Abstract
We have proposed a new model of intestinal sugar absorption in which high sugar concentrations promote rapid insertion of the facilitative transporter GLUT2 into the brush-border membrane so that absorptive capacity is precisely regulated to match dietary intake during the assimilation of a meal. However, location of GLUT2 at the brush border by immunocytochemistry has been problematical. We report that control of rapid GLUT2 trafficking and the use of an antibody to a sequence within the large extracellular loop of GLUT2 permits localization of GLUT2 at the brush border. To reveal brush-border GLUT2 fully, it is necessary to digest the sugar chain at the glycosylation site close to the antigenic site. In this way, we have demonstrated by immunocytochemistry PKC-dependent changes in the regulation of brush-border GLUT2 in rat jejunum that correspond to those seen by Western blotting. The functional and immunocytochemical data are now reconciled.
Keywords: intestine, sugar transport, fructose, GLUT5, SGLT1
We have proposed a new model for sugar absorption across the brush-border membrane of the rat small intestine. When intestine is challenged with high concentrations of glucose, the facilitative transporter GLUT2 is rapidly activated and inserted into the brush-border membrane. Regulation of the GLUT2-facilitated component of absorption involves a PKC-dependent pathway that is activated by glucose transport through SGLT1 (Helliwell et al. 2000a; Kellett and Helliwell 2000; Helliwell et al. 2003). Inhibition of SGLT1 with phloridzin diminishes the level of GLUT2 at the brush-border membrane and so inhibits the facilitated as well as the active component. SGLT1 is therefore seen to exert an important control function in addition to its established functions as scavenger and transporter (Kellett 2001). Regulation also involves PI 3-kinase, ERK, and p38 signaling pathways (Helliwell et al. 2000b) and is altered in experimental diabetes (Corpe et al. 1996). The ability to detect regulation of the facilitated component depends crucially on the design of the perfusion experiment because regulation is observed in low (physiological) but not in high stress perfusions (Helliwell and Kellett 2002).
GLUT2 is a high-Km, high-capacity transporter, which displays a normal Michaelis-Menten-type saturation response in basolateral membrane vesicles. However, the activation and rapid insertion of GLUT2 into the brush-border membrane results in a cooperative response by which absorptive capacity is matched precisely to dietary intake such that GLUT2 affords the major route of absorption at high glucose concentrations (Kellett and Helliwell 2000). In this model, because GLUT2 transports not only glucose but also fructose (Cheeseman 1993), it follows that fructose absorption across the brush-border membrane is mediated not only by GLUT5, which is highly specific for fructose, but also by GLUT2 (Helliwell et al. 2000a,b; Au et al. 2002).
A feature of the GLUT2-facilitated component model of sugar absorption is that GLUT2 traffics very rapidly (t1/2 ∼ a few minutes) to and from the brush-border membrane in response to the presence or absence, respectively, of glucose or effectors of the intracellular signaling pathways in the perfusate. In addition, and of equal importance, the intrinsic activity of GLUT2 is rapidly regulated over a nine-fold range in response to the same stimuli (Helliwell et al. 2000b). Of particular note, when intestine is excised, most of the GLUT2 traffics rapidly away from the brush-border membrane because of the loss of influence of activating hormones or sugars. Moreover, the minority of GLUT2 that remains has diminished intrinsic activity. Failure to control the trafficking away or inactivation of GLUT2 provides one reason why a role for GLUT2 in brush-border membrane absorption has been previously overlooked. However, two very recent studies in which trafficking and inactivation were controlled by the use of ice-cold conditions have shown that a GLUT2-mediated transport component can be readily detected in membrane vesicles in rat (Au et al. 2002) and mice (Dr. E. Brot-Laroche, personal communication). Similarly, the cytochalasin B-sensitive glucose transport system BBS2 in guinea pig brush-border membrane vesicles (Brot-Laroche et al. 1986,1988) and also in pig (Dr. E. Brot-Laroche, personal communication) seems likely to be GLUT2.
