Abstract
Reexposure to viruses is assumed to strengthen humoral and cellular immunity via the secondary immune response. We studied the effects of frequent exposure to viral infectious challenges on immunity. Furthermore, we assessed whether repetitive exposures to varicella-zoster virus (VZV) elicited persistently high immune responses. Blood samples from 11 pediatricians and matched controls were assessed at 3 time points and 1 time point, respectively. Besides the assessment of general immunity by means of measuring T-cell subset percentages, antibody titers and gamma interferon (IFN-γ)/interleukin 2 (IL-2)-producing T-cell percentages against adenovirus type 5 (AdV-5), cytomegalovirus (CMV), tetanus toxin (TT), and VZV were determined. Pediatricians had lower levels of circulating CD4+-naive T cells and showed boosting of CD8+ effector memory T cells. Although no effect on humoral immunity was seen, repetitive exposures to VZV induced persistently higher percentages of IFN-γ-positive T cells against all VZV antigens tested (VZV glycoprotein E [gE], VZV intermediate-early protein 62 [IE62], and VZV IE63) than in controls. T cells directed against latency-associated VZV IE63 benefitted the most from natural exogenous boosting. Although no differences in cellular or humoral immunity were found between the pediatricians and controls for AdV-5 or TT, we did find larger immune responses against CMV antigens in pediatricians. Despite the high infectious burden, we detected a robust and diverse immune system in pediatricians. Repetitive exposures to VZV have been shown to induce a stable increased level of VZV-specific cellular but not humoral immunity. Based on our observations, VZV IE63 can be considered a candidate for a zoster vaccine.
INTRODUCTION
The secondary immune response in humans, elicited after reexposure to a virus or other pathogens, can reinforce the quantity and quality of the immune response against the challenging pathogen. However, the existence of a saturation level or even exhaustion after repetitive natural exposures has not been sufficiently studied in humans. Also, some authors have discussed the existence of competition between pathogens in regard to humoral immunity (1, 2). The question thus remains how individuals with frequent exposures to different pathogens and repetitive exposures to some pathogens maintain a healthy immune system in balance. In particular, the ubiquitous varicella-zoster virus (VZV) presents a challenging dilemma, as stated by Hope-Simpson (3), who hypothesized that reexposure to VZV might postpone the reactivation of VZV, herpes zoster. As such, simulation programs have predicted temporary increases in herpes zoster incidence after the introduction of a childhood widespread VZV vaccination program (4–6). Although a live attenuated VZV vaccine is currently universally given to children in several countries (e.g., United States, Germany, and Australia), much debate regarding the suitability of such a program remains (5, 7–46), and a recent multidisciplinary systematic review has concluded that so-called exogenous boosting exists, but the true extent of this is yet to be determined (47).
In this exploratory study, we have set forth the following goals. First, we aimed to describe the general effects of frequent infectious exposures in pediatricians on their humoral and cellular immune responses. Second, we set out to examine virus-specific effects of repeated exposures, particularly for VZV.
MATERIALS AND METHODS
Study design and subjects.
Eleven pediatricians (age range, 33 to 60 years; mean age, 42 years; 7 women) comprising a high-exposure (HE) group donated venous blood samples at three different time points: winter (24 February to 16 March 2012) (HE-WIN), spring (11 May to 8 June 2012) (HE-SPR), and summer (3 to 19 July 2012) (HE-SUM). Information regarding chickenpox exposure frequencies in pediatricians is shown in Table 1 (as recorded in their study journals). Eleven age (±1 year)- and gender-matched normally exposed healthy control individuals (CO) with no known exposure to chickenpox during the past 2 years donated venous blood samples at a single time point (2 to 20 July 2012). Following recently proposed guidelines, venipuncture was performed at fixed sampling sites (48). This study was approved by the ethics board of the University Hospital Antwerp, Antwerp, Belgium. Written informed consent was obtained from all study participants.
TABLE 1.
Monthly chickenpox exposure frequencies in pediatriciansa
| Data | No. of exposures in: |
||||
|---|---|---|---|---|---|
| February | March | April | May | June | |
| Range | 1–5 | 0–10 | 2–15 | 2–23 | 1–12 |
| Median | 2 | 5 | 5 | 9 | 5 |
| No. of respondents | 4 | 7 | 7 | 7 | 9 |
All pediatricians had an exposure to chickenpox within, at most, 11 weeks prior to the first sampling point, but mostly within a few weeks.
White blood cell count determination.
One fresh EDTA tube per sample was analyzed using an automated hemocytometer (Sysmex XS-1000i) in order to obtain the total white blood cell and differential counts.
Blood processing and flow cytometric analyses.
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque Plus gradient separation (Amersham Biosciences, Uppsala, Sweden) from freshly obtained heparinized blood. The freezing of PBMC was performed in 90% fetal bovine serum (FBS) (Gibco Invitrogen, Ghent, Belgium) supplemented with 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich, Steinheim, Germany). Filled cryovials were put into Nalgene cryoboxes before freezing at −80°C, and afterwards, the cells were stored in liquid nitrogen. Thawing of the cells for batch analysis was done in a water bath at 37°C. After washing with preheated (37°C) medium supplemented with 10% FBS, flow cytometric analysis was performed. For this, PBMC were left unstimulated or stimulated overnight in the presence of GolgiPlug (BD Biosciences) with (i) phytohemagglutinin (PHA) plus ionomycin, (ii) VZV intermediate-early protein 62 (IE62) PepMix (JPT, Berlin, Germany), (iii) VZV IE63 PepMix (JPT), (iv) VZV glycoprotein E (gE) PepMix (JPT), (v) tetanus toxin (TT) mix (PANATecs, Heilbronn, Germany), (vi) cytomegalovirus (CMV) pp65 peptide mix (NIH, Bethesda, MD), or (vii) human adenovirus type 5 (AdV-5) penton PepMix (JPT). Per condition, 1 × 10E6 PBMC per ml Iscove's modified Dulbecco's medium (Gibco Life Technologies, Ghent, Belgium) plus 10% FBS were stimulated using 1 μg peptide/ml. Afterwards, the cells were washed and stained using the antibody panel as described in Table 2. In doing so, the cell subsets were determined based on membrane expression levels of CD3, CD4, CD8, CD45RA, and CCR7 (see Fig. S1 in the supplemental material for the gating strategy). Next, the cells were fixed and permeabilized using fluorescence-activated cell sorting (FACS) lysing solution and FACS permeabilizing solution 2 (BD Biosciences), according to the manufacturer's protocol, before staining to determine intracellular expression levels of gamma interferon (IFN-γ) and interleukin 2 (IL-2). Nonreactive isotype-matched antibodies were used as controls for IL-2, IFN-γ, and CCR7. Flow cytometric analysis was performed on a BD FACSAria II flow cytometer (BD Biosciences). If possible, 1,000,000 events were measured. The FlowJo software version 9.6.2 (Tree Star, Inc., Ashland, OR) was used for cytometric data analysis. As suggested recently, absolute cell counts were obtained by multiplying the ratio of marker-positive cells to thawed, viable, and single cells with the number of lymphocytes plus monocytes per volume unit (49). However, we noted no major differences between the analyses based on the relative and absolute data.
TABLE 2.
