Abstract
Brucella taxonomy is perpetually being reshuffled, at both the species and intraspecies levels. Biovar 7 of Brucella abortus was suspended from the Approved Lists of Bacterial Names Brucella classification in 1988, because of unpublished evidence that the reference strain 63/75 was a mixture of B. abortus biovars 3 and 5. To formally clarify the situation, all isolates previously identified as B. abortus bv. 7 in the AHVLA and ANSES strain collections were characterized by classical microbiological and multiple molecular approaches. Among the 14 investigated strains, including strain 63/75, only four strains, isolated in Kenya, Turkey, and Mongolia, were pure and showed a phenotypic profile in agreement with the former biovar 7, particularly agglutination with both anti-A/anti-M monospecific sera. These results were strengthened by molecular strategies. Indeed, genus- and species-specific methods allowed confirmation that the four pure strains belonged to the B. abortus species. The combination of most approaches excluded their affiliation with the recognized biovars (biovars 1 to 6 and 9), while some suggested that they were close to biovar 3.These assays were complemented by phylogenetic and/or epidemiological methods, such as multilocus sequence analysis (MLSA) and variable-number tandem repeat (VNTR) analysis. The results of this polyphasic investigation allow us to propose the reintroduction of biovar 7 into the Brucella classification, with at least three representative strains. Interestingly, the Kenyan strain, sharing the same biovar 7 phenotype, was genetically divergent from other three isolates. These discrepancies illustrate the complexity of Brucella taxonomy. This study suggests that worldwide collections could include strains misidentified as B. abortus bv. 7, and it highlights the need to verify their real taxonomic position.
INTRODUCTION
Brucellosis is a major worldwide zoonosis. This disease affects domestic and wild mammals, causing abortion and reduced fertility. The infection is transmitted to humans by animals through direct contact with contaminated animal fluids or indirectly through ingestion of unpasteurized milk products. Despite surveillance and eradication programs recommended by the World Health Organization (WHO), the Food and Agricultural Organization (FAO), and the World Organization for Animal Health (OIE), the disease remains endemic in many regions of the world (1). Accordingly, brucellosis is of serious public health importance and causes substantial losses to livestock producers and international trade for herds in areas where it is enzootic.
Brucella, the causal agent of brucellosis, is a genus of Gram-negative, nonmotile, mostly oxidase- and urease-positive, nonencapsulated, and facultative intracellular bacteria. On the basis of genetic criteria (DNA-DNA hybridization and 16S rRNA sequence comparison), the genus Brucella belongs to the family Rhizobacteriaceae, class Alphaproteobacteria, within the phylum Proteobacteria (2). Classification of these bacteria has been based primarily on phenotypic and biochemical methods and host preference (Table 1). On this basis, the Brucella genus currently contains 10 species, most with a preferential host: B. abortus infects cattle, B. suis is normally associated with swine, B. melitensis infects mainly sheep and goats, B. ovis seems to be responsible for a specific infection of sheep, B. canis is associated with dogs, B. neotomae is from desert wood rats, B. ceti and B. pinnipedialis infect marine mammals (3), B. microti has been isolated from the common vole (Microtus arvalis) (4, 5), and while B. inopinata was isolated from a human infection, its reservoir remains unknown (6). Additional novel strains, such as unnamed strains isolated from baboons (7, 8), foxes (9), frogs (10), and rodents (11), have been described recently, and ongoing updates on the Brucella taxonomy are expected in the near future.
TABLE 1.
Differential characteristics of the Brucella species and biovarsa
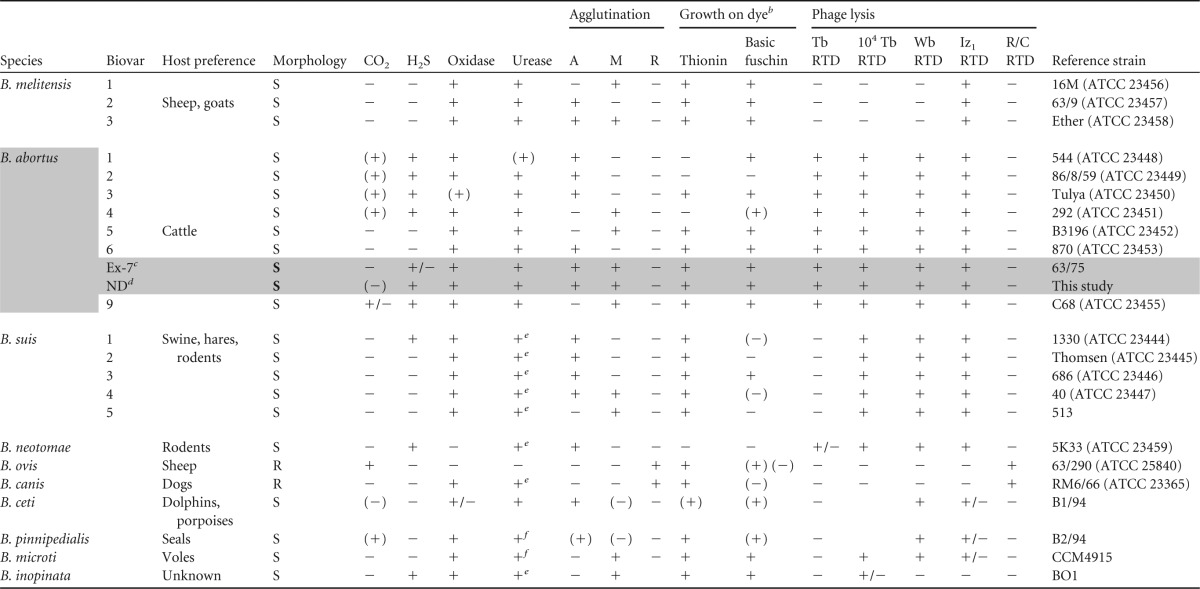
Data are from references 28 and 37 and http://www.the-icsp.org/taxa/Brucellalist.htm. R and S, rough and smooth colonial morphology, respectively; CO2, CO2 requirement; H2S, H2S production; agglutination, agglutination with monospecific A, M, and R (rough) antisera. +, growth or lysis; −, no growth or no lysis; +/−, partial lysis; (+), most strains positive; (−), most strains negative.
Dye concentration of 20 μg · ml−1 in serum dextrose medium (1/50,000).
Withdrawn (ICSP).
ND, not yet designed in the Brucella systematics.
Strong intensity.
Low intensity.
Some species are subdivided into biovars, i.e., B. melitensis bv. 1 to 3, B. abortus bv. 1 to 6 and 9, and B. suis bv. 1 to 5. Prior to 1986, the B. abortus species included 8 biovars (1 to 7 and 9), biovar 8 having been deleted from Brucella nomenclature in 1978 by the International Committee on Systematics of Prokaryotes (ICSP) (http://www.the-icsp.org/taxa/Brucellalist.htm) because no authentic isolate of this biovar had been reported for many years and no reference strain was available (12). From 1977, strain 63/75, also designated type strain NCTC 10506 or ATCC 23454, was considered the B. abortus bv. 7 reference strain (13) (Table 1). Between 1986 and 1988, following successful cloning, Verger et al. (INRA, Nouzilly, France) suggested that strain 63/75 consisted of a mixture of B. abortus bv. 3 and bv. 5 strains (14), so biovar 7 was suspended from the Approved Lists of Bacterial Names Brucella classification in 1986 by the International Subcommittee on the Taxonomy of Brucella (ISTB) (14) until the situation could be clarified.