However, one notable piece of evidence was seemingly at variance with the battery of evidence that GLUT2 can be present at the brush-border membrane. Thorens et al. (1988) were the first to clone GLUT2 and to establish its localization in intestine and kidney by immunocytochemistry (ICC). Using an antibody raised to the C-terminal sequence of GLUT2, they detected GLUT2 exclusively at the basolateral membrane in rat duodenum and in kidney proximal tubule (Thorens et al. 1990a,b). GLUT2 was not observed at the brush-border membrane in either intestine or kidney. Therefore, the only direct demonstration that GLUT2 could be present at the brush-border membrane was by cell surface biotinylation (Helliwell et al. 2000a). Very recently, however, in an ICC study using an antibody to a sequence within the large extracellular loop of GLUT2, Au et al. (2002) have reported strong labeling at the terminal web related to a light glucose-induced labeling of the brush-border membrane in rat jejunum. We have therefore addressed the question of why, since the functional data are so clear, it has not been possible to visualize GLUT2 clearly at the brush-border membrane by ICC. The answer has enabled us to demonstrate strong, specific labeling of GLUT2 at the brush-border membrane.
Materials and Methods
Animals
All procedures used conformed to the UK Animals (Scientific Procedures) Act 1986. Male Wistar rats (240–260 g) were fed ad libitum on standard Bantin and Kingman (Hull, UK) rat and mouse diet with free access to water.
Perfusion Experiments
Rats were anesthetized by an IP injection of a mixture of 1.0 ml Hypnorm (Janssen; High Wycombe, UK) and 0.4 ml Hypnovel (Roche; Welwyn, UK) per kg. Jejunum was then perfused in vitro as described previously (Helliwell et al. 2000a). PKC activators and inhibitors take some time to become effective in whole tissue and there is a limit to the time of viability for isolated loops in vitro. Initially, therefore, jejunum was first perfused luminally in vivo with 5 mM d-fructose (plus 1 mM β-hydroxybutyrate as an energy source) to allow drugs time to work while maintaining viability. The perfusion system was a gas-segmented, single-pass system with perfusate and gas flow rates of 0.75 and 0.38 ml min−1, respectively. Jejunum was perfused with 200 nM PMA for 30 min or with 2 μM chelerythrine for 45 min. When they were perfused in combination, PMA was added to the perfusate 15 min after the inhibitors. At the end of the in vivo treatment period, the cannulated loop was then excised and perfused in vitro using the gas-segmented, recirculated flow system: 5 mM d-fructose and the combination of drugs at the end of in vivo treatment period were perfused luminally through the jejunum for 30 min. The flow rates of perfusate and gas were 7.5 and 3.4 ml min−1, respectively. Perfusion was switched to a second reservoir of the same perfusate containing PLP fixative (2% paraformaldehyde/0.075 M lysine/0.01 M sodium m-periodate in PBS; McLean and Nakane 1974). Tissue was fixed for 15 min at a perfusion rate of 0.75 ml min−1.
Immunocytochemistry
After initial fixation, jejunum was excised and washed in 10 ml fixative to remove surplus blood on the outside. Segments approximately 2 cm long were slit longitudinally on a glass plate and 2-mm squares then incubated in fresh fixative overnight at 4C. The squares were transferred to PBS containing 20% sucrose and incubated overnight at 4C. The fixed and cryoprotected squares were then embedded in OCT in foil cups, frozen in isopentane, and stored at −80C.
The protocol for detection of antigen was as follows. Seven-μm sections were placed in a Coplin jar containing citrate buffer (0.01 M citric acid plus 0.01 M trisodium citrate, pH 6.0) and microwaved for 10 min at 450 W. The slides were cooled, washed in running tapwater, and then rinsed in deionized water and finally in PBS. Sections were blocked in blocking buffer (4% v/v Vector S-100 goat serum, 1% w/v BSA, 1% w/v gelatin, and 0.1% Triton X-100 in PBS) overnight in a humidifying chamber maintained at 4C. Slides were brought slowly to room temperature so that the blocking buffer gently melted, and sections were incubated with primary antibody at the stated concentration and time in a humidifying chamber. After washing slides three times for 10 min in PBS/blocking buffer (1:1, v/v), sections were incubated in FITC-conjugated goat anti-rabbit IgG (1:100; Sigma, Dorset, U.K.) for 1 hr and slides were again washed three times for 10 min in PBS. Sections were mounted in Vectashield (Vector Laboratories; Burlington, CA) under a sealed coverslip and stored in the dark at 4C. Fluorescence micrographs were taken using a BioRad (Hercules, CA) MRC 1000 confocal microscope.
In some instances, when sections were to be probed with extracellular loop antibody, sugars were digested away before microwaving by use of recombinant N-glycosidase F (Roche). Sections were incubated at 37C with glycosidase at 150 U ml−1 in PBS for a period not less than 2 hr but often overnight for convenience. To demonstrate specificity of labeling, some sections were treated with antibody that had been neutralized by incubation for 1 hr with an excess of antigenic peptide (antibody to peptide 1:1 v/v, peptide 50 μg ml−1).
