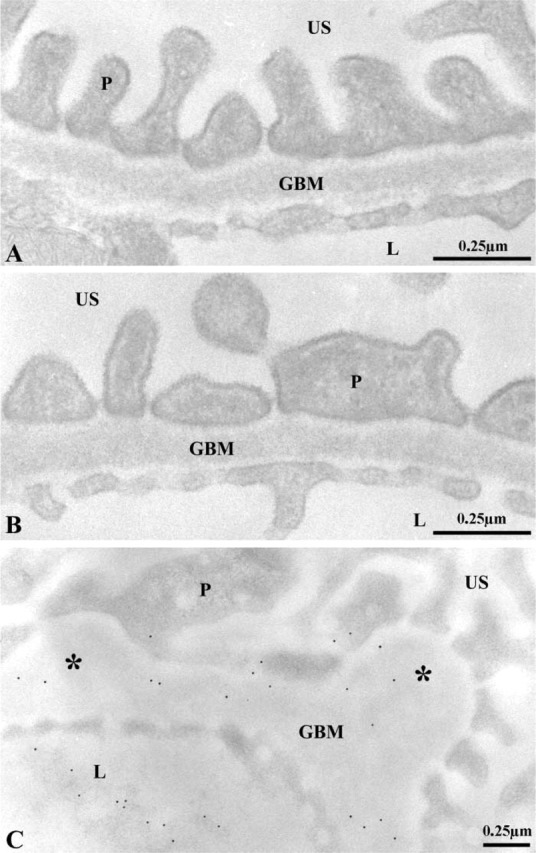
Morphological features of the glomerular wall of the wild-type (A) and entactin-1-null homozygous animals (B,C). Osmium-fixed, Epon-embedded tissues from the wild-type (A) and the homozygous (B) animals reveal GBMs with their characteristic morphology. No major morphological difference can be found beside slight increases in thickness, as determined by the morphometrical evaluations (Table 2). The endothelial cells of the blood capillaries and the epithelial cells delineating the urinary space (US) appear normal. (C) Lowicyl-embedded tissue from the entactin-1-null homozygous animal display GBM focal nodules (∗), a morphological characteristic frequently observed in the transgenic animals. L, capillary lumen.
Table 2.
Thickness of the glomerular basement membrane (nm ± SEM)
| Genotype | |||
|---|---|---|---|
| Wild-type animals (+/+) | Heterozygous animals (+/−) | Homozygous animals (−/−) | |
| 246.79 ± 1.42 | 278.10 ± 1.75 a | 299.45 ± 2.13 a | |
Significantly different from the wild-type value (p<0.05).
Figure 8.
Immunocytochemical localization of endogenous albumin in entactin-1-null animals (A,B). Intense immunolabeling by gold particles is present in lysosomal structures (Ly) of podocytes (A) and tubule epithelial cells (B). This was not encountered in tissues of the wild-type animals. m, mitochondrion; mv, microvilli.
Impact on Other Major GBM Components
We then evaluated the distribution of other basement membrane components such as type IV collagen and laminin, to detect any changes in the molecular configuration of the basement membranes. Using antibodies recognizing type IV collagen and laminin, respectively, in a broad array of human and mouse basement membranes, we revealed the correspondent basement membrane components. As demonstrated in Figure 3 and 4 with the corresponding histograms, the distribution of type IV collagen immunolabeling and that of laminin do not appear to be altered in basement membranes of transgenic mice in spite of the lack in entactin-1 (Figure 3 and 4). Type IV collagen immunolabeling retained its largely subendothelial distribution (Figure 3), and laminin immunolabeling was homogeneously distributed throughout the entire thickness of the basement membrane (Figure 4). Control experiments confirmed the specificity of these immunolabelings as reported previously (Desjardins and Bendayan 1989) (results not shown).
Figure 3.