The genetic homogeneity of the Brucella genus (DNA homology of >90%) initially strongly hindered the development of molecular tools for species and biovar identification. The rrs polymorphism is applicable for genus identification but does not allow differentiation between Brucella species (there is almost 100% identity in the 16S rRNA sequences) (15). To date, the most relevant genus identification technique is a real-time PCR (RT-PCR), which targets bcsp31, IS711, and per (16). For species identification, molecular approaches must target other loci. Polymorphism of omp (outer membrane protein) genes, mainly the deletion of omp31 in B. abortus, is useful for identifying genetic variants (17). Other multiplex PCR assays are available, i.e., AMOS PCR (18), which discriminates B. abortus (biovars 1, 2, and 4), B. melitensis (biovars 1 to 3), B. ovis, and B. suis (only biovar 1), and Bruce-ladder (8 target genes), which differentiates between the classical, vaccine and marine Brucella species (19, 20). In addition, molecular typing methods with greater discriminatory power, such as multilocus variable-number tandem repeat analysis 16 (MLVA-16) (21, 22), variable-number tandem repeat 21 (VNTR-21) (23), and extended multilocus sequence analysis 21 (MLSA-21) (24; A. M. Whatmore, unpublished data) can be used to further subdivide species, giving insight into phylogenetic, taxonomic, and/or epidemiological links between different terrestrial and marine strains.
The withdrawal of B. abortus bv. 7 from Brucella systematics remains poorly understood or simply ignored within the scientific community. Indeed, the literature still abounds in typing studies that include the former biovar 7 reference (presumably mixed) strain (5, 25–27). Moreover, several laboratories still count in their strain collections some isolates probably misidentified as B. abortus bv. 7. Therefore, we proposed to determine if, as for the reference strain 63/75, these isolates were a mixture of various biovars or whether they constituted a new biovar with its own characteristics. The aim of our study was to clarify the situation concerning B. abortus bv. 7 by both conventional and molecular approaches, including RT-PCR, omp polymorphism, Bruce-ladder, AMOS-ERY PCR, IS711 fingerprinting, MLSA-21, VNTR-21, and MLVA-16, and thus provide recommendations on the future taxonomic status of this biovar.
MATERIALS AND METHODS
Bacterial strains.
A total of 14 strains, including strains historically labeled B. abortus bv. 7 from the AHVLA and ANSES Brucella collections and the reference strain 63/75, were analyzed. The Brucella strains used in this study are listed in Table 2. In order to exclude the possibility of a mixed culture of different biovars, the strains were subjected to three successive cloning isolations (probability of a mixed colony = 10−9) (J.-M. Verger, personal communication).
TABLE 2.
Strains identified as Brucella abortus bv. 7 in the AHVLA and ANSES collections and their phenotypic characteristicsa
| Isolate | AHVLA no. | Yr | Host | Origin | Pure strain no. | Genus | Species | Biovar | Morphology | CO2 | H2S | Oxidase | Urease | Agglutination |
Dye sensitivityb |
Phage lysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | M | R | T1 | T2 | F1 | F2 | Tb RTD | Wb RTD | Iz RTD | R/C RTD | ||||||||||||||
| 07-994-2401 | 84/35 | 1984 | ND | Poland | 07-994-2401 | Brucella | abortus | 4 | S | + | + | + | + | − | + | − | − | − | + | + | + | + | + | − |
| 07-994-2402 | 62/05 | 1962 | ND | ND | 07-994-2402 | Brucella | abortus | 3 | S | + | + | + | + | + | − | − | − | + | + | + | + | + | + | − |
| 07-994-2403 | 84/31 | 1984 | ND | ND | 07-994-2403a | Brucella | abortus | 1 | S | − | + | + | + | + | − | − | − | − | + | + | + | + | + | − |
| 07-994-2403b | Brucella | melitensis | 1 | S | − | − | + | + | − | + | − | + | + | + | + | − | − | + | − | |||||
| 07-994-2404 | 84/37 | 1984 | ND | ND | 07-994-2404a | Brucella | abortus | 1 | S | − | + | + | + | + | − | − | − | − | − | + | + | + | + | − |
| 07-994-2404b | Brucella | abortus | 4 | S | + | + | + | + | − | + | − | − | − | + | + | + | + | + | − | |||||
| 07-994-2405 | 84/40 | 1984 | ND | ND | 07-994-2405a | Brucella | abortus | 4 | S | + | + | + | + | − | + | − | − | − | + | + | + | + | + | − |
| 07-994-2405b | Brucella | abortus | 1 | S | − | + | + | + | + | − | − | − | − | − | + | + | + | + | − | |||||
| 07-994-2406 | F6/5/05 | 2005 | Bovine | ND | 07-994-2406 | Brucella | abortus | 3 | S | + | + | + | + | + | − | − | + | + | + | + | + | + | + | − |
| 07-994-2407 | 84/32 | 1984 | Human | ND | 07-994-2407a | Brucella | abortus | 1 | S | − | + | + | + | + | − | − | − | − | − | + | + | + | + | − |
| 07-994-2407b | Brucella | abortus | 4 | S | + | + | + | + | − | + | − | − | − | + | + | + | + | + | − | |||||
| 07-994-2408 | 63/59 | 1963 | ND | Poland | 07-994-2408 | Brucella | abortus | 1 | S | − | + | + | + | + | − | − | − | − | + | + | + | + | − | |
| 07-994-2409 | 63/66 | 1963 | ND | Poland | 07-994-2409 | Brucella | abortus | 5 | S | − | − | + | + | − | + | − | − | − | + | + | + | + | + | − |
| 07-994-2410 | 63/75c | 1963 | ND | Poland | 07-994-2410a | Brucella | abortus | 3 | S | − | + | + | + | + | − | − | + | + | + | + | + | + | + | − |
| 07-994-2410b | Brucella | abortus | 5 | S | − | − | + | + | − | + | − | − | + | + | + | + | + | + | − | |||||
| 07-994-2411 | 63/294 | 1963 | Bovine | Kenya | 07-994-2411 | Brucella | abortus | ND | S | − | + | + | + | + | + | − | + | + | + | + | + | + | + | − |
| 03-4923-239-D | 2003 | Bovine | Turkey | 03-4923-239-D | Brucella | abortus | ND | S | +/− | + | + | + | + | + | − | + | + | + | + | + | + | + | − | |
| 99-9971-135d | 1988 | Bovine | Mongolia | 99-9971-135 | Brucella | abortus | ND | S | − | + | + | + | + | + | − | + | + | + | + | + | + | + | − | |
| 99-9971-159 | 1993 | Bovine | Mongolia | 99-9971-159 | Brucella | abortus | ND | S | − | + | + | + | + | + | − | + | + | + | + | + | + | + | − | |
R and S, rough and smooth colonial morphology, respectively; CO2, CO2 requirement; H2S, H2S production; agglutination, agglutination with monospecific A, M, and R (rough) antisera. +, growth or lysis; −, no growth or no lysis; +/−, partial lysis. ND, not determined.
T, thionin; F, basic fuchsin; T1 and F1: dye concentration of 10 μg · ml−1 in serum dextrose medium (1/25,000); T2 and F2, dye concentration of 20 μg · ml−1 in serum dextrose medium (1/50,000).
Former B. abortus bv. 7 reference strain.
Proposed reference strain for B. abortus bv. 7.
