Abstract
The study of symbiotic nitrogen transfer in soil has largely focused on nitrogen-fixing bacteria. Vascular plants can lose a substantial amount of their nitrogen through insect herbivory. Previously, we showed that plants were able to reacquire nitrogen from insects through a partnership with the endophytic, insect-pathogenic fungus Metarhizium robertsii. That is, the endophytic capability and insect pathogenicity of M. robertsii are coupled so that the fungus acts as a conduit to provide insect-derived nitrogen to plant hosts. Here, we assess the ubiquity of this nitrogen transfer in five Metarhizium species representing those with broad (M. robertsii, M. brunneum, and M. guizhouense) and narrower insect host ranges (M. acridum and M. flavoviride), as well as the insect-pathogenic fungi Beauveria bassiana and Lecanicillium lecanii. Insects were injected with 15N-labeled nitrogen, and we tracked the incorporation of 15N into two dicots, haricot bean (Phaseolus vulgaris) and soybean (Glycine max), and two monocots, switchgrass (Panicum virgatum) and wheat (Triticum aestivum), in the presence of these fungi in soil microcosms. All Metarhizium species and B. bassiana but not L. lecanii showed the capacity to transfer nitrogen to plants, although to various degrees. Endophytic association by these fungi increased overall plant productivity. We also showed that in the field, where microbial competition is potentially high, M. robertsii was able to transfer insect-derived nitrogen to plants. Metarhizium spp. and B. bassiana have a worldwide distribution with high soil abundance and may play an important role in the ecological cycling of insect nitrogen back to plant communities.
INTRODUCTION
Despite its atmospheric abundance, nitrogen gas (N2) is not directly available as a source of nitrogen to plants. Free-living or symbiotic soil microbes fix N2 and produce nitrogen-containing compounds that are directly utilized by plants (1). Traditionally, the paradigm of symbiotic nitrogen transfer to plants has focused on nitrogen-fixing bacteria such as Rhizobium. However, once this nitrogen is fixed and transferred to the plant, it can be lost, primarily through microbial mineralization of decaying vegetation or insect herbivory. With respect to mineralized nitrogen, more than 90% of land plants are able to form symbiotic relationships with soil fungi, and several of these fungi are able to transfer nitrogen to plants (2). Mycorrhizal fungi, specifically from the phylum Glomeromycota, have been shown to provide plants with nitrogen obtained from the soil in exchange for plant-derived carbon (3).
Up to 31% of plant nitrogen in an ecosystem can be lost to insects through herbivory (4). Recently, we illustrated a strategy whereby plants can reacquire nitrogen previously lost to herbivorous insects through an association with an endophytic, insect-pathogenic fungus (EIPF) (5). In the soil, the EIPF Metarhizium robertsii infected and killed an insect, formed an endophytic relationship with a plant, and subsequently transferred insect-derived nitrogen directly to the plant. This strategy suggested the possibility that other endophytic, insect-pathogenic fungal species, such as species of Beauveria and Lecanicillium, are also able to transfer nitrogen from infected insects to plants.
Here, we tested the potential ubiquity of insect-derived nitrogen transfer in four plant species (representing monocots and dicots) via five species in the genus Metarhizium, representing broad-range insect pathogens (M. robertsii, M. guizhouense, M. brunneum) and narrow-range insect pathogens (M. flavoviride and M. acridum), as well as Beauveria bassiana and Lecanicillium lecanii. These fungi are found worldwide, from arctic to tropical ecosystems (6), with up to 106 propagules per gram of soil (7), and the broad-range insect pathogens can infect up to 200 different species of insects (8). We measured insect-derived nitrogen transfer by these fungi in soybean (Glycine max), haricot bean (Phaseolus vulgaris), switchgrass (Panicum virgatum), and wheat (Triticum aestivum). We also tested insect-derived nitrogen transfer to plants by M. robertsii under field conditions. The results suggested that all of the Metarhizium species and B. bassiana were able to transfer significant amounts of insect-derived nitrogen to plants. Endophytic association by these fungi increased overall plant productivity. Also, in natural settings, where competition is potentially high, Metarhizium is an effective conduit for the transfer of insect-derived nitrogen to plants.
MATERIALS AND METHODS
Sources of fungi and plant material. (i) Fungal cultures.
Metarhizium robertsii strain 2575, M. acridum strain 7486, M. flavoviride strain 9358, Beauveria bassiana strain 252, and Lecanicillium lecanii strain 313 were obtained from the U.S. Department of Agricultural Research Service Collection of Entomopathogenic Fungal Cultures, Ithaca, NY. M. brunneum strain 43a-2i and M. guizhouense strain B77-ai were isolated from the field and cultured in the laboratory (9). Aspergillus flavus strain 6982 was obtained from the University of Alberta Microfungus Collection and Herbarium, Edmonton, Alberta, Canada. M. robertsii, M. guizhouense, B. bassiana, and L. lecanii fungi carrying plasmids expressing green fluorescent protein (GFP) were used for confocal micrographs. The construction of the GFP-expressing plasmids, as well as transformation of Metarhizium species, have been previously described (10). Stock cultures were grown at 27°C in potato dextrose agar (PDA; Difco laboratories, Mississauga, Ontario, Canada).
(ii) Plant material.
Phaseolus vulgaris (haricot bean, cultivar ‘Soldier') and Panicum virgatum (switchgrass, cultivar ‘Blackwell') seeds were obtained from OSC Seeds, Waterloo, Ontario, Canada. Triticum aestivum (winter wheat, cultivar ‘Accipiter') seeds were obtained from Sprout Master, Elmvale, Ontario, Canada. Glycine max (soybean, cultivar ‘Harosoy') seeds were obtained from the Agriculture Canada research station Southern Crop Protection and Food Research Centre London, London, Ontario, Canada.
Seed sterilization and plating.