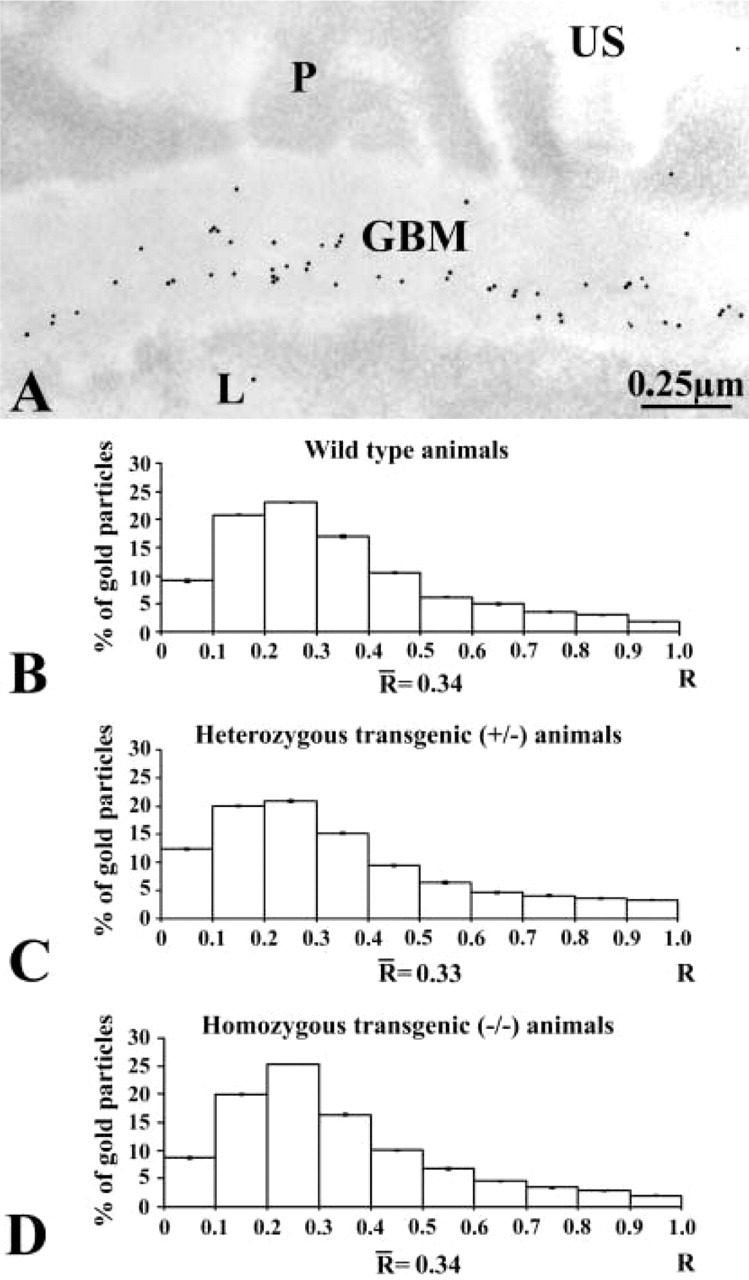
Immunocytochemical localization of type IV collagen. (A) Immunogold labeling in an entactin-1-null homozygous animal tissue. The gold particles revealing type IV collagen antigenic sites are restricted to the GBM, with a concentration in the subendothelial side. US, urinary space; L, capillary lumen. (B-D) Histograms of the distributions of type IV collagen labeling across the GBM of the wild-type (B), heterozygous (C), and homozygous (D) animal tissues. The three distributions indicate the preferential location of type IV collagen in the subendothelial side of the GBM. No changes are detected among the groups of animals.
Figure 4.
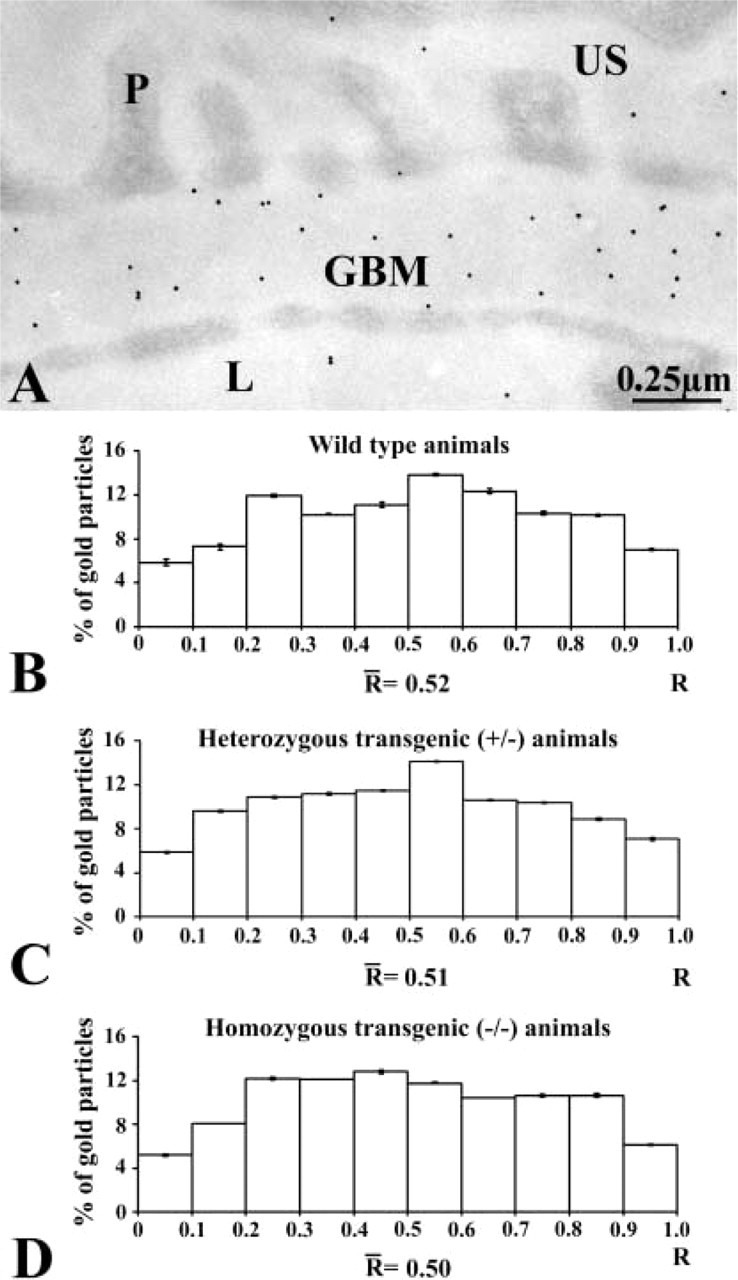
Immunocytochemical localization of laminin. (A) Immunogold labeling of an entactin-1-null heterozygous animal tissue. The gold particles revealing laminin antigenic sites are restricted to the GBM with a rather homogeneous distribution across the GBM. (B-D) Histograms of the distributions of laminin labeling across the GBM of the wild-type (B), heterozygous (C), and homozygous (D) animals. The three histograms display similar distributions, with no changes among the groups of animals.