The reference strains B. abortus bv. 1 strain 544, B. abortus bv. 2 strain 86/8/59, B. abortus bv. 3 strain Tulya, B. abortus bv. 4 strain 292, B. abortus bv. 5 strain B3196, B. abortus bv. 6 strain 870, and B. abortus bv. 9 strain C68 and the B. abortus bv. 1 vaccine strains S19, S99, and RB51, as well other reference strains, such as B. melitensis bv. 1 strain 16M, B. melitensis bv. 3 strain Ether, B. suis bv. 1 strain 1330, B. suis bv. 4 strain 40, and B. ovis strain 63/290, were included in this study as controls for phenotypic typing and/or for molecular analysis.
Analysis of phenotypic characteristics.
Pure cloned strains were characterized using the conventional Brucella typing methods, as previously described, i.e., CO2 requirement, H2S production, urea hydrolysis, oxidase test, agglutination with monospecific sera (anti-A, anti-M, and anti-R), dye sensitivity (basic fuchsin and thionin), and phage typing (Tbilisi [Tb], 104 Tb, Weybridge [Wb], Izatnagar1 [Iz1], and R/C) (28).
Molecular analysis. (i) DNA preparation.
Molecular tools were applied only on pure clones according to classic biotyping description of biovar 7. Genomic DNA was extracted using the High Pure PCR template preparation kit (Roche Diagnostics, France) according to the manufacturer's instructions.
(ii) PCR analysis and typing methods.
(a) Real-time PCR. To confirm the genus Brucella, the RT-PCR assay which targets bcsp31, IS711, and per was performed on the genomic DNA as previously described (16).
(b) omp fingerprinting. Polymorphism of the omp31, omp2a, and omp2b genes was studied by PCR assays as previously described (29). Furthermore, restriction fragment length polymorphism (RFLP)-PCR was applied to various outer membrane protein-encoding genes (omp31/AvaII and/HaeIII, omp2a/NcoI and/StyI, and omp2b/KpnI and EcoRI) as previously described (30, 31).
(c) Bruce-ladder multiplex PCR. Bruce-ladder is species specific, and all the strains and biovars from the same Brucella species give the same profile. Pure clones obtained in our study were characterized by the Bruce-ladder multiplex PCR as previously described (19).
(d) AMOS-ERY PCR assay. For the identification and discrimination of the B. abortus strains of biovars 1, 2, and 4 from other B. abortus biovars, the S19 vaccine strain, and other species, the B. abortus species-specific (BaSS) PCR assay, also designated AMOS-ERY PCR, was performed following previously described approaches (18, 32).
(e) IS711 fingerprinting. Restriction profiles of the insertion sequence IS711 (EcoRI and EcoRI plus DdeI) were investigated as previously described (33, 34).
(f) MLSA-21. Analysis by extended multilocus sequence analysis (MLSA) of the 21 distinct sequence fragments covering more than 10.2 kb of genome was performed according to previously described procedures (5, 24). Each allele at each locus gives an arbitrary numerical designation (sequence type [ST]). A representative strain of each genotype was used for phylogenetic analysis. Phylogenetic trees were constructed in MEGA (35) with the concatenated sequence data of the 21 loci using the neighbor-joining algorithm and the Jukes-Cantor model.
(g) VNTR-21 assay. The diversity of the isolates highlighted in this study was analyzed by the VNTR-21 method, based on the examination of 21 loci, including some of those described in the HOOF-Prints assay (36), as previously described (23).
(h) MLVA-16 assay. The selected pure strains, as well the B. abortus reference strains, were characterized by MLVA-16, using 16 genetic markers, as previously described (22). Fragment sizes converted to repeat unit numbers were imported into BioNumerics v6.6 as a character data set. The obtained MLVA patterns were compared with the Brucella2012 MLVA database, hosted by University Paris-Sud (Orsay, France) (http://mlva.u-psud.fr/mlvav4/genotyping/). According to the speed of molecular evolution, weights were assigned to the distinct panels (weights of 2, 1, and 0.1 per locus for panel 1, panel 2A, and panel 2B, respectively).
A minimum spanning tree (MST) was constructed to compare the pure strain genomes within the network comprising 714 Brucella isolates of distinct species (MLVA-16 patterns are available in the Brucella2010 database, http://mlva.u-psud.fr/mlvav4/genotyping/). The MST results were shown by using a logarithmic scaling. To compare the strains of interest among other B. abortus isolates, a cluster analysis was performed using the unweighted-pair group method (UPGMA) algorithm with categorical coefficient.
RESULTS
Cultures and biotyping.
Fourteen strains, including the reference strain 63/75, previously identified as B. abortus bv. 7 (in particular due to their A+M+ serotype) were subjected to 3 successive cloning isolations. The characteristics of the Brucella cultures obtained are presented in Table 2. Five strains were found to consist of mixed cultures of different B. abortus biovars or a mixture of different Brucella species (Table 2). Three field strains, isolated in 1984, were found to represent a mixture of B. abortus bv. 1 (A+M−) and bv. 4 (A−M+), while, as expected, the former reference strain 63/75 was not a pure strain but was confirmed as a combination of two B. abortus biovars, biovar 3 (A+M−) and biovar 5 (A−M+), as described previously (14). Furthermore, one strain was characterized as a mixture of different species, B. abortus bv. 1 (A+M−) and B. melitensis bv. 1 (A−M+). In addition, five other strains that had historically been deposited in strain collections as biovar 7 were recharacterized as pure cultures of B. abortus bv. 1, 3, 4, or 5, with characteristics different from those expected for the former biovar 7.
Finally, only four strains, all isolated from cattle, were found to represent B. abortus pure clones with characteristics which did not conform to the B. abortus profiles of any recognized biovar (biovars 1 to 6 and 9): one strain isolated in 1963 in Kenya (07-994-2411, designated K), two strains isolated in Mongolia in 1988 and 1993 (99-9971-135 and 99-9971-159, respectively, designated M1 and M2, respectively), and one strain isolated in Turkey in 2003 (03-4923-239D, designated T). Their particular profile conformed to the differential characteristics of the former biovar 7, particularly agglutination with both anti-A and anti-M monospecific sera (Tables 1 and 2) (37).
Molecular analysis.
Accurate species-specific PCR methods (RT-PCR, Bruce-ladder, AMOS-ERY PCR, omp polymorphism, and IS711 fingerprinting) were performed separately to identify the species to which the four pure strains belonged and to exclude their affiliation with the recognized biovars (biovars 1 to 6 and biovar 9). These assays were complemented by phylogenetic and/or epidemiological methods, such as extended MLST, VNTR-21, and MLVA-16.
The pure strains M1, M2, T, and K, conforming to the former biovar 7, were analyzed by RT-PCR. The cycle threshold (CT) values indicated strong positive reactions (data not shown). The RT-PCR assay confirmed that these clones belonged to the Brucella genus. Likewise, the obtained Bruce-ladder pattern designated these pure strains M1, M2, T, and K as B. abortus (data not shown). This result was confirmed by omp31 PCR (Fig. 1a), which evidenced the deletion of this gene for the species B. abortus. The omp2a PCR polymorphism (Fig. 1b) confirmed the thionin resistance of the four strains, in agreement with the results of the biotyping (Table 2), showing that these B. abortus strains were not biovar 1, 2, or 4. In parallel, in AMOS-ERY PCR (Fig. 1c), the pattern for the four strains showed uniquely the ery bands (1.2 kb), confirming the genus Brucella and showing that the biovar was different from B. abortus bv. 1, 2, and 4, which show an additional specific 0.5-kb band (Fig. 1c).
FIG 1.
