Abstract
Under laboratory conditions, the biocontrol potential of Steinernema thermophilum was tested against eggs and larval stages of two important lepidopteran insect pests, Helicoverpa armigera and Spodoptera litura (polyphagous pests), as well as Galleria mellonella (used as a model host). In terms of host susceptibility of lepidopteran larvae to S. thermophilum, based on the LC50 36 hr after treatment, G. mellonella (LC50 = 16.28 IJ/larva) was found to be more susceptible than S. litura (LC50 = 85 IJ/larva), whereas neither host was found to be significantly different from H. armigera (LC50 = 54.68 IJ/larva). In addition to virulence to the larval stages, ovicidal activity up to 84% was observed at 200 IJ/50 and 100 eggs of H. armigera and S. litura, respectively. To our knowledge this is the first report of entomopathogenic nematode pathogenicity to lepidopteran eggs. Production of infective juvenile (IJ) nematodes/insect larva was also measured and found to be positively correlated with rate of IJ for H. armigera (r = 0.990), S. litura (r = 0.892), as well as G. mellonella (r = 0.834). Both Phase I and Phase II of symbiotic bacteria Xenorhabdus indica were tested separately against neonates of H. armigera and S. litura by feeding assays and found to be virulent to the target pests; phase variation did not affect the level of virulence. Thus S. thermophilum as well as the nematode’s symbiotic bacteria applied separately have the potential to be developed as biocontrol agents for key lepidopteran pests.
Keywords: entomopathogenic nematode, Helicoverpa armigera, ovicidal activity, Phase I and Phase II virulence, Spodoptera litura
Safety and environmental issues surrounding the use of chemical insecticides has led to an emphasis on developing alternative control measures such as entomopathogens and their products. Entomopathogenic nematodes (EPNs) (Rhabditida: Steinernematidae and Heterorhabditidae) occur naturally in soil environments and locate their host in response to chemical and physical cues (Shapiro-Ilan et al., 2012a). Species in these two families have been effectively used as biological insecticides in pest management programs (Grewal et al., 2005) as they are considered nontoxic to humans, relatively specific to their target pest(s) and can be applied with standard pesticide equipment (Shapiro-Ilan et al., 2006). Infective juveniles (IJ) nematodes search for and enter the host, then release symbiotic bacteria into the haemocoel; the bacteria is the primary agent responsible for killing the host (usually within 24 to 48 hr). Xenorhabdus spp. are entomopathogenic bacteria symbiotically associated with EPNs belonging to the family Steinernematidae. These bacteria are carried monoxenically within a bi-lobed vesicle in the anterior portion of the intestine of nonfeeding free-living infective stage nematodes (Bird and Akhurst, 1983; Martens et al., 2003). Once inside the host, the bacterial symbionts create a favorable environment for the nematode to propagate by suppressing the immune protein of the insect (Gotz et al., 1981) and providing nutrition for the development and reproduction of nematodes (Poinar, 1983).
EPNs can be used as inundative or inoculative biological control agents or the proteinaceous toxins produced by their symbionts can be transferred to or/and expressed in crop plants or other microorganisms (Shapiro-Ilan et al., 2012b). The IJ of EPNs are compatible with other biological and chemical pesticides, fertilizers, and soil amendments (Krishnayya and Grewal, 2002). The symbiotic association of bacteria with nematode makes it challenging for the insect to develop resistance.
The bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) is a global polyphagous pest imposing crop damage in India worth $1 billion (USD) annually and it attacks more than 200 crop species belonging to 45 families (Choudhury et al., 2010). In India, this insect occurs as a major pest in many economically important crops including cotton, pigeonpea, chickpea, tomato, okra, and blackgram. The tobacco armyworm Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) is also an economically important polyphagous pest in India that causes considerable economic loss to many vegetable and field crops. This pest attacks more than 112 species of cultivated crops and causes severe losses of between 10% and 30% depending on the crop (Ferry et al., 2004; Baskar et al., 2011). The greater wax moth, Galleria mellonella L. (Lepidoptera: Pyralidae), a major pest of bee honeycombs widely used as a model organism in laboratories studying EPNs being highly susceptible.
In developing biocontrol programs using EPNs, one mechanism to increase the chance of success is to screen novel nematode species or strains for potential efficacy against particular target pests (Shapiro-Ilan et al., 2012b). Nematodes that are native to the region associated with the target pests may be more suitable for biocontrol purposes because they will likely to be adapted to the climate and local environmental conditions and because regulatory issues are minimized when using endemic strains or species. Steinernema thermophilum is indigenous to India (Ganguly and Singh, 2000) and is thus adapted to local agro-climatic conditions, e.g., the nematode is capable of infecting insects at a wide range of temperatures (20°C to 35°C) including higher temperatures that are encountered in the region of the target pests (H. armigera and S. litura) (Ganguly and Singh, 2000). In this study, we investigated the biocontrol potential of S. thermophilum, against two major pests of India, i.e., H. armigera and S. litura, and one model insect G. mellonella. Specifically, virulence of the nematode and associated bacterial symbiont (tested separately) was determined in insect eggs and larval stages.
Materials and Methods
Test Insects:
Larvae of H. armigera and S. litura were collected from the field and cultured on a chickpea-based semi-synthetic diet in the laboratory as described by Nagarkatti and Prakash (1974), and modified by Kalia et al. (2001) at 27 ± 2°C and 60 ± 5% relative humidity (RH). The adult moths were offered 10% honey solution fortified with multivitamins throughout their egg-laying period. In the present study, lab-reared eggs, neonates, and last instars were used.
G. mellonella was cultured in controlled laboratory conditions on semi-synthetic diet ingredients (wheat [Triticum aestivum] flour, 64 g; wheat bran, 64 g; maize [Zea mays] powder, 47.5 g; milk powder, 64 g; dried yeast powder, 47.5 g; wax powder, 12 g; honey, 75 ml; and glycerine, 75 ml) at 27 ± 2°C and 60 ± 5% RH. Adult moths were provided with 10% honey solution fortified with multivitamins in mating jars. In the mating jars, butter paper folded in a fan shape was provided for egg laying.
Nematodes:
S. thermophilum Ganguly & Singh, 2000 was obtained from the Division of Nematology, IARI, New Delhi, and maintained at the Insect Physiology laboratory, Division of Entomology, IARI, New Delhi, in double-distilled water (ddw) at 10°C to 15°C. It was described based on morphological characters of its five life stages (Ganguly and Singh, 2000) as well as molecular parameters (Ganguly and Pandey, 2006). Nematodes were propagated by passage through last instar larvae H. armigera and S. litura. The IJ were harvested using White traps (White, 1927).