Semiquantitative analysis of relative changes in the intensity of brush-border membrane labeling was determined using a Flowgen AlphaImager 1200 analysis system (Alpha Innotech; San Leandro, CA); image analysis was performed using AlphaEase v5.5 software. A series of small squares (120 pixels each) down the target membrane were framed by the software for intensity measurement. The brush-border membrane in sections of perfused intestine was clearly separated at most points from the cell by the tight junction, which appeared as black background. Each square therefore enclosed a section of membrane that was specifically brush border, surrounded on both sides by pure background, and gave a corresponding intensity value. Subtraction of the intensity of an identical-sized square of adjacent background gave a value for the total intensity within that section membrane. For any single section, the mean value of the intensity of labeling of a given brush-border sample was obtained from a set of 50 squares distributed roughly evenly around a villus. The enhancement in GLUT2 levels induced by PMA was expressed as the ratio of the intensities in PMA-treated and non-treated jejunum. This method does not work for basolateral membranes because the cytosol contains many GLUT2-containing vesicles and there is therefore no pure background surrounding the target section of membrane.
Primary Antibodies
Primary polyclonal antibodies were raised in rabbit to the following sequences: residues 508–522 KATVQMEFLGSSETV-COOH at the C-terminal of rat GLUT2 (raised in York); residues 40–55 SHYRHVLGVPLDDRKA in the first extracellular loop of human GLUT2 (from Chemicon International; Temecula, CA); residues 40–55 SHYRHVLGVPLDDRRA in the first extracellular loop of rat GLUT2 (raised by Research Genetics, Carlsbad, CA for York); residues 489–502 EKELNDLPPATREQ at the C-terminal of rat GLUT5 raised in York. BLAST searches of the NCBI and SWISS PROT databases against each sequence revealed only the expected protein, either GLUT2 or GLUT5. Rat GLUT2 C-terminal antibody was affinity-purified against C-terminal peptide immobilized on an ELISA plate. Rat GLUT2 extracellular loop antibody from York was affinity-purified according to the manufacturer's instructions using a Pierce (Rockford, IL) SulfoLink Kit, by which antigenic peptide was immobilized through a cysteine residue attached to the N-terminal of the peptide. Affinity-purified antibodies were used neat and antisera at either 1:100 or 1:20 dilution, as stated.
Membrane Vesicle Preparation
Jejuna from two rats were perfused as described above. Every stage of the vesicle preparation was performed at 0–4C to prevent changes in trafficking after the intestine had been excised as described by Helliwell et al. (2000a). Immediately after perfusion, each jejunum was flushed with ice-cold buffered mannitol (20 mM imidazole buffer, pH 7.5, containing 250 mM mannitol and 0.1 mM PMSF). The jejunum was then placed on an ice-cold glass plate and slit longitudinally so that the muscle of the jejunum flattened out on the cold plate. Mucosal scrapings were taken with an ice-cold glass slide and homogenized immediately at 4C in buffered mannitol using a Kinematica Polytron homogenizer (four 30-sec bursts using the large probe at setting 7). The rest of the preparation and its detailed characterization for purity are as described in Corpe et al. (1996). Protein was determined using a BCA (biconchininic acid) assay kit with bovine serum albumin as standard (Pierce Perbio; Chester, UK). Enrichment of sucrase activity in these highly purified preparations ranged from 16- to 20-fold. There was no significant enrichment of Na+/K+-ATPase activity.
