Abstract
This study was undertaken to clarify the controversy in the literature about pancreatic localization of the cholecystokinin (CCK) CCKA and CCKB receptors. With antibodies used by other investigators, we first established their specificity by Western blotting, indirect immunofluorescence, and confocal microscopy with each antibody's peptide antigen. Co-localization assays between the CCK receptors and the pancreatic hormones insulin, glucagon, and somatostatin revealed that the CCKA RAbs 1122 and R1-2 recognized insulin and glucagon cells in rat, pig, and human pancreas but not in the somatostatin cells. Conversely, the three CCKB RAbs tested, 9262, 9491, and GR4, identified the somatostatin cells. Abs 9491 and GR4 occasionally co-localized with glucagon, a feature that never occurred with Ab 9262. Finally, the specificity of Ab 9262 for the pancreatic CCKB R was confirmed in six different species. It co-localized with somatostatin but never with glucagon in these species. Our data suggest the use of Abs 1122 and 9262 to specifically identify and localize pancreatic CCKA and CCKB receptors, respectively. Confusion in the literature may result from the lack of specificity of most antibodies used, as established in this study.
Keywords: pancreas, CCKA-CCKB receptor localization, islets of Langerhans
Although cholecystokinin (CCK) and its analogues are recognized as potent secretagogues of pancreatic enzyme secretion in rodents (Jensen et al. 1980; Maouyo and Morisset 1995), their physiological effects on islet hormone physiology are not well defined. In the rat, Otsuki et al. (1979) demonstrated in vivo that release of insulin and glucagon were stimulated only at supramaximal doses of cerulein, a CCK analogue. In perfused rat pancreas, CCK enhanced glucose-induced insulin secretion, with no effect at low-perfusate glucose concentrations. Furthermore, glucagon release remained unaffected (Martindale et al. 1982). In perfused porcine pancreas, CCK-4 and CCK-8 induced dose-dependent stimulation of pancreatic α-, β-, and δ-cell secretion, possibly via neural pathways (Hermansen 1984), with the C-terminal tetrapeptide of CCK the most potent stimulus (Rehfeld et al. 1980). Somatostatin release by the perfused canine pancreas occurred only at pharmacological concentrations of gastrin and CCK octapeptide (Ipp et al. 1977). Data obtained from isolated rat pancreatic islets indicated that CCK-8 can reverse the inhibitory effect of glucose on glucagon secretion, sensitize the β-cells to the insulinotropic effect of glucose, and enhance the effect of glucose on somatostatin release at a concentration compatible with CCKB receptor occupation (Verspohl and Ammon 1987).
Debate also exists regarding the type of CCK receptor involved in the control of pancreatic hormone release. In rats, the CCKA receptor antagonist MK329 significantly reduced insulin and glucagon secretion in response to a protein-rich meal (Rossetti et al. 1987), as well as CCK-stimulated insulin release from isolated rat islets (Zawalich et al. 1988). Conversely, the same CCKA receptor antagonist failed to inhibit meal-stimulated insulin and glucagon secretion in human male volunteers. A potential difference in human pancreatic CCKA receptor structure, compared to that of other species, may be responsible for such differences (Liddle et al. 1990).
One would have thought that the availability of specific antibodies raised against the CCKA and CCKB receptor subtypes could help to identify the precise localization of each CCK receptor subtype in pancreatic endocrine cells. Unfortunately, the initial studies using such antibodies also resulted in controversies. By binding studies and storage phosphor imaging, Tang et al. (1996) established that the human pancreas predominantly expresses the CCKB receptor, which is diffusely distributed throughout the exocrine pancreas. In contrast, by confocal microscopy with a CCKB receptor antibody raised by Tarasova et al. (1995) against the third extracellular loop of the human receptor, Saillan-Barreau et al. (1999) claimed that the CCKB receptors present in human adult pancreas co-localize with glucagon in α-cells but not in acinar cells. This observation was later confirmed in the rat pancreas with a different antibody obtained from the Center for Ulcer Research and Education (CURE) and raised against peptides 418–429 of the rat CCKB receptor (Rooman et al. 2001). By immunohistochemistry (IHC) with an antibody raised in chicken against the N-terminal region (1–15) of the human CCKA receptor, Schweiger et al. (2000) demonstrated in the pig pancreas that the glucagon α-cells possess the CCKA but not the CCKB receptor. Our own study with antibody 9262 from CURE, recognizing the N-terminal 42–55 amino acids of the dog CCKB receptor, strongly indicated that this receptor co-localizes with somatostatin in δ-cells of rat, mouse, human, and pig pancreas, with no recognition of acinar cells (Morisset et al. 2000).
A recent study suggested that CCK might be used as a potential treatment for type 2 diabetes because of its antidiabetogenic action in human (Ahren et al. 2000). Furthermore, a chronic cerulein and secretin treatment in rats led to increased pancreatic content of somatostatin and glucagon, with no effect on insulin (Yamada et al. 1983). If such treatments are to be envisaged in the near future, it becomes very important to establish which CCK receptor subtype is involved for selection of the proper CCK agonist, as well as its dosage, to respect the fundamental biochemical characteristics of each receptor subtype.
According to the species studied, the antibodies used, and the designed experimental conditions, the CCKB receptors were observed all over the human pancreas (Tang et al. 1996), specifically on human (Saillan-Barreau et al. 1999) and rat (Rooman et al. 2001) pancreatic glucagon cells or on human, rat, mouse, and pig pancreatic somatostatin cells (Morisset et al. 2000). This study was therefore undertaken to shed some light on the existing controversy. Our objectives were primarily (a) to validate the specificity of the antibodies previously used to identify the CCKA and CCKB receptor subtypes, (b) to localize these receptors on rat endocrine pancreatic cells, and (c) to confirm that the CCK receptors' localization found in the rat endocrine pancreas reflects that in other species. These objectives will be met using Western blotting, immunofluorescence, and confocal microscopy techniques.
Materials and Methods
Tissue Preparation
Once excised, tissues were quickly frozen in liquid nitrogen and later processed for Western blotting analysis or embedded in Tissue-Tek optimal cutting temperature (OCT) 4583 compound (Sakuta Fine Tek; Torrance, CA) and frozen in liquid nitrogen for indirect immunofluorescence studies. Samples of rat, human, dog, calf, pig, and horse pancreas were so collected. Human pancreatic tissues were obtained either from normal elective pregnancy terminations or surgical samples. These studies were approved by the Institutional Human Subject Review Board. The animal studies were performed according to our institutional animal care policies.