Bioassays with Steinernema thermophilum:
Last instar H. armigera, S. litura, and G. mellonella were exposed individually in 12 well sterile plates (2.5-cm-diam. × 2-cm-depth, each well was padded with filter paper, Whatman No.1) to IJ suspended in 100 μl of sterile water applied at different rates i.e., 0, 5, 10, 20, 50, 100, and 200 IJ of S. thermophilum. Each test insect had 10 replicates per concentration and each treatment was repeated thrice, so 210 larvae per test insect were used. Incubation was performed at a constant temperature of 27 ± 2°C, and 60 ± 5% RH. Mortality was recorded at every 12 hr till 48 hr and afterward every 24 hr until pupation or 100% mortality was attained in most of the treatments, whatever was earlier. Mortality data at 36 hr as well as 48 hr was analyzed to calculate median lethal concentrations (LC50). Median lethal time (LT50) was calculated for the different concentrations.
Ovicidal activity assays:
Freshly laid eggs from mating jars of H. armigera and S. litura were used to test for ovicidal activity in S. thermophilum. Fifty eggs of H. armigera and 100 eggs of S. litura per replicate were kept in each plastic container (5-cm-diam. × 2-cm depth), which had a filter paper lining. These eggs were overlaid with 100 μl of sterile water containing 0, 20, 50, 100, or 200 IJ. Each treatment was replicated thrice thus 750 to 1,500 eggs were used per test insect. The treated as well as control eggs were kept at a constant temperature of 27 ± 2°C and 60 ± 5% RH. Hatching was recorded every 24 hr until 96 hr. The number of unhatched and hatched eggs in each replicate was counted and LC50s were calculated after 96 hr of treatment.
Production of Steinernema thermophilum IJ:
In the above experiment (Bioassays with S. thermophilum), ten nematode infected larvae were randomly selected and removed from plates, rinsed in ddw and transferred individually on to White traps. The IJ were harvested every 3 d up to a period of 30 d. Three replicates from pooled harvest/concentration were used to calculate the total number of IJ produced per ten larvae. The average yield/larva was then determined accordingly.
Isolation of symbiotic bacteria:
Last instar larvae of H. armigera were exposed individually in sterile plates (5-cm-diam. × 2-cm-depth) lined with Whatman No.1 filter paper to 20 IJs of S. thermophilum suspended in 100 μl ddw. After 24 hr of exposure, fresh haemolymph was collected by making a lesion in a proleg, in precooled centrifuge tubes. Then 10 μl of this haemolymph was streaked on NBTA plates (Nutrient agar 7, Bromothymol blue 0.025; triphenyl-2,3,4-tetrazolium chloride 0.04 gl-1). Plates were incubated at 28°C for 24 hr and primary form (Phase I) was differentiated from secondary form (Phase II) on the basis of standard characteristics (e.g., blue colony for Phase I and red colony for Phase II) (Akhurst, 1980). Phase I and II colonies were purified by subculturing thrice on NBTA plates and subsequently inoculated in 250-ml Luria broth. The inoculated broth was incubated at 28°C and 200 rpm for 24 hr. Culture broth was centrifuged at 8,000 rpm for 15 min at 4°C. Bacterial cell counts were made using ‘Neubaur Haemocytometer’ (Germany) and the number of bacterial cells/ml calculated. A stock of suspension of 2 × 1010 cell ml-1 was prepared and appropriate serial dilutions were made as required by diluting with ddw for bioassays.
Bioassay with Phase I and Phase II of symbiotic bacteria Xenorhabdus indica:
Both Phase I and II of symbiotic bacteria X. indica were tested separately against neonates of H. armigera and S. litura by feeding assays using diet incorporation methodin plastic containers (5-cm-diam. × 2-cm-depth) with 0.1 ml of solution incorporated at rates of 10, 1 × 102, 1 × 104, 1 × 106, or 1 × 108 bacterial cells/gm of diet. Each treatment was replicated thrice. In the control, the diet was incorporated with 0.1 ml of ddw alone. Ten neonates were released into each container, i.e., per replicate. A minimum of 180 neonates were used for each bioassay. Mortality was recorded every 24 hr till 96 hr, and LC50 was calculated after 96 hr of treatment.
Statistical analysis:
The mortality data were analyzed using maximum likelihood program for probit analysis (Ross, 1977). The LC50s in terms of lJs/larva or bacterial cells/gm diet or IJ/50 or 100 eggs, and LT50s in terms of hr for different bioassays were considered significantly different if their 95% fiducial limits (FL) did not overlap. The percentage mortalities of test insects were taken up to 8 d at different intervals, i.e., at 12, 24, 36, 48, 72, 96, 120, 144, 168, and 192 hr. Data were corrected for control mortality (Abbott, 1925) and arc-sine transformed when required to meet assumptions of normality and homogeneity of variances. Treatment effects for IJ production and ovicidal data were determined by one way analysis of variance (ANOVA) (SAS 9.2, SAS Institute Inc., Cary, NC). Probability less than or equal to 5% (p value < 0.05) was accepted as statistically significant. Correlation between the parameters viz., LT50 vs. concentration, production vs. concentration and yield of IJ vs. inoculums was determined by regression analysis.
Results
Bioassays with Steinernema thermophilum:
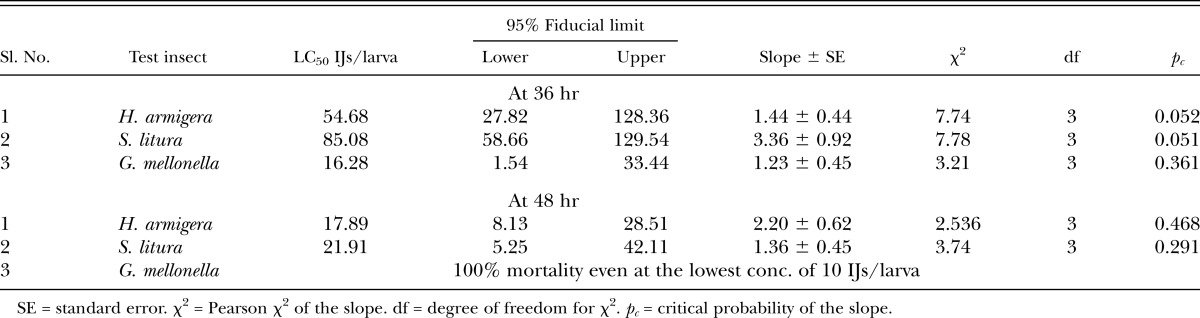
In terms of host susceptibility of lepidopteran larvae to S. thermophilum, based on the LC50 36 hr after treatment, G. mellonella (LC50 = 16.28 IJ/larva) was found to be more susceptible than S. litura (LC50 = 85 IJ/larva), whereas neither host was found to be significantly different from H. armigera (LC50 = 54.68 IJ/larva). Similarly at 48 hr, LC50 values vary from 17.89 IJ/larva (H. armigera ) to 21.91 IJ/larva (S. litura) but not found to be significantly different (Table 1).