Western Blotting
SDS-PAGE and Western blotting were also performed as described previously using ECL (Enhanced ChemiLuminescence) detection (Corpe et al. 1996). Immunoblotting was performed using polyclonal antibodies as specified above. Quantitation of Western blots (or areas of brush-border membrane in micrographs; see above) was performed using a Flowgen AlphaImager 1200 analysis system with Alpha-Ease v5.5 software (see above). The level of GLUT2 determined in vesicle preparations from jejunum was expressed relative to that in control preparations and presented as mean ± SEM. The linear range of intensity response in ECL photographs was established using a 20-fold range of the amount of an actin standard (2–40 μg). Background correction was made using an area of blank film defined by the template drawn round the corresponding band in the blot. Under these circumstances, the response was linear (correlation coefficient 0.996) for integrated density values ranging from 13,024 to 465,029. As far as possible, exposures for measurement of GLUT2 relative amounts were such that the intensity values fell within the middle third of the linear response range. The same loading of 15 μg protein was used for all samples. Comparison of relative levels of GLUT2 was made on a protein basis to minimize potential complications that might be caused by the trafficking of other proteins in response to the same stimuli that affect GLUT2 trafficking. A monoclonal antibody to the last 18 residues at the C-terminal of PKC βII was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Results
We have previously reported (Helliwell et al. 2000a) that PMA (200 nM) stimulates fructose absorption in isolated loops in vitro by 60% as result of a fourfold increase in the level of GLUT2 (determined with antibody to the C-terminal of rat GLUT2) and in the magnitude of the GLUT2-mediated component of fructose absorption. GLUT5 levels and the GLUT5-mediated component of absorption are unchanged. The changes in transport and GLUT2 levels induced by PMA were mediated by the activation of PKC βII and blocked by the PKC inhibitor chelerythrine (2 μM). Figure 1 shows Western blots of GLUT2 in brush-border membrane vesicles from rat jejunum perfused with 5 mM fructose and different combinations of PMA and chelerythrine. The results confirm our previous findings with C-terminal antiserum. Of particular relevance for ICC studies, however, the important new result in Figure 1 is that antiserum to the extracellular loop of GLUT2 detects a similar response to that for the GLUT2 C-terminal antibody with the same samples. The increase in GLUT2 levels induced by PMA was 3.9- ± 0.6-fold (n=12; p<0.001) determined with C-terminal antibody and 3.5- ± 0.8-fold (n=6; p<0.001) determined with extracellular loop antibody. The bands revealed by both antisera are abolished when antisera are first neutralized by incubation with an excess of the corresponding antigenic peptide, confirming the specificity of the antibodies (data not shown; but see Figure 2). The level of GLUT2 at the brush-border membrane as determined by either C-terminal or extracellular loop antibodies correlates with the activation of PKC βII as monitored by the translocation of PKC βII (Figure 1).
Figure 1.
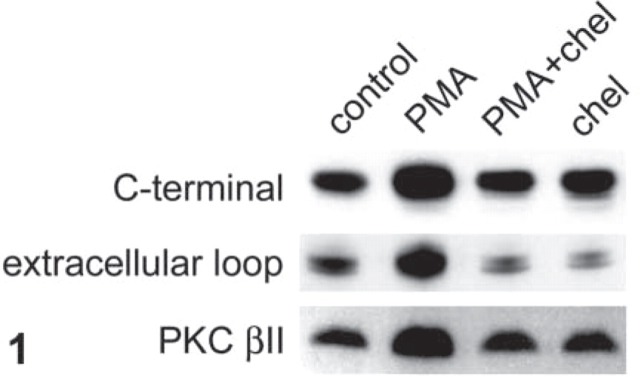
PMA increases GLUT2 levels at the brush-border membrane of rat jejunum by a PKC-dependent pathway. The jejunum of an anesthetized rat was perfused for 30 min in vivo and then in vitro for 30 min with 5 mM D-fructose, either alone (control) or in the presence of the PKC activator PMA (200 nM). When present, the PKC inhibitor, chelerythrine (chel, 2 μM) was added to the perfusate 15 min before the PMA. After preparation of brush-border membrane vesicles, vesicle protein (15 μg) was separated on 10% SDS-PAGE gels, transblotted onto nitrocellulose, and Western-blotted for GLUT2 using either C-terminal or extracellular loop antisera to rat GLUT2, or a C-terminal monoclonal antibody to PKC βII. For further details see Materials and Methods.
Figure 2.