Seeds were surface sterilized before use in order to prevent any unwanted fungal or bacterial growth. Seeds were immersed in sterile distilled water for 30 min in a 50-ml capped plastic tube and subsequently immersed in a 4% sodium hypochlorite solution three times for 5 min. After each sodium hypochlorite wash, seeds were rinsed with sterile distilled water. Seeds were then placed in 15% hydrogen peroxide for 10 min and subsequently washed three times with sterile distilled water to remove all hydrogen peroxide. Seeds were kept overnight at 4°C to allow for synchronization of growth and then plated on water agar and kept at 25°C for a photoperiod of 16 h a day for a minimum of 7 days in order to obtain seedlings. The seedlings were then placed in soil microcosms and harvested 1, 7, 14, and 28 days after placement in the soil microcosms.
Injection and infection of Galleria mellonella (wax moth larvae).
G. mellonella larvae were injected with 10 μl of a 5% [15N]ammonium sulfate solution through the rear proleg using a sterile syringe. After 48 h, live larvae were infected with fungal conidia by agitation for 2 min on a 10-day-old conidiating fungal culture of one of the Metarhizium species, B. bassiana, L. lecanii, or A. flavus. The insects were then placed into the petri dish portion of the soil microcosm.
Soil sampling and plating.
Soil was taken from microcosms containing G. mellonella larvae infected with Metarhizium robertsii, B. bassiana, L. lecanii, or A. flavus and plant seedlings. Soil was sampled 0.5 cm from the plant root at a depth of 2 cm. One gram of soil was sampled from 5 separate plants every 2 days. Soil samples were suspended in 0.5 ml of a 1% peptone solution, vortexed, and plated on selective PDA that contained 9.75 g potato dextrose agar, 0.125 g cycloheximide, 0.05 g chloramphenicol, 0.125 g 65% Dodine, and 0.0025 g crystal violet (all weights per 250 ml). The samples were spread with a plate spreader and kept at 27°C for 10 days. After 10 days of growth, CFU were counted.
Microcosm setup.
A 7-cm-diameter hole was cut in the lid of a plastic petri dish (9 cm in diameter), which was then covered with a 30-μm mesh and adhered with silicon glue. Plants were grown in plastic garden pots (10 cm in height by 15 cm in diameter). The garden pots and the modified petri dishes were sterilized with UV light for 2.5 h prior to use. Once sterilized, the petri dishes were filled with sterile soil and five fungus-infected, 15N-injected larvae and sealed with parafilm. The petri dishes were then placed in the pots at a depth of 8 to 9 cm and covered with sterile soil, completing the microcosm. The pots were filled with soil to 1 cm from the top, and a seedling was planted in each. Only the fungi were able to penetrate the mesh and move across both compartments of the microcosm. Plants were watered daily with sterile distilled water and once a week with 50 ml of 50% MMN solution {0.05 g CaCl2, 0.025 g NaCl, 0.05 g KH2PO4, 0.5 g (NH4)2PO4, 0.15 g MgSO4·7H2O, 1 mg FeCl3·6H2O, 5 g glucose monohydrate, 10 ml trace element solution [3.728 g KCl, 1.546 g H3BO3, 0.845 g MnSO4·H2O, 0.05 g ZnSO4·7H2O, 0.0125 g CuSO4, 0.05 g (NH4)6Mo7O24·4H2O per 1 liter] per 1 liter}. In total, 250 soil microcosms were set up, representing all experimental and control treatments.
Preparation of samples for 15N analysis.
Above-ground plant tissue (stems and leaves) was removed at 7-day intervals subsequent to placement in soil microcosms and was dried at 60°C. The plant material was then crushed into a fine powder using a mortar and pestle. The comminuted plant material was encapsulated in 4-mm by 4-mm tin cups and analyzed for 15N content by using an NOI-5 emission spectrophotometer.
Plant growth measurements.
Plants were grown under the experimental conditions described above. In plants grown with the fungus alone, conidia from 10-day-old fungal cultures were used to inoculate the soil within the petri dish section of soil microcosms. In insect-only treatments, insects were placed in the petri dish section of the microcosm without fungus. After 4 days and then every 4 days for 16 days, plants were harvested, washed with sterile distilled water to remove soil, dried at 60°C for 24 to 48 h, and sectioned for weighing. Plant roots and leaves were removed from the whole plant with a sterile scalpel. The plant growth parameters measured were the leaf weight of haricot bean (Phaseolus vulgaris), root weights of haricot bean (Phaseolus vulgaris), switchgrass (Panicum virgatum), and wheat (Triticum aestivum), and whole-plant weights of haricot bean (Phaseolus vulgaris), switchgrass (Panicum virgatum), and wheat (Triticum aestivum).
Confocal microscopy of plant root associations with GFP-labeled fungus.
The associations between the various fungi and plant roots were visualized microscopically. Roots were observed using confocal microscopy to confirm association of EIPF by root colonizing. Plants were grown under the experimental conditions described above. Whole roots were harvested at 7 days and washed with sterile distilled water to partially remove soil. The roots were then cut into 5-cm sections and placed on glass slides for visualization. Plant tissue was examined using a Leica DMIRE2 inverted display confocal microscope utilizing an argon-krypton laser operated at excitation wavelengths of 480 ± 10 nm and 518 nm.
Field experiment.
The experimental plot (ca. 100 m2) was on an old field (near St. Catharines, ON, Canada) predominantly comprised of clay soil and dominated by three grass species, timothy (Phleum pratense), smooth bromegrass (Bromus inermis), and orchard grass (Dactylis spp.). Within the field site, the petri dishes, modified with 30- or 1-μm mesh covers, were buried approximately 15 cm below the surface. The 1-μm mesh was used in control treatments since Metarhizium is unable to migrate through this mesh. The treatments in the modified petri dishes were as follows: 15N-labeled insects plus M. robertsii with 30-μm mesh (experimental), 15N-labeled insects plus M. robertsii with 1-μm mesh, 15N-labeled insects with 1-μm mesh, and 15N-labeled insects with 30-μm mesh. Samples (ca. 50 g) of grass collected at distances of 5, 10, and 30 cm from the center of each buried petri dish were harvested at 1, 7, 14, and 28 days.
