Abstract
Studies were undertaken to manufacture a multivalent Shigella inactivated whole-cell vaccine that is safe, effective, and inexpensive. By using several formalin concentrations, temperatures, and incubation periods, an optimized set of inactivation conditions was established for Shigella flexneri 2a, S. sonnei, and S. flexneri 3a to produce inactivated whole cells expressing a full repertoire of Ipa proteins and lipopolysaccharide (LPS). The inactivation conditions selected were treatment with 0.2% formalin (S. flexneri 2a and 3a) or 0.6% formalin (S. sonnei) for 48 h at 25°C. Vaccine formulations prepared under different inactivation conditions, in different doses (10E5, 10E7, and 10E9 cells), and with or without the inclusion of double-mutant heat-labile toxin (dmLT) were evaluated in mice. Two intranasal immunizations with ≥10E7 inactivated whole cells resulted in high levels of anti-Invaplex and moderate levels of LPS-specific IgG and IgA in serum and in lung and intestinal wash samples. Addition of dmLT to the vaccine formulations did not significantly enhance humoral immunogenicity. Minimal humoral responses for IpaB, IpaC, or IpaD were detected after immunization with inactivated whole Shigella cells regardless of the vaccine inactivation conditions. In guinea pigs, monovalent formulations of S. flexneri 2a of 3a or S. sonnei consisting of 10E8, 10E9, or 10E10 cells were protective in a keratoconjunctivitis assay. A trivalent formulation provided protection against all three serotypes (S. flexneri 2a, P = 0.018; S. flexneri 3a, P = 0.04; S. sonnei, P < 0.0001). The inactivated Shigella whole-cell vaccine approach incorporates an uncomplicated manufacturing process that is compatible with multivalency and the future development of a broadly protective Shigella vaccine.
INTRODUCTION
Shigellosis continues to be a leading cause of diarrheal disease in many parts of the developing world (1, 2). Preventative measures such as improved sanitation, education, and nutrition are often difficult to implement in large part because of infrastructure and funding deficiencies. Prophylactic vaccines may overcome the disease burden and increasing antibiotic resistance of the most prevalent Shigella serotypes in the most susceptible population (under 5 years of age) if effective. A similar scenario exists for cholera, but recent progress has indicated that an inexpensive oral vaccine can be produced that has a high level of protection (3–5). The success of the cholera vaccine is in large part due to the use of simple technology (inactivated whole cells) to manufacture the vaccine and awareness of the antigens required for protective immunity.
The Shigella vaccines under development span a spectrum of approaches and antigens (6–10). Almost all Shigella vaccines include the O-specific lipopolysaccharide (LPS), which is considered a protective antigen (11), but this antigen restricts vaccine efficacy to only homologous or cross-reactive serotypes. In theory, broad coverage with an LPS-based vaccine can be achieved by including LPS from the five serotypes (Shigella flexneri 2a, S. flexneri 3a, S. flexneri 6, S. sonnei, and S. dysenteriae 1) that are the most prevalent and demonstrate some level of cross-reactivity with other prevalent serotypes (12). Conserved proteins such as the invasion plasmid antigens (IpaB, IpaC, and IpaD) or OmpA are also dominant antigens recognized by the immune system after natural infection and are attractive vaccine components because of inherent structural similarities within all Shigella species (13–15). A vaccine that stimulates an immune response, presumably a mucosal response, to both LPS and the conserved Ipa proteins would mimic the specificity of the immune response observed after natural infection. Two categories of Shigella vaccine candidates that have the potential to stimulate such a comprehensive immune response are live-attenuated and inactivated whole-cell vaccines.
Inactivated whole-cell Shigella vaccines including heat-killed, acetone-killed, and formalin-inactivated bacteria have been evaluated in several studies encompassing small animals, nonhuman primates, and humans (8, 10, 16). Protection is consistently observed in various animal models for all inactivation methods, which has justified interest in this approach as a promising vaccine for shigellosis. Furthermore, current good manufacturing practices (cGMP) manufacture of formalin-inactivated S. sonnei with an uncomplicated manufacturing process permitted clinical evaluation in human volunteers (17) in which both a mucosal and a systemic immune response to Shigella antigens was induced after oral immunization. Building upon the encouraging results obtained with S. sonnei, our strategy was to produce successful monovalent vaccines for S. flexneri 2a and 3a and S. sonnei, followed by evaluation of a trivalent, inactivated whole-cell Shigella vaccine formulated by combining all of the monovalent vaccine products. In addition, to enhance the immunogenicity of the inactivated whole cells, a strong mucosal adjuvant (double-mutant heat-labile toxin [dmLT]) was also evaluated. These studies addressed the effectiveness of the trivalent vaccine against heterologous and homologous challenges and also monitored the immune response to specific antigens in animals receiving monovalent vaccines in contrast to animals immunized with the trivalent formulation.
(This work was presented in part at the Vaccines for Enteric Diseases Conference, Cannes, France, September 2011.)
MATERIALS AND METHODS
Growth of Shigella species.
For S. flexneri 2a (strain 2457T, lot 1617) and S. flexneri 3a (strain J17B, lot 1654), a single Congo red-positive colony was inoculated into a flask of tryptic soy broth (TSB, nonanimal origin; EMD Chemicals Inc., Gibbstown, NJ; 100 ml) and incubated for 5 h at 37 ± 1°C with agitation at 200 rpm. At 5 h, 50 ml of the culture was aseptically inoculated into a 6-liter fermentation vessel (BioFlo110; New Brunswick Scientific Ltd., Edison, NJ) containing 5 liters of TSB. For S. sonnei (strain Moseley, lot 1618), two form I Congo red colonies suspended in TSB were used to directly inoculate the fermentor broth. The fermentation parameters were agitation at 200 rpm, 37°C, air sparging at 5 liters/min, and 0.01% antifoam 204 (Sigma, St. Louis, MO). The dissolved oxygen was started at 100%, and the pH was initially set at 7.0, but neither of these parameters was controlled during the run. After 16 to18 h (final optical density at 600 nm [OD600] of 1.5 ± 0.5), the fermentor culture was harvested into sterile, 4-liter screw-cap bottles. The harvested culture was collected by centrifugation at 6,000 × g for 30 min at 4°C and suspended in 500 ml Hanks balanced salt solution (HBSS, without phenol red, with calcium chloride and magnesium chloride; GIBCO/Invitrogen, Carlsbad, CA) at 25°C. The suspended cells were adjusted to a final OD600 of 18 by adding HBSS. Preliminary experiments indicated that consistent, complete formalin inactivation was achieved when a standardized cell density (OD600 of 18) was used during formalin treatment. Viability and antigen expression of Ipa proteins and LPS were monitored in the starter culture at the end of the 5-h growth period (S. flexneri 2a and 3a) and at the end of fermentation (see below).
Formalin inactivation.
The HBSS-suspended cells were placed in sterile 1-liter screw-cap flasks for formalin inactivation. A control consisting of cells treated with HBSS only was included to monitor the stability of the antigens during the inactivation process. For S. flexneri 2a and 3a, the final formalin (37% formaldehyde; J. T. Baker Ltd., Phillipsburg, NJ) concentration during the inactivation step was 0.2% (vol/vol). For S. sonnei, it was necessary to increase the formalin concentration to 0.6% (vol/vol) to achieve complete inactivation. During inactivation, the flasks were placed in a shaking water bath (model G76; New Brunswick Scientific Ltd., Edison, NJ) at 25°C with agitation at 200 rpm for 48 h. Samples were removed for determination of viability and antigen stability at 0, 1, 2, 4, 6, 8, 10, 12, 24, and 48 h (see below). At the end of the 48-h inactivation period, the treated cell suspensions were centrifuged at 6,000 × g for 30 min at 4°C. The pellets were resuspended in phosphate-buffered saline (PBS) and centrifuged as described above. The final pellets were resuspended in PBS, and the volume was adjusted to 300 ml with PBS. The turbidity of the cell suspension was measured and adjusted to a final OD600 of 23 (17) by adding the required amount of PBS. Larger volumes of cells were collected at 24 and 48 h of inactivation to allow analyses of immunogenicity and efficacy in small animals (see below). Shigella whole cells (SWC) were serially diluted in PBS and enumerated by microscopic counts in a Petroff-Hausser counting chamber. Parallel particle counts of ViaCheck beads (Bang Laboratories, Inc., Fishers, IN) were used to validate the counting procedure. The final formalin-inactivated SWC vaccines were stored in sterile polypropylene bottles at 4°C.
Shigella viability assessment.
Bacteria collected at the end of the fermentation period and during the various steps of inactivation were checked for viability by plating serial 10-fold dilutions of the bacteria on tryptic soy agar (TSA; Remel, Lenexa, KS) plates in triplicate. Cultures and treated bacteria were also plated on TSA-Congo red to determine the stability of the Congo red phenotype. Plates were incubated at 37°C and read at 24 and 48 h.
