Abstract
The increasing tolerance to currently used fungicides in both clinical and agricultural areas is of great concern. The nonconventional light-based approach of antimicrobial photodynamic treatment (APDT) is a promising alternative to conventional fungicides. We evaluated the effects of APDT with four phenothiazinium derivatives (methylene blue [MB], new methylene blue N [NMBN], toluidine blue O [TBO], and the novel pentacyclic phenothiazinium photosensitizer [PS] S137) on conidia of three fungal species (Colletotrichum acutatum, Colletotrichum gloeosporioides, and Aspergillus nidulans). The efficacy of APDT with each PS was determined, initially, based on photosensitizer MICs. Additionally, the effects of APDT with two selected PSs (NMBN and S137) on survival of conidia were evaluated. The subcellular localization of the PS in C. acutatum conidia was determined. The effects of photodynamic treatments on leaves of the plant host Citrus sinensis were also investigated. APDT with S137 showed the lowest MIC. MICs for S137 were 5 μM for the three fungal species when a fluence of 25 J cm−2 was used. APDT with NMBN (50 μM) and S137 (10 μM) resulted in a reduction in the survival of the conidia of all species of approximately 5 logs with fluences of ≥15 J cm−2. Washing of the conidia before light exposure did not prevent photodynamic inactivation. Both NMBN and S137 accumulated in cytoplasmic structures, such as lipid bodies, of C. acutatum conidia. No damage to orange tree leaves was observed after APDT.
INTRODUCTION
The control of plant-pathogenic fungal species faces some of the problems that have been observed in the related clinical area, including the selection of antifungal-tolerant strains and the relatively few classes of currently available and effective fungicides (1–5). Additionally, contamination of agricultural products and the environment due to overuse and/or inappropriate use of fungicides is a matter of major concern.
Colletotrichum is a large genus of ascomycete fungi containing several species that are common pathogens of a wide array of crops and noncultivated plant species (6, 7). Colletotrichum acutatum and Colletotrichum gloeosporioides are among the most pathogenic species of this genus and cause economically important losses of temperate, subtropical, and tropical fruits worldwide (7). Disease symptoms range from fruit rot to shoot, leaf, and flower blight. Common hosts include many eudicotyledonous plants such as strawberry, apple, citrus, and stone fruits (6).
During the asexual stage of their life cycles, C. acutatum and C. gloeosporioides produce abundant unicellular hyaline conidia (6, 7). Conidia are specialized structures responsible for the dispersion, environmental persistence, and/or host infection of many fungal species (6, 8–10). After being produced, Colletotrichum conidia remain attached to each other by a mucilage and are dispersed over short distances by rain splash after the mucilage has been dissolved by water, so rates of infection are usually highest during the wettest periods of the growing season (7).
Current management strategies for these fungi are based mainly on the intensive use of fungicides. Even so, control failure is common because of the selection of tolerant strains (1–5, 11). Increasing tolerance to currently used fungicides has stimulated the development of new strategies to control pathogenic fungi. Antimicrobial photodynamic treatment (APDT) is an alternative and promising antifungal discovery platform that can be used to control localized mycoses in animal hosts or to kill fungi in the environment (12–22). The approach is based on the use of a photosensitizer (PS) that binds to the surface or preferentially accumulates in the target fungal cell (13, 14, 17, 20). Subsequent exposure of the PS to light of an appropriate wavelength starts a photochemical process that produces several reactive oxygen species (ROS), such as peroxides and singlet oxygen, leading to nonspecific oxidative damage and causing the subsequent death of the fungal cells without significant harm to the host (14, 19, 20). In comparison with currently used fungicides, the multiple and variable targets of reactive oxygen species reduce the chance of selecting tolerant microorganisms. An additional advantage of APDT is that unlike many conventional fungicides that kill only metabolically active cells, it is able to kill both metabolically active and dormant or quiescent structures such as conidia (16, 20).
Previous studies have reported the use of APDT with different PSs to kill fungi of several genera, including Aspergillus, Candida, Cryptococcus, Metarhizium, and Trichophyton (15–17, 20). Data regarding the photodynamic inactivation of plant-pathogenic fungi are scarce, and most of those studies were performed by using natural compounds as PSs. Additionally, as far as we know, none of those studies evaluated the effects of APDT on the plant host. DiCosmo et al. (23) reported the in vitro effects of APDT on Alternaria alternata, Aspergillus niger, Cladosporium variable, Colletotrichum spp., Rhizopus nigricans, Pythium aphanidermatum, and Saprolegnia spp. using naturally occurring thiophene derivatives. Similarly, Bourque et al. (24) reported in vitro APDT against Fusarium culmorum and Aspergillus flavus using phenylheptatriyne from Bidens pilosa. Interestingly, Lukšienė et al. (25) also described APDT against Aspergillus flavus, Trichothecium roseum, Fusarium avenaceum, and Rhizopus oryzae using the standard anticancer photodynamic treatment (PDT) agent hematoporphyrin dimethyl ether as a PS.
Prerequisites for using APDT to control plant-pathogenic fungi include the identification of effective PSs against different fungal species and the evaluation of side effects of the treatment on the host plants. The objective of this initial study was to assess the efficacies of APDT with four phenothiazinium derivatives (methylene blue [MB], new methylene blue N zinc chloride double salt [NMBN], toluidine blue O [TBO], and the novel pentacyclic phenothiazinium photosensitizer S137) on conidia of the plant-pathogenic fungi C. acutatum and C. gloeosporioides and on conidia of the model ascomycete Aspergillus nidulans. These derivatives have already been shown by us to be highly effective photosensitizers against Candida and Trichophyton spp. in vitro (15, 16). In an attempt to improve the understanding of the mechanisms involved in conidial photoinactivation, subcellular photosensitizer localization and the effects of treatment on the cell structures of C. acutatum conidia were investigated. The effects of APDT with all PSs on the leaves of sweet orange (Citrus sinensis) were also determined.
MATERIALS AND METHODS
Fungal species and strains.
Colletotrichum acutatum strain CA 142 from citrus was obtained from the Plant Pathogenic Fungi Collection of the Department of Phytopathology and Nematology (Escola Superior de Agricultura Luiz de Queiroz, University of São Paulo, Piracicaba, Brazil). C. gloeosporioides strain CPC 20935 from avocado was obtained from the Centraalbureau voor Schimmelcultures (CBS) (Utrecht, Netherlands). Aspergillus nidulans strain ATCC 10074 was obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA).
Photosensitizers.
MB (catalog number M9140), NMBN (catalog number 202096), and TBO (catalog number T3260) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). The novel pentacyclic phenothiazinium photosensitizer S137 was synthesized as previously described (26). Stock solutions of all the PSs were prepared with phosphate-buffered saline (PBS) (pH 7.4) at concentrations 10-fold higher than the highest concentration used. The solutions were stored in the dark at −20°C for up to 2 weeks. Dilutions were prepared with PBS (pH 7.4). Chemical structures of all the PSs are shown in Fig. S1 in the supplemental material.
Visible light source and light measurements.
