Abstract
The human endolymphatic duct (ED) and sac of the inner ear have been suggested to control endolymph volume and pressure. However, the physiological mechanisms for these processes remain obscure. We investigated the organization of the periductal interstitial connective tissue cells and extracellular matrix (ECM) in four freshly fixed human EDs by transmission electron microscopy and by immunohistochemistry. The unique surgical material allowed a greatly improved structural and epitopic preservation of tissue. Periductal connective tissue cells formed frequent intercellular contacts and focally occurring electron-dense contacts to ECM structures, creating a complex tissue network. The connective tissue cells also formed contacts with the basal lamina of the ED epithelium and the bone matrix, connecting the ED with the surrounding bone of the vestibular aqueduct. The interstitial connective tissue cells were non-endothelial and non-smooth muscle fibroblastoid cells. We suggest that the ED tissue network forms a functional mechanical entity that takes part in the control of inner ear fluid pressure and endolymph resorption.
Keywords: basal lamina, intercellular adhesion, cell-ECM contacts, interstitial fluid pressure, Ménière's disease
The human endolymphatic duct (ED) and sac (ES), together with the cochlea and the vestibular organs, constitute the membranous labyrinth of the inner ear. The membranous labyrinth with the organ of hearing and balance forms an extracellular compartment containing a unique fluid, the endolymph. The ED forms an epithelial tube coursing in the vestibular aqueduct of the petrous bone, connecting the ES with the rest of the membranous labyrinth. The vestibular aqueduct is surrounded by many small bone channels, many containing blood vessels, as well as the accessory canal with the vein of the vestibular aqueduct (Ogura and Clemis 1971; Wilbrand et al. 1974; Rask-Andersen et al. 1981b). This vein empties its blood into the intracranial venous sinus. The ED, with its single-layer epithelium is embedded in a loose interstitial connective tissue (CT). The epithelium consists of flattened cuboidal or columnar cells with apical microvilli and extensive basal infoldings covered by a basal lamina (Rask-Andersen et al. 1981b; Friberg et al. 1984). In the human periductal CT, a few capillaries but no arteries, arterioles, muscular veins, or true lymph vessels have been found (Wackym et al. 1986).
Several findings support the theory that the ED and ES are important for the regulation of endolymph pressure and volume (Kimura and Schuknecht 1965; Rask-Andersen et al. 2000; Salt and DeMott 2000). It is believed that the pressure is balanced by high compliance of the “walls” of these structures and the volume to be controlled by both regulation of endolymph resorption and secretion. The ES has been suggested to be mainly phagocytotic, acting as a local organ involved in the immune defense of the inner ear, and to be involved in the degradation of waste products (Rask-Andersen and Stahle 1980; Tomiyama and Harris 1986; Altermatt et al. 1990). The ED, on the other hand, is believed to be responsible for the majority of resorption of water and solutes and equilibration of ions of the endolymph (Rask-Andersen et al. 1981a; Bagger-Sjöbäck and Rask-Andersen 1986; Wackym et al. 1986).
In this investigation, human ED tissues were serially sectioned for analysis by light and transmission electron microscopy to establish a morphological background for the potential physiological functions of the interstitial connective tissue surrounding the ED. We focused on the structure of the periductal CT and on relationships between CT cells and the local extracellular matrix (ECM). In addition, immunohistochemical (IHC) stainings were carried out to characterize the periductal CT cells.
Materials and Methods
Patient Material
Four human endolymphatic ducts were dissected out during skull base surgery on patients suffering from meningioma. Ethical approval for the procedure and the use of the human ED biopses was given by an institutional committee on human experimentation. During the translabyrinthine procedure the EDs, together with a small portion of bone, were obtained by use of a diamond burr. The structure was fixed directly in 3% buffered glutaraldehyde for TEM or in 4% paraformaldehyde for IHC stainings. The tissues were decalcified in 0.1 M Na-EDTA for 2 weeks.
Light and Transmission Electron Microscopy
The tissues were rinsed in saline, stained with 1% osmium tetroxide, and finally dehydrated in graded ethanols and embedded in Epon for semithin and thin sections. One ED was cut transversely from the vestibular orifice and every section was mounted on a coverglass and stained with toluidine blue. At every tenth μm a thicker section was taken and mounted on an Epon plastic column for further thin sectioning. Thin sections were cut with a diamond knife, attached to one-hole grids, and stained with uranyl acetate and lead citrate. The thin sections were viewed in a Jeol 100 SX electron microscope. Specimens were analyzed by both light and transmission electron microscopy at regular intervals throughout the entire length of the EDs.
