Abstract
Trichoderma parareesei and Trichoderma reesei (teleomorph Hypocrea jecorina) produce cellulases and xylanases of industrial interest. Here, the anamorphic strain T6 (formerly T. reesei) has been identified as T. parareesei, showing biocontrol potential against fungal and oomycete phytopathogens and enhanced hyphal growth in the presence of tomato exudates or plant cell wall polymers in in vitro assays. A Trichoderma microarray was used to examine the transcriptomic changes in T6 at 20 h of interaction with tomato plants. Out of a total 34,138 Trichoderma probe sets deposited on the microarray, 250 showed a significant change of at least 2-fold in expression in the presence of tomato plants, with most of them being downregulated. T. parareesei T6 exerted beneficial effects on tomato plants in terms of seedling lateral root development, and in adult plants it improved defense against Botrytis cinerea and growth promotion under salt stress. Time course expression patterns (0 to 6 days) observed for defense-related genes suggest that T6 was able to prime defense responses in the tomato plants against biotic and abiotic stresses. Such responses undulated, with a maximum upregulation of the jasmonic acid (JA)/ethylene (ET)-related LOX1 and EIN2 genes and the salt tolerance SOS1 gene at 24 h and that of the salicylic acid (SA)-related PR-1 gene at 48 h after T6 inoculation. Our study demonstrates that the T. parareesei T6-tomato interaction is beneficial to both partners.
INTRODUCTION
Trichoderma is a genus of filamentous fungi that includes strains used as biocontrol agents in agriculture due to their antagonistic abilities against phytopathogenic fungi and oomycetes (1). The biocontrol mechanisms are based on the production of antibiotics (2, 3) and/or hydrolytic enzymes as well as competition for nutrients (4). In addition, Trichoderma strains are also able to stimulate defense responses in plants, inducing resistance to biotic and abiotic stresses, and to promote plant growth (5–7). Several studies have reported the responses of plants to the presence of a root-colonizing Trichoderma biocontrol strain (8–12), and investigations describing the Trichoderma transcriptomic changes elicited by plants using macroarray (13, 14) and microarray (15, 16) analyses have been performed with biocontrol species.
Trichoderma reesei (teleomorph Hypocrea jecorina) is widely used in the enzyme industry for the production of cellulases (17–19), and it has also been used as a cell factory to express numerous recombinant proteins (20). While the T. reesei holomorph includes most of the teleomorphs found on dead wood, Trichoderma parareesei has recently been described as a new species, in which sexual reproduction does not occur, and T. parareesei encompasses all of the extremely efficient cellulase-producing strains formerly isolated as T. reesei from soil (21). T. parareesei likely resembles the ancestor that gave rise to T. reesei (22), and it displays all the properties of an environmental opportunist: it shows faster growth on a wider spectrum of carbon sources than T. reesei, produces a much higher number of propagules on a greater variety of carbon sources, is well adapted to various lighting conditions, and displays stronger antagonistic potential in dual-confrontation tests against a variety of plant-pathogenic fungi (21). Both species are cellulolytic and xylanolytic and are grouped with a third sister species, Trichoderma gracile, to form an independent clade within the major Longibrachiatum clade of Hypocrea/Trichoderma, which also includes a close clade with the facultative opportunistic human pathogens Hypocrea orientalis and Trichoderma longibrachiatum (23). Trichoderma pseudokoningii is another interesting species within the clade Longibrachiatum with the ability to inhibit mycorrhizal spore germination (24), produce peptaibols able to induce defense responses in plants (25), and trigger apoptosis in phytopathogenic fungi (26). Compared to species belonging to other clades of Hypocrea/Trichoderma, with proven biocontrol abilities and beneficial effects on plants (27), neither T. reesei nor T. parareesei has been considered relevant for biocontrol, although the mycoparasitic ability of T. parareesei has recently been demonstrated (21). To date, no studies on the interaction of these two species with living plants have been reported.
The aim of the present study was to analyze the biocontrol potential of T. parareesei T6 in in vitro assays against the oomycete Pythium irregulare, the basidiomycete Rhizoctonia solani, and the ascomycete Botrytis cinerea as well as the mutualistic interaction between this fungus and tomato plants. A Trichoderma high-density oligonucleotide (HDO) microarray was used to examine the transcriptomic profiles in T. parareesei T6 at 20 h of interaction with tomato plants and to compare them with the transcriptomic profiles previously obtained for the known biocontrol strains of Trichoderma hamatum, Trichoderma harzianum, and Trichoderma virens in interactions with tomato plants under identical experimental conditions. Physiological parameters and expression changes of plant defense marker genes linked to biotic and environmental stresses were used to analyze the response of the tomato plants to T. parareesei T6. This paper reports that the T. parareesei-tomato interaction is beneficial to both partners and exerts long-term positive effects on seedlings or adult plants in terms of systemic plant defense against B. cinerea and increased lateral root development and growth promotion under salt stress conditions.
MATERIALS AND METHODS
Microorganisms and tomato seeds.
Trichoderma parareesei (formerly T. reesei) IMI 113135 (CABI Bioscience, Egham, United Kingdom), referred to as T6, was grown on potato dextrose agar medium (PDA) (Difco-Becton, Dickinson, Sparks, MD) and stored at −80°C in 30% glycerol. Spores from 7-day-old PDA plates were harvested by adding 5 ml of water to the plates and scraping the culture with a rubber spatula. These suspensions were filtered through a double layer of cheesecloth to separate large mycelial fragments from conidia. Spore concentrations were calculated using a counting chamber, and suspensions were used to inoculate the medium.
Rhizoctonia solani 19, Botrytis cinerea B05.10, and the oomycete Pythium irregulare (provided by R. Solano, CNB-CSIC, Madrid, Spain) were used as plant-pathogenic microorganisms in antagonism assays. These strains were stored at 4°C in PDA plugs suspended in sterile water (R. solani and P. irregulare) and at −80°C in 30% glycerol (B. cinerea).
Tomato seeds (Solanum lycopersicum “Marmande”) were sterilized in 2% sodium hypochlorite for 20 min and washed thoroughly in sterile distilled water before use.
DNA extraction, conventional PCR amplification, and sequencing.
T6 mycelium for the DNA extraction was obtained from a potato dextrose broth (PDB) (Difco-Becton, Dickinson) culture incubated at 28°C and 200 rpm for 2 days. The mycelium was collected by filtration, washed with distilled water, frozen, and lyophilized. Fungal genomic DNA was isolated according to previously described protocols (28).
Amplification of a fragment of the tef1 (encoding translation elongation factor 1-α) and cal1 (encoding calmodulin) genes was carried out with the primer pairs EF1-728F/EF1-LLErev and CAL-228F/CAL-737R, respectively, as described previously (29). The las1 gene, which encodes the orthologue of an essential nuclear protein regulating bud formation and morphogenesis in Saccharomyces cerevisiae, was amplified using the LAS1 fw/LAS1 rev primers, as described previously (21). The PCR products were purified from agarose gels using the NucleoSpin Extract II kit (Macherey-Nagel, Düren, Germany), according to the manufacturer's protocol. PCR fragments were sequenced in an ABI 377 Prism sequencer (Applied Biosystems, Foster City, CA).
Antagonism assays.
In vitro confrontation assays between T6 and the pathogens P. irregulare and R. solani on PDA plates and B. cinerea on malt extract agar (MEA) (Difco-Becton, Dickinson) plates were carried out in triplicate as previously described (2). Plates were incubated at 28°C, and the behavior of T6 against each pathogen was examined visually until the Trichoderma strain had overgrown or surrounded the pathogen colony.
