Abstract
ExpA (GacA) is a global response regulator that controls the expression of major virulence genes, such as those encoding plant cell wall-degrading enzymes (PCWDEs) in the model soft rot phytopathogen Pectobacterium wasabiae SCC3193. Several studies with pectobacteria as well as related phytopathogenic gammaproteobacteria, such as Dickeya and Pseudomonas, suggest that the control of virulence by ExpA and its homologues is executed partly by modulating the activity of RsmA, an RNA-binding posttranscriptional regulator. To elucidate the extent of the overlap between the ExpA and RsmA regulons in P. wasabiae, we characterized both regulons by microarray analysis. To do this, we compared the transcriptomes of the wild-type strain, an expA mutant, an rsmA mutant, and an expA rsmA double mutant. The microarray data for selected virulence-related genes were confirmed through quantitative reverse transcription (qRT-PCR). Subsequently, assays were performed to link the observed transcriptome differences to changes in bacterial phenotypes such as growth, motility, PCWDE production, and virulence in planta. An extensive overlap between the ExpA and RsmA regulons was observed, suggesting that a substantial portion of ExpA regulation appears to be mediated through RsmA. However, a number of genes involved in the electron transport chain and oligogalacturonide metabolism, among other processes, were identified as being regulated by ExpA independently of RsmA. These results suggest that ExpA may only partially impact fitness and virulence via RsmA.
INTRODUCTION
The virulence of soft rot phytopathogens such as Pectobacterium wasabiae strain SCC3193 depends on the coordinated production and secretion of plant cell wall-degrading enzymes (PCWDEs), the main virulence factors of this pathogen (1–6). These factors are regulated in part by quorum-sensing signals, two-component regulatory systems (TCS) responding to environmental signals such as those from plants, and posttranscriptional mechanisms (1, 6–8). In SCC3193, a central TCS required for virulence is the ExpS/ExpA pair, and expA mutants are rendered avirulent. The expS gene encodes a sensor kinase that is activated by environmental signals that are currently uncharacterized, although acidic plant compounds have been postulated as candidates (1, 7). The expA gene encodes the DNA binding response regulator that converts the ExpS signal into modifications in downstream gene expression, including activation of genes for PCWDEs (7). The ExpS/ExpA TCS has homologues in many gammaproteobacteria, such as the GacA/GacS regulatory system in certain Pseudomonas subspecies, BarA/SirA in Salmonella enterica, and BarA/UvrY in Escherichia coli, all of which have been linked to the positive control of metabolism and virulence factors, similar to ExpS/ExpA (8–22).
The ExpS/ExpA TCS regulates large parts of the transcriptome of Pectobacterium via modulation of a posttranscriptional system consisting of RsmA, an RNA-binding protein that promotes RNA decay or stabilization, and a positive small regulatory RNA encoded by rsmB, which modulates the activity of RsmA (5, 7, 16, 23–25). The ExpS/ExpA TCS and its homologues in many gammaproteobacteria appear to primarily induce the transcription of genes encoding these small nontranslated RNAs (sRNAs); these include rsmB in P. wasabiae and Pectobacterium carotovorum and rsmX, rsmY, and rsmZ in Pseudomonas syringae DC3000 or Pseudomonas fluorescens (20, 25–29). RsmA homologues in related gammaproteobacteria include CsrA in E. coli and S. enterica serovar Typhimurium and RsmA and RsmE in P. fluorescens (7, 15, 17, 25, 28, 28, 30–32). RsmA has been proposed to be a key downstream component in the ExpS/ExpA regulatory network and has been suggested as the main channel through which the homologous GacA/GacS systems operate in some phytopathogens (7, 15–17, 28, 32, 33).
Previous studies of the ExpS/ExpA TCS in P. wasabiae SCC3193 have shown that it is a positive regulator of virulence related traits, particularly PCWDE production and secretion (5, 7, 15). Accordingly, the negative regulation of these virulence-related genes in Pectobacterium by RsmA has been well documented (7, 8, 15, 24, 25, 28). Recently, we conducted a microarray analysis of the impact of an RsmA mutant on the global gene expression pattern in P. wasabiae SCC3193 (23). The resulting data revealed increased transcription of a number of genes involved in virulence, as well as fermentation and glycogen metabolism in the rsmA mutant. This prompted us to address the question of whether all control of gene expression by ExpA is channeled through RsmA or whether there are ExpA-regulated genes that are controlled independently of RsmA. There is evidence that in the gammaproteobacteria Pseudomonas aeruginosa and P. fluorescens, GacA activates the transcription of several sRNA-encoding genes, and each sRNA affects gene expression in a manner that is dependent on or independent of RsmA (34). There are also studies suggesting that GacA binds DNA regions upstream of virulence-related genes, affecting their expression directly (17). To further examine the network of ExpA-mediated regulation in Pectobacterium, we characterized the transcriptome in rsmA and expA single mutants along with an expA rsmA double mutant, utilizing microarrays. Microarray results were confirmed by qPCR analysis of selected genes and phenotypic assays for PCWDE production, motility, virulence, and growth. Based on the microarray data, the majority of the expA and double-mutant regulons were found to overlap the RsmA regulon. Thus, our results demonstrate that ExpA indeed controls expression of the majority of genes through RsmA but also exerts control of a number of genes affecting virulence and fitness through RsmA-independent mechanisms. These ExpA-controlled genes are involved in oligogalacturonide transport and metabolism, electron transport, and energy metabolism. Furthermore, virulence regulators such as KdgR (6, 15, 35) and CadC (36, 37) were found to be controlled by ExpA as well.
MATERIALS AND METHODS
Bacterial strains and mutant construction.
The P. wasabiae expA mutant strain used in this study was described in previous work (5). An rsmA deletion mutant and an rsmA expA double mutant were constructed using the lambda red recombination method (38), permitting the replacement of the rsmA gene in wild-type P. wasabiae or in the already existing expA mutant SCC3060 with a chloramphenicol resistance marker from plasmid pKD3 (5). The same method was used to replace the phn cluster with the antibiotic cassette, resulting in a phnGHIJKLMNP mutant. Mutants were checked using primers for two PCRs as described in the original lambda red recombination protocol, as well as by utilizing sequencing. Primers utilized in P. wasabiae mutant and vector construction are listed in Tables S1 and S2 in the supplemental material, and the strains used in this study are listed in Table S3 in the supplemental material. A complemented strain of the P. wasabiae expA mutant was constructed by PCR amplification using the primers BSexpAF and BSexpAR (see Tables S1 and S2 in the supplemental material) and cloning the resulting product into the Bluescript SK plasmid (Stratagene).
Bacterial growth.
The P. wasabiae wild type and mutants were cultured in liquid minimal medium with 0.4% polygalacturonic acid (PGA) as well as in liquid LB medium. Ampicillin (100 mg/liter), kanamycin (20 mg/liter), and chloramphenicol (20 mg/liter) were used when appropriate (7, 39). For the construction of growth curves, the bacteria were cultured in 30 ml of medium in 300-ml Erlenmeyer flasks, and the optical density at 600 nm (OD600) was measured at different time points. All bacterial incubation was performed with 200-rpm rotation at 28°C. At least three biological replicates were used for each strain to construct the growth curves. The experiments were repeated at least three times.
RNA extraction and preparation for microarray hybridization.
P. wasabiae strains were cultured in a liquid minimal medium (39) supplemented with 0.4% PGA as the carbon source at 28°C and 200 rpm. PGA has been used in previously published studies investigating the induction of enzyme production in P. wasabiae. Because the rsmA mutant and the double mutant grow at significantly decreased rates compared to the wild type and expA mutants, we decided to perform transcriptome analysis at different growth phases. Three separate biological replicates for each of the four strains were grown to late logarithmic and early stationary phase. RNA from the cultures was isolated based on a previously published extraction protocol (40). The total RNA was then treated with the RNeasy MinElute (Qiagen) cleanup kit to remove genomic DNA and other possible contaminants. For the purified total RNA, we first used the Ambion MICROBExpress kit, followed by the Ambion MessageAmp II-Bacteria kit (all according to the manufacturer's instructions), to synthesize aminoallyl-UTP-containing antisense RNA (aRNA) from purified mRNA. The aRNA from each biological replicate of the four strains was coupled with one of three different dyes: HyPer 5 (Amersham), Cy3 (Amersham), and Alexa Fluor 488 (Invitrogen). Dye coupling was performed as described in our previous microarray study of SCC3193 (23).
Microarray hybridization and analysis.
The microarray platform and the hybridization procedure for RNA samples were the same as those described by Kõiv et al. (23). Normalization and gene expression were analyzed using the Bioconductor and Limma packages provided for the R language. Print-tip LOESS was used for normalization of samples within the individual arrays on the slide, and subsequently quantile normalization (41, 42) was used for samples between the different arrays. The array contained three 60-mer probes per gene, encompassing 4,571 open reading frames (ORFs) of P. wasabiae SCC3193. As a cutoff to determine significant differences in gene expression, we used a log2 change of ≥0.5-fold or ≤−0.5-fold and a false discovery rate (FDR) value of ≤0.05. Gene expression comparisons between the strains resulting from this study are listed in Data Sets S1 to S7 in the supplemental material.
Validation of microarray data via quantitative real-time RT-PCR.
The data from the microarray analysis were verified by quantitative real-time RT-PCR of several genes of interest. Fresh RNA samples were collected from the growth phases of interest for all strains involved in the microarray analysis, using the method of RNA isolation described for the microarray experiment. Total RNA was used to synthesize cDNA via the Superscript VILO cDNA synthesis kit (Life Technologies) following the manufacturer's instructions. Samples were then analyzed using LightCycler 480 SYBR green I master mix (Roche) as the PCR master mix, according to the manufacturer's instructions. Two housekeeping genes were used as reference genes, ffh (W5S_1021) and recA (W5S_1006), which have been used as references for this type of experiment (43, 44). The method used for data handling and calculation of fold change was the 2−ΔΔCT method (45). Relative gene expression ratios less than 1.0 were transformed to the negative inverse of the ratio. The genes that were analyzed by qPCR along with the primers are listed in Table S1 in the supplemental material.
