Abstract
Using 16S rRNA gene sequencing analysis, we examined the bacterial diversity and the presence of opportunistic bacterial pathogens (i.e., Campylobacter and Helicobacter) in red knot (Calidris canutus; n = 40), ruddy turnstone (Arenaria interpres; n = 35), and semipalmated sandpiper (Calidris pusilla; n = 22) fecal samples collected during a migratory stopover in Delaware Bay. Additionally, we studied the occurrence of Campylobacter spp., enterococci, and waterfowl fecal source markers using quantitative PCR (qPCR) assays. Of 3,889 16S rRNA clone sequences analyzed, the bacterial community was mostly composed of Bacilli (63.5%), Fusobacteria (12.7%), Epsilonproteobacteria (6.5%), and Clostridia (5.8%). When epsilonproteobacterium-specific 23S rRNA gene clone libraries (i.e., 1,414 sequences) were analyzed, the sequences were identified as Campylobacter (82.3%) or Helicobacter (17.7%) spp. Specifically, 38.4%, 10.1%, and 26.0% of clone sequences were identified as C. lari (>99% sequence identity) in ruddy turnstone, red knot, and semipalmated sandpiper clone libraries, respectively. Other pathogenic species of Campylobacter, such as C. jejuni and C. coli, were not detected in excreta of any of the three bird species. Most Helicobacter-like sequences identified were closely related to H. pametensis (>99% sequence identity) and H. anseris (92% sequence identity). qPCR results showed that the occurrence and abundance of Campylobacter spp. was relatively high compared to those of fecal indicator bacteria, such as Enterococcus spp., E. faecalis, and Catellicoccus marimammalium. Overall, the results provide insights into the complexity of the shorebird gut microbial community and suggest that these migratory birds are important reservoirs of pathogenic Campylobacter species.
INTRODUCTION
Migratory birds are often suggested as vectors of infectious diseases due to their potential for spreading pathogens over large geographical distances (1–3). Shorebirds have among the longest migration routes of animals worldwide and are known to congregate in large flocks, often numbering over 10,000 individuals (4, 5). During their migration, which on an annual basis may comprise over 10,000 miles, shorebirds visit many different habitats, including a number of refueling or staging sites. As these birds live and forage intensively and in high densities in these staging habitats, it is reasonable to propose that such sites play an important role in further spreading of microorganisms due to the close contact with conspecifics and other migratory bird species. As some of these microorganisms can be pathogenic, such interactions could have important water monitoring as well as public health ramifications. Indeed, a wide variety of bird species have been found to harbor human and avian pathogens, among them gulls (6–9), geese (9–11), terns (9), psittacines (12), corvids (7), raptors (6), songbirds (4, 7), and shorebirds (8–10). Some of the human and avian pathogens identified thus far are bacteria such as Escherichia coli (3, 11), Streptococcus spp. (7), Salmonella spp. (8), Helicobacter spp. (12), Campylobacter spp. (1, 5, 13), and Yersinia spp. (14), protozoan parasites such as Cryptosporidium spp. (2), and viral pathogens such as avian influenza (9), avian paramyxoviruses (8), and West Nile virus (15, 16).
Shorebirds have largely been ignored as potential sources of fecal pollution, possibly due to their small size and relatively short stay at staging sites. At one particular staging site in Delaware Bay, every spring thousands of shorebirds aggregate to refuel for their migration to the Canadian Arctic. For an average of 3 weeks, these birds intensively use the beaches around the bay to forage on eggs of the horseshoe crab Limulus polyphemus. These birds have been observed to defecate every 5 min (17), resulting in a continuous contribution of fecal matter to their environment. Delaware Bay lies between the heavily populated coastal areas of Delaware and New Jersey, and during spring and summer it is extensively used for recreational activities. As a result, shorebirds may impact the microbial water quality of recreational areas within this bay through fecal deposition and increase human health risks if shorebirds indeed harbor human pathogens.
To date, little is known about the gastrointestinal microbiota of shorebirds. This information is relevant to determine the potential risks associated with them and critical to the development of tools that can help us assess their fecal loads in recreational waters. To our knowledge, only one study has focused on the identification of gut microbiota diversity in shorebirds. Using 16S rRNA gene sequencing analysis, Santos et al. (18) studied the cloacal bacterial composition of the shorebird species common redshank (Tringa tetanus), black-winged stilt (Himantopus himantopus), and black-tailed godwit (Limosa limosa). Despite the relatively small number of R2A isolates (n = 240) and clone (n = 58) sequences examined, they identified several genera, such as Campylobacter/Helicobacter, Escherichia, Staphylococcus, and Legionella. Some species of these genera include potential human pathogens. It should be noted that other studies have shown that birds are reservoirs of Campylobacter spp. (19), and that as a group these bacteria may be part of some birds' commensal microbiota (20). While there is no evidence of Campylobacter strains pathogenic to birds, several studies have detected species considered pathogenic to humans, such as C. jejuni, C. coli, and C. lari (21, 22). As these bacterial species can cause severe gastroenteritis in humans, deposition of these and other pathogens via shorebird fecal pollution in coastal areas poses a potential human health threat. Besides the potential role of shorebirds as vectors of human disease, it is important to gain insight into the gut microbiota composition of shorebirds to determine their link, if any, to the health of the shorebird population. Specifically, numbers of many migratory shorebird species are declining at alarming rates, and to assess the effect of disease on population numbers, it is imperative to investigate the diversity of their gut microbiota.
The primary goals of this study are (i) to characterize fecal bacterial diversity and interspecific bacterial variation of three shorebird species, red knot (Calidris canutus), ruddy turnstone (Arenaria interpres), and semipalmated sandpiper (Calidris pusilla), during the spring migration staging period in Delaware Bay and (ii) to examine the occurrence of potentially pathogenic members of the Epsilonproteobacteria class, specifically Campylobacter and Helicobacter species. We addressed these objectives by identifying the compositions of the bacterial communities found in fecal samples collected from shorebirds in Delaware Bay during the spring migration staging period through use of 16S and 23S rRNA gene-based analyses.
MATERIALS AND METHODS
Sample collection and DNA extraction.
