Abstract
Near infrared spectroscopy (NIRS) has been frequently used to assess intra-muscular oxygenation past few decades. In recent years, refinement of NIRS hardware and algorithms used to convert changes in optical absorption to changes in concentration improved the validity of oxygenated haemoglobin (HbO2), deoxyhaemoglobin (Hb), myoglobin (Mb) and the oxidised copper compound of cytochrome aa3, (cyt aa3) measurements. Subsequently, the use of NIRS to study the muscle oxygenation profiles during various types of exercise and to monitor differences in oxygenation levels in patients under various pathological conditions is being increased. It would be thought-provoking to combine future muscle studies with MRS and/or electromyography techniques to improve the understanding of intramuscular oxygenation.
Key Words: Near infrared spectroscopy, muscle oxygenation
Abstract
Geçtiğimiz birkaç on-yıldan beri kas-içi oksijenlenmeyi değerlendirmede “near infrared spektroscopy” (NIRS) yöntemi sıklıkla kullanılmaktadır. Son yıllarda NIRS cihazlarının ve bunlarda kullanılan optik absorbsiyon değişikliklerini konsantrasyon farklarına çeviren algoritmaların gittikçe gelişmesi oksijenlenmiş hemoglobin (HbO2), oksijensiz hemoglobin (Hb), miyoglobin (Mb) ve okside bakır bileşiği olan sitoktom aa3, (cyt aa3) ölçümlerinin güvenilirliğini arttırmıştır. Böylece NIRS farklı egzersiz tiplerinde kas içi oksijenlenme profillerini ortaya koymanın yanı sıra değişik patolojik bozuklukları olan hastalarda oksijenlenme düzeylerini gözlemede kullanılması giderek artmaktadır. Gelecekteki kas çalışmalarında NIRS ile beraber MRS veya elektromiyografi tekniklerini birleştirmek kas içi oksijenlenmenin anlaşılmasını geliştirmede yeni çarpıcı düşüncelerin oluşmasına yol açabilir.
Introduction
In human body, muscle cell is the leading oxygen consumer during aerobic energy production process i.e. oxidative metabolism. The extraction of O2 from blood by muscle cells and the blood flow dictate the rate of O2 utilization (QO2). Near infrared spectrophotometry (NIRS) provides a relatively new technology capable of continuous non-invasive monitoring of changes in tissue (O2 stores and O2 availability at the cellular level) (Piantadosi et al., 1986; Hampson et al., 1987; Jöbsis-Vandervliet et al., 1988). It works on the fact that biological tissues are relatively transparent to light of the near infra-red region of the spectrum (wavelength between 700-l000 nm) and therefore NIRS can be used to measure muscle oxygenation up to 8 cm of tissue (Wilson et al., 1989; Chance et al., 1992). Light in the visible region (wavelength between 450-700nm), on the other hand, is strongly absorbed in tissue and therefore fails to penetrate more than approximately 1 cm of tissue.
In biological tissues, there are compounds whose absorption of light is oxygenation-status dependent. Such compounds in muscle tissue are oxygenated haemoglobin (HbO2), deoxyhaemoglobin (Hb), myoglobin (Mb) and the oxidised copper compound of cytochrome aa3, (cyt aa3, the terminal member of the mitochondrial cytochrome chain). Information can be obtained about the oxygenation state of the tissue by determining the concentration of these variables. The near infra-red (NIR) absorption characteristics of the tissue therefore give information about the presence of these compounds within it.
The absorbence of deoxygenated Hb peaks at a wavelength of 760 nm, while that of oxygenated Hb is highest at 850 nm. The isobestic point for the oxygenated and deoxygenated forms of Hb occurs at 798 nm (Chance et al., 1992; Mancini et al., 1994). Since the near infrared absorption spectrum of Mb overlaps with that of Hb, changes in tissue saturation monitored at 760 nm and 850 nm are attributed to the desaturation of both Hb and Mb (De Blasi et al., 1993). Studies on isolated muscle preparations have indicated that approximately 65%-75% of the deoxygenation observed during exercise is due to the release of oxygen by Hb, while the balance is due to that released by Mb (Wilson et al., 1989; Chance et al., 1992).
In practice, absorption at several different wavelengths is measured and an algorithm is used to convert changes in optical absorption to changes in concentration (Seiyama et al., 1988; Wilson et al., 1989). In doing so, it is possible to display the changes occurred in the concentrations of different variables of interest (e.g. HbO2, Hb, HbT-total hemoglobin-, representative response are presented in Figure 1) during experiment graphically, and, an event marker allows specific points (e.g. onset or end of exercise and MVC, etc) to be recorded for future reference.