Antibody panel for PBMC staining
| Target | Fluorochromea | Clone | Company |
|---|---|---|---|
| IFN-γ | FITC | B27 | BD |
| CCR7 | PE | 150503 | BD |
| CD3 | PE-Texas Red | S4.1 | Life Technologies |
| CD45RA | PE-Cy7 | HI100 | BD |
| LIVE/DEAD | Violet | Life Technologies | |
| IL-2 | APC | 5344.111 | BD |
| CD8 | Pacific orange | 3B5 | Life Technologies |
| CD4 | APC-H7 | RPA-T4 | BD |
FITC, fluorescein isothiocyanate; PE, phycoerythrin; APC, allophycocyanin.
Determination of antibody responses.
Blood serum samples were frozen at −80°C until batch analysis. All serological analyses were performed for both IgM and IgG. For the analysis of VZV-specific antibodies, two methods were used, a VZV IgM/G glycoprotein-based enzyme-linked immunosorbent assay (gpELISA) (Serion, Germany) and VZV-infected cell lysate on XL Liaison using a DiaSorin kit (USA). IgG directed against CMV pp150, pp28, p38, and p52 and IgM against CMV pp150, p52 (RoCO, Basel, Switzerland), AdV-5 hexon-specific IgM/G (Demeditec, Germany), and tetanus toxin-specific IgM/G (Demeditec) titers were determined as well. The positivity thresholds used were those given by the manufacturers.
Data management and statistics.
Hematocytological cell counts and IgG titers against the different pathogens were compared between the different sampling points (HE-WIN, HE-SPR, HE-SUM, and CO). For the flow cytometric analysis, only the time points HE-SPR and HE-SUM were retained for further analysis due to a low viability in the PBMC from HE-WIN.
First, T-cell subset percentages of CD3+ T cells were compared between the different sampling groups (HE-SPR, HE-SUM, and CO). The CD3+ CD4+ and CD3+ CD8+ subsets were further subtyped as CCR7+ CD45RA+ (naive T cells [Tna]), CCR7+ CD45RA− (central memory T cells [Tcm]), CCR7− CD45RA− (effector memory T cells [Tem]), and CCR7− CD45RA+ (effector T cells [Tef]). Next, the percentages of IFN-γ- or/and IL-2-producing T-cell subtypes were calculated per stimulus by subtracting the percentage from the unstimulated samples. Fisher's exact test for contingency tables, comparing stimulated with unstimulated samples, was used to retain positive differences, with a P value of <0.05 (one-sided). Comparisons with a P value of >0.05 but with <2,000 cells in the parent group were left blank due to the low cell count (465/4,752 blanks with 144 due to one unusable PBMC sample in the HE-SUM group). The proportions of antigen-responsive individuals per sampling group and per antigen were compared with the seroprevalence per sampling group and per pathogen. The comparison was not formally made between AdV-5 hexon antibody and penton cytokine responses due to the broader range of responses against AdV-5 hexon IgG by different adenovirus types compared to those against the AdV-5 penton peptide mix. Individuals were considered immune to VZV or CMV if they had a positive serological or cytokine response against VZV or CMV, respectively. All individuals were considered immune against TT (due to vaccination), and for the cellular analyses against AdV-5 penton, only individuals with positive cytokine responses were considered immune to AdV-5. The proportions of antigen-responsive individuals were compared per antigen between the three sampling groups. Next, antigen-stimulus-induced IFN-γ-producing total T-cell and T-cell subset percentages were calculated per pathogen for the pathogen-immune individuals. Comparisons were made between antigens and between the sampling groups. Spearman's correlations were calculated between the VZV IgG titers and VZV IE62, VZV IE63, and VZV gE responses. Although our main focus was directed toward the antiviral cytokine IFN-γ, a sensitivity analysis included the combination of IFN-γ- and IL-2-producing cells as well.
All comparative statistical analyses were performed using either a two-sided t test (paired or unpaired) when the variable was deemed normally distributed, or a nonparametric test (Wilcoxon signed-rank or Mann-Whitney rank-sum test) if not. A nonparametric test was used if nonnormality was concluded by the Shapiro-Wilk statistic for at least one of the distributions, and otherwise both a t test and a nonparametric test were used. However, if the nonparametric test proved to have more power to detect differences than did the t test (an indication of nonnormality), the results from the nonparametric test were used. In addition, all statistical analyses based on cytokine-producing cell counts were performed using nonparametric tests due to the skewed distributions of these counts. Matlab 2012b and SPSS 20 were used for the analyses. The results were considered significant if there was a P value of <0.05 or tended to be significant if the P value was >0.05 and <0.10.
RESULTS
Comparison of white blood cell counts.
The total white blood cell count tended to be higher in the HE-SUM (summer sampling of pediatricians) group than in the HE-SPR (spring sampling of pediatricians), HE-WIN (winter sampling of pediatricians), and CO (control group) groups (factor 1.1, P = 0.066; factor 1.1, P = 0.079; factor 1.2, P = 0.034, respectively). The neutrophil count was higher in the HE-SUM group than in the HE-SPR group (factor 1.2, P = 0.028). The monocyte count tended to be higher in the HE-SUM group than in the HE-WIN group (factor 1.2, P = 0.053).
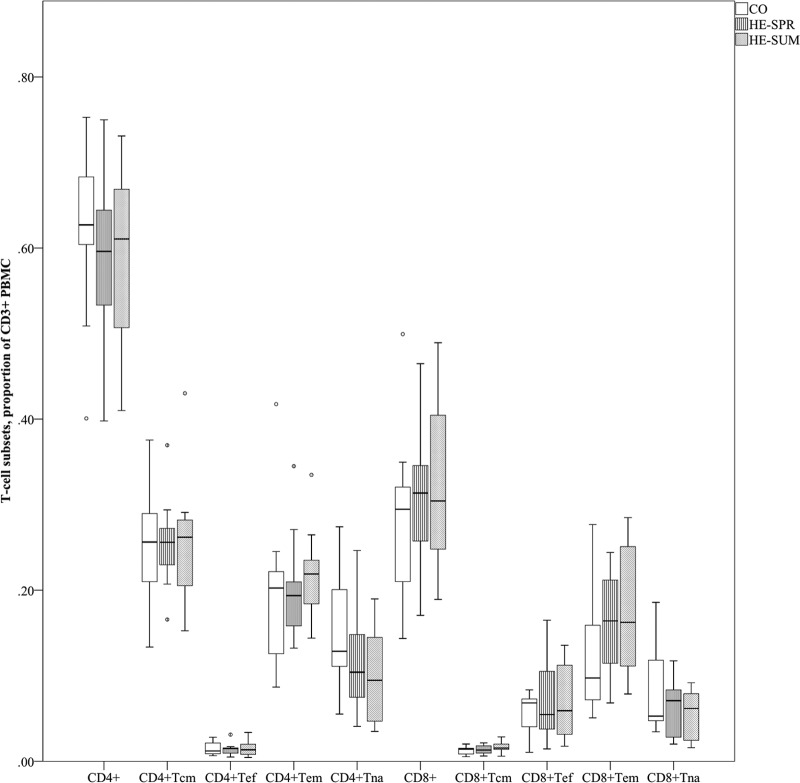
Comparison of T-cell subset percentages (Fig. 1).
FIG 1.