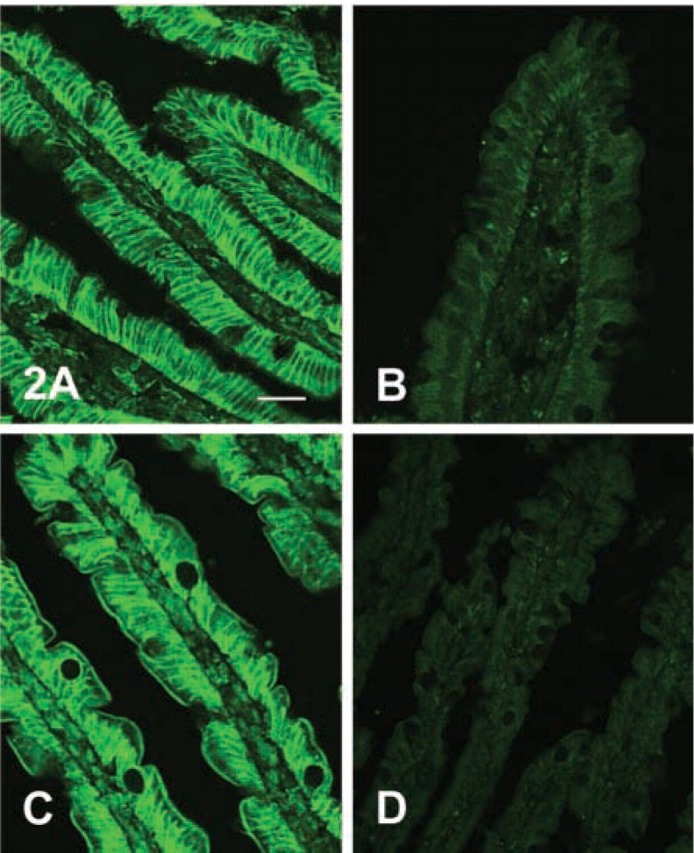
Antiserum to the large extracellular loop but not the C-terminal of GLUT2 detects brush-border membrane GLUT2 in sections of whole jejunum. Rat jejunum was perfused with 5 mM fructose and 200 nM PMA in vitro and then fixed by perfusion with PLP fixative as described in Materials and Methods. Seven-μm sections were then blocked, labeled with GLUT2 antiserum raised in rabbit, and visualized with an FITC-conjugated secondary antibody. Images captured by a confocal microscope were obtained at ×40 magnification: microscope settings for images in A and B were the same. (A) Affinity-purified antibody to residues 508–522 at the C-terminal of rat GLUT2 (1:100 dilution in PBS) and the control with antibody preabsorbed with antigenic peptide (B). (C) Antiserum to residues 40–55 within the large extracellular loop of human GLUT2 obtained from Chemicon International. (1:100 dilution in PBS; C) and the control with antiserum preabsorbed with antigenic peptide (D). Bar = 50 μm.
Figure 2 shows labeling of sections of rat jejunum perfused with 5 mM fructose in the presence of 200 nM PMA, so that GLUT2 insertion into the brush-border membrane is maximal. Figure 2A shows extensive staining of the basolateral membrane with affinity-purified antibody to the C-terminal of rat GLUT2 (dilution 1:100 in PBS). Staining is specific because preabsorption of antiserum with excess antigenic peptide blocks labeling (Figure 2A). However, the brush-border membrane shows no labeling.
Because no labeling was observed with C-terminal antibody, we investigated whether labeling could be detected with an antibody to part of the extracellular loop between transmembrane regions 1 and 2 of GLUT2. Initially we used a commercially available antibody to residues 40–55 of human GLUT2, which differs from the corresponding rat sequence only in the penultimate C-terminal residue (K54R) and cross-reacts with rat tissue. Figure 2B shows labeling with this antibody (dilution 1:100 in PBS) of sections of rat jejunum perfused with 5 mM fructose in the presence of 200 nM PMA. In sharp contrast to the C-terminal antiserum, there is intense labeling not only of the ba-solateral membrane but also of the brush-border membrane. Staining of both the brush-border and the basolateral membranes is blocked by preincubation of antiserum with excess antigenic peptide (Figure 2B).
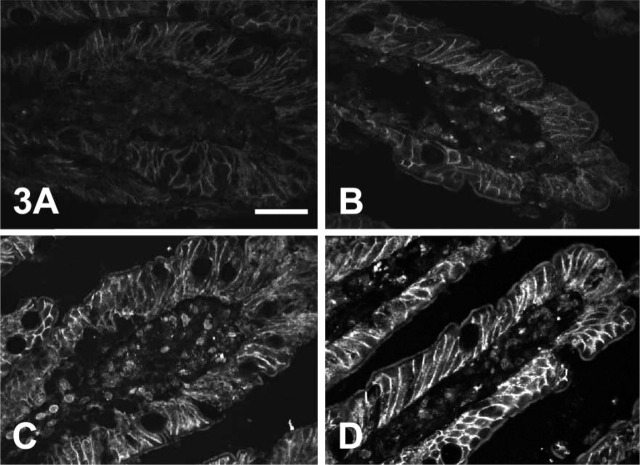
Although GLUT2 was readily detected in the brush-border membrane using the extracellular loop antiserum, we were unable at this point to demonstrate satisfactorily by ICC changes in the brush-border levels of GLUT2 that corresponded to those induced by PMA as revealed in Western blots. In particular, although we could detect some differences in labeling between jejunum perfused with fructose or fructose plus PMA, we could not detect differences similar to those in Western blots without losing the ability to detect brush-border GLUT2 in fructose-perfused jejunum. Because the putative glycosylation site at residue N62 is just 7 residues to the C-terminal of the antigenic peptide sequence for the extracellular loop antibody, we wondered whether the complex sugar chain might interfere with antibody binding. Because we were attempting to compare semiquantitatively ICC and Western blotting data, we used affinity-purified antibody to rat C-terminal GLUT2, prepared from the antiserum used for blots. Figure 3 shows the effects of digestion observed at ×40 magnification. The top images (Figure 3A and 3B) show sections not treated with glycosidase. Both show baso-lateral labeling, which appears somewhat more intense in the PMA-perfused section. Brush-border labeling is seen in the section from PMA-perfused jejunum (Figure 3B) but is not seen in the section from control jejunum (Figure 3A). However, pretreatment of sections with glycosidase produces a marked improvement in GLUT2 detection (bottom images, Figure 3C and 3D). Thus, Figure 3C shows a section of