Table 1.
Toxicity of Steinernema thermophilum against last instar Helicoverpa armigera, Spodoptera litura, and Galleria mellonella.
LT50 values for all the test insects were found to be rate dependent (Table 2). In H. armigera rates of 20, 50, and 100 IJ/larva were found to be at par with each other, whereas rate of 200IJ/larva was found to be significantly different from all other rates within this species. The lowest rate of 10 IJ/larva in S. litura was unable to attain 50% mortality, whereas 20IJ/larva was found to be significantly different from 50, 100, and 200 IJ/larva. In G. mellonella LT50 was found to be at par for all the treatment ranges from 10 IJ/larva to 200 IJ/larva. When comparing virulence among the host insects, the LT50 of G. mellonella was found to be at par with H. armigera at the highest rate of 200IJ/larva and at the 100 IJ rate yet both insects differed significantly with S. litura at these rates (41.40 hr). At the lowest rate of 10 IJ/larva, the LT50 of G. mellonella (33.66 hr) was found to be significantly different from H. armigera (65.20 hr). At 20 IJs, there were no differences in LT50 among the hosts, whereas at the 50 IJ rate G. mellonella was more susceptible than S. litura but at par with H. armigera. G. melonella was found to be more susceptible than H. armigera at the 10 IJ rate. In all the test insects LT50 was found to be negatively correlated with the rate of application (Table 2).
Table 2.
LT50 values calculated from dosage response assays conducted with Steinernema thermophilum against last instar Helicoverpa armigera, Spodoptera litura, and Galleria mellonella.
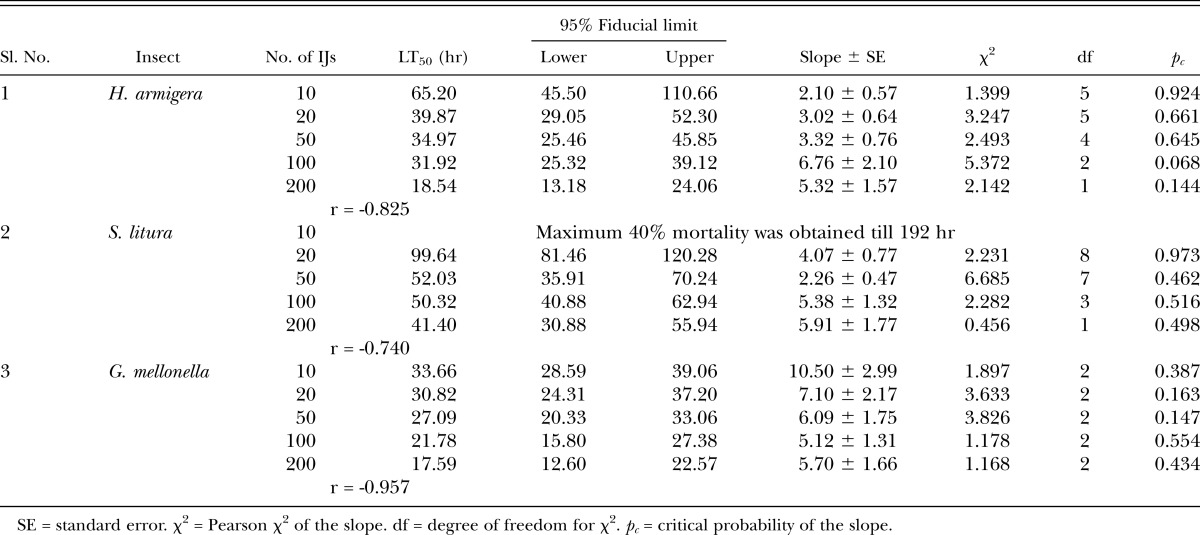
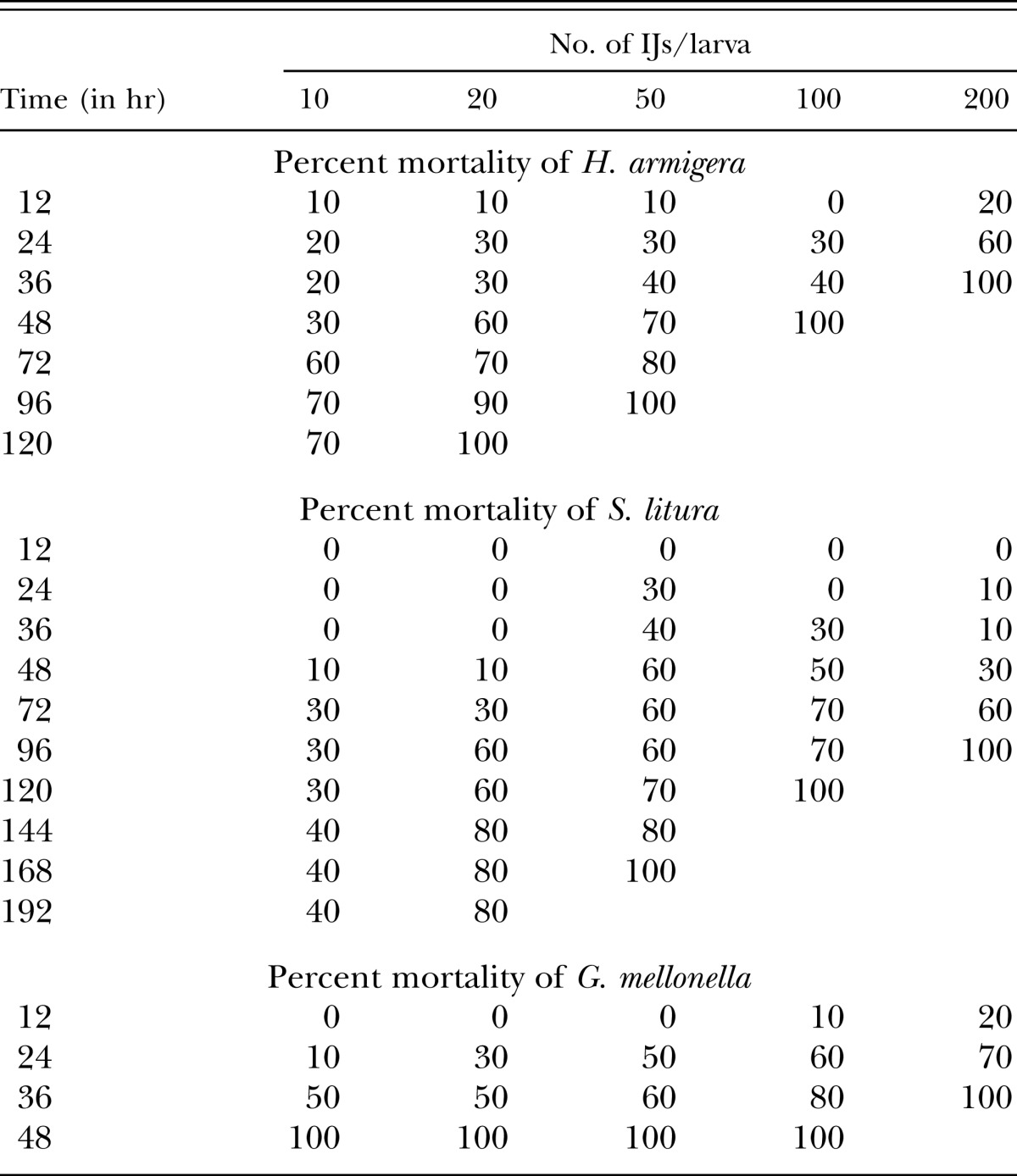
Last instar of H. armigera and G. mellonella attained 100% mortality at 200 IJ/larva as early as 36 hr. However in G. mellonella 100% mortality was observed even at the lowest concentration of 10 IJ/larva within 48 hr (Table 3). On other hand in case of S. litura, even higher concentration viz., 200 IJ/larva and 100 IJ/larva had taken longer time, i.e., 96 hr and 120 hr, respectively, to kill 100% of the larvae. At the lowest concentration, 10 IJ/larva, only 70% and 40% mortality was attained even after 120 and 192 hr exposure in H. armigera and S. litura, respectively. Last instar H. armigera is nearly as susceptible as the model host G. mellonella.