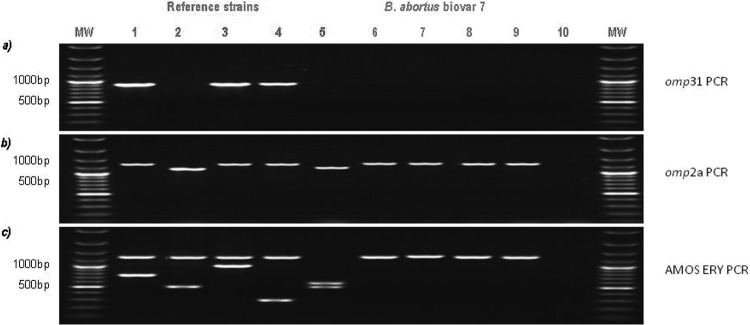
PCR analysis of Brucella reference strains and the 4 field B. abortus bv. 7 strains. Lanes: 1, B. melitensis bv. 1 strain 16M; 2, B. abortus bv. 1 strain 544; 3, B. ovis 63/290; 4, B. suis bv. 1 strain 1330; 5, B. abortus bv. 1 vaccine strain S19; 6, B. abortus bv. 7 field strain T; 7, B. abortus bv. 7 field strain M1; 8, B. abortus bv. 7 field strain M2; 9, B. abortus bv. 7 field strain K; 10, negative control. (a and b) omp31 and omp2a polymorphism, respectively, for strains M1, M2, T, and K investigated in this study and the reference strains (29). (c) Discrimination between the investigated strains and the reference strains as determined by AMOS-ERY PCR assay (18).
With regard to IS711 fingerprinting (data not shown), the isolates M1, M2, and T shared an identical profile by both EcoRI and EcoRI-plus-DdeI digestion. The EcoRI profile was identical to that seen with some biovar 5, 6, and 9 isolates, but the EcoRI-plus-DdeI profile was unique to these three isolates. The restriction profiles of strain K were distinct from the profiles of the other three isolates using both the EcoRI and EcoRI-plus-DdeI approaches. Furthermore, the omp RFLP showed that the same three isolates (M1, M2, and T) shared an identical profile, mirroring the relationship determined by IS711 fingerprinting (data not shown).
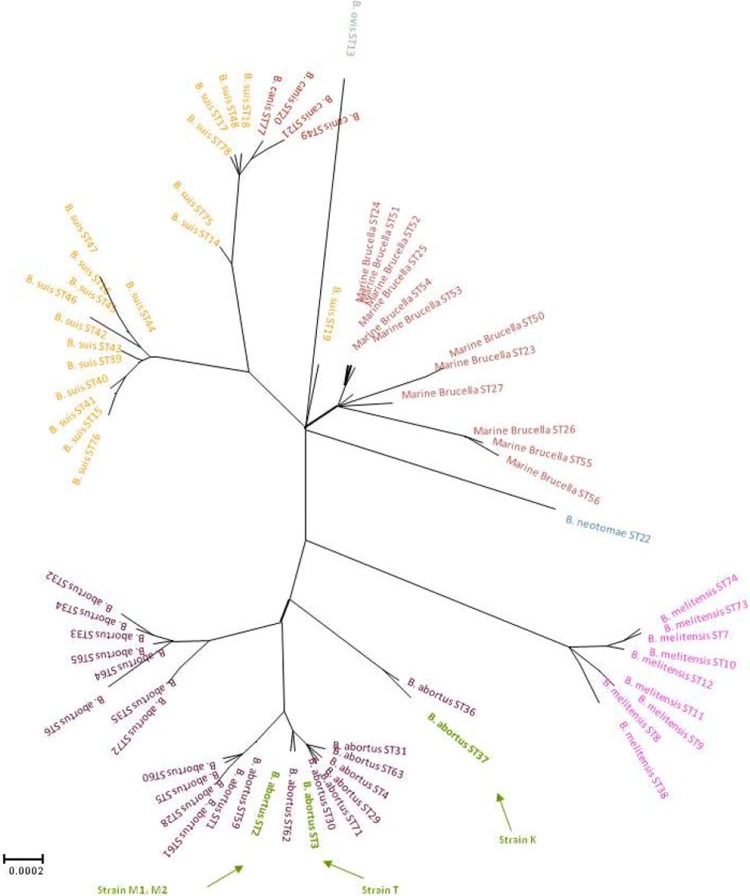
The MLSA-21 scheme indicated that the four field isolates belonged to three distinct sequence types (STs) (Fig. 2). The two Mongolian isolates M1 and M2 share an identical ST (ST2), along with many non-African B. abortus bv. 3 strains. Strain T was a member of a distinct ST, ST3. However, genotype ST3 is very closely related to ST2, possessing only one discriminating SNP in gpd in over 10.2 kb of sequence. This ST has not been described in any other isolate to date. In contrast, strain K, which represented the sole member to date of ST37, is distantly related to the above isolates and indeed is rather divergent from most other B. abortus strains. The only other closely related isolate is the sole representative of ST36, also a field isolate from Africa.
FIG 2.
Unrooted phylogenetic reconstruction of relationships between 440 Brucella isolates representing distinct species and biovars by extended MLSA. The tree was constructed with the concatenated sequence data of the 21 loci (>10.2 kb) using the neighbor-joining algorithm with the Jukes-Cantor model. Brucella species are distinguished by different colors. Strains M1, M2, T, and K investigated in this study are designated by arrows (24; Whatmore, unpublished data).
The VNTR-21 genotypes of the M1, M2, T, and K isolates were compared with those of B. abortus reference and vaccine strains (Table 3). Isolates M1 and M2 from Mongolia were closely related and shared an identical profile with isolate T at the six loci used for taxonomic resolution (VNTR14, VNTR21, VNTR27, VNTR24, VNTR7, and VNTR26) (23). In contrast, strain K appeared to be distantly related to these three isolates and possessed a unique profile, not observed in any other isolate to date, at these six loci.
TABLE 3.
