Abstract
We performed a detailed analysis of mouse cytochrome P450 2A5 (CYP2A5) expression by in situ hybridization (ISH) and immunohistochemistry (IHC) in the respiratory tissues of mice. The CYP2A5 mRNA and the corresponding protein co-localized at most sites and were predominantly detected in the olfactory region, with an expression in sustentacular cells, Bowman's gland, and duct cells. In the respiratory and transitional epithelium there was no or only weak expression. The nasolacrimal duct and the excretory ducts of nasal and salivary glands displayed expression, whereas no expression occurred in the acini. There was decreasing expression along the epithelial linings of the trachea and lower respiratory tract, whereas no expression occurred in the alveoli. The hepatic CYP2A5 inducers pyrazole and phenobarbital neither changed the CYP2A5 expression pattern nor damaged the olfactory mucosa. In contrast, the olfactory toxicants dichlobenil and methimazole induced characteristic changes. The damaged Bowman's glands displayed no expression, whereas the damaged epithelium expressed the enzyme. The CYP2A5 expression pattern is in accordance with previously reported localization of protein and DNA adducts and the toxicity of some CYP2A5 substrates. This suggests that CYP2A5 is an important determinant for the susceptibility of the nasal and respiratory epithelia to protoxicants and procarcinogens.
Keywords: CYP2A5, immunohistochemistry, in situ hybridization, olfactory toxicant, dichlobenil, methimazole, olfactory neuroepithelium, Bowman's glands, salivary gland, respiratory metaplasia
Certain members of the cytochrome P450 (CYP) 2A subfamily catalyze the biotransformation of many protoxicants and procarcinogens commonly found in the environment (Lang and Pelkonen 1999). In particular, the mouse CYP2A5 and the orthologous rat CYP2A3 and human CYP2A13 and CYP2A6 appear to have a major role in the bioactivation of such compounds. Evidence suggests that the tissue-selective expression of these enzymes could explain the organotropism of N-nitrosodiethylamine and aflatoxin B1 in esophageal and hepatic cancers, respectively (Kirby et al. 1994; Pinto et al. 2001; Godoy et al. 2002).
According to several reports, the CYP2A enzymes are present at high levels in the respiratory tract. For example, CYP2A3 is reported to be highly expressed in the rat nasal mucosa (Bereziat et al. 1995; Thornton-Manning et al. 1997; Gu et al. 1998). Su and co-workers (1996) have demonstrated expression of CYP2A5 in the mouse olfactory mucosa and lung, whereas CYP2A12 was not detected at these sites (Ding et al. 1996). In the human nasal mucosa and respiratory tract there is only low expression of CYP2A6, whereas the expression of CYP2A13 is more pronounced (Getchell et al. 1993; Fernandez-Salguero and Gonzalez 1995; Koskela et al. 1999; Dahlin et al. 2000; Ding and Kaminsky 2003).
Several substrates of the CYP2A enzymes appear to be toxic and carcinogenic for the nasal and respiratory epithelia. Coumarin, the major model substrate of CYP2A5 and CYP2A6, has recently been demonstrated to be an olfactory toxicant (Zhuo et al. 1999). CYP2A5 has also been reported to catalyze the metabolism of the potent olfactory toxicant dichlobenil (Eriksson and Brittebo 1991; Gu et al. 1998), the tobacco-specific procarcinogen and olfactory toxicant NNK[4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone] and the procarcinogens aflatoxin B1 and N-nitrosodi-ethylamine (Brittebo et al. 1981; Liu et al. 1996; Gu et al. 1998; Felicia et al. 2000).
The CYP levels in extrahepatic tissues are usually low and do not markedly influence the overall metabolism of drugs and chemicals in the body. Instead, the high expression of CYP2A enzymes in the nasal mucosa, along with the organ-specific damage induced by some of their substrates at this site, suggests that these enzymes may play a role in the mechanism of toxicity of these compounds. To better understand the possible role of the CYP2A5 in organ-selective metabolism and toxicity, the cellular sites of expression of the enzyme in the mouse respiratory tissues have been examined by ISH and IHC.
Because the CYP2A5 level in the liver is usually increased after exposure to various hepatotoxicants, we have also examined the effects of two olfactory toxicants on the CYP2A5 expression pattern in the olfactory mucosa (Honkakoski et al. 1988; Kojo et al. 1991,1998; Donato et al. 2000). Dichlobenil is known to induce permanent changes, such as respiratory metaplasia and fibrosis, in the lamina propria of the dorsomedial part of the olfactory region (Bergman et al. 2002). Methimazole is less potent but induces a more widespread necrosis in the neuroepithelium and Bowman's glands. However, this damage is rapidly repaired.
In addition, the effects of two typical hepatic CYP2A5 inducers, pyrazole and phenobarbital, on the morphology and CYP2A5 expression pattern in the olfactory mucosa were investigated in a mouse strain known to respond to these compounds. Phenobarbital is a transcriptional activator of CYP2A5, whereas pyrazole induces a protein, heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1), which stabilizes the CYP2A5 mRNA and increases the expression by increasing the half-life of mRNA (Thulke-Gross et al. 1998).
The results of the present investigation revealed a distinct expression of CYP2A5 in specific cell types in the upper and lower respiratory tract, the excretory ducts of salivary and nasal glands, and the nasolacrimal duct epithelium. The expression of CYP2A5 is in accordance with the previously suggested role of the enzyme in organ-specific toxicity of some protoxicants and may indeed determine the susceptibility of cells and tissues to compounds activated by CYP2A5. Furthermore, olfactory toxicants induced characteristic changes in the expression pattern, whereas hepatic CYP2A5 inducers did not change the expression pattern in the olfactory mucosa.