Table 3.
Percentage mortality of last instar Helicoverpa armigera, Spodoptera litura, and G. mellonella exposed to different concentrations of the infective juveniles of the Steinernema thermophilum.
Ovicidal activity assays:
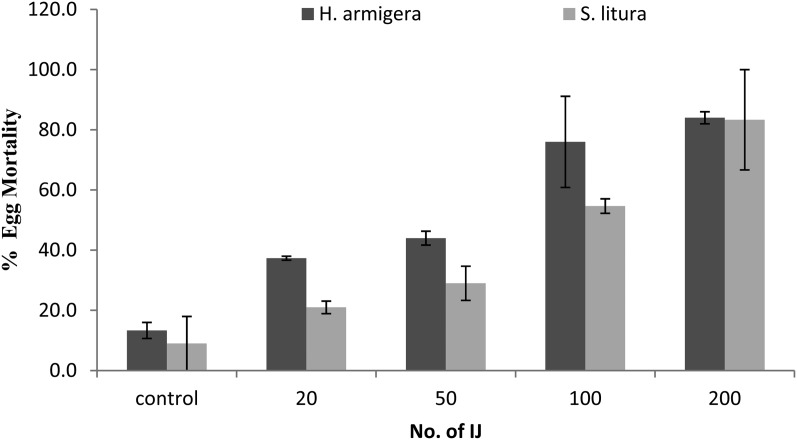
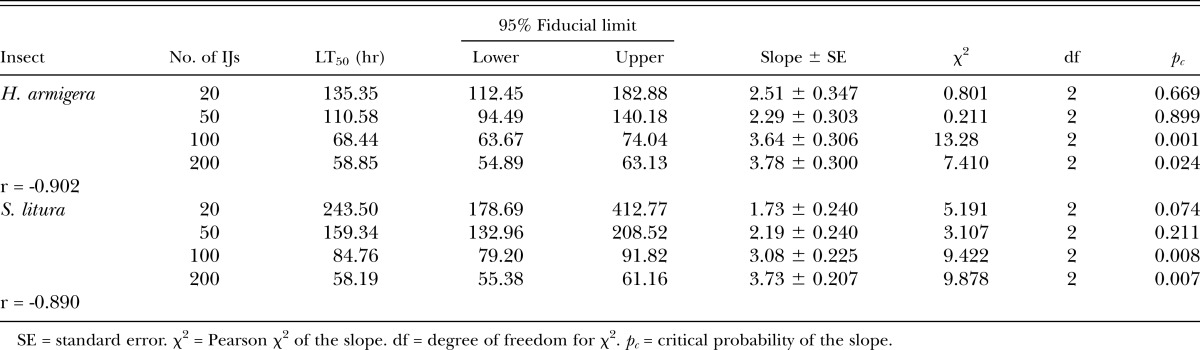
The highest egg mortality (84%) was observed at 200 IJ/50 eggs of H. armigera and 200IJ/100 eggs of S. litura, respectively (Fig. 1). Low mortality was observed in the control, i.e., 9% and 14% was observed in H. armigera and S. litura, respectively (Fig. 2). There is a significant effect of IJ concentration on egg infection in both H. armigera (F4,14 = 10.11, p < 0.0015) and S. litura (F4,14 = 10.57, p < 0.0013) with a positive correlation of 0.97 and 0.91, respectively. Perusal of data in Table 4 shows that LC50 for H. armigera (120 IJ/150 eggs, pc = 0.002) and S. litura (130 IJ/300 eggs, pc = 0.045) is at par (Table 5). Specifically, LT50 was found to be negatively correlated with concentration of IJ/larva in H. armigera (r = -0.902) as well as S. litura (r = -0.890).
Fig. 1.
Mean percentage mortality (± SE) of eggs of Helicoverpa armigera and Spodoptera litura by different infective juvenile (IJ) concentrations of Steinernema thermophilum.