Immunohistochemical Stainings
The decalcified ED tissues were frozen in liquid nitrogen and cut transversely in 6-μm sections with a cryostat. The IHC staining was carried out according to standard protocols (Sundberg et al. 2001). Briefly, nonspecific binding was blocked with 20% non-immune serum from the secondary antibody species in PBS supplemented with 0.2% bovine serum albumin (BSA) and 0.1% Tween-20 for 30 min. The sections were incubated with primary antibodies diluted in PBS with 0.2% BSA and 0.1% Tween-20 and supplemented with 4% of non-immune serum for 30 min. The anti-human vimentin (clone Vim3B4, dilution 1:400), the anti-human desmin (1:100), the anti-human macrophage CD68 (1:100), the anti-human fibroblast (clone 5B5, 1:200), and the anti-human CD31 endothelial cell (1:100) monoclonal antibodies were purchased from DAKO (Glostrup, Denmark). The anti-human α-smooth muscle actin (1:50) and the anti-pancytokeratin (1:400) monoclonal antibodies were purchased from Sigma-Aldrich (St Louis, MO). The anti-human fibro-blast-specific antibody AS02 (1:600) was purchased from Dianova (Hamburg, Germany). After washing twice with PBS with 0.2% Tween-20 and once with PBS, the sections were incubated for 30 minutes with biotinylated F(ab')2 fragments of rabbit anti-mouse and swine anti-rabbit immunoglobulins, respectively, diluted 1:250 (DAKO) and then washed again. The staining was performed using the avidin-biotin complex method (Vectastain ABC-elite kit; Vector, Burlingame, CA) with diaminobenzidine (DAB substrate kit; Zymed, S. San Francisco, CA) as the peroxidase substrate. Sections were counterstained with Mayer's hematoxylin, dehydrated in graded ethanols, and mounted in Entellan.
Results
General Morphology of the Periductal Connective Tissue
The use of directly fixed surgical material instead of postmortem material greatly improved EM preservation of the tissue. The cells showed less vacuolation and organellar structures appeared more distinct and preserved. In addition, the ECM showed a more homogeneous texture with fewer empty areas and less vacuolation.
The periductal connective tissue was of the loose interstitial type, containing collagen fiber bundles (Figure 1 and 2D). In the subepithelial area, particularly in the mid and distal parts of the ED where the stretched epithelium formed protuberances into the lumen of the duct, the CT was very loose and CT cells were sparse (Figure 1C). The collagen fibers appeared in bundles, which ran in several directions but mostly parallel to the duct (Figure 2B and 2C). The CT cells formed a network via cytoplasmic branches, which ramified and connected to other cell ramifications via small membrane densifications (Figures 2A-2D and 2F). Each individual CT cell therefore made contact with three or four other CT cells in the 2D pictures (Figure 2A and 2B). The tissue cells also formed electron-dense contacts with adjacent collagen fiber bundles or other ECM components, forming a network between cells and ECM fibers (Figure 2D and 2E). In addition, the cytoplasmic processes of the CT cells often formed direct physical connections to the basal lamina underlying the epithelial cells (Figure 3) and to the bone matrix of the surrounding vestibular aqueduct (Figure 4F).
Figure 1.
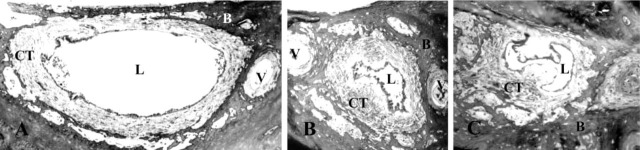
Light microscopy of the human endolymphatic duct (ED) at three different intervals: proximal (A), intermediate (B), and distal (C) ED. The specimen was cut transversely and the light microscopy pictures show the vestibular aqueduct with the ED embedded in loose connective tissue. Note the veins of the vestibular aqueduct and the many bone channels containing blood vessels embedded in loose connective tissue. L, lumen of the ED; CT, connective tissue; B, bone matrix; and V, vein of the vestibular aqueduct.
Figure 2.