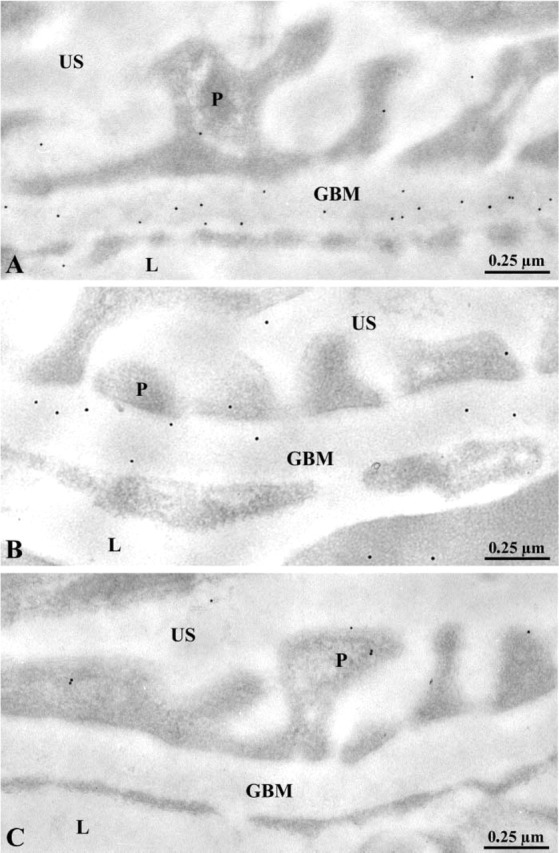
The distribution of the anionic sites, also an indication of the basement membrane molecular organization, was evaluated by using the poly-l-lysine-gold (PLG) approach (Russo et al. 1993). On applying the PLG complex, the gold particles were found distributed along the plasma membrane of the epithelial and endothelial cells of the glomerular wall (Figure 5). The foot processes of the podocytes, in particular, were decorated by the gold particles. In the GBM, the labeling followed a bimodal distribution, with concentrations in both the lamina rara and a weak labeling in the central part of the lamina densa (Figure 5A). Few gold particles were seen in the cell cytoplasm. In tissues of entactin-1-null mice, the distribution of the labeling underwent some changes, with significant decreases of the labeling in the subendothelial side of the GBM or lamina rara interna (Figure 5B). This was confirmed by morphometrical evaluations and the histograms presented in Figure 5. No difference was detected between tissues of heterozygous and homozygous animals (Figure 5).
Figure 5.
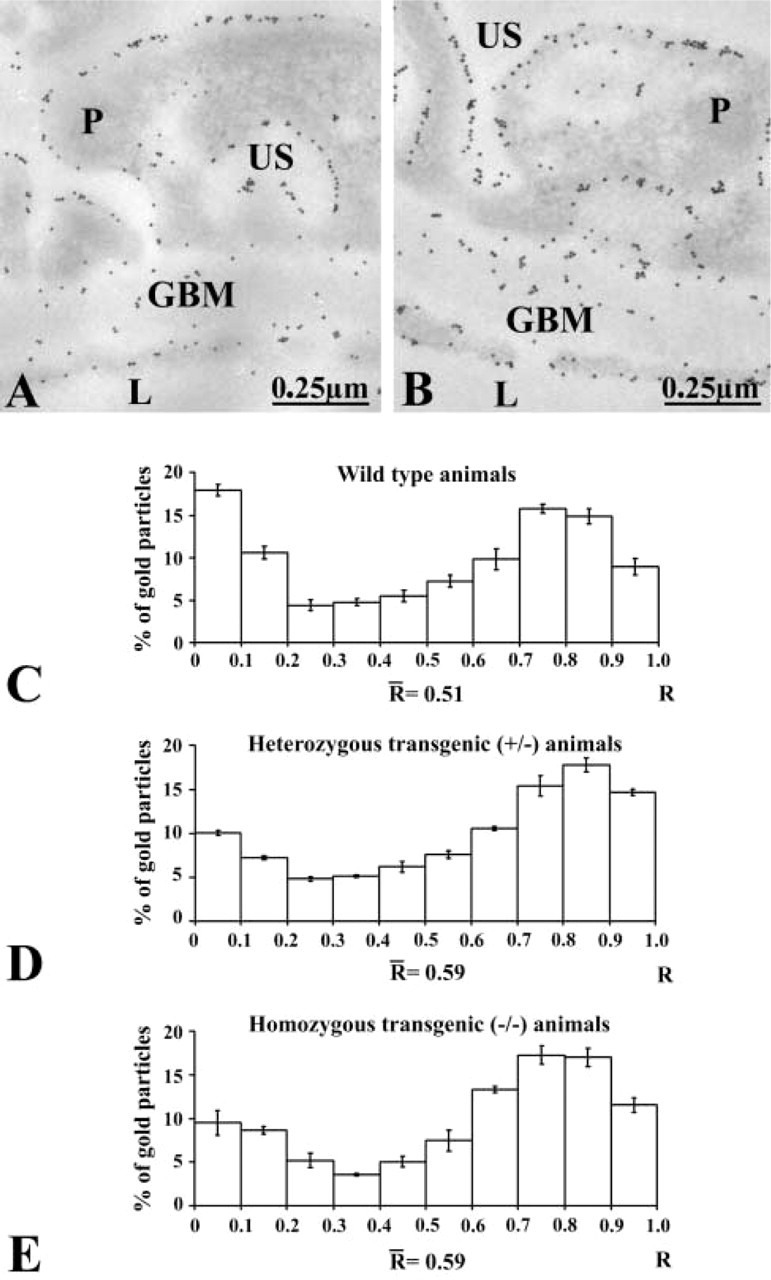
Distribution of the anionic charges across the glomerular wall of wild-type (A) and entactin-1-null homozygous (B) animal tissues as revealed by poly-l-lysine-gold. The gold particles are located along the plasma membranes of the endothelial and epithelial cells or podocytes (P). They are also present across the GBM. However, the labeling appears to be less intense on the subendothelial side of the GBM for the entactin-1-null homozygous animal (B). (C-E) Histograms of the distributions of the charges across the GBM in the wild-type (C), heterozygous (D), and homozygous (E) animals. In tissues of the wild-type animals the labeling is concentrated in the subendothelial and subepithelial sides of the GBM. In the transgenic animals the labeling on the subendothelial side decreases significantly.
Impact on ECM Receptors, the Integrins