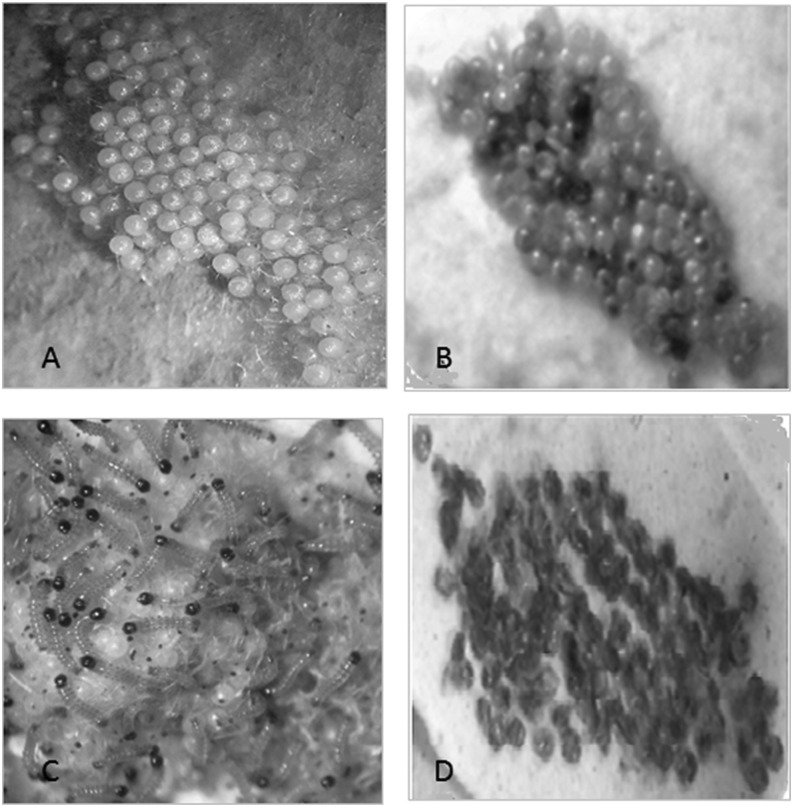
Fig. 2.
Ovicidal activity of Steinernema thermophilum against eggs of Spodoptera litura A. Control after 24 h. B. Treatment after 24 h. C. Control after 96 h. D. Treatment after 96 h.
Table 4.
Ovicidal activity of Steinernema thermophilum in Helicoverpa armigera and Spodoptera litura.
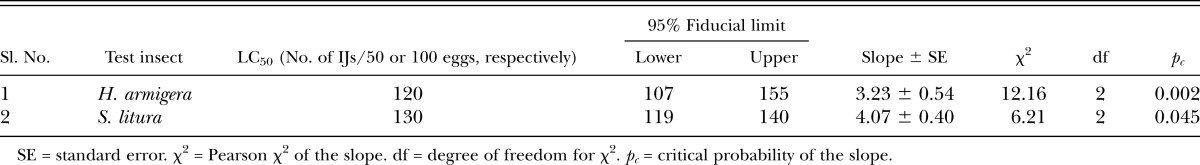
Table 5.
LT50 values calculated from dosage response assays conducted with Steinernema thermophilum against eggs of Helicoverpa armigera and Spodoptera litura.
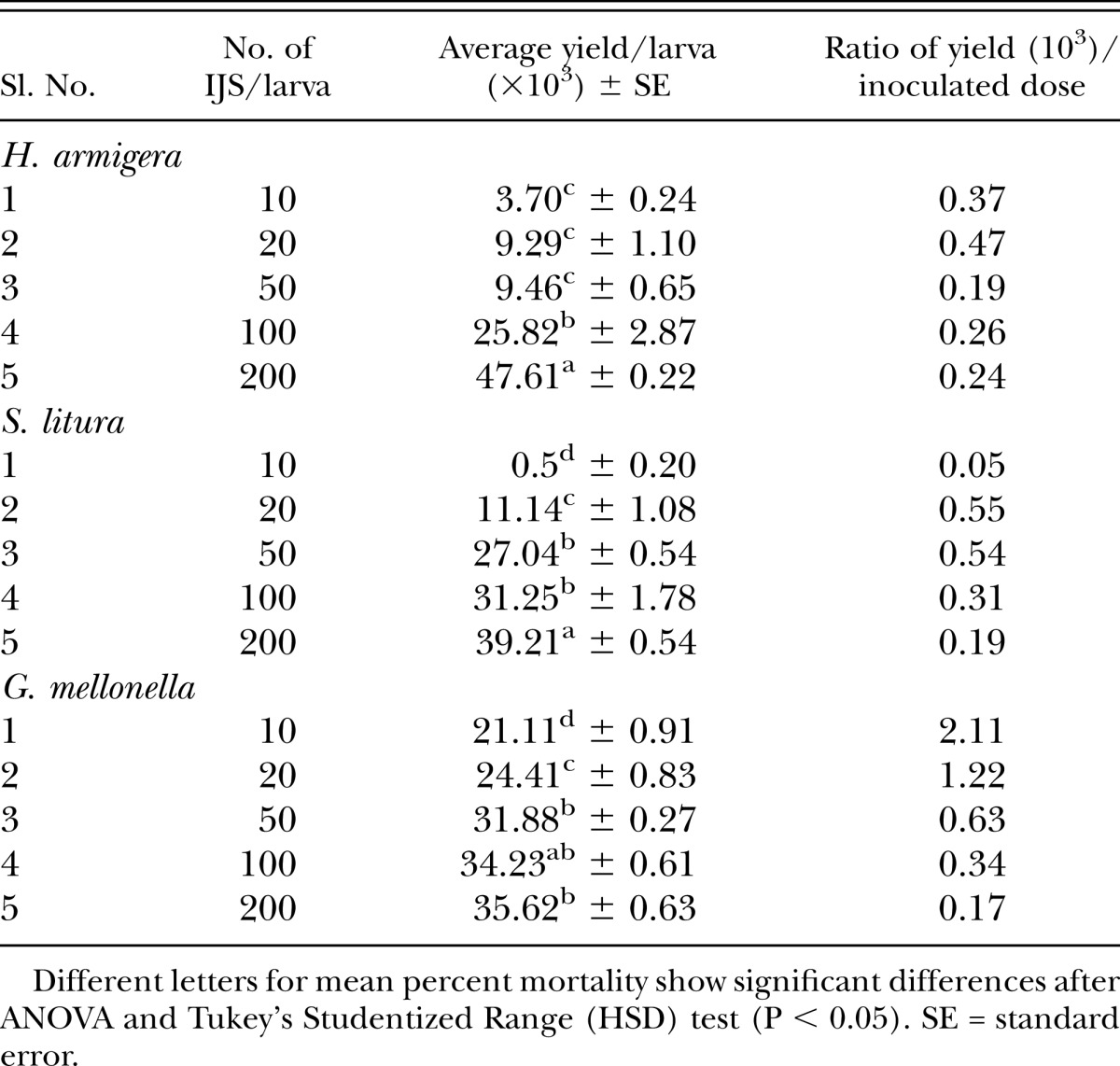
Production of IJ of Steinernema thermophilum:
Following host mortality, the emerging IJ from host cadavers were collected and counted during a 30-d period. Data in Table 6 show a significant effect of concentration upon average yield of IJ/larva, although H. armigera average yield did not differ at 10, 20, and 50 IJ/larva. However, a significant difference between 100 IJ/ larva (25.8 × 103IJ/larva) and 200 IJ/larva (47.6 × 103 IJ/larva) was observed (F4,14 = 161.39, p < 0.0001). For S. litura (F4,14 = 113.72, p < 0.0001), IJ yield (39.2 × 103 IJ/larva) was found to be highest at 200 IJ/larva and found to be significantly different from other concentrations. Conversely yield at 10 and 20 IJ/larva (0.5 × 103 and 11.14 × 103, respectively) as well as 50 and 100 IJ/larva was found to be at par. In case of G. mellonella, yield was found to be at par at 50, 100, and 200 IJ/larva, significantly different at 10 and 20 IJ/larva (F4,14 = 85.67, p < 0.0001). Production of IJ was positively correlated with the IJ concentrations for H. armigera (r = 0.990), S. litura (r = 0.871) as well as G. mellonella (r = 0.834). Though, yield/inoculum ratio was negatively correlated with the concentrations for H. armigera (r = -0.561), S. litura (r = -0.955) as well as G. mellonella (r = -0.795).