Growth assays on cellophane sheets and 10-kDa-cutoff dialysis cellulose membranes were carried out on PDA (for P. irregulare and R. solani) or MEA (for B. cinerea) plates as previously described (2). Each pathogen was tested in triplicate. Growth diameters were measured after 72 h for P. irregulare and R. solani and after 120 h for B. cinerea. The results are expressed as the percentage of growth inhibition of each pathogen by T6, with respect to the mean colony diameters of each pathogen grown alone.
Trichoderma-plant interaction in hydroponic culture for microarray experiments.
T. parareesei-tomato hydroponic cultures were carried out as previously described (15). Briefly, T6 was cultured in minimal medium (MM) (30) containing 2% glucose at 28°C with stirring for 48 h. The mycelium was then collected, washed, and used to inoculate Phytatray II boxes (Sigma-Aldrich, Madrid, Spain) containing 2-week-old tomato plants in Murashige-Skoog (MS) medium (Duchefa Biochemie BV, Haarlem, The Netherlands) supplemented with 1% (wt/vol) sucrose, pH 5.7. The cultures were maintained at 25°C and 80 rpm for 20 h. Control mycelia were grown under the same conditions but in the absence of tomato plants. After this period, the T6 mycelium was collected by filtration (the mycelium on the plant roots was recovered with a direct jet of water), washed, frozen, and lyophilized. The RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA), following the manufacturer's instructions, and then purified using the RNeasy MinElute Cleanup kit (Qiagen, Hilden, Germany).
The microarray design, RNA labeling, hybridizations, and data acquisition were performed by Roche-NimbleGen (Madison, WI), as recently reported (15). Digitization of the fluorescent signals emitted after the hybridization was performed using an Axon GenePix 4000B scanner with NimbleScan 2.3 software, and then the images and the raw probe intensity values obtained from 6 microarrays examined (three replicates obtained from the “off-and-on” presence [i.e., absence or presence] of tomato plants) were analyzed. The signal intensities from these arrays were normalized with the Robust Multiple-Chip Analysis (RMA) algorithm (31). The normalized values for each probe obtained from the 6 microarrays were scaled in the range of 0 to 1 to compensate for sequence-specific sensitivity. The processed data for the different probes within a probe set were summed to produce an expression measure. Finally, a multiclass significance analysis of microarray (SAM) test was carried out on the expression values using a false-discovery rate (FDR) of 0.10. The analysis was performed using the Gene Spring GX program through R software. The transcripts showing significant differential expression (fold change [FC] of ≥2 and FDR of 0.10) were annotated using Gene Ontology (GO) terms (32), which were based on the BLAST definitions, applying an E value level of <10−5.
Real-time PCR quantification of transcripts.
cDNAs were synthesized from 1 μg of total RNA with an oligo(dT) primer, using the Transcriptor first-strand cDNA synthesis kit (TaKaRa, Tokyo, Japan) according to the manufacturer's protocol, and then 1 μl of the cDNA was used in the subsequent PCR.
Real-time PCRs were carried out with an AB Prism 7000 sequence detection system (Applied Biosystems) in a total volume of 10 μl using the Brilliant SYBR green QPCR master mix (Roche, Penzberg, Germany) and a final primer concentration of 300 nM each. Real-time PCRs were performed with the cDNAs of four pooled biological replicates for each condition, with the exception of two pooled biological replicates used to analyze plant defense gene expression from in vitro cultures. All PCRs were performed in triplicate, and the primers used are given in Table S1 in the supplemental material. The amplification protocol consisted of an initial denaturation step (10 min at 95°C) followed by 40 cycles of denaturation (30 s at 95°C), annealing (1 min 60°C), and extension (1 min 72°C). The β-tubulin and actin genes were used as Trichoderma and tomato endogenous controls, respectively. Data are expressed using the 2−ΔΔCT method (33).
Growth of T. parareesei in the presence of plant polymers.
Mycelial plugs with a diameter of 0.5 cm of T. parareesei T6 were placed on the center of petri plates containing MM (30) supplemented with 1.5% agar plus 0.25% carboxymethylcellulose (CMC) (Sigma-Aldrich), polygalacturonic acid (PGA) (Sigma-Aldrich), xylan (Sigma-Aldrich), or pectin (Sigma-Aldrich). MM plates supplemented only with agar were used as controls. The growth of T. parareesei was measured after 4 days of incubation at room temperature. Experiments were performed in triplicate.
Growth of T. parareesei in the presence of plant exudates.
Plant exudates were obtained by placing tomato seeds inside Phytatray II boxes (Sigma-Aldrich) on a sterile gauze sheet over a sterile stainless steel screen (20 seeds per box), holding them 1 cm above 100 ml of MS medium supplemented with 1% (wt/vol) sucrose, pH 5.7. The boxes were kept in a plant growth chamber under conditions of 40% humidity, 22°C, and a 16-h light/8-h dark photoperiod for 12 days. Boxes containing sterile MS–1% sucrose medium without tomato seeds were maintained under the above-described conditions for use as controls. The culture media were filtered through sterile filter paper. Aliquots of medium (120 μl) from plant exudates or the control were placed in the wells of E plates (96 wells per plate), and then a batch of 2,000 T6 conidia in a total volume of 10 μl water was added to each well and the plates were incubated at 28°C for 120 h. Fungal growth was calculated by measuring absorbance at 570 nm and subtracting the respective absorbance basal values, which corresponded to wells containing only plant exudates or MS–1% sucrose without T6 conidia. Experiments were performed in triplicate.
Effect of T. parareesei on tomato plant growth.
An in vitro assay was carried out to analyze the effect of T6 on tomato seedlings. Fungal densities of 1 × 106 spores were inoculated by placing the spores at the opposite ends of MS–1% sucrose medium plates, supplemented with 0.8% agar (pH 5.7) and containing 3-day-old germinated tomato seedlings (5 seedlings per plate). Plates were cultured in a growth chamber under conditions of 40% humidity, 24°C, and a 16-h light/8-h dark photoperiod. MS–1% sucrose plates containing only tomato seedlings, without Trichoderma spores, were used as controls. Experiments were performed in triplicate, and the plates were photographed at 4 days after T6 inoculation.
Salt stress tolerance assays were carried out as indicated above but with 0, 10, or 25 mM NaCl added to the MS–1% sucrose medium. Plants were grown for 7 days before T6 inoculation and photographed 2 days later.
The ability of T6 to promote the growth of tomato plants was also evaluated in in vivo assays, as previously described (15). To analyze the salt stress tolerance of tomato plants grown from T6-treated seeds or untreated seeds (control), 4-week-old plants were treated daily with 5 ml of either water or 100 mM NaCl for 10 days, and measurements of stem height and main root length were taken at this time.
Quantification of marker genes in tomato.
Defense marker gene expression was checked by real-time PCR in tomato seedlings grown under in vitro and in vivo conditions. In in vitro assays, sterilized tomato seeds were sown on 15-cm petri plates containing MS–1% sucrose supplemented with 0.8% agar (pH 5.7) and incubated for 1 week as indicated above. T6 conidia were inoculated on MS–1% sucrose plates containing 7-day-old germinated tomato seedlings (6 seedlings per plate), as indicated above. MS–1% sucrose plates containing only tomato seedlings, without conidia, were used as controls. The aerial part of each tomato seedling was collected at 6 days after inoculation with T6 or water (control), and it was used for RNA extraction with TRIzol reagent. Marker genes representative of salicylic acid (SA) (isochorismate synthase 1 [ICS1] and pathogenesis-related protein 1 [PR-1]), jasmonic acid (JA) (lipoxygenase 1 [LOX1]) and ethylene (ET) (ethylene-insensitive protein 2 [EIN2]) signaling pathways and abiotic stress responses (ascorbate peroxidase [APX1], abscisic acid [ABA]-responsive element binding protein 2 [AREB2], and salt overly sensitive 1 [SOS1]) were analyzed by real-time PCR. In in vivo assays, 4-week-old tomato plants grown in pots incubated in a greenhouse at 22 ± 4°C, as previously described (15), were irrigated with 2 ml of a 1 × 106 T6 conidia/ml or 2 ml water (control). The aerial part of each tomato plant was sampled at 0, 8, 24, and 48 h and 6 days and used for RNA extraction. ICS1, PR-1, LOX1, EIN2, AREB2, and SOS1 gene expression was analyzed using real-time PCR. The primer pairs used for real-time PCR of these genes, under the conditions indicated above, are shown in Table S1 in the supplemental material.