Materials and Methods
Chemicals
AdvanTaq DNA polymerase was purchased from Clontech Laboratories (Palo Alto, CA). Biotinylated goat anti-rabbit IgG, diaminobenzidine tetrahydrochloride (DAB), and streptavidin-HPR complex were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and also from Dakopatts AB (Älvsjö, Sweden). Bovine serum albumin (BSA) and pyrazole were purchased from Sigma-Aldrich Sweden AB (Stockholm, Sweden). Pertex was obtained from Histolab Product AB (Gothenburg, Sweden). Alkaline phosphatase-conjugated Fab fragment of sheep anti-DIG antibodies was obtained from Roche Diagnostics Scandinavia AB (Bromma, Sweden). Phenobarbital was purchased from Apoteket AB (Stockholm, Sweden). 2,6-Dichlorobenzonitrile (dichlobenil) was purchased from Aldrich Chemie (Steinheim, Germany) and methimazole was purchased from Sigma (St Louis, MO). Dimethyl sulfoxide (DMSO) was purchased from Merck (Darmstadt, Germany).
Antibody and Probe
A polyclonal antibody against the mouse CYP2A5 was raised in rabbits as previously described (Lang et al. 1989). The specificity of the antibody was tested by Western blotting and immunoinhibition analyses using mouse liver microsomes. The immunoblotting confirmed the presence of a major band corresponding to CYP2A5 (49.5 kD), while the immunoinhibition revealed that the highly homologous CYP2A4 with the same molecular mass was also recognized. It is not known if the antibody crossreacts with CYP2A12 or CYP2G1. The pUC9 plasmid containing CYP2A5 cDNA, used to produce the ISH probes, was kindly provided by Dr. Negishi (LRDT, NIEHS; Research Triangle Park, NC) (Squires and Negishi 1988).
Animals
Male (n = 3) and female (n = 3) NMRI mice were obtained from B&K Universal (Stockholm, Sweden). The mice were 9–10 weeks old and their weight ranged from 37 to 41 g (males) and from 30 to 32 g (females). In the study on olfactory toxicants, the female mice (n = 18) weighed 20–22 g. In addition, male DBA/2J (n = 9) were obtained from Möllegaard Glostrup, Denmark). The DBA/2J mice (20 g body weight) were 7 weeks old. The mice were housed at 22C with a 12-hr light/dark cycle and were given a standard pellet diet and tapwater ad libitum. The animals had at least 1 week of acclimatization. The animal studies were conducted in accordance with the guidelines of the Swedish National Board for Laboratory Animals (CFN) policy LSFS 1988:45. In addition, the studies were approved by the local Ethics Committee for Animal Research.
Untreated Mice
Male (n = 3) and female (n = 3) NMRI mice were anesthetized with gaseous CO2 and exsanguinated. Liver, lung, trachea, and salivary glands were excised and fixed in ice-cold 4% phosphate-buffered formaldehyde (pH 7.4). In addition, the entire nasal regions were dissected by removing the eyes, the integument, the lower jaws, and brain from the skull. The nasal passages were then gently perfused with phosphate-buffered formaldehyde via the nasopharyngeal duct. The nasal regions were decalcified with 5% EDTA in formaldehyde and cut into two blocks by slicing them transversely, perpendicularly to the hard palate through the first palate ridge of the mouse nasal cavity (Young 1981). The tissue blocks were embedded in low melting temperature paraffin. Transverse tissue sections (4 μm) were taken through the nose on levels 2, 3, and 4 according to the system of Young (1981) and were used for IHC and for ISH.
Effects of the Olfactory Toxicants Dichlobenil and Methimazole
Female NMRI mice were injected IP on days 0 and 3 with dichlobenil (25 mg/kg; n = 5) or methimazole (50 mg/kg; n = 5). Control mice were injected IP with DMSO (n = 4) or saline (n = 4). Four days or 2 weeks after the first administration the mice were anesthetized with gaseous CO2 and exsanguinated. The nasal region was excised, fixed, decalcified, and embedded in paraffin. Paraffin sections were used for IHC and histology. Sections used for histology were stained with hematoxylin-eosin or PAS (periodic acid-Schiff reagent).
Effects of the Hepatotoxicants Pyrazole and Phenobarbital
Male DBA/2J mice were injected IP with pyrazole (180 mg/kg; n = 3) three times (0, 24, 48 hr) or phenobarbital (80 mg/kg; n = 3). Control mice (n = 3) were injected IP with saline. At 24 hr after the last injection the mice were anesthetized with gaseous CO2 and exsanguinated. The nasal region and liver were excised and processed for IHC and histology as described above.
Immunohistochemistry
CYP2A5 was localized using the immunoperoxidase procedure with the streptavidin-horseradish peroxidase complex and DAB as the chromogen. Tissue sections were deparaffinized with xylene and hydrated gradually through a graded alcohol series (99.5%, 95%, and 70%). After washes with PBS and 3% Triton X-100 in PBS (PBS-T), to quench endogenous peroxidase activity, the sections were incubated for 30 min with 1% H2O2 in PBS-T. Nonspecific binding was blocked with 4% BSA in PBS for 1 hr. The sections were incubated overnight in a humidified chamber with the primary antibody (dilution 1:700) anti-CYP2A5. The next day the sections were rinsed and washed three times in PBS and PBS-T. The sections were then incubated for 1 hr with biotinylated secondary antibody (biotinylated goat anti-rabbit IgG) and for 30 min with streptavidin-HRP conjugate. The CYP2A5 was localized by the development of substrate-chromogen mixture (DAB). Some of the sections were counterstained with Gill's hematoxylin and were mounted immediately with Pertex. Staining specificity was appraised by substitution of the primary antibody with rabbit normal serum and of the secondary antibody with goat-normal serum.