control jejunum taken from the same block as that in Figure 3A, and it is clear that the effect of glycosidase treatment is to reveal GLUT2 at the brush-border membrane. The intensity of labeling at the basolateral membrane is also increased markedly. Similar changes on glycosidase treatment are seen for sections of PMA-perfused jejunum taken from the same block as the section in Figure 3B when Figure 3B and 3D are compared. GLUT5, which transports fructose and is also present in the brush-border membrane, does not traffic to the membrane in response to PMA and was therefore used as a control for potential nonspecific changes caused by deglycosylation in the GLUT2 experiments. Digestion of glycosylation sugars had no effect on the labeling of GLUT5 when probed with C-terminal antibody in sections taken from jejunum perfused with either fructose or fructose plus PMA (data not shown). Brush-border membrane labeling of glycosidase-treated sections of jejunum from control (Figure 3C) and PMA-treated (Figure 3D) jejunum using rat GLUT2 extracellular loop affinity-purified antibody was then analyzed semiquantitatively using AlphaEase software as described in Materials and Methods. Over four sets of matched micrographs, the labeling of brush-border membrane in PMA-treated jejunum was 2.9- ± 0.4-fold greater than in control jejunum. The differences in labeling were therefore now similar to those seen in Western blots (Figure 1).
Figure 3.
Digestion of glycosylation sugars reveals GLUT2 at the brush-border membrane. Rat jejunum was perfused with 5 mM fructose in the absence (A,C) and presence (B,D) of 200 nM PMA in vitro and then fixed by perfusion with PLP fixative as described in Materials and Methods. Seven-μm sections were then incubated overnight with PBS alone (A,B) or N-endoglycosidase F (150 U ml−1 in PBS; C,D). Sections were then blocked, labeled with affinity-purified antibody to residues 40–55 within the large extracellular loop of rat GLUT2, and visualized with an FITC-conjugated secondary antibody. Images captured by a confocal microscope were obtained at ×40 magnification. An approximately linear intensity response was obtained by setting the fluorescence saturation limit on the most intense image (D) and the background limit on the least intense image (A). Bar = 50 μm.
Discussion
We have demonstrated that GLUT2 can be detected by ICC at the brush-border membrane of rat small intestine using an antibody to residues 40–55 within the large extracellular loop. The ability to do so depends critically on an understanding of the regulation of GLUT2 insertion in conjunction with a number of technical considerations.
As outlined above, a key feature of our new model of intestinal sugar absorption is that the GLUT2-mediated component of either glucose or fructose absorption is regulated by the rapid trafficking of GLUT2 (t1/2 ∼ a few minutes) to and from the brush-border membrane. The latter is the case when intestine is excised for in vitro studies, such as ICC, and the influences of endogenous hormones and nutrients in vivo that promote GLUT2 insertion into the membrane are lost. It is therefore essential to control the level of GLUT2 at the brush-border membrane. In this study we chose to demonstrate the regulation of the trafficking of GLUT2 in response to PMA in rat jejunum by comparing jejunum perfused with 5 mM fructose in the presence and absence of PMA. To allow time for PMA to dissolve in the membrane, while at the same time maintaining the viability of the perfusion, jejunum was first perfused in vivo for a period of the 30 min either with PMA or without (control). Perfusion was then continued in vitro for 30 min, which allowed ample time for GLUT2 to be lost from the membrane in the absence of PMA (Helliwell et al. 2000a). Over the same 30-min period in vitro, PMA treatment caused the retention of GLUT2 at the brush-border membrane by maintaining activation of PKC βII. At this point the tissue was fixed by perfusion with PLP fixative.