VNTR-21 genotypes for the four B. abortus pure strains phenotypically identified with a biovar distinct from biovars 1 to 6 and 9 in comparison with the reference strains
| Strain | B. abortus biovar or designation | Genotypea |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hoof 1 | Hoof 2 | Hoof 3 | Hoof 4 | Hoof 5 | Hoof 6 | Hoof 7 | Hoof 8 | VNTR2 | VNTR16 | VNTR17 | VNTR5A | VNTR5B | VNTR12A | VNTR12B | VNTR14b | VNTR21b | VNTR27b | VNTR24b | VNTR7b | VNTR26b | ||
| 544 | 1 | 3 | 4 | 6 | 5 | 2 | 2 | 4 | 2 | 3 | 4 | 3 | 5 | 4 | 2 | 13 | 1 | 2 | 1 | 5 | 2 | 4 |
| 86/8/59 | 2 | 7 | 4 | 6 | 2 | 2 | 2 | 11 | 2 | 2 | 3 | 4 | 4 | 5 | 3 | 2 | 1 | 2 | 1 | 5 | 2 | 4 |
| Tulya | 3 | 9 | 4 | 1 | 5 | 7 | 5 | 9 | 2 | 10 | 3 | 6 | 5 | 4 | 2 | 9 | 1 | 2 | 2 | 5 | 2 | 4 |
| 292 | 4 | 7 | 4 | 5 | 3 | 2 | 2 | 7 | 2 | 2 | 3 | 4 | 4 | 2 | 3 | 5 | 1 | 2 | 1 | 3 | 2 | 4 |
| B3196 | 5 | 6 | 2 | 8 | 6 | 6 | 5 | 10, 11 | 2 | 2 | 4 | 5 | 7 | 10 | 8 | 5 | 1 | 2 | 1 | 5 | 1 | 4 |
| 870 | 6 | 9 | 2 | 4 | 10 | 4 | 2 | 6 | 2 | 2 | 4 | 5 | 6 | 9 | 4 | 13 | 1 | 2 | 1 | 5 | 2 | 4 |
| C68 | 9 | 7 | 2 | 8 | 5 | 2 | 5 | 3 | 2 | 2 | 4 | 5 | 6 | 13 | 4 | 9 | 1 | 2 | 1 | 5 | 1 | 4 |
| S19 | Vaccine strain | 5 | 4 | 4 | 2 | 2 | 2 | 8 | 2 | 2 | 4 | 4 | 5 | 6 | 5, 6, 7 | 5 | 1 | 2 | 1 | 5 | 2 | 4 |
| RB51 | Vaccine strain | 5 | 4 | 4 | 2 | 2 | 2 | 4 | 2 | 2 | 4 | 4 | 7 | 4 | 3 | 5 | 1 | 2 | 1 | 5 | 2 | 4 |
| 07-994-2411c | K | 5 | 3 | 4 | 5 | 6 | 4 | 5 | 5 | 6 | 2 | 3 | 2 | 5 | 4 | 2 | 1 | 2 | 1 | 5 | 2 | 3 |
| 03-4923-239c | T | 9 | 2 | 8 | 4 | 8 | 5 | 8 | 5 | 2 | 5 | 4 | 7 | 4 | 2 | 13 | 1 | 2 | 1 | 1 | 2 | 4 |
| 99-9971-135c | M1 | 15 | 2 | 1 | 4 | 6 | 4 | 10 | 3 | 2 | 5 | 4 | 6 | 7 | 4 | 5 | 1 | 2 | 1 | 1 | 2 | 4 |
| 99-9971-159c | M2 | 13 | 2 | 1 | 4 | 6 | 4 | 9 | 3 | 2 | 5 | 4 | 6 | 6 | 4 | 5 | 1 | 2 | 1 | 1 | 2 | 4 |
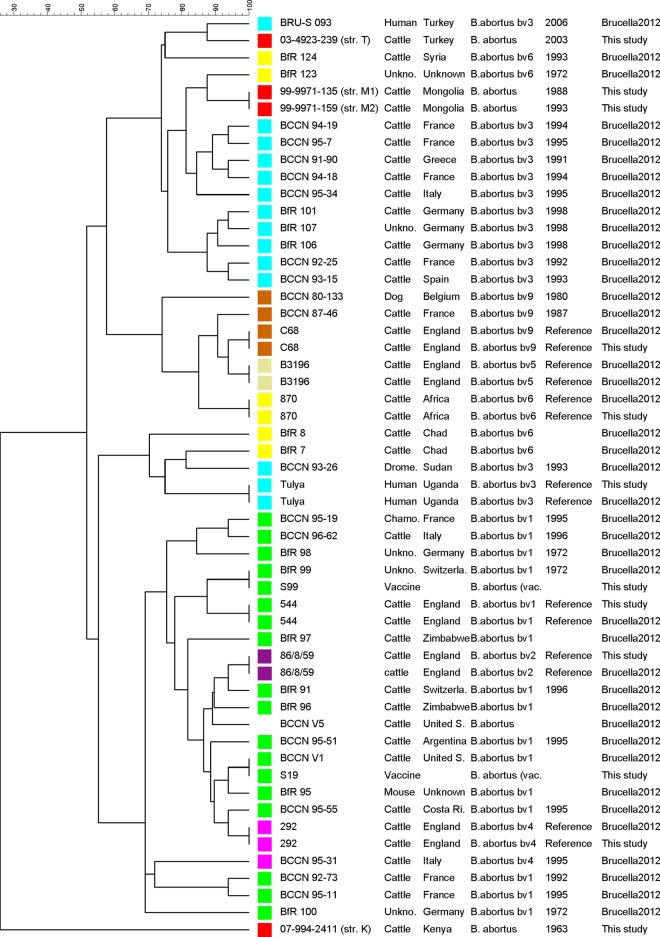
The MLVA patterns obtained for the M1, M2, T, and K isolates (Table 4) were compared with those of 714 Brucella isolates of distinct species (Fig. 3) and with those of the B. abortus reference and vaccine strains (Fig. 4). The MLVA-16 scheme divided the organisms into clusters corresponding to their taxonomic designations. Isolates M1, M2, and T were closely related to the B. abortus members. Interestingly, isolate K, the MLVA-8 profile of which did not correspond to any described pattern in the Brucella2012 database, seemed to be closer to the B. melitensis bv. 1 cluster (only a 1-U difference in bruce42 with MLVA-8 profile of BCCN87-92, a B. melitensis bv. 1 strain isolated in the United States in 1997). Consequently, the MLVA-16 profile of K was very distant from those for other B. abortus genotypes, while strains M1, M2, and T were closer to the B. abortus bv. 3 strains, as also observed with the MLST assay.
TABLE 4.
MLVA-16 genotypes for the four B. abortus pure strains phenotypically identified with a biovar distinct from biovars 1 to 6 and 9 in comparison with the reference strains
| Strain | B. abortus biovar or designation | Host | Yr | Genotypea |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Panel 1 |
Panel 2A |
Panel 2B |
|||||||||||||||||
| bruce06 | bruce08 | bruce11 | bruce12 | bruce42 | bruce43 | bruce45 | bruce55 | bruce18 | bruce19 | bruce21 | bruce04 | bruce07 | bruce09 | bruce16 | bruce30 | ||||
| 544 | 1 | Bovine | 1942 | 4 | 5 | 4 | 12 | 2 | 2 | 3 | 3 | 5 | 21 | 8 | 3 | 5 | 3 | 4 | 5 |
| 86/8/59 | 2 | Bovine | 1959 | 4 | 5 | 4 | 12 | 2 | 1 | 3 | 3 | 6 | 21 | 8 | 3 | 4 | 3 | 3 | 5 |
| Tulya | 3 | Human | 1958 | 3 | 5 | 4 | 11 | 2 | 2 | 3 | 3 | 8 | 20 | 8 | 6 | 5 | 3 | 11 | 5 |
| 292 | 4 | Bovine | 1961 | 4 | 5 | 4 | 12 | 2 | 2 | 3 | 2 | 6 | 21 | 8 | 3 | 4 | 3 | 3 | 5 |
| B3196 | 5 | Bovine | 1959 | 3 | 5 | 3 | 12 | 2 | 2 | 2 | 3 | 7 | 21 | 8 | 6 | 7 | 3 | 3 | 3 |
| 870 | 6 | Bovine | 1959 | 3 | 5 | 3 | 12 | 2 | 2 | 3 | 3 | 7 | 21 | 8 | 3 | 6 | 3 | 3 | 3 |
| C68 | 9 | Bovine | 1958 | 3 | 5 | 3 | 12 | 2 | 2 | 2 | 3 | 7 | 21 | 8 | 6 | 6 | 3 | 3 | 3 |
| S19 | 1 (vaccine) | Bovine | 1943 | 4 | 5 | 4 | 12 | 2 | 2 | 3 | 3 | 6 | 21 | 8 | 3 | 5 | 3 | 3 | 5 |
| S99 | 1 | 1957 | 4 | 5 | 4 | 12 | 2 | 2 | 3 | 3 | 6 | 21 | 8 | 3 | 5 | 3 | 4 | 6 | |
| 07-994-2411b | K | Bovine | 1963 | 2 | 4 | 2 | 12 | 3 | 2 | 3 | 3 | 5 | 22 | 6 | 5 | 2 | 6 | 7 | 5 |
| 03-4923-239b | T | Bovine | 2003 | 4 | 5 | 3 | 12 | 2 | 2 | 3 | 1 | 6 | 21 | 8 | 6 | 7 | 6 | 3 | 3 |
| 99-9971-135b | M1 | Bovine | 1988 | 4 | 5 | 3 | 12 | 2 | 2 | 3 | 1 | 6 | 21 | 8 | 5 | 6 | 4 | 3 | 3 |
| 99-9971-159b | M2 | Bovine | 1993 | 4 | 5 | 3 | 12 | 2 | 2 | 3 | 1 | 6 | 21 | 8 | 5 | 6 | 4 | 3 | 3 |
The 16 investigated loci are organized in agreement with their molecular evolution rate in two panels; panel 1 is useful for species identification, and panel 2 shows a higher discriminatory power (22).