Effect of T. parareesei on the induction of tomato defenses against Botrytis.
Four-week-old tomato plants were irrigated with 2 ml of 1 × 106 T6 conidia/ml or 2 ml water (control). After 3 days, plants were leaf inoculated with B. cinerea B05.10 as previously described (34). The appearance of necrotic spots was assessed at 4 days after inoculation. Values of 0 (absence of reaction) to 7 (high necrotic reaction more than 1.5 cm diameter) were used to indicate the Botrytis lesion index. Data are shown as the mean value ± standard deviation from three experiments.
Accession numbers.
The sequences of the tef1, cal1, and las1 fragments were deposited in the GenBank database under accession numbers KF699130, KF699131, and KF699132, respectively. The microarray data are available at the GEO database with accession number GSE29171.
RESULTS
Molecular characterization of the T6 strain.
Strain T6, formerly identified as T. reesei (34), was subjected to further molecular analysis considering the present taxonomic frame of the Longibrachiatum clade. The sequences of the tef1, cal1, and las1 gene fragments were obtained, and the fourth intron of tef1 and the second and third introns of cal1 served to identify strain T6 as T. parareesei.
Antagonistic activity of the T6 strain.
Plate confrontation experiments between T6 and the pathogens P. irregulare, R. solani, and B. cinerea were carried out at 28°C, and plates were observed after 12 days of incubation (data not shown). The T6 strain antagonized the three pathogens in all the dual cultures, although different behaviors were observed: T6 completely overgrew the colonies of P. irregulare, partially overgrew (35 to 50% surface) the colonies of R. solani, and surrounded the colonies of B. cinerea.
Antagonism assays were also performed by growing T6 on cellophane and cellulose (10-kDa-cutoff) membranes to allow the diffusion of Trichoderma extracellular compounds into the medium. After removal of the membranes containing the mycelium, the effect of hydrolytic enzymes plus metabolites (cellophane) or only metabolites (cellulose) on the growth of the three pathogens was determined. Table 1 summarizes the percentages of growth inhibition of P. irregulare, R. solani, and B. cinerea by T6. It may be observed that the inhibition values for the three pathogens on cellophane were always higher than the inhibition values on cellulose. The highest inhibition values corresponded to P. irregulare on both types of membranes.
TABLE 1.
Colony growth inhibition of Pythium irregulare, Rhizoctonia solani, and Botrytis cinerea by hydrolytic enzymes/metabolites from Trichoderma parareesei T6 grown on cellophane or a 10-kDa-cutoff dialysis membrane for 2 days
| Species (time, h) | % growth inhibitiona on: |
|
|---|---|---|
| Cellophane | Dialysis membrane | |
| P. irregulare (72) | 100.0 ± 0.0 a | 73.4 ± 1.1 a |
| R. solani (72) | 61.8 ± 4.2 b | 33.9 ± 5.9 b |
| B. cinerea (120) | 65.0 ± 6.1 b | 30.1 ± 3.7 b |
Values are the means for three replicates with the corresponding standard deviations. Values followed by different letters are significantly different according to Duncan's test (P < 0.05).
Transcriptional response of T. parareesei at 20 h of interaction with tomato plants.
A transcriptomic analysis using a Trichoderma HDO microarray containing 34,138 probe sets was performed to ascertain the physiological and biochemical changes produced in T. parareesei T6 as consequence of 20 h of growth in the presence of tomato plants in MS–1% sucrose hydroponic cultures. Transcriptomic changes detected in T. parareesei were compared with those observed in a previous study of T. hamatum, T. harzianum, and T. virens interacting with tomato plants under identical experimental conditions (15). A total of 250 probe sets (0.73%) differed significantly (FDR, 0.10) in expression by at least 2-fold after 20 h of fungus-plant interaction. Thirty-three of these were upregulated, whereas 217 were downregulated. A majority of the differentially expressed probe sets (57.6% and 80.6% of the up- and downregulated sets, respectively) belonged to the T. reesei genome.
GO terms were assigned to 132 of the 250 probe sets (18 up- and 114 downregulated probe sets). We then examined whether these differentially expressed probe sets were associated with similar GO categories. This analysis revealed that no categories were significantly overrepresented in T. parareesei T6 in the presence of tomato plants. Accordingly, all the differentially expressed probe sets were analyzed independently and then grouped into several physiological processes, with T6 carbohydrate metabolism and transport being the most affected (Table 2). More detailed information about up- and downregulated probe sets, with indication of their corresponding orthologous numbering in the genomes of T. reesei and T. virens, is given in Table S2 in the supplemental material. The downregulation of a high number of genes involved in the carbohydrate metabolism and nutritional support of the fungus (β-galactosidase, alginate-lyase, β-mannosidase, etc.) and of genes related to basic cell functions (phosphoglycerate mutase, glucose dehydrogenase, 6-phosphofructo-2-kinase, 2-hydroxy-3-oxopropionate reductase, etc.) was observed, indicating that fungal metabolism was decreased in strain T6 after 20 h of interaction with tomato plants. Regarding transport processes, although the upregulation of two probe sets related to ABC transporters and two probe sets related to the major facilitator superfamily (MFS) transporters was detected, we also observed the downregulation of 16 probe sets related to other MFS transporters. In addition, we detected the downregulation of the genes involved in the metabolism of fatty acids and lipids, amino acids and proteins, nucleotides and nucleic acids, vitamins, and inorganic and nitrogen compounds, genes involved in energetic and secondary metabolism, and genes involved in several cellular processes, signaling (e.g., detoxification, adhesion, regulation, etc.), and information storage and processing.
TABLE 2.
Summary of the physiological processes expressed differentially for Trichoderma parareesei T6 in response to the presence of tomato plants in the culture medium in comparison to the basal medium
| Probe set | No. |
|---|---|
| Upregulated | |
| Metabolism | |
| Carbohydrate | 2 |
| Lipid and fatty acid | 1 |
| Secondary | 3 |
| Energy | 1 |
| Cellular processes and signaling | |
| Transport | 4 |
| Posttranslational events | 1 |
| Secretion | 1 |
| Cell wall and membranes | 2 |
| Signaling | 3 |
| Information storage and processing | |
| Transcription | 4 |
| Translation (protein synthesis) | 1 |
| Unknown function | 10 |
| Downregulated | |
| Metabolism | |
| Carbohydrate | 28 |
| Lipid and fatty acid | 7 |
| Amino acid | 5 |
| Protein | 2 |
| Nucleotide and nucleic acid | 6 |
| Vitamin | 3 |
| Secondary | 6 |
| Energy | 9 |
| Inorganic | 3 |
| Nitrogen compounds | 1 |
| Cellular processes and signaling | |
| Transport | 21 |
| Detoxification | 21 |
| Posttranslational events | 8 |
| Adhesion | 1 |
| Cell communication | 1 |
| Cell wall and membranes | 2 |
| Signaling | 6 |
| Regulation | 1 |
| Information storage and processing | |
| Replication | 1 |
| Transcription | 15 |
| Translation (protein synthesis) | 1 |
| Repair | 2 |
| Unknown function | 68 |
Of the 18 upregulated probe sets with assigned GOs, some were related to enzymes involved in the colonization process (endopolygalacturonase) (34), the biosynthesis of secondary metabolites or their precursors (saccharopine dehydrogenase, nonribosomal peptide synthases [NRPSs] and quinate dehydrogenase) (35), nutrient uptake (glucan 1,4-α-glucosidase and phospholipase) (36), and germ tube elongation and hyphal growth (saccharopine dehydrogenase) (37).
