Abstract
Two new species of Pristionchus, P. lucani n. sp. and P. bulgaricus n. sp., are described from France and Bulgaria, respectively. Additionally, new morphological and morphometric data are provided for two previously described species from Europe, P. brevicauda (Kotlán, 1928) Paramonov, 1952 and P. clavus (von Linstow, 1901) Sudhaus and Fürst von Lieven, 2003. A phylogeny including these four species was inferred from a dataset including 26 ribosomal protein-coding genes, sequences of which are original for P. bulgaricus n. sp. and P. clavus. Relationships support a radiation of all sequenced European Pristionchus species from a single, gonochoristic common ancestor, and current knowledge of species ranges supports “western” and “eastern” clades. Similar diagnostic morphologies reflect the close relationships among the new and recharacterized species, especially P. bulgaricus n. sp., P. brevicauda, and P. clavus, although mating tests as well as genetic and phylogenetic separation support their identities as unique species. Our results show that Pristionchus species in Europe are more diverse than typological characters suggest, and thus biological and molecular profiling will be essential for future delimitation of Pristionchus species from the region.
Keywords: biogeography, gonochorism, hermaphroditism, morphology, morphometrics, phylogeny, ribosomal protein genes, taxonomy
The utility of the nematode Pristionchus pacificus Sommer, Carta, Kim, and Sternberg, 1996 as a model for comparative developmental biology stems from the presence of a sophisticated genetics toolkit with a resolved phylogenetic context for the species (Sommer and McGaughran, 2013). To expand the comparative framework for this system, collection efforts have recovered several new species of Pristionchus Kreis, 1932, which are particularly diverse in East Asia (Kanzaki et al. 2012a, 2012b, 2012c, 2013a, 2013b; Ragsdale et al., 2013). Phylogenetic analyses have consistently supported five highly resolved Pristionchus clades reflecting the biogeography of their gonochoristic members. Three of these clades, previously named the elegans, triformis, and pacificus groups (Kanzaki et al., 2013c; Ragsdale et al., 2013), are of East Asian origin, as is the closest known outgroup to Pristionchus, Parapristionchus giblindavisi Kanzaki, Ragsdale, Herrmann, Mayer, Tanaka, and Sommer, 2012c. In contrast, two clades, the maupasi and lheritieri groups, contain gonochoristic species from North America and Europe, respectively. Although previous studies suggested the phylogenetic separation of at least seven putative European species in the lheritieri group (Herrmann et al., 2006a; Mayer et al. 2007, 2009), four of them, Pristionchus sp. 4, Pristionchus sp. 13, Pristionchus sp. 18, and Pristionchus sp. 19, were so far not described or identified.
Confounding species designations in Pristionchus from Europe is the long taxonomic history of the genus on that continent. In their revision of Diplogastridae, Sudhaus and Fürst von Lieven (2003) recognized 27 valid species of Pristionchus, 14 of which were described from Europe. Based on the relative abundance of males reported in the original descriptions, we hypothesize that six of the nominal European Pristionchus species are gonochoristic: P. brevicauda (Kotlán, 1928) Paramonov, 1952; P. clavus (von Linstow, 1901) Sudhaus and Fürst von Lieven, 2003; P. eurycephalus (Völk, 1950) Sudhaus and Fürst von Lieven, 2003; P. lheritieri (Maupas, 1919) Paramonov, 1952; P. migrans (Lespés, 1856) Sudhaus and Fürst von Lieven, 2003; and P. uniformis Fedorko and Stanuszek, 1971. Two of these species, P. lheritieri and P. uniformis, were previously molecularly characterized (Herrmann et al., 2006a) and, together with the hermaphrodite P. entomophagus (Steiner, 1929) Sudhaus and Fürst von Lieven, 2003, formed the well-supported lheritieri group of Pristionchus species (Mayer et al., 2007; Kanzaki et al., 2013c).
In this article, we describe two new European species, P. lucani n. sp. and P. bulgaricus n. sp., which were previously called Pristionchus sp. 4 (Herrmann et al., 2006a) and Pristionchus sp. 18 (Mayer et al., 2009), respectively. In addition, we give detailed morphological data for P. brevicauda and P. clavus, which were previously referred to as Pristionchus sp. 13 (Mayer et al., 2007) and Pristionchus sp. 19 (Mayer et al., 2009), respectively. To make species diagnoses more precise, we present informative morphological characters for three additional species in the lheritieri group, P. entomophagus, P. lheritieri, and P. uniformis. To biologically delimit putatively close species in the group, we have tested their reproductive isolation from one another. Finally, to place the two new and two recharacterized species into a robust phylogenetic framework, we provide and analyze sequences for 26 ribosomal protein-coding genes of P. clavus and P. bulgaricus n. sp.
Materials and Methods
Nematode isolation and cultivation:
Pristionchus lucani n. sp. and P. brevicauda were isolated from stag beetles and soil, respectively, as described previously (Herrmann et al., 2006a; Mayer et al., 2007). Pristionchus bulgaricus n. sp. and P. clavus were isolated from an adult rose chafer (Coleoptera: Scarabaeidae) and soil, respectively. To isolate P. bulgaricus n. sp., host beetles were dissected on a 2.0% agar plate, after which the plate was kept at room temperature for several weeks. Nematodes proliferated on bacteria associated with the host beetle cadavers. Individuals were thereafter transferred to nematode growth medium (NGM) agar plates seeded with Escherichia coli OP50, and have been since kept in laboratory culture on this medium. Individuals of P. clavus were extracted with a Baermann funnel, after which single females were likewise transferred to NGM plates with E. coli OP50.
Mating tests:
Hybrid crosses were performed to test whether the new and recharacterized species were reproductively isolated from each other. Crosses were performed between closely related species, as informed by our phylogenetic analyses, and included six cross types: (i) P. brevicauda × P. clavus; (ii) P. brevicauda × P. bulgaricus n. sp.; (iii) P. brevicauda × P. uniformis; (iv) P. clavus × P. bulgaricus n. sp.; (v) P. clavus × P. uniformis; (vi) P. bulgaricus n. sp. × P. uniformis. Each cross consisted of two virgin (J4) females of one species and five males of the opposite species. Crosses were performed reciprocally and in at least three replicates for each direction. As a control, conspecific crosses were performed between two males and two virgin females placed under the same conditions as for interspecies crosses. All crosses were performed on plates seeded with small bacterial lawns of approximately the same diameter, as grown from 25 μL E. coli OP50 in L-broth.
Morphological observation and preparation of type and voucher material:
Material for morphological observation were provided by 1- to 2-wk-old cultures of P. brevicauda, P. clavus, and the two new species. Observations by light microscopy (LM) and differential interference contrast (DIC) microscopy were conducted using live nematodes. For morphometrics, specimens were mounted on slides with pads of 5% noble agar and 0.15% sodium azide and were additionally relaxed by heat when necessary. For line drawings, specimens were mounted into water on slides with silicon grease and then relaxed by applying gentle heat; this method allowed manipulation of nematode bodies to allow drawing from different aspects (Kanzaki, 2013). To prepare type and voucher material, nematodes were isolated from type or voucher strain cultures, rinsed in distilled water to remove bacteria, heat killed at 95°C, fixed in 5% formalin and processed through a glycerol and ethanol series using Seinhorst’s method (Hooper, 1986). For comparison with the two new and two recharacterized species, original LM and DIC observations were also made on live specimens of P. entomophagus, P. lheritieri, and P. uniformis. Drawings of previously described species include characters, namely in stomatal and male sexual morphology, that are presently most informative for diagnostics in Pristionchus (Kanzaki et al., 2012a, 2012b, 2013a, 2013b, 2013c; Ragsdale et al., 2013).
Molecular characterization and phylogenetic analysis:
Partial SSU rRNA sequences for the two new and two recharacterized species were available in the GenBank database. A fragment of the gene amplified by the primers SSU18A (5’-AAA GAT TAA GCC ATG CAT G-3’) and SSU9R (5’-AGC TGG AAT TAC CGC GGC TG-3’) (Floyd et al., 2002), and which consisted of 479 positions in our alignment, was designated as a diagnostic sequence (see Herrmann et al., 2006a).
The phylogeny was inferred from the partial SSU rRNA gene and 26 ribosomal protein genes. The aligned SSU rRNA fragment consisted of 852 positions. The suite of ribosomal protein genes, originally developed by Mayer et al. (2007) as marker loci for Pristionchus and other diplogastrid species, constituted a dataset of 10,767 aligned coding nucleotides. Genes included in the analysis were: rpl-1, rpl-2, rpl-10, rpl-14, rpl-16, rpl-23, rpl-26, rpl-27, rpl-27a, rpl-28, rpl-30, rpl-31, rpl-32, rpl-34, rpl-35, rpl-38, rpl-39, rps-1, rps-8, rps-14, rps-20, rps-21, rps-24, rps-25, rps-27, and rps-28. Ribosomal protein gene sequences for P. brevicauda and P. lucani n. sp. were available in GenBank. The orthologous sequences for P. bulgaricus n. sp. and P. clavus are original in this study, and except for those of rpl-38 and rpl-39, which were less than 200 nucleotides in length, sequences for these two species have been deposited in GenBank under accession numbers KJ395505-KJ395528 and KJ395529-KJ395552, respectively. All information regarding genes, primers, and PCR conditions is given in Mayer et al. (2007).
The concatenated dataset of the partial SSU rRNA and ribosomal protein genes was aligned using MUSCLE (Edgar, 2004), followed by manual alignment in MEGA5.05 (Tamura et al., 2011), including deletion of ambiguously aligned positions. The phylogeny was inferred under Bayesian and maximum likelihood (ML) optimality criteria, as implemented in MrBayes 3.2.2 (Ronquist et al., 2012) and RAxML v.7.2.8 (Stamatakis, 2006), respectively. Bayesian analyses, which were computationally intensive, were performed on the CIPRES Science Gateway (Miller et al., 2010). In the Bayesian analysis, the alignment was partitioned into one subset containing the partial SSU rRNA gene and into one containing the concatenated set of ribosomal protein genes. The analysis invoked a codon model of substitution with a gamma-shaped distribution for ribosomal protein genes, and it invoked a general time reversible (GTR) model with a gamma-shaped distribution of rates across sites (Γ) for the partial SSU rRNA gene. Bayesian analyses were initiated with random starting trees and were run with four chains for 2.4 × 106 generations. Markov chains were sampled at intervals of 100 generations. The analysis was performed twice. After confirming convergence of runs and discarding the first 1 × 106 generations as burn-in, remaining topologies were used to generate a 50% majority-rule consensus tree with clade credibility values given as posterior probabilities (PP). For the ML analysis, the alignment was partitioned into four subsets: one for the partial SSU rRNA gene and three according to codon position for the concatenated set of ribosomal protein genes. Fifty independent runs with different starting trees were performed for the ML analysis, which invoked a GTR + Γ model. In both Bayesian and ML analyses, model parameters were unlinked across character partitions. Bootstrap support (BS) for the ML tree was evaluated by 1,000 pseudoreplicates.