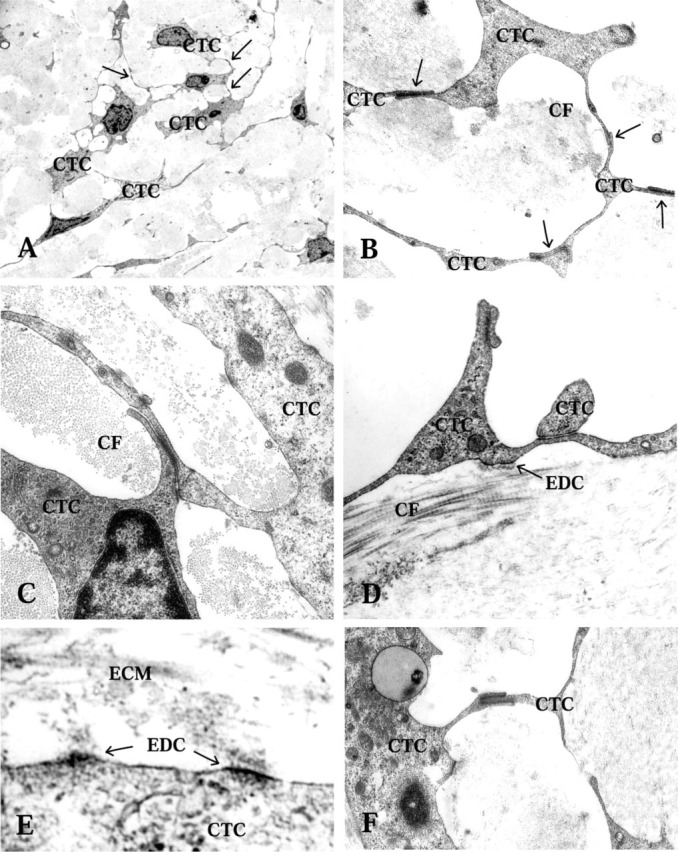
Transmission electron microscopy of CT cells (CTC), intercellular contacts, and electron-dense contacts (EDC) in the periductal CT. (A,B) CTC intercellular contacts are shown with arrows. Collagen fiber bundles (CF) mostly cut transversly between the CTC network. (C) Two juxtapositioned CTCs with an intercellular contact. (D) Collagen fibers (CF) with typical transmission electron microscopy appearance. Note the electron-dense contact between the ECM and the CTC. (E) Electron-dense contacts between CTC and ECM are seen. (F) A CTC cytoplasmic finger-like indentation is seen, with membrane densifications along the adhesion zone.
Figure 3.
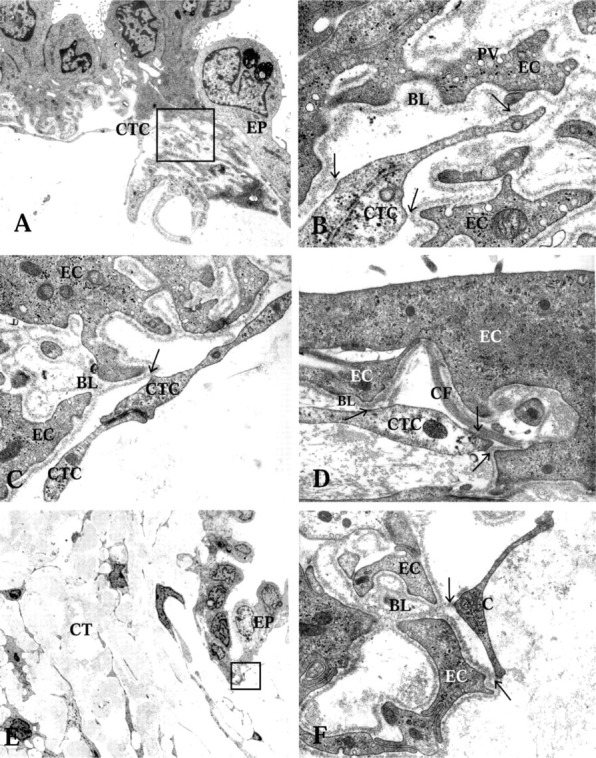
Transmission electron microscopy showing connections between the ED epithelium, the CT cells (CTC), and the ECM. (A) Rugose part of the ED epithelium (EP), showing electron-dense and electron-translucent cells and their extensive basal infoldings. Note the CT cell in close vicinity to the basal aspect of the epithelium. Arrows show physical contacts between the CT cells and the basal lamina. Framed area highlighted in B. (B) High-power transmission electron microscopy of CT cell and basal lamina contact spots. Micro-pinocytotic vesicles (PV) are frequent near the cell membrane of the epithelial cells. (C) High-power transmission electron microscopy of a CT cell and epithelial cell contact. Note the CT cells' intercellular contact. (D) The CT cell and epithelial cell contacts are shown with arrows. Note the collagen fiber bundle (CF) just beneath the epithelium in close contact with the basal lamina (BL). (E) Close relationship between the CT cells and the epithelium. Framed area highlighted in F. Arrows showing CT cell and epithelial cell (EC) contacts.
Figure 4.