Assays for enzyme activities.
We utilized plate assays to estimate cellulolytic and pectinolytic activity in the P. wasabiae strains as described in previous investigation (3), using minimal medium supplemented with 0.4% PGA. For assaying cellulase activity, carboxymethylcellulose sodium (Sigma-Aldrich) was used as the substrate, while polygalacturonic acid sodium salt (Sigma-Aldrich) was used as the substrate for pectinase activity. The diameters of the emerging halos were measured and compared.
Swimming motility assay.
A swimming motility assay was performed with P. wasabiae strains using 0.3% agar in M9 minimal medium plates containing 10 mM sucrose or 0.2% PGA as the carbon source. Cells were cultured in LB until the early stationary phase and then washed with 10 mM MgSO4 solution. Washed cells were then adjusted to an OD600 value of 0.2, and 3 μl of washed culture was subsequently pipetted into the center of the agar plates. At least five replicate plates were used per strain, and the plates were incubated at 28°C for 2 days. The diameter of the swimming halo was measured, and the experiment was repeated at least three times.
Phosphonate sensitivity assay.
The growth of the P. wasabiae wild type and that of mutant strains were tested in liquid minimal medium supplemented with 1 mM glucose and various concentrations (10 μM, 100 μM, and 1 mM) of the phosphonate compounds N-(phosphomethyl)glycine or phosphomycin (Sigma-Aldrich). Bacteria were cultured in 30 ml medium in 300-ml Erlenmeyer flasks with shaking at 200 rpm in 28°C. Phosphonate sensitivity tests were performed on LB agar plates with sterile Whatman paper discs soaked with a 1 mM solution of either phosphonate compound placed in the center.
In planta growth in tobacco seedlings.
The virulence of P. wasabiae strains was tested on tobacco seedlings. Bacterial CFU were counted from inoculated plants at 0, 24, and 48 h postinfection according to a previously utilized protocol for tobacco seedling infections by Nykyri et al. (3). One 24-well plate with a tobacco seedling in each well was used per strain during the infection experiments. At least three biological replicates, each replicate sample consisting of the contents of two combined plants (excluding the roots), were sampled per time point for CFU counts. The experiment was repeated three times. Resulting differences between strains in terms of CFU/plant at the 24 and 48 h time points were statistically confirmed using Microsoft Excel software to calculate 2-tailed Welch's t test, with a P value less than 0.05.
Microarray data accession number.
A detailed microarray experiment description and raw data are available in the GEO database under accession number GSE47545.
RESULTS AND DISCUSSION
Microarrays for the expA-rsmA regulon analysis.
In an effort to expand upon previous gene expression studies of an SCC3193 expA mutant (4, 5, 7) and rsmA mutant (23) by determining the regulatory hierarchical relationship between ExpA and RsmA, a comparative transcriptome analysis utilizing microarrays and real-time RT-PCR was performed. Both ExpA and RsmA in Pectobacterium as well as their homologs in other bacteria are global regulators that control large number of genes. ExpA and RsmA have been indicated to have an opposite effect on the expression of virulence-related and non-virulence-related genes in several bacteria (11, 15, 16, 23, 24, 28, 46, 47). The complexity of the corresponding regulons and their intertwined nature, with RsmA mediating the effects of ExpA on downstream gene expression, make the analysis of these regulons particularly challenging. To facilitate this analysis and to identify potential genes specifically regulated by ExpA, either directly or indirectly, we compared global downstream gene expression in both single mutants and an expA rsmA double mutant by microarray analysis. Double mutants have been increasingly utilized in large-scale microarray experiments to clarify the roles of genes involved in related regulatory functions (48, 49). Initially, the double mutant, the P. wasabiae wild type, and the expA and rsmA single mutants were assessed for their growth phenotypes in liquid minimal medium supplemented with 0.4% PGA (Fig. 1), which has been used to activate the ExpS/ExpA TCS (7). While the expA mutant displayed growth similar to that of the wild type, the maximum cell density of the rsmA mutant was severely reduced, and the expA rsmA double mutant demonstrated a cell density intermediate between those of the single mutants. The drastically impaired growth of the rsmA mutant and the double mutant in both culture media could partially be explained by an uncontrolled overproduction of PCWDEs and other virulence factors reducing the bacterial fitness (23); however, we cannot rule out the possibility that other physiological factors may impact the growth of these mutants. The large impact of an rsmA mutation on growth made the transcriptome analysis particularly challenging, and constructing the growth curves was essential to determining the corresponding growth phases for RNA extraction in the different strains that were used (Fig. 1). In this study, samples for microarray analysis were collected from bacterial cultures grown to late logarithmic phase, as well as to early stationary phase, in which ExpA homologs appear to be most active in gammaproteobacteria (9, 11, 16, 32, 50). The microarray data from early stationary growth phase had a relatively low statistical significance and as such were used only to support data from the logarithmic phase in this study, using the same log2 change but a P value cutoff of ≤0.005. Genes mentioned specifically in this text can be found in Data Set S7 in the supplemental material.
FIG 1.
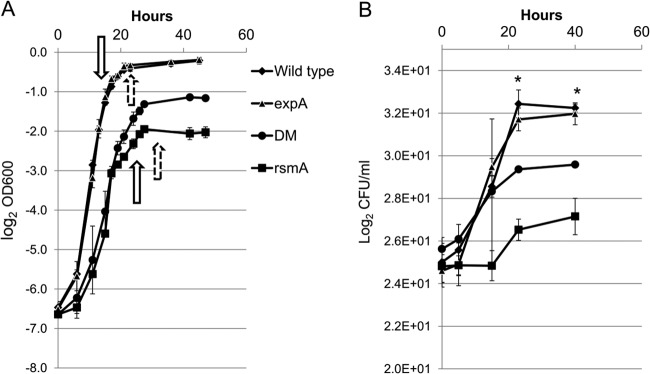
Bacterial strain growth curves in minimal medium. A growth curve was constructed based on cultures of wild-type P. wasabiae and the expA, rsmA, and expA rsmA (DM) mutants grown on minimal liquid medium supplied with 0.4% PGA. The optical density at 600 nm was measured throughout the culture growth, using cultures starting at an OD600 of approximately 0.008. The experiment was performed using three biological replicates for all strains. (A) Arrows indicate the OD600s at the time points at which RNA was extracted from the strains for microarray or qPCR analysis, with dashed arrows indicating early stationary phase. (B) Numbers of CFU per ml were calculated at five time points using biological duplicates of all strains. The statistical significance of mean maximal cell density values according to the CFU/ml measurements was calculated using analysis of variance (ANOVA), and asterisks indicate statistical significance (P < 0.05).
Characterization of the expA regulon in P. wasabiae.
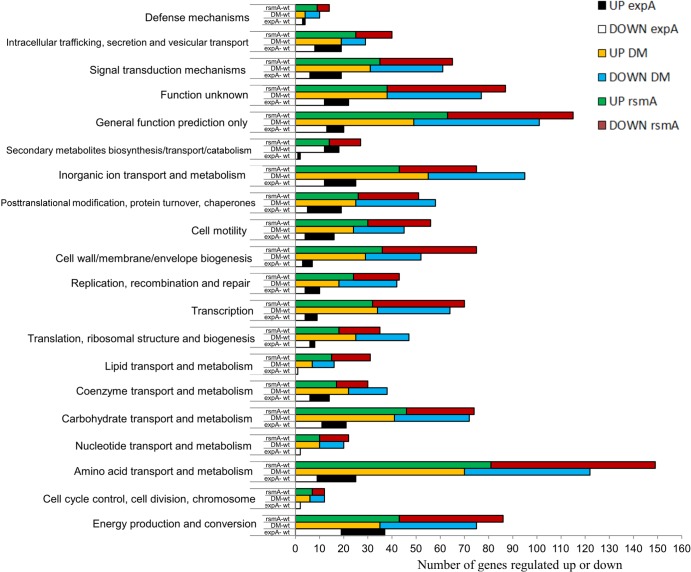
Based on previous transcriptional analyses of expA mutants in Pseudomonas species, as well as from our previous rsmA mutant study, we expected to see an impact on a significant part of the global transcriptome. Virulence-related genes were expected to be downregulated by an expA mutation, while genes involved in glycogen metabolism, the citric acid cycle, and the electron transport chain were expected to be upregulated (11, 23, 34, 49). Microarray analysis from samples of bacteria grown to late logarithmic phase revealed 344 significantly differentially expressed genes out of the 4,571 ORFs present on the array between the expA mutant SCC3060 and the wild type (see Data Set S1 in the supplemental material). According to a COG (clusters of orthologous groups) analysis of the microarray data, the differentially expressed genes in the expA mutant were mainly involved in the following COG categories; energy metabolism, protein turnover, inorganic ion transport and metabolism, and carbohydrate and amino acid metabolism, as well as unknown functions (Fig. 2). This type of global effect of an expA mutation was expected based on evidence from previous studies with expA and rsmB homologues (11, 23, 46, 49). Some of the larger operons of significantly downregulated virulence-related genes in the expA mutant were involved in the general secretion pathway (gspSBCDEFGHIJKLMO operon, W5S_1291 to W5S_1305), the type VI secretion system (T6SS) cluster called T6SS-1 in SCC3193 (3), the T6SS-related hcp and vgrG genes, and PCWDE production. Many of these alterations in gene expression were similar to those found in gacA mutants of Pseudomonas species (11, 34, 46, 51, 52). When more relaxed significance cutoff values (a P value of ≤0.005 instead of an FDR of ≤0.05) were used, the downregulation of genes involved in flagellar biosynthesis (fliRQPONMLKJIHGF, W5S_1790 to W5S_1802) and phosphonate metabolism (phnGHIJKLMNP, W5S_0593 to W5S_0601) was apparent as well in the expA mutant, particularly during stationary growth (see Data Set S4 in the supplemental material).
FIG 2.