We then switched to investigation of plasma membrane receptors known to interact with basement membrane components. Integrins, a large family of transmembrane heterodimeric receptors composed of α- and β-subunits, are involved in cell-to-cell and cell-to-matrix interactions (Ruoslahti 1991). Therefore, using the immunogold approach we evaluated the expression of two integrin subunits, αv and α3, known to act directly as entactin-1 ligands (Dedhar et al. 1992; Dong et al. 1995; Wu et al. 1995; Yi et al. 1998). Immunolabeling for both integrins was revealed at the level of endothelial and epithelial plasma membranes, particularly those facing the GBM (Figure 6). Interestingly, only the labeling for αv was found to change along the endothelial and epithelial plasma membranes (Figure 6). Tissues from the heterozygous and homozygous transgenic mice demonstrated higher labeling density for the αv-sub-unit, whereas those for the α3-subunit remained unchanged (Table 3 and 4). Again, control experiments confirmed the specificity of these labelings (results not shown).
Figure 6.
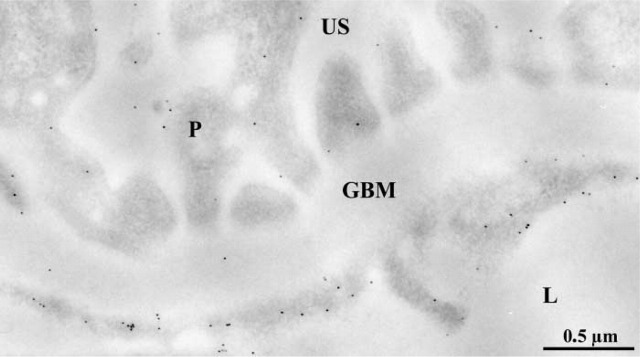
Immunocytochemical localization of the αv-integrin subunit on the glomerular wall of an entactin-1-null animal. Labeling by gold particles is present along the plasma membranes of the endothelial and the epithelial cells or podocytes (P). Both the luminal and abluminal membranes are labeled.
Table 3.
αv-integrin subunit immunocytochemistry (gold particles/μm ± SEM) along the endothelial and epithelial plasma membranes
| Genotype | |||
|---|---|---|---|
| Wild-type group (+/+) | Heterozygous group (+/−) | Homozygous group (−/−) | |
| Epithelial cell abluminal membrane | 0.39 ± 0.03 | 0.46 ± 0.03 a | 0.52 ± 0.02 a , b |
| Endothelial cell abluminal membrane | 0.27 ± 0.02 | 0.36 ± 0.03 a | 0.41 ± 0.02 a , b |
Significantly different from the corresponding wild-type value (p<0.05).
Significantly different from the corresponding heterozygous value (p<0.05).
Table 4.