Initially, we assumed that the reason Thorens et al. (1990a) detected strong GLUT2 labeling of the baso-lateral membranes, but not the brush-border membrane, could be explained by the fact that the duodenum was excised and flushed with PBS (no sugar) before fixation. Such a procedure would allow GLUT2 to be lost rapidly from the brush-border membrane. However, in our present studies designed to increase GLUT2 at the brush-border membrane by use of PMA and fix it there, we also could not detect GLUT2 at the brush-border membrane with C-terminal antibody (Figure 2A). Nevertheless, antibody to residues 40–55 within the large extracellular loop readily recognized GLUT2 at the brush-border membrane as well as at the basolateral membrane (Figure 2B). It is therefore clear that, in addition to the procedural differences, an important factor in the results obtained by Thorens et al. (1990a) and now by us was that C-terminal antibody does not detect brush-border GLUT2 in sections of whole tissue. One possible explanation of the data might have been that the C-terminal of GLUT2 at the brush-border is truncated. However, this possibility is ruled out by the fact that the C-terminal antibody recognizes GLUT2 in Western blots (Figure 1). Sequencing of intestinal GLUT2 by PCR from cDNA using primers corresponding to N- and C-terminal regions did not detect splice variants (Thorens et al. 1990a; E.L. Morgan (York), personal communication). Moreover, because the C-terminal antibody detected GLUT2 in brush-border membrane vesicles, the only likely unresolved possibility for a splice variant remains at the N-terminal. The most likely explanation of the data, therefore, is that the C-terminal is masked in some way, perhaps by a docking protein. The rapid and likely continuing increase in the number of known glucose transporters (Joost et al. 2002) means we cannot preclude the possibility that other transporters will be found to be involved in intestinal sugar absorption.
Masking of the antigenic sequence is a significant factor in the detection of GLUT2 using the extracellular loop antibody raised against residues 40–55. The presence of a complex sugar chain linked to a putative N-linked glycosylation site at residue N62 clearly interferes with the ability of antibody to bind to its target sequence. In this work, we used PLP fixative (McLean and Nakane, 1974). Normally, it would be expected that the periodate would destroy the glycosylation sugars, so that there would be no interference. However, the fixative was used in perfusate containing fructose or fructose plus PMA, so that the level of GLUT2 at the membrane was the same as in the perfusion conditions. The free sugar appears to have protected the glycosylation sugars from oxidation. Therefore, if jejunum were perfused in vitro solely with 5 mM fructose, then the low levels of GLUT2 could not be detected readily (Figure 3A), i.e., the same result superficially as for C-terminal antibody. However, digestion of glycosylation sugars with N-endoglycosidase F revealed detection of brush-border membrane GLUT2 with extracellular loop antibody; compare Figure 3A and 3C. PMA (Figure 3B) induced higher levels of GLUT2 at the brush-border membrane compared with control perfusions; compare Figure 3A and 3B). Digestion of glycosylation sugars from PMA-perfused jejunum also increased labeling markedly (Figure 3B and 3D). The regulation of brush-border GLUT2 by PMA revealed in ICC sections (Figure 3C and 3D) now matched that originally seen only in Western blots (Figure 1).
PMA also increases the intensity of GLUT2 labeling at the basolateral membrane (compare Figure 3A and 3B or 3C and 3D). It therefore appears that upregulation of absorptive capacity at both the brush-border and the basolateral membrane in response to glucose is synchronized to provide coordinated increase in transepithelial transport. This conclusion fits well with the observation that GLP-2 inserts GLUT2 not only into the brush-border membrane (Au et al. 2002) but also into the basolateral membrane (Cheeseman and O'Neill 1998).
GLUT2 protein expression is not confined to the upper third of the villi, a region that is often assumed to effect the bulk of sugar absorption. Labeling is clearly strong and relatively uniform over the upper half of the villi (Figure 2 and 3) but may tail off towards the base of the villi. A significant difference between our results and those of Au et al. (2002) is that the bulk of the apical labeling detected in their experiments was at the terminal web, even in glucose-perfused jejunum. We have seen little labeling at the terminal web. Rather, we detect a black gap between the brush-border and the tips of the underlying lateral membranes. The differences may be accounted for by differences in technique, because they arrested trafficking using ice-cold conditions with final fixation being made on the microscope slide. Nevertheless, in some sections we do see thin lines of fluorescence joining the tips of the lateral membranes, apparently coincident with the terminal web. We also see extensive particulate staining throughout the cell and often immediately in the region underlying the terminal web. Such staining is seen most clearly at high magnification and presumably reflects GLUT2 vesicles ready to cross the tight junction and into the brush-border membrane. These data support the suggestion of Au et al. (2002) that the terminal web plays an important role in GLUT2 trafficking to the membrane. Interestingly, the terminal web is also a major location for PKC βII (Saxon et al. 1994), implying close coordination between control and insertion mechanisms.