Figure 1.
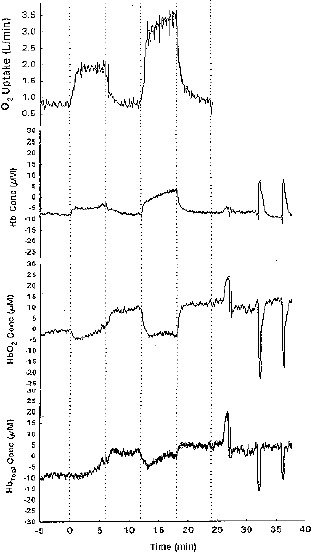
The comparison of VO2, Hb, HbO2 and HbT profiles during moderate and very heavy constant-load exercise.
The reliability and validity of NIRS technology in evaluating muscle oxygenation levels during exercise has been established (Wilson et al., 1989; Mancini et al., 1994; Sako et al., 2001). One of the limitations of NIRS is that oxygen uptake in intact human muscle (mVO2) cannot be calculated because the effective path length of the NIRS signal cannot be readily quantified (Mancini et al., 1994). However, this technique can provide important information about the profile of muscle oxygenation during exercise and recovery. Furthermore, it enables us to better understand the physiological factors influencing exercise performance when used in combination with cardiorespiratory and metabolic measurements.
A number of scientists have therefore used NIRS to study the muscle oxygenation profiles during incremental and square-wave exercise in various forms, such as cycling (Wilson et al., 1989; Chance et al., 1992; Mancini et al 1994; Belardinelli et al 1995a & 1995b; Matsui et al., 1995; Costes et al., 1996; Bhambhani et al., 1997; 1998; 2001), rowing (Chance et al., 1992), arm cranking (Jensen-Urstad et al., 1995; Ogata et al., 2002) and speed skating (Rundell et al., 1997). In addition, the acute changes in muscle oxygenation that occur during isotonic grip exercise (Hamaoka et al., 1996; Kutsuzawa et al., 2001), upper extremity weight-lifting exercise (Tamaki et al., 1994) and isometric contractions of the forearm (Hampson and Piantodosi, 1988; De Blasi et al., 1993; Murthy et al. 1997) and knee extension exercise (Sahlin, 1992; Quarisema et al., 2001) have been documented. In clinical studies this technique has been used to establish differences in respiratory muscle (Mancini et al., 1991) and working muscle (Wilson et al., 1989; Matsui et al., 1995) oxygenation levels between healthy subjects and patients with heart failure . It seems, therefore, that NIRS can be a useful tool in the evaluation of exercise performance.
Muscle oxygenation profiles during sub- and supra-θ1 exercise
Several experiments have been performed using the NIRS technique during muscular exercise in humans in order to gather some information as to what is occurring within the muscle at varying workloads. An extensive analysis of the oxygen supply and demand in near maximal exercise has been performed by Chance et al (1992), with the aim of understanding the post exercise responses in terms of repaying the energy cost incurred. They presented data regarding the changes in deoxygenation profile and blood flow as the work rate increased, and the signals from different muscles were compared simultaneously.
Wilson et al (1989) examined the oxygenation levels in the vastus lateralis muscle during an incremental work test on a cycle ergometer comparing healthy controls against patients with heart failure. They examined the metabolic exercise responses with respect to oxygenation in a group of patients suffering severe exercise intolerance due to their pathological condition. They were able to demonstrate that both patients and controls had similar levels of Hb desaturation when reached exhaustion, but the heart failure patients reached this level sooner than the controls.
To use NIRS technique efficiently for observing the intramuscular oxygenation changes during exercise, it is important to combine it with the breath by breath gas exchange analysis technique. Combining both techniques, Kawaguchi et al (2001) reported that kinetics of peripheral muscle oxygenation reflect systemic VO2. Belardinelli et al (1995a) examined the desaturation response of the vastus lateralis muscle during four periods of steady state cycling at four different work rates. They tested if there was any evidence to support the hypothesis that the slow component of oxygen uptake at heavy work rates is related to the progressive desaturation of haemoglobin, facilitated partly by the Bohr effect consequent to the muscular lactic acidosis. The group was able to demonstrate a decrease in oxygen saturation that occurred gradually in proportion to the increasing work rate. Additionally, at sub-qL work rates the level reached a minimum and stayed either constant or began to return to baseline. However, the level of desaturation continued to fall throughout the period of exercise at supra-qL work rates. In conclusion, these results were considered consistent with the hypothesis by the group.