Strain investigated in this study.
FIG 3.
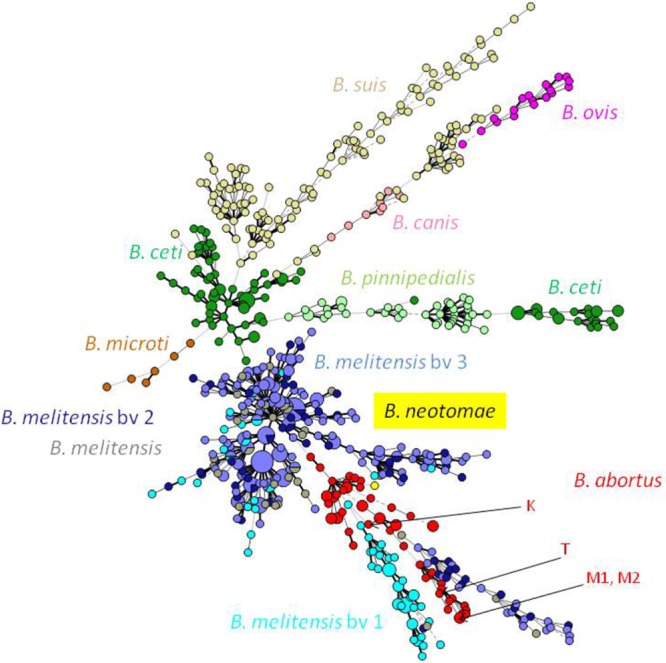
Minimum spanning tree of 718 Brucella isolates, representing the majority of known species/biovars, by MLVA-16. An MST of 718 Brucella isolates of distinct species and biovars was constructed, based on MLVA-16 patterns obtained in this study or available in the Brucella2010 database (http://mlva.u-psud.fr/mlvav4/genotyping/). The MST results are shown by using a logarithmic scaling. Brucella species and B. melitensis biovars are distinguished by different colors. Strains M1, M2, T, and K investigated in this study are designated by arrows.
FIG 4.
Analysis of relationships between 56 B. abortus isolates, representing all biovars, by MLVA-16. Clustering analysis was performed using the UPGMA algorithm with categorical coefficient; each data set (panel 1, panel 2A, and panel 2B) was weighted according to the molecular evolution rates (22). Reference strain genotypes were obtained both from this study and from the Brucella2012 database. B. abortus biovars are distinguished by different colors. Strains M1, M2, T, and K investigated in this study are designated in red. The strain identity, the host, the geographical origin, the biovar, and the corresponding genotype reference are listed in the dendrogram. The bars reflect the percentage of weighted divergence.
DISCUSSION
In the past decades Brucella taxonomy has been hotly debated and has undergone many reorganizations at both the species and intraspecies levels. Indeed, from 1986, on the basis of results of DNA-DNA hybridization (high similarity values up to 99%) and in agreement with Bergey's Manual of Systematic Bacteriology, some scientists proposed that all the Brucella species should be regarded as belonging to a single species, B. melitensis (14). In 2003, ISTB recommendations allowed a return to the pre-1986 taxonomy of the Brucella genus (classical Brucella species with their recognized biovars) (38), and a number of molecular typing and phylogenetic studies illustrating that the classical species correspond to genetically distinct, if closely related, entities (23, 24, 39) have supported this decision.
In the same way, the presence of B. abortus bv. 7, represented by the reference strain 63/75, in Brucella systematics has been the subject of controversy. Indeed, this biovar was suspended from the Approved Lists of Bacterial Names Brucella classification in 1988 because of unpublished evidence that the reference strain 63/75 was a mixture of B. abortus bv. 3 and bv. 5 (14). To formally confirm and clarify the situation with regard to B. abortus bv. 7, all isolates phenotypically identified in the past as B. abortus bv. 7 in the AHVLA and ANSES strain collections were characterized by classical microbiological and multiple molecular approaches.
Among the 14 investigated collection strains, including the reference strain 63/75, 10 conformed to the previous B. abortus bv. 7 characteristics. However, they either were a mixed culture (different B. abortus biovars or a mixture of distinct Brucella species), conferring a typical A+M+ serological profile or were another pure B. abortus biovar, possibly reflecting the loss of another strain that resulted in the originally described A+M+ serological profile. Only four cattle strains, isolated in Kenya (K) in 1963, in Turkey (T) in 2003, and in Mongolia (M1 and M2) in 1988 and 1993, showed a particular profile, previously described as B. abortus bv. 7, characterized mainly by an A+M+ serological pattern.
The molecular techniques (RT-PCR and IS711 fingerprinting) identified these four field strains as members of the Brucella genus. Furthermore, AMOS PCR, Bruce-ladder multiplex PCR, and the polymorphism of the outer membrane genes omp2a, omp2b, and omp31 confirmed that these strains belonged to the B. abortus species but were distinct from the designated biovars (biovars 1 to 6 and biovar 9) and vaccine strains. The phylogenetic and/or epidemiological schemes supported these results.
In agreement with the above data, strains M1, M2, and T were found to represent a genetic cluster, with the allelic profiles, obtained by VNTR-21 and MLVA-16 assays, allowing discrimination of these strains with a characteristic MLVA-11 pattern, closely related to other B. abortus strains, especially with the B. abortus bv. 3 strains. In addition, phylogenetic multilocus sequence analysis placed the isolates as two closely related sequence types (ST2 and ST3) in the same genetically conserved group of B. abortus strains.
Interestingly, strain K, phenotypically identical to isolates M1, M2, and T, is divergent from them on molecular characterization grounds. Indeed, strain K possessed a distinct and novel profile using the VNTR approaches, adjacent to a B. melitensis bv. 1 member, and a unique MLST genotype, related to an African field isolate. While several molecular strategies (Bruce-ladder, omp31 deletion, and AMOS PCR) allowed placement of strain K among B. abortus strains, this strain is unique among isolates examined to date, and genotyping data revealed that this isolate is rather distant from other B. abortus strains. Phenotypic and molecular discrepancies concerning strain K illustrate the complexities of Brucella taxonomy.