α3-integrin subunit immunocytochemistry (gold particles/μm ± SEM) along the endothelial and epithelial plasma membranes
| Genotype | |||
|---|---|---|---|
| Wild-type group (+/+) | Heterozygous group (+/−) | Homozygous group (−/−) | |
| Epithelial cell abluminal membrane | 0.29 ± 0.02 | 0.30 ± 0.02 | 0.24 ± 0.01 |
| Endothelial cell abluminal membrane | 0.21 ± 0.02 | 0.21 ± 0.02 | 0.19 ± 0.01 |
Functional Properties
After establishing the absence of entactin-1 in the basement membranes of transgenic mice and its impact on other major GBM components and plasma membrane integrins, our concern was to evaluate the consequence of this lack on the overall glomerular filtration properties. We therefore revealed the distribution of circulating endogenous albumin through the glomerular wall using a specific antibody and the immunogold approach. Gold particles revealing mouse albumin antigenic sites were found over the flocculent material present in the capillary lumen and over the GBM in the animals of the three groups (Figure 7). In addition, the entactin-1-null homozygous animals displayed labeling in the urinary space (Figure 7C) and very intense labeling in the lysosomes of the glomerular epithelial cells (Figure 8A). Similarly, labeling for albumin was intense in lysosomal structures of the tubule eptithelial cells (Figure 8B). Labeling for albumin across the GBM differed among animals. In tissues of the wild-type animals, labeling for albumin was concentrated in the subendothelial side of the glomerular basement membrane, with few gold particles over the lamina densa, whereas in tissues of the two groups of transgenic mice the distribution of the labeling was rather homogeneous, the gold particles being present throughout the GBM. Morphometric analysis confirmed these observations (Figure 7). The mean “R” value for each group increased from the wild-type group to the heterozygous group and further to the homozygous transgenic group, reflecting a loss of the restricted filtration properties of the GBM in the transgenic animals. Statistical analysis of these distributions, using the Kruskal-Wallis and the Mann-Whitney U-tests, revealed significant differences among the three groups of animals. Here again, the control experiments confirmed the specificity of the albumin labeling as reported previously (Bendayan et al. 1986).
Figure 7.
Immunocytochemical localization of circulating endogenous albumin across the glomerular wall of the wild-type (A), heterozygous (C), and homozygous (E) transgenic mice. Gold particles are located in the capillary lumen (L) and across the GBM. (B,D,F) Distribution of labeling across GBM. The histograms demonstrate changes in these distributions. In the wild-type animals (B) Labeling is rather concentrated on the subendothelial side of the GBM. For the transgenic mice, heterozygous (D) as well as homozygous (F), the labelings display rather homogeneous distributions.
Discussion
The GBM plays key roles in the filtration properties of the glomerulus (Deen et al. 1983; Bendayan et al. 1986; Simpson and Shand 1986; Daniels et al. 1992). Present between the fenestrated endothelium and the foot processes of the epithelial cells, known as podocytes, it acts as a selective sieve by means of its architecture and negative charges, allowing small molecules and fluid to pass through and reach the urinary space (Brenner et al. 1978). In fact, molecules the size of albumin and larger are normally restricted by the GBM (Brenner et al. 1978; Deen et al. 1983; Bendayan et al. 1986; Daniels et al. 1992; Ghitescu et al. 1992; Adal et al. 1995). Several pathologies display alterations of this permselectivity property (Abrahamson 1986; Timpl and Dziadek 1986). Diabetes is probably the best example of this phenomenon. In fact, one of the main pathophysiological features of diabetic complications is the functional alteration of the blood capillary walls, so-called diabetic microangiopathy (Goode et al. 1995; Doucet et al. 2000), which results in increased vascular permeability to macro-molecules (Bendayan and Rasio 1981; Bendayan et al. 1986; Chittenden and Shami 1991; Yamaji et al. 1993; Arshi et al. 2000; Bouchard et al. 2002). The functional properties of the glomerular wall are directly related to the integrity and adequate molecular organization of the components of the GBM. Entactin-1, a sulfated glycoprotein, is present in all basement membranes as one of the major constituents, along with type IV collagen, laminin, and heparan sulfate proteoglycans (Carlin et al. 1981; Chung and Durkin 1990; Yurchenco and Schittny 1990; Paulsson 1992; Weber 1992; Chung et al. 1993; Timpl and Brown 1996). For the GBM, it was found to be distributed throughout the thickness of this extracellular matrix, with a certain concentration in the lamina lucida externa (Desjardins and Bendayan 1989; Katz et al. 1991). Entactin-1 appears to be important for the stabilization of several basement membrane components and should therefore be essential for the overall functional properties of the GBM (Aumailley et al. 1989,1993; Chung and Durkin 1990; Aumailley et al. 1993; Chung et al. 1993). Although type IV collagen and laminin form independent scaffolds, entactin-1 links these two scaffolds to generate a more stable network (Aumailley et al. 1989,1993; Miosge et al. 1999). Entactin-1 also mediates the formation of a complex between laminin and the proteoglycans (Aumailley et al. 1993). In fact it has been reported that type IV collagen, laminin, and proteoglycans bind to one another other through entactin-1 interactions, creating the three-dimensional network characteristic of the basement membranes (Chung and Durkin 1990; Aumailley et al. 1993).
Transgenic entactin-null animals have been recently generated to assess the role of this component in the formation of basement membranes (Murshed et al. 2000; Dong et al. 2002). Murshed and colleagues (2000) did not find modifications in basement membranes formation for the transgenic animals, while Dong and colleagues (2002) found rather subtle changes in particular basement membranes, i.e., those from the brain capillaries and the lens capsule. These two studies raised the possibility of a compensatory role for entactin-2, a protein related to entactin-1. Entactin-2 is present in most basement membranes and exhibits the same domains as entactin-1, but having different binding affinities with the major constituents of the basement membrane. However, a recent work has reported that entactin-2 is not essential for basement membrane formation or maintenance (Schymeinsky et al. 2002), although some findings revealed complementary actions of both entactins (Salmivirta et al. 2002).