Results
Mating tests:
Almost all pairwise interspecific crosses of all combinations of the four species characterized herein failed to produce viable F1 hybrids, whereas intraspecific control crosses produced offspring. In one replicate of one interspecific cross, which was between P. uniformis females and P. bulgaricus n. sp. males, one hybrid second-stage juvenile (J2) emerged but did not develop to adulthood. Given the rarity of this event, particularly when considering that other pairs of unique Pristionchus species can easily produce F1 hybrids (Herrmann et al., 2006b; Kanzaki et al., 2012a), the species in this and all other crosses are considered to be biologically isolated from each other.
Morphology of the new and recharacterized species:
The two new and two recharacterized species are similar in their morphology, reflecting their close relationship to each other. Therefore, to avoid redundancy, the morphology common to all four species is given first, followed by species-specific characters and diagnoses for each species.
Adults:
The body is cylindrical and stout. The body cuticle is thick, with fine annulation and clear longitudinal striations. The lateral field consists of two lines, which are only weakly distinguishable from the body striation. The head bears six short and papilliform labial sensilla. Four small, papilliform cephalic papillae are present in males, as typical for diplogastrid nematodes. Lips are not offset. Amphidial apertures are located at the level of the posterior end of the cheilostomatal plates. Stomatal dimorphism is present, with stenostomatous (“narrow mouthed”) and eurystomatous (“wide mouthed”) forms occurring in both males and females. The dorsal pharyngeal gland could be clearly observed, penetrating the dorsal tooth to the gland opening. The corpus of the pharynx is 1.5 times as long as the postcorpus (isthmus and basal bulb). The procorpus is very muscular and stout, occupying one-half to two-thirds of corresponding body width. The metacorpus is very muscular, forming a well-developed median bulb. The isthmus is narrow and not muscular. The basal bulb is glandular. The pharyngo-intestinal junction could be clearly observed and is well developed. The nerve ring usually surrounds the middle or a slightly more anterior part of the isthmus. The excretory pore is not conspicuous and has a position that varies between slightly anterior to the basal bulb and pharyngo-intestinal junction or sometimes further posterior; the excretory duct extends anteriad and is reflexed back to the position of the pore. The hemizonid could not be clearly observed. Deirids are slightly posterior to pharyngo-intestinal junction. A “postdeirid” (or “prephasmid”) associated with a gland-like cell was observed, being located at about the midpoint between vulva and anus in the female and about one-fifth body length from tail tip in male. Several other pores are located along the body and are either slightly left or right of lateral between body striations, and their positions are inconsistent among individuals; observation by LM could confirm three to six for males and seven to eight for females.
Stenostomatous form:
The cheilostom consists of six per- and interradial plates. The anterior end of each plate is rounded and elongated to stick out from the stomatal opening, thereby forming a small flap. The gymnostom is short, its cuticular ring-like anterior end overlapping the cheilostom internally (medially). The dorsal gymnostomatal wall is slightly thickened compared with the ventral side. The stegostom bears: a large, conspicuous, flint-shaped (or inverted “V”-shaped) dorsal tooth; in the right subventral sector, a crescent-shaped or half-circular ridge with one or occasionally two small cusps; a crescent-shaped left subventral plate, often with an apparent longitudinal cleavage midway along its breadth, which is host to a ridge with three small peaks. The dorsal tooth is sclerotized at its surface.
Eurystomatous form:
The cheilostom is divided into six distinct per- and interradial plates. The anterior end of each plate is rounded and elongated to stick out from the stomatal opening, thereby forming a small flap. The gymnostom is short, its cuticular ring-like anterior end overlapping the cheilostom internally (medially). The stegostom bears: a large, heavily sclerotized, claw-like dorsal tooth; a large claw-like right subventral tooth; in the left subventral sector, a cuticular plate that appears split midway along its length and supports three basic denticles, which may be further divided into smaller apices anteriorly, thereby comprising three (if undivided) to eight small peaks. The dorsal and right subventral teeth are movable; the left subventral denticles are immovable.
Male:
The body is ventrally arcuate and strongly ventrally curved at the tail region when killed by heat. The testis is single, running along the ventral side of body, with the anterior part reflexed to the right side. Spermatogonia are arranged in about four to eight rows in the reflexed part; larger, well-developed spermatocytes are, depending on age of males, arranged in fewer rows or sometimes distally one row in the anterior two-thirds of the main (i.e., nonreflexed) branch; mature amoeboid spermatids are arranged in multiple rows in the remaining, proximal part of the gonad. The vas deferens is not clearly separated from other parts of the gonad. Three (two subventral and one dorsal) cloacal gland cells are present at the distal end of testis and intestine. The spicules are paired and separate, slender and smoothly curved in ventral view, and adjacent to each other for the distal third of their length, each