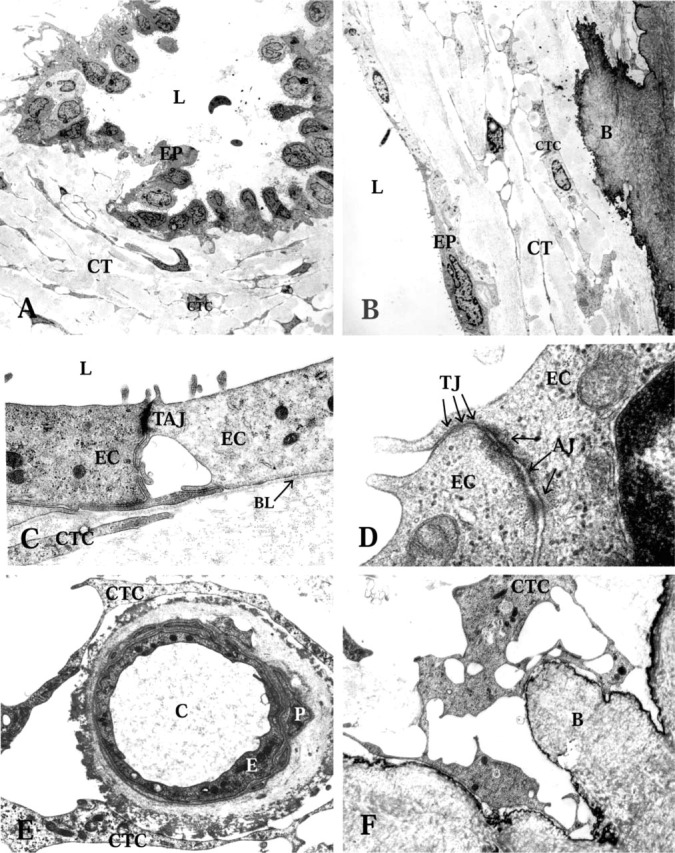
Transmission electron microscopy of the endolymphatic duct (ED) epithelium and the periductal connective tissue (CT). EP, ED epithelium; L, lumen of the ED; CTC, connective tissue cells. (A) Folded epithelium with basal cell processes extending into the surrounding CT, in the intermediate part of the human ED. (B) Flat epithelium with underlying CT in the proximal part of the human ED. Note the interconnecting CT cells of the periductal tissue. B, bone matrix. (C) Flat epithelium with apical microvilli (MV), and intercellular tight and adherens junctions (TAJ). EC, epithelial cell; BL, basal lamina. (D) High-power transmission electron microscopy of intercellular contacts between the epithelial cells. TJ, tight junctions; arrows show three junctional strands; AJ, adherens junctions. (E) A capillary of the periductal CT. E, endothelial cell; P, pericyte; C, lumen of capillary. (F) A CT cell making contacts with the bone matrix (B) and adjacent cell.
The periductal CT contained few capillaries. The CT cells formed frequent contacts with the outer matrix of the capillary wall (Figure 4E). No lymph vessels or larger blood vessels could be observed. However, several bone channels harboring blood vessels were observed near the vestibular aqueduct and the ED (Figure 1). These vessels were surrounded by a loose CT, which at different levels formed a continuum with the periductal CT.
General Morphology of the ED Epithelium
All four EDs were funnel-shaped and wide in the proximal portion near the vestibular orifice (Figure 1A). The ED narrowed continuously until it was transformed into the ES (Figure 1C), in agreement with earlier observations. In the proximal portion, the ED consisted mostly of a flat, thin epithelial layer (Figure 1A and 4B). However some cuboidal or low columnar epithelial cell types could be found (Figures 1A and 4A). In the mid and distal parts of the duct, most of the epithelial cells consisted of cuboidal or low columnar epithelial cells (Figure 1B and 1C). From the mid to the distal part of the duct, the epithelium also showed some large polyp-like formations. At certain sites these formations took up most of the luminal surface and the epithelial cells appeared stretched or were flattened (Figure 1C). The epithelial cells were interconnected by tight junctions and adherens junctions (Figure 4C). The tight junctions were of the shallow type, with only one to four parallel junctional strands (Figure 4D), in agreement with results from studies on the ES (Bagger-Sjöbäck and Rask-Andersen 1986). In the zonula adherens, typical desmosomes were present (Figure 4D). The columnar cells were electron-translucent or electrondense, as described earlier (Wackym et al. 1986). Both cell types had extensive basal infoldings covered by a basal lamina (Figure 3A and 3B). The epithelial cell membrane facing the basal lamina contained many micro-pinocytotic vesicles (Figure 3B). Long, slender basal cytoplasmic epithelial cell protrusions formed deep projections into the surrounding loose CT. These protrusions were always covered by a basal lamina.