Belardinelli et al. (1995b) also studied the intramuscular oxygenation response profile of incremental exercise test. They particularly studied the lactate threshold (qL), testing whether any relation between intramuscular oxygenation profile and the qL exists. Therefore, they investigated the hypothesis that lactic acidosis causes a shift in the haemoglobin dissociation curve and is closely related to such a break point in this curve. The lactate threshold was estimated by a V-slope plot and the time at which the qL occurred was analysed for significant events in the desaturation of the vastus lateralis muscle. As discussed, one limitation of NIRS work is that without a specific experiment to determine the differential path-length the results are not quantitative, and thus cannot be compared between individuals. As a result of this the group scaled all NIRS results to the maximum and minimum readings that they recorded. From this experiment the group were able to show that with progressive increase in work rate the level of oxygenation within the muscle decreased. Significantly, they were able to show that the desaturation curve consisted of two components, with an increase in the rate of desaturation occurring very slightly before the detection of the qL at the mouth. They proposed that this accelerated desaturation marks the onset of the lactic acidosis.
Bhambhani et al. (1997) reported evidence for significant changes in the NIRS signal at the lactate threshold. They designed a study, using NIRS and breath by breath analysis, in which subjects performed incremental exercise tests where the same rate of increment was used in each experiment. This is in contrast to the study of Belardinelli et al. (1995b) in which different rates of incrementation had been used. The group demonstrated four phases of oxygenation in an incremental exercise test, with the notable absence in about half of their experiments, of the components that Belardinelli et al. (1995b) have reported. The group also argued that at the onset of exercise there is a transitional increase in the signal for oxygen saturation, attributable to an increase in the blood supply to the working muscles. However, as the exercise progresses the blood pool in the vastus lateralis muscle begins to become increasingly desaturated, until it reaches a minimal level, at or near exhaustion. Critically, these investigators state that as the level of saturation falls after an initial increase, it is the point at which it crosses its baseline level that is significant “crossing point” - although it is not clear what this actually represents physiologically. The lactate threshold was estimated by a computer, using the V slope method and the group has shown that the qL occurs at or just after the point where the near infra-red signal crosses the baseline.
Chance et al (1992) and Matsui et al (1995) also have documented the NIRS trend exercise as a four-phase response. They reported in phase I, there was an immediate, rapid increase in absorbency from the resting baseline values at the onset of zero load exercise, implying an increase in muscle oxygenation relative to this level. In phase II there was a steady decline in tissue absorbency that continues beyond the resting baseline value as the power increased, suggesting a decrease in muscle oxygenation relative to this value. In phase III, absorbency readings tend to level off with increasing power output until VO2peak is attained. In phase IV (during recovery), there was a very rapid increase in the absorbency that extends above the maximum values observed in phase I. This response, which has been attributed to hyperaemia (Chance et al., 1992), tends to level off after the first 2-3 minutes into the recovery phase.
The results of all these experiments are encouraging as they show the potential of NIRS to present a non-invasive method for detecting a number of events in exercise testing as long as the appropriate device is chosen to evaluate the intramuscular oxygenation profile. However, these studies used the “Runman” which provides a single output estimate of the near infra-red assessment: it is not possible, for example, using this device to detect the influence of the increase in blood volume per se on the oxygenation profile (Chance et al., 1992; MacDonald et al., 1999). It is advisable, therefore, to choose a NIRO 500 (Hamamatsu) device, which enables to monitor continuously the deoxyhaemoglobin, oxyhaemoglobin and total haemoglobin concentrations in the interrogation field of the muscle throughout the experiment.
Conclusion
Interestingly, almost all of the above studies concentrated on lower extremities, exclusively on vastus lateralis. It is generally assumed that only leg muscles are working and the upper extremities are resting during leg exercise cycling. To date, there is only a few study focused on the upper extremity oxygenation profile during leg exercise cycling (e.g. Özyener et al., 1999). Hence, the “resting” situation of the upper extremities remains to be demonstrated. Especially, any extra work done by the upper body could contribute to the emergence of the VO2 slow component and provide important insight into the energetics of muscle.
It is salient to note, therfore, that NIRS could play an important role to enhance scientific understanding of oxidative metabolism in healthy muscle as well as the pathological effects on damaged muscle tissue. To combine future muscle studies with MRS and/or electromyography techniques could provide valuable insights into muscle energetics and mechanisms coupling intramuscular oxygenation with pulmonary oxygen uptake.