Islet Isolation
Rats (300–350 g) were anesthetized with Somnotol (MTC Pharmaceuticals; Cambridge, ONT, Canada) prepared as a 6-mg mL−1 solution and given at 1 ml per 100 g bw. The pancreatic islets were isolated using the collagenase digestion method of Lacy and Kostianovsky (1967). Briefly, after ligation of the distal pancreatic duct and cannulation of the common bile duct, the pancreas was perfused with 20 ml of Hanks' Balanced Salt Solution (HBSS) (Gibco BRL; Gaithersburg, MD) followed by dissection of the organ and digestion in HBSS containing 15 mg collagenase/g pancreas (Sigma; St Louis, MO) and 0.5 mg DNase I/g pancreas (Sigma) at 37C by manual shaking. After washing in HBSS, the islets were collected on a discontinuous Ficoll 400 gradient (Fluka; Buchs, Switzerland). Harvested islets were washed three times with HBSS containing 10% FBS and handpicked under a dissecting microscope. Approximately 600–750 islets were obtained from each pancreas. The islets were then cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, penicillin (100 U/mL−1), streptomycin (100 μg mL−1) and 11 mM glucose. On the next day the medium was changed and the glucose concentration was reduced to 5.5 mM. Islets were used 48 hr after their purification.
Gel Electrophoresis and Immunoblotting
This procedure was performed as described by Morisset et al. (2000). The dilution of each antibody used is indicated in the legend to Figure 1.
Figure 1.
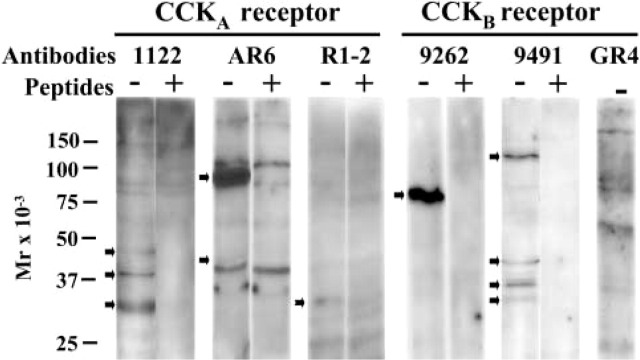
Specificity of the different CCKA and CCKB receptor antibodies established by Western blotting. Rat pancreatic membranes (30 μg) were used for Western blotting analysis with antibodies 1122 (1:10,000), AR6 (1:1000), R1-2 (1:1000), 9262 (1:10,000), 9491 (1:1000), and GR4 (1:10,000). Specificity was established by preincubation of each primary antibody for 2 hr at RT with 40 μg/mL−1 of the corresponding peptide antigen, with the exception of antibody GR4 for which the peptide antigen was unavailable. Ab, antibody; P, peptide antigen.
Indirect Immunofluorescence
Frozen pieces of pancreatic tissue embedded in OCT were cut into 3–4-μm slices on a JUNG FRIGOCUT 2800 N cryostat (Leica; Montreal, PQ, Canada), spread on glass slides, and air-dried for 30 min at 37C. Tissue sections were fixed for 45 min at 4C in 2% formaldehyde in PBS, pH 7.4, washed in PBS, and permeabilized with 0.25% Triton X-100 for 5 min, and washed for 5 min in PBS. The remaining aldehyde groups were quenched in 100 mM glycine in PBS (10 min at RT) and after one wash, for 5 min in PBS, nonspecific binding was blocked with 2% donkey serum in PBS for 1 hr or with 20% serum for the Tarasova antibody. Slides were incubated overnight with the different antibodies at 4C and washed three times for 5 min, followed by FITC-labeled secondary antibody for 1 hr at RT. After three more washes in PBS, slides were mounted in Fluoroguard medium (BioRad; Montreal, PQ, Canada) and viewed with a Nikon Eclipse E1000 fluorescence microscope with the appropriate filter combinations.
Immunohistochemistry and Image Analysis by Confocal Microscopy
Islets were plated on 20-mm glass coverslips precoated with 0.01% poly-l-lysine (Sigma) and briefly rinsed in PBS. They were then fixed for 10 min at RT in 2% paraformaldehyde and saturated for 10 min at RT in sodium borohydride 2 mg/mL−1. After several rinses in PBS, islets were permeabilized for 30 min at RT in PBS, 0.4% Triton X-100, 7% normal donkey serum. After medium aspiration, islets were in cubated in PBS, 0.4% Triton X-100, 1.4% normal donkey serum for 12 hr at 4C with the primary antibody used at the following dilution: CCKBR 9262 (1:1000), 9491 (1:500); GR4 (1:1000); CCKAR 1122 (1:200); somatostatin (10 μg/mL−1); insulin (1:50); glucagon (1:500). For specificity evaluation, peptides or hormones were preincubated at 40 μg/mL−1 for 2 hr at RT with their respective antibodies. Then the islets were incubated with the appropriate secondary antibody (fluorochrome-conjugated FITC 2 μg/mL−1 and rhodamine 4 μg/mL−1 in PBS, 0.4% Triton X-100, 1.4% normal donkey serum for 12 hr at 4C. The immunostained islets were examined with an Olympus Fluoview Confocal Scanning System (Olympus America; New York, NY) mounted on an Olympus IX70 microscope. The FITC and rhodamine fluophores were imaged using a 488-nm excitation and a green bandpass emission filter (510–550-nm) and a 568-nm excitation and a red bandpass emission filter (585–610-nm), respectively.
Antibodies Used
The peptide sequences selected to raise each antibody against the CCKA and CCKB receptor are listed in Table 1, along with their location on the receptor's molecule. The CCKA receptor antibodies AR5 (1122) and AR6 are two rabbit polyclonals, generously given by Dr. Marie-Luise Kruse (Kiel, Germany). The R1-2 CCKAR antibody is a chicken polyclonal given by Schweiger and Amselgruber (Stuttgart, Germany). Antibodies 9262 and 9491 are rabbit polyclonals raised against the CCKBR; both were provided by CURE/Gastroenteric Biology Center, Antibody/RIA Core, NIH grant # DK41301. The GR4 antibody is also a rabbit polyclonal against the CCKBR and purchased from Boston Biologicals (Boston MA). This is the only antibody for which we were unable to obtain the peptide antigen. The glucagon monoclonal antibody (MAb), clone K79bB10, was purchased from Sigma (St Louis, MO). The insulin antibody (2D11-H5) was from Santa Cruz Technology (Santa Cruz, CA) and the somatostatin (Barbar) antibody, further purified by affinity chromatography, was a gift from Dr. Brazeau (Université de Montréal, Montréal, Canada). As second antibodies, the following were used: with antibodies 9262, 9491 and 1122, Alexa Fluor 488 donkey anti-rabbit IgG, 2 μg/mL−1; with Barbar, Alexa Fluor 546 donkey anti-goat IgG, 2 μg/mL−1; with insulin and glucagon, we used either Alexa Fluor® 488 donkey anti-mouse IgG, 2 μg/mL−1 from Molecular Probes (Eugene, OR) or donkey anti-mouse IgG rhodamine-conjugated, 4 μg/mL−1 from Santa Cruz with R1-2, Alexa Fluor 488 donkey anti-chicken IgG, 2 μg/ml−1 from Molecular Probes.