Immunohistochemical Stainings of the Periductal CT Cells and the ECM
The CT cells, as well as the endothelium of large vessels in the bone channels, were vimentin-positive (Figure 5A; Table 1). The endothelial cells showed stronger homogeneous vimentin staining, whereas the CT cells displayed a granular staining pattern. In addition, some but not all epithelial cells were vimentin-positive, with a staining pattern varying from homogeneous to granular. The endothelial cells of the periductal capillaries and larger vessels in the neighboring bone channels were CD31-positive (Figure 5B; Table 1). The CT cells and the ED epithelium were negative for α-smooth muscle actin and desmin antibodies (Table 1). Both the CT cells and the ED epithelial cells were stained positive by the anti-human fibroblast antibody (Figure 5C; Table 1). Again, the CT cells presented a more granular staining pattern. The anti-human fibroblast-specific antibody showed a strong homogeneous staining of a majority of the ductal epithelial cells and a weaker staining of some but not all CT cells (Figure 5D; Table 1). The epithelial cells exclusively were stained positive with the anti-pan-cytokeratin antibody (Figure 5E; Table 1). A few cells in the periductal tissue and in the CT surrounding the vessels of the bone channels were stained specifically with the macrophage CD68 antibody (Figure 5F; Table 1). Positive cells were found in immediate contact with the epithelium as well as in the larger veins. Control experiments with secondary antibodies alone and normal mouse serum were negative (data not shown). Positive controls of the anti-fibroblast antibodies on human skin showed no staining of the epidermis but specific staining of fibroblasts in dermis (data not shown).
Figure 5.
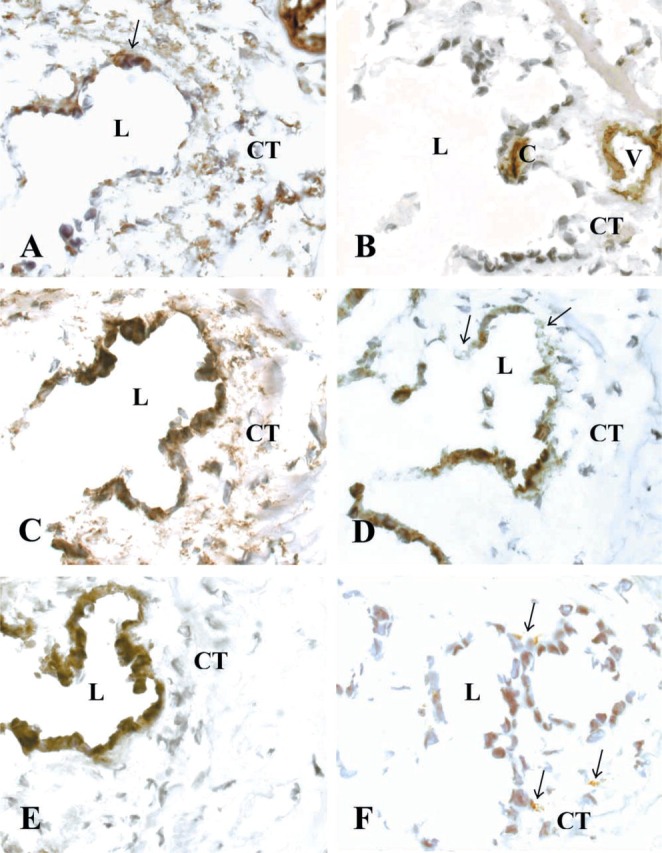
Immunohistochemical stainings of the transversely cut human ED with surrounding interstitial CT. L, lumen of the ED; CT, periductal connective tissue; V, vein of the vestibular aqueduct; and C, capillary. Cryostat sections were stained with (A) anti-human vimentin (arrows show positive epithelial cells), (B) anti-human CD31 endothelial cell, (C) anti-human fibroblast (clone 5B5), (D) anti-human fibroblast-specific AS02 (arrow shows negative epithelial cells), (E) anti-pan cytokeratin, and (F) anti-human macrophage CD68 antibodies (arrows show positive cells).
Table 1.
Immunohistochemical staining of the human endolymphatic duct a
| Antibodies | Epithelial cells | Connective tissue cells | Endothelial cells | Macrophages |
|---|---|---|---|---|
| Vimentin | + | ++ | ++ | − |
| CD31 endothelia | − | − | ++ | − |
| CD68 macrophage | − | − | − | + |
| Pan-cytokeratin | ++ | − | − | − |
| Fibroblast (AS02) | + | + | − | − |
| Fibroblast (5B5) | ++ | ++ | − | − |
| α-Smooth muscle actin | − | − | − | − |
| Desmin | − | − | − | − |
Immunohistochemical staining of the human ED with the surrounding interstitial connective tissue, using antibodies against cell type markers. ++, a majority of cells of the listed cell types were stained positive; +, some but not all of the cells were positive; -, cells were negative.