All light (light-emitting diode [LED] and solar radiation) measurements were performed by using a cosine-corrected irradiance probe (CC-3-UV; Ocean Optics, Dunedin, FL, USA) screwed onto the end of an optical fiber coupled to a USB4000 spectroradiometer (Ocean Optics, Dunedin, FL). Red light was provided by an array of 600 LEDs with an emission peak at 634 nm (LED600). The LED array was made in-house by using a 5-mm, high-brightness, 7,000- to 8,000-mcd LED (ZX Lecomp, São Paulo, Brazil) and allowed homogenous illumination of the surface of a 96-well microtiter plate. The integrated irradiance in the red region of the visible spectrum (550 to 750 nm) was 9.2 mW cm−2. Light was measured inside the well, at the sample level, to reduce interference.
Solar spectral irradiance was measured under a clear sky at midday on 8 May 2013 (midautumn) and 5 June 2013 (late autumn). In midautumn, integrated irradiances in the red region of the visible spectrum and in the UV spectrum (290 to 390 nm) were approximately 20.7 and 3.7 mW cm−2, respectively, and in late autumn, irradiances were approximately 18 and 2.9 mW cm−2, respectively. LED and midday solar visible (400- to 790-nm) spectral irradiances are shown in Fig. 1A, and solar UV spectral irradiance (280 to 400 nm) is shown in Fig. 1B. Figure 1C shows the absorption spectra of the dyes (10 μM in PBS [pH 7.4]) measured with an Ultraspec 2100 Pro UV-visible spectrophotometer (GE Healthcare, Germany).
FIG 1.
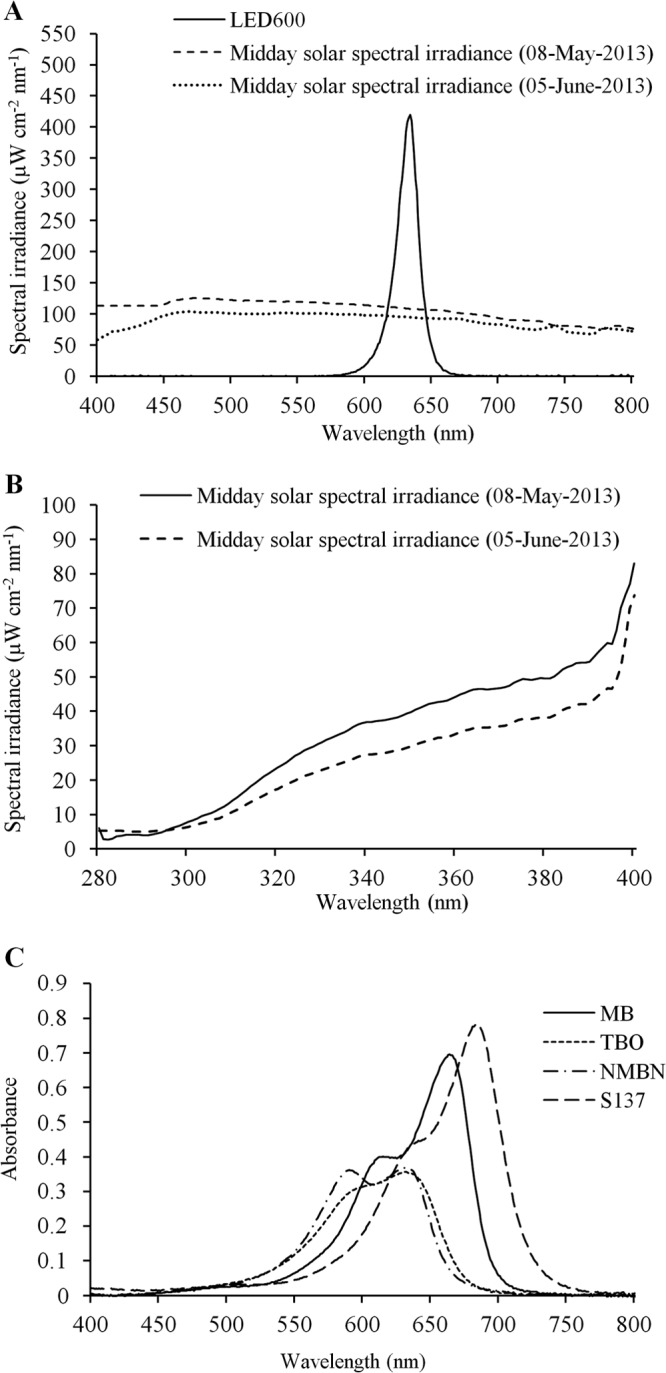
Spectral irradiances of light sources. (A) LED system and midday solar visible (400- to 800-nm) spectral irradiances. (B) Midday solar UV (280- to 400-nm) spectral irradiance. (C) Visible absorption spectra for the photosensitizers (10 μM) employed in this study.
Fungal growth and production of conidia.
All fungi were grown on 23 ml Difco potato dextrose agar (Becton, Dickinson and Company, Sparks, MD, USA) supplemented with 1 g liter−1 Bacto yeast extract (BD) (PDAY) in petri dishes (90 by 10 mm) at 28°C for 5 days with 12-h (dark/light) photoperiods. Conidia were carefully scraped from the colonies and suspended in a 0.01% (vol/vol) Tween 80 (Sigma-Aldrich) solution. The concentration of conidia was determined with a hemocytometer, and appropriate dilutions were made with the same solution.
Evaluation of APDT efficacies on fungal conidia based on PS MIC.
MIC-based experiments were performed, as previously described (15, 16), to determine the most effective PS and the optimized conditions for APDT. Experiments were performed in 96-well flat-bottomed microtiter plates (TPP, Switzerland). Fifty microliters of the fungal cell suspension and 50 μl of the PS solution (MB, NMBN, TBO, or S137) were added to each well. The final concentration of the conidia in the mixture was 4 × 104 cells ml−1; the final concentrations of MB, NMBN, and TBO were 0, 1, 2.5, 5, 10, 12.5, 25, 50, 75, 100, and 200 μM; and the final concentrations of S137 were 0, 0.5, 1, 2.5, 5, 10, 12.5, 25, 30, 40, 50, and 75 μM. Plates were held in the dark for 30 min at 28°C and exposed to light fluences of 5, 10, 15, 20, 25, and 30 J cm−2 using the LED array as a light source or alternatively kept in the dark to provide dark controls. Fluences resulted from exposures of 9, 18, 27, 36, 45, and 54 min, respectively. After the exposures, 100 μl of RPMI 1640 culture medium (Gibco, Invitrogen Corporation, NY, USA) (2-fold concentrate) was added to each well, and plates were incubated at 28°C. Growth was evaluated after 48, 72, and 96 h by visual inspection when the MICs were determined (see Fig. S2 in the supplemental material). The MIC was considered the minimal PS concentration (for each fluence) in which total growth inhibition was achieved. Three independent experiments were performed.
Effect of APDT with NMBN or S137 and red light on conidial survival.