We captured birds by canon net in Delaware Bay between 13 and 27 May 2011. A total of 40, 35, and 22 fecal pellets were collected from red knots, ruddy turnstones, and semipalmated sandpipers, respectively. Upon capture, birds were color banded to ensure individual recognition and placed in individual cardboard boxes lined with sterile waxed paper for collection of feces. Fecal pellets were preserved on site in sterile 2-ml collection tubes prefilled with 96% ethanol. Samples were stored at 4°C until further analyses. A total of 12 water samples were also collected from three locations, Port Mahon, Misillion Harbor, and Broadkill Beach, presumed to be impacted by shorebirds. Water samples (250 ml) were filtered onto polycarbonate membranes (0.4-μm pore size, 47-mm diameter) (GE Water and Process Technologies, Trevose, PA) at the Delaware Biotechnology Institute, University of Delaware, Lewes, DE, and stored at −80°C. The membrane filters and fecal samples were shipped overnight frozen to the U.S. EPA laboratory (Cincinnati, OH). Fecal samples were spun down for 3 min at 14, 000 rpm and washed three times with one ml of sterile molecular biology-grade water (Fisher Scientific, Pittsburgh, PA) to further remove the ethanol (23). DNA extractions were performed on the ethanol-free fecal pellets and membrane filters using Mo Bio PowerLyzer PowerSoil kits according to the manufacturer's protocol (MO BIO Laboratories, Carlsbad, CA). DNA extracts were stored at −20°C until further processing.
All sampling procedures were in compliance with animal welfare laws of the United States, and all permits necessary for shipping and handling of biological samples were obtained.
Cloning and sequencing analyses.
A total of 18 DNA extracts from three bird species (six samples for each bird species) were used as templates in PCR assays using domain-specific Bacteria (eubacteria) 16S rRNA gene primers 8F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 785R (5′-ACTACCRGGGTATCTAATCC-3′), as described elsewhere (8, 24). In addition, the class-specific epsilonproteobacterial 23S rRNA gene primers EPS_23S_1325F (5′-TCGTCATCGTAGGGTTAG-3′) and EPS_23S_1763R (5′-GTAAACAGTCGGGAGGGA-3′) were used specifically for the amplification of Campylobacter and Helicobacter against 18 fecal and three water samples (25). PCR amplifications were performed in 25 μl using the polymerase TaKaRa Ex Taq (TaKaRa Bio Inc.) in a Bio-Rad Tetrad2 Peltier thermal cycler (Bio-Rad, Hercules, CA) under the following cycling conditions: one initial denaturation step at 95°C for 5 min and then 30 cycles of 1 min at 95°C, 1 min at 56°C or 55°C (for the domain Bacteria or Epsilonproteobacteria, respectively), and 1 min at 72°C. PCR products were visualized in 1.5% agarose gels using GelStar nucleic acid gel stain (Lonza, Rockland, ME).
Domain Bacteria and class Epsilonproteobacteria PCR products were cloned into pCR4 TOPO vector and transformed into TOPO10 chemically competent E. coli cells as described by the manufacturer (Invitrogen, Carlsbad, CA). The clone libraries were developed from PCR products generated using fecal and water DNA extracts. Individual E. coli clones were subcultured into 300 μl of Luria Broth containing 50 μg/ml ampicillin and screened for inserts using M13 PCR. The PCR products were sequenced using an Applied Biosystems Prism 3730XL DNA analyzer (Children's Hospital DNA Core Facility, Cincinnati, OH). Raw sequences were processed using Sequencher software (Gene Codes, Ann Arbor, MI). Chimeric sequences detected using Bellerophon (26) were not included in further analyses. Sequences were submitted to Greengenes for alignment using the nearest alignment space termination algorithm (27, 28). The clone libraries were compared using the Ribosomal Database Project (RDP) naive Bayesian rRNA classifier, version 2.5, with a 95% confidence threshold (29). For 16S rRNA gene sequences, homology searches of DNA sequences in the GenBank (NR) database were undertaken with the National Center for Biotechnology Information (NCBI) BLASTn application (http://www.ncbi.nlm.nih.gov/BLAST/). Phylogenetic trees for shorebird excreta and water sample sequences were constructed using the neighbor-joining method. 16S and 23S rRNA gene sequences obtained from the GenBank database were used as bacterial reference sequences. Bootstrap values were based on 1,000 replications.
qPCR assays.
Five TaqMan quantitative PCR (qPCR) assays targeting Campylobacter spp. (30), Enterococcus spp. (31), Enterococcus faecalis (32), E. faecium (33), and E. casseliflavus (33) were performed against water and fecal DNA extracts in 25-μl reaction mixtures containing 1× TaqMan universal PCR master mix with AmpErase uracil-N-glycosylase (Applied Biosystems, Foster City, CA) and 0.2 μg/μl bovine serum albumin (Table 1). Tenfold dilutions of each DNA extract were used to test for PCR inhibition (21, 34). The amplification protocol involved an initial incubation at 50°C for 2 min to activate uracil-N-glycosylase, followed by 10 min of incubation at 95°C to activate AmpliTaq Gold enzyme and then 40 cycles of 95°C for 15 s and the optimum annealing temperature for 1 min (Table 1). The qPCR assays were performed using a 7900 HT fast real-time sequence detector (Applied Biosystems, Foster City, CA). All assays were performed in duplicate in MicroAmp optical 96-well reaction plates with MicroAmp optical caps (Applied Biosystems, Foster City, CA). PCR data were analyzed using ABI's Sequence Detector software (version 2.2.2). Four independent standard curves for each qPCR assay were generated by plotting threshold cycle (CT) values against the number of target copies corresponding to serially diluted plasmid standards purchased from IDT (Coralville, IA). The target copy numbers (T) were estimated by the equation T = [D/(PL × 660)] × 6.022 × 1023, where D (g/μl) is plasmid DNA concentration and PL (bp) is plasmid length. Each standard curve was generated from at least five 10-fold plasmid dilutions performed in triplicate. Percent amplification efficiencies were calculated according to the instrument manufacturer's instructions (Applied Biosystems). Two no-template controls per PCR plate were used to check for cross-contamination.
TABLE 1.