Discussion
We demonstrate that the interstitial connective tissue (CT) cells surrounding the human ED make morphologically discernable intercellular contacts, in addition to the electron-dense contacts between CT cell processes and ECM fibers. It has been previously reported that human fibroblasts in the dermis form functional gap junctions (Salomon et al. 1988). The electrondense contacts resemble morphologically close contacts or focal adhesions, characteristically seen at the basal side of cultured fibroblasts (Chen and Singer 1982). Furthermore, we show that this interstitial connective tissue network makes direct contact with the basal lamina of the ED epithelium and with the surrounding bone matrix of the vestibular aqueduct, forming a continuous tissue network. The connective tissue network probably helps to keep the ED lumen open. In addition, this organization is compatible with a function of the network to control tissue swelling.
In this study we show that the interstitial CT cells are non-endothelial, non-smooth muscle mesenchymal cells, based on the fact that they stained negative for the vascular endothelial cell marker CD31, α-smooth muscle actin, and desmin, but were vimentin-positive. Vimentin is an intermediate filament protein present in cells of mesenchymal origin. Furthermore, based on the fact that CT cells were stained positive by the fibroblast-specific (AS01) antibody and anti-fibroblast antibody (clone 5B5), it is reasonable to conclude that these cells are fibroblastoid cells. The anti-human fibroblast antibody reacts specifically with prolyl 4-hydroxylase, an enzyme of collagen synthesis, in CT cells and myoepithelial cells. The anti-human fibro blast-specific antibody recognizes fibroblasts of different origin but also weakly stains kidney tubule epithelial cells (Saalbach et al. 1996).
The ED epithelium appears to consist of different cell types because the anti-vimentin and fibroblast-specific antibodies stained some but not all epithelial cells. This is consistent with findings with transmission electron microscopy, in which the columnar epithelial cells have an electron-translucent or electrondense appearance. The vimentin-positive epithelial cell type appears to be of mesenchymal origin because it contains vimentin intermediate filaments. Kidney tubule epithelium develops from mesenchyme (Ekblom 1989). It may be that one or more cell types of the ED epithelia are derived from a similar mesenchyme-to-epithelium transition. Both kidney tubule epithelia and ED epithelia are important for water and ion regulation. Bauwens et al. (1991) found co-expression of vimentin and cytokeratin in the epithelium of the human ED and ES, probably reflecting a dual origin of the cells constituiting the epithelium of the endolymphatic duct and sac. These data further support our findings.
Available data suggest that there is an electrolyte shift along the length of the ED, because the ES endolymph, unlike that of the inner ear, contains a high concentration of sodium but is low in potassium (Miyamoto and Morgenstern 1979). The transepithelial flow of water is probably driven by the osmotic potential formed by such an active equilibration of ions. The epithelium of the human ED, with its conspicuous polarity, probably helps to maintain a proper ion balance between the endolymph and the interstitium. Na+/K+-ATPase has earlier been localized in the epithelium of the ES in guinea pig but only at low concentration in the guinea pig ED (Ichiyama et al. 1994). However, the structure of the epithelial cells, with the extensive basal infoldings, suggests the presence of an active metabolic mechanism for equilibration of electrolytes at this site. The gradual change in epithelial structure along the length of the ED may indicate that more passive mechanisms are initially involved in the transepithelial flow, whereas distally active mechanisms may be more important.
New concepts in physiology suggest that loose interstitial connective tissue, with its fibroblasts and pericytes, actively and dynamically controls interstitial fluid pressure and thereby tissue fluid volume (Rubin et al. 1996; Reed et al. 2001; Wiig et al. 2003). It is tempting to speculate that the ED connective tissue network, via intercellular and cell-ECM contacts, participates in the control of interstitial fluid pressure in the periductal tissue (Figure 6). A pressure gradient may be necessary for the transport of water and solutes from the ED lumen, through the CT, and into the draining veins of the neighboring bone channels. A slow flow from the membranous labyrinth to the intracranial veins through the CT network may form an important pathway for both regulation of inner ear pressure and endolymph outflow. In addition, there are still controversies concerning the degree of longitudinal flow under normal and pathological conditions (Salt 2001). Under normal conditions, the flow appears to be nonexistent or extremely low. On the other hand, under pathological conditions leading to increased pressure and volume of endolymph, this flow may be substantial.
Figure 6.