Table 6.
Production of Steinernema thermophilum IJs in last instar Helicoverpa armigera, Spodoptera litura, and Galleria mellonella.
Bioassay with Phase I and Phase II of symbiotic bacteria Xenorhabdus indica:
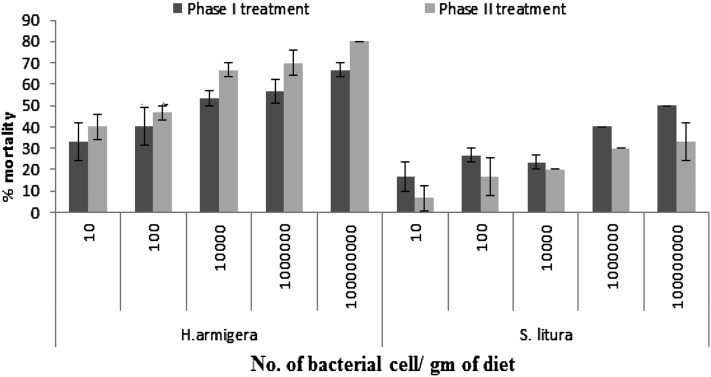
X. indica was pathogenic to H. armigera and S. litura. In comparing the susceptibility between hosts, H. armigera was found to be more susceptible to X. indica (both phases) than S. litura on the 4th and 7th day assessments (Table 7). The virulence of Phase I bacteria compared with Phase II bacteria did not differ in each of the host except in S. litura, for which 50% mortality could not be obtained by in the Phase II treatment by the 4th day even at the highest concentration. However, Phase II of symbiotic bacteria X. indica was found to be more virulent than Phase I against neonates of H. armigera (F9,29 = 7.24, p < 0.0001) and vice versa for S. litura (F9,29 = 5.70, p < 0.0006) (Fig. 3).
Table 7.
Efficacy of Phase I and Phase II of symbiotic bacteria Xenorhabdus indica against neonates of Helicoverpa armigera and Spodoptera litura after 4 and 7 d of treatment.
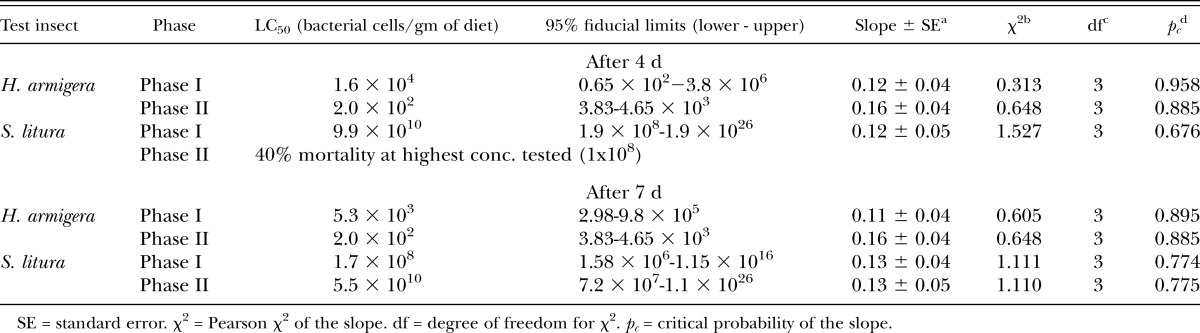
Fig. 3.
Mean percentage mortality (± SE) of neonates of Helicoverpa armigera and Spodoptera litura by Phase I and Phase II of symbiotic bacteria Xenorhabdus indica on 7 d after treatment.
Discussion
Virulence of S. thermophillum to two major polyphagous pests was determined and results indicate that susceptibility of H. armigera is similar to G. mellonella. In contrast S. litura was found to be less susceptible than G. mellonella. Numerous studies have made use of these assays to evaluate the efficacy of EPNs against various insect pests and also found a positive relationship between the dose of IJ and host mortality as found in the present study (Forschler and Nordin, 1988; Glazer and Navon, 1990; Sims et al., 1992; Peters and Ehlers, 1994; Bhatnagar et al., 2004; Phan et al., 2005; Ali et al., 2008; Adiroubane et al., 2010; Yadav and Lalramliana, 2012). S. thermophilum caused 100% mortality in H. armigera and G. mellonella within 36 hr through direct exposure of 200 IJ, which agrees with the studies of Ali et al., 2008. In contrast, in S. litura 100% mortality was observed later compared with the other two hosts tested. The reason for delayed mortality may be based on an inability of the IJs to penetrate the host or perhaps enzymes or toxins related to the symbiotic bacterium (X. indica) are less potent in S. litura relative to the other hosts. Thus, similar to numerous other studies, we observed that the efficacy of the EPNs against insect hosts varies with insect and nematode species (Shapiro-Ilan et al., 2002; Ansari et al., 2006).
Although it is not surprising that larvae were susceptible to S. thermophilum, a novel finding was that the EPN has the ovicidal activity as it was observed that IJ kills the eggs of H. armigera and S. litura by penetrating them. In S. litura eggs were laid in groups so single IJ was capable of penetrating and killing more eggs (2.31 eggs) as compared with H. armigera (1.29 eggs), where eggs were laid sparsely. LT50 values for both the test insects were found to be rate dependent. When comparing the two hosts, LT50s were similar at all rates except at the 100 IJ treatment in which H. armigera was virulent than S. litura. Other studies showed a lack of impact of EPN to insect eggs (De Doucet et al., 1998; Kim et al., 2004). However, Shahina et al. (2009) reported ovicidal activity of Heterorhabditis bacteriophora and S. siamkayai against Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae). Our finding of ovicidal activity by EPN intensifies their potential as biological control agents against lepidopteran pests.