In Situ Hybridization
Amplification of 1 ng of CYP2A5 cDNA was performed by the polymerase chain reaction (PCR) in a total volume of 50 μl, which contained sense and reverse primers (0.25 μM), 10 × PCR buffer (50 mM KCl, 10 mM Tris-HCl, 0.1% Triton X-100, and 2.5 mM MgCl2), dNTPs (0.2 μM), and 1 μl of DNA polymerase. The double-stranded DNA, including in its sequence the T7 promoter for posterior in vitro transcription of the antisense probe, was amplified using the 5′-TAATACGACTCACTATAGGGAGATGCCATA-3′ and 5′-ACCGCCACCATGCTGACCTCAGGA-3′ primers. Similarly, the primers for the sense probe were 5′-TAATACGACTCACTATAGGGAGAATGCTTGACCTCAGGACTCCT-3′ and 5′-TGCCATAAATAATATCTACT-3′ primers. These primers bind with the nucleotides 1670–1689 (T7 primers) and 1456–1475 of the CYP2A5 cDNA (Squires and Negishi 1988). The samples were incubated at 94C for 4 min and then amplified during 40 cycles (94C for 15 sec; 60C for 30 sec; 68C for 30 sec) with a final extension at 68C for 7 min. Amplified cDNA was ethanol-precipitated and dissolved in a final volume of 10 μl diethyl pyrocarbonate-treated water (DEPC). Single-stranded RNA probes (sense and antisense) labeled with digoxigenin-UTP were synthesized by in vitro transcription using T7 polymerase. ISH was carried out on paraffin-sectioned material according to the protocol described by Wilkinson (1992). In brief, the sections were dewaxed, rehydrated, and then pretreated with proteinase K (10 μg/ml in PBS) for 7 min. After fixation in 4% paraformaldehyde in PBS, the sense and antisense probes, diluted in hybridization buffer (50% formamide, 5 × SSC, 50 μg/ml yeast RNA, 1% SDS, and 50 μg/ml heparin), were added. After hybridization overnight at 65C, the sections were washed in 4 × SSC, followed by washes in 50% formamide, 5 × SSC, 1% SDS, and in 50% formamide and 2 × SSC at 65C. Sections were finally washed in 25 mM Tris-HCl, pH 7.5, containing 140 mM NaCl and 1% Tween-20. The pre-treatment and application of the alkaline phosphatase-conjugated Fab fragments of sheep anti-DIG antibodies, as well as the color development, were performed according to the manufacturer's recommendation (Roche Diagnostics Scandinavia). After color development, the sections were washed in PBS, dehydrated in 99.5% ethanol, cleared with xylene, and mounted in Pertex. Sections of the liver, nose, trachea, and lung were hybridized with the sense probe of CYP2A5 as negative controls.
Evaluation
The HC staining was performed twice in at least five sections per tissue for untreated NMRI mice and was performed three times with at least two sections per tissue for NMRI mice treated with test chemical or vehicle. The IHC staining was also performed twice in at least six sections per tissue for DBA/2J mice treated with test chemical or vehicle. The in situ staining was evaluated in one male and one female NMRI mouse in at least two sections per tissue except for the salivary glands, for which only one female mouse was evaluated. The evaluation of the staining was performed independently by two persons. The tissue sections used for IHC and ISH were photographed in a Leitz DM RXE microscope (Leica) using Nomarski differential interference technique with a digital camera (Hamamatsu; Tokyo, Japan). Images were processed in Adobe Photoshop 6.0 and Adobe Illustrator 10.
Results
Untreated Mice
Table 1 describes the evaluation of the cellular staining for CYP2A5 mRNA and protein in various tissues of NMRI mice. A general pattern of staining was clearly observed in various tissues among the individuals. In addition, there were no major qualitative differences in the staining pattern between male and female mice, except for a somewhat higher level of staining of the female liver. In the nasal passages, distinct staining for CYP2A5 mRNA and protein was detected in the apical part of the sustentacular cells and in the excretory duct and acini of Bowman's glands in the olfactory mucosa, and also in the transitional epithelium (Figures 1A and 2A). In the lateral parts of the olfactory region, the staining of the sustentacular cells and Bowman's glands was less intense.
Table 1.
Summary of immunohistochemistry and in situ hybridization of CYP2A5 in selected mouse tissues a
| Tissue | CYP2A5 protein | CYP2A5 mRNA |
|---|---|---|
| Olfactory mucosa | ||
| Sustentacular cells | ||
| Apical part | ++ + | + + + |
| Foot process | ++ | − |
| Bowman's glands | ||
| Excretory ducts | ++ | + + + |
| Acini | ++ | + + + |
| Olfactory neurons | − | − |
| Basal cells | − | − |
| Axon bundles | − | − |
| Nasal respiratory mucosa | ||
| Respiratory epithelium | − | − |
| Transitional epithelium | + | + |
| Seromucous glands | ||
| Excretory ducts | ++ | + + |
| Acini | − | − |
| Nasal tissues | ||
| Lateral nasal gland | ||
| Excretory ducts | + | + |
| Acini | − | − |
| Maxillary nasal gland | ||
| Excretory ducts | ++ | + + |
| Acini | − | − |
| Maxillary sinus epithelium | − | − |
| Nasolacrimal duct epithelium | ++ | + + |
| Respiratory tract | ||
| Tracheal epithelium | ++ + | + + + |
| Bronchial epithelium | + + | + + |
| Bronchiolar epithelium | + | + |
| Lung parenchyma | − | − |
| Salivary glands | ||
| Sublingual gland | ||
| Excretory ducts | ++ | + |
| Acini | − | − |
| Submandibular gland | ||
| Excretory ducts | + | − |
| Acini | − | |
| Liver | ||
| Centrilobular region | + + (female) | + + (female) |
| + (male) | + (male) | |
| Midzonal region | − | − |
| Periportal region | − | − |
+ + +, intense staining; ++, moderate staining; +, weak staining; -, no staining.
Figure 1.