Summary of oligonucleotide primers and probes for qPCR assays
| Target organism(s), assay, and primer/probe namea | Primer/probe sequence (5′ to 3′) | Tab (°C) | Sizec (bp) |
|---|---|---|---|
| Campylobacter spp. | |||
| Camp2 | |||
| campF2 | CACGTGCTACAATGGCATAT | 58 | 108 |
| campR2 | GGCTTCATGCTCTCGAGTT | ||
| campP2 | FAM-CAGAGAACAATCCGAACTGGGACA-BHQ1 | ||
| Enterococcus spp. | |||
| Entero1 | |||
| ECST748F | AGAAATTCCAAACGAACTTG | 60 | 92 |
| ENC854R | CAGTGCTCTACCTCCATCATT | ||
| GPL813TQ | FAM-TGGTTCTCTCCGAAATAGCTTTAGGGCTA-TAMRA | ||
| Enterococcus faecalis | |||
| Faecalis1 | |||
| FaecalF | CGCTTCTTTCCTCCCGAGT | 60 | 143 |
| FaecalR | GCCATGCGGCATAAACTG | ||
| FaecalP | 6FAM-CAATTGGAAA GAGGAGTGGCGGACG-TAMRA | ||
| Enterococcus casseliflavus | |||
| Casseli1 | |||
| CasselF | GGAGCTTGCTCCACCGAA | 60 | 132 |
| CasselR | TTTCTTCCATGCGGAAAATAGT | ||
| CasselP | 6FAM-CGAACGGGTGAGTAACACGTGGGTAA-TAMRA | ||
| Enterococcus faecium | |||
| Faecium1 | |||
| CiumF | TTCTTTTTCCACCGGAGCTT | 60 | 141 |
| CiumR | AACCATGCGGTTTYGATTG | ||
| CiumP | 6FAM-AGTAACACGTGGGTAACCTGCCCATCAGA-TAMRA | ||
| Catellicoccus marimammalium | |||
| Gull2-SYBR green | |||
| Forward | TGCATCGACCTAAAGTTTTGAG | 64 | 412 |
| Reverse | GTCAAAGAGCGAGCAGTTACTA |
Entero1 targets the 23S rRNA gene, whereas the other assays target the 16S rRNA gene. The Faecium1 assay amplified E. faecium, E. hirae, and E. mundtii strains, and the Casseli1 assay amplified E. casseliflavus and E. gallinarum. FAM, 6-carboxyfluorescein; BHQ1, black hole quencher 1; TAMRA, 6-carboxytetramethylhrodamine.
Optimum annealing temperatures (Ta) determined using temperature gradient PCR.
Approximate product size determined from in silico data.
The gull2 SYBR green-based qPCR assay targeting the 16S rRNA gene of Catellicoccus marimammalium (8) was conducted against fecal and water samples using aliquots from respective DNA extracts (2 μl) as templates (Table 1). This marker has been detected in waters presumed to be affected by gull fecal contamination (21, 35). Reaction mixtures (25 μl) contained 1× Power SYBR green master mix (Applied Biosystems, Foster City, CA), 0.2 μg/μl bovine serum albumin, and 0.2 μM (final concentration) primers. The amplification protocol involved incubation at 50°C for 2 min, followed by 95°C for 10 min and then 40 cycles of 95°C for 15 s and 64°C for 1 min with a fluorescence read, followed by a melting curve analysis from 60 to 90°C in 0.1-degree increments. Equipment and data analysis were performed as described above. Dissociation curves were examined to determine the presence of potential primer-dimers and other nonspecific reaction products. Signal intensity values were recorded for those reactions showing one corresponding amplification peak within the disassociation curves. In addition, PCR products were visualized in 1.5% agarose gels to confirm the size of amplification products.
Nucleotide sequence accession numbers.
Sequences of different representative taxa obtained in this study were deposited in GenBank under accession numbers KC993229 to KC993796.
RESULTS AND DISCUSSION
Taxonomic and phylogenetic analyses of bacterial 16S rRNA gene sequences.
Most fecal samples (i.e., 80 to 91%) from red knot, ruddy turnstone, and semipalmated sandpiper birds were positive with the general eubacterial assay (data not shown). Six eubacterium-positive samples from each bird species were selected for constructing clone libraries. A total of 1,097, 1,345, and 1,447 clone sequences were analyzed to determine the identities of excreta bacteria of red knots, ruddy turnstones, and semipalmated sandpipers, respectively. Excluding unclassified sequences or those classified as unknown, a total of 27 bacterial genera were represented in the shorebird clone libraries (Table 2). Specifically, 23, 9, and 14 bacterial genera were identified for ruddy turnstones, red knots, and semipalmated sandpipers, respectively. The bacterial community of these three shorebird species was mostly composed of populations closely related to the classes Bacilli, Clostridia, Fusobacteria, and Epsilonproteobacteria, whereas few sequences were classified as the class Bacteroidia. The relatively low abundance of Bacteroidia in shorebird excreta is consistent with other avian studies (8, 35–39) and further suggests that members of this class are not ideal targets in avian microbial source tracking studies.
TABLE 2.
Distribution of 16S rRNA genes in the clone library of shorebird excreta from Delaware Bay
| Class and genus | No. of clones (% total clones) |
||
|---|---|---|---|
| Ruddy turnstone (n = 1,097) | Red knot (n = 1,345) | Semipalmated sandpiper (n = 1,447) | |
| Actinobacteria | |||
| Microbacterium | 3 | —a | — |
| Corynebacterium | 1 | — | — |
| Ilumatobacter | 2 | — | — |
| Nocardioides | 1 | — | — |
| Williamsia | 1 | — | — |
| Rothia | 2 | — | — |
| Unclassified genus | 9 | — | — |
| Bacilli | |||
| Bacillus | 336 (30.6) | 600 (44.6) | 700 (48.4) |
| Catellicoccus | 209 (19.1) | 214 (15.9) | 70 (4.8) |
| Tumebacillus | 18 | 1 | 6 |
| Paenibacillus | 23 | — | 12 |
| Lysinibacillus | 17 | 14 | 188 (13.0) |
| Oceanobacillus | 3 | — | — |
| Unclassified Enterococcaceae | 5 | 4 | — |
| Unclassified Bacillaceae | 21 | 26 | 1 |
| Bacteroidia | |||
| Bacteroides | 5 | — | 1 |
| Unclassified Bacteroidales | 1 | — | — |
| Clostridia | |||
| Butyricicoccus | 2 | 1 | 5 |
| Tissierella | 1 | — | — |
| Acetivibrio | 1 | — | 1 |
| Blautia | — | — | 5 |
| Phascolarctobacterium | — | — | 10 |
| Unclassified Clostridiales | 89 (8.1) | 15 | 79 (5.5) |
| Unclassified Ruminococcaceae | 14 | 2 | 2 |
| Deferribacteres | |||
| Mucispirillum | 5 | — | 4 |
| Mollicutes | |||
| Ureaplasma | — | 3 | — |
| Erysipelotrichi | |||
| Coprobacillus | 4 | — | — |
| Unclassified Erysipelotrichaceae | 4 | 15 | 1 |
| Fusobacteria | |||
| Cetobacterium | 78 (7.1) | 225 (16.7) | 23 (1.6) |
| Unclassified Fusobacteriaceae | 79 (7.2) | 15 | 73 (5.0) |
| Alphaproteobacteria | |||
| Phaeobacter | 18 | — | — |
| Loktanella | 3 | — | — |
| Unclassified Rhizobiales | 7 | 1 | 6 |
| Unclassified Rhodobacteraceae | 14 | 4 | 9 |
| Epsilonproteobacteria | |||
| Campylobacter | 55 (5.0) | 125 (9.3) | 27 (1.9) |
| Helicobacter | 19 (1.7) | 26 (1.9) | — |
| Caldilineae | |||
| Caldilinea | — | — | 5 |
—, not found.