In addition to a positive correlation between mortality and rate of the IJ applied, the present study also showed a positive correlation between the reproductive yield and the concentration of IJ used against H. armigera, S. litura, and G. mellonella. These results concur with Poinar and Thomas (1967), Reardon et al. (1986), and Abdel-Razek (2006). In contrast, Shapiro-Ilan et al. (2002) did not detect an impact of IJ inoculation rate on yield of progeny. Production of EPNs in the host can play a crucial role in their persistence in the environment because of recycling and thus affect the effectiveness of pest control applications (Harlan et al., 1971; Georgis and Hague, 1981). Furthermore, reproductive capacity is a critical component for optimum efficiency in commercial in vivo production ventures.
The basis of pathogenesis in EPNs is largely because of their symbiotic bacteria, which are carried in the alimentary tract of the IJ (Thomas and Poinar, 1979). According to Akhurst and Dunphy (1993) most species of Xenorhabdus are highly pathogenic when injected, e.g., with an LD50 of less than 20 cells. However, data on oral infection is lacking and hence this study was undertaken to determine toxicity of X. indica per os. We observed pathogenicity stemming from per os infection, but the level of virulence was affected by phase variation. Phase II bacteria of X. indica were found to be less effective than Phase I against neonates of H. armigera, which concurs with previous studies on X. nematophila (Volgyi et al., 1998), whereas an opposite trend in virulence was observed in our study for S. litura. Thus, efficacy of the two bacterial phases apparently varies among different insect hosts. Kumar et al. (2013) reported efficacy of a purified protease from X. indica in suppressing neonates of H. armigera. Similarly, Mahar et al. (2004) demonstrated the possibility of applying either bacterial suspensions containing cells of X. nematophila or cell-free solutions containing bacterial metabolites to control larvae of P. xylostella. This study extends previous finding by demonstrating that the EPN complex and symbiotic bacteria alone have the potential to be developed as biocontrol agents against key lepidopteran pests such as H. armigera and S. litura.
Acknowledgments
The authors acknowledge World Bank–funded NAIP-ICAR project (IARI-70:13) for sponsoring this research project. We are grateful to Director, Indian Agricultural Research Institute, New Delhi, for providing infrastructure and facilities for carrying out these studies.
Literature Cited
- Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–267. [Google Scholar]
- Abdel-Razek AS. Infectivity prospects of both nematodes and bacterial symbionts against cotton leafworm, Spodoptera littoralis (Biosduval) (Lepidoptera: Noctuidae) Journal of Pest Science. 2006;79:11–15. [Google Scholar]
- Adiroubane D, Tamilselvi R, Ramesh V. Efficacy of Steinernema siamkayai against certain crop pests. Journal of Biopesticides. 2010;3:180–185. [Google Scholar]
- Akhurst RJ. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. Journal of General Microbiology. 1980;121:303–309. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Dunphy GB. 1993. Tripartite interactions between symbiotically associated entomopathogenic bacteria (Xenorhabdus spp.) and nematodes (Steinernematide and Heterorhabditidae) and their insect hosts. Pp. 1–23 in N. E. Beckage, S. N. Thompson, and B. Federici, eds. Parasites and pathogens of insects, Vol. 2. New York: Academic Press. [Google Scholar]
- Ali SS, Pervez R, Hussain A, Ahmad R. Susceptibility of three lepidopteran pests to five entomopathogenic nematodes and in vivo mass production of these nematodes. Archives of Phytopathology and Plant Protection. 2008;41:300–304. [Google Scholar]
- Ansari MA, Shah FA, Moens TM. Field trials against Hoplia philanthus (Coleoptera: Scarabaeidae) with a combination of an entomopathogenic nematode and the fungus Metarhizium anisopliae CLO 53. Biological Control. 2006;39:453–459. [Google Scholar]
- Baskar K, Sasikumar S, Muthu C, Ignacimuthu S. Bioefficacy of Aristolochia tagala Cham. against Spodoptera litura Fab (Lepidoptera: Noctuidae) Saudi Journal of Biological Sciences. 2011;18:23–27. doi: 10.1016/j.sjbs.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A, Shinde V, Bareth SS. Evaluation of entomopathogenic nematodes against white grub, Maladerains anabilis Brenske. Journal of Integrated Pest Management. 2004;50:285–289. [Google Scholar]
- Bird AF, Akhurst RJ. The nature of the intestinal vesicle in nematodes of the family Steinernematidae. International Journal of Parasitology. 1983;13:599–606. [Google Scholar]
- Choudhury RA, Rizvi PO, Sayed MP, Mehdi H, Ghalib RM. Antifeedant response of two medicinal plants against Helicoverpa armigera (Hubner) (Lepidotera: Noctuidae) on chickpea, Cicer arietinum. Middle-East Journal of Scientific Research. 2010;5:329–335. [Google Scholar]
- De Doucet MMA, Miranda MB, Bertolotti MA. Infectivity of entomogenous nematodes (Steinernematidae and Heterorhabditidae) to Pediculus humanuscapitis De Geer (Anoplura: Pediculidae) Fundamental and Applied Nematology. 1998;21:13–16. [Google Scholar]
- Ferry N, Edwards MG, Gatehouse JA, Gatehouse AMR. Plant–insect interaction: Molecular approaches to insect resistance. Current Opinion in Biotechnology. 2004;15:155–161. doi: 10.1016/j.copbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Forschler BT, Nordin GL. Comparative pathogenicity of selected entomogenous nematodes to the hardwood borers, Prionoxystus robiniae (Lepidoptera: Cossidae) and Megacyllene robiniae (Coleoptera: Cerambycidae) Journal of Invertebrate Pathology. 1988;52:343–347. [Google Scholar]
- Ganguly S, Pandey J. Molecular characterization of Steinernema thermophilum based on RFLP and sequence of ITS region of rDNA. Indian Journal of Nematology. 2006;36:115–121. [Google Scholar]
- Ganguly S, Singh LK. Steinernema thermophilum sp. n. (Rhabditida: Steinernematidae) from India. International Journal of Nematology. 2000;10:183–191. [Google Scholar]
- Georgis R, Hague NGM. A neoaplectanid nematode in the larch sawfly Cephalcia larieiphila (Hymenoptera: Pamphilidae) Annals of Applied Biology. 1981;99:171–177. [Google Scholar]