To determine if any residual viable Shigella cells were present in the final formalin-treated preparations, 10 tubes each of TSB (Remel, Lenexa, KS) and thioglycolate broth (Remel, Lenexa, KS) were inoculated with 0.1 ml of the final inactivated SWC preparations. The tubes were incubated for 14 days at 37°C, and the OD600 of each tube was measured daily with a spectrophotometer (Spectronic 20+; Thermo Electron Corp., Madison, WI). Tubes showing increased absorbance were subcultured on Hektoen enteric agar and then analyzed to determine the Shigella serotype.
Antigen expression.
Western blot assays were used to monitor the expression and antigenicity of IpaB, IpaC, IpaD, and LPS in the formalin-treated shigellae. Monoclonal antibodies (MAbs) to specific Ipa proteins (13, 18) were used for Western blot assays. Bacteria (0.5 to 1.0 ml) collected at the end of the 5-h seed culturing period and the fermentor incubations and at indicated time points during formalin inactivation were centrifuged, and the resulting cell pellet was lysed with sample buffer in preparation for Western blot assays (19).
Viability after formalin treatment.
After formalin treatment, log dilutions of the cell suspensions were plated onto TSA plates in triplicate for colony counting. Untreated cells suspended in HBSS were also counted. All of the TSA plates were incubated at 37°C, and the colony counts were first determined at 24 h of incubation and then verified again at 48 h because of the delayed growth characteristics of formalin-treated cells. At each time point, 0.5 ml of the suspension was processed for SDS-PAGE analysis.
Murine immunogenicity and protection study to compare inactivation procedures and the use of dmLT as a mucosal adjuvant.
Groups of female 4- to 6-week-old BALB/cByJ mice (5 to 20/group) were purchased from Jackson Laboratories (Bar Harbor, ME) and intranasally immunized on days 0 and 14 with one of three different doses (10E5, 10E7, or 10E9 SWC/dose) of S. flexneri 2a whole cells (Sfl2aWC) inactivated for either 24 or 48 h with 0.2% formalin at 25°C. Separate groups of mice were immunized with the same doses of Sfl2aWC mixed with 5 μg dmLT (20). Control groups were immunized intranasally with saline, dmLT alone, or S. flexneri 2a Invaplex (19) on the same immunization schedule as above. For intranasal immunization, mice were anesthetized with a mixture of xylazine HCl and ketamine HCl and placed in dorsal recumbency, and 25 μl of the vaccine was applied in four to six droplets to the external nares with a micropipette. Blood was collected on days 0, 28, and 49. Lung and intestinal wash samples were collected on day 21 to investigate mucosal antibody responses. Mice were challenged intranasally on day 35 with 1.6 × 107 CFU S. flexneri 2a 2457T as previously described (21). Mice were monitored daily for morbidity (e.g., labored breathing, hunched posture, ruffled fur, weight loss, and lethargy) and death for a total of 14 days postchallenge. Mouse studies were conducted under IACUC-approved protocol 11-BRD-27. This research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animal experiments involving animals and adheres to principles stated in the 1996 edition of the NRC Guide for the Care and Use of Laboratory Animals.
Immunogenicity and protection study with guinea pigs to evaluate monovalent Sfl2aWC vaccine and trivalent SWC vaccine.
Male (Hartley strain) guinea pigs (6 to 12/group) weighing 150 to 200 g were purchased from Charles River Laboratories (Frederick, MD). Guinea pigs were immunized intranasally on days 0, 7, and 14. Blood samples were collected from the lateral ear vein on days 0, 21, and 42. Ocular wash samples and fecal pellets were collected on days 0 and 21.
In the monovalent study, guinea pigs were immunized intranasally with a total volume of 100 μl containing Sfl2aWC (10E8, 10E9, or 10E10 Sfl2aWC/dose) either with or without dmLT (10 μg). Control groups were immunized intranasally with dmLT (10 μg) or saline. In the trivalent study, groups were immunized intranasally with Sfl3aWC (10E8, 10E9, or 10E10 SWC/dose) with each dose delivered either alone or combined with Sfl2aWC and SsWC (each at 10E10 SWC/dose). Other groups were immunized with Sfl2aWC or SsWC (10E10 SWC/dose) or saline (negative control). Guinea pigs were challenged ocularly on day 28 at 1.5 × 10E8 CFU/eye with S. flexneri 2a 2457T (monovalent study) or at 2.5, 2.7, or 2.3 × 10E8 CFU/eye with S. flexneri 2a 2457T, S. flexneri 3a J17B, or S. sonnei 53G, respectively (trivalent study), as previously described (22). After inoculation, the eyes were observed daily for 5 days for the occurrence of keratoconjunctivitis. The degree of inflammation and keratoconjunctivitis was scored on a scale of 0 to 3 established by Hartman et al. (22). Guinea pig studies were conducted under IACUC-approved protocol IB03-10.
Sample collection and processing.
Blood from mice and guinea pigs was collected onto Whatman no. 1 filter paper and stored at 4°C until eluted with Tris-buffered casein prior to analysis by enzyme-linked immunosorbent assay (ELISA). Lung and intestinal lavage fluids were collected from individual mice to assess mucosal antibody levels as previously described (23). Ocular wash samples were collected from individual anesthetized guinea pigs by instilling 100 μl of sterile 0.9% saline onto the eye. The fluid was removed and reapplied three times before being transferred to a 1-ml Nunc CryoTube and stored on wet ice. Wash samples were collected from both eyes, combined in a single tube, and stored at −30°C until assayed by ELISA for antigen-specific IgA.
Immunological analysis.
Antigen-specific endpoint titers of IgG and IgA antibodies to Shigella LPS, Shigella Invaplex, purified recombinant IpaB, IpaC, and IpaD, and dmLT in serum and mucosal wash samples were determined by ELISA as previously described (23). The LPS antigens of S. flexneri 2a and 3a and S. sonnei were prepared by the Westphal procedure (24). Invaplex for each serotype and recombinant IpaB, IpaC, and IpaD antigens were purified as previously described (25, 26). The Invaplex antigen is used to measure antibodies reactive with a native invasin complex consisting of Ipa proteins (IpaB, IpaC, and IpaD) and LPS. The dmLT was manufactured by the WRAIR pilot bioproduction facility under cGMP conditions.
Statistical analyses.
All statistical analyses were completed in Prism (version 5.0; GraphPad). Comparisons of serum endpoint titers between groups was accomplished by a two-way analysis of variance (ANOVA) with Bonferroni's post hoc test of log-transformed titers. Mucosal immune responses of different treatment groups were analyzed in a one-way ANOVA of log-transformed titers. Differences between protection levels postchallenge in the murine and guinea pig models were assessed with Fisher's exact test. Correlations between ocular IgA titers and protection from keratoconjunctivitis were determined by first calculating the composite disease score of an individual guinea pig by adding the disease score recorded on day 5 for the left and right eyes. The disease score of each animal challenged in only one eye (saline control group) was calculated by doubling the disease score in the challenged eye. The disease scores of individual animals and the respective ocular IgA titers were then evaluated with a Spearman statistical test, and the two-tailed P value was calculated. For correlation analysis, endpoint titers were log transformed for calculations and graphed with a linear best-fit line.
RESULTS
Inactivation of Shigella.
The overarching goal was to determine the inactivation conditions (temperature, formalin concentration, and inactivation time) that preserved antigenicity while ensuring complete inactivation and maintaining a simple, inexpensive manufacturing process. The antigenicity of specific antigens (IpaB, IpaC, IpaD, and LPS) was evaluated by Western blot assays with MAbs. Qualitative changes such as diminished MAb reactivity and/or changes in the antigen migration patterns in the gels were considered undesirable consequences of the inactivation treatment used. The most important criterion for a successful inactivation process was the efficient and effective killing of shigellae. With cell suspensions standardized for cell density (OD600 of 18), any condition that did not result in 100% inactivation was not evaluated further.
Over the course of several experiments, it was determined that incubation of shigellae with formalin at 4°C diminished the killing process (see Table S1 in the supplemental material). Viable shigellae were detected after incubation with up to 1.0% formalin for 6 h at 4°C. At a lower formalin concentration (0.2%), viable shigellae were still detected after 72 h of treatment at 4°C. Although prolonged incubations at 4°C eventually resulted in complete killing of the treated shigellae, the length of time required for 100% inactivation was unreasonable for manufacture. For this reason, 4°C was not used as an inactivation temperature. In contrast, inactivation experiments conducted at 37°C resulted in rapid killing (100% killing after 4 h of exposure to 0.2% formalin) (see Table S1 in the supplemental material) but also reduced the reactivity of the MAbs with protein antigens. At 25°C, low concentrations of formalin (0.2% for S. flexneri and 0.6% for S. sonnei; see Table S2 in the supplemental material) achieved complete killing after 4 to 12 h of incubation (see below).
Protein antigenicity after formalin inactivation.
As the Ipa proteins are major protein antigens recognized by infected humans and are highly conserved across all Shigella species, it was desirable to maintain their presence and reactivity in the inactivated whole-cell vaccine preparations. The ability of formalin treatment to cross-link proteins may have a negative effect on the antigenicity of the proteins (17). Previous studies have indicated that formaldehyde treatment (1%, 72 h, room temperature) of S. sonnei yielded a safe, inactivated whole-cell preparation that stimulated immune responses to LPS and IpaC after the immunization of human volunteers (17).