Table 1.
Peptide sequences used to develop the different CCKA, CCKB receptor antibodies
| Antibodies | Peptide sequences | Receptor region | Species | Ref | |
|---|---|---|---|---|---|
| CCKAR | |||||
| AR5 (1122) | CGVRGEVGEEEDGRTIRALL | C-term | 410-429 | Rat | Morys-Wortmann et al. 1996 |
| AR6 a | MSHSPARQHLVESSRMDVVDG | N-term | 1-21 | Rat | – |
| R1-2 | MDVVDSLLVNGSNIT | N-term | 1-15 | Human | Schweiger et al. 2000 |
| CCKBR | |||||
| 9262 | YPRLRGAGTRELELA | N-term | 42-55 | Dog | Helander et al. 1997 |
| 9491 | PRARPQPLPDED | C-term | 418-429 | Rat | Rooman et al. 2001; Helander et al. 1997 |
| GR4 | RAFDGPGAHRALSGAP | 3rd extracellular loop | 356-472 | Human | Tarasova et al. 1995 |
We are the first investigators to publish on this antibody.
Results
Specificity of the CCK Receptor Subtype Antibodies
Before any attempt is made to clarify the precise cellular localization of the pancreatic CCK receptor protein subtypes and the hormones, one must first establish the specificity of the different antibodies to be used. These important verifications were indeed performed with Western blotting, indirect immunofluorescence, and confocal microscopy. The specificity of each antibody was therefore tested against the peptide or hormone initially used to raise it.
As shown in Figure 1, Western blotting analysis of rat pancreatic membrane proteins indicates that the 1122 CCKAR antibody recognized three specific receptor proteins of ∼32, ∼40, and ∼48 kD, whose immunoreactivities were abolished by an excess of the immunizing peptide. However, the AR6 antibody also identified the CCKAR as proteins of ∼45 and 78–80 kD, whose specificity was confirmed by preabsorption with excess antigen. The CCKAR R1-2 antibody developed by Schweiger et al. (2000) lightly recognized several proteins, of which one, of ∼32 kD, appears specific because absorption of the antibody to its peptide antigen displaced this single band.
The search for the pancreatic CCKBR protein also compared three different antibodies. As also shown in Figure 1, the 9262 antibody specifically recognized a 78–80 kD protein whose immunoreactivity was totally blocked by preabsorption of the antibody to its antigen. Conversely, the 9491 antibody recognized at least four different CCKBR proteins of ∼35, 38, 45, and 120 kD, all displaced by preabsorption of this antiserum to its antigen. Finally, the GR4 CCKBR antibody developed by Tarasova et al. (1995) also identified at least four proteins. The technical data sheet from Boston Biologicals stated that this GR4 antibody identifies a 78-kD CCKBR protein, which can be seen on this gel. Unfortunately, we were unable to get this antibody's antigen to confirm its specificity.
In addition to the Western blotting technique, the specificity of the different CCKA and CCKB receptor antibodies was also verified by indirect immunofluorescence and confocal microscopy. As shown in Figure 2A, antibody 1122, raised against a portion of the rat CCKAR, gave highly positive immunostaining in purified rat islets of Langerhans. The specificity of this reaction was clearly established by preabsorption of the antibody to its peptide antigen. Data obtained with the R1-2 antibody, raised against the human CCKAR, are less convincing. Indeed, the value of this antibody was investigated from serial sections of the pig and human pancreas because Schweiger et al. (2000) used this antibody to localize the CCKAR in the pig pancreas. Furthermore, it was raised against a portion of the human CCKAR. As shown in Figure 2A, this antibody did not give a clear identification of the receptor in pancreas of both species. Furthermore, the lack of specificity was confirmed by failure of the peptide antigen to prevent any specific staining. These data strongly contrast with those obtained on rat purified islet with antibody 1122.
Figure 2.
Specificity of the different CCKA and CCKB receptor antibodies established by indirect immunofluorescence or confocal microscopy. (A) Serial sections of pig and human pancreas and a purified rat islet were incubated overnight at 4C with the CCKAR primary antibodies R1-2 (1:30) and 1122 (1:200). Bar = 100 μm. (B) Rat purified islets were also incubated overnight at 4C with the CCKBR primary antibodies 9262 (1:1000), 9491 (1:500), and GR4 (1:1000). Specificity was established by preincubation of each primary antibody for 2 hr at RT with 40 μg/mL−1 of the corresponding peptide antigen with the exception of antibody GR4 for which the peptide antigen was unavailable. P, peptide antigen. Bar = 50 μm.
The specificity of the three antibodies used to localize the CCKBR was also tested on isolated rat pancreatic islets. As shown in Figure 2B, recognition of the CCKBR on the purified islets by the 9262 antibody is very specific because its binding is totally abolished by preabsorption to its peptide antigen. On similar islet preparations, the 9491 antibody showed less specificity because its peptide antigen did not totally prevent binding. Finally, the GR4 antibody of Tarasova et al. (1995) recognized many structures in the islets, but we were unable to ascertain its specificity because it was impossible to obtain the peptide antigen. The specificity of the pancreatic hormone antibodies was recently reported using serial sections of a single islet from calf pancreas (Morisset et al. 2003) and confirmed on rat pancreatic islets (Julien et al. 2002b).
Co-localization of the Pancreatic CCKA Receptor with Three Islet Hormones
To estimate co-localization of the CCKAR with three islet hormones, purified rat islets and sections of pig and human pancreas were double stained with the 1122 or R1-2 antibodies along with the insulin, glucagon, or somatostatin antibodies as markers of β-, α-, and δ-cells, respectively. The PP cells were not investigated in this study because we could not find clean antibodies for confocal microscopy. As shown in Figure 3A, the 1122 antibody clearly indicates by confocal microscopy that the rat CCKAR co-localizes with insulin and glucagon on purified islets. The somatostatin cells, however, do not appear to carry this receptor subtype. By indirect immunofluorescence (Figure 3B), the R1-2 antibody applied to serial sections of pig and human pancreas failed to give any specific signal on acinar cells. However, this receptor can be identified on pig and human glucagon cells and on pig insulin cells, more specifically when images from the receptor and hormones are superposed as in “Merge.”