Based on previous MIC experiments, the optimized conditions for APDT were established, and the effects of the treatments on the survival of C. acutatum, C. gloeosporioides, and A. nidulans conidia were evaluated. Five hundred microliters of the conidial suspension and 500 μl of the PS solution (NMBN or S137) were added to a 1.5-ml tube (polypropylene; Axygen Scientific, CA, USA). Final concentrations of conidia and PS in the mixtures were 2 × 106 cells ml−1 and 50 μM NMBN or 10 μM S137, respectively. Tubes were held in the dark for 30 min at 28°C, and after that, they were divided into two groups. In one group, conidia were washed to remove unbound PS, and in the other group, cells were not washed before light exposure. To remove unbound PS, cells were washed three times (centrifuged at 5,000 × g for 5 min and resuspended in Tween 80 solution [0.01%, vol/vol]). Nonwashed and washed conidia (200 μl; 2 × 106 cells ml−1) were placed into a 96-well flat-bottomed microtiter plate and exposed to fluences of 0 J cm−2 (dark control), 15 J cm−2, 20 J cm−2, and 25 J cm−2. After light exposure, suspensions were removed and serially diluted 10-fold in PBS to give dilutions of 10−1 to 10−3 times the original concentration, and 50 μl was spread onto the surface of 5 ml of PDAY medium containing 0.08 g liter−1 of deoxycholic acid sodium salt (Fluka, Italy) in petri dishes (60 by 15 mm). At this concentration, the salt makes the colonies grow more slowly and more compactly, allowing the evaluation of fungal growth over several days. Three replicate dishes were prepared for each treatment in each of three independent experiments. The dishes were incubated in the dark at 28°C. After 24 h, CFU were counted daily at a ×8 magnification for up to 7 days. The effects of the different treatments on conidial survival were determined based on survival fractions, as previously described (15, 16). For each trial, the light effect was calculated as M−P+L/M−P−L, where M−P+L is the mean number of CFU of the three replicate dishes in which conidia were exposed to light only and M−P−L is the mean number of CFU of the replicates in which conidia were not treated with the PS or exposed to light; the PS effect (dark toxicity) was calculated as M+P−L/M−P−L, where M+P−L is the mean number of CFU of the replicate dishes in which conidia were treated with the PS but not exposed to light; and the APDT effect was calculated as M+P+L/M−P−L, where M+P+L is the mean number of CFU of the replicate dishes in which conidia were treated with the PS and exposed to light.
Effects of APDT with NMBN or S137 and full-spectrum solar radiation on conidial survival.
To examine the efficacy of photodynamic treatment under field conditions, we also evaluated the effects of APDT with NMBN or S137 and sunlight on conidial survival. One milliliter of the conidial suspension and 1 ml of PS solution (NMBN or S137) were added to a 10-ml glass tube (diameter, 13 mm) (Schott GL18). Final concentrations of conidia and PS in the mixtures were 2 × 106 cells ml−1 and 50 μM NMBN or 10 μM S137, respectively. Tubes were held in the dark for 30 min at 28°C, and the mixtures were transferred onto 24-well flat-bottomed microtiter plates. To avoid contamination, plates were covered with a solar radiation-transparent material (0.13-mm-thick premium cellulose triacetate; Liard Plastics, Salt Lake City, UT) and exposed to sunlight for 1 and 2 h floating in water at 22°C ± 2°C. Three different types of control plates were prepared in all experiments: (i) control plates in which conidia were exposed to solar radiation but not treated with a PS (−P+L), (ii) control plates in which conidia were treated with a PS but were protected from solar radiation during exposure (plates were wrapped in aluminum foil) (+P−L), and (iii) control plates in which conidia were not treated with a PS and were protected from solar radiation (−P−L). The effects of the treatments on conidial survival were determined as described above. The solar radiation effect was calculated as M−P+L/M−P−L, the PS effect (dark toxicity) was calculated as M+P−L/M−P−L, and the APDT effect was calculated as M+P+L/M−P+L. Two experiments with NMBN were performed on 16 April 2013, and two were performed on 23 April 2013 (midautumn). The same was done with S137 on 13 and 19 June 2013 (late autumn). All the experiments were performed under a clear sky between 11 a.m. and 1 p.m. in Ribeirão Preto, SP, Brazil (21°10′S latitude at a 560-m elevation).
Evaluation of NMBN and S137 efficacies in APDT after exposure to solar radiation.
The exposure of photosensitizers to high irradiances can reduce their activity. We evaluated the effects of exposures to full-spectrum solar radiation on NMBN and S137 absorption spectra and on their efficacy in killing C. acutatum conidia in APDT. The efficacy of APDT with NMBN and S137 was evaluated after both PSs had been previously exposed to solar radiation for up to 12 h. Ten milliliters of NMBN and S137 solutions (100 μM and 20 μM, respectively) was placed into petri dishes (60 by 15 mm) whose lids were replaced by premium cellulose triacetate. Plates were exposed to solar radiation for 0, 0.5, 1, 3, 6, 9, and 12 h floating in water at 22°C ± 2°C. Two solutions of each PS were exposed under a clear sky on 15 April 2013 (early autumn). At the end of the exposures, the volumes of the solutions were readjusted to 10 ml with PBS (pH 7.4). All subsequent dilutions were also made with PBS. Five hundred microliters of the PS solution (NMBN or S137) and 500 μl of the conidial suspension were added to a 1.5-ml tube. Final concentrations of conidia and the PS in the mixtures were 2 × 106 cells ml−1 and 50 μM NMBN or 10 μM S137, respectively. Tubes were held in the dark for 30 min at 28°C, and after this, nonwashed conidia (300 μl; 2 × 106 cells ml−1) were placed into a 96-well flat-bottomed microtiter plate and exposed to fluences of 0 J cm−2 (dark control) and 15 J cm−2, using the LED array as the light source. The effect of APDT on conidial survival was determined as described above. Two experiments were performed with each of the PS-exposed solutions.
Statistical analysis.
The effects of the APDT with NMBN or S137 and red light on conidial survival were assessed by one-way analysis of variance (ANOVA). A posttest was performed by using orthogonal contrasts (27). The effects of APDT with NMBN or S137 and solar radiation on conidial survival (field experiments) were compared by using a mixed linear model. This model is used for the analysis of data on which the responses are grouped and the assumption of the independence between observations in the same group is not adequate (28). The posttest was performed by using orthogonal contrasts. The level of significance was 0.05 (P < 0.05). All analyses were carried out by using PROC MIXED in SAS version 9.0 (SAS Institute, Inc., Cary, NC, USA).
Conidial microscopy studies.
Microscopic studies were performed to determine the subcellular localization of the PS. Conidia of C. acutatum (2 × 106 conidia ml−1) were treated with NMBN (10, 50, and 100 μM) or S137 (1, 10, 50, and 100 μM) and washed or not washed before being observed at a ×1,000 magnification by using a Leica DMD5000 B fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany). In an attempt to identify the cellular structures in which the PS accumulated, conidia were also stained with the dye Sudan III (0.5% [wt/vol] in 2:8 distilled water-ethanol) and the fluorescent dyes 4′,6-diamidino-2-phenylindole (DAPI) (Sigma) (3.5 mM in distilled water) and FM4-64 (Invitrogen) (8 μM in a 1% [vol/vol] dimethyl sulfoxide [DMSO] solution), which have affinity for lipids, genetic material, and cellular membranes, respectively. Conidia were treated with each of the three dyes, kept in the dark for 30 min, and washed three times with PBS (5,000 × g for 5 min) before being observed at a ×1,000 magnification.