RESULTS
Nitrogen transfer to plants by Metarhizium species. (i) Soybean (Glycine max).
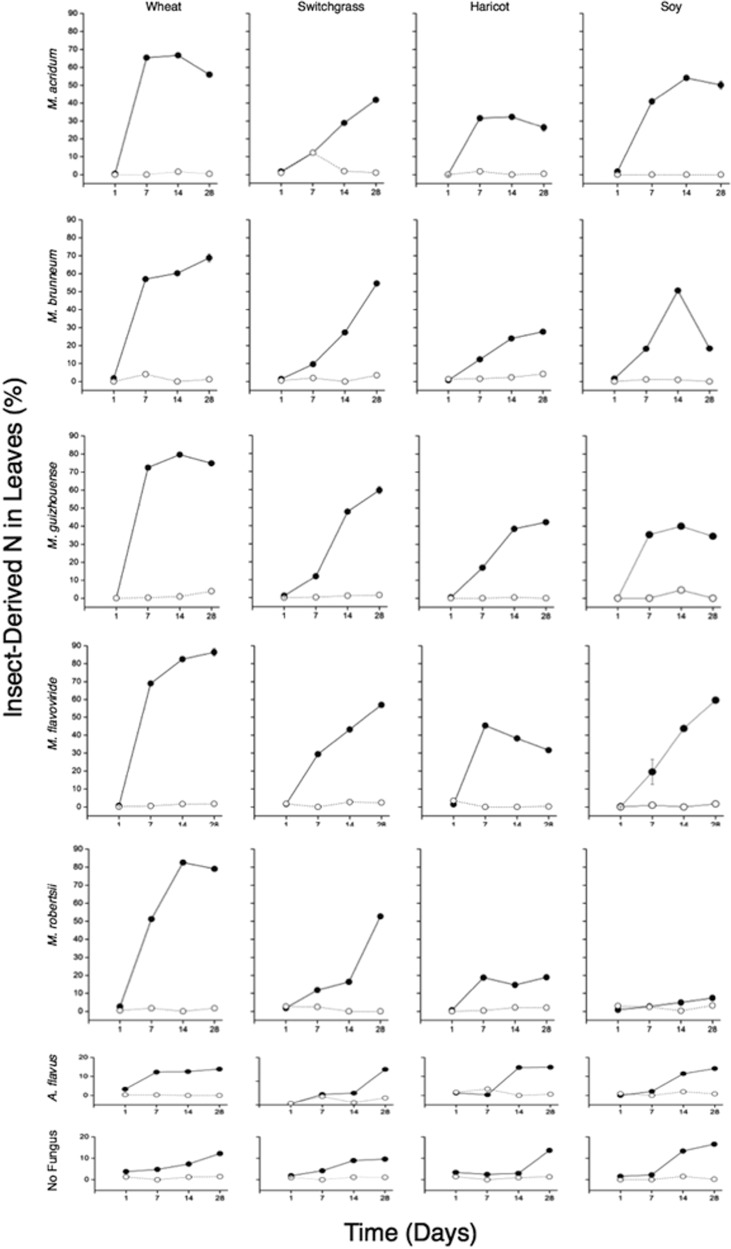
After 14 days of soybean growth in soil microcosms containing Metarhizium-infected, 15N-injected wax moth larvae, the insect-derived nitrogen levels in soybean ranged from 9% (M. robertsii) to 55% (M. acridum) (Fig. 1). At 28 days, M. flavoviride had transferred the highest levels of insect-derived nitrogen to soybean roots, with insect-derived nitrogen constituting 60% of the total plant nitrogen content (Fig. 1). With the exception of soybean plants grown in the presence of M. robertsii-infected, 15N-injected larvae, soybean plants with Metarhizium showed significantly greater levels of 15N incorporation than soybean plants grown with 15N-injected but uninfected wax moth larvae at 14 and 28 days (analysis of variance [ANOVA], P < 0.05).
FIG 1.
Percentages of plant nitrogen derived from 15N-injected wax moth larvae by five species of the endophytic, insect-pathogenic fungus Metarhizium (M. robertsii, M. acridum, M. guizhouense, M. brunneum, and M. flavoviride). Four plant species were used, wheat (Triticum aestivum), switchgrass (Panicum virgatum), soybean (Glycine max), and haricot bean (Phaseolus vulgaris). Results are means of three separate trials done in duplicate. Solid circles and open circles represent treatments with and without wax moth larvae, respectively. Amounts of insect-derived nitrogen in leaves were determined by NOI-5 emission spectrophotometer. Results were analyzed using ANOVA. Standard deviations not shown are less than 1% of the means.
The 15N incorporation into soybean plants under the experimental conditions was also compared to that in soybean plants harvested from microcosms that contained the opportunistic insect-pathogenic but nonendophytic fungus Aspergillus flavus. Again, with the exception of soybean plants grown in microcosms with M. robertsii-infected, 15N-injected insects, all experimental plants analyzed had significantly higher levels of 15N incorporation (ANOVA, P < 0.05).
(ii) Haricot bean (Phaseolus vulgaris).
All species of Metarhizium were able to transfer significant amounts of insect-derived nitrogen to haricot beans at 14 and 28 days. Plants grown in the presence of M. guizhouense-infected, 15N-injected wax moth larvae were found to contain the highest levels of insect-derived nitrogen, while M. robertsii was found to have the lowest levels of 15N incorporation (Fig. 1). However, all plants grown in soil microcosms containing 15N-labeled, Metarhizium-infected larvae had significantly higher levels of 15N incorporation than plants grown in the presence of uninfected, 15N-injected insects or with A. flavus (ANOVA, P < 0.05).
(iii) Switchgrass (Panicum virgatum).
After 14 and 28 days, all species of Metarhizium were able to transfer significant levels of insect-derived nitrogen to switchgrass. Plants grown in microcosms containing M. guizhouense had the highest 15N content (48% and 62% of total plant nitrogen at days 14 and 28, respectively) (Fig. 1). At 14 and 28 days, all switchgrass grown in the presence of Metarhizium and 15N-injected insects had significantly higher levels of 15N content than plants grown in the presence of uninfected, 15N-injected insects or A. flavus (ANOVA, P < 0.05).