Antigen sensitivity to formalin was evaluated for each Shigella strain. Over the course of 72 h, a gradual reduction in reactivity with Ipa-specific MAbs was evident across the three serotypes evaluated. For S. sonnei, the MAb reactivity of IpaD in whole cells treated with 0.6, 0.8, or 1.0% formalin was comparable to that of untreated preparations for treatment times of up to 12 h (see Fig. S1 in the supplemental material). At 24 h, cells treated with 0.8 or 1.0% formalin had diminished MAb recognition of IpaD with the protein band that comigrated with the full-size IpaD protein. After 72 h of formalin treatment, the IpaD band at 37 kDa was barely visible in the 0.8 or 1.0% formalin-treated S. sonnei cells and was diminished in the 0.6% formalin-treated cells as well. In addition, with all of the concentrations of formalin treatment used, the appearance of higher-molecular-weight forms of IpaD were evident at all of the incubation times used, suggesting oligomerization of IpaD or cross-linking with other unidentified proteins. Similar results for IpaD were also observed with S. flexneri 2a (data not shown) and 3a (see Fig. S2 in the supplemental material) cells treated with formalin. In addition, IpaB and IpaC for all serotypes also showed decreased recognition by specific MAbs after formalin treatment for prolonged times (see Fig. 2; see Fig. S1 in the supplemental material and reference 2). Western blot assay results indicated that higher concentrations of formalin did not alter the LPS Western blot assay profile (data not shown). Because of the apparent alteration of the Ipa proteins' antigenicity, lower concentrations of formalin appeared more favorable for the maintenance of the Ipa proteins' antigenic structure.
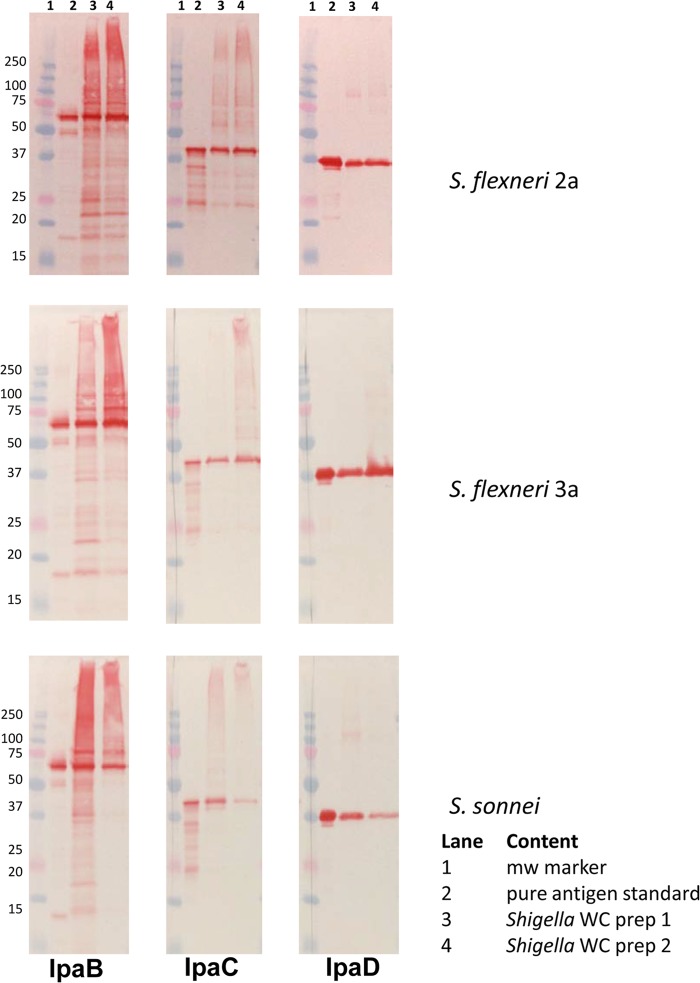
FIG 2.
Antigen content of formalin-inactivated Shigella whole cells. Whole-cell lysates of two separate preparations (lanes 3 and 4 in each panel) of S. flexneri 2a and 3a and S. sonnei cells treated with 0.2% formalin (S. flexneri 2a and 3a) or 0.6% formalin (S. sonnei) at 25°C for 48 h were evaluated by Western blot assays for IpaB, IpaC, and IpaD. Each formalin-treated preparation has a MAb-reactive antigen that is comparable in size to a purified antigen, indicating that the antigens are present in the inactivated whole-cell preparations.
Final product conditions and characterization.
The studies described above identified formalin concentrations and temperatures that achieved the complete inactivation of shigellae and maintenance of the Ipa protein and LPS antigens. For final vaccine preparation, shigellae were inactivated at 25°C for 48 h. The formalin concentrations used for inactivation were 0.2% for S. flexneri strains and 0.6% for S. sonnei. Figure 1 shows the inactivation curves for S. flexneri 2a and 3a and S. sonnei. The amount of time required for inactivation was 4 to 12 h (Fig. 1), depending on the strain. Maintenance of IpaB, IpaC, and IpaD antigenicity was possible under these conditions (Fig. 2A to C). LPS reactivity in Western blot assays was also maintained and in general was not affected by the formalin concentration or temperature (data not shown). Vaccine preparations made under these conditions were used as monovalent vaccines or included in multivalent formulations for immunogenicity and efficacy experiments with mice and guinea pigs. Inactivation preparations at 24 and 48 h were selected for immunogenicity studies with mice. The extended inactivation times (24 and 48 h) were used to ensure that all of the bacteria were killed.
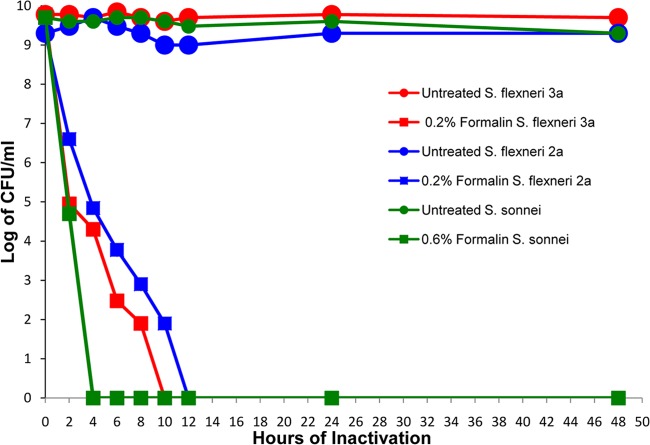
FIG 1.
Inactivation of S. flexneri 2a and 3a and S. sonnei with formalin. Shigellae were incubated with 0.2% formalin (S. flexneri 2a and 3a) or 0.6% formalin (S. sonnei) and sampled out to 48 h to determine viability. HBSS-treated control preparations (untreated) of each organism were also monitored at each time point. Complete inactivation of S. flexneri 2a was achieved at 12 h, complete inactivation of S. flexneri 3a was achieved at 10 h, and complete inactivation of S. sonnei was achieved at 4 h. The viability of the untreated controls remained constant for the duration of the 48-h experiment. Viability was determined by enumerating CFU on TSA plates.
Comparison of monovalent Sfl2aWC inactivated with 0.2% formalin for either 24 or 48 h in a murine model.
The murine model was used to evaluate the immunogenicity and protective efficacy of vaccination with Sfl2aWC. Groups of mice were intranasally immunized on days 0 and 14 with increased doses of Sfl2aWC inactivated with 0.2% formalin for either 24 or 48 h. In addition, other groups were intranasally immunized with comparable doses of inactivated Sfl2aWC combined with the mucosal adjuvant dmLT to determine if enhanced immunogenicity could be achieved with the adjuvant.
Blood collected on days 0, 28, and 49 was assayed by ELISA to determine serum IgG and IgA endpoint titers. Antigen-specific titers of samples collected on day 0 were low to undetectable (≤90) in all of the groups. Groups immunized with dmLT or saline also had low Shigella antigen-specific titers on day 28. Overall, there were no significant differences in antigen-specific serum endpoint titers between groups intranasally immunized with comparable doses of Sfl2aWC inactivated with 0.2% formalin for 24 or 48 h (Fig. 3). Furthermore, the addition of dmLT to the Sfl2aWC preparation did not result in higher Shigella antigen-specific serum endpoint titers, but 100% of the mice that received dmLT did seroconvert with high levels of anti-dmLT serum IgG and IgA (data not shown). The antigen-specific response directed to IpaB (serum IgG) and to Invaplex (serum IgA) was significantly higher in groups immunized with Sfl2aWC than in those immunized with Sfl2aWC delivered with dmLT. With respect to serum IgG and IgA responses directed to IpaB, LPS, and Invaplex, immunization with 10E9 Sfl2aWC resulted in significantly higher endpoint titers than immunization with 10E5 or 10E7 Sfl2aWC. IpaC- and IpaD-specific serum IgG and IgA levels were generally below the limit of detection on day 28 in all of the groups assayed. Antibodies directed to LPS, Invaplex, IpaB, IpaC, and IpaD were detected in postchallenge serum samples from surviving mice collected on day 46, except for animals mock immunized with saline (data not shown). In general, antigen-specific serum IgG and IgA titers increased from day 28 to day 46 in vaccinated animals. The increase in titers on day 46 was also observed in animals that did not have detectable antigen-specific IgG or IgA on day 28, indicating that the immunizations successfully primed the humoral immune response.