COG classification of the gene expression data comparing mutants to the wild type. Clusters of orthologous groups (COG) classification of genes whose expression was significantly different in the expA mutant, rsmA mutant, and expA rsmA double mutant (DM) compared to the wild type (wt) was based on the late-logarithmic-growth-phase microarray data. The graphs show the numbers of genes within each group on the x axis and COG category on the y axis. The bars are color coded to show what comparison they belong to.
In contrast to the reduced expression of several major virulence genes in the expA mutant, the microarray data analysis revealed an increased expression of genes involved in glycogen metabolism (glgPACXB, W5S_4290 to W5S_4294) and fumarate reductase biosynthesis (frdABCD, W5S_4092 to W5S_4095), the nitrate reductase-encoding operon narIJHGKXL (W5S_2536 to W5S_2542), the nitrite reductase-encoding nirBD (W5S_4204 to W5S_4205), and the operon encoding cytochrome bo oxidase synthesis (W5S_3266 to W5S_3270). The nar and nir clusters encode proteins for the utilization of nitrate and nitrite as alternative electron transporters in anaerobic environments (53), which could affect survival in the plant apoplast and, thus, propagation and disease development. Furthermore, expression of kdgR (W5S_2118) was decreased in the expA mutant, while cadC (W5S_0954), which is involved in survival at low pH in other bacteria (54), was upregulated. Our microarray data for genes identified as differentially expressed in the expA mutant are supported by corresponding data for homologous genes identified as belonging to the gacA regulon in related species, such as P. fluorescens Pf-5 and Pectobacterium atrosepticum (11, 46, 49). In conclusion, we have characterized the regulon controlled by ExpA in SCC3193 and confirmed that genes involved in a variety of virulence-related and non-virulence-related functions were affected by ExpA inactivation (see Data Sets S1 and S4 in the supplemental material).
Identification of the overlap between the ExpA and RsmA regulons.
Since RsmA has been identified as a main component of the ExpS/ExpA regulatory pathway, it seemed likely that large parts of their respective regulons would overlap and be regulated in opposing ways. In particular, genes encoding virulence factors such as secretion systems, PCWDE, and motility were expected to be regulated in an opposing fashion, as indicated in earlier investigations (7, 15, 23). Out of the total 4,571 ORFs present on the array, 1,274 genes had significantly altered expression in the rsmA mutant relative to the wild type, and 1,211 had significantly different expression between the double mutant and the wild type (see Data Sets S2 and S3 in the supplemental material), demonstrating a significantly larger impact for RsmA on the transcriptome than ExpA. The expected opposing up- and down-regulation between ExpA and RsmA according to the microarray data is summarized in Venn diagrams (Fig. 3; see also Data Sets S1 to S6 in the supplemental material). COG categorization of the array data (Fig. 3) indicated that most of the genes affected in terms of expression in the rsmA mutant were involved in energy turnover, unknown functions, and amino acid and inorganic ion transport and metabolism, with the double mutant exhibiting regulation of the same groups. The regulation of genes within the mentioned groups by rsmA corresponds with evidence from previous studies of P. wasabiae and related bacteria. The gene expression data for the expA mutant compared to the wild type revealed a similar emphasis on certain COG classifications, but with a smaller overall impact on the number of genes affected. This similarity of relative COG regulation in expA and rsmA mutants on the overall transcriptome impact corresponds to the idea of ExpA modulating gene expression in ways that are both dependent on and independent of RsmA.
FIG 3.
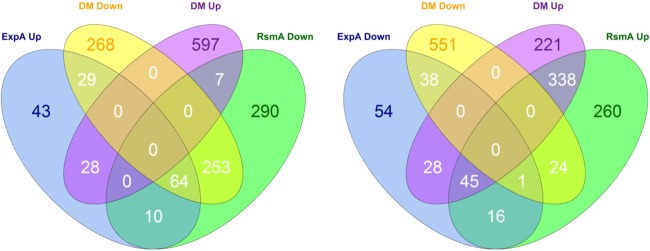
Venn diagrams of gene expression data illustrating ExpA and RsmA regulon overlaps. Venn diagrams comparing significantly (FDR of ≤0.05; log2 change of ≥0.5-fold or ≤−0.5-fold) up- and downregulated genes between the expA and rsmA mutant, as determined by microarray analysis, using the expA rsmA double mutant (DM) as a separator. The diagrams show the degree to which the regulons overlap between expA and rsmA based on the model of their opposing effects on the expression of many genes. “Up” and “Down” indicate the groups of genes upregulated or downregulated in the mutant in comparisons of the expA mutant to the wild type (ExpA), the rsmA mutant to the wild type (RsmA), and the expA rsmA double mutant to the wild type.
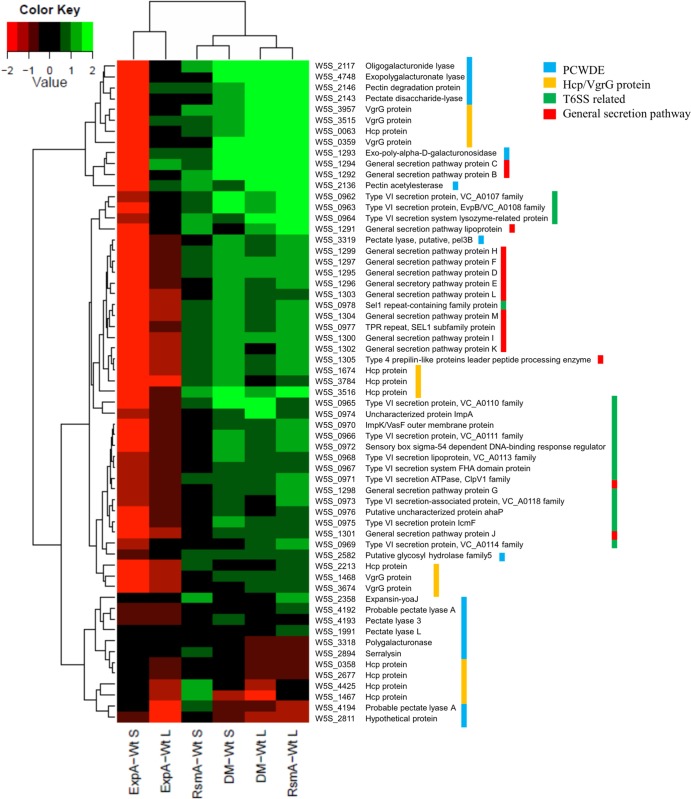
Many of the genes identified as upregulated in the rsmA mutant (and often in the double mutant as well) but downregulated in the expA mutant encode virulence-related functions such as secretion and motility or central metabolism (sdhABCD, encoding succinate dehydrogenase, and sucABCD, encoding succinyl CoA synthetase; W5S_3088 to W5S_3095). In contrast, genes downregulated in the rsmA mutant and the double mutant but upregulated in the expA mutant are involved in metabolism; for example, these include the previously described frdABCD, glgABCPX, nar, and nir operons. Gene expression data from early stationary phase suggested that downregulation of genes encoding PCWDEs, the T6SS-related proteins VgrG and Hcp, and proteins involved in flagellar biosynthesis was more prevalent in the stationary phase in the expA mutant (Fig. 4; see also Data Set S2 in the supplemental material), with an opposite effect being observed in the rsmA mutant. Some of the genes involved in virulence aspects and metabolism, as well as those potentially regulated by ExpA independently of RsmA (as identified in the microarray data), were selected and further confirmed via qPCR (Table 1).
FIG 4.
Heat map of virulence-related genes according to microarray data. The heat map of unfiltered microarray results shows various plant cell wall-degrading enzymes, secretion systems, and related proteins seen to be affected in comparisons of the expA mutant to wild-type SCC3193 (ExpA-wt), the rsmA mutant to the wild type (RsmA-wt), and the double mutant to the wild type (DW-wt) at the late logarithmic phase (L) and stationary phase (S). Most of these genes were not significantly affected in ExpA-wt comparisons at the late logarithmic phase (meaning an FDR value of >0.05 or a log2 fold change between −0.5 and 0.5), but the ExpA-wt results are included because they show a general trend that is supported by microarray data for the samples from the stationary growth phase. The color key translates observed changes to a red-green color gradient in the heat map. Color bars on the right indicate which virulence-related functional group the gene belongs to.
TABLE 1.
Quantitative real time RT-PCR verification of genes with significantly altered expression in late-logarithmic-phase microarray data
| NCBI locus tag | Gene | Fold changea |
|||||
|---|---|---|---|---|---|---|---|
| qPCR |
Microarray |
||||||
| expA/wt | DM/wt | rsmA/wt | expA/wt | DM/wt | rsmA/wt | ||
| W5S_0060 | hcp | −1.5 | 4.2 | 3.3 | NAg | 3.4 | 3.18 |
| W5S_0593 | phnG | −1.1 | 3.7 | 11.9 | NA | 4.1 | 17.5 |
| W5S_0599 | phnM | −1.4 | ND | ND | NA | 3.9 | 17.8 |
| W5S_0965 | A0110 | −7.6 | 10.2 | 16.9 | −2.9 | NA | 1.8 |
| W5S_1006 | recA | Ref | Ref | Ref | NA | NA | NA |
| W5S_1009 | rsmA | 1.5 | <10−4 | <10−4 | NA | −45.4 | −1.4 |
| W5S_1021 | ffh | Ref | Ref | Ref | NA | NA | NA |
| W5S_2118 | kdgR | −5.5 | −18.8 | 5.6 | −1.67 | −2.12 | NA |
| W5S_2139 | togB | −1.8 | −1.5 | 4.8 | −2.0 | −1.9 | NA |
| W5S_2142 | togM | −3.9 | −1.6 | 1.9 | −2.6 | −2.0 | NA |
| W5S_2539 | narG | 1.4 | −3.3 | −3.8 | 2.8 | −6.4 | −5.0 |
| W5S_2933 | moaE | 2.4 | −1.7 | −1.3 | 2.4 | NA | NA |
| W5S_3266 | cyoA | 1.7 | 3.5 | −1.6 | 3.5 | 4.0 | NA |
| W5S_3268 | cyoC | 3.0 | 5.5 | −1.8 | 2.7 | 2.6 | NA |
| W5S_4193 | Pel 3 | 1.9 | 3.7 | 11.9 | 2.0 | 15.5 | 11.0 |
| W5S_4205 | nirD | 1.5 | −5.7 | −7.9 | 2.4 | −11.2 | −6.5 |
| W5S_4748 | pelX | −4.7 | −1.6 | −1.7 | −2.9 | −1.7 | −2.2 |
| No NCBI tagb | rsmB | −205 | −146.7 | −9 | NA | NA | NA |
Fold change was calculated via the 2−ΔΔCT method, negatively inverting values of <1.0, for the expA mutant compared to the wild type (wt), the expA rsmA double mutant (DM) compared to the wild type, and the rsmA mutant compared to the wild type. ND, not done; NA, not available from microarray data due to cutoff parameters; Ref, reference gene used for the qPCR gene expression analysis.