To confirm the microarray results, quantitative real-time PCR was performed to analyze the expression of 9 genes: 2 that were upregulated in the T6-tomato interaction (encoding an ABC transporter and a cellulose-signaling related protein) and 7 that were downregulated (encoding a phosphoglycerate mutase, a dehydrogenase reductase, an alcohol dehydrogenase, two MFS transporters, a cytochrome P450 monooxygenase, and a steroid monooxygenase). Expression of these genes correlated well with the data from the microarray experiments (see Fig. S1 in the supplemental material).
Effect of tomato plants on the growth of T. parareesei.
An in vitro assay was performed to evaluate the influence of different plant polymers on the growth of T. parareesei T6. Fungal growth was significantly enhanced, in comparison with that on MM plates, when 0.25% xylan or pectin polymers were added to the culture medium, with the largest colony size being observed in cultures containing pectin (Table 3). T6 growth in the presence of tomato plant exudates was also evaluated on 96-well E plates, as described in Materials and Methods. Tomato exudates significantly favored fungal growth compared to growth in MS–1% sucrose medium (control) (see Fig. S2 in the supplemental material).
TABLE 3.
Colony diameter of Trichoderma parareesei T6 on solid MM with 2% glucose and solid MM with 2% glucose plus 0.25% carboxymethylcellulose, polygalacturonic acid, xylan, or pectin
| Medium | Mycelial growth diameter (cm)a |
|---|---|
| MM | 2.79 ± 0.39 a |
| MM + 0.25% pectin | 6.57 ± 0.37 b |
| MM + 0.25% PGA | 3.15 ± 0.66 a |
| MM + 0.25% CMC | 3.42 ± 0.42 a |
| MM + 0.25% xylan | 5.10 ± 0.36 c |
Values are the means for three replicates with the corresponding standard deviations. Values followed by different letters are significantly different according to Duncan's test (P < 0.05).
Effect of T. parareesei on tomato development.
We studied the effect of T6 on the development of tomato seedling roots using an in vitro assay. This strain favored seedling root development, increasing the number and length of the lateral roots compared to those under the control condition (Fig. 1).
FIG 1.
Effect of Trichoderma parareesei T6 on tomato seedlings. Three-day-old germinated tomato seedlings grown on MS medium supplemented with 1% (wt/vol) sucrose and 0.8% agar (pH 5.7) were inoculated with water (A) and T. parareesei T6 (B). Photographs were taken at 4 days after inoculating 1 × 106 T6 conidia.
In vivo assay results revealed that there were no significant differences in stem and root lengths of 4-week-old tomato plants between the two conditions (Table 4). Plants from T6 conidium-treated seeds had stem and root lengths of 28.1 ± 2.9 and 25.8 ± 3.9 cm, respectively, and plants from water-treated seeds (control) had stem and root lengths of 27.4 ± 3.6 and 25.7 ± 3.5 cm, respectively.
TABLE 4.
Effect of Trichoderma parareesei T6 and salt stress on the growth of tomato plants
| Plants | Length (cm)a |
|||
|---|---|---|---|---|
| Stem |
Root |
|||
| Water | NaCl | Water | NaCl | |
| Control | 27.45 ± 3.56 b | 23.33 ± 4.65 a | 25.75 ± 3.54 b | 21.41 ± 3.76 a |
| T6 inoculated | 28.14 ± 2.89 b | 28.69 ± 2.71 b | 25.85 ± 3.90 b | 29.71 ± 1.85 c |
Root and stem length values correspond to 4-week-old tomato plants developed from untreated (control) or T. parareesei T6-treated seeds, which were treated daily with 5 ml of either water (water irrigation) or 100 mM NaCl (salt treatment) for 10 days. Values are the means for two biological replicates and 28 seeds per condition, with the corresponding standard deviations. In each experimental data set (stem or root), values followed by different letters are significantly different according to Duncan's test (P < 0.05).
Effect of T. parareesei on defense marker gene expression in tomato plants.
To test whether the tomato response to T. parareesei T6 involved the differential activation of systemic defense-related genes, we analyzed markers of the SA (ICS1 and PR-1), JA (LOX1), and ET (EIN2) pathways and markers of the abiotic stress response (APX1, AREB2, and SOS1) using real-time PCR in tomato samples from the in vitro and in vivo assays.
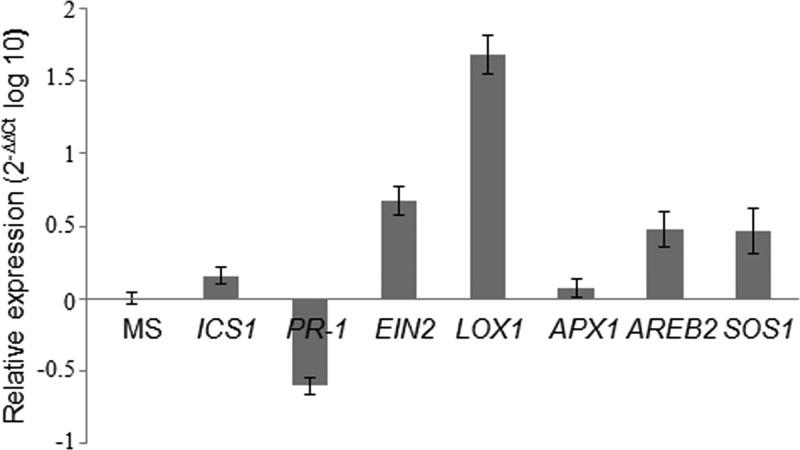
Marker gene expression in tomato seedlings from in vitro assays is shown in Fig. 2. In comparison with the control, tomato seedlings inoculated with T6 showed significantly increased expression levels of the EIN2 and LOX1 genes, whereas the SA response PR-1 gene expression level was reduced and the SA biosynthesis ICS1 gene was not significantly affected. These results show that the JA and ET signaling pathways were induced at 6 days after T6 inoculation in 7-day-old tomato seedlings, which indicates an induced systemic resistance (ISR) response provoked by T. parareesei. At the same time, two genes related to plant defense to abiotic stress conditions were upregulated in tomato seedlings (Fig. 2): the expression levels of the AREB2 and SOS1 genes, which are, respectively, related to ABA responses and salt tolerance, were increased in tomato seedlings challenged with T6. However, the expression of the oxidative stress tolerance APX1 marker gene was not significantly affected in tomato seedlings inoculated with T6. These results were complemented with a time course in vivo assay carried out on 4-week-old tomato plants to detect defense responses at shorter interaction times. The expression of marker genes in the aerial part of tomato plants sampled at 0, 8, 24, and 48 h and 6 days after T6 inoculation was analyzed (Fig. 3). Depending on the genes, an undulating expression response (alternately up and down) was observed along the time course study. Maximum expression peaks were detected for LOX1, EIN2, ICS1, and SOS1 at 24 h, while PR-1 showed a maximum expression peak at 48 h. In all cases, upregulation of these genes was observed in the 6-day samples. AREB2 displayed a different expression pattern, with a maximum at 8 h, which coincided with the low expression levels of EIN2 and ICS1.
FIG 2.
Quantitative real-time PCR of the ICS1, PR-1, EIN2, LOX1, APX1, AREB2, and SOS1 genes in tomato seedlings inoculated with Trichoderma parareesei T6. Total RNA was extracted from the aerial part of tomato seedlings at 6 days after inoculation with water (control) or 1 × 106 T6 conidia on plates containing 7-day-old seedlings. The actin gene was used as a control. Values correspond to relative measurements against their respective transcripts in control plates (2−ΔΔCT = 1) and are expressed as log10 relative quantification (2−ΔΔCT). Error bars represent standard deviations of the mean values for two biological replicates.