(iv) Wheat (Triticum aestivum).
At 14 days, all species of Metarhizium transferred significant levels of insect-derived nitrogen to wheat. Plants grown in microcosms with 15N-injected insects infected with M. robertsii showed the highest level of 15N incorporation (82% of total nitrogen). Plants grown with M. brunneum showed the lowest levels of 15N incorporation (61%) (Fig. 1).
At 28 days, 88% of total wheat nitrogen was insect derived when the wheat was grown with 15N insects and M. flaviviridae, while wheat grown with M. acridum had the lowest levels of 15N incorporation (58%) (Fig. 1). Wheat grown in the presence of 15N-injected insects alone or in the presence of 15N-injected insects infected by A. flavus showed significantly less 15N incorporation than wheat grown under all experimental treatments (ANOVA, P < 0.05).
Beauveria bassiana and Lecanicillium lecanii. (i) Insect-derived nitrogen transfer to switchgrass (Panicum virgatum) and haricot bean (Phaseolus vulgaris).
After switchgrass and haricot bean plants had grown for 28 days in the presence of B. bassiana and 15N-injected insects, the insect-derived nitrogen content in the plants was significantly higher than in plants grown in the presence of uninfected, 15N-injected wax moth larvae alone (ANOVA, P < 0.05) (Fig. 2). Plants grown with A. flavus did not contain more than 12% 15N, which was significantly lower than the amount in plants grown under experimental conditions (ANOVA, P < 0.05).
FIG 2.
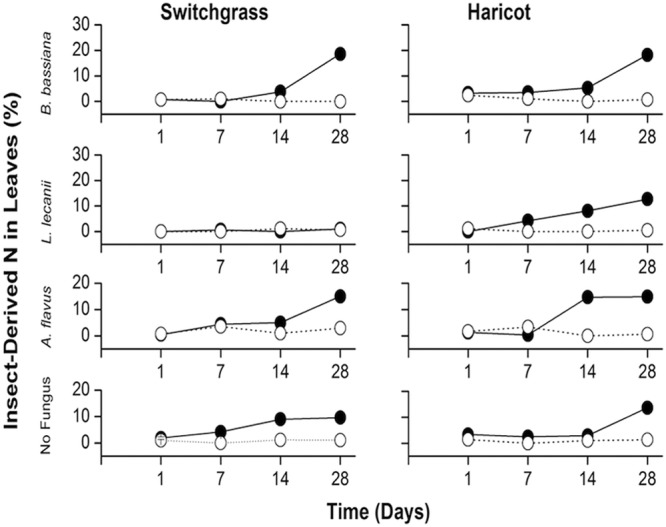
Percentages of plant nitrogen derived from 15N-injected wax moth larvae by the endophytic, insect-pathogenic fungi Beauveria bassiana and Lecanicillium lecanii. Two plant species were used, switchgrass (Panicum virgatum) and haricot bean (Phaseolus vulgaris). Results are means of three separate trials done in duplicate. Solid circles and open circles represent treatments with and without wax moth larvae, respectively. Amounts of insect-derived nitrogen in leaves were determined by NOI-5 emission spectrophotometer. Results were analyzed using ANOVA. Standard deviations not shown are less than 1% of the means.
At 7, 14, and 28 days, switchgrass and haricot bean plants grown in the presence of L. lecanii-infected, 15N-injected insects did not have significantly higher 15N content than plants grown in the presence of 15N-injected insects alone or plants grown in microcosms containing A. flavus-infected, 15N-injected wax moth larvae (ANOVA, P < 0.05) (Fig. 2).
(ii) Soil samples.
Soil samples from microcosms containing insects infected with Metarhizium, Beauveria, Lecanicillium, and Aspergillus were taken at 0, 2, 4, 6, and 8 days at a depth of 2 cm and a distance of 0.5 cm from the plant root. Metarhizium and Beauveria were found within 0.5 cm of the plant root at 4 days with an average of 15 and 3.5 CFU/gram, respectively. L. lecanii and A. flavus were found within 0.5 cm of the plant root at 6 days at an average of 13 and 29 CFU/gram of soil, respectively (all results are the means of five separate trials) (Fig. 3).
FIG 3.
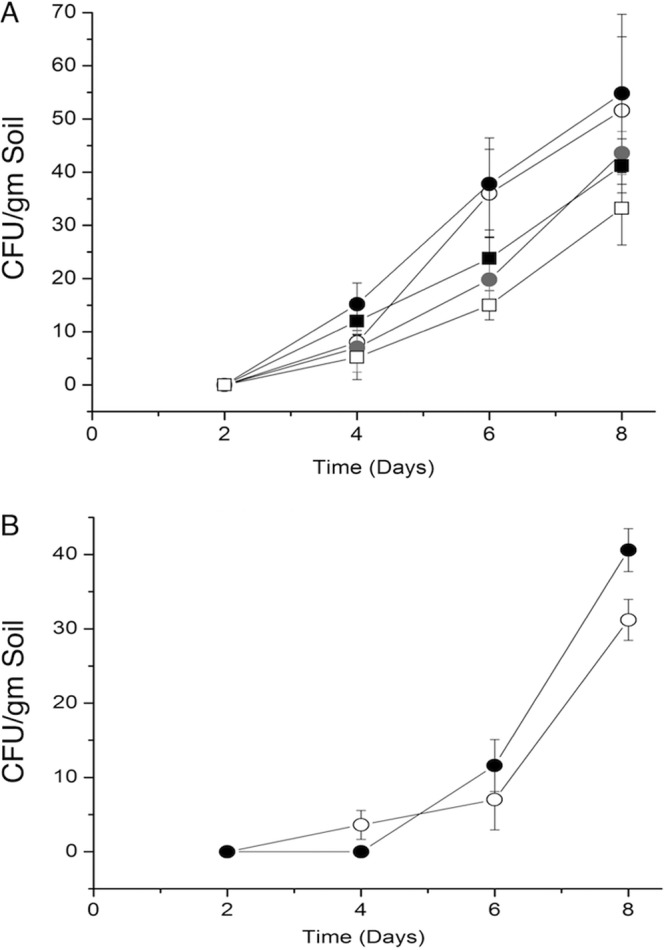
Time course of fungal propagules found per gram of soil after placement of infected insects. Soil was sampled within 0.5 cm from plant roots at a depth of 2 cm. Soil was plated on selective medium, and CFU were counted. (A) CFU of Metarhizium species per gram of soil. Open circles, M. brunneum; black circles, M. robertsii; gray circles, M. flavoviride; black squares, M. acridum; open squares, M. guizhouense. (B) CFU of Beauveria bassiana and Lecanicillium lecanii per gram of soil. Open circles, B. bassiana; closed circles, L. lecanii. Standard deviations of the means are shown. n = 5 for each time point.