The gene encoding RsmB has not been annotated as such in the NCBI database.
The microarray data indicated that genes in the T2SS and one of the two T6SS operons (T6SS-1) were downregulated in the expA mutant but upregulated in the rsmA mutant and the double mutant (Fig. 4; also, see Data Sets S1 to S6 in the supplemental material), in accordance with previous RsmA gene expression data for P. wasabiae (23). Inactivation of these secretion systems has been shown to reduce virulence of Pectobacterium species (3, 23, 28, 55, 56). This ExpA/RsmA regulation of T6SS-related genes is supported by data from previous studies of Pectobacterium and Pseudomonas species (11, 23, 46, 49). T6SS-associated hcp and vgrG genes were also found to be slightly downregulated in the expA mutant, including W5S_1674, but upregulated in the rsmA mutant. However, when the stationary-phase data were analyzed using a P value of <0.005, the contrasting regulation by ExpA for this type of gene was apparent. These Hcp and VgrG proteins may have roles in the T6SS, perhaps either as effectors or as structural components (2, 57). Overall, the virulence-related secretion systems appear to be affected by ExpA through the RsmA pathway.
In related gammaproteobacteria, both ExpA and RsmA have been shown to impact flagellar biosynthesis and motility, which are important virulence factors (5, 6, 11, 23, 47, 49, 58). The microarray data in this study and previous studies revealed that inactivation of rsmA indeed has a positive impact on the expression of flagellar biosynthesis- and motility-related flh, flg, and fli operons in SCC3193 (23, 32, 50), while an expA mutation negatively impacted expression of flagellum-related genes (see Data Sets S1 to S6 in the supplemental material). Notably, the cheABRWYZ genes, which encode the proteins that have a central role in coupling sensory receptors to the flagellar rotor system (59, 60), along with motAB, which are involved in torque generation of the flagellar rotor (61), were upregulated in rsmA and the double mutant, while the expA mutant displayed significant downregulation of several chemotaxis-related genes (W5S_1696, W5S_1733, and W5S_4020). In previous work on SCC3193 (23), we observed that an rsmA inactivation mutant had increased flagellation but showed no motility on swimming motility agar plates. The expA mutant also exhibited a decrease in swimming halo size (approximately 31 mm in diameter) compared to the wild type (approximately 39 mm in diameter), as well as a lack of a defined halo edge, when the motility agar was supplemented with PGA as the carbon source but not when sucrose was the carbon source. The expA complementation strain and the vector control confirmed this finding (Fig. 5). The expA complementation strain demonstrated an increased halo size (approximately 34 mm). This result supports the effects seen on the expA mutant expression of motility- and chemotaxis-related genes in the microarray results, revealing the impaired swimming motility of both the expA and rsmA mutants and suggesting different regulation of certain aspects of motility.
FIG 5.
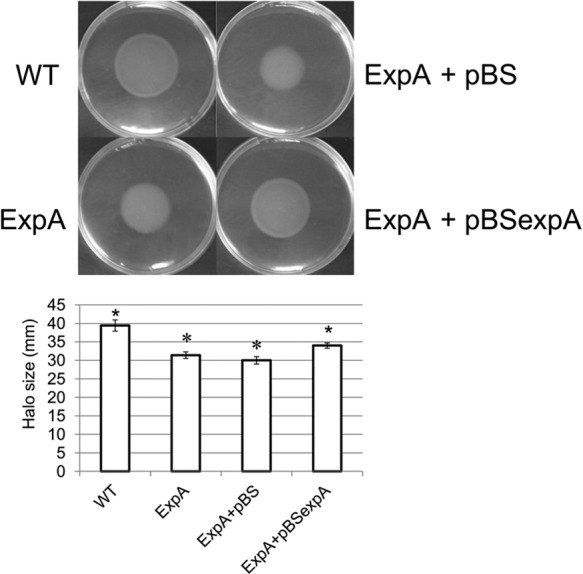
ExpA impacts motility in PGA supplemented medium. Photographs show results from bacterial swimming motility plate assays. Plates were made from 0.3% (wt/vol) agar in M9 minimal medium supplemented with 0.2% PGA as the carbon source. Bacteria from the P. wasabiae wild type (WT), the expA mutant (ExpA), the expA mutant with complementation vector (ExpA + pBSexpA), and the vector control (ExpA + pBS) were grown overnight and subsequently pipetted onto the center of the plates. Plates were incubated for 2 days at 28°C, with at least five technical replicate plates per strain, and the experiment was repeated at least 3 times. Five-pointed asterisks indicate P values of 0.01 or less for comparison of the mean diameter of one strain to the other strains, using one-way ANOVA with the Scheffe post hoc test. However, diameters of the ExpA and ExpA+pBS vector control strains were not significantly different from each other (P < 0.01), as indicated by six-pointed asterisks.
The rsmA and double mutants demonstrated significantly upregulated expression of genes in the phnGHIJKLMNP operon, as well as associated genes W5S_0590 to W5S_0592, during the logarithmic growth phase, compared to the wild type. In contrast, the expA mutant demonstrated downregulation of this operon at the stationary phase (see Data Sets S1 to S4 in the supplemental material). The microarray results were supported by qPCR data for phnG and phnM (Table 1). There are studies that have suggested that genes involved in phosphonate transport and metabolism are affected during bacterial interactions with plant tissue (62, 63). A P. wasabiae deletion mutant of this operon was made to test whether virulence was affected, possibly due to reduced phosphorus utilization by the mutant, but we did not observe any impact on virulence in tobacco seedlings or potato tubers (data not shown). A phosphonate sensitivity assay with phosphonate-containing compounds suggested that high concentrations (over 100 mM) of N-(phosphomethyl)glycine or phosphomycin inhibited bacterial growth in liquid media. However, no difference in sensitivity was detected between the wild type and the phn operon mutant (data not shown). The microarray data suggest that ExpA seems to regulate this type of alternative phosphorus uptake via RsmA, possibly affecting phosphonate utilization or survival inside and outside hosts (64, 65).
According to the array data, there was a significantly greater impact on the global transcriptome during the logarithmic growth phase in the rsmA mutant than the expA mutant compared to the wild type. Aside from the negative regulation of virulence-related traits already mentioned, positive RsmA regulation was observed for genes involved in LPS synthesis, cell division, the enterobacterial common antigen (ECA) cluster, and the glyoxylate shunt (see Data Set S2 in the supplemental material). In conclusion, the overall transcriptome analysis data in this study suggest that via RsmA, ExpA regulates the expression of many genes within nutrient transport and metabolism, either directly or indirectly, and acts as a positive regulator of many genes involved in virulence (Fig. 6). It seems probable that this is also reflected in phenotypes such as cell culture growth, cell size, and overall fitness. This observation is bolstered by previous observations from gene expression studies of the homologous gacA-rsmA pathway in related bacteria such as P. atrosepticum and E. coli (8, 11, 23, 24, 31, 46–49).
FIG 6.
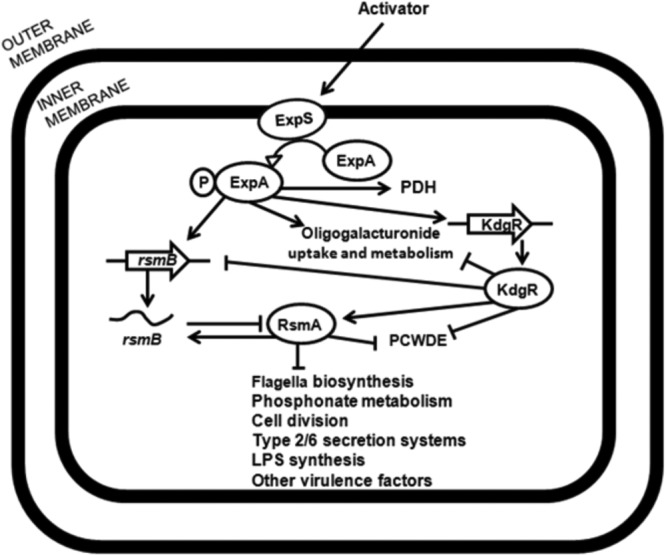
Regulation model of ExpA and RsmA in Pectobacterium wasabiae. A putative conclusive regulatory scheme of rsmA and expA in SCC3193 for various functions and operons, possibly related to virulence functions, is shown. This model is based on the results from the microarray analysis, virulence assays, exoenzyme production assays, and growth curves. Arrows indicate positive regulation, direct or indirect, while truncated lines indicate negative regulation, direct or indirect. PDH, pyruvate dehydrogenase genes pdhCDR and aceE.
Identification of genes regulated by ExpA independently of RsmA.