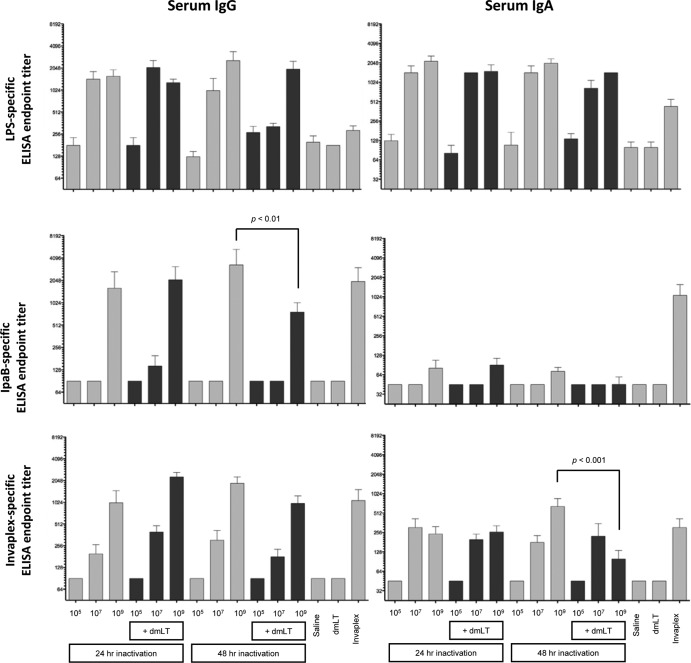
FIG 3.
Shigella antigen-specific serum IgG and IgA endpoint titers on day 28 after the intranasal immunization of mice with Sfl2aWC inactivated with 0.2% formalin for either 24 or 48 h and formulated with or without dmLT. Blood was collected on day 28 (2 weeks after the second and final immunization) and assayed by ELISA for S. flexneri 2a LPS (top panels)-, IpaB (middle panels)-, and S. flexneri 2a Invaplex (bottom panels)-specific IgG (left panels) and IgA (right panels). Comparisons between groups were accomplished by two-way ANOVA of log-transformed titers.
Lung and intestinal wash samples were collected on day 21 (1 week after the second vaccination) and assayed by ELISA for Shigella antigen-specific antibody responses. Immunization with saline or 10E5 Sfl2aWC (inactivated for 24 or 48 h) did not induce detectable Shigella-specific lung IgA (Fig. 4). Inclusion of dmLT to the Sfl2aWC vaccine (10E5-cell dose) did not increase the magnitude of the Shigella-specific responses. In contrast, groups immunized with 10E7 or 10E9 Sfl2aWC with or without dmLT mounted a lung IgA response against Invaplex and S. flexneri 2a LPS that was significantly higher (P < 0.05) than that of the saline and dmLT control groups.
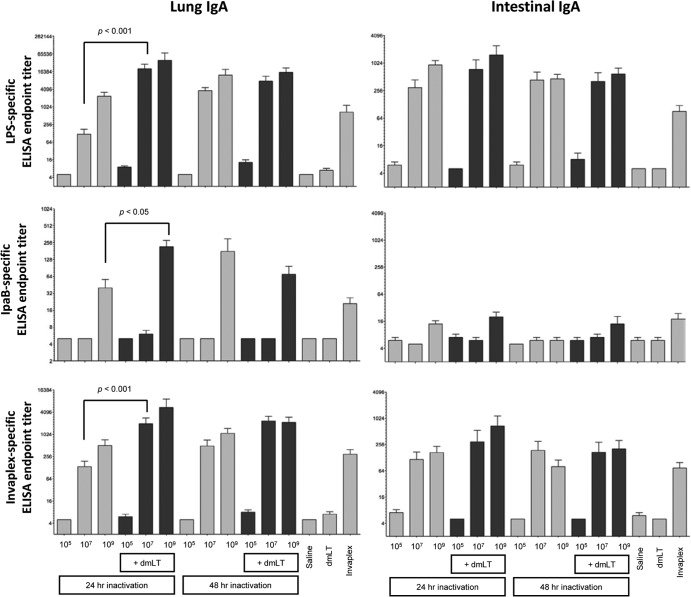
FIG 4.
Shigella antigen-specific lung and intestinal IgA endpoint titers on day 21 after the intranasal immunization of mice with Sfl2aWC inactivated with 0.2% formalin for either 24 or 48 h and formulated with or without dmLT. Mucosal wash samples collected on day 21 (1 week after the second and final immunization) were assayed by ELISA for LPS (top panels)-, IpaB (middle panels)-, and Invaplex (bottom panels)-specific lung IgA (left panels) and intestinal IgA (right panels). Comparisons between groups were accomplished by one-way ANOVA of log-transformed titers.
Groups immunized with 10E9, but not 10E5 or 10E7, Sfl2aWC inactivated for 24 or 48 h and administered with or without dmLT mounted a lung IgA response to IpaB. The addition of dmLT to the 24-h-inactivated 10E9-Sfl2aWC vaccine raised the anti-IpaB titer significantly above that induced in mice receiving only 10E9 Sfl2aWC (Fig. 4). Interestingly, the same adjuvant effect was not achieved with the 48-h-inactivated vaccine preparation. Levels of IpaC- and IpaD-specific lung IgA were undetectable (titers of ≤10) in all of the immunized groups (data not shown). In summary, minimal differences in antigen-specific lung IgA were apparent in groups immunized with Sfl2aWC (24 h) and those immunized with Sfl2aWC (48 h) delivered with or without dmLT.
Antigen-specific IgA was also assessed in intestinal wash samples collected on day 21 (Fig. 4). Immunization with 10E5 Sfl2aWC with or without dmLT did not induce Shigella-specific intestinal IgA titers above those of saline or dmLT control titers. Groups immunized with 10E7 or 10E9 Sfl2aWC (both 24- and 48-h inactivation preparations) mounted an intestinal IgA response specific for Invaplex and S. flexneri 2a LPS significantly higher than that of saline controls. However, no differences in antigen-specific IgA were noted between the 24- and 48-h-inactivated vaccine preparations or between formulations prepared with or without dmLT. Intestinal IgA responses directed to Ipa proteins were minimal, with only animals receiving 10E9 Sfl2aWC (24 h) plus dmLT showing an anti-IpaB response higher than that of dmLT control groups, whereas the IgA titers to IpaC and IpaD were undetectable (data not shown).
Protective efficacy of Sfl2aWC in mice after a lethal challenge with S. flexneri 2a 2457T.
Mice were challenged intranasally on day 35 (3 weeks after the second and final immunization) with a lethal dose (1.5 × 10E7 CFU) of S. flexneri 2a 2457T. Morbidity and death were monitored for 14 days postinfection (Table 1). None (0/15) of the saline-immunized mice survived the challenge, whereas nonspecific protection was observed in dmLT-immunized mice (9/15; 64% protection; P < 0.0002 compared to saline). Significant protection (>93%) was achieved after immunization with 10E7 and 10E9 Sfl2aWC (24- and 48-h inactivation preparations) compared to the saline control group. Groups immunized with 10E5 Sfl2aWC (24 and 48 h) were not protected (P ≥ 0.4828) compared to the saline control group. Groups immunized with 10E5, 10E7, and 10E9 24-h-inactivated Sfl2aWC combined with dmLT were not significantly protected from a challenge compared to the dmLT control group (P values of 0.7, 0.08, and 0.16, respectively). Similarly, the group immunized with 10E5 Sfl2aWC (48-h inactivation) combined with dmLT was not significantly protected compared to the dmLT control group (P = 0.08). In contrast, groups immunized with 10E7 and 10E9 Sfl2aWC (48-h inactivation) were significantly protected compared to the dmLT control group (P values of 0.017 and 0.041, respectively).
TABLE 1.
Protective efficacy of a monovalent Sfl2aWC vaccine in mice challenged with a lethal dose of S. flexneri 2a (2457T)
| Treatment (inactivation time [h], dose [no. of cells])a | No. of survivors/total no. of animals | % protectionb |
P valuec vs: |
|
|---|---|---|---|---|
| Saline | dmLT | |||
| Sfl2aWC (24, 10E5) | 2/15 | 13 | NSd | |
| Sfl2aWC (24, 10E7) | 15/15 | 100 | <0.0001 | |
| Sfl2aWC (24, 10E9) | 15/15 | 100 | <0.0001 | |
| Sfl2aWC + dmLT (24, 10E5) | 8/15 | 53 | NS | |
| Sfl2aWC + dmLT (24, 10E7) | 14/15 | 93 | 0.0801 | |
| Sfl2aWC + dmLT (24, 10E9) | 13/14 | 93 | 0.1647 | |
| Sfl2aWC (48, 10E5) | 1/15 | 7 | NS | |
| Sfl2aWC (48, 10E7) | 14/15 | 93 | <0.0001 | |
| Sfl2aWC (48, 10E9) | 14/14 | 100 | <0.001 | |
| Sfl2aWC + dmLT (48, 10E5) | 8/14 | 57 | NS | |
| Sfl2aWC + dmLT (48, 10E7) | 15/15 | 100 | 0.0169 | |
| Sfl2aWC + dmLT (48, 10E9) | 13/13 | 100 | 0.0407 | |
| Saline | 0/15 | 0 | 0.0002 | |
| dmLT | 9/14 | 64 | 0.0002 | |
| Invaplex | 14/15 | 93 | <0.0001 | |
Animals were challenged intranasally with 1.5 × 107 CFU of S. flexneri 2a (2457T). Animals were observed for 14 days postinfection. Survivors were bled and euthanized.