A total of 280 operational taxonomic units (OTUs) (97% sequence similarity) were detected in our fecal samples. Shorebird species showed high variability in fecal OTU composition, with 97, 58, and 81 unique OTUs for ruddy turnstones, red knots, and semipalmated sandpipers (Fig. 1). Only 15 OTUs were shared by all three species, but these 15 OTUs accounted for 55% of all sequences. These results indicate that our three shorebird species share a core gut microbiota, which consisted predominantly of Bacillus spp. (42%). Despite the high number of unique OTUs per shorebird species, these OTUs represented a relatively low abundance of 3.1 to 10.8% of all sequences. One hypothesis for the origin of the large shared fraction of bacterial species among these shorebird species is the similar diet and habitat the birds encounter in Delaware Bay, and that species-specific gut microbes are acquired elsewhere. Alternatively, it is possible that Bacilli spp. are selectively retained year-round due to the gut selective pressure.
FIG 1.

Venn diagram for the shared and nonshared OTUs among red knots (REKN), ruddy turnstones (RUTU), and semipalmated sandpipers (SESA). Sequences were assigned to contigs (boldface) by 97% similarity. Data in parentheses represent percentages of the total number of sequences that the respective contigs contains.
For the Bacilli, more than 50% of all shorebird sequences were classified as Bacillus or Catellicoccus; moreover, these bacterial groups were detected in all of the clone libraries (n = 18) from all three shorebird species. Most Catellicoccus-like sequences showed 95% sequence identity to C. marimammalium (Fig. 2), which is detected predominantly in gull feces (8, 35). Only one sequence, from ruddy turnstones, was identical to that of C. marimammalium. The latter species is rarely detected in avian species other than gulls, but it should be noted that there are significantly fewer studies on avian bacterial diversity than on, for example, mammalian hosts. The fact that shorebirds staging in Delaware Bay forage in close proximity to several gull species might provide a route for interspecific transmission of C. marimammalium. However, most shorebird Catellicoccus-like sequences were different from those identified in the clone libraries of other migratory birds, such as the sandhill crane and snow goose (i.e., <96% identity) (39). Thus, novel Catellicoccus-like species seem to be common bacteria in shorebird and waterfowl intestinal microbiota. Their wide distribution and relative abundance suggest that as a group, Catellicoccus spp. interact with the host at some level. Interestingly, genome analysis of C. marimammalium has suggested that this bacterium is transitioning from a commensal into an obligate symbiont (40). Genome sequences of additional isolates are needed to determine if other Catellicoccus-like species have similar reduced metabolic pathways that are compatible with a symbiotic lifestyle. Such evidence will further support the use of this bacterial group as targets for development of tracking markers based on fecal sources.
FIG 2.
Phylogenetic analysis of bacterial 16S rRNA gene sequences from shore bird fecal samples. The tree was constructed using the neighbor-joining method, and bootstrap values were based on 1,000 replications. The scale bar corresponds to 0.02 changes per nucleotide.
Besides the two dominant genera within the Bacilli, 13% (188/1,447) of the sequences from semipalmated sandpipers were classified as Lysinibacillus, a species which is commonly found in soil or near plant roots and is known as an insect pathogen. However, the sequences were detected in only one of six semipalmated sandpiper clone libraries, suggesting that Lysinibacillus members are not common intestinal bacteria of semipalmated sandpiper, or that their densities are lower than the detection limits of the sequencing approach used in this study. Cetobacterium and Fusobacterium were identified as the main genera within the class Fusobacteria for all three shorebird species (Fig. 2 and Table 2). Most sequences within the class Clostridia were classified at less than the family level (i.e., Clostridiales order, unclassified), suggesting that many of these represent novel bacterial assemblages.
A relatively high prevalence of opportunistic human pathogens within the class Epsilonproteobacteria was observed in the general eubacterial clone libraries of all of the shorebird species tested in this study (Fig. 2 and Table 2). Specifically, Campylobacter spp. were detected in all three bird species, whereas Helicobacter-like sequences were detected in the ruddy turnstone and red knot clone libraries but not in the semipalmated sandpiper samples. Based on the latter results, we developed and analyzed Epsilonproteobacteria-specific clone libraries to further identify the species diversity associated with these two genera.
Species diversity of Campylobacter and Helicobacter.