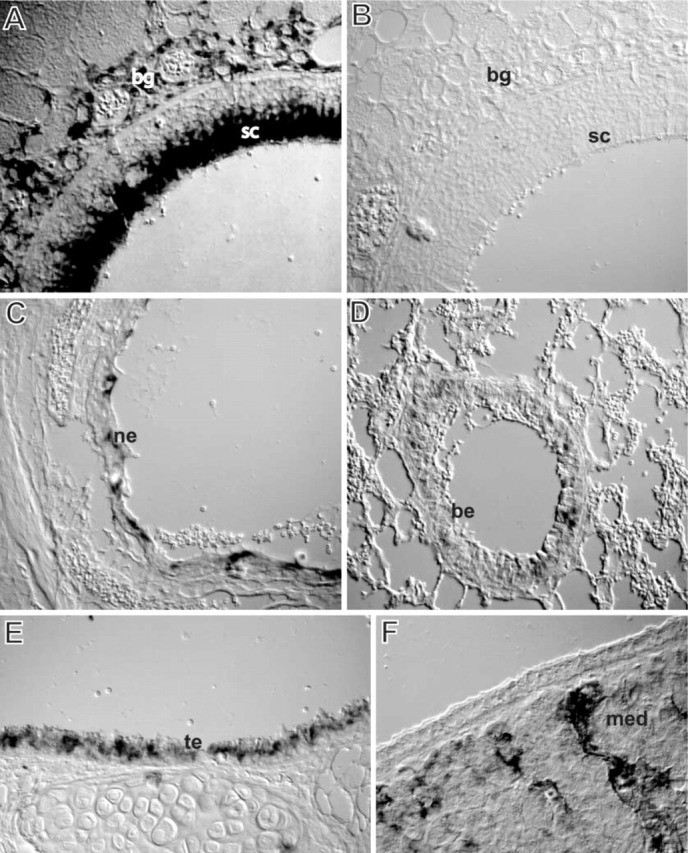
In situ hybridization of CYP2A5 mRNA in selected tissues of NMRI mice. (A) Olfactory mucosa (dorsal meatus): intense staining of the apical part of the sustentacular cells and of the Bowman's glands. (B) Olfactory mucosa (dorsal meatus): control (sense probe) no staining. (C) Nasolacrimal duct: staining of the squamous epithelium. (D) Lung: staining of the bronchiolar columnar epithelium but no staining of the alveolar part. (E) Trachea: staining of the columnar epithelium. (F) Maxillary nasal gland: staining of the excretory ducts but no staining of the acini. bg, Bowman's glands; sc, sustentacular cell; ne, nasolacrimal duct epithelium; be, bronchiolar epithelium; te, tracheal epithelium; med, maxillary nasal gland excretory duct. Original magnification ×400. Nomarski differential interference technique was used on tissue sections that had not been counterstained.
Figure 2.

Immunohistochemical staining of CYP2A5 in selected tissues of NMRI mice. (A) Olfactory mucosa (dorsal meatus): intense staining of the apical part of the sustentacular cells and their foot processes and of the Bowman's glands and their excretory ducts. (B) Olfactory mucosa (dorsal meatus): control (substitution of the primary antibody with normal serum) no staining. (C) Nasolacrimal duct: staining of the squamous epithelium. (D) Sublingual salivary gland: staining of the excretory ducts but no staining of acini. Original magnification ×400. (E) Trachea: staining of the columnar epithelium. (F) Lung: staining of the bronchiolar columnar epithelium but no staining of the alveolar part. Original magnification ×200. Nomarski differential interference technique was used on tissue sections that had not been counterstained. bg, Bowman's glands; sc, sustentacular cell; ne, nasolacrimal duct epithelium; sed, sublingual gland excretory duct; te, tracheal epithelium; be, bronchiolar epithelium.
In the basal part of the olfactory epithelium there was distinct IHC staining, most likely corresponding to the foot processes of the sustentacular cells, whereas there was no in situ staining at this site (Figures 1A and 2A). Otherwise, there was an almost identical localization of CYP2A5 mRNA and protein, indicating that regulation of expression of the enzyme at these sites is pretranslational and that the staining is specific.
The nasal respiratory mucosa presented no or weak staining in the epithelium and in the excretory ducts of seromucous glands in the lamina propria. There was also staining of excretory ducts of nasal glands around the maxillary sinuses and in the nasolacrimal duct epithelium (Figures 1C and 2C). In the lower respiratory tract there was staining of the apical parts of the columnar epithelium of the trachea (Figures 1E and 2E), bronchi, and bronchioles (Figures 1D and 2F). Staining of the apical part of the epithelium corresponded to non-ciliated Clara cells protruding into the lumen. Staining of the columnar epithelium decreased along the respiratory airways and the lowest level of staining occurred in the bronchioles. There was also staining of the striated and excretory ducts in the salivary glands (Figure 2D) and in the centrilobular region of the liver (data not shown).
No staining was observed in the lung parenchyma of the lung (Figure 1D), the nasal-associated lymphoid tissues, squamous epithelium of the oral cavity, acini of nasal glands around the maxillary sinuses, and acini of salivary glands. In addition, staining was not observed in the tissues after omission of the primary or the secondary antibody for the IHC studies and the use of the sense probe for the ISH (examples shown in Figures 1B and 2B).
Effects of the Olfactory Toxicants Dichlobenil and Methimazole
Dichlobenil. As previously reported, dichlobenil selectively targeted the dorsomedial part of the olfactory region whereas the lateral part was undamaged (Bergman et al. 2002). Four days after exposure to dichlobenil, the olfactory epithelium was thin and disorganized in the dorsomedial part of the olfactory region. The Bowman's glands were necrotic or had disappeared. At 2 weeks after administration there was an atypical ciliated respiratory-like epithelium and frequent invaginations of respiratory-like epithelium appeared in the dorsomedial region. In addition, there was fibrosis and the Bowman's glands had disappeared in the dorsomedial region. In the vehicle-treated controls the olfactory mucosa was intact.
In the present study, 4 days after treatment with dichlobenil (Figure 3D) there was staining for CYP2A5 in some cells in the damaged and disorganized epithelium in the dorsomedial part of the olfactory region. There was no staining of the necrotic Bowman's glands in the lamina propria. Intact Bowman's glands in the undamaged lateral part of the olfactory region displayed weak staining, similar to that of control. At 2 weeks after administration, there was staining of the atypical respiratory-like epithelium and of the invaginations into the lamina propria. The stained cells were columnar. There was no staining of the fibrotic lamina propria in the dorsomedial part of the olfactory region (Figure 3F). Furthermore, there was no increased staining of the epithelium or glands in the border zone between damaged and undamaged mucosa 2 weeks after administration. In vehicle-treated NMRI mice (Figure 3B) the staining for CYP2A5 protein was similar to that observed in untreated NMRI mice.
Figure 3.