- Glazer I, Navon A. Activity and persistence of entomoparasitic nematodes tested against Heliothis armigera (Lepidoptera: Noctuidae) Journal of Economic Entomology. 1990;83:1795–1800. [Google Scholar]
- Gotz P, Boman A, Boman HG. Interactions between insect immunity and an insect-pathogenic nematode with symbiotic bacteria. Proceedings of the Royal Society B: Biological Sciences. 1981;212:333–350. [Google Scholar]
- Grewal PS, Ehlers R-U, Shapiro-Ilan DI. 2005. Nematodes as biocontrol agents. New York: CABI Publishing. [Google Scholar]
- Harlan DP, Dutky SR, Padgett GR, Mitchell JA, Shaw ZA, Barlett FJ. Parasitism of Neoaplectana dutkyi in white-fringed beetle larvae. Journal of Nematology. 1971;3:280–283. [PMC free article] [PubMed] [Google Scholar]
- Kalia V, Chaudhari S, Gujar GT. Changes in haemolymph constituents of American Bollworm, Helicoverpa armigera (Hübner), infected with nucleopolyhedrovirus. Indian Journal of Experimental Biology. 2001;39:1123–1129. [PubMed] [Google Scholar]
- Kim HH, Choo HY, Kaya HK, Lee DW, Lee SM, Jeon HY. Steinernema carpocapsae (Rhabditida: Steinernematidae) as a biological control agent against the fungus gnat Bradysia agrestis (Diptera: Sciaridae) in propagation houses. Biocontrol Science and Technology. 2004;14(2):171–183. [Google Scholar]
- Krishnayya PV, Grewal PS. Effect of neem and selected fungicides on viability and virulence of the entomopathogenic nematode Steinernema feltiae. Biocontrol Science Technology. 2002;12:259–266. [Google Scholar]
- Kumar P, Singh S, Dutta D, Singh N, Sharma G, Ganguly S, Kalia V, Nain L. Extracellular novel metalloprotease from Xenorhabdus indica and its potential as an insecticidal agent. Journal of Microbiology and Biotechnology. 2013 doi: 10.4014/jmb.1306.06062. doi:10.4014/jmb.1306.06062. [DOI] [PubMed] [Google Scholar]
- Mahar AN, Munir M, Elawad S, Gowen SR, Hague NGM. Microbial control of diamondback moth, Plutella xylostella L. (Lepidoptera: Yponomeutidae) using bacteria (Xenorhabdus nematophila) and its metabolites from the entomopathogenic nematode Steinernema carpocapsae. Journal of Zhejiang University Science. 2004;5:1183–1190. doi: 10.1631/jzus.2004.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Heungens K, Goodrich-Blair H. Early colonization events in the mutualistic association between Steinernema carpocapsae nematodes and Xenorhabdus nematophila bacteria. Journal of Bacteriology. 2003;185:3147–3154. doi: 10.1128/JB.185.10.3147-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti S, Prakash A. Rearing of Heliothis armigera (Hübner) on an artificial diet. Technical Bulletin of the Commonwealth Institute of Biological Control. 1974;17:169–173. [Google Scholar]
- Peters A, Ehlers R-U. Susceptibility of leather jackets (Tipulapaludosa and Tipulaoleracea; Tipulidae: Nematocera) to the entomopathogenic nematode Steinernema feltiae. Journal of Invertebrate Pathology. 1994;63:163–171. [Google Scholar]
- Phan KL, Tirry L, Moens M. Pathogenic potential of six isolates of entomopathogenic nematodes (Rhabditidia: Steinernematidae) from Vietnam. Biocontrol. 2005;50:477–491. [Google Scholar]
- Poinar GO. 1983. The natural history of nematodes. Prentice-Hall, NJ: Englewood Cliffs. [Google Scholar]
- Poinar GO, Thomas GM. The nature of Achromobactor nematophilus as an insect pathogen. Journal of Invertebrate Pathology. 1967;9:510–514. [Google Scholar]
- Reardon RC, Kaya HK, Fusco RA, Lewis FB. Evaluation of Steinernema feltiae and Steinernema bibionis (Rhabditida: Steinernematidae) for suppression of Lymantria dispar (Lepidoptera: Lymantidae) in Pennsylvania. USA. Agriculture Ecosystems & Environment. 1986;15:1–9. [Google Scholar]
- Ross GES. 1977. Maximum likelihood programme. The numerical algorithms Gr. Rothamsted Experiment Station, UK: Harpenden. [Google Scholar]
- Shahina F, Gulsher M, Javed S, Khanum TA, Bhatti MA. Susceptibility of different life stages of red palm weevil, Rhynchophorus ferrugineus, to entomopathogenic nematodes. International Journal of Nematology. 2009;19:232–240. [Google Scholar]
- Shapiro-Ilan DI, Gouge DH, Koppenhöfer AM. 2002. Factors affecting commercial success: Case studies in cotton, turf and citrus. Pp. 333–356 in R. Gaugler, ed. Entomopathogenic nematology. New York: CABI Publishing. [Google Scholar]
- Shapiro-Ilan DI, Gough DH, Piggott SJ, Patterson FJ. Application technology and environmental considerations for use of entomopathogenic nematodes in biological control. Biological Control. 2006;38:124–133. [Google Scholar]
- Shapiro-Ilan DI, Bruck DJ, Lacey LA. 2012a. Principles of epizootiology and microbial control. Pp. 29–72 in F. E. Vega and H. K. Kaya, ed. Insect pathology, 2nd ed. Amsterdam: Elsevier. [Google Scholar]
- Shapiro-Ilan DI, Campbell JF, Lewis EE, Kim-Shapiro DB. Directional movement of entomopathogenic nematodes in response to electrical field: Effects of species, magnitude of voltage, and infective juvenile age. Journal of Invertebrate Pathology. 2012b;109:34–40. doi: 10.1016/j.jip.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Sims SR, Downing AA, Pershing JC. Comparison of assays for determination of entomogenous nematode infectivity. Journal of Nematology. 1992;24:271–274. [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Poinar GO. Xenorhabdus gen. nov., a genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. International Journal of Systematic Bacteriology. 1979;29:352–360. [Google Scholar]
- Volgyi A, Fodor A, Szentirmai A, Forst S. Phase variation in Xenorhabdus nematophilus. Applied Environmental Microbiology. 1998;64:1188–1193. doi: 10.1128/aem.64.4.1188-1193.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. A method for obtaining infective nematode larvae from culture. Science. 1927;66:302–303. doi: 10.1126/science.66.1709.302-a. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Lalramliana Evaluation of the efficacy of three indigenous strains of entomopathogenic nematodes from Meghalaya, India against mustard sawfly, Athalia lugens proxima Klug (Hymenoptera: Tenthredinidae) Journal of Parasitic Disease. 2012;36:175–180. doi: 10.1007/s12639-012-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]