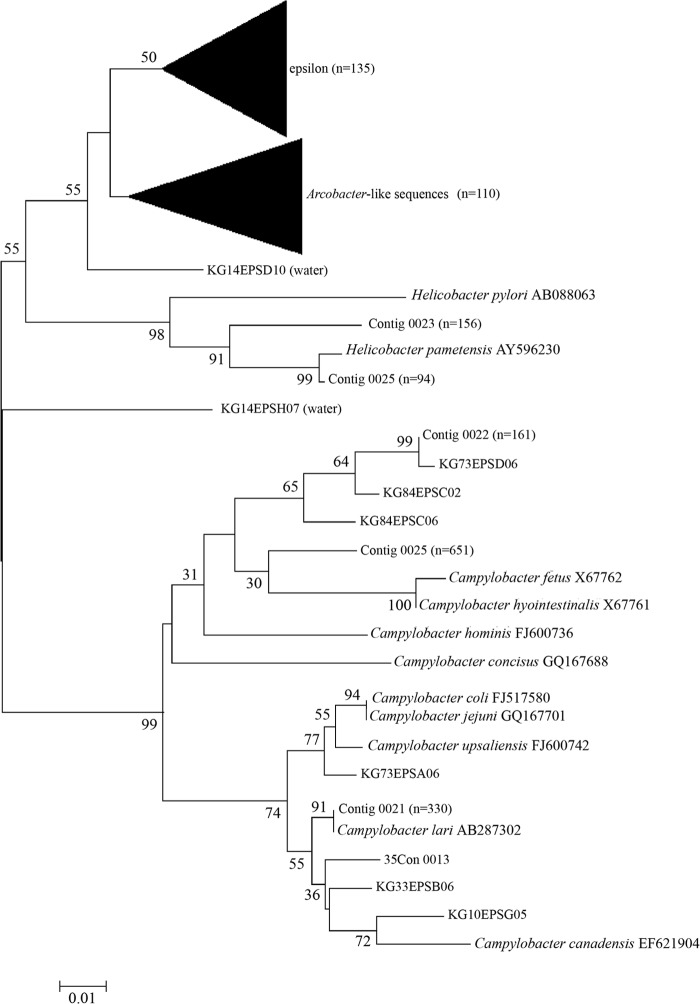
Epsilonproteobacteria were detected in 43, 43, and 41% of fecal samples from red knots, ruddy turnstones, and semipalmated sandpipers, respectively, using the class-specific PCR assay. Six samples of each bird species that were positive for Epsilonproteobacteria were selected for constructing epsilonproteobacterial 23S rRNA gene clone libraries. A total of 1,414 epsilonproteobacterial clone sequences were obtained from shorebird excreta, and most sequences were closely related to Campylobacter spp. or Helicobacter spp. (Fig. 3 and Table 3). Campylobacter species sequences represented more than three times the number of Helicobacter species sequences in all three shorebirds. Additionally, 241 sequences were obtained from water samples. Neither Campylobacter spp. nor Helicobacter spp. were identified in the water samples; most epsilonproteobacteria water sequences were classified as Arcobacter-like bacteria or unclassified epsilonproteobacteria (Fig. 3).
FIG 3.
Phylogenetic analysis of epsilonproteobacterial 23S rRNA gene sequences from shorebird fecal and water samples. The tree was constructed using the neighbor-joining method, and bootstrap values were based on 1,000 replications. The scale bar corresponds to 0.02 changes per nucleotide.
TABLE 3.
Distribution of epsilonproteobacterial 23S rRNA genes in the clone library of shorebird excreta from Delaware Bay
| Epsilonproteobacteria contig | NCBI data (accession no.; % coverage, % identity) | No. of clones (% total clones) |
||
|---|---|---|---|---|
| Ruddy turnstone (n = 378) | Red knot (n = 533) | Semipalmated sandpiper (n = 503) | ||
| 21 | Campylobacter lari (AB287302.2; 99, 100), C. jejuni (GQ167701.1; 99, 97), C. coli (FJ517580.1; 99, 97) | 145 (38.4) | 54 (10.1) | 131 (26.0) |
| 22 | C. fetus (CP000487.1; 100, 91), C. hominis (CP000776.1; 100, 91) | 161 (42.6) | 0 | 0 |
| 25 | C. fetus (CP000487.1; 100, 93), C. hyointestinalis (X67761.1; 100, 92), C. hominis (CP000776.1; 100, 90) | 0 | 399 (74.9) | 252 (50.1) |
| 23 | Helicobacter anseris (DQ418749.1; 100, 92), H. pametensis (AY596230.1; 100, 92), H. pylori (CP002980.1; 100, 87) | 60 (15.9) | 0 | 96 (19.1) |
| 41 | H. pametensis (AY596230.1; 100, 99), H. pylori (CP002980.1; 100, 87) | 1 | 75 (14.1) | 18 (3.6) |
| 26 | Uncultured Epsilonproteobacteria (HQ729157.1; 97, 97), Sulfurovum sp. (AP009179.1; 99, 96), Arcobacter sp. (AP012048.1; 100, 86) | 2 | 4 | 0 |
Campylobacter species have a broad host range, including many birds (41) and mammals (42). In particular, C. jejuni, C. coli, and C. lari are known to be pathogenic species causing human gastroenteritis worldwide (43, 44). In this study, C. lari (>99% identity) was identified in 38.4%, 10.1%, and 26.0% of clone sequences from ruddy turnstones, red knots, and semipalmated sandpipers, respectively (Table 3). However, other pathogenic species of Campylobacter, such as C. jejuni and C. coli, were not detected in any of three bird excreta (Fig. 3). In bacterial 16S rRNA clone libraries, more sequences of Campylobacter spp. were associated with red knots than the other two bird species (Table 2), but more than 70% of sequences in epsilonproteobacterial clone libraries retrieved from red knots were classified as nonpathogenic species (Table 3). Pathogenic Campylobacter species have been detected frequently in waterfowl, such as gulls, Canada geese, Whistling swans, sandhill cranes, and snow geese (20, 21, 22). Lu et al. (21) reported that most Campylobacter sequences identified from gull excreta were novel, whereas fewer than 2% of isolates were classified as C. jejuni and C. lari. More recently, C. jejuni was identified as the most dominant Campylobacter species in sandhill crane excreta (22). Besides pathogenic species, the vast majority of Campylobacter sequences obtained from the shorebird clone libraries showed 90 to 93% sequence identity to C. fetus, C. hyointestinalis, and C. hominis and may represent novel species (Table 3).
No sequences closely related to Helicobacter pathogenic species, such as H. pylori, were detected in any of the clone libraries (Table 3). H. pametensis was identified (i.e., >99% identity) in 14.1% and 3.6% of clone sequences from red knots and semipalmated sandpipers, respectively, whereas most Helicobacter 16S rRNA gene sequences detected in ruddy turnstone samples (i.e., 15.9%) showed 92% sequence identity to H. anseris.
qPCR assays.