% protection = [% death control − % death vaccinees)/% death control] × 100. Mice immunized with Sfl2aWC vaccines formulated with or without dmLT were compared to either mice immunized with dmLT or saline, respectively.
Fisher's exact test was used.
NS, not statistically significant.
Immunogenicity and protective efficacy of a monovalent Sfl2aWC vaccine and assessment of dmLT as an adjuvant in guinea pigs.
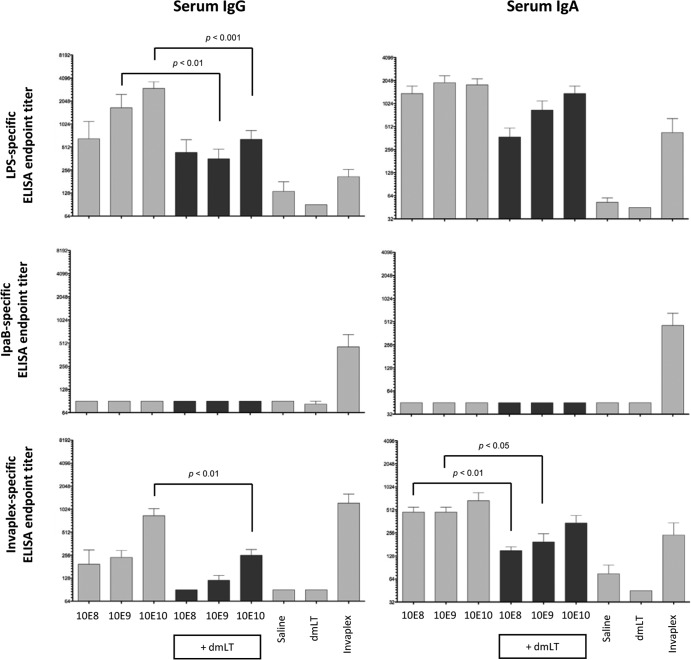
No significant differences in immunogenicity or protective efficacy in mice were noted between vaccine preparations inactivated for either 24 or 48 h. Solid protection in mice, maintenance of antigenicity for all key antigens, and less risk of residual viable bacteria suggested that the 48-h inactivation treatment with 0.2% formalin would be suitable for eventual clinical development and was therefore used in all further studies. In guinea pigs, three vaccine doses formulated with or without dmLT were used to evaluate the immunogenicity and efficacy of the 48-h-inactivated vaccine. Groups of guinea pigs were intranasally immunized on days 0, 7, and 14 with 10E8, 10E9, or 10E10 Sfl2aWC delivered with or without dmLT (10 μg). Controls consisted of separate groups immunized with saline (negative control), dmLT (adjuvant control), or S. flexneri 2a InvaplexNAT (positive control).
Blood collected on days 0, 21, and 42 was assayed by ELISA for serum IgG and IgA specific for S. flexneri 2a Invaplex, LPS, IpaB, IpaC, IpaD, and dmLT. Preimmune samples were negative (titers of ≤90) in all assays (data not shown). Modest serum titers of IgG and IgA directed to S. flexneri 2a LPS and Invaplex were detected in blood samples collected on day 21 from guinea pigs immunized with 10E8, 10E9, and 10E10 Sfl2aWC (Fig. 5). All of the groups immunized with dmLT mounted an anti-dmLT serum IgG response (data not shown). Animals immunized with saline, dmLT, or Sfl2aWC with or without dmLT did not mount a detectable IgG or IgA antibody response to IpaB, IpaC, or IpaD after vaccination, whereas guinea pigs immunized with S. flexneri 2a Invaplex seroconverted (≥4-fold increase in titer over baseline) to IpaB (100%) and IpaD (17%). Similar to the results achieved in the mouse studies, dmLT did not enhance the Shigella-specific serum IgG or IgA response when delivered intranasally along with Sfl2aWC, and in several instances, the Shigella-specific response was significantly lower when dmLT was added to the vaccine. Interestingly, the LPS-specific IgA titers did not increase significantly after the second immunization or after a challenge in groups immunized with Sfl2aWC, with titers on days 21 (after vaccination 3) and 42 (postchallenge) at similar levels, whereas groups immunized with Sfl2aWC combined with dmLT had significantly higher titers on day 42 (postchallenge) than on day 21 (data not shown).
FIG 5.
Shigella antigen-specific serum IgG and IgA endpoint titers on day 21 after the intranasal immunization of guinea pigs with monovalent Sfl2aWC inactivated with 0.2% formalin for 48 h and formulated with or without dmLT. Blood was collected on day 21 (1 week after the third and final immunization) and assayed by ELISA for LPS (top panels)-, IpaB (middle panels)-, and Invaplex (bottom panels)-specific serum IgG (left panels) and serum IgA (right panels). Comparisons between groups were accomplished by two-way ANOVA of log-transformed titers.
Ocular wash samples were collected from individual guinea pigs on day 0 prior to immunization and on day 21, 1 week after the third and final immunization, and assayed for IgA specific for LPS, Invaplex, IpaB, IpaC, and IpaD by ELISA. Samples collected from all of the groups on day 0 and from saline- and dmLT-inoculated animals on day 21 (Fig. 6) had minimal Shigella antigen-specific ocular IgA (titers of ≤10). Furthermore, all of the groups immunized intranasally with Sfl2aWC or with Sfl2aWC combined with dmLT had low to undetectable levels of IgA specific for IpaB, IpaC, and IpaD. In contrast, all of the animals immunized with Sfl2aWC with or without dmLT had ocular IgA specific for S. flexneri 2a LPS and Invaplex, with little evidence of a dose response.
FIG 6.
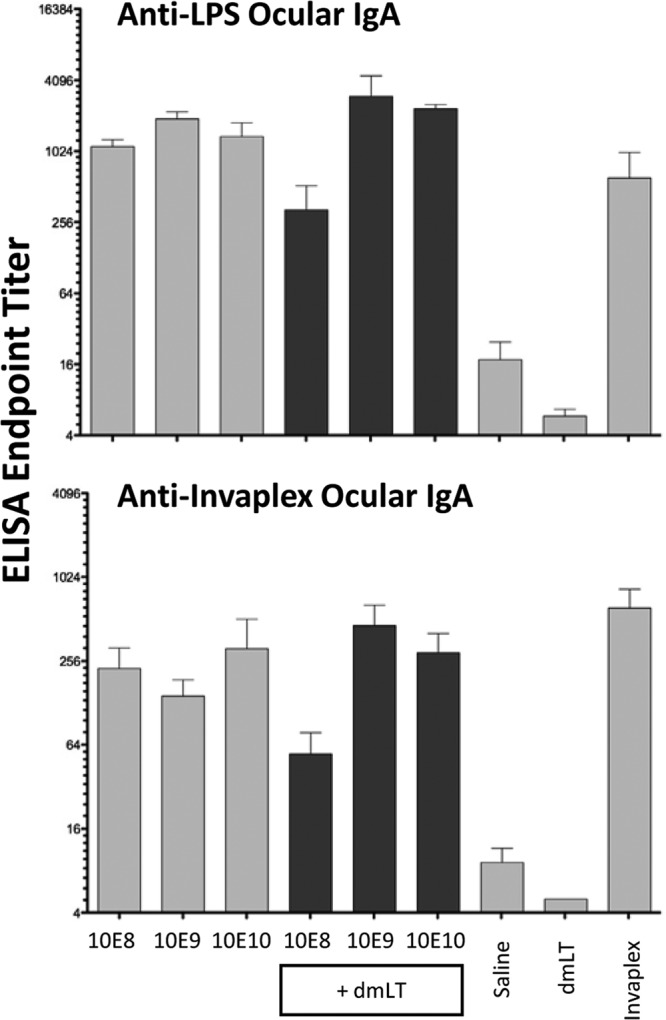
Shigella antigen-specific ocular IgA endpoint titers after two intranasal immunizations of guinea pigs with monovalent inactivated Sfl2aWC formulated with or without dmLT. Ocular wash samples were collected 1 week after the third vaccination (day 21) and assayed by ELISA for LPS (top panel) and Invaplex (bottom panel) IgA endpoint titers. Comparisons between groups were accomplished by two-way ANOVA of log-transformed titers.