Immunohistochemical staining of CYP2A5 in the olfactory mucosa of NMRI mice treated with the olfactory toxicants dichlobenil or methimazole. (A) Vehicle-treated (saline) control. Olfactory mucosa (dorsal meatus): intense staining of the apical part of the sustentacular cells and their foot processes and of the Bowman's glands and their excretory ducts in the lamina propria. (B) Vehicle-treated (DMSO) control. Olfactory mucosa (dorsal meatus): intense staining of the apical part of the sustentacular cells and their foot processes. Intense staining of the Bowman's glands and their excretory ducts in the lamina propria. (C) Four days after treatment with methimazole (2 × 50 mg/kg). Olfactory mucosa (dorsal meatus): weak staining of the damaged olfactory epithelium and no staining of the damaged lamina propria. (D) Four days after treatment with dichlobenil (2 × 25 mg/kg). Olfactory mucosa (dorsal meatus): weak staining of the thin and disorganized epithelium. No staining in the damaged lamina propria. (E) Two weeks after treatment with methimazole (2 × 50 mg/kg). Olfactory mucosa (dorsal meatus): sustentacular cells are stained as well as columnar cells in the disorganized epithelium in the dorsomedial part. The staining of the Bowman's glands is similar to control. (F) Two weeks after treatment with dichlobenil (2 × 25 mg/kg). Olfactory mucosa (dorsal meatus): staining of the atypical respiratory-like epithelium and invaginations into the lamina propria. No staining of the fibrotic lamina propria. bg, Bowman's glands; sc, sustentacular cell; in, invagination; ep, epithelium. Original magnification ×400. Nomarski differential interference technique was used on tissue sections that had not been counterstained.
Methimazole. As previously reported, there was a thin and disorganized ol-factory epithelium in the ol factory region 4 days after administration of methimazole (Bergman et al. 2002). Most of the Bowman's glands had disappeared but a few scattered glands were observed. After 2 weeks the epithelium in the dorsomedial part was thin and disorganized and included cysts containing a homogeneous material, whereas the epithelium in the lateral part of the olfactory region was restored. In the lamina propria there were many Bowman's glands with an intact appearance. In the vehicle-treated controls the olfactory mucosa was intact.
In the present study, at 4 days after treatment with methimazole (Figure 3C) there was only weak staining for CYP2A5 in some cells of the regenerating olfactory epithelium but no staining of the necrotic lamina propria. At 2 weeks after administration (Figure 3E), staining was present in sustentacular cells in the regenerated olfactory epithelium and in some columnar cells of the disorganized epithelium in the dorsomedial part. The stained cells were focally distributed and in some regions the sustentacular cells did not display any stained foot processes. Most of the Bowman's glands were stained but scattered acini displayed no staining. In vehicle-treated NMRI mice (Figure 3B), the staining for CYP2A5 protein was similar to that observed in untreated NMRI mice.
Effects of the Hepatic CYP2A5 Inducers Pyrazole and Phenobarbital
Histological evaluation revealed that the olfactory mucosa of DBA/2J mice pretreated with pyrazole or phenobarbital was similar to that of saline-treated control animals. In all animals there was an intense PAS reaction of the contents of the Bowman's glands. In the livers of pyrazole- and phenobarbital-pretreated mice there was a decreased PAS reaction (marker for glycogen depletion) in the centrilobular parts. The decrease was most marked in the pyrazole-treated mice. In the saline-treated controls there was a homogeneous reaction for PAS throughout the liver.
The staining for CYP2A5 protein in the olfactory mucosa was not increased in the pyrazole- or phenobarbital-treated mice compared to the vehicle-treated controls. In the pyrazole-treated mice there was markedly increased staining of the CYP2A5 protein in the centrilobular region of the liver, especially in cells adjacent to the damaged centrilobular region. In the phenobarbital-treated mice there was only slightly increased staining of the centrilobular region of the liver. In the saline-treated DBA/2J mice, the localization of CYP2A5 protein in the nasal region and liver was similar to that observed in NMRI mice.
Discussion
The results of the present investigation revealed a highly specific expression of CYP2A5 mRNA and protein in the upper and lower respiratory tract, the excretory ducts of salivary and nasal glands, and in the nasolacrimal duct epithelium. There was a predominant expression of CYP2A5 in the olfactory mucosa; the apical parts of the sustentacular cells and the acini and excretory ducts of Bowman's glands expressed CYP2A5 mRNA and protein. Interestingly, there was no staining for mRNA in the basal part of the olfactory epithelium, although the protein was present at this site, suggesting a transfer of CYP2A5 protein from the apical part of the sustentacular cells to the foot processes. Pretreatment with the hepatic CYP2A5 inducers pyrazole and phenobarbital did not change the expression pattern of the enzyme in the olfactory mucosa, whereas the olfactory toxicants dichlobenil and methimazole induced characteristic changes in the expression pattern at this site.
The predominant expression of CYP2A5 in the mouse olfactory mucosa is in agreement with previous studies demonstrating that CYP2A5 is a major CYP in this tissue (Walters et al. 1993; Thornton-Manning et al. 1997; Gu et al. 1998). It cannot be excluded that the antibody or the cRNA probe used in this study might crossreact with CYP2A12 and CYP2A4. However, these isoforms are reported to be expressed at much lower levels than CYP2A5 in the mouse olfactory mucosa (Ding et al. 1996). Therefore, the signals detected in the olfactory mucosa are a bona fide reflection of the CYP2A5 level, with only a minor contribution of other CYP2A isoforms. No data are available on the cellular expression of CYP2A12 and CYP2A4 in other sites of the respiratory tract. Gu and co-workers (1998) have reported that several antibodies against CYP2A also react with CYP2G1. This has, however, not been determined for the antibody used in this study.
The predominant expression of CYP2A5 and some minor CYP forms in the olfactory mucosa may be of importance for the protection of neurons in the olfactory pathways against chemically induced damage (Adams et al. 1991; Chen et al. 1992). A rapid biotransformation would increase the clearance of airborne compounds in the neuroepithelium but might also lead to a decreased transfer of potential neurotoxicants to the brain via the olfactory pathways. The olfactory neurons have dendrites projecting into the olfactory mucus and axons projecting directly into the olfactory bulb. This connection may therefore be a portal of entry of drugs and toxicants into the brain, thus circumventing the blood-brain barrier. A significant uptake of some drugs and chemicals has been reported in the ipsilateral olfactory bulb after a unilateral intranasal instillation (Dahlin et al. 2000; Bergstrom et al. 2002).