The definition of Brucella species is based only on characteristics of lysotyping and urease and oxidase hydrolysis (40). In agreement with these phenotypic criteria, the four field isolates M1, M2, T, and K might be classified in the species B. abortus. Nevertheless, their profile does not coincide with any designated B. abortus biovars (biovars 1 to 6 and biovar 9), but it matches the criteria of the former biovar 7. In addition, these data are partially congruent with molecular approaches. On the basis of polyphasic taxonomy results, taking into account available phenotypic and genotypic data, we propose that the B. abortus biovar 7 could be reintroduced into the Brucella classification, with as a reference strain the oldest isolate (99-9971-139), isolated in Mongolia in 1988. However, as with the existing B. abortus bv. 3 scenario, where a number of studies have shown that this biovar includes genetically distinct clusters (27, 41), the results here highlight the limitations of a classification system based on phenotype alone. The “clustering” of genetically unrelated strains clearly limits the value of biotyping as a typing or epidemiological tool, as equally does the finding that the ST2 type is shared by many biovar 3 isolates. Such observations strengthen the argument for moving toward a molecularly based classification, which, in the scenario described here, would readily separate the genetically distinct but phenotypically identical strain K.
Conclusion.
The aim of our study was to clarify the situation concerning B. abortus biovar 7 by both conventional and molecular typing approaches. This consensus approach confirmed that worldwide collections could possess some strains misidentified as B. abortus bv. 7, a biovar suspended from the Approved Lists of Bacterial Names Brucella classification, and highlighted the need to verify the taxonomic position of these strains.
Our study allowed the identification of four clonal B. abortus strains among 14 strains investigated, with specific phenotypic criteria corresponding to the criteria defining the former biovar 7. The phenotypic data results were strengthened by molecular strategies. This polyphasic investigation allows us to propose the reintroduction of biovar 7 into the Brucella classification, with three representative strains (M1, M2, and T) and strain 99-9971-135 as the potential reference strain. However, the study also highlighted the existence of (i) genetically unrelated strains sharing the same biovar 7 phenotype and (ii) non-biovar 7 isolates that share the same genotype, which could compromise the reintroduction of this biovar.
ACKNOWLEDGMENTS
We are grateful to María-Laura Boschiroli for critically reviewing the manuscript and supportive comments that improved the paper, to Moulay-Ali Cherfa for technical support, and to Gabriela Vecchio for logistic expertise. We also acknowledge the valuable help of Jean-Michel Verger and Maggy Grayon (formerly at INRA, Nouzilly, France) for the strain cloning methodology. We thank A. Yondondorj (Mongolian Research Institute of Veterinary Medicine, Ulanbaatar, Mongolia) and S. Erdenlig (Pendik Veterinary Control Institute, Pendik, Turkey), who isolated the Mongolian and Turkish strains, respectively.
Footnotes
Published ahead of print 20 December 2013
REFERENCES
- 1.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91–99. 10.1016/S1473-3099(06)70382-6 [DOI] [PubMed] [Google Scholar]
- 2.Williams KP, Sobral BW, Dickerman AW. 2007. A robust species tree for the alphaproteobacteria. J. Bacteriol. 189:4578–4586. 10.1128/JB.00269-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster G, Osterman BS, Godfroid J, Jacques I, Cloeckaert A. 2007. Brucella ceti sp nov and Brucella pinnipedialis sp nov for Brucella strains with cetaceans and seals as their preferred hosts. Int. J. Syst. Evol. Microbiol. 57:2688–2693. 10.1099/ijs.0.65269-0 [DOI] [PubMed] [Google Scholar]
- 4.Scholz HC, Hubalek Z, Sedlacek I, Vergnaud G, Tomaso H, Al Dahouk S, Melzer F, Kämpfer P, Neubauer H, Cloeckaert A, Maquart M, Zygmunt MS, Whatmore AM, Falsen E, Bahn P, Göllner C, Pfeffer M, Huber B, Busse HJ, Nöckler K. 2008. Brucella microti sp. nov., isolated from the common vole Microtus arvalis. Int. J. Syst. Evol. Microbiol. 58:375–382. 10.1099/ijs.0.65356-0 [DOI] [PubMed] [Google Scholar]
- 5.Al Dahouk S, Hofer E, Tomaso H, Vergnaud G, Le Fleche P, Cloeckaert A, Koylass MS, Whatmore AM, Nöckler K, Scholz HC. 2012. Intraspecies biodiversity of the genetically homologous species Brucella microti. Appl. Environ. Microbiol. 78:1534–1543. 10.1128/AEM.06351-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholz HC, Nöckler K, Göllner C, Bahn P, Vergnaud G, Tomaso H, Al Dahouk S, Kämpfer P, Cloeckaert A, Maquart M, Zygmunt MS, Whatmore AM, Pfeffer M, Huber B, Busse HJ, De BK. 2010. Brucella inopinata sp. nov., isolated from a breast implant infection. Int. J. Syst. Evol. Microbiol. 60:801–808. 10.1099/ijs.0.011148-0 [DOI] [PubMed] [Google Scholar]
- 7.Schlabritz-Loutsevitch NE, Whatmore AM, Quance CR, Koylass MS, Cummins LB, Dick EJ, Jr, Snider CL, Cappelli D, Ebersole JL, Nathanielsz PW, Hubbard GB. 2009. A novel Brucella isolate in association with two cases of stillbirth in non-human primates—first report. J. Med. Primatol. 38:70–73. 10.1111/j.1600-0684.2008.00314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappas G. 2010. The changing Brucella ecology: novel reservoirs, new threats. Int. J. Antimicrob. Agents. 36:S8–S11. 10.1016/j.ijantimicag.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 9.Hofer E, Revilla-Fernandez S, Al Dahouk S, Riehm JM, Nockler K, Zygmunt MS, Cloeckaert A, Tomaso H, Scholz HC. 2012. A potential novel Brucella species isolated from mandibular lymph nodes of red foxes in Austria. Vet. Microbiol. 155:93–99. 10.1016/j.vetmic.2011.08.009 [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg T, Hamann HP, Kaim U, Schlez K, Seeger H, Schauerte N, Melzer F, Tomaso H, Scholz HC, Koylass MS, Whatmore AM, Zschöck M. 2012. Isolation of potentially novel Brucella spp. from frogs. Appl. Environ. Microbiol. 78:3753–3755. 10.1128/AEM.07509-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiller RV, Gee JE, Frace MA, Taylor TK, Setubal JC, Hoffmaster AR, De BK. 2010. Characterization of novel Brucella strains originating from wild native rodent species in North Queensland, Australia. Appl. Environ. Microbiol. 76:5837–5845. 10.1128/AEM.00620-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Committee on Systematic Bacteriology, Subcommittee on Taxonomy of Brucella. 1978. Minutes of the meeting, 4 and 5 September 1978 Int. J. Syst. Bacteriol. 32:260–261 [DOI] [PubMed] [Google Scholar]
- 13.Meyer ME, Morgan WJB. 1973. Designation of neotype strains and of biotype reference strains for species of the genus Brucella—Meyer and Shaw. Int. J. Syst. Bacteriol. 23:135. 10.1099/00207713-23-2-135 [DOI] [Google Scholar]
- 14.International Committee on Systematic Bacteriology, Subcommittee on the Taxonomy of Brucella. 1988. Report of the meeting, 5 September 1986 Int. J. Syst. Bacteriol. 38:450–452. 10.1099/00207713-38-4-450 [DOI] [PubMed] [Google Scholar]