Protective efficacy of monovalent Sfl2aWC vaccine in guinea pigs after an ocular challenge with S. flexneri 2a 2457T.
All of the animals were challenged ocularly 2 weeks after the third immunization with S. flexneri 2a 2457T (1.5 × 108 CFU/eye). Mice were monitored for disease for 5 days postinfection, and each eye was scored daily (Table 2). Of the 10 eyes scored in saline-immunized animals, all 10 eyes were positive (score of ≥2.0), with a mean group score of 3.0 on day 5. In addition, the dmLT control group also had nine of nine eyes scored as positive, with a mean group score of 3.0 on day 5. Significant protection from disease compared to saline control groups was achieved in groups immunized with 10E8 (P = 0.0017), 10E9 (P = 0.0053), or 10E10 (P = 0.0152) Sfl2aWC, with mean group scores of 1.0, 1.2, and 1.2, respectively. In contrast, protection from disease was obtained only when groups were immunized with 10E9 Sfl2aWC combined with dmLT (P = 0.0396; mean group score of 1.5). Groups immunized with 10E8 (P = 1.0; mean group score of 2.1) or 10E10 (P = 0.0964; mean group score of 1.5) combined with dmLT were not protected (P > 0.05) against disease on day 5.
TABLE 2.
Protection against ocular challenge with S. flexneri 2a 2457T after intranasal immunization of guinea pigs with monovalent Sfl2aWC vaccine delivered with or without dmLT
| Treatment (dose [no. of cells])a | Day 3 data |
Day 5 data |
||||||
|---|---|---|---|---|---|---|---|---|
| No. positive/total | % protectionb | P valuec | Mean disease scored | No. positive/total | % protectionb | P valuec | Mean disease scored | |
| Sfl2aWC (10E8) | 4/12 | 67 | 0.0017 | 1.1f | 4/12 | 67 | 0.0017 | 1.0f |
| Sfl2aWC (10E9) | 5/12 | 58 | 0.0053 | 1.2f | 5/12 | 58 | 0.0053 | 1.2f |
| Sfl2aWC (10E10) | 4/12 | 67 | 0.0017 | 0.9f | 6/12 | 50 | 0.0152 | 1.2f |
| Sfl2aWC + dmLT (10E8) | 11/12 | 2 | NSh | 2.3 | 11/12 | 8 | NS | 2.1g |
| Sfl2aWC + dmLT (10E9) | 6/12 | 44 | 0.0152 | 1.8g | 7/12 | 42 | 0.0396 | 1.5f |
| Sfl2aWC + dmLT (10E10) | 5/12 | 53 | 0.0053 | 1.4f | 8/12 | 33 | NS | 1.5f |
| Saline | 10/10 | 3.0 | 10/10 | 3.0 | ||||
| dmLT | 9/10e | 3.0 | 9/9 | 3.0 | ||||
| S. flexneri 2a Invaplex | 5/12 | 58 | 0.0053 | 1.6f | 8/12 | 33 | NS | 1.5f |
Animals were intraocularly challenged with 1.5 × 108 CFU/eye of S. flexneri 2a 2457T lot 1617 on day 29 and observed for 5 days postinfection with each eye scored daily. A positive response was a score of ≥2.0 according to Hartman et al. (22).
% protection = [% positive control − % positive vaccinees)/% positive control] × 100. guinea pigs immunized with Sfl2aWC vaccines formulated with or without dmLT were compared to either guinea pigs immunized with dmLT or saline, respectively.
Fisher's exact test was used. Guinea pigs immunized with Sfl2aWC vaccines formulated with or without dmLT were compared to groups immunized with either dmLT or saline, respectively.
Results were obtained with the Kruskal-Wallis test with Dunn posttest setting alpha at 0.05. Guinea pigs immunized with Sfl2aWC vaccines formulated with or without dmLT were compared to groups immunized with either dmLT or saline, respectively.
One animal was euthanized 24 h postinfection because of lethargy.
P < 0.001.
P = 0.01.
NS, not statistically significant.
Immunogenicity and efficacy of a trivalent (Sfl2aWC, SsWC, and Sfl3aWC) vaccine in guinea pigs.
Immunogenicity and protective-efficacy studies were conducted with guinea pigs to evaluate a bivalent (Sfl2aWC and SsWC) vaccine with 10E10 Sfl2aSWC delivered intranasally with 10E8, 10E9, or 10E10 SsWC. Results from the bivalent study (data not shown) indicated that a dose of 10E10 Sfl2aWC combined with a dose of 10E10 SsWC induced immune responses directed to both Shigella serotypes and protected against an ocular challenge with either S. flexneri 2a 2457T or S. sonnei 53G.
Evaluation of a trivalent vaccine was accomplished with the successful bivalent formulation and the addition of various doses of S. flexneri 3a to complete the trivalent formulation. Groups of guinea pigs were immunized with either monovalent SWC vaccines or a trivalent SWC vaccine consisting of Sfl2aWC and SsWC (10E10 cells each) combined with 10E8, 10E9, or 10E10 Sfl3aWC. Two weeks after the third and final immunization, guinea pigs were challenged ocularly with S. flexneri 2a 2457T, S. flexneri 3a J17B, or S. sonnei 53G to evaluate homologous and heterologous protection.
Serum antibodies directed to purified LPS prepared from the corresponding Shigella serotypes used for immunization (Fig. 7), IpaB, IpaC, and Invaplex (data not shown) were measured in samples collected on days 0, 21, and 42 (postchallenge). Samples collected on day 0 from all of the animals and from saline control guinea pigs on days 0 and 21 had undetectable Shigella-specific antibodies. In addition, antibodies directed at IpaB, IpaC, IpaD, and LPS purified from Shigella strains heterologous to the immunizing strain serotype were undetectable in the assays.
FIG 7.
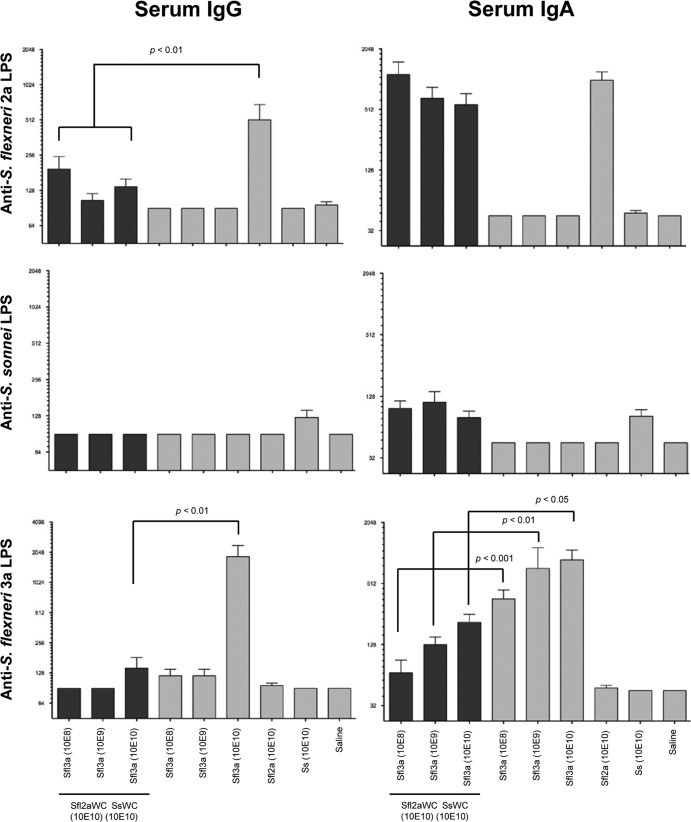
Shigella antigen-specific serum IgG and IgA endpoint titers on day 21 after the intranasal immunization of guinea pigs with trivalent (Sfl2aWC, SsWC, and Sfl3aWC) vaccine. Blood was collected on day 21 (1 week after the third vaccination) and assayed by ELISA for S. flexneri 2a LPS (top panel), S. sonnei LPS (middle panels), and S. flexneri 3a LPS (bottom panel) serum IgG (left panels) and serum IgA (right panels) endpoint titers. Comparisons between groups were accomplished by two-way ANOVA of log-transformed titers.
Serum IgG endpoint titers specific for LPS from S. flexneri 2a and 3a were significantly higher in groups immunized with monovalent SWC vaccines than in those immunized with the trivalent vaccine formulations (Fig. 7). LPS-specific serum IgA endpoint titers were comparable in groups immunized with monovalent vaccines than in groups immunized with trivalent vaccines against S. flexneri 2a and S. sonnei, but titers of antibodies to S. flexneri 3a LPS were significantly higher in groups immunized with monovalent Sfl3aWC than in groups immunized with the trivalent vaccines. Minimal levels of anti-S. sonnei LPS serum antibodies could be detected in guinea pigs immunized with other monovalent or trivalent SWC vaccine formulations.