Previous studies have demonstrated a high rate of metabolic activation of some CYP2A5 and CYP2A3 substrates, such as the herbicide dichlobenil and the tobacco-specific carcinogen NNK, into reactive intermediates in the rodent olfactory mucosa (Eriksson and Brittebo 1991; Ding et al. 1996; Gu et al. 1998). Light microscopic autoradiography studies have revealed a preferential localization of protein adducts of radio-labeled dichlobenil and NNK in Bowman's glands (Tjalve et al. 1985; Brandt et al. 1990). Furthermore, IHC studies have reported a selective localization of NNK DNA adducts in these glands (Van Benthem et al. 1994). The alkylation of vital macromolecules in the Bowman's glands may result in a changed or impaired function leading to detrimental consequences, and both dichlobenil and NNK are reported to initially damage these glands (Belinsky et al. 1987; Brandt et al. 1990).
The present study revealed that pretreatment with the olfactory toxicants dichlobenil and methimazole induced characteristic changes in the olfactory expression pattern of CYP2A5. At 4 days after treatment with dichlobenil or methimazole there was no expression of CYP2A5 in the damaged Bowman's glands in the lamina propria. At 2 weeks after treatment with dichlobenil there was no expression of the enzyme in the fibrotic lamina propria. In contrast, 2 weeks after treatment with methimazole the expression of CYP2A5 in the regenerated Bowman's glands was similar to that in the controls, confirming that methimazole-induced effects at this site are reversible.
Interestingly, our study also revealed that there was distinct CYP2A5 expression in the damaged and disorganized epithelium 4 days after the administration of dichlobenil and methimazole. At 2 weeks after treatment with methimazole, the neuroepithelium was still thin and disorganized in the dorsomedial part. CYP2A5 was expressed in sustentacular cells in the restored part and in columnar cells in the disorganized epithelium. A slow recovery of CYP2A expression in the sustentacular cells has also been observed in rats 4–6 weeks after treatment with the reversible olfactory toxicant methyl bromide (Schwob et al. 1995). However, no recovery of CYP2A expression was reported in methyl bromide-induced respiratory-like epithelium in the olfactory region of rats (Schwob et al. 1995). In contrast, the present results demonstrated that the dichlobenil-induced respiratory-like epithelium expressed CYP2A5. At 2 weeks after treatment with dichlobenil there was expression of CYP2A5 in the atypical respiratory-like epithelium and in invaginations of the epithelium into the lamina propria. In olfactory mucosa recovering from a toxic insult, the sustentacular cells have been suggested to originate from both the Bowman's glands and basal cells (Huard et al. 1998). Because no intact Bowman's glands were present in the fibrotic lamina propria, these glands are most likely not stem cells for the respiratory-like epithelium expressing CYP2A5 in dichlobenil-treated mice. These respiratory-like cells could thus originate from undamaged basal cells, but the molecular mechanisms and lineage relationships involved in the respiratory metaplasia are unclear. In addition, because the respiratory epithelium of vehicle-treated mice showed negligible expression of CYP2A5, it seems less likely that the CYP2A5-expressing cells in the respiratory-like epithelium are related to the columnar cells in the normal respiratory epithelium.
Recent studies have indicated that the induction of hepatic CYP2A5 by typical inducers is not due to the compounds per se but instead is related to hepatotoxic effects (Gilmore et al. 2003). The present results demonstrated that the hepatic CYP2A5 inducers pyrazole and phenobarbital neither damaged the olfactory mucosa nor changed the expression of CYP2A5 at this site. The lack of induction may be related to the lack of toxicity of these compounds in the olfactory mucosa. Gilmore and co-workers (2003) have suggested that an increased CYP2A5 expression in areas adjacent to damaged hepatocytes may be related to oxidative injury in these cells. Increased CYP2A6 expression has also been found in areas adjacent to liver tumors (Kojo et al. 1998). Previous studies have revealed a distinct border zone with markedly increased neurogenesis between damaged and undamaged parts of the olfactory region 2 weeks after exposure to the olfactory toxicant dichlobenil (Bergman et al. 2002). However, there was no increase in expression of CYP2A5 in the neuroepithelium or Bowman's glands in this border zone of dichlobenil-treated mice.
Site-selective and region-specific differences are often reported for CYPs in the respiratory tissues. The present study also revealed a decreasing expression of CYP2A5 mRNA and protein along the epithelial linings of the trachea, bronchi, and bronchioles. CYP2A5 expression was not observed in the lung alveolar region, indicating that alveolar cells, endothelial cells, and macrophages do not express this enzyme. The metabolism of CYP2A5 substrates is generally low in the lung, but it should be noted that a distinct localization of protein adducts of some CYP2A5/3 substrates, dichlobenil and NNK, in the epithelium of the trachea and bronchi/bronchioles of rodents has been reported (Bakke et al. 1988). NNK is also known to induce tumors in the lung of rodents (Belinsky et al. 1990).
The present study also revealed a weak expression in the excretory ducts of seromucous glands in the nasal septum as well as in sublingual and submandibular glands, whereas no staining occurred in the acini. There was also CYP2A5 expression in the squamous epithelium in the nasolacrimal duct and weak expression in the excretory ducts of the glands around the maxillary sinuses. Little is known about the metabolism and effects of chemicals in excretory ducts. In the epithelium of the rat nasolacrimal duct, a selective localization of NNK has been reported (Lofberg et al. 1982), suggesting that a biotransformation can occur at this site.
In summary, the results of the present study demonstrated a cell-specific expression of CYP2A5 mRNA and protein in the mouse respiratory tract, excretory ducts of nasal and salivary glands, and the nasolacrimal duct epithelium. In the nasal region differential expression was observed. There was marked expression in sustentacular cells and Bowman's glands in the olfactory mucosa, whereas in the respiratory mucosa there was no or only weak expression. Typical hepatic CYP2A5 inducers did not change the expression pattern in the olfactory mucosa. In contrast, olfactory toxicants induced characteristic changes. The damaged Bowman's glands did not express CYP2A5, whereas the damaged epithelium expressed the enzyme. The results also revealed a decreasing expression of CYP2A5 along the epithelial linings of trachea and lower respiratory tract. The cell-specific expression of CYP2A5 indicates that this CYP isoform plays a key role in determining the susceptibility of the respiratory tract after exposure to air- and blood-borne protoxicants and procarcinogens. Indeed, previous reports have demonstrated a cell-specific localization of protein and DNA adducts of some CYP2A5 substrates in the respiratory tract.