Effects of PDT on leaves of orange trees (Citrus sinensis).
Plants used were about 1.5 m tall. Five microliters of MB, TBO, NMBN, and S137 at a concentration of 50 μM was spotted on the adaxial surface of the leaves. After application of the PS, plants were kept outdoors under a natural sunlight regimen. Plants were visually evaluated for damage to the leaves daily for up to 15 days. Experiments were conducted in April and November 2012 in Ribeirão Preto, SP, Brazil. Three experiments were performed.
Microscopic studies of orange tree leaves.
Microscopic studies were performed to determine if the PS penetrated the leaves after being spotted onto the leaf adaxial surface. Ten microliters of a 100 μM solution of the PSs NMBN and S137 was spotted onto the adaxial surface, and the leaves were kept at 24°C until the PS had dried. After this, leaves were cross-cut in the spotted area by using a table microtome (Roble, Rolemberg & Bhering, Belo Horizonte, Brazil). Histological inspection was carried out at a ×200 magnification by using a Leica DM5000 B microscope. Sections of the leaves were treated with both NMBN and S137 in order to determine the appearance of the leaf tissues had the PS penetrated and spread throughout the structure.
RESULTS
Evaluation of APDT efficacies against C. acutatum, C. gloeosporioides, and A. nidulans conidia based on MIC.
Exposure to red light in the absence of the PS did not inhibit the growth of any species regardless of the fluence applied (data not shown). Treatments with MB, NMBN, and TBO in the absence of light, at concentrations of up to 200 μM (which was the highest concentration tested), did not inhibit the growth of any of the species (Table 1). S137 was more toxic in the dark and inhibited the growth of all three species at 75 μM (Table 1). Treatment with each of the PSs at all fluences inhibited the growth of all species (the only exceptions were MB and TBO at a fluence of 5 J cm−2 against C. gloeosporioides). The MICs for all PSs and fluences are shown in Table 1. While the MICs varied among both PSs and species, for most of the treatments, the MIC decreased with increasing fluence. S137 was the most effective PS for all the species at all fluences. APDT with S137 inhibited the growth of all species at 5 μM and 25 J cm−2.
TABLE 1.
MIC of PSs MB, TBO, NMBN, and S137 for Colletotrichum acutatum, C. gloeosporioides, and Aspergillus nidulansa
| Species | PS | MIC (μM) at fluence of: |
||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 J cm−2 | 10 J cm−2 | 15 J cm−2 | 20 J cm−2 | 25 J cm−2 | 30 J cm−2 | ||
| C. acutatum | MB | >200 | 200 | 200 | 100–200 | 75–200 | 100–200 | 50–200 |
| TBO | >200 | 200 | 200 | 50–100 | 75–100 | 75–100 | 12.5–75 | |
| NMBN | >200 | 100 | 75 | 50 | 25–50 | 25–50 | 10–50 | |
| S137 | 75 | 25 | 10–12.5 | 5–12.5 | 5–10 | 5 | 5 | |
| C. gloeosporioides | MB | >200 | >200 | 200 | 200 | 100–200 | 100–200 | 75–200 |
| TBO | >200 | >200 | 200 | 50–150 | 50–100 | 75–100 | 12.5–75 | |
| NMBN | >200 | 200 | 75–100 | 50 | 25–75 | 12.5–75 | 10–50 | |
| S137 | 75 | 12.5–50 | 5–12.5 | 5–12.5 | 5–10 | 5 | 5 | |
| A. nidulans | MB | >200 | 75–200 | 50–200 | 75 | 75 | 75–100 | 50–100 |
| TBO | >200 | 75 | 25–75 | 25–75 | 10–25 | 10–25 | 5–12.5 | |
| NMBN | >200 | 50–100 | 50 | 50 | 25–50 | 25–75 | 12.5–25 | |
| S137 | 75 | 25 | 10–12.5 | 5–12.5 | 5–10 | 5 | 5 | |
Results are MIC ranges from three independent experiments.
Effects of APDT with NMBN or S137 and red light on conidial survival.
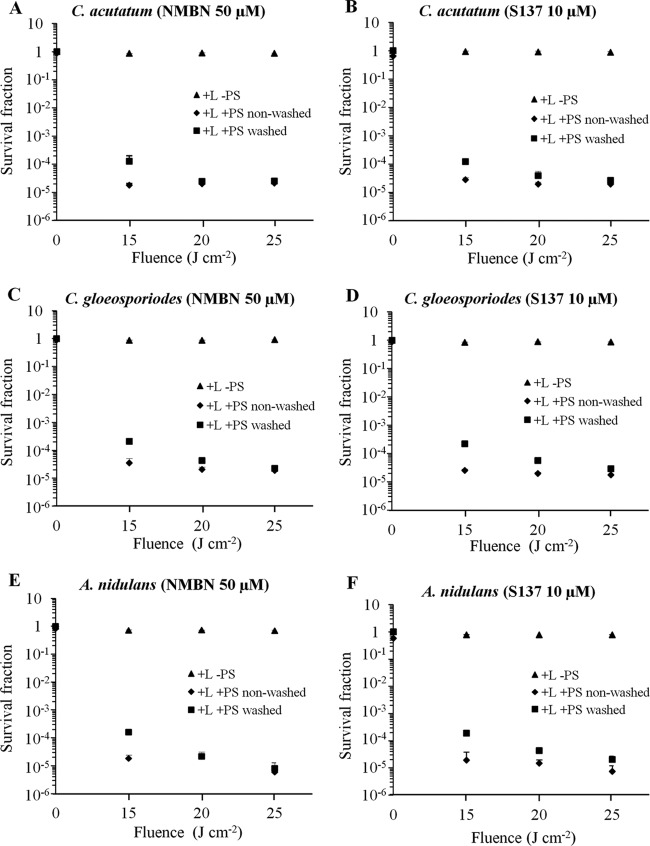
The effects of APDT with NMBN and S137 on the three species are shown in Fig. 2. Conidia were incubated with the PS (50 μM NMBN and 10 μM S137) for 30 min and washed or not washed before being exposed to fluences of 15, 20, and 25 J cm−2. Exposure to light alone (in the absence of PS) did not kill conidia of any species (P > 0.05 for all fungal species and fluences) (Fig. 2). In the absence of light (i.e., dark toxicity), NMBN at 50 μM did not kill conidia of any species either (P > 0.05 for all species). Treatment with only S137 at 10 μM killed approximately 28, 14, and 30% of the conidia of C. acutatum, C. gloeosporioides, and A. nidulans, respectively (P = 0.05, 0.23, and 0.07 for C. acutatum, C. gloeosporioides, and A. nidulans, respectively). Photodynamic killing of conidia was observed for both PSs and for all species at all fluences (P < 0.01 for all treatment comparisons) (Fig. 2). APDT with NMBN (50 μM) or with S137 (10 μM) and at a fluence of 15 J cm−2 resulted in an approximately 5-log reduction in the survival of conidia of all three species (which is the maximum reduction that could be determined with the experimental design). For both PSs and for all species (for both washed and nonwashed conidia), no difference was found in the survival fractions when fluences of ≥15 J cm−2 were used (P > 0.69 for all treatment comparisons). This happened because for most of the treatments, the maximum reduction that can be detected by the experiments (5-log reduction) had already been reached using low fluence (15 J cm−2). For comparison, in a field experiment, a fluence of 15 J cm−2 would be reached after 12 and 14 min of exposure to solar radiation at noon on 8 May (midautumn) and 5 June (late autumn), respectively. Washing of the conidia to remove unbound PS before light exposure reduced the effect of APDT for some of the treatments (i.e., both PSs at a fluence of 15 J cm−2 [P < 0.01 for the three species]) but not for others (Fig. 2).