Control guinea pigs immunized with saline and challenged with S. flexneri 2a or 3a or S. sonnei were ≥88% Sereny positive 5 days postchallenge, with group mean disease scores of ≥2.6 (Table 3). Similarly, animals immunized with the monovalent SWC vaccines and challenged with a heterologous Shigella serotype were 100% Sereny positive on day 5, with a mean disease score of ≥2.9, demonstrating a lack of heterologous protection. In contrast, guinea pigs immunized with monovalent vaccines at a dose of 10E10 cells were significantly protected against serotype-homologous challenges with S. flexneri 3a (P = 0.0053), S. sonnei (P < 0.0001), and S. flexneri 2a (P = 0.0002). Guinea pigs immunized with the trivalent SWC vaccine (10E10-cell dose for all SWC) and challenged with S. flexneri 2a (P = 0.0186) or S. sonnei (P < 0.0001) were also significantly protected at levels comparable to those of their counterparts immunized with the monovalent SWC vaccine.
TABLE 3.
Results of ocular challenge after intranasal immunization of guinea pigs with trivalent Sfl2aWC, Sfl3aWC, and SsWC vaccines
| Challenge and treatment strains (SWC dose [no. of cells]) | Day 3 data |
Day 5 data |
||||||
|---|---|---|---|---|---|---|---|---|
| No. positive/totala | % Protectionb | P valuec | Mean disease scored | No. positive/total | % Protection | P value | Mean disease score | |
| S. flexneri 3a J17B (2.7 × 10E8 CFU/eye) | ||||||||
| Sfl2a (10E10) + Ss (10E10) + Sfl3a (10E8) | 12/12 | 0 | NSe | 2.5 | 12/12 | 0 | NS | 2.7 |
| Sfl2a (10E10) + Ss (10E10) + Sfl3a (10E9) | 5/12 | 58 | 0.0053 | 1.3f | 7/12 | 42 | 0.0396 | 1.5 |
| Sfl2a (10E10) + Ss (10E10) + Sfl3a (10E10) | 7/12 | 42 | 0.0325 | 1.6 | 8/12 | 33 | NS | 1.7 |
| Sfl3a (10E8) | 2/12 | 83 | 0.0001 | 0.8g | 3/12 | 75 | 0.0005 | 0.9g |
| Sfl3a (10E9) | 0/12 | 100 | <0.0001 | 0.5g | 3/12 | 75 | 0.0005 | 0.9g |
| Sfl3a (10E10) | 4/12 | 67 | 0.0017 | 0.8g | 5/12 | 58 | 0.0053 | 1.0g |
| Saline | 10/10 | 3.0 | 10/10 | 3.0 | ||||
| Sfl2a (10E10) | 10/10 | 0 | NS | 2.9 | 10/10 | 0 | NS | 3.0 |
| Ss (10E10) | 10/10 | 0 | NS | 2.9 | 10/10 | 0 | NS | 2.9 |
| Sfl2a (10E10) + Ss (10E10) + Sfl3a (10E10) | 0/12 | 100 | 0.0015 | 0.2g | 0/12 | 100 | <0.0001 | 0.4g |
| S. sonnei 53G (2.3 × 10E8 CFU/eye) | ||||||||
| Ss (10E10) | 0/12 | 100 | 0.0015 | 0.0g | 0/12 | 100 | <0.0001 | 0.0g |
| Saline | 6/9 | 2.3 | 8/9 | 2.6 | ||||
| Sfl3a (10E10) | 10/10 | 0 | NS | 2.4 | 10/10 | 0 | NS | 2.4 |
| Sfl2a (10E10 | 9/10 | 0 | NS | 2.7 | 9/10 | 1 | NS | 2.6 |
| S. flexneri 2a 2457T (2.5 × 10E8 CFU/eye) | ||||||||
| Sfl2a (10E10) + Ss (10E10) + Sfl3a (10E10) | 4/12 | 67 | 0.0046 | 1.2f | 6/12 | 50 | 0.0186 | 1.3f |
| Sfl2a (10E10) | 0/12 | 100 | <0.0001 | 0.3g | 2/12 | 83 | 0.0002 | 0.5g |
| Saline | 9/9 | 2.9 | 9/9 | 2.9 | ||||
| Sfl3a (10E10) | 10/10 | 0 | NS | 3.0 | 10/10 | 0 | NS | 3.0 |
| Ss (10E10) | 10/10 | 0 | NS | 3.0 | 10/10 | 0 | NS | 3.0 |
Animals were intraocularly challenged on day 28 and observed for 5 days postinfection, and each eye was scored daily. A positive response was a score of ≥2.0 according to Hartman et al. (22).
% Protection = [% Positive Control − % Positive Vaccinees)/% Positive Control] × 100.
Comparisons with animals inoculated with saline were done with Fisher's exact test.
Results were obtained with the Kruskal-Wallis test with Dunn posttest setting alpha at 0.05.
NS, not statistically significant.
P = 0.01.
P <0.0001.
Interestingly, guinea pigs immunized with the monovalent Sfl3aWC vaccine at a dose of 10E8, 10E9, or 10E10 cells were significantly protected from disease when challenged with S. flexneri 3a (P ≤ 0.0053) whereas guinea pigs immunized with trivalent vaccine containing 10E8 (P = 1.0) or 10E10 (P = 0.0867) Sfl3aWC were not protected. Immunization with the trivalent vaccine containing 10E9 Sfl3aWC induced a significant level of protection against S. flexneri 3a (P = 0.0396), albeit at less robust levels than those achieved by immunization with monovalent Sfl3aWC vaccines.
Intranasal immunization with trivalent SWC induces Shigella-specific ocular IgA that correlates with protection against keratoconjunctivitis after an ocular Shigella challenge.
Ocular wash samples were collected from individual guinea pigs on day 0, prior to immunization and after the last immunization (day 28) and assayed by ELISA for endpoint titers of antibodies directed to LPS purified from the three Shigella serotypes used for immunization (Fig. 8). Ocular wash samples collected on day 0 from all of the groups had undetectable levels of LPS-specific IgA (data not shown), and minimal ocular antibodies to heterologous LPS (geometric mean titers of <16) were induced. Immunization with monovalent Sfl2aWC (10E10) elicited S. flexneri 2a LPS-specific ocular IgA at levels comparable to those produced by immunization with trivalent SWC vaccines delivered with 10E9 and 10E10 Sfl3aWC but significantly higher (P < 0.01) than the endpoint titers induced after immunization with the trivalent vaccine formulation containing 10E8 Sfl3aWC. Similarly, monovalent Sfl3aWC vaccines (10E9 and 10E10 cells) elicited S. flexneri 3a LPS-specific ocular IgA levels comparable to those produced by trivalent vaccine formulations containing 10E9 and 10E10 Sfl3aWC, but significantly lower S. flexneri 3a LPS-specific endpoint titers were achieved after immunization with trivalent SWC vaccine (10E8 cells) than after immunization with monovalent Sfl3aWC vaccine (10E8 cells). There was no significant difference in the titers elicited by the monovalent SsWC vaccine and the trivalent SWC vaccine.
FIG 8.
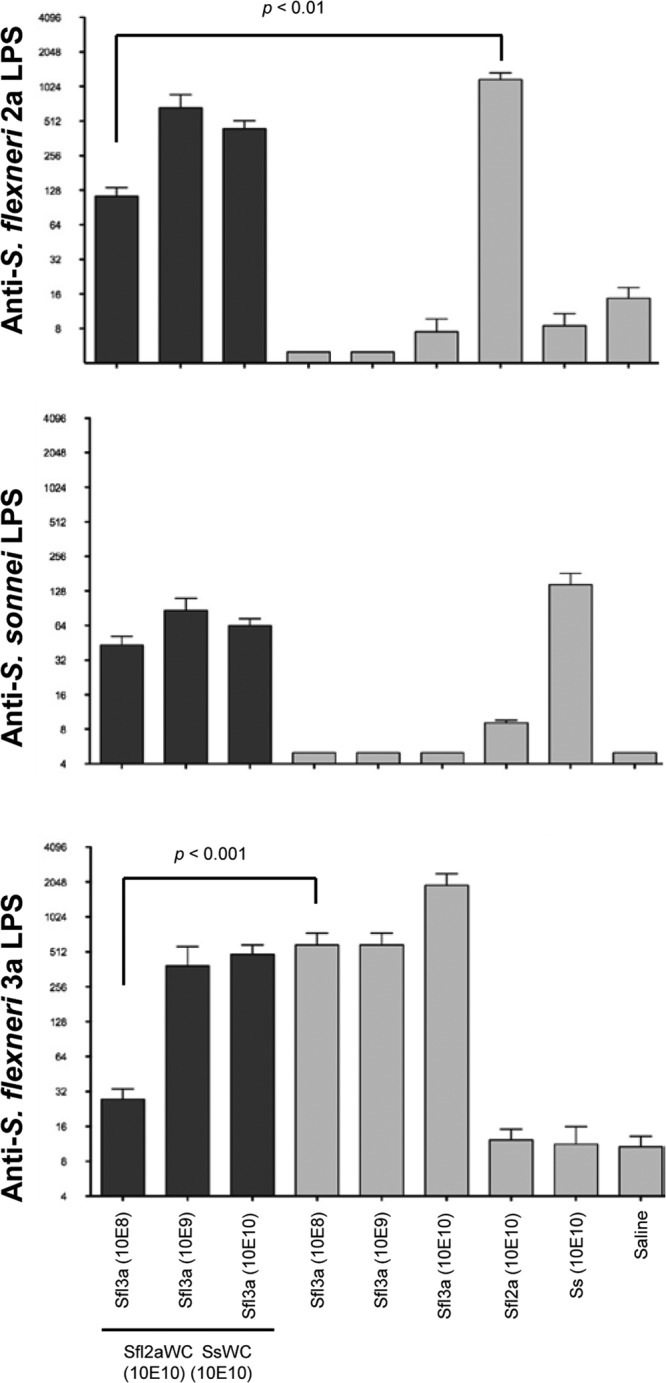
Shigella LPS-specific ocular IgA endpoint titers on day 21 after the intranasal immunization of guinea pigs with monovalent or trivalent vaccine formulations. Ocular wash samples were collected on day 21 (1 week after the third vaccination) and assayed by ELISA for S. flexneri 2a LPS (top panel), S. sonnei LPS (middle panels), and S. flexneri 3a LPS (bottom panel) IgA endpoint titers. Comparisons between groups were accomplished by two-way ANOVA of log-transformed titers.