Comparison of T-cell subset percentages (of CD3+ PBMC). Distribution of T-cell subsets is presented per sampling as a box plot (with medians as horizontal lines, interquartile range as boxes, ranges as vertical lines, and outliers as dots). Data for CD4+ Tna, CO versus HE-SPR (P = 0.076); for CD4+ Tna, CO versus HE-SUM (P = 0.041); for CD8+ Tna, HE-SPR versus HE-SUM (P = 0.092); for CD8+ Tem, CO versus HE-SPR (P = 0.076); and for CD8+ Tem, CO versus HE-SUM (P = 0.099). All other P values are >0.10.
The CD4+ CCR7+ CD45RA+ (CD4+ Tna) percentage was lower in the HE-SUM group than in the CO group (factor 1.6, P = 0.041). The CD4+ Tna percentage also tended to be lower in the HE-SPR group than in the CO group (factor 1.4, P = 0.076). In addition, we found that the CD8+ Tna percentage tended to be lower in the HE-SUM group versus the HE-SPR group (factor 1.1, P = 0.092). The CD8+ CCR7− CD45RA− (CD8+ Tem) percentage tended to be higher in the HE-SPR and HE-SUM groups than in the CO group (factor 1.3, P = 0.076; factor 1.4, P = 0.099, respectively). No differences were found for the total CD4+, total CD8+, CCR7+ CD45RA− (Tcm), or CCR7− CD45RA− (Tef) percentages.
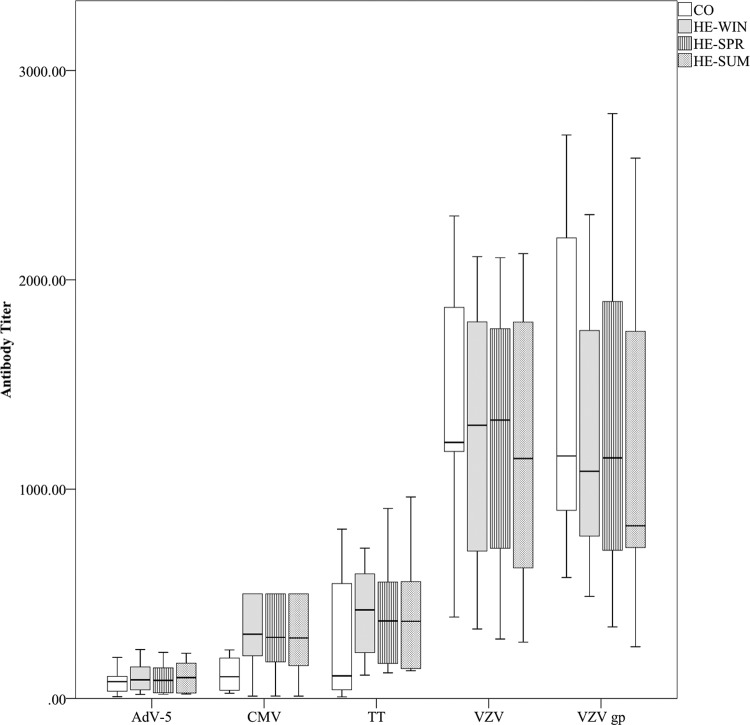
Antibody detection against common pathogens.
Pathogen seropositivity remained constant at all time points in the HE groups. All study participants were VZV IgG seropositive (for both the VZV gpELISA and VZV cell lysate). All HE samples were AdV-5 hexon seropositive, whereas the IgG titer was negative for one CO participant and unequivocal for another. For CMV only, a tendency (P = 0.085) toward more IgG positive individuals was shown in the HE samples (9/11) than in the CO samples (4/11) (Fig. 2 and Table 3).
FIG 2.
Antibody detection against common pathogens. IgG titers per pathogen (AdV-5 hexon [IU/ml], CMV [IU/ml], TT [IU/100 ml], VZV cell lysate [mIU/ml], VZV gp [mIU/ml]) and per sampling group are presented using a box plot (with medians as horizontal lines, interquartile ranges as boxes, ranges as vertical lines, and outliers as dots). Data for VZV cell lysate, HE-WIN versus HE-SUM (P = 0.090). All other P values are >0.10.
TABLE 3.
Humoral and cellular immunity against common pathogensa
| Pathogen | Cellular antigen | Group | No. of individuals with positive responses/total no. of individuals for: |
P for IgG vs CMI | CMI |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
P for: |
No. of individuals with responsive cells/total no. of individuals for: |
P for CD4+ vs CD8+ | |||||||||
| IgG | CMI | CO vs HE-SPR | CO vs HE-SUM | HE-SPR vs HE-SUM | CD4+ cells | CD8+ cells | |||||
| AdV-5 | AdV-5 penton | CO | 9/10 | 9/11 | NSb | NS | NS | 6/11 | 6/10 | NS | |
| HE-SPR | 11/11 | 11/11 | 7/11 | 9/11 | NS | ||||||
| HE-SUM | 11/11 | 10/10 | 9/10 | 6/10 | NS | ||||||
| CMV | CMV pp65 | CO | 4/11 | 10/11 | 0.024 | NS | NS | NS | 8/10 | 6/10 | NS |
| HE-SPR | 9/11 | 11/11 | NS | 10/11 | 9/11 | NS | |||||
| HE-SUM | 9/11 | 10/10 | NS | 9/10 | 9/10 | NS | |||||
| Tetanus | TT | CO | 10/11 | 6/11 | NS | 0.036 | 0.036 | NS | 6/11 | 3/11 | NS |
| HE-SPR | 11/11 | 11/11 | NS | 8/11 | 5/11 | NS | |||||
| HE-SUM | 11/11 | 10/10 | NS | 10/10 | 5/10 | 0.033 | |||||
| VZV | VZV gE | CO | 11/11 | 8/11 | NS | NS | NS | NS | 6/11 | 3/10 | NS |
| HE-SPR | 11/11 | 9/11 | NS | 9/11 | 3/11 | 0.016 | |||||
| HE-SUM | 11/11 | 9/10 | NS | 9/10 | 4/10 | 0.058 | |||||
| VZV | VZV IE62 | CO | 11/11 | 11/11 | NS | NS | NS | NS | 10/11 | 7/11 | NS |
| HE-SPR | 11/11 | 11/11 | NS | 9/11 | 9/11 | NS | |||||
| HE-SUM | 11/11 | 10/10 | NS | 10/10 | 9/10 | NS | |||||
| VZV | VZV IE63 | CO | 11/11 | 6/11 | 0.036 | 0.036 | NS | NS | 5/11 | 5/11 | NS |
| HE-SPR | 11/11 | 11/11 | NS | 8/11 | 9/11 | NS | |||||
| HE-SUM | 11/11 | 9/10 | NS | 8/10 | 6/10 | NS | |||||
Shown is memory against pathogens using either humoral (IgG) or cell-mediated immunity (CMI) (IFN-γ- or/and IL-2-producing T cells) as proxy. Fisher's exact test was used to compare immunity as assessed by IgG or CMI (against an antigen) for three sampling groups (P values of <0.10 are shown). CMI is compared among the three groups (P values of <0.10 are shown). For each antigen, the presence of CD4+ and CD8+ IFN-γ or/and IL-2 responses are assessed and compared with each other per antigen and per group (P values of <0.10 are shown).
NS, nonsignificant.
None of the pathogens' IgG titers indicated statistically meaningful differences between the sampling groups, although the VZV cell lysate titer had a tendency to be higher in the HE-WIN group than in the HE-SUM group (factor 1.1, P = 0.090), and the CMV IgG titer showed a trend toward higher values in the HE groups (Fig. 2). We note that several CMV IgG titers in the HE groups reached the experimental upper limit.