FIG 2.
Effects of APDT on conidial survival. Shown is conidial photodynamic inactivation of Colletotrichum acutatum (A and B), C. gloeosporioides (C and D), and Aspergillus nidulans (E and F) with red light and NMBN or S137, respectively. Conidia were incubated with the PS for 30 min and washed or not washed before light exposures. Error bars are standard deviations of three independent experiments.
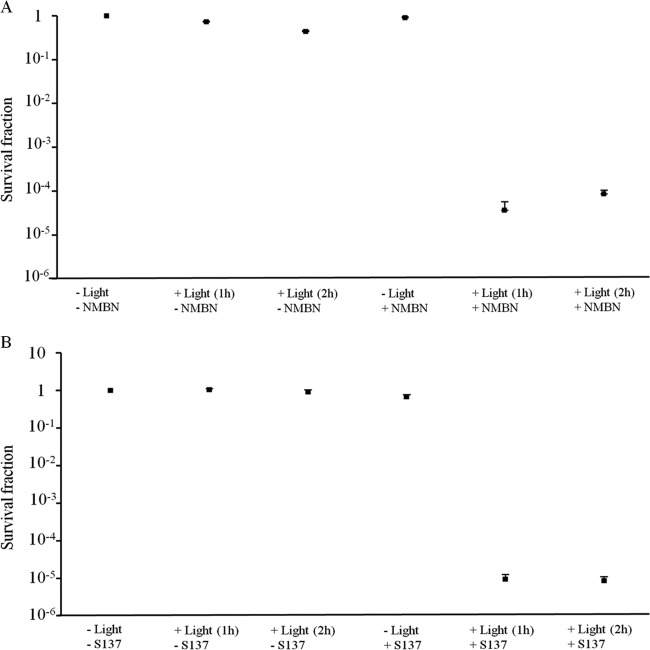
Effect of APDT with NMBN or S137 and full-spectrum solar radiation on survival of C. acutatum conidia.
Conidia were incubated with the PS (50 μM NMBN or 10 μM S137) for 30 min before being exposed to solar radiation for 1 and 2 h. Exposure to solar radiation alone (in the absence of PS) for 1 and 2 h killed approximately 27% and 43% the conidia of C. acutatum, respectively, during the experiments performed in the middle of autumn (P < 0.01 for both 1 and 2 h of exposure) but did not kill the conidia in experiments performed 2 months later, in late autumn (P = 0.56 and 0.11 for 1 and 2 h of exposure, respectively), when the UV irradiances were lower (Fig. 1B). In the absence of light (dark toxicity), NMBN (50 μM) had no effect on conidial survival (P = 0.36), and S137 (10 μM) killed approximately 33% of the conidia (P < 0.01). The effects of APDT with NMBN (50 μM) or S137 (10 μM) and solar radiation are shown in Fig. 3A and B, respectively. APDT with both PSs was highly effective in killing conidia and resulted in a 5-log reduction in conidial survival after both 1 and 2 h of exposure.
FIG 3.
Conidial photodynamic inactivation of Colletotrichum acutatum with NMBN (A) or S137 (B) and solar radiation. Conidia were incubated with the PS for 30 min before exposures to natural sunlight for 1 and 2 h. Error bars are standard deviations of four experiments.
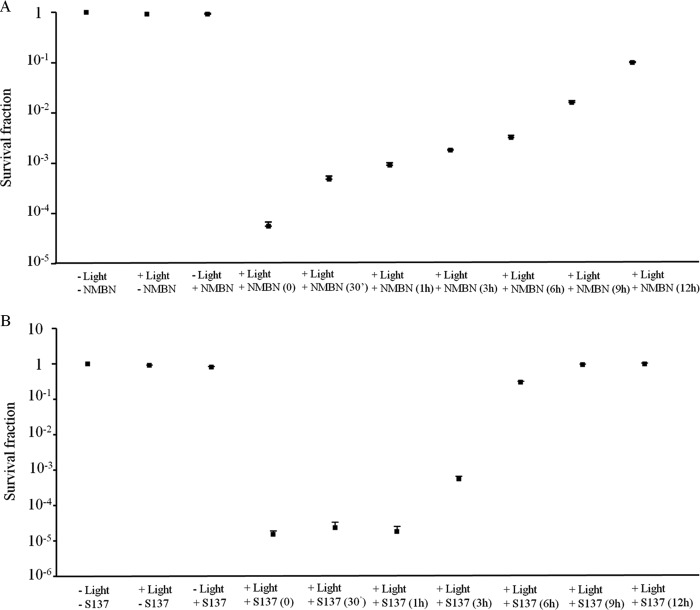
Effects of full-spectrum solar radiation on NMBN and S137 absorption spectra and efficacy as PSs in APDT.
Exposures of NMBN and S137 to solar radiation changed their absorption spectra (see Fig. S3 in the supplemental material) and reduced their effectiveness in APDT (Fig. 4). Both modifications were related directly to exposure time.
FIG 4.
Conidial photodynamic inactivation of Colletotrichum acutatum with red light and NMBN (A) or S137 (B) previously exposed to full-spectrum solar radiation. Error bars are standard deviations of four experiments.
Conidial microscopy studies.
Conidia of C. acutatum were treated with NMBN and S137, and microscopic studies were performed to determine the subcellular localization of the PS. Both NMBN and S137 penetrated the conidia and accumulated cytoplasmic vesicles. Colocalization of PSs with the dyes Sudan III and FM4-64 indicated that both PSs accumulated in lipid bodies (Fig. 5 and 6), structures that occupy a large portion of the cytoplasm of young conidia of Colletotrichum. However, the presence of the PS in other membranous structures, such as small vacuoles, is a possibility that cannot be excluded. Treatment with high S137 concentrations (≥50 μM) caused cytoplasmic disorganization and the formation of large vesicles in the cytoplasm of the conidia (Fig. 6). Washing of the conidia did not remove the PS from their interior, showing that they were strongly bound to lipid bodies or membranous structures (Fig. 5 and 6).
FIG 5.
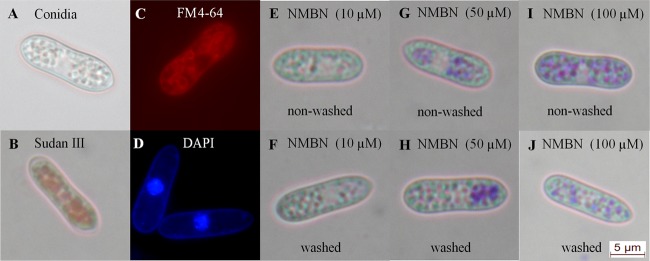
(A to D) Nonstained Colletotrichum acutatum conidia (A) and conidia stained with Sudan III (0.5%) (B), FM4-64 (8 μM) (C), and DAPI (3.5 mM) (D). (E to J) Conidia were also treated with 10 μM (E and F), 50 μM (G and H), and 100 μM (I and J) NMBN and not washed (E, G, and I) or washed to remove unbound PS (F, H, and J).