An important aspect of this study was to elucidate whether ExpA operates only via RsmA in its regulation or whether potential differences in their regulons could be identified. As expA mutants do not exhibit phenotypes that are completely opposite to those of rsmA mutants regarding motility, growth, or virulence on axenically grown tobacco, an incomplete overlap of their regulons seemed plausible. the data suggest that over 10% of the 344 genes found to be differentially expressed between the wild type and the expA mutant were affected by ExpA, directly or indirectly, independently of RsmA (Table 2). The genes were not found to be significantly affected when the rsmA mutant was compared to the wild type or when the double mutant was compared to the expA mutant. These genes were not affected, as indicated by the data from our previous microarray study of rsmA mutants (23) when the same normalization and statistical procedures were applied to all the data. The 37 genes, both up and downregulated, included homologs of the togMNAB operon (W5S_2139 to W5S_2142) (66), which were found here to be expressed less in the expA mutant than in the wild type. The togMNAB operon has been found to have a redundant role in oligogalacturonide transport from the periplasm to the cytoplasm in the related soft rot phytopathogen Dickeya dadantii (66). According to growth curves (Fig. 1), there was no effect on the expA mutant growth rate in PGA-supplemented minimal medium despite lower expression of this operon. Furthermore, the data for the expA mutant revealed reduced expression of W5S_0817, which encodes a similar protein of the TogT oligogalacturonide transporter in D. dadantii. TogT acts as a secondary transporter in addition to togMNAB. Mutating the togM gene has been shown to reduce the virulence of D. dadantii on chicory leaves (66), while mutating both togM and togT resulted in a more pronounced loss of virulence and reduced survival in minimal medium with galacturonic acid as the sole carbon source. The togMNAB operon has been implicated as negatively regulated by kdgR (66), a regulatory gene that was found to be downregulated only in the expA mutant. The KdgR protein is a negative regulator of PCWDEs and virulence factors in general (1, 15, 66–68). Despite the reduced expression of kdgR in the expA mutant, the togMNAB was still downregulated, suggesting a higher hierarchical position for ExpA compared to KdgR in the regulation of these genes. Additionally, W5S_2143, which encodes the cytoplasmic protein PelW, was downregulated in the expA mutant and appeared to be influenced by ExpA independently of RsmA.
TABLE 2.
Genes identified in microarray data analysis as affected by an expA mutation independently of an rsmA mutation
| NCBI locus tag | Gene name | Log2 FC, expA/wta | FDRb | Description |
|---|---|---|---|---|
| W5S_0002 | NA | 0.514 | 0.01 | DNA polymerase III, beta subunit |
| W5S_0389 | NA | 0.944 | 0.00 | IS407A, transposase OrfA |
| W5S_0399 | exbD | −0.501 | 0.03 | TonB system transport protein ExbD |
| W5S_0685 | dnaG | 0.637 | 0.00 | DNA primase |
| W5S_0817 | melB | −0.577 | 0.01 | Oligogalacturonide transporter |
| W5S_0954 | cadC | 0.591 | 0.00 | Transcriptional regulator, CadC |
| W5S_0955 | NA | 0.517 | 0.01 | Putative exported protein |
| W5S_1120 | NA | −0.610 | 0.00 | EAL domain containing protein involved in flagellar function |
| W5S_1458 | uvrC | −1.916 | 0.00 | UvrABC system protein C |
| W5S_1502 | NA | −0.998 | 0.00 | BpiB05 |
| W5S_1546 | NA | 0.617 | 0.00 | Putative exported protein |
| W5S_2118 | kdgR | −0.738 | 0.00 | Transcriptional regulator KdgR |
| W5S_2139 | togB | −1.024 | 0.00 | Oligogalacturonide ABC transporter |
| W5S_2140 | togA | −0.951 | 0.00 | Carbohydrate ABC transporter ATP-binding protein |
| W5S_2141 | togN | −1.144 | 0.00 | Oligogalacturonide ABC transporter, permease protein |
| W5S_2142 | togM | −1.384 | 0.00 | Oligogalacturonide ABC transporter, permease protein |
| W5S_2143 | pelW | −1.134 | 0.00 | Pectate disaccharide-lyase |
| W5S_2348 | NA | −0.821 | 0.00 | Putative uncharacterized protein |
| W5S_2483 | kdgM | −0.558 | 0.01 | Oligogalacturonate-specific porin |
| W5S_2701 | ccmH | 0.497 | 0.02 | Cytochrome c-type biogenesis protein CcmH |
| W5S_2740 | ppsA | 0.673 | 0.00 | Phosphoenolpyruvate synthase |
| W5S_2741 | ydiA | 0.660 | 0.00 | Putative phosphotransferase YdiA |
| W5S_2967 | NA | −0.504 | 0.02 | Putative uncharacterized protein |
| W5S_2973 | afuA | −0.690 | 0.00 | Ferric iron uptake ABC transporter (FeT) family, periplasmic iron-binding protein |
| W5S_3018 | NA | −0.507 | 0.02 | Acylneuraminate cytidylyltransferase |
| W5S_3224 | cstA | 0.611 | 0.00 | Carbon starvation protein A |
| W5S_3266 | cyoA | 1.797 | 0.00 | Cytochrome o ubiquinol oxidase, subunit II |
| W5S_3267 | cyoB | 1.452 | 0.00 | Cytochrome o ubiquinol oxidase, subunit I |
| W5S_3268 | cyoC | 1.457 | 0.00 | Cytochrome o ubiquinol oxidase, subunit III |
| W5S_3269 | cyoD | 1.191 | 0.00 | Cytochrome o ubiquinol oxidase, subunit IV |
| W5S_3270 | cyoE | 1.311 | 0.00 | Protoheme IX farnesyltransferase |
| W5S_3294 | brnQ | 0.504 | 0.01 | Branched-chain amino acid transport system II carrier protein |
| W5S_3526 | rci | 0.555 | 0.01 | Shufflon-specific recombinase |
| W5S_3888 | NA | −0.753 | 0.00 | Putative hydrolase YtaP |
| W5S_3892 | aceF | −0.525 | 0.01 | Dihydrolipoyl dehydrogenase |
| W5S_3893 | aceE | −0.593 | 0.00 | Dihydrolipoamide acetyltransferase c |
| W5S_4652 | dnaJ | −0.516 | 0.02 | Putative heat shock protein, DnaJ like |
Fold change in the Pectobacterium wasabiae expA mutant compared to the wild type. NA, not applicable.
False discovery rate.
The operons encoding cytochrome o ubiquinol oxidase (cyoABCDE, W5S_3266 to W5S_3270; upregulated in the expA mutant) and for pyruvate dehydrogenase (pdhCDR and aceE, W5S_3892 to W5S_3895; downregulated in the expA mutant) were also affected by ExpA independently of RsmA. The effects observed on genes involved in these two pathways were similar to those observed for gacA mutants of Pseudomonas species (11, 46). Cytochrome o ubiquinol oxidase is the terminal oxidase of the electron transport chain during anaerobic growth. Results obtained with Pseudomonas putida mutants have suggested that the cyoABCD operon is involved in catabolic repression and affects the redox state of the cell (69), which may also affect virulence in bacteria (70–72). The PDH pyruvate dehydrogenase complex coded by pdhCD and aceE has been documented in E. coli to have a role in connecting glycolysis with the citric acid cycle, and the pdhR gene has been shown to negatively regulate the cyoABCD operon and other operons involved in energy metabolism (73, 74). In conclusion, the overall gene expression data suggest that both regulators control the expression of virulence-related genes as well as genes involved in general metabolism. However, the data indicate that the ExpA regulon is partially independent of RsmA, as the double mutant behaves similarly to the expA mutant, regardless of RsmA presence. As such, expA may very well be an independent virulence regulator as well as working through the rsmAB pathway in SCC3193.
PCWDE gene regulation and phenotypes.
Previous studies indicated the opposing effects of expA and rsmA mutants on PCWDE biosynthesis in P. wasabiae, P. carotovorum, P. fluorescens, Pseudomonas chlororaphis, and S. Typhimurium (11, 15, 16, 23, 24, 48, 49). Consequently, we decided to elucidate this aspect of virulence using our mutants to investigate the regulation of Pel-, Peh-, Cel-, and Prt-encoding genes. Our microarray analysis of this gene type revealed an upregulation of PCWDE genes such as celV (W5S_2582) and pel123 (W5S_3319 and W5S_4192 to W5S_4194) in the rsmA mutant and the double mutant during the late logarithmic growth phase, with opposing regulation exhibited by expA at stationary growth phase (see Data Sets S1 to S6 in the supplemental material). Plate assays were utilized to visualize enzymatic activity to confirm the microarray data. These plate tests indicated that both the rsmA mutant and the double mutant demonstrated higher activity of pectolytic enzymes and cellulase in the medium, while the expA mutant produced no detectable pectolytic enzyme or cellulase activity in the medium (Fig. 7). The reason for these phenotypes was indicated by the microarray results, in which the double mutant and rsmA mutant strains showed enhanced expression of a number of genes involved in PCWDE biosynthesis, including those encoding various pectate lyases, an expansin, and a polygalacturonase, along with genes involved in T2SS (see Data Sets S2 and S3 in the supplemental material). In contrast, the expA mutant demonstrated a few significantly downregulated PCWDE-encoding genes during the logarithmic growth phase, with an even more noticeable reduction in expression at the stationary growth phase (see Data Sets S1 and S4 in the supplemental material). During logarithmic growth, however, two genes encoding products involved in the breakdown of plant cell wall derivatives appeared to be exceptions to the model of ExpA regulation via RsmA; the gene encoding pectate lyase 3 (W5S_4193) had increased expression in all mutant strains compared to the wild type, and pelX (W5S_4748) had reduced expression in all mutants. These effects were confirmed by qPCR (Table 1). In conclusion (Fig. 6), expA seems to control PCWDE production through its interaction with rsmA, as the double mutant and rsmA mutant have similar phenotypes and transcriptome effects. However, gene expression data also suggest that ExpA regulates the expression of some PCWDE-related genes independently of RsmA. In addition, our qPCR analysis showed that rsmB was downregulated in all mutant strains compared to the wild type, particularly the double mutant, which is supported by studies showing that rsmB RNA is stabilized and maintained in part by RsmA itself (25).
FIG 7.