FIG 3.
Relative expression analysis of defense-related genes in 4-week-old tomato plants inoculated with Trichoderma parareesei. Total RNA from the aerial part of tomato plants inoculated with strain T6 was subjected to real-time PCR to quantify six genes related to different plant defense responses: ICS1 (SA biosynthesis), PR-1 (SA signaling pathway), EIN2 (ET signaling pathway), LOX1 (JA signaling pathway), AREB2 (ABA signaling pathway), and SOS1 (salt tolerance). The actin gene was used as a control. Values correspond to relative measurements against their respective uninoculated controls (2−ΔΔCT = 1) and are expressed as log10 relative quantification (2−ΔΔCT). Error bars represent standard deviations of the mean values for three technical replicates from a cDNA of four pooled biological replicates.
Effect of T. parareesei on induction of tomato defense against Botrytis.
When 4-week-old tomato plants were inoculated with T6 and were leaf inoculated with B. cinerea 3 days later, the necrotic spots produced by this pathogen showed a significantly lower mean lesion index than that observed in plants not treated with T. parareesei (see Fig. S3 in the supplemental material).
Effect of T. parareesei on salt-stressed tomato plants.
In vitro assays revealed that under 10 or 25 mM NaCl stress, tomato seedlings inoculated with T6 developed more and longer lateral roots than uninoculated seedlings (see Fig. S4 in the supplemental material). Although seed germination decreased from 80% to 40% when NaCl was increased from 10 to 25 mM in the medium, the beneficial effect of T6 on lateral root development was observed even at the highest salt concentration.
When T6-treated, 4-week-old tomato plants were assayed in vivo for salt tolerance, the stem height and root length values were significantly higher than those measured in the control plants (Table 4). In addition, the T6-treated plants displayed a lower degree of chlorophyll bleaching, indicative of a better tolerance to this abiotic stress, than that observed in the untreated plants (see Fig. S5 in the supplemental material).
DISCUSSION
In recent years, molecular plant-Trichoderma interactions have been explored in different species and plant systems (5, 7). Most studies have been carried out with known biocontrol strains, and with a few exceptions, such as T. pseudokoningii (25), they have left the abilities of Longibrachiatum clade members practically untouched. The present study attempted to fill that gap by analyzing the mutualistic interactions between T. parareesei and tomato plants in terms of fungal transcriptomic changes and hyphal growth as well as plant stimulation of defense and development.
Mycoparasitism is an ancestral property of the genus Trichoderma, and genomic data indicate that T. reesei partially lost the ability to antagonize, parasitize, or even kill other fungi (38, 39). A recent study has confirmed the reduced mycoparasitic potential of T. reesei compared with those of the strongly mycoparasitic species T. atroviride and T. virens (40). Despite the close genetic position of T. reesei and T. parareesei, T. parareesei has kept an antagonistic potential (21). In the present study, in vitro assays carried out with strain T6 against three phytopathogens confirm the antagonistic abilities of T. parareesei. As would be expected due to the powerful production of cellulolytic enzymes by T. parareesei, the greatest antagonistic control was detected against the oomycete P. irregulare, a pathogen with a cell wall composed mainly of cellulose. Although similar R. solani and B. cinerea growth inhibition by T6 was recorded in the cellophane or cellulose membrane assays, different mycoparasitic behaviors were observed in the dual cultures, as R. solani colonies were overgrown while B. cinerea colonies were surrounded by T6. Interestingly, four strains of T. parareesei had previously shown a conserved pattern of mycoparasitic activity against B. cinerea, overgrowing the colonies of this pathogen (21).
The rhizosphere is among the most common ecological niches for Trichoderma spp., and it provides opportunities for both biotrophy and saprotrophy on plant roots (39). As could be expected from a rhizosphere-competent fungus, T. parareesei T6 showed increased growth when plant polymers were added to MM or a liquid medium containing root exudates. The significant increase in T6 growth that was detected in the presence of pectin, xylan, or other substrates released from the mucigel supports the notion that root-derived nutrients are attractors for T. parareesei to colonize the rhizosphere and develop interactions with roots as a symbiotic lifestyle with plants. The ecophysiological adaptation to the rhizosphere niche is in agreement with the carbon source utilization plasticity described for this species (21). Sucrose is a main component of root exudates and can be used as a carbon source by T. parareesei, as previously observed (21) and now confirmed in the present work, with T6 growth in MS–1% sucrose.
To facilitate comparisons with other Trichoderma species, a T6 transcriptomic analysis was performed at 20 h of interaction with tomato plants, using the same Trichoderma microarray system and experimental conditions as in a previous study with strains of T. hamatum, T. harzianum, and T. virens colonizing tomato roots (15). A major limitation of this microarray approach was the DNA chip used; although it contained 374,874 probes representing a total of 34,138 gene transcripts from 14 Trichoderma strains, it lacked T. parareesei probe sets, which could lead to false-negative results. However, the complete genome of T. reesei, the closest species to T. parareesei, was deposited in the DNA chip. In this sense, among the 250 probe sets differing significantly in expression, 57.6% of those upregulated genes and 80.6% of those downregulated genes were orthologous to T. reesei. Another limiting factor may be the standardization of culture conditions in the fungus-plant interactions when the objective is to analyze transcriptomic changes in the fungus. While T. parareesei was inoculated in a chemically defined MS–1% sucrose medium as a control, mycelia for the test condition were obtained in a complex medium in the presence of tomato seedlings in MS–1% sucrose plus the modification of the MS medium and sucrose content due to the germination of tomato seeds sowed 15 days before plus the exudates released from the seedlings. Thus, the appropriate amount of nutrients available for fungal growth to achieve adequate control was difficult to estimate. This limitation would not affect comparisons with previous Trichoderma microarrays performed under the same conditions.
In T6, relatively few genes were upregulated, and the number of differentially expressed probe sets was very different from those obtained for the three known biocontrol strains in which upregulated probe sets were predominant (15). Among these, carbohydrate metabolism and transport processes were overrepresented in T. hamatum, T. harzianum, and T. virens during their interactions with tomato plants, whereas no GO categories were significantly overrepresented in T6 at 20 h under identical conditions. Unlike the marked metabolic upregulation observed in T. harzianum (15), T6 displayed the downregulation of a large number of genes involved in carbohydrate metabolism, nutritional support, and basic cell functions, indicating that carbohydrate flow between the fungus and the plant was significantly low at 20 h of interaction between these two partners. The predominance of downregulated transport genes suggests that after hyphal root attachment and colonization, T. parareesei T6 nutrient uptake would be limited, at least at an early stage, as also described for T. hamatum during interaction with tomato plants (15). However, these results are not in agreement with the upregulation of transport genes detected in the transcriptomes of T. harzianum and T. virens in interaction with plants (14–16). Despite the low metabolism of T6 at 20 h, this strain was able to increase the lateral root development of tomato seedlings in the first 2 to 4 days after application; this increase was similar to the increases observed with T. hamatum- or T. harzianum-inoculated tomato seedlings (15). This would certainly mean that a positive interaction occurs between T6 and the tomato plant. Although the fungus is somewhat inactive metabolically at 20 h, two ABC transporters and two MFS transporters were upregulated in tomato-colonizing T6. Proteomic and macroarray studies have reported that an ABC transporter is also upregulated in T. atroviride and T. harzianum when the fungi interact with plants (9, 14).
Interestingly, the reduced number of upregulated probe sets included a gene orthologous to Thpg1, encoding a T. harzianum endopolygalacturonase involved in efficient root colonization and the induction of plant defenses (34). We also observed the upregulation of a probe set corresponding to a phospholipase. This class of enzymes is ubiquitous and is involved in diverse cellular responses, including membrane disruption processes during host cell invasion (41). Recently, the upregulation of a T. harzianum phospholipase A2 during the colonization of tomato-germinating seeds has been reported (36).