Pathogen-specific T cells.
Although CMV-specific IgG titers were detected in only 4/11 CO samples, we detected CMV pp65-specific T cells in 10/11 CO samples when using IFN-γ or/and IL-2 intracellular cytokine staining upon stimulation (P = 0.024). In addition, we observed that CMV positivity in the HE samples reached 100% while using cellular immunology instead of serology as a measurement tool. We also noted only 6/11 CO samples having VZV IE63-specific T cells (P = 0.036), whereas all CO and HE samples had responses to VZV IE62. The percentage of samples with cellular responses to VZV gE T cells ranged between 73 and 90%. The percentage of individuals with TT-specific T cells was significantly lower in CO (6/11) samples than in the HE-SPR (11/11, P = 0.036) and HE-SUM (10/10, P = 0.036) samples. CD4+ and CD8+ responses were observed against all antigens tested (Table 3).
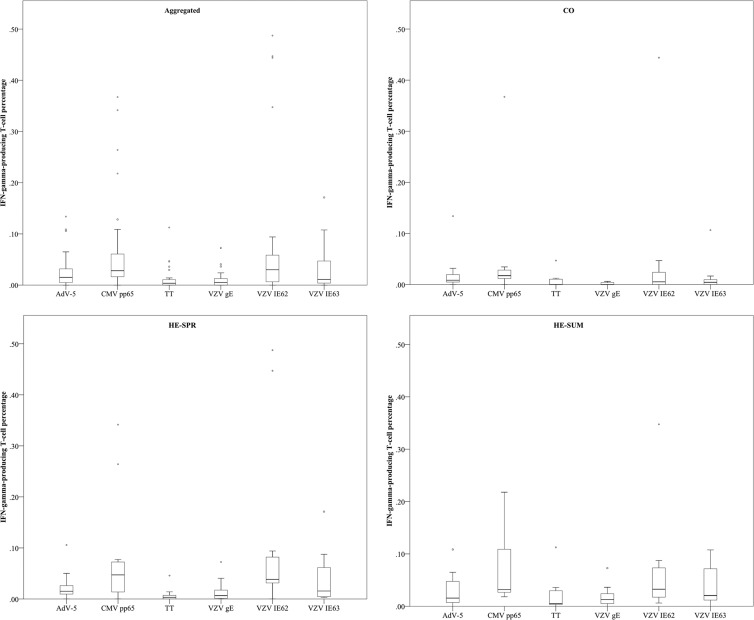
Comparing IFN-γ T-cell percentages between antigens and between groups.
Altogether, we noted that CMV pp65- and VZV IE62-specific T-cell responses were the most abundant, although some differences existed between the CO and HE samples (Fig. 3 and 4).
FIG 3.
Comparing IFN-γ-producing T-cell percentages (of CD3+ PBMC) between antigens. Box plot representations (with medians as horizontal lines, interquartile ranges as boxes, ranges as vertical lines, and outliers as dots) are used for the six different antigens (AdV-5, adenovirus type 5 penton; CMV, cytomegalovirus; TT, tetanus toxin; VZV, varicella-zoster virus) per sampling condition (aggregated, aggregate of all sampling groups; CO, control group for high-exposure group; HE-SPR, high-exposure group in spring; HE-SUM, high-exposure group in summer). For P values, see text.
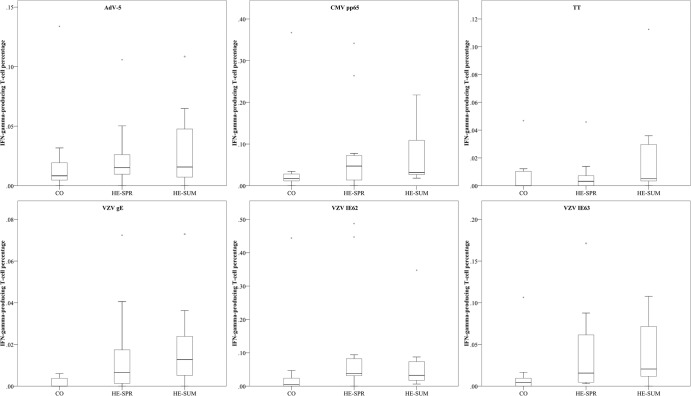
FIG 4.
Comparing IFN-γ-producing T-cell percentages (of CD3+ PBMC) between sampling groups. Box plot representations (with medians as horizontal lines, interquartile ranges as boxes, ranges as vertical lines, and outliers as dots) are used for the three different sampling groups for each antigen (AdV-5, adenovirus type 5 penton; CMV, cytomegalovirus; TT, tetanus toxin; VZV, varicella-zoster virus). Data for CMV pp65, CO versus HE-SUM (P = 0.024); for VZV gE, CO versus HE-SPR (P = 0.028); for VZV gE, CO versus HE-SUM (P = 0.019); for VZV IE62, CO versus HE-SPR (P = 0.023); for VZV IE62, CO versus HE-SUM (P = 0.034); for VZV IE63, CO versus HE-SPR (P = 0.064); and for VZV IE63, CO versus HE-SUM (P = 0.030). All other P values are > 0.10.
The control individuals had more CMV pp65-specific T cells than T cells against VZV gE (P < 0.001), VZV IE63 (P = 0.020), or TT (P < 0.001). Also, specific T cells against AdV-5 penton and VZV IE62 were more prevalent than those against VZV gE (P = 0.0039 and P = 0.079, respectively). Similarly, pediatricians had higher CMV pp65-specific T-cell responses both during spring and summer than against VZV gE (P = 0.042 and P = 0.0052, respectively) or TT (P = 0.0024 and P = 0.015, respectively). Comparable to the controls, VZV IE62 responses were more frequent than VZV gE responses in both the HE-SPR (P = 0.020) and HE-SUM (P = 0.044) groups. However, VZV IE62 responses were also more frequent than TT responses in both the HE-SPR (P = 0.0031) and HE-SUM (P = 0.024) groups and more frequent than AdV-5 penton responses during the spring (P = 0.049). We also noted that during the spring, TT-specific T-cell responses were less frequent than those against AdV-5 penton (P = 0.011) and VZV IE63 (P = 0.0063).
Overall, we found positive correlations between the percentages of T cells against VZV IE62 and VZV IE63 (data not shown). Aggregating the different groups also showed a minor correlation between the percentages of T cells against VZV gE, VZV IE62, and VZV IE63 (data not shown).
The antigen-specific analysis between the sampling groups (Fig. 4) found the percentage of CMV-specific T cells to be higher in the HE-SUM group than in the CO (P = 0.024) group. All VZV-specific T-cell percentages were lower in controls than in highly exposed individuals (both spring and summer): VZV IE62 was lower in the CO group than in the HE-SPR (P = 0.023) and HE-SUM (P = 0.034) groups, VZV IE63 was lower in the CO group than in the HE-SPR (P = 0.064) and HE-SUM (P = 0.030) groups, and VZV gE was lower in the CO group than in the HE-SPR (P = 0.028) and HE-SUM (P = 0.0019) groups. No differences between the sampling groups were found for AdV-5 penton or TT.
Analysis of within-individual distribution of antigen-specific T-cell subsets.