Figure 3.
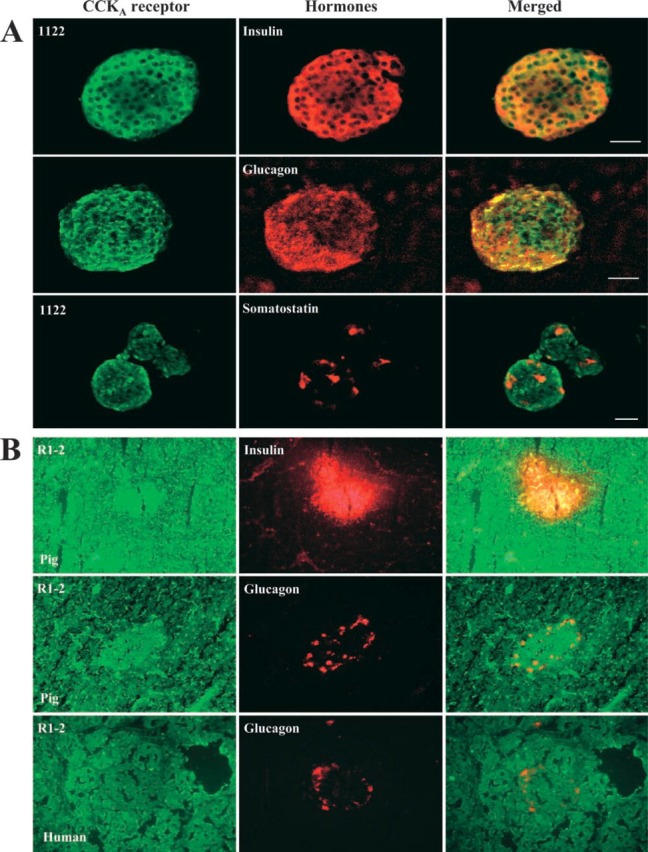
Co-localization of the pancreatic CCKA receptor with three islets' hormones. (A) Purified rat islets were incubated overnight at 4C with the CCKAR antibody 1122 (1:200), insulin (1:50), glucagon (1:500), and somatostatin (10 μg/mL−1) antibodies. Co-localization was established by confocal microscopy as described in Materials and Methods. Bars −100 μm; –, 50 μm. (B) Pig and human pancreatic tissue sections were incubated overnight at 4C with the CCKAR antibody R1-2 (1:30), insulin (4 μg/mL−1, 1:50) and glucagon (1:500) antibodies. Co-localization was established by indirect immunofluorescence as described in Materials and Methods. Magnification ×40. In Figure 3B, we use the same frozen pieces of pig and human pancreatic tissue as in Figure 2A; they are serial sections.
Co-localization of the Pancreatic CCKB Receptor with Three Islet Hormones
The debate about the pancreatic localization of the CCKBR resides on its presence either on glucagon cells (Saillan-Barreau et al. 1999; Rooman et al. 2001) or on somatostatin cells (Morisset et al. 2000). One reason for this controversy may be the specificity of the antibodies used, as shown in Figure 1 and 2B. To confirm this possibility, we investigated the three antibodies previously used and tested them on purified rat islets. As shown in Figure 4A, all CCKBR antibodies identified the receptor on some somatostatin cells; the most consistent and accurate recognition was from antibody Ab9262. In this picture, one can easily see that antibodies 9491 and GR4 are much less specific than 9262, because both of them located the receptor all across the islets unlike 9262, which is much more selective. With regard to the glucagon cells (Figure 4B), antibody 9262 clearly indicates that CCKAR is absent from these cells. However, the diagnosis from antibodies 9491 and GR4 is much less secure. Indeed, co-localization of the CCKBR with glucagon can be seen with both antibodies, but it is irregular and seems to occur 50% of the time after observation of several islets.
Figure 4.
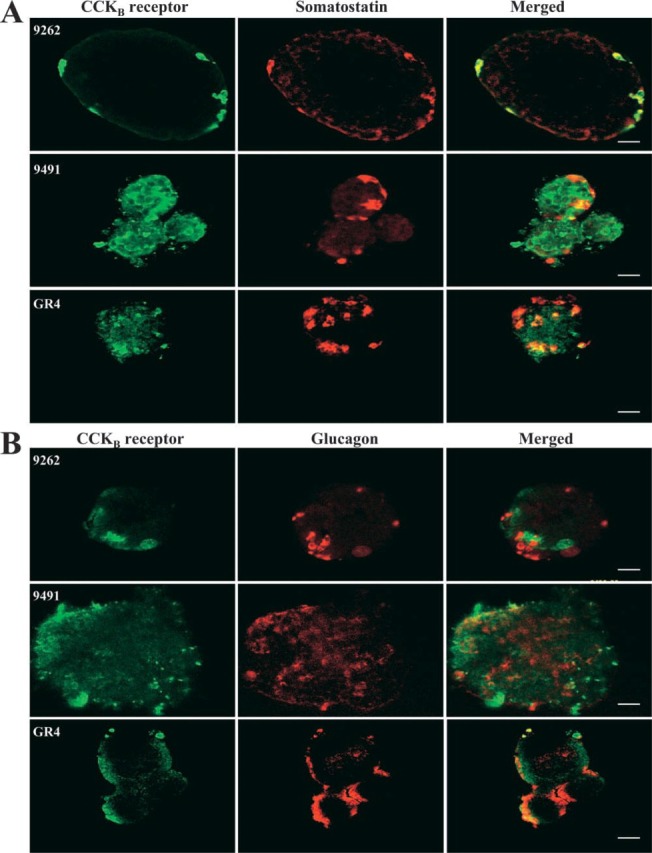
Co-localization of the pancreatic CCKB receptor with somatostatin and glucagon. Rat purified islets were incubated overnight at 4C with the CCKBR antibody 9262 (1:1000) and the somatostatin antibody (10 μg/mL−1, A) or with the glucagon antibody (1:500, B). Co-localization was established by confocal microscopy as described in Materials and Methods. Bars: A = 100 μm; B = 50 μm.
Co-Localization of the Pancreatic CCKB Receptor with Two Islet Hormones in Different Species
Data from the previous figure indicated that the CCKBR antibody 9262 appears to be the most specific to co-localize this receptor with somatostatin. To strengthen this observation, we reinvestigated this selective co-localization of CCKBR-somatostatin compared with glucagon in the pancreas of six different species. The first major observation obtained from serial sections and double staining of these pancreatic tissues clearly indicates that this receptor subtype is absent from acinar cells surrounding the islets in all six species investigated (Figure 5 and 6). The most important and convincing feature emerging from this experiment is that, in all six species, the CCKBR co-localizes with somatostatin (Figure 5), with no match with glucagon (Figure 6) when antibody 9262 is used. Also, as shown in Figure 6, the horse pancreas appears to have many fewer glucagon cells than the other species examined.