Another upregulated probe set showed homology with the chimeric enzyme spermidine synthase/saccharopine dehydrogenase, which is involved in spermidine biosynthesis and lysine metabolism and plays a role in the alpha-aminoadipic acid pathway. Increasing levels of spermidine are required by Aspergillus nidulans for the transitions from germ tube to hyphal growth mode to tissue differentiation and secondary metabolite production (37). Saccharopine dehydrogenase activity is involved in the step from lysine to pipecolic acid, which can be used by NRPSs as a substrate to synthesize secondary metabolites. Curiously, an NRPS of T6 was upregulated in interactions with the tomato plant, as well as a quinate dehydrogenase, which plays a major role in the shikimate pathway for aromatic amino acids and SA biosynthesis. The chorismate mutase gene, which also is involved in the shikimate pathway, was upregulated in T6 in interaction with tomato roots (E. Pérez, unpublished data).
Although it could be expected that hydrophobin and hydrophobin-like (ceratoplatanin) proteins related to fungal interaction with the environment (42), root colonization (43), and the induction of systemic defense in plants (44, 45) would be upregulated in T. parareesei, hydrophobin downregulation due the presence of plants has also been reported in T. virens (15).
As observed in the mycelia of the three known biocontrol strains, it was evident that the T6 mycelium was attached to the roots at 20 h after inoculation. The relatively low metabolism detected in T6 during the plant-fungus interaction, with respect to its control, could be the result of the tomato plants modifying the composition of the medium. It may be difficult to compare the T6 interaction with other Trichoderma-plant interactions because the optimal growth temperature range described for T. parareesei (22) is higher than that of the biocontrol species of Trichoderma.
In contrast to a report that both T. parareesei and T. reesei inhibited the growth of garden cress (Lepidium sativum) seedlings in plate assays (22), our in vitro results for the T6-tomato interaction show a typical auxin effect on increased lateral root development (Fig. 1), as has already been demonstrated for other Trichoderma spp. (46), and also show systemic modifications in the plant, observed as expression changes of genes related to defense against pathogens and abiotic stresses (Fig. 2 and 3). The discrepancy between the studies could be due to different experimental approaches. Root colonization by Trichoderma triggers a systemic response signaled by JA/ET in a way similar to that described for rhizobacteria (47). Systemic defenses induced by Trichoderma are also signaled by SA (48, 49), though they vary depending on the plant genetic background, the interaction time, and the strain and inoculum size (5, 50). In the present study, the in vitro assay showed that T. parareesei triggers defense responses against pathogens and salt stress in tomato seedlings. The time course in vivo analysis showed that such responses undulate in the tomato plants, which is compatible with the model proposed in Arabidopsis, where temporal phases of hormone signaling underpin the establishment of systemic immunity (51). Expression peaks observed at 24 or 48 h for JA/ET or SA signaling gene markers are in agreement with a previously reported model of mutual antagonism between the SA and JA/ET signaling pathways (52).
Because in vitro and in vivo growth are two markedly different systems with distinct experimental conditions, gene expression comparisons between these two assays should be cautiously interpreted. Nevertheless, with the exception of PR-1 expression, the expression of the tested genes (ICS1, EIN2, LOX1, and AREB2) was significantly upregulated at 6 days in both systems.
A previous study of the T. harzianum-Arabidopsis interaction (12) showed an initial downregulation of SA-related genes, followed by an increase in their expression; this agrees with the PR-1 expression pattern observed in the tomato plants during the first 48 h after T6 inoculation. The relatively low PR-1 expression at 8 and 24 h and the ICS1 expression increase at 24 h, followed by a marked PR-1 expression increase at 48 h, would support the hypothesis that these patterns take place to limit the penetration of the fungus into the first few layers of root cortical cells, as previously described for other Trichoderma spp. (14, 53).
In accordance with previous studies in which root application of T. harzianum or T. atroviride was able to reduce the leaf lesion area caused by B. cinerea (49, 54, 55), T. parareesei T6 also triggered ISR against this foliar pathogen.
The beneficial effects of Trichoderma are more apparent in plants subjected to some type of stress (7), although the mechanisms controlling multiple plant stress factors are still being explored. Tomato seed treatment with T. harzianum confers protection against oxidative damage, alleviating the biotic, abiotic, and physiological stresses of germinating seeds and seedlings (56). We had observed the upregulation of several genes related to responses against abiotic stresses in Arabidopsis after 24 h of incubation in the presence of T. harzianum (12), and a recent study reported that Trichoderma can induce the expression of APX1 in salt-stressed Arabidopsis plants (57). The slight APX1 upregulation observed in in vitro assays and the absence of significant expression changes in the time course study (data not shown) could be due to the absence of salt-stressed plants in these assays. However, the salt tolerance SOS1 gene (58) displayed an undulating expression pattern, similar to that observed for LOX1 (Fig. 3), which could agree with the salt tolerance observed in tomato plants that were inoculated with T6 (Table 4; see Fig. S4 and S5 in the supplemental material).
AREB2 is a transcription factor that requires ABA for full activation of the response and tolerance to salt stress (59), and the transcription factor EIN2 is a cross-link node in the ET, ABA, and stress signaling pathways (60). The time course expression pattern of AREB2 observed in T6-challenged tomato plants was mutually antagonistic with the patterns detected for EIN2 and ICS1. An explanation for this behavior could be that EIN2 acts as a negative regulator of ABA biosynthesis during early growth under normal conditions (60), and in turn, a suppressive effect of ABA on the SA signaling pathway has been previously reported (61). This could also explain why T. parareesei T6-treated tomato plants subjected to salt stress showed better growth than uninoculated tomato plants, because an EIN2 downregulation induced by salt stress could lead to an increase in ABA levels.
The antagonistic potential and the positive effects of T6 on plants observed in the present study could suggest the use of T. parareesei in plant protection as a biocontrol agent and/or as a plant-beneficial microbe. However, because T. parareesei has an optimal growth temperature of between 35 and 40°C and is closely related to human pathogens (i.e., T. longibrachiatum), risk assessment studies are needed before considering any in vivo application of this species in commercial agriculture. In any case, the present study will serve as a basis for further investigations into biocontrol and plant biostimulation of the so-far-neglected Trichoderma species within the major Longibrachiatum clade.
Supplementary Material
ACKNOWLEDGMENTS
Research project funding was from Spanish Government projects MICINN (AGL2009-13431-C02-01) and MINECO (AGL2012-40041-C02-01). Narciso M. Quijada was supported by a fellowship from the Spanish Ministry of Education, Esclaudys Pérez by a Spanish Foreign Office AECID award, and Sara Domínguez by a fellowship from the Regional Government of Castile & Leon.
Footnotes
Published ahead of print 10 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03375-13.