(iii) GFP confocal imaging.
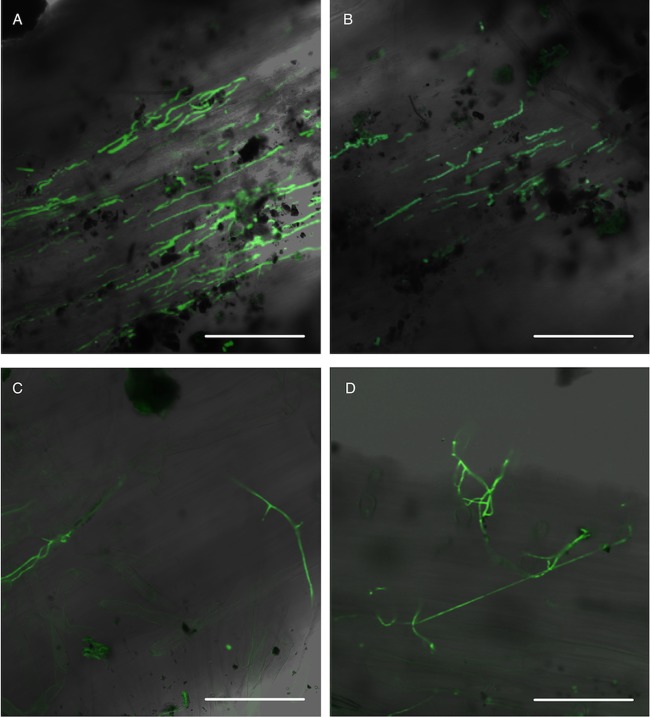
GFP-expressing M. guizhouense, M. robertsii, B. bassiana, and L. lecanii hyphae were observed on root surfaces of haricot bean (Phaseolus vulgaris) at 7 days (Fig. 4). These images, as well as the soil samples, indicated that after 1 week, M. guizhouense and M. robertsii were able to migrate to the plant and form root associations more efficiently than either B. bassiana or L. lecanii.
FIG 4.
Confocal micrographs obtained after plants had grown in soil microcosms with wax moth larvae and Metarhizium expressing GFP for 7 days. GFP micrographs of EIPF associating with plant roots at 7 days are overlaid over bright-field images. Magnification, ×400. Bars represent 10 μm. (A) Metarhizium guizhouense. (B) Metarhizium robertsii. (C) Beauveria bassiana. (D) Lecanicillium lecanii.
(iv) Nitrogen transfer under field conditions.
In field experiments, M. robertsii transferred insect-derived nitrogen to three grass species, timothy (Phleum pratense), smooth bromegrass (Bromus inermis), and orchard grass (Dactylis spp.). There was a progressive decline in the amount of insect-derived nitrogen incorporated by these grasses harvested further away from the soil-implanted source, the Metarhizium-infected, 15N-injected insects. After 28 days, the 15N contents in these grass species were 26.2%, 13.7%, and 1.0% at 5, 10, and 30 cm, respectively, from the Metarhizium-infected-, 15N-injected-insect sources (Fig. 5). The controls included insects only, as well as Metarhizium-infected, 15N-injected insects that were contained separate from plants by a 1-μm mesh. The 15N contents in field grass samples harvested after 28 days at 5 and 10 cm from the Metarhizium-infected-, 15N-injected-insect sources were significantly greater than the 15N contents in plants grown under all control treatments and harvested at the same time point at the same distances (ANOVA, P < 0.05).
FIG 5.
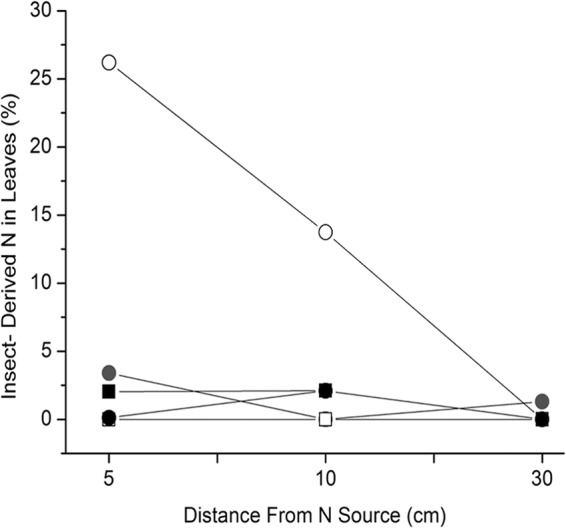
Percentages of plant nitrogen derived from 15N-injected wax moth larvae by the endophytic, insect-pathogenic fungus M. robertsii under field conditions. Three dominant grass species were present in the field site, timothy (Phleum pratense), smooth bromegrass (Bromus inermis), and orchard grass (Dactylis spp.). Open circles, 15N-labeled, M. robertsii-infected insects under 30-μm mesh; black squares, 15N-labeled, M. robertsii-infected insects under 1-μm mesh; closed circles, 15N-labeled, uninfected insects under 1-μm mesh; gray circles, 15N-labeled, uninfected insects under 30-μm mesh; white squares, grass-only controls. Results are means of two trials done in duplicate. Standard deviations are less than 1% of the means.