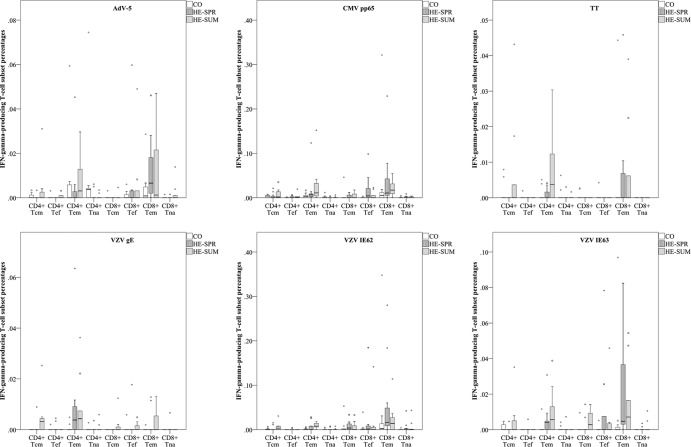
The CO, HE-SPR, and HE-SUM antigen-specific T-cell subset proportions showed for all antigens tested that IFN-γ-producing T cells mainly had a CD4+ Tem, CD8+ Tem, or combined Tem subset. Noteworthy are the CD4+ Tem dominance for VZV gE and the CD8+ Tem dominance for VZV IE62 (Fig. 5; see also Table S1 in the supplemental material).
FIG 5.
Comparing differences in IFN-γ-producing T-cell subsets between sampling groups. Box plot representations (with medians as horizontal lines, interquartile ranges as boxes, ranges as vertical lines, and outliers as dots) are used to compare T-cell subset percentages (of CD3+ PBMC) for the three different sampling groups. See Table S1 in the supplemental material for P values.
Combining IFN-γ- and IL-2-specific T-cell responses.
In general, we noted that the inclusion of IL-2 did not have a major effect on the results obtained with IFN-γ alone. However, the proportion of CD4+ Tcm responses was increased with the inclusion of IL-2 for all antigens tested (see Fig. S2 and Table S1 in the supplemental material).
DISCUSSION
This exploratory study was designed to analyze the cellular and humoral immune responses at different time points (winter, spring, and summer) in a group with high exposure to infections (represented by pediatricians) and to compare these to single time point responses (summer) in a group with what we considered a normal exposure load (non-MDs, nonteachers, etc.). The aim was to analyze both the general indices of immune and antigen-specific responses, particularly against VZV but also against CMV, adenovirus type 5, and tetanus toxin.
The total white blood cell count was about 10% to 20% higher for the high-exposure group during the summer than in the winter and spring and than in the normal-exposure group. The neutrophil and monocyte counts in the high-exposure group were higher during the summer than during the spring and winter, respectively. Importantly, we did not find any differences for the lymphocyte counts. These results might imply that highly exposed individuals have an upregulated innate immune system during seasons with frequent infectious challenges that is reflected in higher white blood cell counts in the peripheral circulation (on “standby”) at moments with less infectious challenges.
We found that the highly infectious burden was associated with a loss of CD4+ CCR7+ CD45RA+ (naive) T cells in pediatricians. This was contrasted with a trend toward a higher prevalence of CD8+ CCR7− CD45RA− (effector memory) T cells during the summer, possibly still due to the antigen-specific proliferation during the winter and spring.
CMV IgG seroprevalence was higher in pediatricians than in the controls, which was in accordance with another study that documented higher CMV seroprevalence in child caretakers (50). The CMV IgG titer suggested higher values for the high-exposure groups than the normal-exposure group. Both natural (8, 9, 16, 38) and vaccine-based reexposures (51–53) to VZV have been shown to majorly increase VZV-specific antibody titers. The pediatricians in our study, however, did not have large differences in VZV-specific antibody titers. Both higher VZV-specific cellular immunity and higher secretory immunoglobulin A (sIgA) levels in saliva (13) might explain these observations. We note, however, that more specific VZV antibody titrations (see Jenke et al. [54]) focused on VZV IE62 or VZV IE63 epitopes might show the differences between pediatricians and controls that are currently averaged out by the large VZV glycoprotein response.
Previous studies have identified several VZV antigens capable of eliciting cellular immune responses (55–57). VZV gE, the most abundant VZV glycoprotein, and VZV IE63, the immediate-early phosphoprotein most likely involved in VZV reactivation (58–60), have been shown to elicit CD4+ T-cell responses (57, 58). The immediate-early phosphoprotein VZV IE62, however, is known to elicit CD8+ T-cell responses as well (55). Our study identified both CD4+ and CD8+ responses against all antigens, albeit not in all samples (which also might be influenced by HLA types). Noteworthy was our finding of a relatively low prevalence (6/11 samples) of T-cell responses against VZV IE63 in the normal-exposure group but not in the high-exposure group (11/11 and 9/10 samples, respectively). Overall, both VZV IE62- and VZV IE63-specific IFN-γ-producing T-cell responses were more frequent than those against VZV gE. However, VZV-specific T-cell responses were modestly correlated with each other although most pronounced between VZV IE62 and VZV IE63. We noted that the VZV-specific cellular and humoral immune responses were not correlated with each other.
Terada and colleagues (11) documented higher responder cell frequencies in pediatricians after stimulation with VZV cell culture antigens. Using state-of-the-art techniques, such as flow cytometry and peptide mixes, we found that all three VZV-specific antigens tested consistently elicited higher IFN-γ-producing T-cell percentages in the high-exposure groups than in the normal-exposure group. In general, CD4+ and CD8+ IFN-γ-producing T cells were dominated by the absence of the expression of CCR7 and CD45RA markers giving them an effector memory phenotype. Our combined results imply that frequent reexposure to VZV stimulates the proliferation of T cells against all VZV antigens. These findings further support the exogenous boosting hypothesis formulated by Hope-Simpson (3), suggesting that reexposure to VZV has a protective effect against herpes zoster. The low prevalence of VZV IE63-specific cellular responses in the normal-exposure group also suggests a faster decline in the memory response against this protein, which has been implicated in VZV reactivation (58–60). The lower incidence of herpes zoster in pediatricians found in some studies (12), although not all (34), might thus be caused by more frequent boosting of the cellular immune responses against VZV IE63. Our results also emphasize the existence of VZV-specific CD8+ responses, including a CD8+ dominance against VZV IE62. We note that our antigens consisted of overlapping 15-mer peptide mixes designed to stimulate both CD4+ and CD8+ T-cell responses, particularly when used on thawed PBMC (61). In contrast, many studies in the past, and importantly, the Zostavax vaccine immunogenicity studies, used VZV cell lysate as a stimulus (11, 16, 38, 53, 62–65). Thus, any change in the CD8+ component against VZV might have been missed. Also, the more recently published GlaxoSmithKline (GSK) VZV gE subunit vaccine immunogenicity study used 20-mer peptide mixes that might be too long to detect CD8+ T-cell responses (52).