FIG 6.
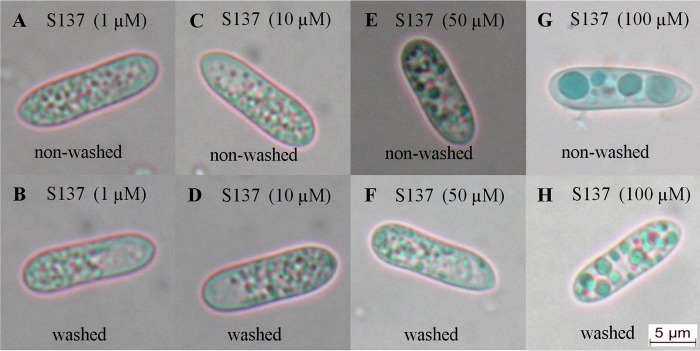
Colletotrichum acutatum conidia treated with 1 μM (A and B), 10 μM (C and D), 50 μM (E and F), and 100 μM (G and H) S137 and not washed (A, C, E, and G) or washed to remove unbound PS (B, D, F, and H).
Effects of PDT on leaves of orange trees.
APDT with the phenothiazinium PS did not cause any damage to the adult leaves of orange trees (Citrus sinensis) (see Fig. S4 in the supplemental material), nor did it cause damage to other structures of the plant, such as young leaves and flowers (data not shown).
Microscopic studies of orange tree leaves.
Comparison of Fig. S5A to S5D in the supplemental material (in which PS solutions were spotted onto the leaf adaxial surface before the leaves had been sectioned) and Fig. S5E to S5H in the supplemental material (in which leaf sections were stained with the PS) shows that the phenothiazinium PS used in the present study did not cross the leaf cuticle.
DISCUSSION
The need to overcome deficiencies in conventional antimicrobial strategies has stimulated the investigation of alternative approaches, such as APDT, to control pathogenic microorganisms in both the clinical and agricultural areas. APDT has been used to treat human mycoses caused mainly by fungi of the genera Candida and Trichophyton (15, 16, 18, 29, 30). It is thus logical that novel applications of APDT are explored, including its use to control plant pathogens.
Phenothiazinium photosensitizers are among the most studied PSs, and novel derivatives with improved photochemical and photophysical characteristics have been synthesized (26, 31–33). APDT with novel MB derivatives such as NMBN and S137 has been shown to be highly effective against human-pathogenic fungi of the genera Candida and Trichophyton (15, 16, 29). There are no data regarding the effects of APDT with these novel PSs on plant-pathogenic fungi or on their plant hosts. In the present study, we evaluated the efficacies of in vitro APDT with four phenothiazinium derivatives in killing of conidia of C. acutatum, C. gloeosporioides, and A. nidulans. The efficacies were established initially by determining the MIC of each PS for each fluence. For the two PSs selected in this study (NMBN and S137), we also evaluated conidial survival after APDT with red light emitted by LEDs and with full-spectrum natural solar radiation. As the MIC experiments were less time-consuming and allowed the evaluation of several treatments simultaneously, this initial approach was convenient to establish the optimized conditions for APDT (15, 16). S137 was the most effective PS, being associated with the lowest MICs for all three species. A concentration of 10 μM at light fluences of ≥20 J cm2 was enough to completely inhibit the growth of all fungi. S137 also presented the highest dark toxicity. The improved performance of S137 and NMBN in comparison with the lead compound MB was previously observed in vitro for both Candida spp. and Trichophyton spp. (15, 16, 29).
APDT with red light and with both NMBN and S137 was very effective in killing conidia of the three species, as observed in the survival-based experiments. Conidia of Colletotrichum and Aspergillus are different in biochemical (i.e., pigmentation) and morphological (i.e., cell size) characteristics that were previously associated with tolerance to photodynamic treatment (20). For example, conidia of Colletotrichum are hyaline and much larger than the dark-green-pigmented conidia of A. nidulans (see Fig. S6 in the supplemental material). Despite these differences, both types of conidia were efficiently killed by APDT. Treatment with NMBN (at 50 μM and a fluence of 15 J cm−2) resulted in an approximately 5-log reduction in survival of the conidia of all species. APDT with S137 was even more effective in killing conidia: a concentration of 10 μM at a fluence of 15 J cm−2 resulted in the same reduction in conidial survival. APDT continued to be very effective even when conidia were washed before light exposure. This is due to the PS either being firmly attached to the cell surface or being internalized by the conidia (20).
Microscopic studies showed that NMBN and S137 were taken up by C. acutatum conidia and accumulated in cytoplasmic vesicles. PS entry and accumulation occurred upon contact with the conidia and were independent of light exposure. The colocalization of the PSs with the dyes Sudan III and FM4-64, which have affinity for lipids and membranes, respectively, indicates that the PSs accumulate in lipid bodies and in small vacuoles. The numerous lipid bodies, ranging from 0.4 to 2.0 μm in diameter, are the most abundant reserve in young, ungerminated conidia of Colletotrichum spp. and dominate the cytoplasmic volume (34, 35). Conidial treatment with S137 at concentrations of ≥50 μM disorganized the cytoplasm and induced the formation of large vesicles (Fig. 6). It is suggested that this is the reason for the higher dark toxicity of S137.
Unlike some currently used fungicides that act only on metabolically active cells and/or are only fungistatic, APDT with NMBN and S137 killed the conidia of all three species. Previously, we demonstrated that APDT with these PSs also kills conidia of Trichophyton mentagrophytes and T. rubrum (16). As far as we know, there is no evidence indicating that conidia or other dormant or quiescent fungal structures are more tolerant to APDT than metabolically active cells. However, additional studies need to be carried out to determine the effect of APDT on other potentially tolerant fungal structures such as the melanized Colletotrichum species appressoria.
APDT to control plant pathogens in the field would be applied under conditions very different from the controlled conditions used in the clinic to treat localized mycoses. Instead of lasers and LEDs, the sun would be the light source. The broad emission spectra and high irradiances in visible and UV spectra (Fig. 1A and B) enable solar radiation to excite both visible-light-activated PSs such as phenothiazinium and UV-activated PSs such as the naturally occurring furanocoumarins and thiophenes. Additionally, light cycles are long and repeated daily. In the present study, we have shown that APDT with both NMBN and S137 and 1 h of solar exposure completely photoinactivated conidia of C. acutatum. The use of APDT to eliminate conidia on sporulating lesions and/or recently dispersed conidia would dramatically reduce the inoculum in the treated area. Reduction of the initial inoculum is among the strategies used to control plant diseases (36–38).