smoothly tapering to a pointed distal end. Each spicule is smoothly ventrally arcuate in lateral view, giving the spicule about 100° curvature. Each spicule has at its anterior end an ovoid manubrium, sometimes three times longer than wide; the spicule blade (i.e., lamina/calomus complex) is ventrally expanded, but sometimes only slightly; the lamina smoothly tapers to a pointed distal end. The gubernaculum is about one-third of the spicule in length, and it is broad anteriorly such that the dorsal wall is slightly recurved and the dorsal and ventral walls separate at a 45° angle at their posterior ends. The dorsal side of the gubernaculum possesses a single membranous process directed anteriad and a lateral pair of more sclerotized processes directed anteriad and ventrad. In lateral view, the processes are arranged such that the anterior part of the gubernaculum shows two serial curves separated by a process directed acutely anteriad, whereby the larger anterior curvature opens at just less than one-half of the gubernaculum length from the posterior end, and the terminal curvature is one-fourth or less of the gubernaculum length; the posterior part of the gubernaculum forms a short tube, about one-third of the gubernaculum length, and envelops the spicules. The cuticle is thick around the tail region, falsely appearing as a narrow leptoderan bursa in ventral view. The cloacal opening is slit-like in ventral view. One small, ventral, single genital papilla (vs) is present on the anterior cloacal lip. Nine pairs of genital papillae are present. Papillae v1-ad (nomenclature follows Sudhaus and Fürst von Lieven, 2003; see Fig. 1) are of almost equal size, and are large and conspicuous; v5 and v6 are very small, sometimes difficult to observe by LM; v7 and pd are small but larger than v5 and v6, i.e., intermediate between v1-ad and v5/v6 in size. The tip of each v6 papilla is split into two small papilla-like projections. Papillae v1-ad, v7, and pd are papilliform and cone-shaped, borne directly by body; v5 and v6 are each shrouded at the base by a socket-like structure. Species-specific papillae arrangements are given below, but the following characters are common to all four species treated herein: v1 and v3 are separated by one cloacal body width, v3 and v4 are separated by about half a cloacal body width, and pd is posterior to but sometimes overlapping v7. Excluding the terminal spike, the tail is about 2 cloacal body widths long. A bursa or bursal flap is absent. The tail is conical, with a long spike, which has a filiform distal end.
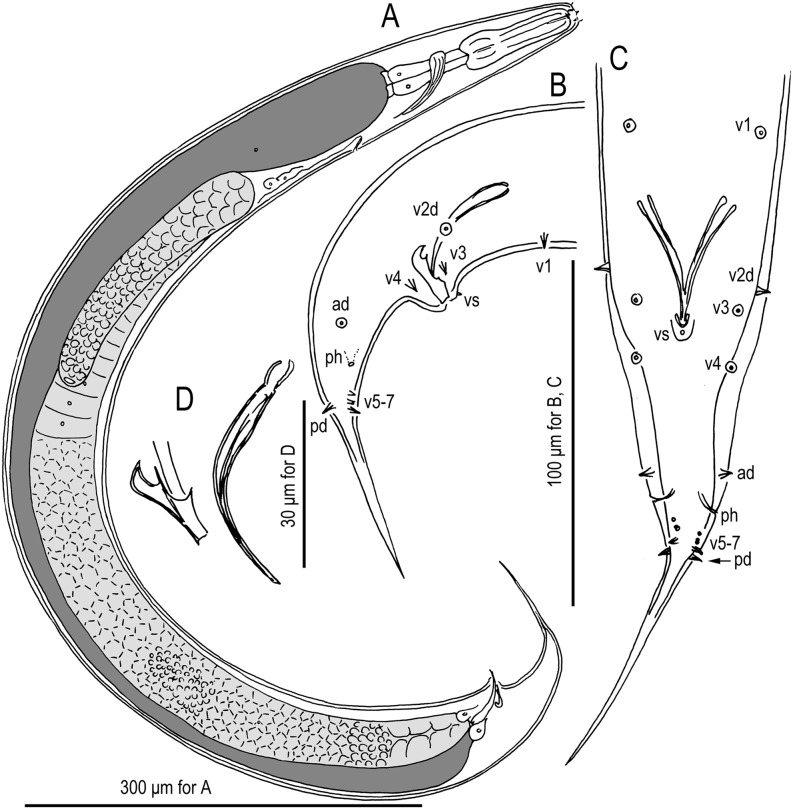
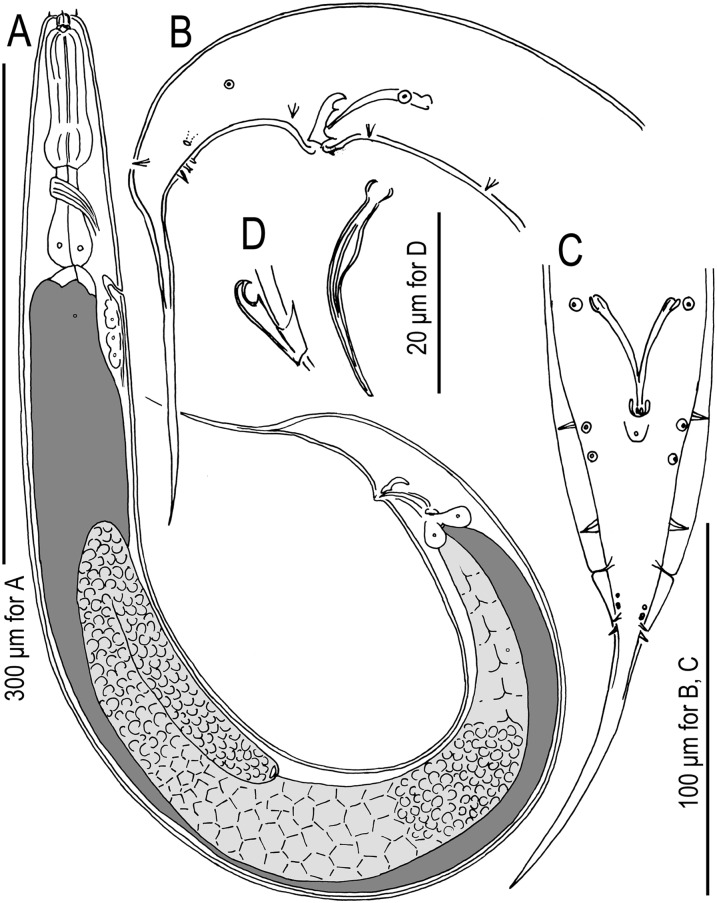
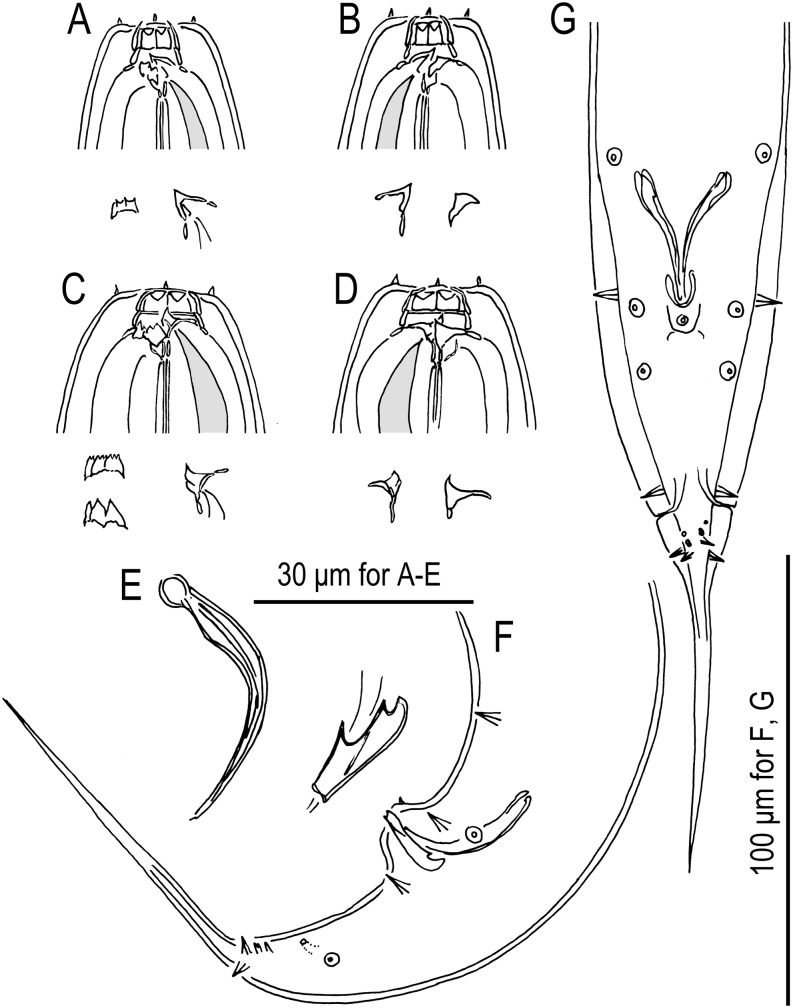
Fig. 1.
Males of Pristionchus lucani n. sp. (strain RS5050). A. Whole body, right lateral view. B. Tail region, right lateral view. C. Tail region, ventral view. D. Gubernaculum and spicule, right lateral view. Nomenclature for male genital papillae (C, D) follows Sudhaus and Fürst von Lieven (2003).
Female:
The gonad is didelphic and amphidelphic. Each gonadal system is arranged from the vulva and vagina as: uterus, oviduct, and ovary. The anterior gonad is to the right of the intestine, with the uterus and oviduct extending ventrad and anteriad on the right of intestine, and has a totally reflexed (= antidromous reflexion) ovary that extends dorsad on the left of intestine. Oocytes are mostly arranged in multiple rows, sometimes more than five, in the distal two-thirds of the ovary and are arranged in a single row in the remaining one-third of the ovary, the distal tips of each ovary reaching the oviduct of opposite gonadal branch. The oviduct serves as a spermatheca, and sperm can be observed in the distal part of oviduct, close to the ovary. Eggs are in the single- to multiple-cell stage or are even further developed in the proximal part of the oviduct (i.e., uterus). A receptaculum seminis could not be observed. The dorsal wall of the uterus is thickened at the level of the vulva and appears dark by LM observation. Four vaginal glands are present but obscure. The vagina is perpendicular to the body surface and is surrounded by sclerotized tissue. The vulva is slightly protuberant in lateral view and is pore-like in ventral view. The rectum is about one anal body width long, and a well-developed sphincter muscle surrounds the intestinal-rectal junction. Three (two subventral and one dorsal) anal glands are present at the intestinal/rectal junction but are not obvious. The anus is in the form of a dome-shaped slit, and the posterior anal lip is slightly protuberant. The phasmids are about two anal body widths posterior to the anus. The tail is long, its distal end being filiform to long and conical.
Pristionchus lucani* n. sp. = Pristionchus sp. 4 apud Hermann et al. (2006a) and Mayer et al. (2007, 2009)
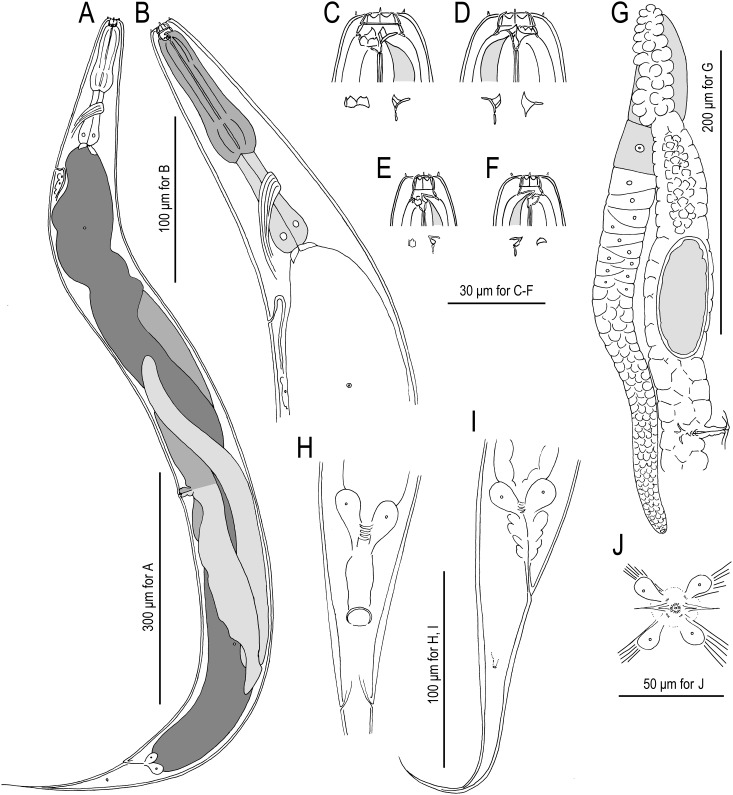
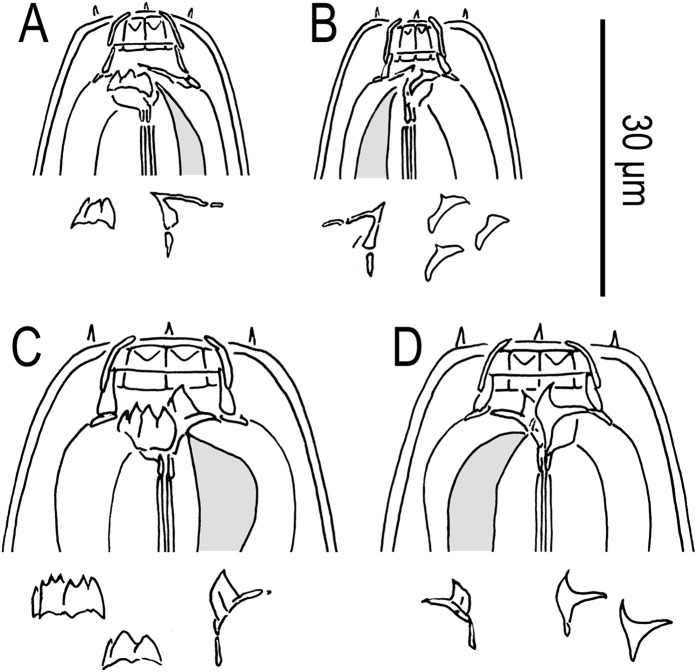
Fig. 2.
Females of Pristionchus lucani n. sp. (strain RS5050). A. Whole body, left lateral view. B. Neck region, left lateral view. C. Stomatal region of eurystomatous form, left lateral view, showing left subventral plate and dorsal tooth below. D. Stomatal region of eurystomatous form, right lateral view, showing dorsal tooth and right subventral tooth below. E. Stomatal region of stenostomatous form, left lateral view, showing left subventral denticles and dorsal tooth below. F. Stomatal region of stenostomatous form, right lateral view, showing dorsal tooth and right subventral ridge below. G. Anterior gonadal branch, right lateral view. H. Tail region, ventral view. I. Tail region, right lateral view. J. Vulva, ventral view.
Measurements: See Table 1.