Biography

Fadɪl ÖZYENER
Employement
Lecturer and Researcher, Uludag Univ Med School, Dept of Physiology, TUR
Degrees
MD, Univ. of Istanbul, TUR, 1987. Specialization, Univ of Uludag, TUR, 1994. PhD, Univ. Of London, UK, 2000
Research interest
Pulmoner gas kinetics and ventilation during exercise. Fibrinolytic activity during and after exertion.
E-mail: fozyener@uludag.edu.tr
References
- Belardinelli R., Barstow T.J., Porszasz J., Wasserman K. (1995a) Skeletal muscle oxygena-tion during constant work rate exercise. Medicine & Science in Sports & Exercise 27: 512-519 [PubMed] [Google Scholar]
- Belardinelli R., Barstow T.J., Porszasz J., Wasserman K. (1995b) Changes in skeletal muscle oxygenation during incremental exercise measured with near infra-red spectroscopy. Medicine & Science in Sports & Exercise 70: 487-492 [DOI] [PubMed] [Google Scholar]
- Bhambhani Y., Buckley S., Susaki T. (1997) Detection of ventilatory threshold using near infrared spectroscopy in men and women. Medicine & Science in Sports & Exercise 29 (3): 402-409 [DOI] [PubMed] [Google Scholar]
- Bhambhani Y., Maikala R., Buckley S. (1998) Muscle oxygenation during incremental and intense anaerobic cycle exercise in young men and women. European Journal of .Applied Physiology 78: 422-431 [DOI] [PubMed] [Google Scholar]
- Bhambhani Y., Maikala R., Esmail S. (2001) Oxygenation trends in vastus lateralis muscle during incremental arm and leg exercise in men and women. European Journal of .Applied Physiology 84: 547-546 [DOI] [PubMed] [Google Scholar]
- Chance B., Dait M.T., Zhang C., Hamaoka T., Hagerman F. (1992) Recovery from exercise induced desaturation in the quadriceps muscles of the elite competitive rowers. American Journal Physiology 262: C766-C775 [DOI] [PubMed] [Google Scholar]
- Costes F., Jean-Claude B., Feasson L., Busso T., Geyssant A., Denis C. (1996) Comparison of muscle near-infrered spectroscopy and femoral blood gases during steady-state exercise in humans. Journal of Applied Physiology, 80: 1345-1350 [DOI] [PubMed] [Google Scholar]
- De Blasi R.A., Cope M., Elwell C., Safoue F., Ferrari M. (1993) Noninvasive measurement of human forearm oxygen consumption by near infrared spectroscopy. European Journal of Applied Physiology 67: 20-25 [DOI] [PubMed] [Google Scholar]
- Hamaoka T., Iwane H., Shimomitsu T., Katsumura T., Murase N., Nishio S., Osada T., Kurosawa Y., Chance B. (1996) Noninvasive measures of oxidative metabolism on working human muscles by near-infrared spectroscopy. Journal of Applied Physiology 81: 1410-1417 [DOI] [PubMed] [Google Scholar]
- Hampson N.B., Jöbsis-VanderVliet F.F., Piantadosi C.A. (1987) Skeletal muscle oxygen availability during respiratory acid-base disturbances in cats. Respiroratory Physiology 70: 143-158 [DOI] [PubMed] [Google Scholar]
- Hampson N.B., Piantadosi C.A. (1988) Near infrared monitoring of human skeletal muscle oxygenation during forearm ischemia. Journal of Applied Physiology 64: 2449-2457 [DOI] [PubMed] [Google Scholar]
- Jensen-Urstad M., Hallback I., Sahlin K. (1995) Effect of hypoxia on muscle oxygenation and metabolism during arm exercise in humans. Clinical Physiology 15: 27-37 [DOI] [PubMed] [Google Scholar]
- Jöbsis-VanderVliet F.F., Piantadosi C.A., Sylvia A.L., Lucas S.K., Keizer J.H. (1988) Near infrared monitoring of cerebral oxygen sufficiency: I. Spectra of cytochrome oxidase. Neurological Research 10: 7-17 [DOI] [PubMed] [Google Scholar]
- Kutsuzawa T., Shıoya S., Kurita D., Haida M., Yamabayashı T. (2001) Effects of age on muscle energy metabolism and oxygenation in the forarm muscles. Medicine & Science in Sports & Exercise 33 (6): 901-906 [DOI] [PubMed] [Google Scholar]