The presence of Campylobacter spp., Enterococcus spp., and C. marimammalium was detected in the fecal samples of the shorebird species tested in this study using qPCR assays. Campylobacter spp. were more prevalent in shorebird excreta (i.e., 44%; n = 97), than Enterococcus spp. (12%) and C. marimammalium (13%) (Table 4). Ryu et al. (33) reported a relatively high prevalence of Enterococcus spp. using genus- and species-specific qPCR assays in avian species such as gull, duck, swan, chicken, turkey, pelican, and guinea fowl. In contrast, relatively low prevalences and concentrations of Enterococcus spp. and E. faecalis were observed in fecal samples from three shorebird species tested in this study, whereas E. faecium and E. casseliflavus were not detected in any of the samples. These results suggest that enterococci are not good indicators of microbial water quality for monitoring waters fecally affected by some shorebird species.
TABLE 4.
Detection of fecal indicator bacteria in shorebird excreta and water samples by qPCR assays
| Sample type | Shorebird species or source | No. of samples | Bacterial species distribution (no. [%]) |
|||||
|---|---|---|---|---|---|---|---|---|
| Campylobacter spp. | Catellicoccus marimammalium | Enterococcus spp. | Enterococcus faecalis | Enterococcus faecium | Enterococcus casseliflavus | |||
| Feces | Ruddy turnstone | 35 | 14 | 4 | 2 | 4 | 0 | 0 |
| Feces | Red knot | 40 | 19 | 5 | 8 | 7 | 0 | 0 |
| Feces | Semipalmated sandpiper | 22 | 10 | 4 | 2 | 2 | 0 | 0 |
| Feces | Total | 97 | 43 (44) | 13 (13) | 12 (12) | 13 (13) | 0 | 0 |
| Estuarine water | Delaware beaches | 12 | 11 (92) | 11 (92) | 7 (58) | 0 | 0 | 0 |
Interestingly, 13 of 97 shorebird fecal samples were positive for the gull2 assay, which targets the 16S rRNA gene of C. marimammalium (Table 4). This result is consistent with a previous study that reported low levels of cross-amplification of the gull2 assay with shorebird excreta (35). It should be noted that the signal intensities of the gull2 assay in shorebird fecal samples (Fig. 4) were several orders of magnitude lower than those in gull fecal samples (35). Most Catellicoccus-like sequences identified from shorebirds showed 95% sequence identity to C. marimammalium. While the presence of sequences closely related to C. marimammalium may be responsible for the observed cross-amplification with shorebird fecal samples, in silico analysis of the sequences obtained in this study showed that most sequences have several mismatches when aligned to the gull2 primers (data not shown). The presence of such mismatches, the low signal intensity associated with the fecal samples, and the relatively high annealing temperature (i.e., 64°C) used for the assay suggest that C. marimammalium is present in very small amounts in those birds that tested positive with the gull2 assay.
FIG 4.
Mean copy numbers of fecal indicator bacteria in shorebird excreta and water samples. To calculate mean concentrations, values below the detection limit were treated as zero. Error bars represent one standard deviation.
Most of the water samples (i.e., 92%; n = 12) were positive for Campylobacter spp. Moreover, Campylobacter spp. had the highest qPCR signal intensity among the tested fecal bacteria (Table 4 and Fig. 4). Overall, the qPCR results are comparable to those of the phylogenetic analyses that show Campylobacter spp. to be more prevalent than Enterococcus spp. in the tested shorebird excreta. Interestingly, of the 241 epsilonproteobacterial water clones analyzed, none of the sequences were classified as Campylobacter-like sequences but as either Arcobacter-like bacteria or unclassified Epsilonproteobacteria (Fig. 3). The lack of Campylobacter-like sequences suggests that deeper sequence coverage is needed for the identification of the Campylobacter species present in these environmental waters. Thus, while the approach used in this study provided insight on the occurrence of potentially relevant fecal bacterial groups in the water samples tested, future studies should consider using next-generation sequencing platforms (e.g., Illumina MiSeq), as they will allow us to examine more samples at a higher sequencing depth. C. marimammalium and Enterococcus spp. were detected in 11 and 7 water samples, respectively. While the relative concentration of C. marimammalium to that of Campylobacter spp. was very low in shorebird excreta, there was a relatively high occurrence and intensity of C. marimammalium qPCR signals in the tested water samples (Fig. 4). It is possible that differences in water survival rates explain the differences in overall levels of qPCR signals among the fecal bacteria tested in this study. In the case of C. marimammalium, a more reasonable explanation is that there are other fecal pollution sources associated with these environmental waters. Indeed, during the sampling collection, large numbers of gulls were present at the collection sites. High densities of C. marimammalium have been reported for gull feces (35), in turn suggesting that gulls are the primary source of this particular fecal bacterial species at these sites.
In summary, we studied the fecal bacterial diversity of three shorebird species and found that they harbor a relatively diverse gut microbial community composed of more than 30 different genera. Despite the differences in their fecal bacterial community structure, more than half of our bacterial sequences were found in all three species. For example, members of the bacterial classes Bacilli, Clostridia, Fusobacteria, and Epsilonproteobacteria were among the most abundant species in all three shorebirds. The high numbers of Bacilli and low numbers of Bacteroidia found in our study are consistent with other avian studies focusing on shorebirds, waterfowl, and poultry. These results suggest interactions between the avian host and its gut microbial community that promote the selection of some bacterial groups over others, a phenomenon that has been previously noted in mammals (24). While this study provides potential insights into shorebird gut microbiota, it is still unclear what factors affect interspecies variation. Clearly, additional studies on the relationship between dynamics of gut microbial ecology and environmental conditions, such as habitat and food sources, migration conditions, and climate changes, are needed. These studies need to identify gut microbiota over a large number of shorebird species as well as over a larger geographical range.
From a public health standpoint, the data indicate that these shorebirds are sources of fecal bacteria relevant to fecal monitoring (enterococci), to fecal source identification (C. marimammalium), and to health risks (Campylobacter spp.). However, relatively low abundances of pathogens also suggest that large numbers of shorebirds are needed to have an impact on the water quality. This is the case in the Delaware Bay during migratory bird stopovers. Similarly, detection of pathogenic bacteria, such as C. jejuni associated with migratory bird fecal loads, will be less likely before and after the migratory season. Lastly, while currently available C. marimammalium-based gull assays might be used to determine the presence of bacterial loads in migratory shorebird feces, the development of additional assays is needed to better estimate fecal loads of each of these shorebird species.