Figure 5.
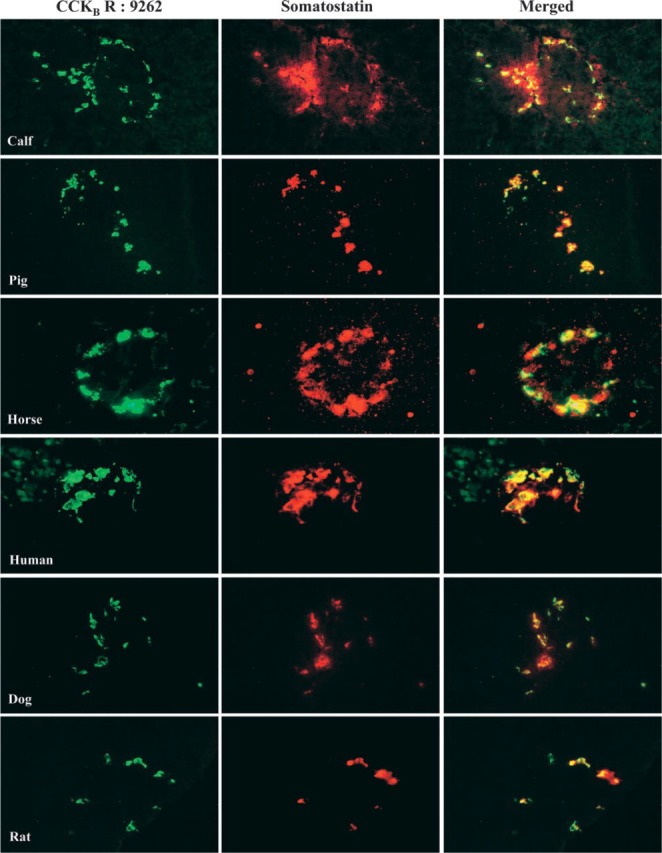
Co-localization of the pancreatic CCKB receptor with somatostatin in six different species. Calf, pig, horse, human, dog, and rat pancreatic tissue sections were incubated overnight at 4C with the CCKB antibody (1:500) and somatostatin (10 μg/mL−1) antibodies. Co-localization was established by indirect immunofluorescence as described in Materials and Methods. Original magnification ×40.
Figure 6.
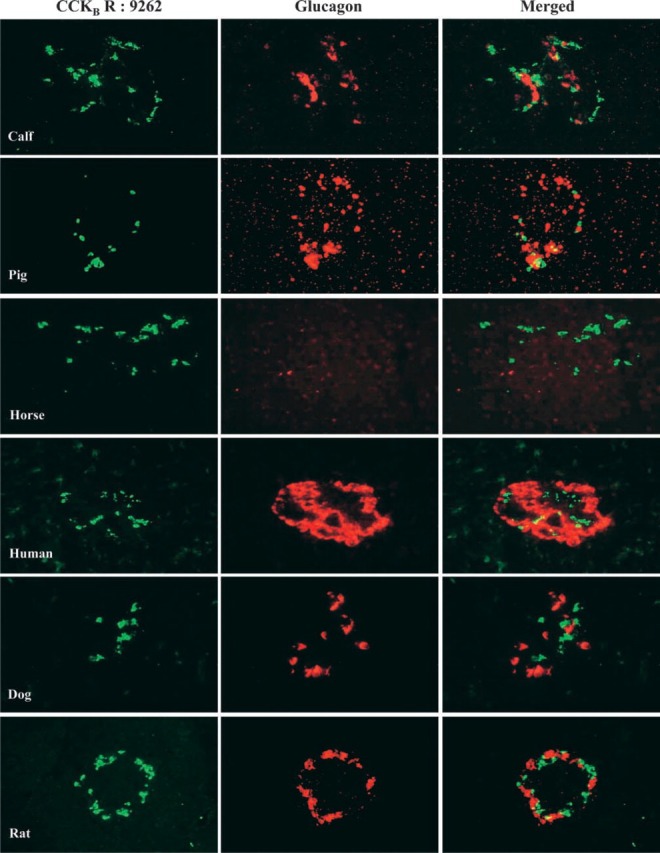
Co-localization of the CCKB receptor with glucagon in six different species. Calf, pig, horse, human, dog, and rat pancreatic tissue sections were incubated overnight at 4C with the CCKBR (1:500) and glucagon (1:500) antibodies. Co-localization was established by indirect immunofluorescence as described in Materials and Methods. Original magnification ×40.
Discussion
Careful examination of the published literature points to a controversy on the pancreatic cellular distribution of the CCK receptor subtypes. Our understanding of the problem suggests that this dilemma arose from antibody selection and improper control of antibody specificity. In an effort to understand the problems, we present an investigation on pancreatic distribution of the CCKA and CCKB receptors in six different species after a systematic evaluation of previously used antibodies to characterize their specificity and their potential use for Western blotting, indirect immunofluorescence, and confocal microscopy.
Our first approach to test the antibodies' specificity involved Western blotting of pancreatic membrane proteins, with preincubation of each antibody with its peptide antigen. The CCKAR antibody 1122 recognized three specific receptor proteins, which have been previously described (Julien et al. 2002a). The AR6 antibody identified two specific CCKAR proteins as major (78–80-kD) and minor (45-kD) bands. This is a first report for this antibody. The R1-2 antibody developed by Schweiger et al. (2000) gave a poor performance, with the identification of a faint band at around 32 kD. Specificity of this CCKAR antibody was previously established either by serial dilution of the antibody or by dot-blot assay (Schweiger et al. 2000), but never on pancreas homogenates or membranes.
Among the CCKBR antibodies tested, the 9262 from CURE identified a specific 78–80-kD protein that we had previously described (Morisset et al. 2000). Also from CURE, the 9491 antibody bound four different specific proteins. Four similar rat pancreatic proteins were recently described by Rooman et al. (2001), but their specificity from preabsorption of this antiserum with an excess of the immunizing peptide was not presented. Finally, because of lack of peptide antigen we (this study) and Saillan-Barreau et al. (1999) were unable to document and appreciate the specificity of the GR4 antibody developed by Tarasova et al. (1995). However, the blot enabled us to guess the recognition of at least four different proteins. In summary, this technique indicates that the most specific antibodies are 1122 and AR6 for the CCKAR and 9262 and 9491 for the CCKB subtype.