The exposure of NMBN and S137 to solar radiation reduced their effectiveness in APDT; i.e., APDT with NMBN and S137 previously exposed to solar radiation for 3 h resulted in a reduction in conidial survival of approximately 3 logs instead of the 5 logs achieved with the nonexposed PSs. Despite the intense PS inactivation caused by solar radiation, NMBN was still able to kill 90% of C. acutatum conidia even after 12 h of exposure to full-spectrum sunlight. It should be emphasized that the experiments to evaluate the effect of solar radiation on PS activity were performed under harsh conditions (i.e., PSs in solution were exposed directly to solar radiation on clear days in a tropical site during early autumn). It may be speculated that under milder conditions, the environmental persistence of the PSs would be increased. The photodegradation of the PSs prevents their accumulation in the environment but may increase the number of applications necessary to control the fungus. However, frequent applications are also required to control Colletotrichum spp. with conventional fungicides. As conidia are dispersed by rain splash, currently used fungicides are usually reapplied after each rain episode. In the case of postbloom fruit drop, up to nine applications are not uncommon during the flowering season to control the disease, depending upon its incidence and the rainfall (39, 40).
In the clinic, the selective toxicity of APDT can be increased by both topical application of the PS and light exposure restricted to the lesion. This cannot happen in field experiments because both the microorganism and host will be exposed to light and the PS. Thus, selectivity would have to be achieved in other ways. In the present study, no damage was observed for orange tree leaves treated with the PSs and exposed to solar radiation. One of the factors that may explain the lack of damage is that the PS did not cross the leaf cuticle. As the reactive species generated during PDT have very short half-lives, their diffusion is limited, and therefore, damage is restricted to structures close to the PS (18). As the PS remained outside the cuticle, which is approximately 4 μm thick, the internal leaf structure was not damaged by the PDT. Thus, fungi on the leaf surface that are in contact with the PS may be killed without damaging the leaf.
To control pathogens, the PS would be applied in the environment and in large areas. This would require the use of environmentally safe PSs. The phenothiazinium derivative methylene blue, the lead compound in this work, has a suitable human toxicity profile, being used routinely for malignancy tracing and the treatment of methemoglobinemia, in both cases at concentrations hundreds of times higher than those required to kill microorganisms (31). Other PSs, such as the above-mentioned furanocoumarins and thiophenes, are produced naturally by plants of several genera and may act as protection against infections caused by plant-pathogenic fungi or bacteria (23, 24, 41).
Our initial study demonstrated that in vitro APDT with the phenothiazinium PS in combination with artificial or natural solar radiation was highly effective in killing conidia of Colletotrichum spp. We also demonstrated that APDT did not damage the plant host. In order to explore the real potential of APDT as an alternative to the intensive use of conventional fungicides, further studies are necessary to determine the effectiveness of APDT in planta under field conditions and the environmental impact of these new approaches as well as to establish the appropriate formulations and delivery systems for the selected PS.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Carlos Alberto de Oliveira for supplying the LED600 and to Carlos Artério Sorgi for technical assistance with fluorescence microscopy.
This work was supported by grant 2012/15204-8 from the State of São Paulo Research Foundation (FAPESP). We sincerely thank FAPESP for an M.S. fellowship to H.D.D.M.
Footnotes
Published ahead of print 20 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02788-13.
REFERENCES
- 1.Deising HB, Reimann S, Pascholati SF. 2008. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 39:288–295. 10.1590/S1517-838220080002000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong FP, de la Cerda KA, Hernandez-Martinez R, Midland SL. 2008. Detection and characterization of benzimidazole resistance in California populations of Colletotrichum cereale. Plant Dis. 92:239–246. 10.1094/PDIS-92-2-0239 [DOI] [PubMed] [Google Scholar]
- 3.Wong FP, Midland SL. 2007. Sensitivity distributions of California populations of Colletotrichum cereale to the DMI fungicides propiconazole, myclobutanil, tebuconazole, and triadimefon. Plant Dis. 91:1547–1555. 10.1094/PDIS-91-12-1547 [DOI] [PubMed] [Google Scholar]
- 4.Peres NAR, Souza NL, Peever TL, Timmer LW. 2004. Benomyl sensitivity of isolates of Colletotrichum acutatum and C. gloeosporioides from citrus. Plant Dis. 88:125–130. 10.1094/PDIS.2004.88.2.125 [DOI] [PubMed] [Google Scholar]
- 5.Andrivon D, Ramage K, Guérin C, Lucas JM, Jovan B. 1997. Distribution and fungicide sensitivity of Colletotrichum coccodes in French potato-producing areas. Plant Pathol. 46:722–728. 10.1046/j.1365-3059.1997.d01-60.x [DOI] [Google Scholar]
- 6.Peres NAR, Timmer LW, Adaskaveg JE, Correll JC. 2005. Lifestyles of Colletotrichum acutatum. Plant Dis. 89:784–796. 10.1094/PD-89-0784 [DOI] [PubMed] [Google Scholar]
- 7.Wharton PS, Diéguez-Uribeondo J. 2004. The biology of Colletotrichum acutatum. An. Jardin Bot. Madrid 61:3–32 http://rjb.revistas.csic.es/index.php/rjb/article/viewFile/61/60 [Google Scholar]
- 8.Nascimento É, da Silva SH, Marques ER, Roberts DW, Braga GUL. 2010. Quantification of cyclobutane pyrimidine dimers induced by UVB radiation in conidia of the fungi Aspergillus fumigatus, Aspergillus nidulans, Metarhizium anisopliae and Metarhizium robertsii. Photochem. Photobiol. 86:1259–1266. 10.1111/j.1751-1097.2010.00793.x [DOI] [PubMed] [Google Scholar]
- 9.Barros BHR, da Silva SH, Marques ER, Rosa JC, Yatsuda AP, Roberts DW, Braga GUL. 2010. A proteomic approach to identifying proteins differentially expressed in conidia and mycelium of the entomopathogenic fungus Metarhizium anisopliae. Fungal Biol. 114:572–579. 10.1016/j.funbio.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 10.Braga GUL, Destéfano RHR, Messias CL. 1999. Oxygen consumption by Metarhizium anisopliae during germination and growth on different carbon sources. J. Invertebr. Pathol. 74:112–119. 10.1006/jipa.1999.4872 [DOI] [PubMed] [Google Scholar]
- 11.Peres NA, Seijo TE, Turechek WW. 2010. Pre- and post-inoculation activity of a protectant and a systemic fungicide for control of anthracnose fruit rot of strawberry under different wetness durations. Crop Prot. 29:1105–1110. 10.1016/j.cropro.2010.05.010 [DOI] [Google Scholar]