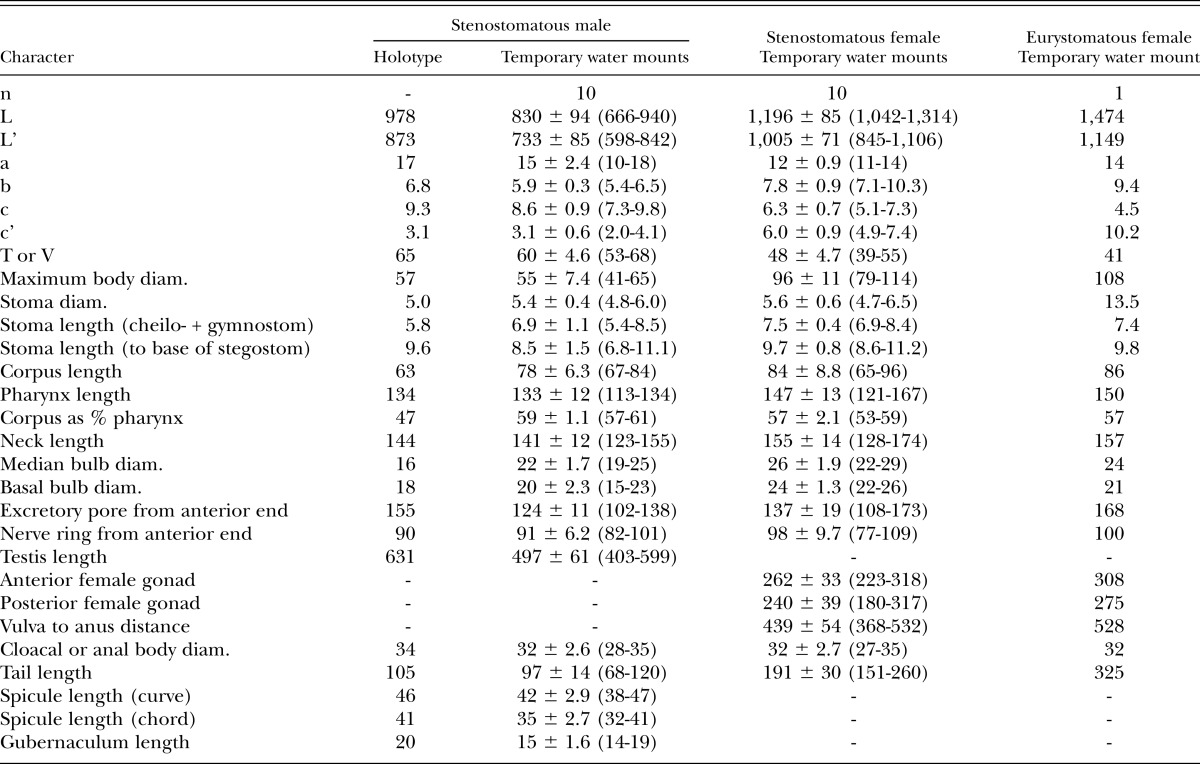
Table 1.
Morphometrics of stenostomatous male holotype (in glycerin) and male and female specimens of Pristionchus lucani n. sp. (temporary water mounts). All measurements made in μm and given in the form: mean ± sd (range).
Male:
Genital papillae and phasmids are arranged as <v1, v2d, v3, C, v4, ad, Ph, (v5, v6, v7), pd> (= <P1, P2d, P3, C, P4, P5d, Ph, (P6, P7, P8), P9d> in the homology-agnostic nomenclature of Kanzaki et al., 2012a), whereby v2d is clearly anterior to v3 and Ph is midway between ad and v5. The tail spike is relatively short, being shorter than the distance from the cloaca to the root of the tail spike (i.e., the observable constriction just posterior to the most posterior genital papillae).
Diagnosis and relationships:
Besides its generic characters, P. lucani n. sp. is diagnosed by the male genital papillae arranged as <v1, v2d, v3, C, v4, ad, Ph, (v5, v6, v7), pd whereby v1 and v3 are separated by one cloacal body width, v2d is clearly anterior to v3, Ph is midway between ad and v5, and pd is posterior to or overlapping v7. Pristionchus lucani n. sp. is distinguished from the phylogenetically closest known species, P. lheritieri, by a male tail spike that is shorter vs. longer than the distance from the cloaca to the root of the tail spike (Fig. 3) and by its unique SSU rRNA sequence, which differs from that of P. lheritieri n. sp. by 5 nucleotide positions in a diagnostic 479-bp fragment of the gene.
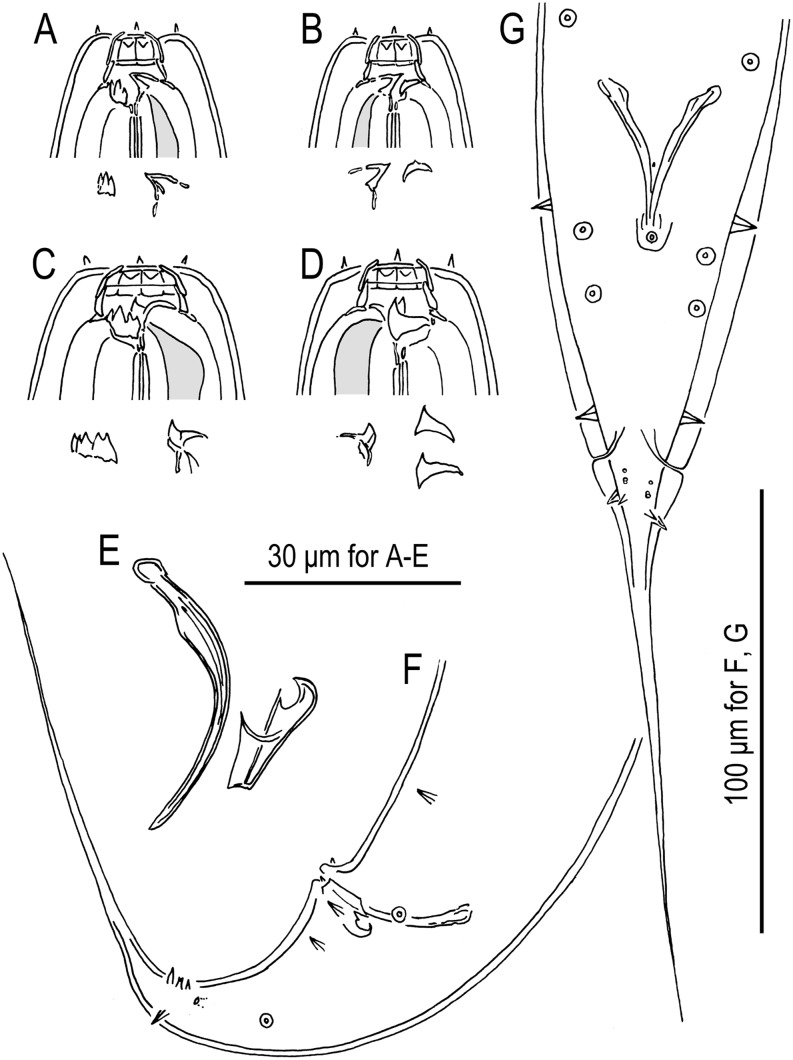
Fig. 3.
Pristionchus lheritieri (Maupas, 1919) Paramonov, 1952 (strain SB245). A. Stomatal region of stenostomatous female, left lateral view, showing left subventral denticles and dorsal tooth below. B. Stomatal region of stenostomatous female, right lateral view, showing dorsal tooth and right subventral ridge below. C. Stomatal region of eurystomatous female, left lateral view, showing left subventral plate and dorsal tooth below. D. Stomatal region of eurystomatous female, right lateral view, showing dorsal tooth (left) and two variants of right subventral tooth (right) below. E. Spicule and gubernaculum, left lateral view. F. Tail region of male, left lateral view. G. Tail region of male, ventral view.
Type host (carrier) and locality:
The culture from which the type specimens were obtained was originally isolated from two of 14 examined stag beetles of the species Lucanus cervus L. (Coleoptera: Lucanidae) collected close to Vailhauquès, France, by M. Herrmann on 15 June 2004.
Type material, type strain, and nomenclatural registration:
Holotype stenostomatous male (accession number 31373), five paratype males, and five paratype females have been deposited in the University of California Riverside Nematode Collection (UCRNC), CA. Four paratype males and four paratype females have been deposited in the Swedish Natural History Museum, Stockholm, Sweden. Four paratype males and three paratype females have been deposited in the Natural History Museum Karlsruhe, Germany. The type strain is available in living culture and as frozen stocks under culture code RS5050 in the Department of Evolutionary Biology, Max Planck Institute (MPI) for Developmental Biology, Tübingen, Germany and can be provided to other researchers on request. The new species binomial has been registered in the ZooBank database (zoobank.org) under the identifier 1FFD92E0-2829-4BEB-BA9E-E22D196DE90B.
Pristionchus bulgaricus* n. sp.
= Pristionchus sp. 18 apud Mayer et al. (2009)
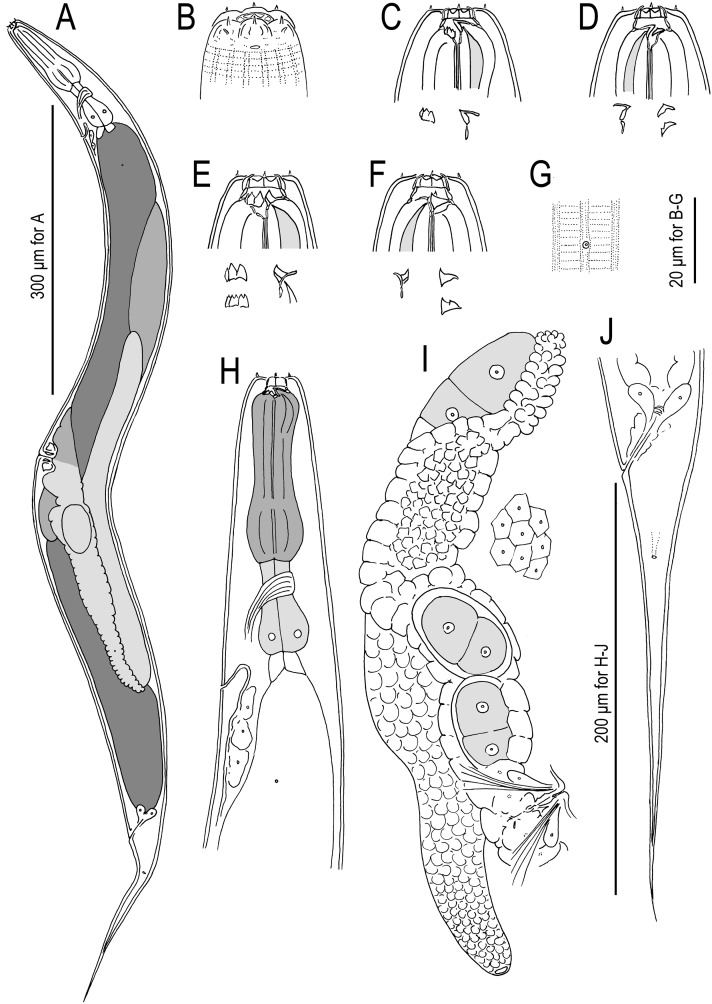
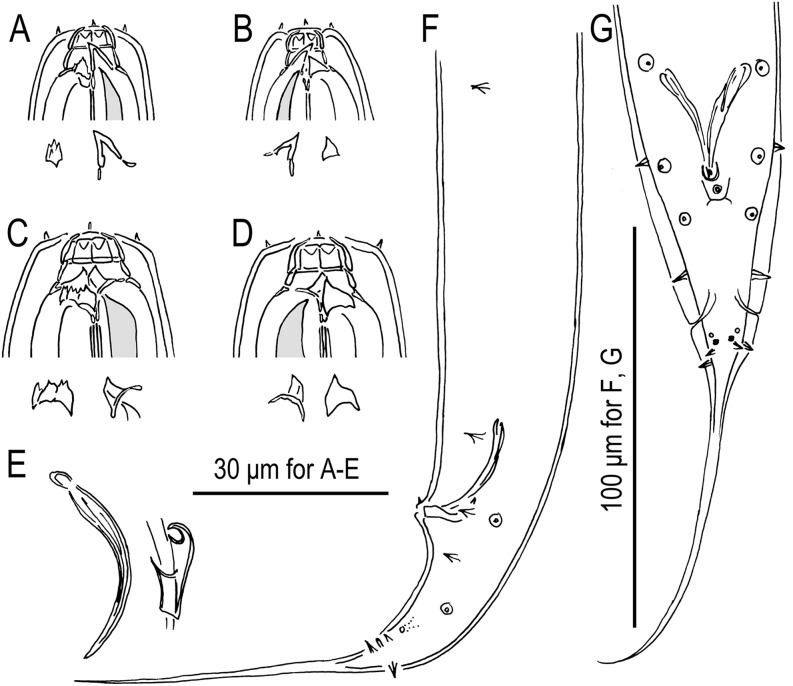
Fig. 4.