One can be concerned with these Western blots prepared from total pancreas to evaluate the specificity of our CCKA and CCKB receptor antibodies because these preparations include exocrine and endocrine pancreatic cells. However, with the CCKA receptor Ab1122 and the CCKB receptor Ab9262, the most specific in this study, we recently established the presence of the CCKA and CCKB receptors in membranes of purified rat islets with molecular weights comparable to those described here (Julien et al. 2002a). The other puzzling feature of this Western blotting study deals with the different molecular weights observed with the same antibody and with different ones. We do not have a clear explanation for this phenomenon, but we can say that it has also been observed by others, as indicated above. The possibility that one antibody detects various glycosylated forms of the receptor, whereas another may detect degraded forms or splice variants of each receptor protein, is among the potential explanations. Different subcellular locations of the same receptor isoforms have also been reported (Watson et al. 1998). The degradation option is the least plausible because all blots were performed from the same membrane preparation.
The specificity of all antibodies used was also confirmed by indirect immunofluorescence or confocal microscopy assay. The 1122 CCKAR antibody labeling of the purified rat islet was totally displaced by preincubation with its peptide. Conversely, such specificity could not be demonstrated with the R1-2 antibody, as shown in Figure 2A. In our hands, this CCKAR antiserum raised against a portion of the human receptor and used on pig pancreas (Schweiger et al. 2000) showed total lack of specificity on human pancreas, whose receptor it represents, and on pig pancreas, on whom it was previously positively tested (Schweiger et al. 2000). With their R1-2 antibody, we had to superimpose the CCKAR Ab with insulin and glucagon to visualize the presence of this receptor on these two cell types, as shown in Figure 3B. We cannot make any comparison with the data of Schweiger et al. because they did not challenge the streptavidin-biotin-horseradish peroxidase complex with their peptide antigen.
The observation that binding of the CCKBR antibody 9262 to purified rat islets was totally displaced by its peptide antigen confirms its specificity, first established by Western blotting. This specificity was also acknowledged on an islet obtained from serial sections of calf pancreas, demonstrating the versatility of this antibody (Morisset et al. 2003). The 9491 antibody also exhibited some specificity because its labeling of the rat islet was not totally displaced by its peptide antigen. We were therefore not quite as successful as Rooman et al. (2001), who showed in their study that immunoreactivity was completely abolished from monolayers of pancreatic duct-like cells and from paraffin sections of rat pancreatic tissue after preincubation of their antibody with its antigen and the streptavidin-biotin method. Unlike antibodies 9262 and 9491, the antiserum GR4, despite its sequence homology among species, remains the least specific because it labeled the entire rat islet. This image differs totally from Saillan-Barreau's observation stressing a selective recognition of islet CCKBR from serial sections of human pancreas. Unfortunately, like us, they did not have the antiserum peptide antigen to establish this antibody's specificity, but rather used preimmune serum (Saillan-Barreau et al. 1999). Finally, we previously established that the antibodies we selected to identify insulin, glucagon, and somatostatin were very specific because preincubation with each hormone totally displaced their binding to serial sections of a single calf islet (Morisset et al. 2003) and to isolated rat pancreatic islets (Julien et al. 2002a). In all previous studies aimed to co-localize the CCKA or CCKBR with the pancreatic hormones, none clearly established the specificity of their hormone antibodies, especially glucagon and insulin (Saillan-Barreau et al. 1999; Schweiger et al. 2000; Rooman et al. 2001).
Once the specificity of the CCKR subtypes and islet hormones was clearly established, we then concentrated on the problem's knot: the controversial co-localization of the two CCKR with three pancreatic hormones. With purified rat islets and antibody 1122, it was clear that the CCKAR co-localizes with insulin and glucagon and not with somatostatin. Comparable co-localizations of the CCKAR with insulin and glucagon were also established with the R1-2 antibody on pig pancreas and with glucagon on human pancreas only when images of receptors and hormones were superposed, because the R1-2Ab could not clearly identify the CCKAR on pig and human serial sections of their pancreatic tissue. These data with the 1122 antibody agree with our previous report on the presence of the CCKAR on the rat insulin cells obtained from pancreas sections (Bourassa et al. 1999). This important demonstration of the presence of CCKAR on β- and α-cells can support the insulinotropic and glucagonotropic action of CCK previously demonstrated in vivo and on isolated rat and pig pancreas (Rehfeld et al. 1980; Karlsson and Ahren 1992) and the presence of this receptor on rat β cells by autoradiography (Sakamoto et al. 1985). Our data obtained with the R1-2 antibody confirm those of Schweiger et al. (2000) on co-localization of CCKAR and glucagon on pig pancreas. However, contrary to their data, we were also able to clearly extend this co-localization with insulin. We cannot yet explain this discrepancy except that we used the same CCKAR antibody.
Our approach to understanding the discrepancies in the literature regarding the localization of the CCKBR on pancreatic endocrine cells was to compare the efficiency of the three antibodies previously used: 9491 by Rooman et al. (2001), GR4 by Saillan-Barreau et al. (1999), and 9262 by us (Morisset et al. 2000) on purified rat islets under similar experimental conditions. Data obtained from this experiment clearly indicate that all three antibodies co-localized the CCKBR with somatostatin, with antiserum 9262 being the most accurate and specific. Helander et al. (1997) were the first to demonstrate that, in dog and guinea pig stomach antrum and corpus, 97% of the cells with CCKBR recognized by Ab9262 co-localized with somatostatin. Antibodies 9491 and GR4 seldom matched with glucagon cells under our conditions. Data obtained with Ab9491 cannot be compared to those of Rooman et al. (2001) because, as indicated by the authors, “staining for glucagon in consecutive sections showed that CCKBR was localized in the α-cells (not shown).” Similarly, in our hands, Ab GR4 recognized more rat somatostatin than glucagon cells, an observation totally different from the selective and unique co-localization CCKBR glucagon described in human pancreas by Saillan-Barreau et al. (1999). We cannot yet explain this difference, unless tissue fixation in Bouin's solution (Saillan-Barreau et al. 1999) preserves cell structures and intracellular elements better than frozen tissue in OCT 4583 as we did. This would contradict the comment by Rehfeld et al. (1980) that “embedding of formaldehyde-fixed tissue in resins or paraffin abolished immunoreactivity. Hence post-embedding staining was not possible.” Can we play the species difference card? This option does not appear to stand because the 9262 antibody specifically recognized the CCKBR on somatostatin cells, and not on glucagon cells, in all species we have thus far examined.