- 12.Vera DMA, Haynes MH, Ball AR, Dai T, Astrakas C, Kelso MJ, Hamblin MR, Tegos GP. 2012. Strategies to potentiate antimicrobial photoinactivation by overcoming resistant phenotypes. Photochem. Photobiol. 88:499–511. 10.1111/j.1751-1097.2012.01087.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai T, Fuchs BB, Coleman JJ, Prates RA, Astrakas C, St Denis TG, Ribeiro MS, Mylonakis E, Hamblin MR, Tegos GP. 2012. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front. Microbiol. 3:120. 10.3389/fmicb.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calzavara-Pinton P, Rossi MT, Sala R, Venturini M. 2012. Photodynamic antifungal chemotherapy. Photochem. Photobiol. 88:512–522. 10.1111/j.1751-1097.2012.01107.x [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues GB, Dias-Baruffi M, Holman N, Wainwright M, Braga GUL. 2013. In vitro photodynamic inactivation of Candida species and mouse fibroblasts with phenothiazinium photosensitizers and red light. Photodiagnosis Photodyn. Ther. 10:141–149. 10.1016/j.pdpdt.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues GB, Ferreira LKS, Wainwright M, Braga GUL. 2012. Susceptibilities of the dermatophytes Trichophyton mentagrophytes and T. rubrum microconidia to photodynamic antimicrobial chemotherapy with novel phenothiazinium photosensitizers and red light. J. Photochem. Photobiol. B 116:89–94. 10.1016/j.jphotobiol.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues GB, Primo FL, Tedesco AC, Braga GUL. 2012. In vitro photodynamic inactivation of Cryptococcus neoformans melanized cells with chloroaluminum phthalocyanine nanoemulsion. Photochem. Photobiol. 88:440–447. 10.1111/j.1751-1097.2011.01055.x [DOI] [PubMed] [Google Scholar]
- 18.Smijs TGM, Pavel S. 2011. The susceptibility of dermatophytes to photodynamic treatment with special focus on Trichophyton rubrum. Photochem. Photobiol. 80:197–202. 10.1111/j.1751-1097.2004.tb00071.x [DOI] [PubMed] [Google Scholar]
- 19.St Denis TG, Dai T, Izikson L, Astrakas C, Anderson RR, Hamblin MR, Tegos GP. 2011. All you need is light. Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2:509–520. 10.4161/viru.2.6.17889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzales FP, da Silva SH, Roberts DW, Braga GUL. 2010. Photodynamic inactivation of conidia of the fungi Metarhizium anisopliae and Aspergillus nidulans with methylene blue and toluidine blue. Photochem. Photobiol. 86:653–661. 10.1111/j.1751-1097.2009.00689.x [DOI] [PubMed] [Google Scholar]
- 21.Donnelly RF, McCarron PA, Tunney MM. 2008. Antifungal photodynamic therapy. Microbiol. Res. 163:1–12. 10.1016/j.micres.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 22.Jori G. 2006. Photodynamic therapy of microbial infections: state of the art and perspectives. J. Environ. Pathol. Toxicol. Oncol. 25:505–519. 10.1615/JEnvironPatholToxicolOncol.v25.i1-2.320 [DOI] [PubMed] [Google Scholar]
- 23.DiCosmo F, Towers GHN, Lam J. 1982. Photo-induced fungicidal activity elicited by naturally occurring thiophene derivatives. Pest. Manag. Sci. 13:589–594. 10.1002/ps.2780130604 [DOI] [Google Scholar]
- 24.Bourque G, Arnason JT, Madhosingh C, Orr W. 1985. The photosensitization of the plant pathogen Fusarium culmorum by phenylheptatriyne from Bidens pilosa. Can. J. Bot. 63:899–902 [Google Scholar]
- 25.Lukšienė L, Pečiulytė D, Lugauskas A. 2004. Inactivation of fungi in vitro by photosensitization: preliminary results. Ann. Agric. Environ. Med. 11:215–220 http://www.aaem.pl/pdf/11215.pdf [PubMed] [Google Scholar]
- 26.Wainwright M, Meegan K, Loughran C. 2011. Phenothiazine photosensitisers. IX. Tetra- and pentacyclic derivatives as photoantimicrobial agents. Dyes Pigm. 91:1–5. 10.1016/j.dyepig.2011.02.001 [DOI] [Google Scholar]
- 27.Montgomery DC. 2000. Design and analysis of experiments, 5th ed. Wiley, Hoboken, NJ [Google Scholar]
- 28.Schall R. 1991. Estimation in generalized linear models with random effects. Biometrika 78:719–727. 10.1093/biomet/78.4.719 [DOI] [Google Scholar]
- 29.Dai T, Bil de Arce VJ, Tegos GP, Hamblin MR. 2011. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob. Agents Chemother. 55:5710–5717. 10.1128/AAC.05404-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzales FP, Maisch T. 2012. Photodynamic inactivation for controlling Candida albicans infections. Fungal Biol. 116:1–10. 10.1016/j.funbio.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 31.Wainwright M, Smalley H, Scully O, Lotfipour E. 2012. Comparative photodynamic evaluation of new phenothiazinium derivatives against Propionibacterium acnes. Photochem. Photobiol. 88:523–526. 10.1111/j.1751-1097.2011.01021.x [DOI] [PubMed] [Google Scholar]
- 32.Wainwright M, Brandt SD, Smith A, Styles A, Meegan K, Loughran C. 2010. Phenothiazinium photosensitizers. VII. Novel substituted asymmetric N-benzylphenothiaziniums as photoantimicrobial agents. J. Photochem. Photobiol. B 99:74–77. 10.1016/j.jphotobiol.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 33.Wainwright M, Meegan K, Loughran C, Giddens RM. 2009. Phenothiazinium photosensitisers. VI. Photobactericidal asymmetric derivatives. Dyes Pigm. 82:387–391. 10.1016/j.dyepig.2009.02.011 [DOI] [Google Scholar]
- 34.Barbosa AC, do Carmo AE, Graf L, Tomaz R, de Souza CF, Mendes J, Randi MAF, Buchi D, Schadeck RJG. 2006. Morphology and lipid body and vacuole dynamics during secondary conidia formation in Colletotrichum acutatum: laser scanning confocal analysis. Can. J. Microbiol. 52:117–124. 10.1139/w05-104 [DOI] [PubMed] [Google Scholar]
- 35.Mims CW, Richardson EA, Clay RP, Nicholson RL. 1995. Ultrastructure of conidia and the conidium aging process in the plant pathogenic fungus Colletotrichum graminicola. Int. J. Plant Sci. 156:9–18. 10.1086/297223 [DOI] [Google Scholar]
- 36.Vander Plank JE. 1975. Principles of plant infection. Academic Press, London, United Kingdom [Google Scholar]
- 37.Agrios GN. 2005. Plant pathology, 5th ed. Academic Press, San Diego, CA [Google Scholar]
- 38.Zulfiqar M, Brlansky RH, Timmer LW. 1996. Infection of flower and vegetative tissues of citrus by Colletotrichum acutatum and C. gloeosporioides. Mycologia 88:121–128. 10.2307/3760791 [DOI] [Google Scholar]
- 39.Timmer LW, Zitko SE. 1992. Timing of fungicide applications for control of postbloom fruit drop of citrus in Florida. Plant Dis. 76:820–823. 10.1094/PD-76-0820 [DOI] [Google Scholar]
- 40.de Goes A, Garrido RBO, Reis RF, Badassari RB, Soares MA. 2008. Evaluation of fungicide applications to sweet orange at different flowering stages for control of postbloom fruit drop caused by Colletotrichum acutatum. Crop Prot. 27:71–76. 10.1016/j.cropro.2007.04.007 [DOI] [Google Scholar]
- 41.Binns SE, Purgina B, Bergeron C, Smith ML, Ball L, Baum BR, Arnason JT. 2000. Light-mediated antifungal activity of Echinacea extracts. Planta Med. 66:241–244. 10.1055/s-2000-8573 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.