Pristionchus bulgaricus n. sp. (strain RS5283). A. Whole body, left lateral view. B. Lip region of male, lateral view. C. Stomatal region of stenostomatous female, left lateral view, showing left subventral denticles and dorsal tooth below. D. Stomatal region of stenostomatous female, lateral view, showing dorsal tooth (left) and two variants of right subventral ridge (right) below. E. Stomatal region of eurystomatous female, left lateral view, showing two variants of left subventral plate (left) and dorsal tooth (right) below. F. Stomatal region of eurystomatous female, right lateral view, showing dorsal tooth (left) and two variants of right subventral tooth (right), including that with two cusps, below. G. Deirid and body surface, lateral view. (H) Neck region of female, left lateral view. (I) Anterior female gonadal branch, right lateral view, also showing cells of uterine wall (right). (J) Tail region of female, left lateral view.
Fig. 5.
Males of Pristionchus bulgaricus n. sp. (strain RS5283). A. Whole body, right lateral view. B. Tail region, right lateral view. C. Gubernaculum and spicule, right lateral view. D. Tail region, ventral view.
Measurements: See Table 2.
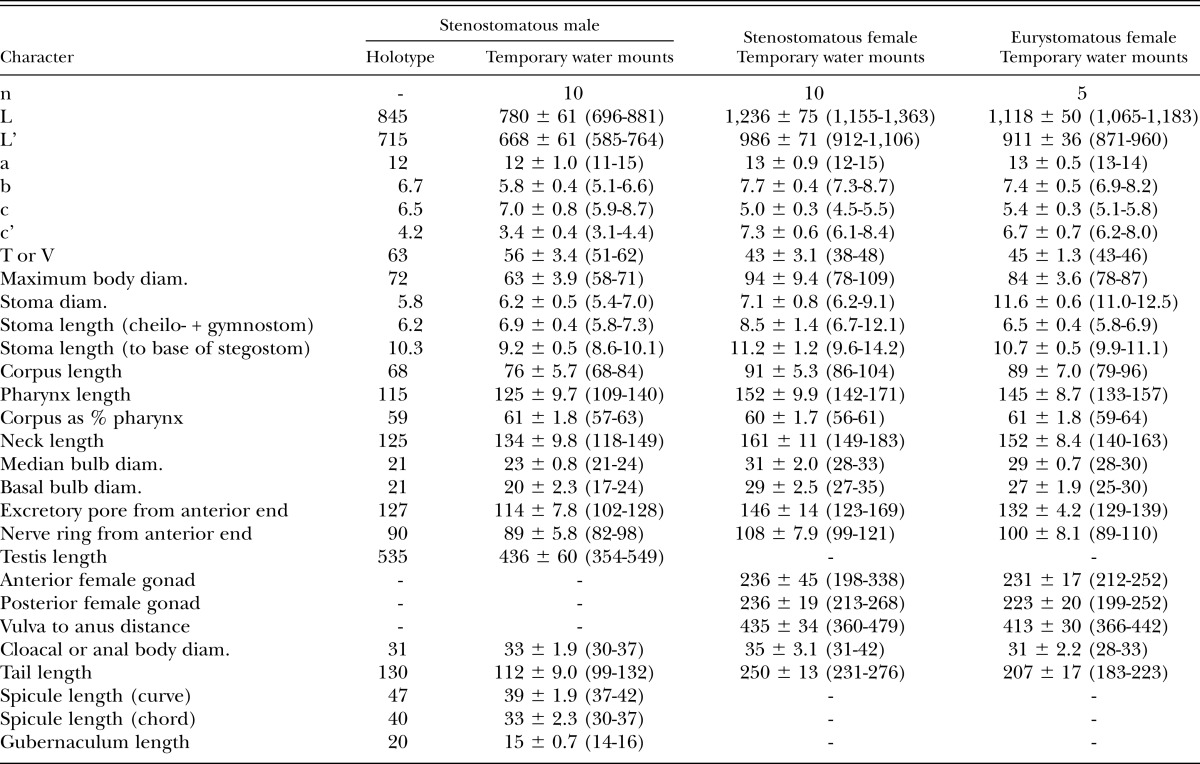
Table 2.
Morphometrics of stenostomatous male holotype (in glycerin) and male and female specimens of Pristionchus bulgaricus n. sp. (temporary water mounts). All measurements made in μm and given in the form: mean ± sd (range).
Eurystomatous form:
The right subventral tooth often but not always has two distinct cusps, the more ventral of the cusps being shorter than the apical cusp that is typical of Pristionchus nematodes.
Male:
Genital papillae and phasmids are arranged as <v1, (v2d, v3), C, v4, ad, Ph, (v5, v6, v7), pd> (= <P1, (P2d, P3), C, P4, P5d, Ph, (P6, P7, P8), P9d> in the nomenclature of Kanzaki et al., 2012a), whereby v2d is close to or at the same level as v3 and ad is anterior, such that Ph is midway between ad and v5 or closer to v5. The tail spike is as long or longer than the distance from the cloaca to the root of the tail spike.
Diagnosis and relationships:
Besides its generic characters, P. bulgaricus n. sp. is diagnosed by a eurystomatous right subventral tooth that often has two distinct cusps and by the male genital papillae arranged as <v1, (v2d, v3) C, v4, ad, Ph, (v5, v6, v7), pd> whereby v1 and v3 are separated by one cloacal body width, v2d is close to or at the same level as v3, Ph is midway between ad and v5 or closer to v5, and pd is posterior to or overlapping v7. Pristionchus bulgaricus n. sp. is distinguished from the phylogenetically closest known species, P. brevicauda, P. clavus, and P. uniformis, by reproductive isolation, namely the inability to produce viable F1 hybrids with those species. It is distinguished from the phylogenetically close P. entomophagus (Fig. 6) by a gonochoristic vs. hermaphroditic mode of reproduction. Pristionchus bulgaricus n. sp. is further distinguished from all four of the other species above by the frequent presence of two cusps on the right subventral tooth. It differs from P. clavus by ad being anterior, such that Ph is midway between ad and v5 or even relatively closer to v5 vs. ad being far posterior and thus very close to Ph. The new species is distinguished from P. uniformis by: the shape of the dorsal tooth in the stenostomatous form, which, as typical for Pristionchus nematodes, does not extend past the gymnostom vs. being relatively large and extending into the cheilostom; a stenostomatous right subventral ridge that is, as typical for Pristionchus nematodes, semicircular vs. high and narrow (Fig. 7). Finally, P. bulgaricus n. sp. is diagnosed by its unique SSU rRNA sequence, which differs from that of from P. brevicauda, P. clavus, and P. uniformis by at least six nucleotide positions in a diagnostic 479-bp fragment of the gene.
Fig. 6.
Stomatal region of Pristionchus entomophagus (Steiner, 1929) Sudhaus and Fürst von Lieven, 2003 (strain RS0144) hermaphrodites. A. Stenostomatous form, left lateral view, showing left subventral denticles and dorsal tooth below. B. Stenostomatous form, right lateral view, showing dorsal tooth (left) and three variants of right subventral ridge (right) below. C. Eurystomatous form, left lateral view, showing two variants of left subventral plate (left) and dorsal tooth (right) below. D. Eurystomatous form, right lateral view, showing dorsal tooth (left) and two variants of right subventral tooth (right) below.
Fig. 7.
Pristionchus uniformis Fedorko and Stanuszek, 1971 (strain RS0141). A. Stomatal region of stenostomatous female, left lateral view, showing tall, narrow left subventral ridge and large dorsal tooth below. B. Stomatal region of stenostomatous female, right lateral view, showing large dorsal tooth and pointed right subventral ridge below. C. Stomatal region of eurystomatous female, left lateral view, showing left subventral plate and dorsal tooth below. D. Stomatal region of eurystomatous female, right lateral view, showing dorsal tooth and right subventral tooth below. E. Spicule and gubernaculum, left lateral view. F. Tail region of male, left lateral view. G. Tail region of male, ventral view.
Type host (carrier) and locality:
The culture from which the type specimens were obtained was originally isolated from one of 20 examined rose chafers of the species Cetonia aurata L. collected close to Tabachka, Bulgaria, by M. Herrmann on 24 May 2007.
Type material, type strain, and nomenclatural registration:
Holotype stenostomatous male (31375), five paratype males, and five paratype females have been deposited in the UCRNC, CA. Four paratype males and four paratype females have been deposited in the Swedish Natural History Museum, Stockholm, Sweden. Four paratype males and four paratype females have been deposited in the Natural History Museum Karlsruhe, Germany. The type strain is available in living culture and as frozen stocks under culture code RS5283 in the Department of Evolutionary Biology, MPI Developmental Biology and can be provided to other researchers on request. The new species binomial has been registered in the ZooBank database under the identifier B94D35AC-FE5A-43EA-A7B0-04FE8CF409D6.