Acknowledgments
Supported by The Swedish Research Council (EBB) and The Italian National Council of Research (EP).
Literature Cited
- Adams DR, Jones AM, Plopper CG, Serabjit—Singh CJ, Philpot RM. (1991) Distribution of cytochrome P-450 monoxygenase enzymes in the nasal mucosa of hamster and rat. Am J Anat 190:291–298 [DOI] [PubMed] [Google Scholar]
- Bakke JE, Larsen GL, Struble C, Feil VJ, Brandt I, Brittebo EB. (1988) Metabolism of 2,6-dichlorobenzonitrile, 2,6-dichlorothiobenzamide in rodents and goats. Xenobiotica 18:1063–1075 [DOI] [PubMed] [Google Scholar]
- Belinsky SA, Foley JF, White CM, Anderson MW, Maronpot RR. (1990) Dose-response relationship between O6-methylguanine formation in Clara cells and induction of pulmonary neoplasia in the rat by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res 50:3772–3780 [PubMed] [Google Scholar]
- Belinsky SA, Walker VE, Maronpot RR, Swenberg JA, Anderson MW. (1987) Molecular dosimetry of DNA adduct formation and cell toxicity in rat nasal mucosa following exposure to the tobacco specific nitrosamine 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone and their relationship to induction of neoplasia. Cancer Res 47:6058–6065 [PubMed] [Google Scholar]
- Bereziat JC, Raffalli F, Schmezer P, Frei E, Geneste O, Lang MA. (1995) Cytochrome P450 2A of nasal epithelium: regulation and role in carcinogen metabolism. Mol Carcinogen 14:130–139 [DOI] [PubMed] [Google Scholar]
- Bergman U, Ostergren A, Gustafson AL, Brittebo B. (2002) Differential effects of olfactory toxicants on olfactory regeneration. Arch Toxicol 76:104–112 [DOI] [PubMed] [Google Scholar]
- Bergstrom U, Franzén A, Sjoblom M, Brittebo EB. (2002) Drug targeting to the brain: transfer of picolinic acid to the brain along the olfactory pathways. J Drug Target 10:469–478 [DOI] [PubMed] [Google Scholar]
- Brandt I, Brittebo EB, Feil VJ, Bakke JE. (1990) Irreversible binding and toxicity of the herbicide dichlobenil (2,6-dichlorobenzonitrile) in the olfactory mucosa of mice. Toxicol Appl Pharmacol 103:491–501 [DOI] [PubMed] [Google Scholar]
- Brittebo EB, Lofberg B, Tjalve H. (1981) Sites of metabolism of N-nitrosodiethylamine in mice. Chem Biol Interact 34:209–221 [DOI] [PubMed] [Google Scholar]
- Chen Y, Getchell ML, Ding X, Getchell TV. (1992) Immunolocalization of two cytochrome P450 isozymes in rat nasal chemosensory tissue. Neuroreport 3:749–752 [DOI] [PubMed] [Google Scholar]
- Dahlin M, Bergman U, Jansson B, Bjork E, Brittebo E. (2000) Transfer of dopamine in the olfactory pathway following nasal administration in mice. Pharmacol Res 17:737–742 [DOI] [PubMed] [Google Scholar]
- Ding X, Kaminsky LS. (2003) Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol 43:149–173 [DOI] [PubMed] [Google Scholar]
- Ding X, Spink DC, Bhama JK, Sheng JJ, Vaz AD, Coon MJ. (1996) Metabolic activation of 2,6-dichlorobenzonitrile, an olfactory-specific toxicant, by rat, rabbit, and human cytochromes P450. Mol Pharmacol 49:1113–1121 [PubMed] [Google Scholar]
- Donato MT, Viitala P, Rodriguez—Antona C, Lindfors A, Castell JV, Raunio H, Gomez—Lechon MJ, et al. (2000) CYP2A5/CYP2A6 expression in mouse and human hepatocytes treated with various in vivo inducers. Drug Metab Dispos 28:1321–1326 [PubMed] [Google Scholar]
- Eriksson C, Brittebo EB. (1991) Metabolic activation of the herbicide dichlobenil in the olfactory mucosa of mice and rats. Chem Biol Interact 79:165–177 [DOI] [PubMed] [Google Scholar]
- Felicia ND, Rekha GK, Murphy SE. (2000) Characterization of cytochrome P450 2A4 and 2A5-catalyzed 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism. Arch Biochem Biophys 384:418–424 [DOI] [PubMed] [Google Scholar]
- Fernandez—Salguero P, Gonzalez FJ. (1995) The CYP2A gene subfamily: species differences, regulation, catalytic activities and role in chemical carcinogenesis. Pharmacogenetics 5:123–128 [DOI] [PubMed] [Google Scholar]
- Getchell ML, Chen Y, Ding X, Sparks DL, Getchell TV. (1993) Immunohistochemical localization of a cytochrome P-450 isozyme in human nasal mucosa: age-related trends. Ann Otol Rhinol Laryngol 102:368–374 [DOI] [PubMed] [Google Scholar]
- Gilmore WJ, Hartmann G, Piquette—Miller M, Marriott J, Kirby GM. (2003) Effects of lipopolysaccharide-stimulated inflammation and pyrazole-mediated hepatocellular injury on mouse hepatic Cyp2a5 expression. Toxicology 184:211–226 [DOI] [PubMed] [Google Scholar]
- Godoy W, Albano RM, Moraes EG, Pinho PR, Nunes RA, Saito EH, Higa C, et al. (2002) CYP2A6/2A7 and CYP2E1 expression in human oesophageal mucosa: regional and inter-individual variation in expression and relevance to nitrosamine metabolism. Carcinogenesis 23:611–616 [DOI] [PubMed] [Google Scholar]
- Gu J, Zhang QY, Genter MB, Lipinskas TW, Negishi M, Nebert DW, Ding X. (1998) Purification and characterization of heterologously expressed mouse CYP2A5 and CYP2G1: role in metabolic activation of acetaminophen and 2,6-dichlorobenzonitrile in mouse olfactory mucosal microsomes. J Pharmacol Exp Ther 285:1287–1295 [PubMed] [Google Scholar]