In conclusion, we see that to detect GLUT2 at the brush-border membrane properly, three things are necessary. The first is to choose conditions that promote the trafficking of GLUT2 to the membrane and to retain it there by fixation in situ. The second is to use an extracellular loop antibody, and the third is to digest a way the glycosylation sugars to allow the antibody full access to the antigenic site. When these were done, the immunocytochemical data could be reconciled with the extensive functional data.
Acknowledgments
GLK is the recipient of a Leverhulme Trust Fellowship and gratefully acknowledges the support of the Wellcome Trust.
We are grateful to Dr E. Brot-Laroche for helpful comments on the manuscript and for access to unpublished results.
Literature Cited
- Au A, Gupta A, Schembri P, Cheeseman CI. (2002) Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J 367:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot-Laroche E, Dao MT, Alcade I, Delhomme B, Alvarado F. (1988) Independent modulation by food supply of two distinct sodium-activated D-glucose transport systems in guinea pig jejunal brush-border membrane. Proc Natl Acad Sci USA 85:6370–6373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot-Laroche E, Serrano MA, Delhomme B, Alvarado F. (1986) Temperature sensitivity and substrate specificity of two distinct Na+-activated D-glucose transport systems in guinea pig jejunal brush-border membrane vesicles. J Biol Chem 261:6168–6176 [PubMed] [Google Scholar]
- Cheeseman CI. (1993) GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology 105:1050–1056 [DOI] [PubMed] [Google Scholar]
- Cheeseman CI, O'Neill D. (1998) Basolateral D-glucose transport activity along the crypt-villus axis in rat jejunum and upregulation induced by gastric inhibitory peptide and glucagon-like peptide-2. Exp Physiol 83:605–616 [DOI] [PubMed] [Google Scholar]
- Corpe CP, Basaleh MM, Affleck J, Gould GW, Jess TJ, Kellett GL. (1996) The regulation of GLUT5 and GLUT2 activity in the adaptation of intestinal brush-border fructose transport in diabetes. Pflugers Arch [Eur J Physiol] 32:192–201 [DOI] [PubMed] [Google Scholar]
- Helliwell PA, Kellett GL. (2002) The active and passive components of glucose absorption in rat jejunum under low and high perfusion stress. J Physiol (Lond) 544:579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck JA, Kellett GL. (2000a) Stimulation by PMA of fructose transport across the intestinal BBM is mediated by GLUT2 and dynamically regulated by PKC. Biochem J 350:149–154 [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Richardson M, Affleck JA, Kellett GL. (2000b) Regulation of GLUT5, GLUT2 and intestinal brush-border fructose transport by the ERK, p38 and PI 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J 350:163–169 [PMC free article] [PubMed] [Google Scholar]
- Helliwell PA, Rumsby MG, Kellett GL. (2003) Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C βII mediated by phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent pathways. J Biol Chem 278:28644–28650 [DOI] [PubMed] [Google Scholar]
- Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, et al. (2002) Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol 282:E974–976 [DOI] [PubMed] [Google Scholar]
- Kellett GL. (2001) The facilitated component of intestinal sugar absorption. J Physiol (Lond) 531:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellett GL, Helliwell PA. (2000) The diffusive component of glucose absorption is mediated by glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J 350:155–162 [PMC free article] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. (1974) Periodate-lysine-paraformaldehyde fixative: a new fixative for immunoelectron microscopy. J Histochem Cytochem 22:1077–1083 [DOI] [PubMed] [Google Scholar]
- Saxon ML, Zhao X, Black JD. (1994) Activation of protein kinase C isozymes is associated with post-mitotic events in intestinal epithelial cells in situ . J Cell Biol 126:747–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Cheng ZQ, Brown D, Lodish HF. (1990a) Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol 259:C279–285 [DOI] [PubMed] [Google Scholar]
- Thorens B, Lodish HF, Brown D. (1990b) Differential localisation of two glucose transporter isoforms in rat kidney. Am J Physiol 259:C286–294 [DOI] [PubMed] [Google Scholar]
- Thorens B, Sarkar HK, Kabak HR, Lodish HF. (1988) Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney and pancreatic β cells. Cell 55:281–290 [DOI] [PubMed] [Google Scholar]