ACKNOWLEDGMENTS
We thank Nigel A. Clark, Jacquie A. Clark (British Trust for Ornithology), and Kevin S. Kalasz (U.S. Fish and Wildlife Services) for granting us permission to sample shorebirds and for providing logistical assistance in Delaware Bay. We also thank Barbara J. Campbell (University of Delaware) for valuable assistance and use of her laboratory facilities.
This study was funded by grants from the Natural Science and Engineering Council of Canada (PDF-373488-2009) and the Netherlands Organization for Scientific Research (Rubicon 825.09.0190) to D.M.B. and by the U.S. Environmental Protection Agency through its Office of Research and Development.
This work has been subjected to the EPA's administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the EPA; therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
Published ahead of print 10 January 2014
REFERENCES
- 1.Newman SH, Hill NJ, Spragens KA, Janies D, Voronkin IO, Prosser DJ, Yan B, Lei F, Batbayar N, Natsagdorj T, Bishop CM, Butler PJ, Wikelski M, Balachandran S, Mundkur T, Douglas DC, Takekawa JY. 2012. Eco-virological approach for assessing the role of wild birds in the spread of avian Influenza H5N1 along the central Asian flyway. PLoS One 7(2):e30636. 10.1371/journal.pone.0030636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawson B, Robinson RA, Neimanis A, Handeland K, Isomursu M, Agren EO, Hamnes IS, Tyler KM, Chantrey J, Hughes LA, Pennycott TW, Simpson VR, John SK, Peck KM, Toms MP, Bennett M, Kirkwood JK, Cunningham AA. 2011. Evidence of spread of the emerging infectious disease, finch trichomonosis, by migrating birds. EcoHealth 8:143–153. 10.1007/s10393-011-0696-8 [DOI] [PubMed] [Google Scholar]
- 3.Rvachev L, Longini I., Jr 1985. A mathematical-model for the global spread of Influenza. Math. Biosci. 75:3–22. 10.1016/0025-5564(85)90064-1 [DOI] [Google Scholar]
- 4.Clark K, Niles L, Burger J. 1993. Abundance and distribution of migrant shorebirds in Delaware Bay. Condor 95:694–705. 10.2307/1369612 [DOI] [Google Scholar]
- 5.Jorgensen JG, McCarty JP, Wolfenbarger LL. 2008. Buff-breasted sandpiper density and numbers during migratory stopover in the Rainwater Basin, Nebraska. Condor 110:63–69. 10.1525/cond.2008.110.1.63 [DOI] [Google Scholar]
- 6.Camarda A, Circella E, Pennelli D, Madio A, Bruni G, Lagrasta V, Marzano G, Mallia E, Campagnari E. 2006. Wild birds as biological indicators of environmental pollution: biotyping and antimicrobial resistance patterns of Escherichia coli isolated from Audouin's gulls (Larus audouinii) living in the Bay of Gallipoli (Italy). Ital. J. Anim. Sci. 5:287–290. 10.4081/ijas.2006.287 [DOI] [Google Scholar]
- 7.Keller JI, Shriver WG, Waldenstrom J, Griekspoor P, Olsen B. 2011. Prevalence of Campylobacter in wild birds of the mid-Atlantic region, U. S. A. J. Wildl. Dis. 47:750–754. 10.7589/0090-3558-47.3.750 [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Santo Domingo JW, Lamendella R, Edge T, Hill S. 2008. Phylogenetic diversity and molecular detection of bacteria in gull feces. Appl. Environ. Microbiol. 74:3969–3976. 10.1128/AEM.00019-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ksoll WB, Ishii S, Sadowsky MJ, Hicks RE. 2007. Presence and sources of fecal coliform bacteria in epilithic periphyton communities of Lake Superior. Appl. Environ. Microbiol. 73:3771–3778. 10.1128/AEM.02654-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Santo Domingo JW, Hill S, Edge TA. 2009. Microbial diversity and host-specific sequences of Canada goose feces. Appl. Environ. Microbiol. 75:5919–5926. 10.1128/AEM.00462-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, Kassa H, Tischler ML, Xiao LH. 2004. Host-adapted Cryptosporidium spp. in Canada geese (Branta canadensis). Appl. Environ. Microbiol. 70:4211–4215. 10.1128/AEM.70.7.4211-4215.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson CR, Quist C, Lee MD, Keyes KI, Dodson SV, Morales C, Sanchez S, White DG, Maurer JJ. 2000. Genetic relatedness of Salmonella isolates from nondomestic birds in southeastern United States. J. Clin. Microbiol. 38:1860–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco G, Lemus JA, Grande J. 2006. Faecal bacteria associated with different diets of wintering red kites: influence of livestock carcass dumps in microflora alteration and pathogen acquisition. J. Appl. Ecol. 43:990–998. 10.1111/j.1365-2664.2006.01200.x [DOI] [Google Scholar]
- 14.Wither A, Rehfisch M, Austin G. 2005. The impact of bird populations on the microbiological quality of bathing waters. Water Sci. Technol. 51:199–207 [PubMed] [Google Scholar]
- 15.Kilpatrick AM. 2011. Globalization, land use, and the invasion of West Nile virus. Science 334:323–327. 10.1126/science.1201010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulbert S. 2011. West Nile virus: the complex biology of an emerging pathogen. Intervirology 54:171–184. 10.1159/000328320 [DOI] [PubMed] [Google Scholar]
- 17.Rose M, Nol E. 2010. Foraging behavior of non-breeding semipalmated plovers. Waterbirds 33:59–69. 10.1675/063.033.0107 [DOI] [Google Scholar]
- 18.Santos SS, Pardal S, Proenca DN, Lopes RJ, Ramos JA, Mendes L, Morais PV. 2012. Diversity of cloacal microbial community in migratory shorebirds that use the Tagus estuary as stopover habitat and their potential to harbor and disperse pathogenic microorganisms. FEMS Microbiol. Ecol. 82:63–74. 10.1111/j.1574-6941.2012.01407.x [DOI] [PubMed] [Google Scholar]
- 19.Hermans D, Pasmans F, Messens W, Martel A, Van Immerseel F, Rasschaert G, Heyndrickx M, Van Deun K, Haesebrouck F. 2012. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector-Borne Zoonotic Dis. 12:89–98. 10.1089/vbz.2011.0676 [DOI] [PubMed] [Google Scholar]