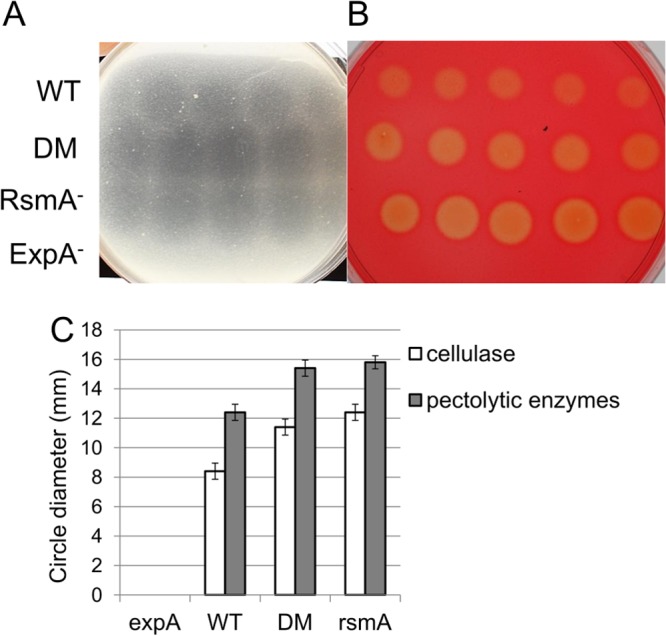
The ExpA RsmA double mutant exhibits cellulase and pectolytic enzyme activities similar to those of the RsmA mutant. Bacteria were grown to stationary phase on liquid minimal medium supplemented with 0.4% PGA. Cells were subsequently pelleted, and the supernatant was diluted 2, 3, and 5 times. The different dilutions were pipetted onto LB plates containing polygalacturonic acid to test for pectolytic enzyme activity (A) or carboxymethylcellulose to test for cellulase activity (B) in 20-μl drops. In the assay, we used five replicate drops per dilution per strain. (C) The diameters of cleared circles on the plates were measured and compared between strains.
Effects of ExpA and RsmA on the virulence of P. wasabiae.
The P. wasabiae expA mutant has been shown to be avirulent, while rsmA mutants have been considered hypervirulent (5, 7, 15, 16, 23, 24, 28). However, these interpretations depend on the type of virulence assays employed; for example, maceration assays measure PCWDE production rather than virulence-associated lifestyle, i.e., the ability to propagate in planta (75). When bacterial growth in planta on tobacco is being assessed as a measure of virulence (Fig. 8), the expA mutant clearly demonstrated impaired virulence, with only slight initial growth inside the plant tissue during the first 48 h and without visible maceration due to a lack of PCWDE biosynthesis. In contrast, the double mutant and the rsmA mutant exhibited significant maceration of plant tissues at the infection site, although this was somewhat greater in the double mutant. As the growth rate was still highly impaired in all mutant strains, they could be considered mutants with reduced virulence. The double mutant proved to be more virulent than either single mutant, although it was still significantly less virulent than the wild type (Fig. 8). It seems possible that this effect is the result of a combination of greater growth of the double mutant, compared to the rsmA mutant, and the almost similar production of many virulence-related proteins, such as PCWDEs. However, the overproduction of virulence determinants along with impaired growth clearly reduces the fitness of these mutants, resulting in a reduced-virulence phenotype. Furthermore, studies of the impact on virulence in rsmA mutants in related gammaproteobacteria have revealed that the situation is more complex than simply exhibiting an opposing phenotype to expA inactivation, and various pathogenicity-related phenotypes have been noted depending on the experiment (16).
FIG 8.
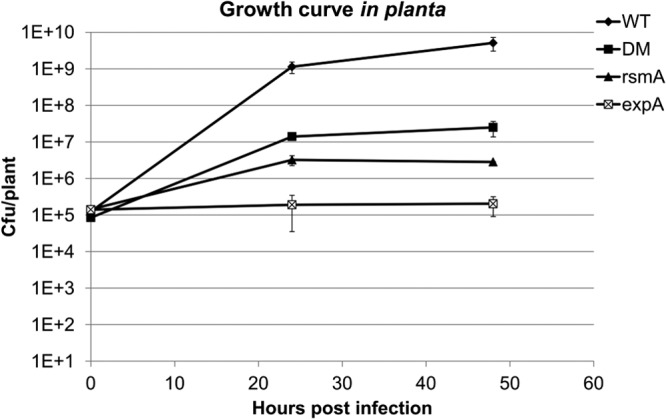
The ExpA RsmA double mutant is more virulent than either of the single mutants during infection on axenically grown tobacco seedlings. The wild-type and expA mutant strains were grown overnight in LB medium, while the double mutant (DM) and rsmA mutant were grown in the same medium for 2 days. These strains were subsequently washed twice in 10 mM MgSO4. The washed cultures were equalized at an OD600 of 0.1, and 1.5 μl was pipetted onto wounds in the leaves of 16-day-old in vitro tobacco seedlings. The plants were grown in sterile 24-well plates, each well containing one plant. For each experimental replicate, one 24-well plate was used for the inoculation of each strain. Inoculated plants were incubated in darkness at room temperature for 0, 24, and 48 h before the bacterial CFU from harvested plants were counted. At least six plants per plate were used to count CFU at each time point and for each strain. In the graph, CFU/plant signifies the number of CFU from the dilution series on LB plates at 0, 24, or 48 h postinfection. All comparisons of numbers of CFU/plant between the strains had a statistical significance of <0.05 using the two-tailed Welch's t test in Microsoft Excel.
It appears that that expA operates through rsmA for many functions, including, as previously established, the PCWDE-biosynthetic pathways, as well as control of secretion systems, chemotaxis, and flagellar biosynthesis, and different metabolic functions. However, ExpA also exerts effects that are separate from those of RsmA on virulence and fitness, leading to the phenotypes we observed in the mutants. It is important to remember, however, that the microarray data as well as the virulence tests and growth assays show that expA also regulates functions involved in PCWDE production, various forms of metabolism, and energy conversion independently of rsmA. In conclusion, these data indeed confirm that ExpA is a positive regulator of a number of central virulence traits such as PCWDE production and secretion, in a regulatory hierarchy above the negative virulence regulators KdgR and RsmA. Furthermore, ExpA appears to have a regulatory role in determining the redox state of the cell as well, positively regulating PGA uptake and metabolism, and yet, mutation of expA causes the bacterial population to exhibit increased growth when RsmA is already deleted in a medium in which PGA is the sole carbon source. This may be related to the upregulation of proton-exporting systems such as cytochrome o ubiquinole oxidase in the expA mutant and may affect the regulation of internal proton levels in the acidic environment.
There are indications that ExpS/ExpA and similar TCS in phytopathogenic enterobacteria are activated by acidic plant compounds such as PGA, o-coumaric acid, and p-coumaric acid (1, 76). ExpA together with KdgR may be part of a complex system controlled by acidity, perhaps by plant compounds, as a bacterial response to plant stimuli. Consequently, ExpA would activate transport and metabolism of plant compounds and subsequently trigger virulence via inhibition of RsmA virulence repression, while KdgR represses virulence, but independently of RsmA, as a response to the absence of 2-keto-3-deoxygluconate. The possible fine-tuning of virulence might be partially due to intracellular activity such as redox potential. Genes involved in redox potential chemistry were observed to be affected in the expA mutant in this study (see Data Set S1 in the supplemental material) and have been inferred to affect virulence in, for example, studies of animal pathogens (71, 72, 77, 78).
Therefore, there is a need for more work to fully map and understand these two regulators and their respective regulons. A schematic model can be presented to summarize the interactions in the global transcriptome controlled by the expA-rsmA pathway in P. wasabiae SCC3193 (Fig. 6). This study provides an approach of transcriptomics combined with microbial genetics and physiology to elucidate regulatory networks with many interacting regulators, highlighting the complexities of the RsmA and ExpA networks and showing how interwoven bacterial functions and regulation can be.
Supplementary Material
ACKNOWLEDGMENTS
We thank Outi Niemi and Petri Törönen (University of Helsinki) for highly valuable technical support.
This work was supported by grants from the Academy of Finland (Center of Excellence program 2006-2011 grants 213509, 129628, 136470, 120821, and 128566), by the University of Helsinki, by Biocentrum Helsinki, by Biocenter Finland, and by the Finnish Doctoral Program in Plant Science. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 17 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03829-13.