- 15.Whatmore AM. 2009. Current understanding of the genetic diversity of Brucella, an expanding genus of zoonotic pathogens. Infect. Genet. Evol. 9:1168–1184. 10.1016/j.meegid.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 16.Bounaadja L, Albert D, Chenais B, Henault S, Zygmunt MS, Poliak S, Garin-Bastuji B. 2009. Real-time PCR for identification of Brucella spp.: a comparative study of IS711, bcsp31 and per target genes. Vet. Microbiol. 137(1–2):156–164. 10.1016/j.vetmic.2008.12.023 [DOI] [PubMed] [Google Scholar]
- 17.Vizcaíno N, Verger JM, Grayon M, Zygmunt MS, Cloeckaert A. 1997. DNA polymorphism at the Omp-31 locus of Brucella spp—evidence for a large deletion in Brucella abortus, and other species-specific markers. Microbiology 143:2913–2921. 10.1099/00221287-143-9-2913 [DOI] [PubMed] [Google Scholar]
- 18.Bricker BJ, Ewalt DR, Olsen SC, Jensen AE. 2003. Evaluation of the Brucella abortus species-specific polymerase chain reaction assay, an improved version of the Brucella AMOS polymerase chain reaction assay for cattle. J. Vet. Diagn. Invest. 15:374–378. 10.1177/104063870301500413 [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Yoldi D, Marín CM, de Miguel MJ, Munoz PM, Vizmanos JL, Lopez-Goñi I. 2006. Multiplex PCR assay for the identification and differentiation of all Brucella species and the vaccine strains Brucella abortus S19 and RB51 and Brucella melitensis Rev1. Clin. Chem. 52:779–781. 10.1373/clinchem.2005.062596 [DOI] [PubMed] [Google Scholar]
- 20.López-Goñi I, García-Yoldi D, Marín CM, de Miguel MJ, Barquero-Calvo E, Guzmán-Verri C, Albert D, Garin-Bastuji B. 2011. New Bruce-ladder multiplex PCR assay for the biovar typing of Brucella suis and the discrimination of Brucella suis and Brucella canis. Vet. Microbiol. 154(1–2):152–155. 10.1016/j.vetmic.2011.06.035 [DOI] [PubMed] [Google Scholar]
- 21.Le Fleche P, Jacques I, Grayon M, Al Dahouk S, Bouchon P, Denoeud F, Nöckler K, Neubauer H, Guilloteau LA, Vergnaud G. 2006. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 6:9. 10.1186/1471-2180-6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Dahouk S, Le Fleche P, Nockler K, Jacques I, Grayon M, Scholz HC, Tomaso H, Vergnaud G, Neubauer H. 2007. Evaluation of Brucella MLVA typing for human brucellosis. J. Microbiol. Methods 69:137–145. 10.1016/j.mimet.2006.12.015 [DOI] [PubMed] [Google Scholar]
- 23.Whatmore AM, Shankster SJ, Perrett LL, Murphy TJ, Brew SD, Thirlwall RE, Cutler SJ, MacMillan AP. 2006. Identification and characterization of variable-number tandem-repeat markers for typing of Brucella spp. J. Clin. Microbiol. 44:1982–1993. 10.1128/JCM.02039-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whatmore AM, Perrett LL, MacMillan AP. 2007. Characterisation of the genetic diversity of Brucella by multilocus sequencing. BMC Microbiol. 7:34. 10.1186/1471-2180-7-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandara B, Merino AL, Rogel MA, Martinez-Romero E. 2001. Limited genetic diversity of Brucella spp. J. Clin. Microbiol. 39:235–240. 10.1128/JCM.39.1.235-240.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marianelli C, Ciuchini F, Tarantino M, Pasquali P, Adone R. 2006. Molecular characterization of the rpoB gene in Brucella species: new potential molecular markers for genotyping. Microbes Infect. 8:860–865. 10.1016/j.micinf.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 27.Valdezate S, Navarro A, Rubio V, Garin-Bastuji B, Albert D, Hernandez P, Alonso PM, Saéz-Nieto JA. 2009. Emergence of a clonal lineage of Brucella abortus biovar 3 in clinical cases in Spain. J. Clin. Microbiol. 47:2687–2688. 10.1128/JCM.00756-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Organization for Animal Health 2009. Bovine brucellosis. In Manual of diagnostic tests and vaccines for terrestrial animals. OIE, Paris, France: http://www.oie.int [Google Scholar]
- 29.Cloeckaert A, Verger JM, Grayon M, Grepinet O. 1995. Restriction site polymorphism of the genes encoding the major 25 Kda and 36 Kda outer-membrane proteins of Brucella. Microbiology 141:2111–2121. 10.1099/13500872-141-9-2111 [DOI] [PubMed] [Google Scholar]
- 30.Al Dahouk S, Tomaso H, Prenger-Berninghoff E, Splettstoesser WA, Scholz HC, Neubauer H. 2005. Identification of Brucella species and biotypes using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). Crit. Rev. Microbiol. 31:191–196. 10.1080/10408410500304041 [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Yoldi D, Marín CM, Lopez-Goñi I. 2005. Restriction site polymorphisms in the genes encoding new members of group 3 outer membrane protein family of Brucella spp. FEMS Microbiol. Lett. 245:79–84. 10.1016/j.femsle.2005.02.026 [DOI] [PubMed] [Google Scholar]
- 32.Bricker BJ, Halling SM. 1995. Enhancement of the Brucella AMOS PCR assay for differentiation of Brucella abortus vaccine strains S19 and RB51. J. Clin. Microbiol. 33:1640–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouahrani S, Michaux S, Widada JS, Bourg G, Tournebize R, Ramuz M, Liautard JP. 1993. Identification and sequence analysis of IS6501, an insertion sequence in Brucella spp. Relationship between genomic structure and the number of IS6501 copies. J. Gen. Microbiol. 139:3265–3273 [DOI] [PubMed] [Google Scholar]
- 34.Ouahrani-Bettache S, Soubrier MP, Liautard JP. 1996. IS6501-anchored PCR for the detection and identification of Brucella species and strains. J. Appl. Microbiol. 81:154–160 [DOI] [PubMed] [Google Scholar]
- 35.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bricker BJ, Ewalt DR, Halling SM. 2003. Brucella ‘HOOF-Prints': strain typing by multi-locus analysis of variable number tandem repeats (VNTRs). BMC Microbiol. 3:15. 10.1186/1471-2180-3-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alton GG, Jones LM, Pietz DE. 1977. La brucellose. Techniques de laboratoire, 2nd ed, FAO/WHO, Geneva, Switzerland [Google Scholar]
- 38.International Committee on Systematic Bacteriology, Subcommittee on the Taxonomy of Brucella. 2006. Minutes of the meeting, 17 September 2003 Int. J. Syst. Evol. Microbiol. 56:1173–1175. 10.1099/ijs.0.64349-0 [DOI] [PubMed] [Google Scholar]
- 39.Wattam AR, Inzana TJ, Williams KP, Mane SP, Shukla M, Almeida NF, Dickerman AW, Mason S, Moriyón I, O'Callaghan D, Whatmore AM, Sobral BW, Tiller RV, Hoffmaster AR, Frace MA, De Castro C, Molinaro A, Boyle SM, De BK, Setubal JC. 2012. Comparative genomics of early-diverging Brucella strains reveals a novel lipopolysaccharide biosynthesis pathway. mBio 3(5):e00246–11. 10.1128/mBio.00246-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alton GG, Jones LM, Angus RD, Verger JM. 1988. Techniques for the brucellosis laboratory. INRA Publications, Paris, France [Google Scholar]
- 41.Ocampo-Sosa AA, Aguero-Balbin J, Garcia-Lobo JM. 2005. Development of a new PCR assay to identify Brucella abortus biovars 5, 6 and 9 and the new subgroup 3b of biovar 3. Vet. Microbiol. 110(1–2):41–51. 10.1016/j.vetmic.2005.06.007 [DOI] [PubMed] [Google Scholar]