- Kawaguchi K., Tabusadani M., Sekikawa Y., Hayashi Y., Onari K. (2001) Do the kinetics of peripheral muscle oxygenation reflect systemic oxygen uptake? European Journal of Applied Physiology 84: 158-161 [DOI] [PubMed] [Google Scholar]
- MacDonald M.J., Tarnopolsky M.A., Green H.J., Hughson R.L. (1999) Comparison of femoral blood gases and muscle near-infrared spectroscopy at exercise onset in humans. Journal of Applied Physiology 86(2): 687-693 [DOI] [PubMed] [Google Scholar]
- Mancini D.M., Ferraro N., Nazzaro D., Chance B., Wilson J.R. (1991) Respiratory muscle deoxy-genation during exercise in patients with heart failure demonstrated with near-infrared spect-roscopy in humans. Journal of the American College of Cardiology 18: 492-498 [DOI] [PubMed] [Google Scholar]
- Mancini D.M., Ferraro N., Nazzaro D., Chance B., Wilson J.R. (1994) Validation of near-infrared spectroscopy in humans. Journal of Applied Physiology 77(6): 2740-2747 [DOI] [PubMed] [Google Scholar]
- Matsui S., Tamura N., Hirakawa T., Kobayashi S., Takekoshi N., Murakami E. (1995) Assessment of working skeletal muscle oxygenation in patients with chronic heart failure. American Heart Journal 129: 690-695 [DOI] [PubMed] [Google Scholar]
- Murthy G., Kahan N.J., hargens A.R., Rempel D.M. (1997) Forearm muscle oxygenation decreases with low levels of voluntary contraction. Journal of Orthopaedic Research 15: 507-511 [DOI] [PubMed] [Google Scholar]
- Ogata H., Yunoki T., Yano T. (2002) Effect of arm cranking on the NIRS-determined blood volume and oxygenation of human inactive and exercising vastus lateralis muscle. European Journal of Applied Physiology 86: 191-195 [DOI] [PubMed] [Google Scholar]
- Ozyener F., Ward S.A., Whipp B.J. (1999) Contribution of arm-muscle oxygenation to the "slow-component" of pulmonary oxygen uptake during leg-exercise cycle ergometry. Proceedings of Journal of Physiology 515: p73 [Google Scholar]
- Piantadosi C.A., Hemstret T.A., Jöbsis-VanderVliet F.F. (1986) Near infrared spectrophotometric monitoring of oxygen distribution to intact brain and skeletal muscle tissues. Critical Care Medicine 14 (8): 698-706 [DOI] [PubMed] [Google Scholar]
- Quaresima V., Homma S., Azuma K. (2001) Calf and shin muscle oxygenation patterns and femoral artery blood flow during dynamic plantar flexion exercise exercise in. European Journal of Applied Physiology 84: 387-394 [DOI] [PubMed] [Google Scholar]
- Rundell K.W., Niola S., Chance B. (1997) Haemoglobin/myoglobin desaturation during speed skating. Medicine & Science in Sports & Exercise 29: 248-258 [DOI] [PubMed] [Google Scholar]
- Sako T., Takafumi H., Hıguchi H., Kurosawa Y., Katsumura T. (2001) Validity of NIR spectroscopy for quantitatively measuring muscle oxidative metabolic rate in exercise. Journal of Applied Physiology 90 : 338-344 [DOI] [PubMed] [Google Scholar]
- Sahlin K. (1992) Non-invasive measurements of O2 availability in human skeletal muscle with near-infrared spectroscopy. International Journal of Sports Medicine 13: S157-S160 [DOI] [PubMed] [Google Scholar]
- Seiyama A., Hazeki O., Tamura M. (1988) Noninvasive quantitative analysis of blood oxygenation in rat skeletal muscle. Journal of Biochemistry 103:419-424 [DOI] [PubMed] [Google Scholar]
- Tamaki T., Uchiyama S., Tamura T., Nokana S. (1994) Changes in muscle oxygenation during weight lifting exercise. European Journal of Applied Physiology 68: 465-469 [DOI] [PubMed] [Google Scholar]
- Wilson J.R., Mancini D.M., McKully K., Ferraro N., Lanoce V., Chance B. (1989) Noninvasive detection of skeletal muscle underperfusion with near infra-red spectroscopy in patients with heart failure. Circulation 80: 1668-1674 [DOI] [PubMed] [Google Scholar]