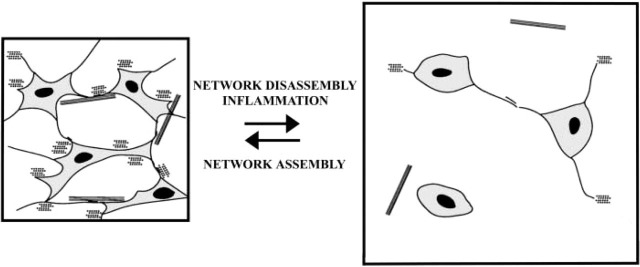
Schematic drawing of a model of the interstitial connective tissue network function and control of tissue swelling. According to this model, the extensive tissue network, formed by CT cells' intercellular contacts and CT cell contacts with the ECM, helps to control interstital fluid pressure by restraining the intrinsic swelling tendency of the ground substance. Inflammation will lead to disorganization of the network structure, probably by affecting the cell-cell and cell-ECM contacts (see Berg et al. 1998) and causing edema to form. Subsequently, the interstitial fluid pressure will increase and lead to impaired endolymph outflow. Eventually endolymphatic hydrops may develop, as seen in patients with Ménière's disease.
Several data support the idea that if the ED and the periductal CT are subjected to an inflammatory process, the normal resorption or outflow of endolymph will be disturbed, and endolymphatic hydrops will develop. Transmission electron microscopy studies on the ES of a patient with active Ménière's disease showed a high number of lymphoid cells in the ES epithelium and in the perisaccular CT, indicating an ongoing inflammatory process (Danckwardt-Lillieström et al. 1997). Patients with intracranial sinus thromboses occasionally develop fluctuating low-tone hearing loss suggestive of endolymphatic hydrops (Jonsson et al. in press). This suggests that altered hemodynamics may contribute to the pathogenesis of endolymphatic hydrops, possibly through a disturbed natural outflow of endolymph (Friberg and Rask-Andersen 2002). Impaired function of the flow- and pressure-regulating periductal interstitial CT might play a role in the etiology of Ménière's disease. It would be desirable to reach the periductal CT with CT-modulating substances to treat the possible cause of the disease.
Acknowledgments
Supported by the Swedish Research Council (3908), the Swedish Cancer Foundation, and Gustaf V's 80-year Foundation.
Literature Cited
- Altermatt HJ, Gebbers JO, Muller C, Arnold W, Laissue JA. (1990) Human endolymphatic sac: evidence for a role in inner ear immune defence. ORL J Otorhinolaryngol Relat Spec 52:143–148 [DOI] [PubMed] [Google Scholar]
- Bagger-Sjöbäck D, Rask-Andersen H. (1986) The permeability barrier of the endolymphatic sac. A hypothesis of fluid and electrolyte exchange based on freeze fracturing. Am J Otol 7:134–140 [PubMed] [Google Scholar]
- Bauwens LJ, De Groot JC, Ramaekers FC, Linthicum F, Veldman JE, Huizing EH. (1991) Differential immunohistochemical detection of cytokeratins and vimentin in the surgically removed human endolymphatic duct and sac. Eur Arch Otorhinolaryngol 248:495–501 [DOI] [PubMed] [Google Scholar]
- Berg A, Ekwall A-KH, Rubin K, Stjernschantz J, Reed RK. (1998) Effect of PGE1, PGI2, and PGF2 alpha analogs on collagen gel compaction in vitro and interstitial pressure in vivo. Am J Physiol 274:H663–671 [DOI] [PubMed] [Google Scholar]
- Chen WT, Singer SJ. (1982) Immunoelectron microscopic studies of the sites of cell-substratum and cell-cell contacts in cultured fibroblasts. J Cell Biol 95:205–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danckwardt-Lillieström N, Friberg U, Kinnefors A, Rask-Andersen H. (1997) “Endolymphatic sacitis” in a case of active Meniere's disease. An ultrastructural histopathologic investigation. Ann Otol Rhinol Laryngol 106:190–198 [DOI] [PubMed] [Google Scholar]
- Ekblom P. (1989) Developmentally regulated conversion of mesenchyme to epithelium. FASEB J 3:2141–2150 [DOI] [PubMed] [Google Scholar]
- Friberg U, Rask-Andersen H, Bagger-Sjöbäck D. (1984) Human endolymphatic duct. An ultrastructural study. Arch Otolaryngol 110:421–428 [DOI] [PubMed] [Google Scholar]
- Friberg U, Rask-Andersen H. (2002) Vascular occlusion in the endolymphatic sac in Meniere's disease. Ann Otol Rhinol Laryngol 111:237–245 [DOI] [PubMed] [Google Scholar]
- Ichiyama I, Adams JC, Kimura RS. (1994) Immunolocalization of Na+/K+-ATPase, Ca2 + -ATPase, calcium-binding proteins and carbonic anhydrase in the guinea pig inner ear. Acta Otolaryngol 114:167–176 [DOI] [PubMed] [Google Scholar]
- Jonsson LÅ, Åström G, Rask-Andersen H. (in press) Sinus thrombosis and labyrinthine dysfunction. A case report.