To additionally prove which cell types express CCKA and CCKB receptors, we might also have investigated the cellular location of their specific mRNA by in situ hybridization. This technique was used by Ohlsson et al. (2000,2001) for the CCKA receptor on rat pancreas, but double labeling has not yet been successfully tried. Furthermore, in these studies data using a sense probe were not presented. However, thus far no one has succeeded in this technique with the CCKB receptor on rodent and human pancreas, and this may result from the low abundance of these receptors' mRNA transcripts. Several studies have indicated that no hybridizing CCKBR mRNA could be identified by Northern blotting from rat (Zhou et al. 1995; Lay et al. 2000; Morisset et al. 2000) or mouse pancreas (Lee et al. 1993). Furthermore, the pancreatic CCKBR mRNA was identified in human pancreas by Northern hybridization (Lee et al. 1993; Pisegna et al. 1992) but not by in situ hybridization (Ji et al. 2001). The technique was successfully used in the human gastric mucosa (Schmitz et al. 2001) for the CCKBR, but this comment from the authors describing one of their images “the acid-secreting parietal cells identified on the haematoxylin and eosin stained section most likely represent the morphological correlate of mRNA CCKBR-expressing cells of the in situ RT-PCR section” let us believe that the co-localization data of CCK receptor-hormone evaluated by confocal microscopy presented in this study are more convincing than co-localization that might result from in situ hybridization.
Finally, to increase the credibility of our data, i.e., that the 9262 CCKBR antibody appears the most specific and the best tool to co-localize with somatostatin in pancreatic 8-cells, we compared its binding on α- and 8-cells obtained from six different species. As shown in Figure 5 and 6, the islets from calf, pig, horse, human, dog, and rat unequivocally exhibited a co-localization of the CCKBR with somatostatin, but not with glucagon. This demonstration suggests that gastrin may be involved with somatostatin metabolism through its CCKBR in all the pancreas of higher mammals, including mouse (Morisset et al. 2000) and rabbit (unpublished data). Recently, Zavros and Shulkes (1997) reported that somatostatin secretion in sheep was stimulated by gastrin, an effect blocked by the CCKBR antagonist L365,260, again in support of gastrin acting on somatostatin metabolism.
This study enables us to recommend that future work on co-localization of the CCKR subtypes with the pancreatic endocrine hormones be performed with antibodies whose specificity has been thoroughly certified. At present, our data suggest that antibodies 1122 and 9262 are the most specific and accurate available to identify the CCKA and CCKBR, respectively. The evidence on which this suggestion is based can be summarized as follows: (a) specificity controls in which specific antisera for insulin, glucagon, somatostatin, CCKA, and CCKBR absorbed with excess purified antigen completely eliminated positive staining of the respective cell types in adjacent section or purified islets; (b) the homogeneity of our finding on CCKBR-somatostatin co-localization in the pancreas of six different species.
Acknowledgments
Supported by grant GP6369 from the Natural Sciences and Engineering Research Council of Canada.
We are grateful to Drs Paul Guilloteau (INRA, Rennes, France) and Alfred Merritt (Veterinary Medical School, Gainesville, FL) for their supply of calf and horse pancreas, respectively. We thank Dr Kruse (Kiel, Germany) for the 1122 and AR6 antibodies and peptides, Drs Schweiger and Amselgruber (Stuttgart, Germany) for their R1-2 antibody and peptide, and Dr Ohning (CURE, Los Angeles, CA) for antibodies 9262, 9491, and peptides, Dr Abou Elela and Mr Catala for use of his confocal microscope and technical assistance, and Dr Danielle Jacques for use of her fluorescence microscope (both are from the Medical School at the University of Sherbrooke). We also thank Ms Christiane Gauvin for secretarial assistance.
Literature Cited
- Ahren B, Holst JJ, Efendic S. (2000) Antidiabetogenic action of cholecystokinin-8 in type 2 diabetes. J Clin Endocrinol Metab 85:1043–1048 [DOI] [PubMed] [Google Scholar]
- Bourassa J, Lainé J, Kruse ML, Gagnon MC, Calvo E, Morisset J. (1999) Ontogeny and species differences in the pancreatic expression and localization of the CCKA receptor. Biochem Biophys Res Commun 260:820–828 [DOI] [PubMed] [Google Scholar]
- Helander HF, Wong H, Poorkhalkali N, Walsh JH. (1997) Immuno-histochemical localization of gastrin/CCK-B receptors in the dog and guinea-pig stomach. Acta Physiol Scand 159:313–320 [DOI] [PubMed] [Google Scholar]
- Hermansen K. (1984) Effects of cholecystokinin (CCK)-4, non-sulfated CCK-8, and sulfated CCK-8 on pancreatic somatostatin, insulin, and glucagon secretion in the dog: studies in vitro. Endocrinology 114:1770–1775 [DOI] [PubMed] [Google Scholar]
- Ipp E, Dobbs RE, Harris V, Arimura A, Vale W, Unger RH. (1977) The effects of gastrin, gastric inhibitory polypeptide, secretin and the octapeptide of cholecystokinin upon immunoreactive somatostatin release by the perfused canine pancreas. J Clin Invest 60:1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RT, Lemp GF, Gardner JD. (1980) Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci USA 77:2079–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. (2001) Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology 121:1380–1390 [DOI] [PubMed] [Google Scholar]
- Julien S, Lainé J, Morisset J. (2002a) Alterations in CCKA and CCKB receptors and somatostatin expression in streptozotocin-diabetic rats. Pancreatology 2:276 [Google Scholar]
- Julien S, Lainé J, Morisset J. (2002b) Expectation of pancreatic physiological responses from CCKB receptor occupation in its natural environment. In Taché Y, Goto Y, Ohning G, Yamada T, eds. Gut Brian Peptides in the New Millennium: A Tribute to John Walsh by His Collaborators. CA 425–436
- Karlsson S, Ahren B. (1992) Cholecystokinin and the regulation of insulin secretion. Scand J Gastroenterol 27:161–165 [DOI] [PubMed] [Google Scholar]
- Lacy PE, Kostianovsky M. (1967) Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16:35–39 [DOI] [PubMed] [Google Scholar]