- 20.Waldenstrom J, On SLW, Ottvall R, Hasselquist D, Olsen B. 2007. Species diversity of campylobacteria in a wild bird community in Sweden. J. Appl. Microbiol. 102:424–432. 10.1111/j.1365-2672.2006.03090.x [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Ryu H, Santo Domingo JW, Griffith JF, Ashbolt N. 2011. Molecular detection of Campylobacter spp. in California gull (Larus californicus) excreta. Appl. Environ. Microbiol. 77:5034–5039. 10.1128/AEM.00018-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Ryu H, Vogel J, Santo Domingo J, Ashbolt N. 2013. Molecular detection of Campylobacter and fecal indicators during the northern migration of sandhill cranes (Grus canadensis) at the Central Platte River. Appl. Environ. Microbiol. 79:3762–3769. 10.1128/AEM.03990-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson IG. 1997. Inhibition and facilitation of nucleic acid amplification. Appl. Environ. Microbiol. 63:3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, Gordon JI. 2008. Evolution of mammals and their gut microbes. Science 320:1647–1651. 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossmassler K, Engel AS, Twing KI, Hanson TE, Campbell BJ. 2012. Drivers of epsilonproteobacterial community composition in sulfidic caves and springs. FEMS Microbiol. Ecol. 79:421–432. 10.1111/j.1574-6941.2011.01231.x [DOI] [PubMed] [Google Scholar]
- 26.Huber T, Faulkner G, Hugenholtz P. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317–2319. 10.1093/bioinformatics/bth226 [DOI] [PubMed] [Google Scholar]
- 27.DeSantis TZ, Hugenholtz P, Keller K, Brodie EL, Larsen N, Piceno YM, Phan R, Andersen GL. 2006. NAST: a multiple sequence alignment server for comparative analysis of 16S rRNA genes. Nucleic Acids Res. 34:W394–W399. 10.1093/nar/gkl244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069–5072. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund M, Nordentoft S, Pedersen K, Madsen M. 2004. Detection of Campylobacter spp. in chicken fecal samples by real-time PCR. J. Clin. Microbiol. 42:5125–5132. 10.1128/JCM.42.11.5125-5132.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig W, Schleifer KH. 2000. How quantitative is quantitative PCR with respect to cell counts? Syst. Appl. Microbiol. 23:556–562. 10.1016/S0723-2020(00)80030-2 [DOI] [PubMed] [Google Scholar]
- 32.Santo Domingo JW, Siefring SC, Haugland RA. 2003. Real-time PCR method to detect Enterococcus faecalis in water. Biotechnol. Lett. 25:261–265. 10.1023/A:1022303118122 [DOI] [PubMed] [Google Scholar]
- 33.Ryu H, Henson M, Elk M, Toledo-Hernandez C, Griffith J, Blackwood D, Noble R, Gourmelon M, Glassmeyer S, Santo Domingo J. 2013. Development of quantitative PCR assays targeting 16S rRNA gene of Enterococcus spp. and their application to the identification of Enterococcus species in environmental samples. Appl. Environ. Microbiol. 79:196–204. 10.1128/AEM.02802-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bustin SA, Vandesompele J, Pfaffl MW. 2009. Standardization of qPCR and RT-qPCR. Genet. Eng. Biotechnol. News 29:40–43 http://hdl.handle.net/1854/LU-876361 [Google Scholar]
- 35.Ryu H, Griffith JF, Khan IUH, Hill S, Edge TA, Toledo-Hernandez C, Gonzalez-Nieves J, Santo Domingo J. 2012. Comparison of gull feces-specific assays targeting the 16S rRNA genes of Catellicoccus marimammalium and Streptococcus spp. Appl. Environ. Microbiol. 78:1909–1916. 10.1128/AEM.07192-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dick LK, Bernhard AE, Brodeur TJ, Santo Domingo JW, Simpson JM, Walters SP, Field KG. 2005. Host distributions of uncultivated fecal Bacteroidales bacteria reveal genetic markers for fecal source identification. Appl. Environ. Microbiol. 71:3184–3191. 10.1128/AEM.71.6.3184-3191.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fogarty LR, Voytek MA. 2005. Comparison of Bacteroides-Prevotella 16S rRNA genetic markers for fecal samples from different animal species. Appl. Environ. Microbiol. 71:5999–6007. 10.1128/AEM.71.10.5999-6007.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeter SN, McDermott CM, Bower PA, Kinzelman JL, Bootsma MJ, Goetz GW, McLellan SL. 2009. Bacteroidales diversity in ring-billed gulls (Larus delawarensis) residing at Lake Michigan beaches. Appl. Environ. Microbiol. 75:1525–1533. 10.1128/AEM.02261-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryu H, Lu J, Vogel J, Elk M, Chavez-Ramirez F, Ashbolt N, Santo Domingo J. 2012. Development and evaluation of a quantitative PCR assay targeting sandhill crane (Grus canadensis) fecal pollution. Appl. Environ. Microbiol. 78:4338–4345. 10.1128/AEM.07923-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weigand MR, Ryu H, Bozcek L, Konstantinidis KT, Santo Domingo JW. 2013. Draft genome sequence of Catellicoccus marimammalium, a novel species commonly found in gull feces. Genome Announc. 1(1):e00019–12. 10.1128/genomeA.00019-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waldenström J, Broman T, Carlsson I, Hasselquist D, Achterberg RP, Wagenaar JA, Olsen B. 2002. Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl. Environ. Microbiol. 68:5911–5917. 10.1128/AEM.68.12.5911-5917.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosef O, Gondrosen B, Kapperud G, Underdal B. 1983. Isolation and characterization of Campylobacter jejuni and Campylobacter coli from domestic and wild mammals in Norway. Appl. Environ. Microbiol. 46:855–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. 2002. Human campylobacteriosis in developing countries. Emerg. Infect. Dis. 8:237–243. 10.3201/eid0803.010233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuel MC, Vugia DJ, Shallow S, Marcus R, Segler S, McGivern T, Kassenborg H, Reilly K, Kennedy M, Angulo F, Tauxe RV. 2004. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996–1999. Clin. Infect. Dis. 38:S165–S174. 10.1086/381583 [DOI] [PubMed] [Google Scholar]