REFERENCES
- 1.Howell CR. 2003. Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 87:4–10. 10.1094/PDIS.2003.87.1.4 [DOI] [PubMed] [Google Scholar]
- 2.Rubio MB, Hermosa R, Reino JL, Collado IG, Monte E. 2009. Thctf1 transcription factor of Trichoderma harzianum is involved in 6-pentyl-2H-pyran-2-one production and antifungal activity. Fungal Genet. Biol. 46:17–27. 10.1016/j.fgb.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 3.Vinale F, Marra R, Scala F, Ghisalberti EL, Lorito M, Sivasithamparam K. 2006. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett. Appl. Microbiol. 43:143–148. 10.1111/j.1472-765X.2006.01939.x [DOI] [PubMed] [Google Scholar]
- 4.Benítez T, Rincón AM, Limón MC, Codón AC. 2004. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 7:249–260 [PubMed] [Google Scholar]
- 5.Hermosa R, Viterbo A, Chet I, Monte E. 2012. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 158:17–25. 10.1099/mic.0.052274-0 [DOI] [PubMed] [Google Scholar]
- 6.Mastouri F, Björkman T, Harman GE. 2012. Trichoderma harzianum enhances antioxidant defense of tomato seedlings and resistance to water deficit. Mol. Plant Microbe Interact. 25:1264–1271. 10.1094/MPMI-09-11-0240 [DOI] [PubMed] [Google Scholar]
- 7.Shoresh M, Harman GE, Mastouri F. 2010. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 48:21–43. 10.1146/annurev-phyto-073009-114450 [DOI] [PubMed] [Google Scholar]
- 8.Alfano G, Ivey MLL, Cakir C, Bos JIB, Miller SA, Madden LV, Kamoun S, Hoitink HAJ. 2007. Systemic modulation of gene expression in tomato by Trichoderma hamatum 382. Phytopathology 97:429–437. 10.1094/PHYTO-97-4-0429 [DOI] [PubMed] [Google Scholar]
- 9.Marra R, Ambrosino P, Carbone V, Vinale F, Woo SL, Ruocco M, Ciliento R, Lanzuise S, Ferraioli S, Soriente I, Gigante S, Turrà D, Fogliano V, Scala F, Lorito M. 2006. Study of the three-way interaction between Trichoderma atroviride, plant and fungal pathogens by using a proteomic approach. Curr. Genet. 50:307–321. 10.1007/s00294-006-0091-0 [DOI] [PubMed] [Google Scholar]
- 10.Segarra G, Casanova E, Bellido D, Odena MA, Oliveira E, Trillas I. 2007. Proteome, salicylic acid, and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum strain T34. Proteomics 7:3943–3952. 10.1002/pmic.200700173 [DOI] [PubMed] [Google Scholar]
- 11.Shoresh M, Harman GE. 2008. The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: a proteomic approach. Plant Physiol. 147:2147–2163. 10.1104/pp.108.123810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morán-Diez E, Rubio B, Domínguez S, Hermosa R, Monte E, Nicolás C. 2012. Transcriptomic response of Arabidopsis thaliana after 24h incubation with the biocontrol fungus Trichoderma harzianum. J. Plant Physiol. 169:614–620. 10.1016/j.jplph.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 13.Bailey BA, Bae H, Strem MD, Roberts DP, Thomas SE, Crozier J, Samuels GJ, Choi Ik-Young, Holmes KA. 2006. Fungal and plant gene expression during the colonization of cacao seedlings by endophytic isolates of four Trichoderma species. Planta 224:1449–1464. 10.1007/s00425-006-0314-0 [DOI] [PubMed] [Google Scholar]
- 14.Chacón M, Rodríguez-Galán O, Benítez T, Sousa S, Rey M, Llobell A, Delgado-Jarana J. 2007. Microscopic and transcriptome analysis of early colonization of tomato roots by Trichoderma harzianum. Int. Microbiol. 10:19–27. 10.2436/20.1501.01.4 [DOI] [PubMed] [Google Scholar]
- 15.Rubio MB, Domínguez S, Monte E, Hermosa R. 2012. Comparative study of Trichoderma gene expression in interactions with tomato plants using high-density oligonucleotide microarrays. Microbiology 158:119–128. 10.1099/mic.0.052118-0 [DOI] [PubMed] [Google Scholar]
- 16.Samolski I, de Luis A, Vizcaíno JA, Monte E, Suárez MB. 2009. Gene expression analysis of the biocontrol fungus Trichoderma harzianum in the presence of tomato plants, chitin, or glucose using a high-density oligonucleotide microarray. BMC Microbiol. 9:217. 10.1186/1471-2180-9-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubicek CP, Mikus M, Schuster A, Schmoll M, Seiboth B. 2009. Metabolic engineering strategies for the improvement of cellulose production by Hypocrea jecorina. Biotechnol. Biofuels 2:19. 10.1186/1754-6834-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nevalainen H, Penttilä M. 2004. Molecular biology of cellulolytic fungi, p 369–390 In Kück U. (ed), The Mycota, 2nd ed, vol II Genetics and biotechnology. Springer-Verlag, Heidelberg, Germany [Google Scholar]
- 19.Penttilä M, Limón C, Nevalainen H. 2004. Molecular biology of Trichoderma and biotecnological applications, p 413–427 In Arora DK. (ed), Handbook of fungal biotechnology. Marcel Dekker, Basel, Switzerland [Google Scholar]
- 20.Nevalainen H, Te'o V, Penttilä M, Pakula T. 2005. Heterologous gene expression in filamentous fungi: a holistic view, p 211–237 In Arora DK, Berka R. (ed), Applied mycology and biotechnology, genes and genomics, vol 5 Elsevier, Amsterdam, Netherlands [Google Scholar]
- 21.Druzhinina IS, Komon-Zelazowska M, Atanasova L, Seidl V, Kubicek CP. 2010. Evolution and ecophysiology of the industrial producer Hypocrea jecorina (anamorph Trichoderma reesei) and a new sympatric agamospecies related to it. PLoS One 5:e9191. 10.1371/journal.pone.0009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atanasova L, Jaklitsch WM, Komon-Zelazowska M, Kubicek CP, Druzhinina IS. 2010. Clonal species Trichoderma parareesei sp. nov. likely resembles the ancestor of the cellulose producer Hypocrea jecorina/T. reesei. Appl. Environ. Microbiol. 76:7259–7267. 10.1128/AEM.01184-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Druzhinina IS, Komon-Zelazowska M, Ismaiel A, Jaklitsch W, Mullaw T, Samuels GJ, Kubicek CP. 2012. Molecular phylogeny and species delimitation in the section Longibrachiatum of Trichoderma. Fungal Genet. Biol. 49:358–368. 10.1016/j.fgb.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez A, Obertello M, Pardo A, Ocampo JA, Godeas A. 2004. Interactions between Trichoderma pseudokoningii strains and the arbuscular mycorrhizal fungi Glomus mosseae and Gigaspora rosea. Mycorrhiza 14:79–84. 10.1007/s00572-003-0240-y [DOI] [PubMed] [Google Scholar]
- 25.Luo Y, Zhang DD, Dong XW, Zhao PB, Chen LL, Song XY, Wang XJ, Chen XL, Shi M, Zhang YZ. 2010. Antimicrobial peptaibols induce defense responses and systemic resistance in tobacco against tobacco mosaic virus. FEMS Microbiol. Lett. 313:120–126. 10.1111/j.1574-6968.2010.02135.x [DOI] [PubMed] [Google Scholar]
- 26.Shi M, Chen L, Wang XW, Zhang T, Zhao PB, Song XY, Sun CY, Chen XL, Zhou BC, Zhang YZ. 2012. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 158:166–175. 10.1099/mic.0.052670-0 [DOI] [PubMed] [Google Scholar]
- 27.Hermosa R, Rubio MB, Cardoza RE, Nicolás C, Monte E, Gutiérrez S. 2013. The contribution of Trichoderma to balancing the costs of plant growth and defense. Int. Microbiol. 16:69–80 [DOI] [PubMed] [Google Scholar]
- 28.Raeder U, Broda P. 1985. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1:17–20. 10.1111/j.1472-765X.1985.tb01479.x [DOI] [Google Scholar]
- 29.Druzhinina IS, Komon-Zelazowska M, Kredics L, Hatvani L, Zsuzsanna Antal, Belayneh T, Kubicek CP. 2008. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology 154:3447–3459. 10.1099/mic.0.2008/021196-0 [DOI] [PubMed] [Google Scholar]
- 30.Penttilä M, Nevalainen H, Ratto M, Salminen E, Knowles J. 1987. A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene 61:155–164. 10.1016/0378-1119(87)90110-7 [DOI] [PubMed] [Google Scholar]