REFERENCES
- 1.Charkowski AO. 2009. Decaying signals: will understanding bacterial-plant communications lead to control of soft rot? Curr. Opin. Biotechnol. 20:178–184. 10.1016/j.copbio.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 2.Liu H, Coulthurst SJ, Pritchard L, Hedley PE, Ravensdale M, Humphris S, Burr T, Takle G, Brurberg M-B, Birch PRJ, Salmond GPC, Toth IK. 2008. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog. 4:e1000093. 10.1371/journal.ppat.1000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nykyri J, Niemi O, Koskinen P, Nokso-Koivisto J, Pasanen M, Broberg M, Plyusnin I, Törönen P, Holm L, Pirhonen M, Palva ET. 2012. Revised phylogeny and novel horizontally acquired virulence determinants of the model soft rot phytopathogen Pectobacterium wasabiae SCC3193. PLoS Pathog. 8:e1003013. 10.1371/journal.ppat.1003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirhonen M, Flego D, Heikinheimo R, Palva ET. 1993. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 12:2467–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirhonen M, Saarilahti H, Karlsson M-B, Palva ET. 1991. Identification of pathogenicity determinants of Erwinia carotovora subsp. carotovora by transposon mutagenesis. Mol. Plant Microbe Interact. 4:276–283. 10.1094/MPMI-4-276 [DOI] [Google Scholar]
- 6.Toth IK, Pritchard L, Birch PRJ. 2006. Comparative genomics reveals what makes an enterobacterial plant pathogen. Annu. Rev. Phytopathol. 44:305–336. 10.1146/annurev.phyto.44.070505.143444 [DOI] [PubMed] [Google Scholar]
- 7.Eriksson ARB, Andersson RA, Pirhonen M, Palva ET. 1998. Two-component regulators involved in the global control of virulence in Erwinia carotovora subsp. carotovora. Mol. Plant Microbe Interact. 11:743–752. 10.1094/MPMI.1998.11.8.743 [DOI] [PubMed] [Google Scholar]
- 8.Cui Y, Chatterjee A, Yang H, Chatterjee AK. 2008. Regulatory network controlling extracellular proteins in Erwinia carotovora subsp. carotovora: FlhDC, the master regulator of flagellar genes, activates rsmB regulatory RNA production by affecting gacA and hexA (lrhA) expression. J. Bacteriol. 190:4610–4623. 10.1128/JB.01828-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumer C, Heeb S, Pessi G, Haas D. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. U. S. A. 96:14073–14078. 10.1073/pnas.96.24.14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitten T, Kinscherf TG, McEvoy JL, Willis DK. 1998. A newly identified regulator is required for virulence and toxin production in Pseudomonas syringae. Mol. Microbiol. 28:917–929. 10.1046/j.1365-2958.1998.00842.x [DOI] [PubMed] [Google Scholar]
- 11.Hassan KA, Johnson A, Shaffer BT, Ren Q, Kidarsa TA, Elbourne LDH, Hartney S, Duboy R, Goebel NC, Zabriskie TM, Paulsen IT, Loper JE. 2010. Inactivation of the GacA response regulator in Pseudomonas fluorescens Pf-5 has far-reaching transcriptomic consequences. Environ. Microbiol. 12:899–915. 10.1111/j.1462-2920.2009.02134.x [DOI] [PubMed] [Google Scholar]
- 12.Rich JJ, Hirano SS, Willis DK. 1992. Pathovar-specific requirement for the Pseudomonas syringae lemA gene in disease lesion formation. Appl. Environ. Microbiol. 58:1440–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. 1997. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol. Microbiol. 24:309–319. 10.1046/j.1365-2958.1997.3291701.x [DOI] [PubMed] [Google Scholar]
- 14.Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, Dove SL, Lory S. 2009. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol. Microbiol. 73:434–445. 10.1111/j.1365-2958.2009.06782.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyytiäinen H, Montesano M, Palva ET. 2001. Global regulators ExpA (GacA) and KdgR modulate extracellular enzyme gene expression through the RsmA-rsmB system in Erwinia carotovora subsp. carotovora. Mol. Plant Microbe Interact. 14:931–938. 10.1094/MPMI.2001.14.8.931 [DOI] [PubMed] [Google Scholar]
- 16.Lapouge K, Schubert M, Allain FH-T, Haas D. 2008. Gac/Rsm signal transduction pathway of gamma-proteobacteria: from RNA recognition to regulation of social behaviour. Mol. Microbiol. 67:241–253. 10.1111/j.1365-2958.2007.06042.x [DOI] [PubMed] [Google Scholar]
- 17.Romeo T, Vakulskas CA, Babitzke P. 2013. Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Environ. Microbiol. 15:313–324. 10.1111/j.1462-2920.2012.02794.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomenius H, Pernestig A-K, Jonas K, Georgellis D, Möllby R, Normark S, Melefors Ö. 2006. The Escherichia coli BarA-UvrY two-component system is a virulence determinant in the urinary tract. BMC Microbiol. 6:27. 10.1186/1471-2180-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pernestig A-K, Melefors Ö, Georgellis D. 2001. Identification of UvrY as the cognate response regulator for the BarA sensor kinase in Escherichia coli. J. Biol. Chem. 276:225–231. 10.1074/jbc.M001550200 [DOI] [PubMed] [Google Scholar]
- 20.Palaniyandi S, Mitra A, Herren CD, Lockatell CV, Johnson DE, Zhu X, Mukhopadhyay S. 2012. BarA-UvrY two-component system regulates virulence of uropathogenic E. coli CFT073. PLoS One 7:e31348. 10.1371/journal.pone.0031348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herren CD, Mitra A, Palaniyandi SK, Coleman A, Elankumaran S, Mukhopadhyay S. 2006. The BarA-UvrY two-component system regulates virulence in avian pathogenic Escherichia coli O78:K80:H9. Infect. Immun. 74:4900–4909. 10.1128/IAI.00412-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahr T, Brüggemann H, Jules M, Lomma M, Albert-Weissenberger C, Cazalet C, Buchrieser C. 2009. Two small ncRNAs jointly govern virulence and transmission in Legionella pneumophila. Mol. Microbiol. 72:741–762. 10.1111/j.1365-2958.2009.06677.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kõiv V, Andresen L, Broberg M, Frolova J, Somervuo P, Auvinen P, Pirhonen M, Tenson T, Mäe A. 2013. Lack of RsmA-mediated control results in constant hypervirulence, cell elongation, and hyperflagellation in Pectobacterium wasabiae. PLoS One 8:e54248. 10.1371/journal.pone.0054248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatterjee A, Cui Y, Liu Y, Dumenyo CK, Chatterjee AK. 1995. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density-sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl. Environ. Microbiol. 61:1959–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee A, Cui Y, Chatterjee AK. 2002. RsmA and the quorum-sensing signal, N-[3-oxohexanoyl]-l-homoserine lactone, control the levels of rsmB RNA in Erwinia carotovora subsp. carotovora by affecting its stability. J. Bacteriol. 184:4089–4095. 10.1128/JB.184.15.4089-4095.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González N, Heeb S, Valverde C, Kay E, Reimmann C, Junier T, Haas D. 2008. Genome-wide search reveals a novel GacA-regulated small RNA in Pseudomonas species. BMC Genomics 9:167. 10.1186/1471-2164-9-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babitzke P, Romeo T. 2007. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr. Opin. Microbiol. 10:156–163. 10.1016/j.mib.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 28.Cui Y, Chatterjee A, Chatterjee AK. 2001. Effects of the two-component system comprising GacA and GacS of Erwinia carotovora subsp. carotovora on the production of global regulatory rsmB RNA, extracellular enzymes, and harpinEcc. Mol. Plant Microbe Interact. 14:516–526. 10.1094/MPMI.2001.14.4.516 [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee A, Cui Y, Yang H, Collmer A, Alfano JR, Chatterjee AK. 2003. GacA, the response regulator of a two-component system, acts as a master regulator in Pseudomonas syringae pv. tomato DC3000 by controlling regulatory RNA, transcriptional activators, and alternate sigma factors. Mol. Plant Microbe Interact. 16:1106–1117. 10.1094/MPMI.2003.16.12.1106 [DOI] [PubMed] [Google Scholar]
- 30.Kay E, Dubuis C, Haas D. 2005. Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc. Natl. Acad. Sci. U. S. A. 102:17136–17141. 10.1073/pnas.0505673102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonas K, Edwards AN, Simm R, Romeo T, Römling U, Melefors Ö. 2008. The RNA binding protein CsrA controls cyclic di-GMP metabolism by directly regulating the expression of GGDEF proteins. Mol. Microbiol. 70:236–257. 10.1111/j.1365-2958.2008.06411.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawhon SD, Frye JG, Suyemoto M, Porwollik S, McClelland M, Altier C. 2003. Global regulation by CsrA in Salmonella typhimurium. Mol. Microbiol. 48:1633–1645. 10.1046/j.1365-2958.2003.03535.x [DOI] [PubMed] [Google Scholar]
- 33.Yang S, Peng Q, Zhang Q, Yi X, Choi CJ, Reedy RM, Charkowski AO, Yang C-H. 2008. Dynamic regulation of GacA in type III secretion, pectinase gene expression, pellicle formation, and pathogenicity of Dickeya dadantii (Erwinia chrysanthemi 3937). Mol. Plant Microbe Interact. 21:133–142. 10.1094/MPMI-21-1-0133 [DOI] [PubMed] [Google Scholar]
- 34.Cha JY, Lee DG, Lee JS, Oh J-I, Baik HS. 2012. GacA directly regulates expression of several virulence genes in Pseudomonas syringae pv. tabaci 11528. Biochem. Biophys. Res. Commun. 417:665–672. 10.1016/j.bbrc.2011.11.124 [DOI] [PubMed] [Google Scholar]
- 35.Hyytiäinen H, Sjöblom S, Palomäki T, Tuikkala A, Palva ET. 2003. The PmrA-PmrB two-component system responding to acidic pH and iron controls virulence in the plant pathogen Erwinia carotovora ssp. carotovora. Mol. Microbiol. 50:795–807. 10.1046/j.1365-2958.2003.03729.x [DOI] [PubMed] [Google Scholar]
- 36.Prosseda G, Carmela Latella M, Barbagallo M, Nicoletti M, Al Kassas R, Casalino M, Colonna B. 2007. The two-faced role of cad genes in the virulence of pathogenic Escherichia coli. Res. Microbiol. 158:487–493. 10.1016/j.resmic.2007.05.001 [DOI] [PubMed] [Google Scholar]
- 37.Lee YH, Kim BH, Kim JH, Yoon WS, Bang SH, Park YK. 2007. CadC has a global translational effect during acid adaptation in Salmonella enterica serovar Typhimurium. J. Bacteriol. 189:2417–2425. 10.1128/JB.01277-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huynh T, Dahlbeck D, Staskawicz B. 1989. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science 245:1374–1377. 10.1126/science.2781284 [DOI] [PubMed] [Google Scholar]