(v) Plant growth.
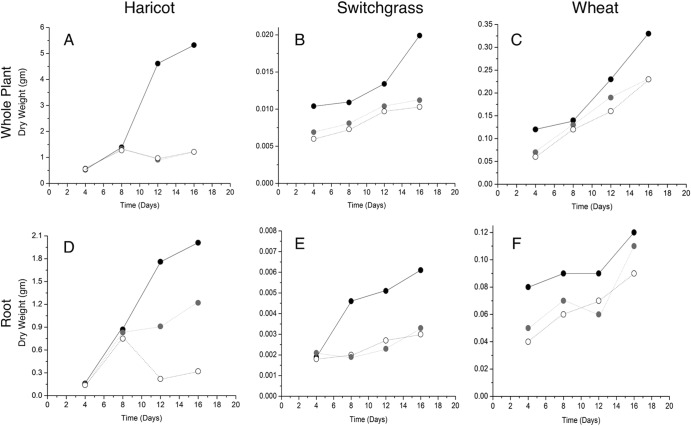
Plant growth parameters were taken every 4 days in order to ascertain the overall health of the plants under various experimental conditions. Bean plants grown in the presence of M. robertsii and infected insect larvae had greater average leaf weight, root weight, and overall plant weight than plants grown with the fungus or the insect alone (ANOVA, P < 0.05) (Fig. 6). In switchgrass and wheat grown in the presence of Metarhizium species and insect larvae, the root weight and whole-plant weight were greater than in plants grown with either the fungus or the insect alone (ANOVA, P < 0.05) (results are the mean of five separate trials) (Fig. 6).
FIG 6.
Plant growth measurements taken every 4 days for 16 days. Three plant species were used in order to ascertain overall plant health under 3 separate growth conditions. Closed circles, plants grown in the presence of Metarhizium-infected insects; open circles, plants grown in the presence of Mertarhizium only; gray circles, plants grown in the presence of insects alone. Whole-plant and root weights of the indicated plants are shown. Standard deviations are less than 1% of the mean.
DISCUSSION
Here, we found that five species of Metarhizium, as well as Beauveria bassiana, were able to endophytically associate with and transfer insect-derived nitrogen to four plant species representing fast-growing monocots and dicots.
B. bassiana shares a number of characteristics with Metarhizium, including a wide insect host range, wide habitat distribution from the arctic to the tropics (11, 12), and shown here, the ability to endophytically associate with several plant species, as well as the ability to transfer insect-derived nitrogen to plants. This overlooked branch of the soil nitrogen cycle represents a potentially large transfer of insect-derived nitrogen to plants. We calculated that there is between ca. 0.47 and 7.44 kg/hectare/year of insect-derived nitrogen provided to plants through EIPF, or approximately 4.7% of the total fixed nitrogen in a given ecosystem (see the supplemental material). This value represents a theoretical potential of EIPF in furnishing plants with insect-derived nitrogen under defined conditions. However, mitigating factors such as plant species and their growth parameters, preferential plant colonization by EIPF, nitrogen requirements, and microbial competition could influence EIPF-mediated nitrogen transfer on an ecosystem-by-ecosystem basis.
The five Metarhizium species studied here have various degrees of insect host specificity. For example, M. robertsii has a broad insect host range and can infect over 200 species of insects, whereas M. acridum is specifically virulent to acridids, such as grasshoppers and locusts (13). Despite the various insect host ranges within the genera of Metarhizium tested here, the strains tested were all able to transfer insect-derived nitrogen to plants using G. mellonella larvae as the host insect (this larva is readily infected by all fungal species tested). These results were intriguing and suggest that the insect host ranges of the Metarhizium species tested and the plant host ranges are not linked. Similarly, other endophytic fungi do not display plant host preferences and broad plant range colonization is a common feature in arbuscular mycorrhizal fungi (14). There are ca. 150 species of arbuscular mycorrhizal fungi and ca. 2,000 species of mycorrhizal fungi overall that are capable of colonizing 300,000 plant species (15). This low fungal species-to-plant host species ratio suggests that most plant-associated fungi do not have fastidious preferences with respect to their plant hosts (14). These plant symbioses, however, show a commonality with respect to the overall positive effect on plant productivity and nutrient uptake (14).
The variability of the insect-derived nitrogen transfer by EIPF to plants found in our study suggests that there may be other ecophysiological criteria with respect to efficient endophytic association and subsequent nitrogen transfer. We have alluded to this in our theoretical calculations for the value of insect-derived nitrogen provided to plants in a given ecosystem. For example, we did not find significant levels of insect-derived nitrogen transfer between M. robertsii and soybean. Potentially, M. robertsii requires specific environmental indicators from soybean in order to transfer nitrogen. Previous work with M. robertsii and soybean suggests that this may be the case. Successful soybean root colonization by M. robertsii alleviated salt-induced oxidative stress in plants (16). While this is a specific example of EIPF mitigating stress to plant partners, it indicates a potential for this unique group of fungi to aid in plant growth, development, and sustainability under a number of fluctuating soil conditions. For example, in soils with high concentrations of heavy metals, such as nickel, chromium, or cobalt, endophytes are able to increase the heavy metal tolerance of plants prone to hyperaccumulation of metals from the soil (17).
The nitrogen uptake efficiency of soybean is related to the rhizospheric pH. In soil environments where the pH is below 6, there is a significant loss of nitrogen uptake by soybean (18). The inability of M. robertsii to furnish soybean with significant levels of insect-derived nitrogen may be directly related to the pH of the soil. M. robertsii can potentially alter the pH of the rhizosphere (19). This pH change can directly affect the activity of H+-ATPase or the proton permeability of the plant cell membrane and consequently influence the amount of nitrogen absorbed by the plant (18).
Beauveria was able to transfer insect-derived nitrogen to plants, albeit at a lower rate than Metarhizium. This is potentially due to the length of time required to colonize plant roots, a variation that has been seen in other plant-associating fungi (20). The fluorescent micrographs suggest that Beauveria showed a lower rate of association than Metarhizium on the plants used in this study (Fig. 4).