When identifying CMV pp65-responsive individuals, intracellular detection of IFN-γ or IL-2 production by T cells upon stimulation with CMV pp65 peptides resulted in more individuals with a memory response against CMV than the serological method. Although the greatest improvement of detection sensitivity was seen in the normal-exposure group (going from 4/11 to 10/11 samples), the proof of concept was noteworthy for the pediatricians, as CMV positivity increased from only 9/11 samples when using IgG as the gold standard to 100% when using intracellular cytokine staining as a diagnostic tool. The increased sensitivity of cellular immunity for memory detection compared to humoral immunity, an important finding for at-risk groups such as transplant patients and pregnant women, was recently already shown for CMV (66) and had already been shown for various pathogens, such as hepatitis C virus (67). CMV pp65-specific IFN-γ-producing T cells were more frequent than those against VZV IE63, VZV gE, TT, and AdV penton. The relatively high prevalence of CMV-specific T cells is consistent with the results from other studies (68, 69). The CMV pp65-specific T-cell frequencies were also higher in samples from pediatricians than in those from the controls. We found no systematic differences in the T-cell frequencies against TT or AdV-5 penton between the pediatricians and controls, although TT-specific IFN-γ- or IL-2-producing CD4+ CCR7− CD45RA− (effector memory) cells were more frequent at both sampling points for pediatricians than for controls.
This exploratory study was limited by its sample size, and consequently, its lower power for finding significant differences that might exist (e.g., in regard to antibody responses). Despite this, several systematic and significant results were found. The limited sample size made it impossible to assess immunity dysregulation caused by CMV, as was recently proposed for VZV (70). Nonetheless, some important conclusions can be made based on this study.
Future work should focus on a reanalysis of the VZV vaccine immunogenicity studies by making use of 15-mer peptide mixes. In addition, our (albeit indirect) support for the importance of VZV IE63 in VZV reactivation might stimulate the development of a herpes zoster vaccine directed at VZV IE63. Future work should analyze the long-term effects of frequent exposures on the specific and nonspecific immune system, keeping in mind that the population in the current study consisted mainly of young individuals.
In conclusion, while comparing immune responses between highly exposed and normally exposed individuals, we observed a transient depletion of naive CD4+ T cells and boosting of CD8+ effector memory T cells. Although no effect on humoral immunity was seen, we found that all VZV-specific antigens tested had persistently higher IFN-γ-producing T-cell percentages after stimulation in the highly exposed than in the normally exposed individuals, thereby illustrating the stimulatory effects of the secondary immune response.
Supplementary Material
ACKNOWLEDGMENTS
We thank all the participants for their participation and M. Claeys for his constructive comments.
The human CMV pp65 peptide pool was obtained through the NIH AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH). This work was supported by grants of the Research Foundation Flanders (project grant, predoctoral fellowship to B.O., postdoctoral fellowships to J.B. and N.C.), the Hercules Foundation-Belgium, and the University of Antwerp (predoctoral fellowship to J.V.D.B.). The funders had no role in study design, data collection or analysis, the decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 15 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00818-13.
REFERENCES
- 1.Amanna IJ, Slifka MK. 2010. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol. Rev. 236:125–138. 10.1111/j.1600-065X.2010.00912.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T, Hiepe F. 2006. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6:741–750. 10.1038/nri1886 [DOI] [PubMed] [Google Scholar]
- 3.Hope-Simpson RE. 1965. The nature of herpes zoster: a long-term study and a new hypothesis. Proc. R. Soc. Med. 58:9–20 [PMC free article] [PubMed] [Google Scholar]
- 4.Bilcke J, van Hoek AJ, Beutels P. 2013. Childhood varicella-zoster virus vaccination in Belgium: cost-effective only in the long run or without exogenous boosting? Hum. Vaccin. Immunother. 9:812–822. 10.4161/jv.23334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brisson M, Edmunds WJ, Gay NJ, Law B, De Serres G. 2000. Modelling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiol. Infect. 125:651–669. 10.1017/S0950268800004714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuette MC, Hethcote HW. 1999. Modeling the effects of varicella vaccination programs on the incidence of chickenpox and shingles. Bull. Math. Biol. 61:1031–1064. 10.1006/bulm.1999.0126 [DOI] [PubMed] [Google Scholar]
- 7.Gershon AA, Steinberg SP, Borkowsky W, Lennette D, Lennette E. 1982. IgM to varicella-zoster virus: demonstration in patients with and without clinical zoster. Pediatr. Infect. Dis. 1:164–167. 10.1097/00006454-198205000-00007 [DOI] [PubMed] [Google Scholar]
- 8.Arvin AM, Koropchak CM, Wittek AE. 1983. Immunologic evidence of reinfection with varicella-zoster virus. J. Infect. Dis. 148:200–205. 10.1093/infdis/148.2.200 [DOI] [PubMed] [Google Scholar]
- 9.Gershon AA, Steinberg SP. 1990. Live attenuated varicella vaccine: protection in healthy adults compared with leukemic children. National Institute of Allergy and Infectious Diseases Varicella Vaccine Collaborative Study Group. J. Infect. Dis. 161:661–666 [DOI] [PubMed] [Google Scholar]
- 10.Garnett GP, Grenfell BT. 1992. The epidemiology of varicella-zoster virus infections: the influence of varicella on the prevalence of herpes zoster. Epidemiol. Infect. 108:513–528. 10.1017/S0950268800050019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terada K, Kawano S, Yoshihiro K, Morita T. 1993. Proliferative response to varicella-zoster virus is inverse related to development of high levels of varicella-zoster virus specific IgG antibodies. Scand. J. Infect. Dis. 25:775–778. 10.3109/00365549309008578 [DOI] [PubMed] [Google Scholar]
- 12.Solomon BA, Kaporis AG, Glass AT, Simon SI, Baldwin HE. 1998. Lasting immunity to varicella in doctors study (L.I.V.I.D. study). J. Am. Acad. Dermatol. 38:763–765. 10.1016/S0190-9622(98)70207-5 [DOI] [PubMed] [Google Scholar]
- 13.Terada K, Niizuma T, Yagi Y, Miyashima H, Kataoka N, Sadahiro T. 2000. Low induction of varicella-zoster virus-specific secretory IgA antibody after vaccination. J. Med. Virol. 62:46–51. [DOI] [PubMed] [Google Scholar]
- 14.Brisson M, Gay NJ, Edmunds WJ, Andrews NJ. 2002. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine 20:2500–2507. 10.1016/S0264-410X(02)00180-9 [DOI] [PubMed] [Google Scholar]
- 15.Thomas SL, Wheeler JG, Hall AJ. 2002. Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet 360:678–682. 10.1016/S0140-6736(02)09837-9 [DOI] [PubMed] [Google Scholar]
- 16.Vossen MT, Gent MR, Weel JF, de Jong MD, van Lier RA, Kuijpers TW. 2004. Development of virus-specific CD4+ T cells on reexposure to varicella-zoster virus. J. Infect. Dis. 190:72–82. 10.1086/421277 [DOI] [PubMed] [Google Scholar]