Pristionchus brevicauda (Kotlán, 1928) Paramonov, 1952 = Pristionchus sp. 13 apud Mayer et al. (2007, 2009)
(Fig. 8)
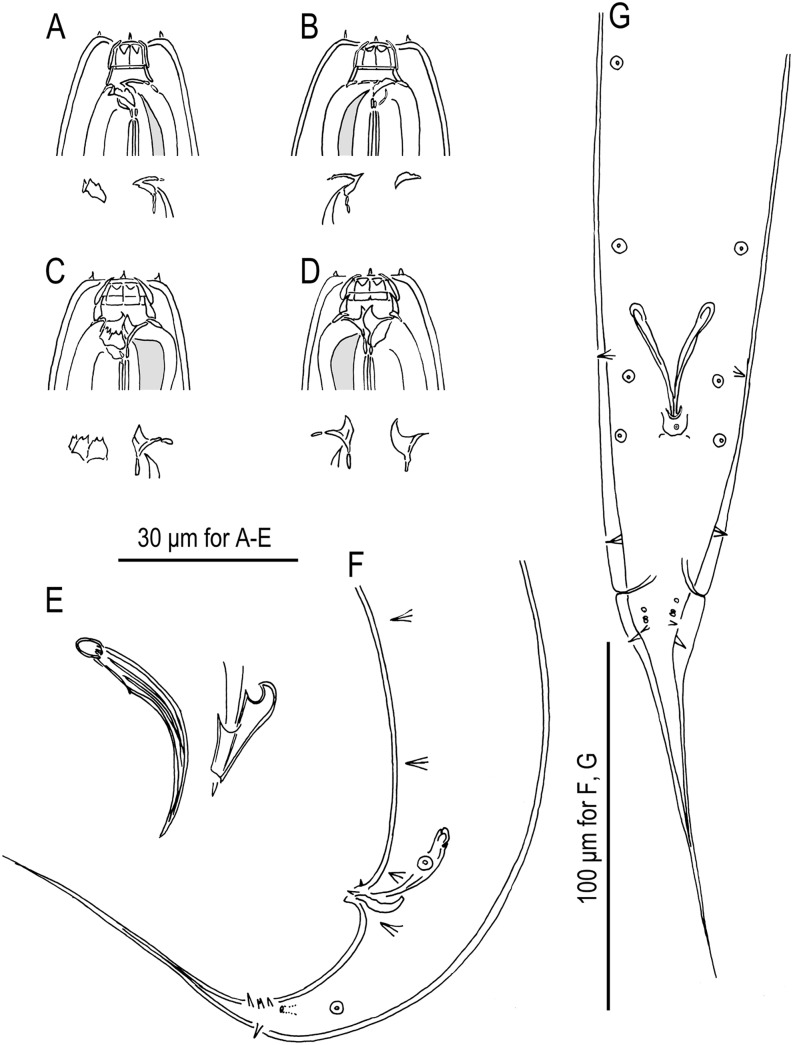
Fig. 8.
Pristionchus brevicauda (Kotlán, 1928) Paramonov, 1952 (strain RS5231). A. Stomatal region of stenostomatous female, left lateral view, showing left subventral denticles and dorsal tooth below. B. Stomatal region of stenostomatous female, right lateral view, showing dorsal tooth and right subventral ridge below. C. Stomatal region of eurystomatous female, left lateral view, showing left subventral plate and dorsal tooth below. D. Stomatal region of eurystomatous female, showing dorsal tooth and right subventral tooth below. E. Spicule and gubernaculum, right lateral view. F. Tail region of male, left lateral view. G. Tail region of male, ventral view.
Measurements:
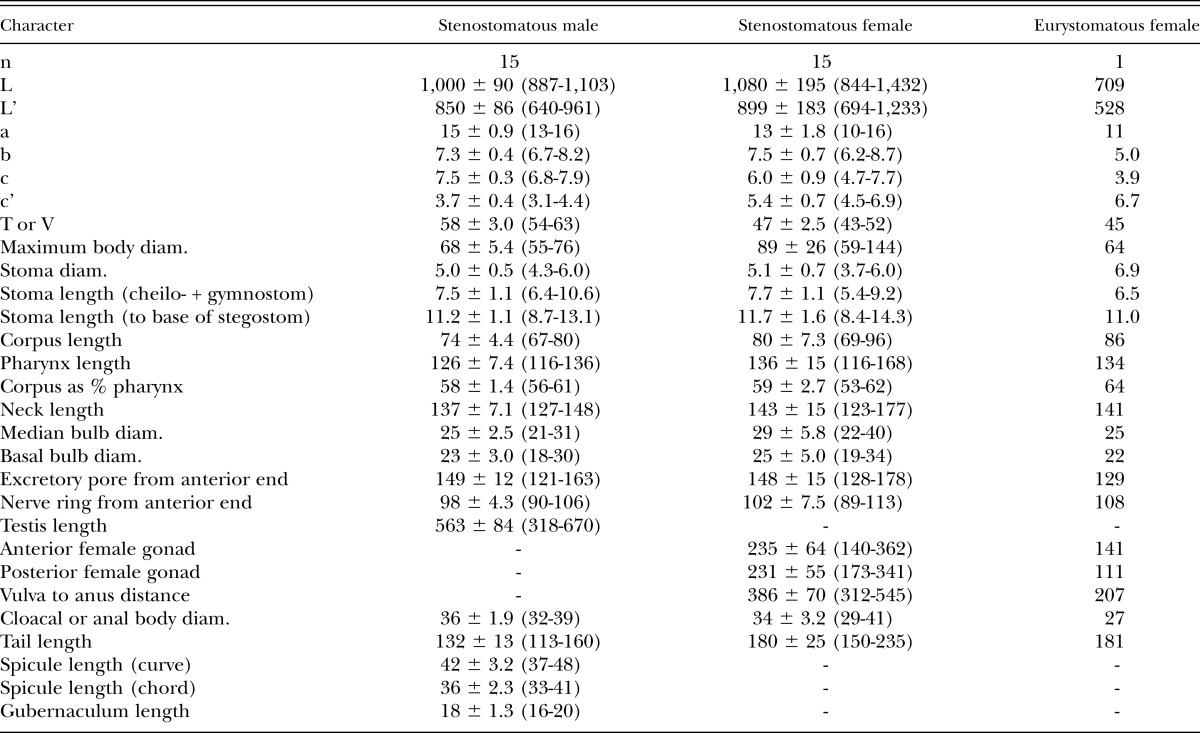
See Table 3. The originally reported body length of P. brevicauda (males 0.4 to 0.5 mm, females 0.5 to 0.6 mm) is less than that of samples from our cultured isolate, but the high plasticity of this trait within populations of Pristionchus species (e.g., Kanzaki et al., 2012a, 2012b, 2012c, 2013a, 2013b, 2013c; present study) discounts its general utility as a diagnostic character. Nevertheless, we do not consider our identification of P. brevicauda as definitive but as a hypothesis subject to testing by future sampling of strains and characters.
Table 3.
Morphometrics of male and female specimens of Pristionchus brevicauda (Kotlán, 1928) Paramonov 1952 (strain RS5231) in temporary water mounts. All measurements made in μm and given in the form: mean ± sd (range).
Male:
Genital papillae and phasmids are arranged as <v1, v2d, v3, C, v4, ad, Ph, (v5, v6, v7), pd> (= <P1, (P2d, P3), C, P4, P5d, Ph, (P6, P7, P8), P9d> in the nomenclature of Kanzaki et al., 2012a), whereby v2d is close to but anterior to v3 and ad is anterior, such that Ph is midway between ad and v5 or closer to v5. Male sexual morphology is identical with what characters are reported in the original description. The tail spike is as long or longer than the distance from the cloaca to the root of the tail spike, also as drawn in the original description.
Diagnosis and relationships:
Besides its generic characters, P. brevicauda is diagnosed by the male genital papillae arranged as <v1, (v2d, v3) C, v4, ad, Ph, (v5, v6, v7), pd> whereby v1 and v3 are separated by one cloacal body width, v2d is close to or at the same level as v3, Ph is midway between ad and v5 or closer to v5, and pd is posterior to or overlapping v7. Pristionchus brevicauda is distinguished from the phylogenetically closest known species, P. bulgaricus n. sp., P. clavus, and P. uniformis, by reproductive isolation, namely the inability to produce viable F1 hybrids with those species. It is distinguished from the phylogenetically close P. entomophagus by a gonochoristic vs. hermaphroditic mode of reproduction. The species differs from P. clavus by ad being anterior, such that Ph is midway between ad and v5 or closer to v6 vs. ad being far posterior and thus very close to Ph. Pristionchus brevicauda is distinguished from P. bulgaricus n. sp. by a eurystomatous right subventral tooth having a single cusp vs. frequently two cusps. It is distinguished from P. uniformis by the shape of the dorsal tooth in the stenostomatous form, which does not extend past the gymnostom vs. being relatively large and extending into the cheilostom and by a stenostomatous right subventral ridge that is semicircular vs. high and narrow. Finally, P. brevicauda is diagnosed by its unique SSU rRNA sequence, which differs from that of from P. bulgaricus n. sp., P. clavus, and P. uniformis by at least nine nucleotide positions in a diagnostic 479-bp fragment of the gene.
Distribution:
The type locality of this species is in Peremarton, Veszprém, Hungary, where it was isolated from dead larvae of Ostrinia nublialis (Hübner, 1796) (Lepidoptera: Pyralidae). The species has since been collected from a soil sample taken close to Piteşti, Romania by M. Herrmann on 19 May 2007.
Voucher material and culture:
Eight female and eight male voucher specimens have been deposited in the UCRNC, CA. The strain used for analysis and observations is available in living culture and as frozen stocks under culture code RS5231 in the Department of Evolutionary Biology, MPI Developmental Biology and can be provided to other researchers on request.
Pristionchus clavus (von Linstow, 1901) Sudhaus and Fürst von Lieven, 2003 = Pristionchus sp. 19 apud Mayer et al. (2009)
(Fig. 9)
Fig. 9.
Pristionchus clavus (von Linstow, 1901) Sudhaus and Fürst von Lieven, 2003 (strain RS5284). A. Stomatal region of stenostomatous female, left lateral view, showing left subventral denticles and dorsal tooth below. B. Stomatal region of stenostomatous female, showing dorsal tooth and right subventral ridge below. C. Stomatal region of eurystomatous female, left lateral view, showing two variants of left subventral plate (left) and dorsal tooth (right) below. D. Stomatal region of eurystomatous female, right lateral view, showing dorsal tooth and right subventral tooth below. E. Spicule and gubernaculum, right lateral view. F. Tail region of male, left lateral view. G. Tail region of male, ventral view.