The importance of entactin-1 in the overall architecture of the basement membrane prompted us to investigate the consequence of its absence on the conformation of the GBM and on the permselectivity properties of the glomerular wall. The approach consisted of the removal of this component from the tissues by genetic engineering followed by morphocytochemical studies of the glomerular wall (Dong et al. 2002). Immunoblotting together with immunocytochemistry at the light microscopic level and in situ hybridization have demonstrated that basement membranes in entactin-1-null mice effectively lack this protein (Dong et al. 2002). We confirmed by quantitative immunoelectron microscopy the absence of this protein in the GBM. Despite full agreement with Dong et al. (2002) of no major morphological alteration of basement membranes in the entactin-1-null mice, we decided to investigate the consequence of the removal of entactin-1 on the functional properties of the GBM. Our analysis made use of different and more sensitive cytochemical techniques. Furthermore, we assessed functional properties of the GBM. We found that entactin-1-null mice exhibit alterations in glomerular filtration. Absence of entactin-1 appears to be sufficient to modify the permselectivity of the glomerular wall. We know that entactin-1 bridges the two independent networks of laminin and type IV collagen, creating a stabilized basement membrane. We found that these two components retained their normal distribution in the GBM despite the absence of entactin-1. Alterations were also detected in the glomerular wall and concerned the thickness of the GBM, the distribution of the anionic charges, and the functional properties. Indeed, the GBM in tissues of entactin-1-null mice showed small but significant thickening and loss of anionic charges on the subendothelial side. The main alteration was the functional one, the glomerular wall being unable to retain albumin. However, despite this leakage of albumin across the glomerular wall, the entactin-1-null mice did not demonstrate any significant proteinuria. This indicates that the epithelial cells along the nephron are efficient enough to reabsorb the filtered albumin. In fact, as illustrated in Figure 8, the glomerular and the tubule epithelial cells of the entactin-1-null animals did exhibit increased number of lysosomes, which contain large amounts of albumin. Such results reflect important endocytotic reabsorptive activities along the nephron.
Integrins are a family of transmembrane receptors composed of α- and β-subunits that interact with components of the extracellular matrix. In the glomerular wall, the α3 and β1 isoforms are the most abundant and represent the main extracellular matrix receptors along the GBM (Kerjaschki et al. 1989; Regoli and Bendayan 1997), suggesting adhesion roles between endothelial as well as epithelial cells and the underly GBM (Adler 1992). This prompted us to investigate the effect of the lack of a major extracellular matrix component, entactin-1, known to be a ligand of the integrins (Dedhar et al. 1992; Dong et al. 1995; Wu et al. 1995; Yi et al. 1998), on the expression of two different integrin subunits by the endothelial and epithelial cells of the glomerular wall. Indeed, the αv-integrin increased along the plasma membrane of both the endothelial and epithelial cells in entactin-1-null mice. Knowing that interactions among components of the extracellular matrix and cell surface integrins mediate a variety of cellular responses, including cell adhesion, cell movement, and signal transduction, we can assume that lack of one ligand, i.e., entactin-1, in the extracellular matrix must affect cellular activities in neighboring cells through integrin relays. For the glomerular wall we can assume that changes in endothelial and epithelial cells might also contribute to the loss of filtration properties.
Acknowledgments
Supported by grants from the Canadian Institutes of Health Research and the Association Diabète Québec to M.B. and by the United States Public Services to A.E.C.
We thank Dr Irène Londoño for her appreciated assistance. This article represents part of the work required for the fulfillment of the M.Sc. program for S.P.L.