The inability of L. lecanii to transfer insect-derived nitrogen to plants, despite its ability to infect insects and form endophytic associations, was surprising, since L. lecanii is similar to Metarhizium and Beauveria with respect to insect host range and endophytic competence (21, 22). We can only speculate as to the reason for this. Perhaps there may be differences in the pathways by which L. lecanii metabolizes insect-derived nitrogen. The metabolic pathways of nutrient assimilation could vary between species of insect-pathogenic fungi (23). With respect to nitrogen, we postulate that insect-derived nitrogen may be directly metabolized by the fungus and not necessarily converted to inorganic nitrogen that can be transferred to plant roots. If this is indeed the case, the symbiotic relationship that exists between L. lecanii and its plant partners may rely on the transfer of other important soil nutrients, such as phosphorus, a relationship that has been observed in some species of mycorrhizal fungi (24).
We observed increased plant health and productivity in plants grown in the presence of an EIPF-infected insect compared to plants grown in the presence of EIPF without an insect, which again had greater health and productivity than plants grown without a fungal partner. Plant photosynthate production is positively correlated with N availability. Plants require substantial amounts of N (2 to 5% N content by dry weight, compared with 0.3 to 0.5% phosphorus content), and well over 50% of leaf N is devoted to photosynthesis (25). Therefore, the acquisition of nitrogen by plants is directly related to their ability to fix carbon via photosynthesis. EIPF can act as a conduit for insect-derived nitrogen availability to plants. Several species of endophytic fungi have been shown to provide plants with nitrogen. Heteroconium chaetospira, a dark septate endophyte, was able to transfer nitrogen to the roots of cabbage plants (26). It was also found that the roots of Ranunculus adoneus colonized by endophytic fungi had increased nitrogen acquisition early in the growing season that resulted in an increased plant survival rate (27).
Our field studies suggest that nitrogen transfer by M. robertsii to plants occurred at high levels in natural environments. Root-colonizing fungi show strong competition for available root space, and this competition is predicated on carbon availability from the plant (28). However, even where competition in the rhizosphere is potentially high, Metarhizium was able to effectively transfer insect-derived nitrogen to plants. This suggests that the plants in our field experiments accommodated colonization by Metarhizium. Metarhizium has also been found in soils at relatively high concentrations compared to the concentrations of other root-colonizing microbes (29).
Metarhizium and Beauveria are ubiquitous endophytic soil fungi isolated from habitats as diverse as the arctic tundra, temperate soil, and tropical environments (6). The worldwide distribution of these fungi and their broad plant host ranges, as well as their ability to infect a broad range of insect species and to contribute to the nitrogen flux in plants, suggest that insect-derived nitrogen transfer to plants mediated by these fungi is a common feature in soil environments worldwide and is an overlooked branch of the global soil nitrogen cycle.
Conclusion.
In most ecosystems, nitrogen is the major limiting factor for plant growth and productivity. Furthermore, insect herbivory exacerbates nitrogen deprivation since it removes nitrogen from plants. Here, we show a novel mechanism by which plants form a symbiotic relationship with the EIPF Metarhizium and Beauveria and reacquire this lost nitrogen. These fungi are globally ubiquitous in soil ecosystems and are able to infect a wide range of insects. Potentially, there are a number of other insect-pathogenic fungi with plant-associative abilities, specifically within the Clavicipitaceae. The potential for nitrogen transfer to plants may extend to fungi like Pochonia and Rotiferophthora that infect insects, nematodes, or rotifers (30). Within the Clavicipitaceae, there are examples of interkingdom host jumping, potentially allowing other arthropod-pathogenic fungi to associate with plants (30). Conversely, most of the zygomycetous fungi that are insect pathogenic are obligate insect pathogens and show no evidence for plant colonization (30). Our results suggest that EIPF play an important role in the ecological cycling of insect nitrogen back to plant communities.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kevin Liao (University of Maryland) for supplying green fluorescent protein-transformed M. guizhouense.
This research was conducted with the assistance of a Natural Sciences and Engineering Research Council of Canada Discovery grant to M.J.B.
Footnotes
Published ahead of print 13 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03338-13.
REFERENCES
- 1.Cocking EC. 2003. Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil 252:169–175. 10.1023/A:1024106605806 [DOI] [Google Scholar]
- 2.Malloch DW, Pirozynski KA, Raven PH. 1980. Ecological and evolutionary significance of mycorrhizal symbioses in vascular plants. Proc. Natl. Acad. Sci. U. S. A. 77:2113–2118. 10.1073/pnas.77.4.2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fellbauma CR, Gachomo EW, Beesetty Y, Choudharib S, Strahan GD, Pferrer PE, Toby Kiers E, Bucking H. 2012. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. U. S. A. 109:2666–2671. 10.1073/pnas.1118650109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovett M, Christenson LM, Groffman PM, Jones CG, Hart JE, Mitchell MJ. 2002. Insect defoliation and nitrogen cycling in forests. Bioscience 52:335–341. 10.1641/0006-3568(2002)052[0335:IDANCI]2.0.CO;2 [DOI] [Google Scholar]