- Kimura RR, Schuknecht HF. (1965) Membranous hydrops in the inner ear of the guinea pig after obliteration of the endolymphatic sac. Pract Otol Rhinol Laryngol 27:343–354 [Google Scholar]
- Miyamoto H, Morgenstern C. (1979) Potassium level in endolymphatic sac of guinea pigs in vivo. Arch Otorhinolaryngol 222:77–78 [DOI] [PubMed] [Google Scholar]
- Ogura Y, Clemis JD. (1971) A study of the gross anatomy of the human vestibular aqueduct. Ann Otol Rhinol Laryngol 80:813–825 [DOI] [PubMed] [Google Scholar]
- Rask—Andersen H, Bredberg G, Lyttkens L, Lööf G. (1981a) The function of the endolymphatic duct—an experimental study using ionic lanthanum as a tracer: a preliminary report. Ann NY Acad Sci 374:11–19 [DOI] [PubMed] [Google Scholar]
- Rask—Andersen H, Bredberg G, Stahle J. (1981b) Structure and function of the endolymphatic duct. In Vosteen K—H, Schuknecht H, Pfaltz CR, Wersall J, Kimura RS, Morgenstern C, Juhn SK, eds. Méniére's Disease: Pathogenesis, Diagnosis and Treatment. International Symposium, Dusseldorf, New York, George Thieme-Verlag, 99–109 [Google Scholar]
- Rask—Andersen H, Kinnefors A, Salt A, Demott JE, Illing R-B. (2000) Homeostatic mechanisms of the inner ear fluids. In Ménière's disease 1999. Proceedings of the 4th International Symposium on Ménière's Disease. Paris, The Hague France Kugler Publications, 59–73 [Google Scholar]
- Rask—Andersen H, Stahle J. (1980) Immunodefence of the inner ear?. Lymphocyte-macrophage interaction in the endolymphatic sac. Acta Otolaryngol 89:283–294 [DOI] [PubMed] [Google Scholar]
- Reed RK, Berg A, Gjerde EA, Rubin K. (2001) Control of interstitial fluid pressure: role of beta1-integrins. Semin Nephrol 21:222–230 [DOI] [PubMed] [Google Scholar]
- Rubin K, Gullberg D, Tomasini—Johansson B, Reed RK, Rydén C, Borg TK. (1996) Molecular recognition of the extracellular matrix by cell surface receptors. In Comper WD, ed. Extracellular Matrix. Molecular Components and Interactions. Vol 2 Reading, UK, Harwood Academic Publishers, 262–309 [Google Scholar]
- Saalbach A, Aneregg U, Bruns M, Schnabel E, Herrmann K, Haustein UF. (1996) Novel fibroblast-specific monoclonal antibodies: properties and specificities. J Invest Dermatol 106:1314–1319 [DOI] [PubMed] [Google Scholar]
- Salomon D, Saurat JH, Meda P. (1988) Cell-to-cell communication within intact human skin. J Clin Invest 82:248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt AN. (2001) Regulation of endolymphatic fluid volume. Ann NY Acad Sci 942:306–312 [DOI] [PubMed] [Google Scholar]
- Salt AN, DeMott JE. (2000) Ionic and potential changes of the endolymphatic sac induced by endolymph volume changes. Hear Res 149:46–54 [DOI] [PubMed] [Google Scholar]
- Sundberg C, Nagy JA, Brown LF, Feng D, Eckelhoefer IA, Manseau EJ, et al. (2001) Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol 158:1145–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama S, Harris JP. (1986) The endolymphatic sac: its importance in inner ear immune responses. Laryngoscope 96:685–691 [DOI] [PubMed] [Google Scholar]
- Wackym PA, Friberg U, Bagger—Sjöbäck D, Rask—Andersen H. (1986) Human endolymphatic duct: possible mechanisms of endolymph outflow. Ann Otol Rhinol Laryngol 95:409–414 [DOI] [PubMed] [Google Scholar]
- Wiig H, Rubin K, Reed RK. (2003) New and active role of the interstitium in control of interstitial fluid pressure: potential therapeutic consequences. Acta Anaesthesiol Scand 47:111–121 [DOI] [PubMed] [Google Scholar]
- Wilbrand HF, Rask—Andersen H, Gilstring D. (1974) The vestibular aqueduct and the paravestibular canal. An anatomic and roentgenologic investigation. Acta Radiol Diagn (Stock) 15:337–355 [DOI] [PubMed] [Google Scholar]