Measurements:
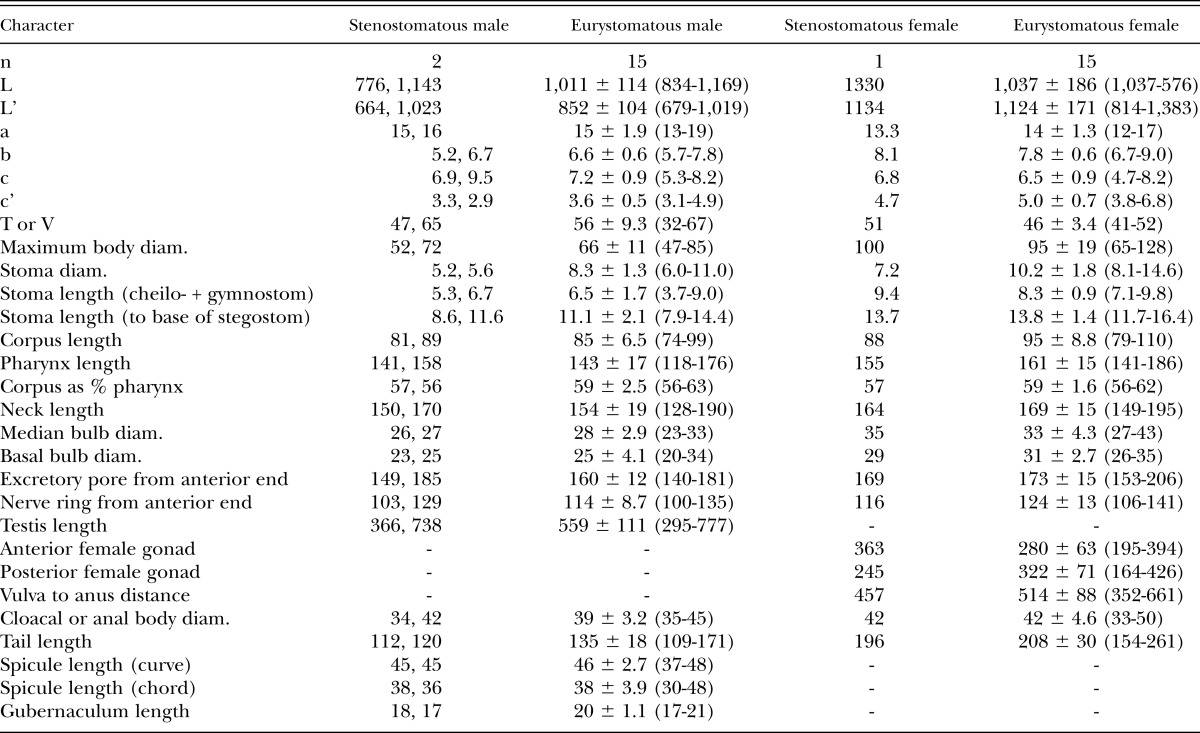
See Table 4. Despite the overlapping of measurements between the types and our own isolates, we do not consider our identification of P. clavus as definitive but, as in the case of P. brevicauda, a hypothesis subject to future testing.
Table 4.
Morphometrics of male and female specimens of Pristionchus clavus (von Linstow, 1901) Sudhaus and Fürst von Lieven, 2003 (strain RS5284) in temporary water mounts. All measurements made in μm and given in the form: mean ± sd (range), except for stenostomatous males, for which all measurements are given.
Male:
Genital papillae and phasmids are arranged as <v1, (v2d, v3), C, v4, (ad, Ph), (v5, v6, v7), pd> (= <P1, (P2d, P3), C, P4, (P5d, Ph), (P6, P7, P8), P9d> in the nomenclature of Kanzaki et al., 2012a), whereby v2d is close to or at the same level as v3 and ad is far posterior, such that Ph is closer to ad than to v5. The tail spike is as long or longer than the distance from the cloaca to the root of the tail spike.
Diagnosis and relationships:
Besides its generic characters, P. clavus is diagnosed by the male genital papillae arranged as <v1, v2d, v3, C, v4, ad, Ph, (v5, v6, v7), pd> whereby v1 and v3 are separated by one cloacal body width, v2d is close to but anterior to v3, ad is far posterior, such that Ph is closer to ad than to v5, and pd is posterior to or overlapping v7. Pristionchus clavus is distinguished from the phylogenetically closest known species, P. brevicauda, P. bulgaricus n. sp., and P. uniformis, by reproductive isolation, namely the inability to produce viable F1 hybrids with those species. Pristionchus clavus is further distinguished from P. brevicauda, P. bulgaricus n. sp., and P. uniformis by ad being far posterior and thus very close to Ph vs. anterior, such that Ph is midway between ad and v5 or even relatively closer to v5. Pristionchus clavus is distinguished from P. bulgaricus n. sp. by a eurystomatous right subventral tooth having a single cusp vs. frequently two cusps. It is distinguished from P. uniformis by the shape of the dorsal tooth in the stenostomatous form, which does not extend past the gymnostom vs. being relatively large and extending into the cheilostom and by a stenostomatous right subventral ridge that is semicircular vs. high and narrow. Pristionchus clavus is distinguished from the phylogenetically close P. entomophagus by a gonochoristic vs. hermaphroditic mode of reproduction. Finally, P. clavus is diagnosed by its unique SSU rRNA sequence, which differs from that of from P. brevicauda, P. bulgaricus n. sp., and P. uniformis by at least nine nucleotide positions in a diagnostic 479-bp fragment of the gene.
Distribution:
The type locality of this species is in Göttingen, Germany, where it was isolated from damaged leaves of Allium vineale L. The species has since been collected from a soil sample taken close to Tabachka, Bulgaria, by M. Herrmann on 24 May 2007.
Voucher material and culture:
Eight female and eight male voucher specimens have been deposited in the UCRNC, CA. The strain used for analysis and observations is available in living culture and as frozen stocks under culture code RS5284 in the Department of Evolutionary Biology, MPI Developmental Biology and can be provided to other researchers on request.
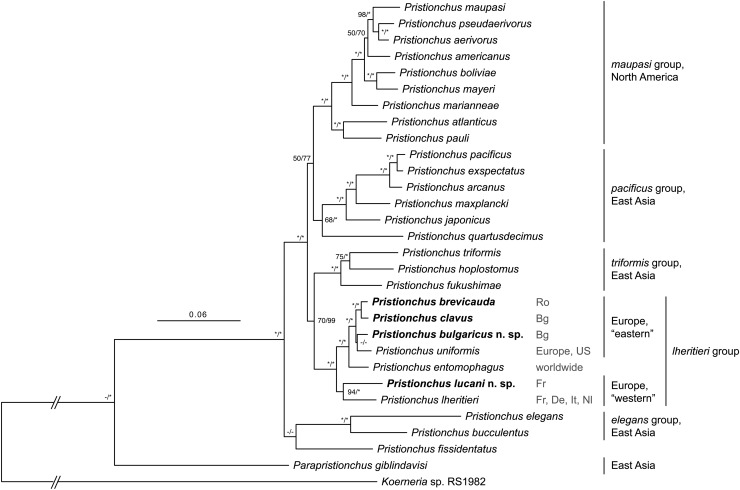
Phylogenetic analysis:
The total concatenated alignment included 4,172 variable and 2,587 parsimony-informative sites. Tree topologies from the ML and Bayesian analyses were nearly identical, so only the ML tree is shown, along with support values from both analyses (Fig. 10). A clade consisting of all six gonochoristic European Pristionchus species and P. entomophagus, together constituting the previously designated lheritieri group of Pristionchus species, was highly supported (100% BS, PP). Furthermore, two major clades within the lheritieri group were resolved. One well-supported clade (94% BS, 100% PP) consisted of P. lucani n. sp. and P. lheritieri; the other inferred clade comprised all other species in the lheritieri group (100% BS, PP). In the latter clade, P. brevicauda and P. clavus were highly supported as sister taxa (100% BS, PP), and P. bulgaricus n. sp. and P. uniformis were sister taxa to this clade. The relationship of lheritieri group to other Pristionchus species was poorly to moderately supported (70% BS, 99% PP): the clade was the sister group to another clade, the triformis group, whose membership comprises the Japanese gonochorists P. fukushimae Ragsdale, Kanzaki, Röseler, Herrmann, and Sommer, 2013; P. hoplostomus Ragsdale, Kanzaki, Röseler, Herrmann, and Sommer, 2013; and the hermaphrodite P. triformis Ragsdale, Kanzaki, Röseler, Herrmann, and Sommer, 2013. Lastly, the discrepancies between the phylogenies inferred under different ML and Bayesian criteria were: the position of P. uniformis, which was poorly supported (< 50% BS, PP) in both analyses and which was inferred to be a sister group to P. bulgaricus n. sp., P. brevicauda, and P. clavus in the Bayesian tree; the position of P. fissidentatus Kanzaki, Ragsdale, Herrmann, and Sommer, 2012b, which was poorly supported (< 50% BS, PP) in both analyses.
Fig. 10.
Phylogenetic relationships of Pristionchus species inferred by maximum likelihood (ML) from a partial SSU rRNA fragment and 26 ribosomal protein-coding genes. The ML tree with the highest log likelihood is shown. The proportion of trees in which the associated taxa clustered together in 1,000 bootstrap pseudoreplicates is shown next to the nodes (left value). Tree topology is identical with that from the Bayesian inference, and posterior probabilities for corresponding nodes are also displayed (right value) on tree. Bootstrap support values above 50% are given. Dashes indicate < 50% BS (left value) or node nonsupport in Bayesian analysis (right value). Asterisks indicate 100% support. Tree is drawn to scale (except for branches connecting to root), with branch lengths measured in the number of substitutions per site. All sequenced gonochoristic species from Europe fall into a single monophyletic clade (lheritieri group) that also includes the hermaphrodite P. entomophagus. Localities for isolates with sequence vouchers are given to right of individual species (Bg: Bulgaria; De: Germany; Fr: France; It: Italy; Nl: Netherlands; Ro: Romania; US: United States). Geographic distributions given to right of clades are for gonochoristic species.
Discussion
Our phylogenetic analysis of four European species of Pristionchus, including two new species, supports a radiation of seven Pristionchus species from a single, gonochoristic common ancestor in the region. Finer biogeographic structure is also apparent from our analysis, which distinguished one clade with species showing relatively western distributions and a second with known ranges extending further east in Europe (Fig. 10). Specifically, the “western” clade included P. lheritieri, which was originally collected in Vire, France, and Walcheren, Netherlands, and has since been isolated in Germany and Italy (e.g., Herrmann et al., 2006a). Similarly, the other species in this clade, P. lucani n. sp., was isolated twice in southern France (Herrmann et al., 2006a).