Literature Cited
- Abrahamson DR. (1986) Recent studies on the structure and pathology of basement membranes. J Pathol 149:257–278 [DOI] [PubMed] [Google Scholar]
- Adal Y, Smith MF, Osicka TM, Comper WD. (1995) Albumin interaction with the glomerular capillary wall in vitro . Kidney Int 47:1031–1038 [DOI] [PubMed] [Google Scholar]
- Adler S. (1992) Characterization of glomerular epithelial cell matrix receptors. Am J Pathol 141:571–578 [PMC free article] [PubMed] [Google Scholar]
- Arshi K, Bendayan M, Ghitescu LD. (2000) Alterations of the rat mesentery vasculature in experimental diabetes. Lab Invest 80:1171–1184 [DOI] [PubMed] [Google Scholar]
- Aumailley M, Battaglia C, Mayer U, Reinhardt D, Nischt R, Timpl R, Fox JW. (1993) Nidogen mediates the formation of ternary complexes of basement membrane components. Kidney Int 43:7–12 [DOI] [PubMed] [Google Scholar]
- Aumailley M, Wiedemann H, Mann K, Timpl R. (1989) Binding of nidogen and the laminin-nidogen complex to basement membrane collagen IV. Eur J Biochem 184:241–248 [DOI] [PubMed] [Google Scholar]
- Bendayan M. (1995) Colloidal gold-post-embedding immunocytochemistry. Prog Histochem Cytochem 29:1–159 [DOI] [PubMed] [Google Scholar]
- Bendayan M, Gingras D, Charest P. (1986) Distribution of endogenous albumin in the glomerular wall of streptozotocin-induced diabetic rats as revealed by high-resolution immunocytochemistry. Diabetologia 29:868–875 [DOI] [PubMed] [Google Scholar]
- Bendayan M, Rasio E. (1981) Hyperglycemia and microangiopathy in the eel. Diabetes 30:317–325 [DOI] [PubMed] [Google Scholar]
- Bouchard P, Ghitescu LD, Bendayan M. (2002) Morpho-functional studies of the blood-brain-barrier in streptozotocin-induced diabetic rats. Diabetologia 45:1017–1025 [DOI] [PubMed] [Google Scholar]
- Brenner BM, Hostetter TH, Humes HD. (1978) Glomerular permselectivity: barrier function based on discrimination of molecular size and charge. Am J Physiol Renal Physiol 234:455–460 [DOI] [PubMed] [Google Scholar]
- Carlin B, Jaffe R, Bender B, Chung AE. (1981) Entactin a novel basal lamina-associated sulfated glycoprotein. J Biol Chem 256:5209–5214 [PubMed] [Google Scholar]
- Chakravarti S, Tam MF, Chung AE. (1990) The basement membrane glycoprotein entactin promotes cell attachment and binds calcium ions. J Biol Chem 265:10597–10603 [PubMed] [Google Scholar]
- Chittenden SJ, Shami SK. (1991) Microangiopathy in the diabetes mellitus. Diabetes Res 17:105–114 [PubMed] [Google Scholar]
- Chung AE, Dong LJ, Wu C, Durkin ME. (1993) Biological functions of entactin. Kidney Int 43:13–19 [DOI] [PubMed] [Google Scholar]
- Chung AE, Durkin ME. (1990) Entactin: structure and function. Am J Respir Cell Mol Biol 3:275–282 [DOI] [PubMed] [Google Scholar]
- Chung AE, Freeman IL, Braginski JE. (1977) A novel extracellular membrane elaborated by a mouse embryonal carcinoma-derived cell line. Biochem Biophys Res Commun 79:859–868 [DOI] [PubMed] [Google Scholar]
- Daniels BS, Hauser EB, Deen WM, Hostetter TH. (1992) Glomerular basement membrane: in vitro studies of water and protein permeability. Am J Physiol 262:R919–926 [DOI] [PubMed] [Google Scholar]
- Dedhar S, Jewell K, Rojiani M, Gray V. (1992) The receptor for the basement membrane glycoprotein entactin is the integrin ά3/β1 . J Biol Chem 267:18908–18914 [PubMed] [Google Scholar]
- Deen WD, Bridges CR, Brenner BM. (1983) Biophysical basis of glomerular permselectivity. J Membr Biol 71:1–10 [DOI] [PubMed] [Google Scholar]
- Desjardins M, Bendayan M. (1989) Heterogeneous distribution of type IV collagen, entactin, heparan sulfate proteoglycans, and laminin among renal basement membranes as demonstrated by quantitative immunocytochemistry. J Histochem Cytochem 37:885–897 [DOI] [PubMed] [Google Scholar]
- Dong LJ, Chen Y, Lewis M, Hsieh JC, Reing J, Chaillet R, Howell K, et al. (2002) Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1. Lab Invest 82:1617–1630 [DOI] [PubMed] [Google Scholar]
- Dong LJ, Hsieh JC, Chung AE. (1995) Two distinct cell attachment sites in entactin are revealed by amino acid substitutions and deletion of the RGD sequence in the cystein-rich epidermal growth factor repeat 2. J Biol Chem 270:15838–15843 [DOI] [PubMed] [Google Scholar]
- Doucet M, Londoño I, Gomez—Pascual A, Bendayan M. (2000) Glomerular basement membrane selective permeability in short-term streptozotocin-induced diabetic rats. Int J Exp Diabetes Res 1:19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin ME, Chakravarti S, Bartos BB, Liu SH, Friedman RL, Chung AE. (1988) Amino acid sequence and domain structure of entactin. Homology with epidermal growth factor precursor and low density lipoprotein receptor. J Cell Biol 107:2749–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L, Desjardins M, Bendayan M. (1992) Immunocytochemical study of glomerular permeability to anionic, neutral and cationic albumins. Kidney Int 42:25–32 [DOI] [PubMed] [Google Scholar]
- Goode NP, Shires M, Crellin DM, Aparicio SR, Davison AM. (1995) Alterations of glomerular basement membrane charge and structure in diabetic nephropathy. Diabetologia 38:1455–1465 [DOI] [PubMed] [Google Scholar]