- Lay JM, Jenkins C, Friis—Hansen L, Samuelson LC. (2000) Structure and developmental expression of the mouse CCK-B receptor gene. Biochem Biophys Res Commun 272:837–842 [DOI] [PubMed] [Google Scholar]
- Lee Y-M, Beinborn M, McBride EW, Lu M, Kolakowski LF, Kopin AS. (1993) The human brain cholecystokinin-B/gastrin receptor. Cloning and characterization. J Biol Chem 268:8164–8169 [PubMed] [Google Scholar]
- Liddle RA, Gertz BJ, Kanayama S, Beccaria L, Gettys TW, Taylor IL, Rushakoff RJ, et al. (1990) Regulation of pancreatic endocrine function by cholecystokinin: studies with MK-329, a non-peptide cholecystokinin receptor antagonist. J Clin Endocrinol Metabolism 70:1312–1318 [DOI] [PubMed] [Google Scholar]
- Maouyo D, Morisset J. (1995) Amazing pancreas: specific regulation of pancreatic secretion of individual digestive enzymes in rats. Am J Physiol 268:E349–359 [DOI] [PubMed] [Google Scholar]
- Martindale R, Levin S, Alfin—Slater R. (1982) Effects of caerulein and bombesin on insulin and glucagon secretion from isolated, perfused rat pancreas. Regul Peptides 3:313–324 [DOI] [PubMed] [Google Scholar]
- Morisset J, Lainé J, Bourassa J, Tessier P, Lessard M, Rome V, Guilloteau P. (2003) Presence and localization of CCK receptor subtypes in calf pancreas. Regul Peptides 111:103–109 [DOI] [PubMed] [Google Scholar]
- Morisset J, Wong H, Walsh JH, Lainé J, Bourassa J. (2000) Pancreatic CCKB receptors: their potential roles in somatostatin release and S-cell proliferation. Am J Physiol 279:G148–156 [DOI] [PubMed] [Google Scholar]
- Morys-Wortmann C, Miller LJ, Nebendahl K, Schmidt WE. (1996) Antisense directed against synthetic peptides recognize the native CCK-A receptor. In Eptor R, ed. Innovation and Perspectives in Solid Phase Synthesis. Fourth International Symposium. Edinburgh, Mayflower Scientific, 489–490 [Google Scholar]
- Ohlsson B, Borg K, Mulder H, Rehfeld JF, Axelson J, Sundler F. (2000) Continuous infusion of cholecystokinin leads to down-regulation of the cholecystokinin-A receptor in the rat pancreas. Scand J Gastroenterol 35:612–618 [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Borg K, Rehfeld JF, Axelson J, Sundler F. (2001) The method of administration of cholecystokinin determines the effects evoked in the pancreas. Pancreas 23:94–101 [DOI] [PubMed] [Google Scholar]
- Otsuki M, Sakamoto C, Maeda M, Yuu H, Morita S, Baba S. (1979) Effect of caerulein on exocrine and endocrine pancreas in the rat. Endocrinology 105:1396–1399 [DOI] [PubMed] [Google Scholar]
- Pisegna JR, deWeerth A, Huppi K, Wank SA. (1992) Molecular cloning of the human brain and gastric cholecystokinin receptor: structure, functional expression and chromosomal localization. Biochem Biophys Res Commun 189:296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld JF, Larsson LI, Goltermann NR, Schwartz TW, Holst JJ, Jensen SL, Morley JS. (1980) Neural regulation of pancreatic hormone secretion by the C-terminal tetrapeptide of CCK. Nature 284:33–38 [DOI] [PubMed] [Google Scholar]
- Rooman I, Lardon J, Flamez D, Schuit F, Bouwens L. (2001) Mitogenic effect of gastrin and expression of gastrin receptors in ductlike cells of rat pancreas. Gastroenterology 121:940–949 [DOI] [PubMed] [Google Scholar]
- Rossetti L, Sclulman GI, Zawalich WS. (1987) Physiological role of cholecystokinin in meal-induced insulin secretion in conscious rats. Studies with L-364,718, a specific inhibitor of CCK-receptor binding. Diabetes 36:1212–1215 [DOI] [PubMed] [Google Scholar]
- Saillan—Barreau C, Dufresne M, Clerc P, Sanchez D, Corominola H, Moriscot C, Guy-Crotte O, et al. (1999) Evidence for a functional role of the cholecystokinin-B/gastrin receptor in the human fetal and adult pancreas. Diabetes 48:2015–2021 [DOI] [PubMed] [Google Scholar]
- Sakamoto C, Goldfine ID, Roach E, Williams JA. (1985) Localization of saturable CCK binding sites in rat pancreatic islets by light and electron microscope autoradiography. Diabetes 34:390–394 [DOI] [PubMed] [Google Scholar]
- Schmitz F, Goke MN, Otte JM, Schrader H, Reimann B, Kruse M-L, Siegel EG, et al. (2001) Cellular expression of CCK-A and CCK-B/gastrin receptors in human gastric mucosa. Regul Peptides 102:101–110 [DOI] [PubMed] [Google Scholar]
- Schweiger M, Erhard MH, Amselgruber WM. (2000) Cell-specific localization of the cholecystokinin A receptor in the porcine pancreas. Anat Histol Embryol 29:357–361 [DOI] [PubMed] [Google Scholar]
- Tang C, Biemond I, Lamers CBHW. (1996) Cholecystokinin receptors in human pancreas and gall bladder muscle: a comparative study. Gastroenterology 111:1621–1626 [DOI] [PubMed] [Google Scholar]
- Tarasova NI, Copeland TD, Farnsworth DW, Wank SA, Hudson EA, Resau JH, Michejda CJ. (1995) Anti-peptide antibodies specific for the gastrin-cholecystokinin-B receptor. Lett Pept Sci 1:221–228 [Google Scholar]
- Verspohl EJ, Ammon HPT. (1987) Cholecystokinin (CCK8) regulates glucagon, insulin, and somatostatin secretion from isolated rat pancreatic islets: interaction with glucose. Pflügers Arch 410:284–287 [DOI] [PubMed] [Google Scholar]
- Watson SA, Clarke PA, Smith AM, Varro A, Michaeli D, Grimes S, Caplin M, et al. (1998) Expression of CCKB/gastrin receptor isoforms in gastro-intestinal tumour cells. Int J Cancer 77:572–577 [DOI] [PubMed] [Google Scholar]
- Yamada T, Brunstedt J, Solomon T. (1983) Chronic effects of caerulein and secretin on the endocrine pancreas of the rat. Am J Physiol 244:G541–545 [DOI] [PubMed] [Google Scholar]
- Zavros Y, Shulkes A. (1997) Cholecystokinin (CCK) regulates somatostatin secretion through both the CCK-A and CCK-B/gastrin receptors in sheep. J Physiol 505:811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich WS, Diaz VA, Zawalich KC. (1988) Stimulatory effects of cholecystokinin on isolated perfused islets inhibited by a potent and specific antagonist L-364,718. Diabetes 37:1432–1437 [DOI] [PubMed] [Google Scholar]
- Zhou W, Povoski SP, Bell RH. (1995) Characterization of cholecystokinin receptors and messenger RNA expression in rat pancreas: evidence for expression of cholecystokinin-A receptors but not cholecystokinin-B (gastrin) receptors. J Surg Res 58:281–289 [DOI] [PubMed] [Google Scholar]