In contrast, species in the “eastern” clade are known mostly from Central and Eastern Europe. Among the gonochorists of this group, the range of P. uniformis is arguably the best characterized, being originally described from Poland but then subsequently recovered multiple times in Europe, as far west as northern France (Herrmann et al., 2006a). Although P. uniformis was also collected in the United States, population genetics analysis supported the colonization of North America to be secondary, presumably in association with migrations of its vector, the Colorado potato beetle (Leptinotarsa decemlineata Say) (D’Anna and Sommer, 2011). Also consistent with an “eastern” clade are the Central and Eastern European distributions of the other species in this clade, namely: the isolation of P. clavus in central Germany and Bulgaria; the type locality of P. brevicauda in Hungary and its subsequent collection in Romania; the type and only known locality of P. bulgaricus n. sp. in Bulgaria. Therefore, although the ranges of species or clades may overlap, they recapitulate a basal divergence in the phylogeny of the lheritieri group. The global distribution of P. entomophagus obscures its precise center of origin, although its nested position in the lheritieri group of Pristionchus species supports its ancestral range to be in Europe.
In addition to the radiation of the lheritieri group, at least one additional incursion into Europe by a gonochoristic species might have occurred. This event is predicted to have happened along some lineage including P. eurycephalus, a species reported from Erlangen, Germany. Although molecular sequence data are still missing for this species, morphological evidence supports it as separate from the lheritieri group and instead akin to species in the maupasi group. Specifically, the male genital papillae arrangement shows a signature of the North American gonochorists P. aerivorus (Cobb in Merrill and Ford, 1916) Chitwood, 1937 and P. pseudaerivorus Herrmann, Mayer, and Sommer, 2006b and the hermaphroditic P. maupasi. In these three species and P. eurycephalus, the position of v3d is clearly posterior to v2, which is equidistant from v1 and v4. In all species of the lheritieri group, as well all other adequately characterized Pristionchus species with the exception of P. fissidentatus, which is unique in several other aspects of male papillae arrangement, v2d is at the same level as or anterior to v3. Re-isolation and molecular profiling of P. eurycephalus will be necessary to test its phylogenetic position and biogeographic history. However, the generally coherent character histories of male papillae arrangement in Pristionchus (Kanzaki et al., 2013c) suggest that P. eurycephalus is nested within the maupasi group, the gonochoristic species of which are all from North America. We therefore hypothesize that this species or an ancestor of it colonized Europe independently of the lheritieri group. The morphological affinity of P. eurycephalus to P. maupasi, which is common in both Europe and North America, raises the possibility of a close relationship between these two species and thus also multiple movements between the Palearctic and Nearctic in the history of the maupasi group.
Finally, supporting a recent radiation of the lheritieri group relative to the history of Pristionchus is the high degree of morphological similarity across the European species thus far sampled, in contrast to the relatively divergent morphotypes found in species with distributions in East Asia (Kanzaki et al., 2012b, 2013a; Ragsdale et al., 2013). Although some characters of stomatal and male sexual morphology can distinguish individual species in the European clade, even these characters are generally conserved. This finding reflects the observation by Fürst von Lieven and Sudhaus (2000) that the stomatal morphology of all gonochoristic species sufficiently characterized at the time of their study to be essentially identical. This general similarity of described gonochoristic species, at least in terms of reported characters, had likewise led to Andrássy’s (1984) proposal to consider all gonochoristic Pristionchus species to be synonymous with P. lheritieri. However, molecular characterization, phylogenetic analysis, and tests for reproductive isolation all support these otherwise cryptic species to be both biologically unique and upon independent evolutionary trajectories (Wiley, 1978; Adams, 1998). The present study thus shows that molecular and biological characters will be essential for discovering and testing the identities of new species of Pristionchus, which is apparently more diverse than typological characters suggest.
Footnotes
The species epithet is the Latin genitive of Lucanus, the type host genus.
The species epithet is the Latin adjective denoting the country of the type locality.
Literature Cited
- Adams BJ. Species concepts and the evolutionary paradigm in modern nematology. Journal of Nematology. 1998;30:1–21. [PMC free article] [PubMed] [Google Scholar]
- Andrássy I. 1984. Klasse Nematoda. Stuttgart, Germany: Gustav Fischer Verlag.
- Chitwood BG. 1937. Cephalic structure and stoma. In B. G. Chitwood and M. B. Chitwood, eds. Introduction to nematology. Baltimore: Monumental Printing Co.
- D’Anna I, Sommer RJ. Pristionchus uniformis, should I stay or should I go? Recent host range expansion in a European nematode. Ecology and Evolution. 2011;1:468–478. doi: 10.1002/ece3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko A, Stanuszek S. Pristionchus uniformis sp. n. (Nematoda, Rhabditida, Diplogasteridae), a facultative parasite of Leptinotarsa decemlineata Say and Melolontha melolontha L. in Poland. Morphology and biology. Acta Parastiologica. 1971;19:95–112. [Google Scholar]
- Floyd R, Abebe E, Papert A, Blaxter M. Molecular barcodes for soil nematode identification. Molecular Ecology. 2002;11:839–850. doi: 10.1046/j.1365-294x.2002.01485.x. [DOI] [PubMed] [Google Scholar]
- Fürst von Lieven A, Sudhaus W. Comparative and functional morphology of the buccal cavity of Diplogastrina (Nematoda) and a first outline of the phylogeny of this taxon. Journal of Zoological Systematics and Evolutionary Research. 2000;38:37–63. [Google Scholar]
- Herrmann M, Mayer WE, Sommer RJ. Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology. 2006a;109:96–108. doi: 10.1016/j.zool.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Mayer WE, Sommer RJ. Sex, bugs and Haldane’s rule: The nematode genus Pristionchus in the United States. Frontiers in Zoology. 2006b;3:14. doi: 10.1186/1742-9994-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DJ. 1986. Handling, fixing, staining and mounting nematodes. Pp. 59–80 in J. F. Southey, ed. Methods for work with plant and soil nematodes. London: Her Majesty’s Stationary Office.
- Kanzaki N. Simple methods for morphological observation of nematodes. Nematological Research. 2013;43:9–13. [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Mayer WE, Sommer RJ. Description of three Pristionchus species (Nematoda: Diplogastridae) from Japan that form a cryptic species complex with the model organism P. pacificus. Zoological Science. 2012a;29:403–417. doi: 10.2108/zsj.29.403. [DOI] [PubMed] [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Mayer WE, Tanaka R, Sommer RJ. Parapristionchus giblindavisi n. gen., n. sp. (Rhabditida: Diplogastridae) isolated from stag beetles (Coleoptera: Lucanidae) in Japan. Nematology. 2012b;14:933–947. [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Röseler W, Sommer RJ. Pristionchus bucculentus n. sp. (Rhabditida: Diplogastridae) isolated from a shining mushroom beetle (Coleoptera: Scaphidiidae) in Hokkaido, Japan. Journal of Nematology. 2013a;45:78–86. [PMC free article] [PubMed] [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Röseler W, Sommer RJ. Two new species of Pristionchus (Nematoda: Diplogastridae) support the biogeographic importance of Japan for the evolution of the genus Pristionchus and the model system P. pacificus. Zoological Science. 2013b;30:680–692. doi: 10.2108/zsj.30.680. [DOI] [PubMed] [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Sommer RJ. Two new species of Pristionchus (Rhabditida: Diplogastridae): P. fissidentatus n. sp. from Nepal and La Réunion Island and P. elegans n. sp. from Japan. Journal of Nematology. 2012c;44:80–91. [PMC free article] [PubMed] [Google Scholar]
- Kanzaki N, Ragsdale EJ, Herrmann M, Susoy V, Sommer RJ. Two androdioecious and one dioecious new species of Pristionchus (Nematoda: Diplogastridae): New reference points for the evolution of reproductive mode. Journal of Nematology. 2013c;45:172–194. [PMC free article] [PubMed] [Google Scholar]
- Kreis HA. Beiträge zur Kenntnis pflanzenparasitischer Nematoden. Zeitschrift für Parasitenkunde. 1932;5:184–194. [Google Scholar]
- Maupas E. Essais d’hybridation chez les nématodes. Bulletin Biologique de la France et de la Belgique. 1919;52:466–498. [Google Scholar]
- Mayer WE, Herrmann M, Sommer RJ. Phylogeny of the nematode genus Pristionchus and implications for biodiversity, biogeography and the evolution of hermaphroditism. BMC Evolutionary Biology. 2007;7:104. doi: 10.1186/1471-2148-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer WE, Herrmann M, Sommer RJ. Molecular phylogeny of beetle associated diplogastrid nematodes suggests host switching rather than nematode-beetle coevolution. BMC Evolutionary Biology. 2009;9:212. doi: 10.1186/1471-2148-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JH, Ford AL. Life history and habits of two new nematodes parasitic in insects. Journal of Agricultural Research. 1916;6:115–127. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November, New Orleans, 1–8.
- Paramonov AA. Opyt ekologicheskoi klassifikatsii fitonematod. Trudy Gelmintologicheskii Laboratorii. Akademia Nauk SSSR (Moskva) 1952;6:338–369. [Google Scholar]
- Ragsdale EJ, Kanzaki N, Röseler W, Herrmann M, Sommer RJ. Three new species of Pristionchus (Nematoda: Diplogastridae) show morphological divergence through evolutionary intermediates of a novel feeding-structure polymorphism. Zoological Journal of the Linnean Society. 2013;168:671–698. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer RJ, McGaughran A. The nematode Pristionchus pacificus as a model system for integrative studies in evoltuionary bioogy. Molecular Ecology. 2013;22:2380–2393. doi: 10.1111/mec.12286. [DOI] [PubMed] [Google Scholar]
- Sommer RJ, Carta LK, Kim SY, Sternberg PW. Morphological, genetic and molecular description of Pristionchus pacificus n. sp. (Nematoda: Neodiplogastridae) Fundamental and Applied Nematology. 1996;19:511–521. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Steiner G. Diplogaster entomophaga n. sp., a new Diplogaster (Diplogasteridae, Nematodes) found on a Pamphilius stellatus (Christ) (Tenthredinidae, Hymenoptera) Zoologischer Anzeiger. 1929;80:143–145. [Google Scholar]
- Sudhaus W, Fürst von Lieven A. A phylogenetic classification and catalogue of the Diplogastridae (Secernentea, Nematoda) Journal of Nematode Morphology and Systematics. 2003;6:43–90. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley EO. The evolutionary species concept reconsidered. Systematic Zoology. 1978;27:17–26. [Google Scholar]