- 17.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. 2005. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J. Infect. Dis. 191:2002–2007. 10.1086/430325 [DOI] [PubMed] [Google Scholar]
- 18.Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. 2005. Incidence of herpes zoster, 1997–2002. Epidemiol. Infect. 133:245–253. 10.1017/S095026880400281X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yavuz T, Ozdemir I, Sencan I, Arbak P, Behçet M, Sert E. 2005. Seroprevalence of varicella, measles and hepatitis B among female health care workers of childbearing age. Jpn. J. Infect. Dis. 58:383–386 [PubMed] [Google Scholar]
- 20.Yih WK, Brooks DR, Lett SM, Jumaan AO, Zhang Z, Clements KM, Seward JF. 2005. The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998–2003. BMC Public Health 5:68. 10.1186/1471-2458-5-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaves SS, Santibanez TA, Gargiullo P, Guris D. 2007. Chickenpox exposure and herpes zoster disease incidence in older adults in the U.S. Public Health Rep. 122:155–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saadatian-Elahi M, Mekki Y, Del Signore C, Lina B, Derrough T, Caulin E, Thierry J, Vanhems P. 2007. Seroprevalence of varicella antibodies among pregnant women in Lyon-France. Eur. J. Epidemiol. 22:405–409. 10.1007/s10654-007-9136-z [DOI] [PubMed] [Google Scholar]
- 23.Bonmarin I, Santa-Olalla P, Lévy-Bruhl D. 2008. Modelling the impact of vaccination on the epidemiology of varicella zoster virus. Rev. Epidemiol. Sante Publique 56:323–331 (In French.) 10.1016/j.respe.2008.07.087 [DOI] [PubMed] [Google Scholar]
- 24.Patel MS, Gebremariam A, Davis MM. 2008. Herpes zoster-related hospitalizations and expenditures before and after introduction of the varicella vaccine in the United States. Infect. Control Hosp. Epidemiol. 29:1157–1163. 10.1086/591975 [DOI] [PubMed] [Google Scholar]
- 25.Valdarchi C, Farchi F, Dorrucci M, De Michetti F, Paparella C, Babudieri S, Spano A, Starnini G, Rezza G. 2008. Epidemiological investigation of a varicella outbreak in an Italian prison. Scand. J. Infect. Dis. 40:943–945. 10.1080/00365540802308449 [DOI] [PubMed] [Google Scholar]
- 26.Toyama N, Shiraki K, Society of the Miyazaki Prefecture Dermatologists 2009. Epidemiology of herpes zoster and its relationship to varicella in Japan: a 10-year survey of 48,388 herpes zoster cases in Miyazaki prefecture. J. Med. Virol. 81:2053–2058. 10.1002/jmv.21599 [DOI] [PubMed] [Google Scholar]
- 27.Brisson M, Melkonyan G, Drolet M, De Serres G, Thibeault R, De Wals P. 2010. Modeling the impact of one- and two-dose varicella vaccination on the epidemiology of varicella and zoster. Vaccine 28:3385–3397. 10.1016/j.vaccine.2010.02.079 [DOI] [PubMed] [Google Scholar]
- 28.Carville KS, Riddell MA, Kelly HA. 2010. A decline in varicella but an uncertain impact on zoster following varicella vaccination in Victoria, Australia. Vaccine 28:2532–2538. 10.1016/j.vaccine.2010.01.036 [DOI] [PubMed] [Google Scholar]
- 29.Donahue JG, Kieke BA, Gargiullo PM, Jumaan AO, Berger NR, McCauley JS, Belongia EA. 2010. Herpes zoster and exposure to the varicella zoster virus in an era of varicella vaccination. Am. J. Public Health 100:1116–1122. 10.2105/AJPH.2009.160002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant KA, Carville KS, Kelly HA. 2010. Evidence of increasing frequency of herpes zoster management in Australian general practice since the introduction of a varicella vaccine. Med. J. Aust. 193:483. [DOI] [PubMed] [Google Scholar]
- 31.Karhunen M, Leino T, Salo H, Davidkin I, Kilpi T, Auranen K. 2010. Modelling the impact of varicella vaccination on varicella and zoster. Epidemiol. Infect. 138:469–481. 10.1017/S0950268809990768 [DOI] [PubMed] [Google Scholar]
- 32.Nelson MR, Britt HC, Harrison CM. 2010. Evidence of increasing frequency of herpes zoster management in Australian general practice since the introduction of a varicella vaccine. Med. J. Aust. 193:110–113 [PubMed] [Google Scholar]
- 33.Rimland D, Moanna A. 2010. Increasing incidence of herpes zoster among veterans. Clin. Infect. Dis. 50:1000–1005. 10.1086/651078 [DOI] [PubMed] [Google Scholar]
- 34.Wu CY, Hu HY, Huang N, Pu CY, Shen HC, Chou YJ. 2010. Do the health-care workers gain protection against herpes zoster infection? A 6-year population-based study in Taiwan. J. Dermatol. 37:463–470. 10.1111/j.1346-8138.2010.00804.x [DOI] [PubMed] [Google Scholar]
- 35.Gaillat J, Gajdos V, Launay O, Malvy D, Demoures B, Lewden L, Pinchinat S, Derrough T, Sana C, Caulin E, Soubeyrand B. 2011. Does monastic life predispose to the risk of Saint Anthony's fire (herpes zoster)? Clin. Infect. Dis. 53:405–410. 10.1093/cid/cir436 [DOI] [PubMed] [Google Scholar]
- 36.Jardine A, Conaty SJ, Vally H. 2011. Herpes zoster in Australia: evidence of increase in incidence in adults attributable to varicella immunization? Epidemiol. Infect. 139:658–665. 10.1017/S0950268810001949 [DOI] [PubMed] [Google Scholar]
- 37.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. 2011. Herpes zoster incidence among insured persons in the United States, 1993–2006: evaluation of impact of varicella vaccination. Clin. Infect. Dis. 52:332–340. 10.1093/cid/ciq077 [DOI] [PubMed] [Google Scholar]
- 38.Ogunjimi B, Smits E, Hens N, Hens A, Lenders K, Ieven M, Van Tendeloo V, Van Damme P, Beutels P. 2011. Exploring the impact of exposure to primary varicella in children on varicella-zoster virus immunity of parents. Viral Immunol. 24:151–157. 10.1089/vim.2010.0031 [DOI] [PubMed] [Google Scholar]
- 39.Salleras M, Domínguez A, Soldevila N, Prat A, Garrido P, Torner N, Borrás E, Salleras L. 2011. Contacts with children and young people and adult risk of suffering herpes zoster. Vaccine 29:7602–7605. 10.1016/j.vaccine.2011.08.023 [DOI] [PubMed] [Google Scholar]
- 40.Tanuseputro P, Zagorski B, Chan KJ, Kwong JC. 2011. Population-based incidence of herpes zoster after introduction of a publicly funded varicella vaccination program. Vaccine 29:8580–8584. 10.1016/j.vaccine.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 41.van Hoek AJ, Melegaro A, Zagheni E, Edmunds WJ, Gay N. 2011. Modelling the impact of a combined varicella and zoster vaccination programme on the epidemiology of varicella zoster virus in England. Vaccine 29:2411–2420. 10.1016/j.vaccine.2011.01.037 [DOI] [PubMed] [Google Scholar]
- 42.Carville KS, Grant KA, Kelly HA. 2012. Herpes zoster in Australia. Epidemiol. Infect. 140:599–600 (Author reply, 600–601). 10.1017/S095026881100149X [DOI] [PubMed] [Google Scholar]
- 43.Chao DY, Chien YZ, Yeh YP, Hsu PS, Lian IB. 2012. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000–2008. Epidemiol. Infect. 140:1131–1140. 10.1017/S0950268811001786 [DOI] [PubMed] [Google Scholar]
- 44.Lasserre A, Blaizeau F, Gorwood P, Bloch K, Chauvin P, Liard F, Blanchon T, Hanslik T. 2012. Herpes zoster: family history and psychological stress-case-control study. J. Clin. Virol. 55:153–157. 10.1016/j.jcv.2012.06.020 [DOI] [PubMed] [Google Scholar]
- 45.Ogunjimi B, Van Damme P, Beutels P. 2012. Zoster in monasteries: some clarification needed. Clin. Infect. Dis. 54:305–306 (Author reply, 306–307). 10.1093/cid/cir841 [DOI] [PubMed] [Google Scholar]