- 31.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf UU, Speed TP. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264. 10.1093/biostatistics/4.2.249 [DOI] [PubMed] [Google Scholar]
- 32.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. 2000. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25:25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−delta delta CT) method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 34.Morán-Diez E, Hermosa R, Ambrosino P, Cardoza RE, Gutiérrez S, Lorito M, Monte E. 2009. The ThPG1 endopolygalacturonase is required for the Trichoderma harzianum-plant beneficial interaction. Mol. Plant Microbe Interact. 22:1021–1031. 10.1094/MPMI-22-8-1021 [DOI] [PubMed] [Google Scholar]
- 35.Strieker M, Tanovic A, Marahiel MA. 2010. Nonribosomal peptide synthetases: structures and dynamics. Curr. Opin. Struct. Biol. 20:234–240. 10.1016/j.sbi.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 36.Mehrabi-Koushki M, Rouhani H, Mahdikhani-Moghaddam E. 2012. Differential display of abundantly expressed genes of Trichoderma harzianum during colonization of tomato-germinating seeds and roots. Curr. Microbiol. 65:524–533. 10.1007/s00284-012-0189-1 [DOI] [PubMed] [Google Scholar]
- 37.Jin Y, Bok JW, Guzman-de-Peña D, Keller NP. 2002. Requirement of spermidine for developmental transitions in Aspergillus nidulans. Mol. Microbiol. 46:801–812. 10.1046/j.1365-2958.2002.03201.x [DOI] [PubMed] [Google Scholar]
- 38.Kubicek CP, Herrera-Estrella A, Seidl-Seiboth V, Martinez DA, Druzhinina IS, Thon M, Zeilinger S, Casas-Flores S, Horwitz BA, Mukherjee PK, Mukherjee M, Kredics L, Alcaraz LD, Aerts A, Antal Z, Atanasova L, Cervantes-Badillo MG, Challacombe J, Chertkov O, McCluskey K, Coulpier F, Deshpande N, von Döhren H, Ebbole DJ, Esquivel-Naranjo EU, Fekete E, Flipphi M, Glaser F, Gómez-Rodríguez EY, Gruber S, Han C, Henrissat B, Hermosa R, Hernández-Oñate M, Karaffa L, Kosti I, Le Crom S, Lindquist E, Lucas S, Lübeck M, Lübeck PS, Margeot A, Metz B, Misra M, Nevalainen H, Omann M, Packer N, Perrone G, Uresti-Rivera EE, Salamov A, Schmoll M, Seiboth B, Shapiro H, Sukno S, et al. 2011. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 12:R40. 10.1186/gb-2011-12-4-r40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, Monte E, Mukherjee PK, Zeilinger S, Grigoriev IV, Kubicek CP. 2011. Trichoderma: the genomics of opportunistic success. Nat. Rev. Microbiol. 9:749–759. 10.1038/nrmicro2637 [DOI] [PubMed] [Google Scholar]
- 40.Atanasova L, Le Crom S, Gruber S, Coulpier F, Seidl-Seiboth V, Kubicek CP, Druzhinina IS. 2013. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genomics 14:121. 10.1186/1471-2164-14-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Istivan TS, Coloe PJ. 2006. Phospholipase A in Gram-negative bacteria and its role in pathogenesis. Microbiology 152:1263–1274. 10.1099/mic.0.28609-0 [DOI] [PubMed] [Google Scholar]
- 42.Seidl-Seiboth V, Gruber S, Sezerman U, Schwecke T, Albayrak A, Neuhof T, von Döhren H, Baker SE, Kubicek CP. 2011. Novel hydrophobins from Trichoderma define a new hydrophobin subclass: protein properties, evolution, regulation and processing. J. Mol. Evol. 72:339–351. 10.1007/s00239-011-9438-3 [DOI] [PubMed] [Google Scholar]
- 43.Viterbo A, Chet I. 2006. TasHyd1, a new hydrophobin gene from the biocontrol agent Trichoderma asperellum, is involved in plant root colonization. Mol. Plant Pathol. 7:249–258. 10.1111/j.1364-3703.2006.00335.x [DOI] [PubMed] [Google Scholar]
- 44.Djonovic S, Pozo MJ, Dangott LJ, Howell CR, Kenerley CM. 2006. Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol. Plant Microbe Interact. 19:838–853. 10.1094/MPMI-19-0838 [DOI] [PubMed] [Google Scholar]
- 45.Seidl V, Marchetti M, Schandl R, Allmaier G, Kubicek CP. 2006. Epl1, the major secreted protein of Hypocrea atroviridis on glucose, is a member of a strongly conserved protein family comprising plant defense response elicitors. FEBS J. 273:4346–4359. 10.1111/j.1742-4658.2006.05435.x [DOI] [PubMed] [Google Scholar]
- 46.Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J. 2009. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 149:1579–1592. 10.1104/pp.108.130369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoresh M, Yedidia I, Chet I. 2005. Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 95:76–84. 10.1094/PHYTO-95-0076 [DOI] [PubMed] [Google Scholar]
- 48.Gallou A, Cranenbrouck S, Declerck S. 2009. Trichoderma harzianum elicits defence response genes in roots of potato plantlets challenged by Rhizoctonia solani. Eur. J. Plant Pathol. 124:219–230. 10.1007/s10658-008-9407-x [DOI] [Google Scholar]
- 49.Salas-Marina MA, Silva-Flores MA, Uresti-Rivera EE, Castro-Longoria E, Herrera-Estrella A, Casas-Flores S. 2011. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 131:15–26. 10.1007/s10658-011-9782-6 [DOI] [Google Scholar]
- 50.Tucci M, Ruocco M, de Masi L, de Palma M, Lorito M. 2011. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 12:341–354. 10.1111/j.1364-3703.2010.00674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Truman WM, Bennett MH, Turnbull CGN, Grant MR. 2010. Arabidopsis auxins mutants are compromised in systemic acquired resistance and exhibit aberrant accumulation of various indolic compounds. Plant Physiol. 152:1562–1573. 10.1104/pp.109.152173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. 2009. Networking by small-molecule hormones in plant inmunity. Nat. Chem. Biol. 5:308–316. 10.1038/nchembio.164 [DOI] [PubMed] [Google Scholar]
- 53.Yedidia I, Benhamou N, Chet I. 1999. Induction of defense responses in cucumber plants (Cucumis sativus L.) by the biocontrol agent Trichoderma harzianum. Appl. Environ. Microbiol. 65:1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Meyer G, Bigirimana J, Elad Y, Höfte M. 1998. Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea. Eur. J. Plant Pathol. 104:279–286. 10.1023/A:1008628806616 [DOI] [Google Scholar]
- 55.Korolev N, David DR, Elad Y. 2008. The role of phytohormones in basal resistance and Trichoderma-induced systemic resistance to Botrytis cinerea in Arabidopsis thaliana. Biocontrol 53:667–683. 10.1007/s10526-007-9103-3 [DOI] [Google Scholar]
- 56.Mastouri F, Björkman T, Harman GE. 2010. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 100:1213–1221. 10.1094/PHYTO-03-10-0091 [DOI] [PubMed] [Google Scholar]
- 57.Brotman Y, Landau U, Cuadros-Inostroza Á, Takayuki T, Fernie AR, Chet I, Viterbo A, Willmitzer L. 2013. Trichoderma-plant root colonization: escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 9:e1003221. 10.1371/journal.ppat.1003221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi H, Ishitani M, Kim C, Zhu JK. 2000. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. U. S. A. 97:6896–6901. 10.1073/pnas.120170197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K. 2000. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. U. S. A. 97:11632–11637. 10.1073/pnas.190309197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. 2010. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 61:672–685. 10.1111/j.1365-313X.2009.04092.x [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Liu C, Li K, Sun F, Hu H, Li X, Zhao Y, Han C, Zhang W, Duan Y, Liu M, Li X. 2007. Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Mol. Biol. 64:633–644. 10.1007/s11103-007-9182-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.