- Gresham HD, Graham IL, Griffin GL, Hsieh JC, Dong L-J, Chung AE, Senior RM. (1996) Domain-specific interactions between entactin and neutrophil integrins. J Biol Chem 48:30587–30594 [DOI] [PubMed] [Google Scholar]
- Katz A, Fish AJ, Kleppel MM, Hagen SG, Michael AF, Butkowski RJ. (1991) Renal entactin (nidogen): isolation, characterization and tissue distribution. Kidney Int 40:643–652 [DOI] [PubMed] [Google Scholar]
- Kerjaschki D, Ojha PP, Susani M, Horvat R, Binder S, Hovorka A, Hillemanns P, et al. (1989) A beta 1-integrin receptor for fibronectin in human kidney glomeruli. Am J Pathol 134:481–489 [PMC free article] [PubMed] [Google Scholar]
- Londoño I, Gingras D, Bendayan M. (in press) Circulating glycated albumin and glomerular anionic charges. Exp Diabetes Res. [DOI] [PMC free article] [PubMed]
- Mann K, Deutzmann R, Aumailley M. (1989) Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO J 8:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Kohfeldt E, Timpl R. (1998) Structural and genetic analysis of laminin-nidogen interaction. Ann NY Acad Sci 857:130–142 [DOI] [PubMed] [Google Scholar]
- Miosge N, Heinemann S, Leissling A, Klenczar C, Herken R. (1999) Ultrastructural triple localization of laminin-1, nidogen-1, and collagen type IV helps elucidate basement membrane structure in vivo. Anat Rec 254:382–388 [DOI] [PubMed] [Google Scholar]
- Miosge N, Sasaki T, Timpl R. (2002) Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol 21:611–621 [DOI] [PubMed] [Google Scholar]
- Murshed M, Smyth N, Miosge N, Karolat J, Krieg T, Paulsson M, Nischt R. (2000) The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol 20:7007–7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M. (1988) The role of Ca2+ binding in the self-aggregation of laminin-nidogen complexes. J Biol Chem 263:5425–5430 [PubMed] [Google Scholar]
- Paulsson M. (1992) Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol 27:93–127 [DOI] [PubMed] [Google Scholar]
- Regoli M, Bendayan M. (1997) Alterations in the expression of the ά3/β1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia 40:15–22 [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. (1991) Integrins. J Clin Invest 87:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo P, Gingras D, Bendayan M. (1993) Poly-L-lysine-gold probe for the detection of anionic sites in normal glomeruli and in idiopathic and experimentally induced nephrosis. A comparative ultrastructural study. Am J Pathol 142:261–271 [PMC free article] [PubMed] [Google Scholar]
- Salmivirta K, Talts JF, Olsson M, Sasaki T, Timpl R, Ekblom P. (2002) Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Exp Cell Res 279:188–201 [DOI] [PubMed] [Google Scholar]
- Schymeinsky J, Nedbal S, Miosge N, Pöschl E, Rao C, Beier DR, Skarnes WC, et al. (2002) Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement formation in mice. Mol Cell Biol 22:6820–6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior RM, Gresham HD, Griffin GL, Brown EJ, Chung AE. (1992) Entactin stimulates neutrophil adhesion and chemotaxis through interactions between its Arg-Gly-Asp (RGD) domain and the leukocyte response integrin. J Clin Invest 90:2251–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson LO, Shand BO. (1986) Glomerular permeability. Clin Sci 71:221–223 [DOI] [PubMed] [Google Scholar]
- Skutelsky E, Roth J. (1986) Cationic colloidal gold—a new probe for the detection of anionic cell surface sites by electron microscopy. J Histochem Cytochem 34:693–696 [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. (1996) Supramolecular assembly of basement membranes. BioEssays 18:123–132 [DOI] [PubMed] [Google Scholar]
- Timpl R, Dziadek M. (1986) Structure, development, and molecular pathology of basement membranes. Int Rev Exp Pathol 29:1–111 [PubMed] [Google Scholar]
- Timpl R, Dziadek M, Fujiwara S, Nowack H, Wick G. (1983) Nidogen: a new self-aggregating basement membrane protein. Eur J Biochem 137:455–465 [DOI] [PubMed] [Google Scholar]
- Weber M. (1992) Basement membrane proteins. Kidney Int 41:620–628 [DOI] [PubMed] [Google Scholar]
- Wu C, Chung AE. (1991) Potential role of entactin in hemostasis. J Biol Chem 266:18802–18807 [PubMed] [Google Scholar]
- Wu C, Chung AE, McDonald JA. (1995) A novel role for ά3/β1 integrins in extracellular matrix assembly. J Cell Sci 108:2511–2523 [DOI] [PubMed] [Google Scholar]
- Yamaji T, Fukuhara T, Kinoshita M. (1993) Increased capillary permeability to albumin in diabetic rat myocardium. Circ Res 72:947–957 [DOI] [PubMed] [Google Scholar]
- Yang Y, Todt JC, Svinarich DM, Qureshi F, Jacques SM, Graham CH, Chung AE, et al. (1996) Human trophoblast cell adhesion to extracellular matrix protein, entactin. Am J Reprod Immunol 36:25–32 [DOI] [PubMed] [Google Scholar]
- Yi XY, Wayner EA, Kim Y, Fish AJ. (1998) Adhesion of cultured human kidney mesangial cells to native entactin: role of integrin receptors. Cell Adhes Commun 5:237–248 [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Schittny JC. (1990) Molecular architecture of basement membranes. FASEB J 4:1577–1590 [DOI] [PubMed] [Google Scholar]