- 5.Behie SW, Zelisko PM, Bidochka MJ. 2012. Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 336:1576–1577. 10.1126/science.1222289 [DOI] [PubMed] [Google Scholar]
- 6.Hajek AE, St Leger RJ. 1994. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 39:293–322. 10.1146/annurev.en.39.010194.001453 [DOI] [Google Scholar]
- 7.Milner RJ. 1991. Biological control of locusts and grasshoppers, p 200–207 In Lomer CJ, Prior C. (ed), Annual review of entomology. CAB International, Wallingford, United Kingdom: [DOI] [PubMed] [Google Scholar]
- 8.Roberts DW, Hajek AE. 1992. Entomopathogenic fungi as bioinsecticides, p 144–159 In Leathan GF. (ed), Frontiers in industrial mycology. Chapman & Hall, New York, NY [Google Scholar]
- 9.Wyrebek M, Huber C, Sasan RK, Bidochka MJ. 2011. Three sympatrically occurring species of Metarhizium show plant rhizosphere specificity. Microbiology 157:2904–2911. 10.1099/mic.0.051102-0 [DOI] [PubMed] [Google Scholar]
- 10.Fang W, Pei Y, Bidochka MJ. 2006. Transformation of Metarhizium anisopliae mediated by Agrobacterium tumefaciens. Can. J. Microbiol. 52:623–626. 10.1139/w06-014 [DOI] [PubMed] [Google Scholar]
- 11.Humber RA. 1992. Collection of entomopathogenic fungal cultures: catalog of strains. Publication no. ARS-110. U.S. Department of Agriculture, Agricultural Research Service, Beltsville, MD [Google Scholar]
- 12.Samson RA, Evans HC, Latgé JP. 1988. Atlas of entomopathogenic fungi. Springer Verlag, Berlin, Germany [Google Scholar]
- 13.Wang C, St Leger RJ. 2005. Developmental and transcriptional responses to host and nonhost cuticles by the specific locust pathogen Metarhizium anisopliae var. acridum. Eukaryot. Cell 4:937–947. 10.1128/EC.4.5.937-947.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klironomos JN, McCune J, Hart M, Neville J. 2000. The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity. Ecol. Lett. 3:137–141. 10.1046/j.1461-0248.2000.00131.x [DOI] [Google Scholar]
- 15.Klironomos JN. 2000. Host-specificity and functional diversity among arbuscular mycorrhizal fungi, p 845–851 In Bell CR, Brylinsky M, Johnson-Green P. (ed), Microbial biosystems: new frontiers. Proceedings of the 8th International Symposium on Microbial Ecology. Atlantic Canada Society for Microbial Ecology, Halifax, Canada http://plato.acadiau.ca/isme/Symposium26/klironomos.PDF [Google Scholar]
- 16.Khan AL, Hamayun M, Khan SA, Kang SM, Shinwari ZK, Kamran M, Rehman S, Kim JG, Lee IJ. 2012. Pure culture of Metarhizium anisopliae LHL07 reprograms soybean to higher growth and mitigates salt stress. World J. Microbiol. Biotechnol. 28:1483–1494. 10.1007/s11274-011-0950-9 [DOI] [PubMed] [Google Scholar]
- 17.Hegg A, Angle JS, Chaney RL. 1990. Effects of vesicular-arbuscular fungi on heavy metal uptake by soybeans. Soil Biol. Biochem. 22:865–869. 10.1016/0038-0717(90)90169-Z [DOI] [Google Scholar]
- 18.Hawkins BJ, Robbins S. 2010. pH affects ammonium, nitrate and proton fluxes in the apical region of conifer and soybean roots. Physiol. Plant. 138:238–247. 10.1111/j.1399-3054.2009.01317.x [DOI] [PubMed] [Google Scholar]
- 19.St Leger RJ, Nelson JO, Screen SE. 1999. The entomopathogenic fungus Metarhizium anisopliae alters ambient pH, allowing extracellular protease production and activity. Microbiology 145:2691–2699 [DOI] [PubMed] [Google Scholar]
- 20.Graham JH, Linderman RG, Menge JA. 1982. Development of external hyphae by different isolates of mycorrhizal Glomus spp. in relation to root colonization and growth of troyer citrange. New Phytol. 91:183–189. 10.1111/j.1469-8137.1982.tb03304.x [DOI] [Google Scholar]
- 21.Krauss U, Hidalgo E, Arroyo C, Piper SR. 2004. Interaction between the entomopathogens Beauveria bassiana, Metarhizium anisopliae and Paecilomyces fumosoroseus and the mycoparasites Clonostachys spp., Trichoderma harzianum and Lecanicillium lecanii. Biocontrol Sci. Technol. 14:331–346. 10.1080/09583150410001665196 [DOI] [Google Scholar]
- 22.Gurulingappa P, McGee PA, Sword G. 2011. Endophytic Lecanicillium lecanii and Beauveria bassiana reduce the survival and fecundity of Aphis gossypii following contact with conidia and secondary metabolites. Crop Prot. 30:349–353. 10.1016/j.cropro.2010.11.017 [DOI] [Google Scholar]
- 23.Marzful GA. 1997. Genetic regulation of nitrogen metabolism in the fungi. Mol. Biol. Rev. 61:17–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George E, Marschner H, Jakobsen I. 1995. Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit. Rev. Biotechnol. 15:257–270. 10.3109/07388559509147412 [DOI] [Google Scholar]
- 25.Field C, Mooney HA. 1986. The photosynthesis-nitrogen relationship in wild plants, p 25–55 In Givnish TJ. (ed), On the economy of plant form and function. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 26.Usuki F, Narisawa K. 2007. A mutualistic symbiosis between a dark septate endophytic fungus, Heteroconium chaetospira, and a nonmycorrhizal plant, Chinese cabbage. Mycologia 99:175–184. 10.3852/mycologia.99.2.175 [DOI] [PubMed] [Google Scholar]
- 27.Mullen RB, Schmidt SK, Jaeger CH. 1998. Nitrogen uptake during snowmelt by the snow buttercup, Ranunculus adoneus. Arct. Alp. Res. 0:121–125 [Google Scholar]
- 28.Pearson JN, Abbott LK, Jasper DA. 1993. Mediation of competition between two colonizing VA mycorrhizal fungi by the host plant. New Phytol. 123:93–98 [DOI] [PubMed] [Google Scholar]
- 29.Bruck DJ. 2004. Natural occurrence of entomopathogens in Pacific Northwest nursery soils and their virulence to the black vine weevil, Otiorhynchus sulcatus (F.) (Coleoptera: Curculionidae). Environ. Entomol. 33:1335–1343. 10.1603/0046-225X-33.5.1335 [DOI] [Google Scholar]
- 30.Humber RA. 2008. Evolution of entomopathogenicity in fungi. J. Invertebr. Pathol. 98:262–266. 10.1016/j.jip.2008.02.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.