- 46.Hales CM, Harpaz R, Joesoef MR, Bialek SR. 2013. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann. Intern. Med. 159:739–745. 10.7326/0003-4819-159-11-201312030-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ogunjimi B, Van Damme P, Beutels P. 2013. Herpes zoster risk reduction through exposure to chickenpox patients: a systematic multidisciplinary review. PLoS One 8:e66485. 10.1371/journal.pone.0066485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogunjimi B, Peeters D, Hens N, Malfait R, Van Tendeloo V, Van Damme P, Beutels P, Smits E. 2012. Sampling site matters when counting lymphocyte subpopulations. PLoS One 7:e41405. 10.1371/journal.pone.0041405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogunjimi B, Hens N, Malfait R, Van Tendeloo V, Smits E. 2013. Creating a robust framework for the analysis of cryopreserved samples in quantitative immunological experiments. J. Immunol. Methods 392:63–67. 10.1016/j.jim.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 50.de Villemeur AB, Gratacap-Cavallier B, Casey R, Baccard-Longère M, Goirand L, Seigneurin JM, Morand P. 2011. Occupational risk for cytomegalovirus, but not for parvovirus B19 in child-care personnel in France. J. Infect. 63:457–467. 10.1016/j.jinf.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 51.Levin MJ, Schmader KE, Gnann JW, McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, Zhao Y, Li X, Chan IS, Annunziato PW, Parrino J. 2013. Varicella-zoster virus specific antibody responses in 50-59-year-old recipients of zoster vaccine J. Infect. Dis. 208:1386–1390. 10.1093/infdis/jit342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leroux-Roels I, Leroux-Roels G, Clement F, Vandepapelière P, Vassilev V, Ledent E, Heineman TC. 2012. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J. Infect. Dis. 206:1280–1290. 10.1093/infdis/jis497 [DOI] [PubMed] [Google Scholar]
- 53.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A, Veterans Affairs Cooperative Studies Program Shingles Prevention Study Investigators 2008. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J. Infect. Dis. 197:825–835. 10.1086/528696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jenke AC, Klein S, Baiker A, Wirth S, Sander M, Noelting C, Boecher O, Vizoso-Pinto MG. 2012. Serologic analysis of the IgG antibody response in children with varicella zoster virus wild-type infection and vaccination. Pediatr. Infect. Dis. J. 31:1148–1152. 10.1097/INF.0b013e31826bac27 [DOI] [PubMed] [Google Scholar]
- 55.Frey CR, Sharp MA, Min AS, Schmid DS, Loparev V, Arvin AM. 2003. Identification of CD8+ T cell epitopes in the immediate early 62 protein (IE62) of varicella-zoster virus, and evaluation of frequency of CD8+ T cell response to IE62, by use of IE62 peptides after varicella vaccination. J. Infect. Dis. 188:40–52. 10.1086/375828 [DOI] [PubMed] [Google Scholar]
- 56.Jones L, Black AP, Malavige GN, Ogg GS. 2007. Phenotypic analysis of human CD4+ T cells specific for immediate-early 63 protein of varicella-zoster virus. Eur. J. Immunol. 37:3393–3403. 10.1002/eji.200737648 [DOI] [PubMed] [Google Scholar]
- 57.Malavige GN, Jones L, Black AP, Ogg GS. 2008. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin. Exp. Immunol. 152:522–531. 10.1111/j.1365-2249.2008.03633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malavige GN, Rohanachandra LT, Jones L, Crack L, Perera M, Fernando N, Guruge D, Ogg GS. 2010. IE63-specific T-cell responses associate with control of subclinical varicella zoster virus reactivation in individuals with malignancies. Br. J. Cancer 102:727–730. 10.1038/sj.bjc.6605542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zerboni L, Sobel RA, Ramachandran V, Rajamani J, Ruyechan W, Abendroth A, Arvin A. 2010. Expression of varicella-zoster virus immediate-early regulatory protein IE63 in neurons of latently infected human sensory ganglia. J. Virol. 84:3421–3430. 10.1128/JVI.02416-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagel MA, Choe A, Traktinskiy I, Cordery-Cotter R, Gilden D, Cohrs RJ. 2011. Varicella-zoster virus transcriptome in latently infected human ganglia. J. Virol. 85:2276–2287. 10.1128/JVI.01862-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maecker HT, Dunn HS, Suni MA, Khatamzas E, Pitcher CJ, Bunde T, Persaud N, Trigona W, Fu TM, Sinclair E, Bredt BM, McCune JM, Maino VC, Kern F, Picker LJ. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27–40. 10.1016/S0022-1759(01)00416-1 [DOI] [PubMed] [Google Scholar]
- 62.Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, Marchese RD, Harbecke R, Williams HM, Chan IS, Arbeit RD, Gershon AA, Schodel F, Morrison VA, Kauffman CA, Straus SE, Schmader KE, Davis LE, Levin MJ, U.S. Department of Veterans Affairs (VA) Cooperative Studies Program Shingles Prevention Study Investigators 2009. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J. Infect. Dis. 200:1068–1077. 10.1086/605611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, Silber JL, MacGregor RR, Chan K, Gershon AA, Levin MJ. 2010. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J. Infect. Dis. 201:1024–1030. 10.1086/651199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, Lang N, Carrillo C, Stanley HA, Caulfield MJ, Weinberg A, Chan IS, Clair J, Smith JG, Marchese RD, Williams HM, Beck DJ, McCook PT, Zhang JH, Johnson G, Oxman MN. 2013. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin. Infect. Dis. 56:1085–1093. 10.1093/cid/cis1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinberg A, Huang S, Song LY, Fenton T, Williams P, Patterson J, Tovar-Salazar A, Levin MJ. 2012. Immune correlates of herpes zoster in HIV-infected children and youth. J. Virol. 86:2878–2881. 10.1128/JVI.06623-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loeth N, Assing K, Madsen HO, Vindeløv L, Buus S, Stryhn A. 2012. Humoral and cellular CMV responses in healthy donors; identification of a frequent population of CMV-specific, CD4+ T cells in seronegative donors. PLoS One 7:e31420. 10.1371/journal.pone.0031420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heller T, Werner JM, Rahman F, Mizukoshi E, Sobao Y, Gordon AM, Sheets A, Sherker AH, Kessler E, Bean KS, Herrine SK, Stevens M, Schmitt J, Rehermann B. 2013. Occupational exposure to hepatitis C virus: early T-cell responses in the absence of seroconversion in a longitudinal cohort study. J. Infect. Dis. 208:1020–1025. 10.1093/infdis/jit270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gillespie GM, Wills MR, Appay V, O'Callaghan C, Murphy M, Smith N, Sissons P, Rowland-Jones S, Bell JI, Moss PA. 2000. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J. Virol. 74:8140–8150. 10.1128/JVI.74.17.8140-8150.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. 2002. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J. Immunol. 169:1984–1992 [DOI] [PubMed] [Google Scholar]
- 70.Ogunjimi B, Theeten H, Hens N, Beutels P. Serology indicates cytomegalovirus infection is associated with varicella-zoster virus reactivation. J. Med. Virol., in press. 10.1002/jmv.23749 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.