- Honkakoski P, Autio S, Juvonen R, Raunio H, Gelboin HV, Park SS, Pelkonen O, et al. (1988) Pyrazole is different from acetone and ethanol as an inducer of the polysubstrate monooxygenase system in mice: evidence that pyrazole-inducible P450Coh is distinct from acetone-inducible P450ac. Arch Biochem Biophys 267:589–598 [DOI] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. (1998) Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol 400:469–486 [PubMed] [Google Scholar]
- Kirby GM, Chemin I, Montesano R, Chisari FV, Lang MA, Wild CP. (1994) Induction of specific cytochrome P450s involved in aflatoxin B1 metabolism in hepatitis B virus transgenic mice. Mol Carcinogen 11:74–80 [DOI] [PubMed] [Google Scholar]
- Kojo A, Heiskanen R, Rytkonen AL, Honkakoski P, Juvonen R, Lang M. (1991) Inducibility of P450Coh by pyrazole and its derivatives. Biochem Pharmacol 42:1751–1759 [DOI] [PubMed] [Google Scholar]
- Kojo A, Viitala P, Pasanen M, Pelkonen O, Raunio H, Juvonen R. (1998) Induction of CYP2A5 by pyrazole and its derivatives in mouse primary hepatocytes. Arch Toxicol 72:336–341 [DOI] [PubMed] [Google Scholar]
- Koskela S, Hakkola J, Hukkanen J, Pelkonen O, Sorri M, Saranen A, Anttila S, et al. (1999) Expression of CYP2A genes in human liver and extrahepatic tissues. Biochem Pharmacol 57:1407–1413 [DOI] [PubMed] [Google Scholar]
- Lang MA, Juvonen R, Jarvinen P, Honkakoski P, Raunio H. (1989) Mouse liver P450Coh: genetic regulation of the pyrazole-inducible enzyme and comparison with other P450 isoenzymes. Arch Biochem Biophys 271:139–148 [DOI] [PubMed] [Google Scholar]
- Lang MA, Pelkonen O. (1999) Metabolism of xenobiotics and chemical carcinogenesis. In Vineis P, Malats N, Lang MA, d' Errico A, Caporaso N, Cuzick J, Boffetta P, eds. Metabolic Polymorphisms and Susceptibility to Cancer. Lyons, IARC, 13–22 [Google Scholar]
- Liu C, Zhuo X, Gonzalez FJ, Ding X. (1996) Baculovirus-mediated expression and characterization of rat CYP2A3 and human CYP2a6: role in metabolic activation of nasal toxicants. Mol Pharmacol 50:781–788 [PubMed] [Google Scholar]
- Lofberg B, Brittebo EB, Tjalve H. (1982) Localization and binding of N'-nitrosonornicotine metabolites in the nasal region and in some other tissues of Sprague-Dawley rats. Cancer Res 42:2877–2883 [PubMed] [Google Scholar]
- Pinto LF, Moraes E, Albano RM, Silva MC, Godoy W, Glisovic T, Lang MA. (2001) Rat oesophageal cytochrome P450 (CYP) monooxygenase system: comparison to the liver and relevance in N-nitrosodiethylamine carcinogenesis. Carcinogenesis 22:1877–1883 [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. (1995) Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol 359:15–37 [DOI] [PubMed] [Google Scholar]
- Squires EJ, Negishi M. (1988) Reciprocal regulation of sex-dependent expression of testosterone 15 alpha-hydroxylase (P-450(15 alpha)) in liver and kidney of male mice by androgen. Evidence for a single gene. J Biol Chem 263:4166–4171 [PubMed] [Google Scholar]
- Su T, Sheng JJ, Lipinskas TW, Ding X. (1996) Expression of CYP2A genes in rodent and human nasal mucosa. Drug Metab Dispos 24:884–890 [PubMed] [Google Scholar]
- Thornton-Manning JR, Nikula KJ, Hotchkiss JA, Avila KJ, Rohrbacher KD, Ding X, Dahl AR. (1997) Nasal cytochrome P450 2A: identification, regional localization, and metabolic activity toward hexamethylphosphoramide, a known nasal carcinogen. Toxicol Appl Pharmacol 142:22–30 [DOI] [PubMed] [Google Scholar]
- Thulke-Gross M, Hergenhahn M, Tilloy-Ellul A, Lang M, Bartsch H. (1998) Pyrazole-inducible proteins in DBA/2 mouse liver bind with high affinity to the 3′-untranslated regions of the mRNAs of coumarin hydroxylase (CYP2A5) and c-jun. Biochem J 331:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjalve H, Castonguay A, Rivenson A. (1985) Microautoradiographic localization of bound metabolites in the nasal cavities of F344 rats treated with the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. J Natl Cancer Inst 74:185–189 [PubMed] [Google Scholar]
- Van Benthem J, Feron VJ, Leeman WR, Wilmer JW, Vermeulen E, den Engelse L, Scherer E. (1994) Immunocytochemical identification of DNA adducts, O6-methylguanine and 7-methylguanine, in respiratory and other tissues of rat, mouse and Syrian hamster exposed to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis 15:2023–2029 [DOI] [PubMed] [Google Scholar]
- Walters E, Buchheit K, Maruniak JA. (1993) Olfactory cytochrome P-450 immunoreactivity in mice is altered by dichlobenil but preserved by metyrapone. Toxicology 81:113–122 [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. (1992) In situ hybridization and the three-dimensional reconstruction of serial sections. In Wilkinson DG, ed. In Situ Hybridization. A Practical Approach. Oxford, IRL Press, 155–171 [Google Scholar]
- Young JT. (1981) Histopathologic examination of the rat nasal cavity. Fund Appl Toxicol 1:309–312 [DOI] [PubMed] [Google Scholar]
- Zhuo X, Gu J, Zhang QY, Spink DC, Kaminsky LS, Ding X. (1999) Biotransformation of coumarin by rodent and human cytochromes P-450: metabolic basis of tissue-selective toxicity in olfactory mucosa of rats and mice. J Pharmacol Exp Ther 288:463–471 [PubMed] [Google Scholar]