- 40.Summers WC. 1970. A simple method for extraction of RNA from E. coli utilizing diethyl pyrocarbonate. Anal. Biochem. 33:460–463. 10.1016/0003-2697(70)90316-7 [DOI] [PubMed] [Google Scholar]
- 41.Bolstad BM, Irizarry RA, Åstrand M, Speed TP. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193. 10.1093/bioinformatics/19.2.185 [DOI] [PubMed] [Google Scholar]
- 42.Smyth GK, Speed T. 2003. Normalization of cDNA microarray data. Methods 31:265–273. 10.1016/S1046-2023(03)00155-5 [DOI] [PubMed] [Google Scholar]
- 43.Kõiv V, Andresen L, Mäe A. 2010. AepA of Pectobacterium is not involved in the regulation of extracellular plant cell wall degrading enzymes production. Mol. Genet. Genomics 283:541–549. 10.1007/s00438-010-0540-9 [DOI] [PubMed] [Google Scholar]
- 44.Takle GW, Toth IK, Brurberg MB. 2007. Evaluation of reference genes for real-time RT-PCR expression studies in the plant pathogen Pectobacterium atrosepticum. BMC Plant Biol. 7:50. 10.1186/1471-2229-7-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 46.Kidarsa TA, Shaffer BT, Goebel NC, Roberts DP, Buyer JS, Johnson A, Kobayashi DY, Zabriskie TM, Paulsen I, Loper JE. 2013. Genes expressed by the biological control bacterium Pseudomonas protegens Pf-5 on seed surfaces under the control of the global regulators GacA and RpoS. Environ. Microbiol. 15:716–735. 10.1111/1462-2920.12066 [DOI] [PubMed] [Google Scholar]
- 47.Wang D, Lee S-H, Seeve C, Yu JM, Pierson LS, III, Pierson EA. 2013. Roles of the Gac-Rsm pathway in the regulation of phenazine biosynthesis in Pseudomonas chlororaphis 30–84. MicrobiologyOpen 2:505–524. 10.1002/mbo3.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monson R, Burr T, Carlton T, Liu H, Hedley P, Toth I, Salmond GPC. 2013. Identification of genes in the VirR regulon of Pectobacterium atrosepticum and characterization of their roles in quorum sensing-dependent virulence. Environ. Microbiol. 15:687–701. 10.1111/j.1462-2920.2012.02822.x [DOI] [PubMed] [Google Scholar]
- 49.Cubitt MF, Hedley PE, Williamson NR, Morris JA, Campbell E, Toth IK, Salmond GPC. 2013. A metabolic regulator modulates virulence and quorum sensing signal production in Pectobacterium atrosepticum. Mol. Plant Microbe Interact. 26:356–366. 10.1094/MPMI-09-12-0210-R [DOI] [PubMed] [Google Scholar]
- 50.Burrowes E, Baysse C, Adams C, O'Gara F. 2006. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 152:405–418. 10.1099/mic.0.28324-0 [DOI] [PubMed] [Google Scholar]
- 51.Lalaouna D, Fochesato S, Sanchez L, Schmitt-Kopplin P, Haas D, Heulin T, Achouak W. 2012. Phenotypic switching in Pseudomonas brassicacearum involves GacS- and GacA-dependent Rsm small RNAs. Appl. Environ. Microbiol. 78:1658–1665. 10.1128/AEM.06769-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng X, de Bruijn I, van der Voort M, Loper JE, Raaijmakers JM. 2013. The Gac regulon of Pseudomonas fluorescens SBW25. Environ. Microbiol. Rep. 5:608–619. 10.1111/1758-2229.12061 [DOI] [PubMed] [Google Scholar]
- 53.Babujee L, Apodaca J, Balakrishnan V, Liss P, Kiley PJ, Charkowski AO, Glasner JD, Perna NT. 2012. Evolution of the metabolic and regulatory networks associated with oxygen availability in two phytopathogenic enterobacteria. BMC Genomics 13:110. 10.1186/1471-2164-13-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kovacikova G, Lin W, Skorupski K. 2010. The LysR-type virulence activator AphB regulates the expression of genes in Vibrio cholerae in response to low pH and anaerobiosis. J. Bacteriol. 192:4181–4191. 10.1128/JB.00193-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reeves PJ, Whitcombe D, Wharam S, Gibson M, Allison G, Bunce N, Barallon R, Douglas P, Mulholland V, Stevens S. 1993. Molecular cloning and characterization of 13 out genes from Erwinia carotovora subspecies carotovora: genes encoding members of a general secretion pathway (GSP) widespread in gram-negative bacteria. Mol. Microbiol. 8:443–456. 10.1111/j.1365-2958.1993.tb01589.x [DOI] [PubMed] [Google Scholar]
- 56.Salmond GPC. 1994. Secretion of extracellular virulence factors by plant pathogenic bacteria. Annu. Rev. Phytopathol. 32:181–200. 10.1146/annurev.py.32.090194.001145 [DOI] [Google Scholar]
- 57.Records AR. 2011. The type VI secretion system: a multipurpose delivery system with a phage-like machinery. Mol. Plant Microbe Interact. 24:751–757. 10.1094/MPMI-11-10-0262 [DOI] [PubMed] [Google Scholar]
- 58.Mulholland V, Hinton JCD, Sidebotham J, Toth IK, Hyman LJ, Perombelon MCM, Reeves PJ, Salmond GPC. 1993. A pleiotropic reduced virulence (Rvi-) mutant of Erwinia carotovora subspecies atroseptica is defective in flagella assembly proteins that are conserved in plant and animal bacterial pathogens. Mol. Microbiol. 9:343–356. 10.1111/j.1365-2958.1993.tb01695.x [DOI] [PubMed] [Google Scholar]
- 59.Yuan J, Branch RW, Hosu BG, Berg HC. 2012. Adaptation at the output of the chemotaxis signalling pathway. Nature 484:233–236. 10.1038/nature10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowsell EH, Smith JM, Wolfe A, Taylor BL. 1995. CheA, CheW, and CheY are required for chemotaxis to oxygen and sugars of the phosphotransferase system in Escherichia coli. J. Bacteriol. 177:6011–6014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blair DF, Berg HC. 1988. Restoration of torque in defective flagellar motors. Science 242:1678–1681. 10.1126/science.2849208 [DOI] [PubMed] [Google Scholar]
- 62.Weir TL, Stull VJ, Badri D, Trunck LA, Schweizer HP, Vivanco J. 2008. Global gene expression profiles suggest an important role for nutrient acquisition in early pathogenesis in a plant model of Pseudomonas aeruginosa infection. Appl. Environ. Microbiol. 74:5784–5791. 10.1128/AEM.00860-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mattinen L, Somervuo P, Nykyri J, Nissinen R, Kouvonen P, Corthals G, Auvinen P, Aittamaa M, Valkonen JPT, Pirhonen M. 2008. Microarray profiling of host-extract-induced genes and characterization of the type VI secretion cluster in the potato pathogen Pectobacterium atrosepticum. Microbiology 154:2387–2396. 10.1099/mic.0.2008/017582-0 [DOI] [PubMed] [Google Scholar]
- 64.Ternan NG, Grath JWM, Mullan GM, Quinn JP. 1998. Review: organophosphonates: occurrence, synthesis and biodegradation by microorganisms. World J. Microbiol. Biotechnol. 14:635–647. 10.1023/A:1008848401799 [DOI] [Google Scholar]
- 65.Kim SY, Ju K-S, Metcalf WW, Evans BS, Kuzuyama T, van der Donk WA. 2012. Different biosynthetic pathways to fosfomycin in Pseudomonas syringae and Streptomyces species. Antimicrob. Agents Chemother. 56:4175–4183. 10.1128/AAC.06478-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hugouvieux-Cotte-Pattat N, Blot N, Reverchon S. 2001. Identification of TogMNAB, an ABC transporter which mediates the uptake of pectic oligomers in Erwinia chrysanthemi 3937. Mol. Microbiol. 41:1113–1123. 10.1046/j.1365-2958.2001.02564.x [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Jiang G, Cui Y, Mukherjee A, Ma WL, Chatterjee AK. 1999. kdgREcc negatively regulates genes for pectinases, cellulase, protease, HarpinEcc, and a global RNA regulator in Erwinia carotovora subsp. carotovora. J. Bacteriol. 181:2411–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tarasova N, Gorshkov V, Petrova O, Gogolev Y. 2013. Potato signal molecules that activate pectate lyase synthesis in Pectobacterium atrosepticum SCRI1043 World. J. Microbiol. Biotechnol. 29:1189–1196. 10.1007/s11274-013-1281-9 [DOI] [PubMed] [Google Scholar]
- 69.Dinamarca MA, Ruiz-Manzano A, Rojo F. 2002. Inactivation of cytochrome o ubiquinol oxidase relieves catabolic repression of the Pseudomonas putida GPo1 alkane degradation pathway. J. Bacteriol. 184:3785–3793. 10.1128/JB.184.14.3785-3793.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alverdy J, Holbrook C, Rocha F, Seiden L, Licheng R, Wu RL, Musch M, Chang E, Ohman D, Suh S. 2000. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host. Ann. Surg. 232:480–489. 10.1097/00000658-200010000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beier D, Gross R. 2006. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 9:143–152. 10.1016/j.mib.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 72.Spellerberg B, Cundell DR, Sandros J, Pearce BJ, Idänpään-Heikkilä I, Rosenow C, Masure HR. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803–813. 10.1046/j.1365-2958.1996.425954.x [DOI] [PubMed] [Google Scholar]
- 73.Ogasawara H, Ishida Y, Yamada K, Yamamoto K, Ishihama A. 2007. PdhR (pyruvate dehydrogenase complex regulator) controls the respiratory electron transport system in Escherichia coli. J. Bacteriol. 189:5534–5541. 10.1128/JB.00229-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Quail MA, Haydon DJ, Guest JR. 1994. The pdhR–aceEF–lpd operon of Escherichia coli expresses the pyruvate dehydrogenase complex. Mol. Microbiol. 12:95–104. 10.1111/j.1365-2958.1994.tb00998.x [DOI] [PubMed] [Google Scholar]
- 75.Wassenaar TM, Gaastra W. 2001. Bacterial virulence: can we draw the line? FEMS Microbiology Lett. 201:1–7. 10.1111/j.1574-6968.2001.tb10724.x [DOI] [PubMed] [Google Scholar]
- 76.Yamazaki A, Li J, Zeng Q, Khokhani D, Hutchins WC, Yost AC, Biddle E, Toone EJ, Chen X, Yang C-H. 2012. Derivatives of plant phenolic compound affect the type III secretion system of Pseudomonas aeruginosa via a GacS-GacA two-component signal transduction system. Antimicrob. Agents Chemother. 56:36–43. 10.1128/AAC.00732-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pancholi V, Chhatwal GS. 2003. Housekeeping enzymes as virulence factors for pathogens. Int. J. Med. Microbiol. 293:391–401. 10.1078/1438-4221-00283 [DOI] [PubMed] [Google Scholar]
- 78.Clair G, Roussi S, Armengaud J, Duport C. 2010. Expanding the known repertoire of virulence factors produced by Bacillus cereus through early secretome profiling in three redox conditions. Mol. Cell Proteomics 9:1486–1498. 10.1074/mcp.M000027-MCP201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.