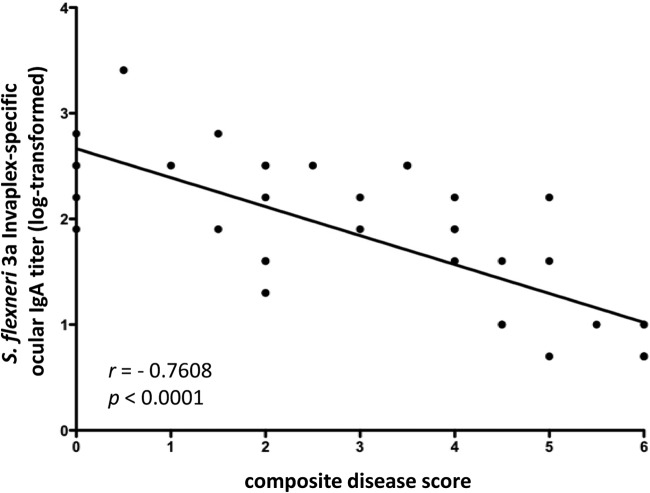
A correlation analysis of ocular IgA endpoint titers of samples collected on day 21 and disease scores was performed (Table 4 and Fig. 9) to determine if the local production of ocular IgA induced after intranasal immunization with SWC was associated with protection against keratoconjunctivitis after a challenge in the guinea pig model. Ocular endpoint titers of IgA directed to Invaplex or LPS homologous to the challenge organism were inversely correlated with disease scores postchallenge. Importantly, a significant correlation with the immune responses directed to LPS and Invaplex from all three Shigella serotypes was seen. The correlations (r values) between disease scores and ocular IgA levels ranged from −0.60 to −0.81 with associated P values of 0.01 to <0.0001. The lower levels of correlation with S. sonnei LPS-specific ocular IgA and disease after a challenge with S. sonnei are consistent with the lower LPS-specific ocular IgA endpoint titers induced after immunization.
TABLE 4.
Correlation of Shigella-specific ocular IgA levels with protection from keratoconjunctivitis in guinea pigs immunized intranasally with SWC vaccinesa
| Challenge strain (no. of guinea pigs) and ocular IgA titer specificity | Spearman r value | P value (two tailed) |
|---|---|---|
| S. flexneri 2a 2457T (16) | ||
| S. flexneri 2a LPS | −0.7866 | 0.0003 |
| S. flexneri 2a Invaplex | −0.8145 | 0.0001 |
| S. sonnei 53G (16) | ||
| S. sonnei LPS | −0.7489 | 0.0008 |
| S. sonnei Invaplex | −0.6012 | 0.0138 |
| S. flexneri 3a J17B (40) | ||
| S. flexneri 3a LPS | −0.7678 | <0.0001 |
| S. flexneri 3a Invaplex | −0.7608 | <0.0001 |
Composite disease scores were calculated as the mean disease scores on day 5 postinfection in the two eyes of SWC vaccinated animals. For saline-inoculated guinea pigs, the disease score in the single challenged eye was multiplied by a factor of two. Composite disease scores and matched antigen-specific ocular IgA titers (day 21) were evaluated for correlations by Spearman's rank order correlation coefficient (r) analysis.
FIG 9.
Shigella-specific ocular IgA is correlated with protection against keratoconjunctivitis in guinea pigs intranasally immunized with SWC vaccines. Guinea pigs were immunized on days 0, 7, and 14 with monovalent or trivalent SWC vaccines. Ocular wash samples collected on day 21 were assayed by ELISA for LPS-specific and Invaplex-specific IgA homologous to the serotype used for a challenge. Guinea pigs were challenged on day 28 with S. flexneri 2a 2457T, S. sonnei 53G, or S. flexneri 3a J17B and monitored for disease for 5 days postinfection. For correlation analysis, LPS-specific ocular IgA endpoint titers (day 21) and composite disease scores on day 5 postinfection were graphed with a linear best-fit line. Spearman's rank order correlation coefficient (r) and P values are indicated for log-transformed data. Representative data from guinea pigs immunized with Sfl3aWC (n = 40) are shown. Similar analyses were accomplished for guinea pigs immunized with SsWC (n = 16) and Sfl2aWC (n = 16).
DISCUSSION
In the absence of clear correlates of immunity, most Shigella vaccine development efforts have been focused on mimicking previously identified antigen-specific immune responses induced after natural infection. This strategy, for the most part, is focused on the O-specific polysaccharide of LPS and several conserved antigens of the type 3 secretion system (IpaB, IpaC, IpaD). Even so, there may be other antigens, such as OmpA (27), not clearly defined at this time and therefore not included in the vaccine development process but whose omission possibly handicaps efforts to create an effective vaccine. Immunization with whole-cell vaccines (live or inactivated) largely overcomes this potential pitfall by delivering a full complement of known and unknown antigens. Vaccine development pathways consider not only research product successes but also theoretical considerations such as extrapolation from successes or failures of other vaccines. Many practical issues, such as the potential for successful mass deployment in developing nations, low cost, and compatibility with existing vaccines and immunization routes, are also part of the product development algorithm. Cost reduction is often a result of simplified manufacturing processes and successful transfer of the processes to developing countries. This was accomplished with the Shanchol cholera vaccine ultimately manufactured in Vietnam after the development of a simplified manufacturing scheme that lowered the cost (3, 5). The potential low cost, simplicity, and oral administration of inactivated whole-cell vaccines are consistent with guidelines facilitating deployment in developing countries.
Several different methods have been used to inactivate virulent Shigella cultures for vaccine formulation that include the use of heat (28, 29), acetone (30), or formalin (16, 17). Formalin treatment of bacterial cultures results in the cross-linking of protein antigens and successful inactivation. Several parameters, such as the formalin concentration, bacterial density, and incubation time and temperature, must be considered to balance complete inactivation and antigen preservation. With both S. flexneri 2a and 3a whole-cell vaccines, inactivation with 0.2% formalin for 48 h at 25°C resulted in complete inactivation and retention of activity with a battery of MAbs directed to protein and LPS antigens. Similar results were achieved with S. sonnei when the formalin concentration was increased to 0.6%. The requirement of a higher formalin concentration to inactivate S. sonnei suggests that surface structures such as LPS may alter the effectiveness of formalin. Few studies have evaluated the effect of formalin on the Ipa proteins. Recently, Camacho et al. (31) indicated that IpaB and IpaC maintained MAb reactivity after treatment with 0.06% formalin and binary ethyleneimine.
Previous clinical evaluation of an SWC vaccine demonstrated intestinal and serum responses directed to LPS and IpaC after multiple oral administrations (17). Studies with oral whole-cell cholera vaccines also indicate that multiple doses are required for optimal immunization (5, 32). The requirement for multiple oral doses of an inactivated vaccine suggests that antigen presentation to the mucosal immune system may benefit from the inclusion of a potent mucosal adjuvant such as dmLT. This has recently been shown specifically for dmLT with a new prototype inactivated whole-cell ETEC vaccine (33). In the present work, vaccine formulations containing dmLT showed minimal improvement or in some cases a decrease of the magnitude of the immune response or in protective efficacy in both the mouse and guinea pig models. Furthermore, oral immunization of mice and rats with SWC combined with dmLT also did not demonstrate enhanced immunogenicity compared to immunization with SWC alone (data not shown). Similar results were previously obtained with guinea pigs when LTR192G (mutant heat-labile toxin) was used as an adjuvant for an orally administered, formalin-inactivated S. sonnei whole-cell vaccine (17). Addition of LTR192G (25 μg) to the whole-cell vaccine did not increase the anti-LPS serum IgG responses before a challenge, nor did it increase the protective efficacy of the vaccine against keratoconjunctivitis. Delivery of the whole-cell vaccine intranasally with LTR192G also did not demonstrate a higher level of efficacy. In contrast, LTR192G was evaluated as an adjuvant with the S. flexneri 2a-Escherichia coli hybrid vaccine strain EcSf2a-3 and heat-killed S. flexneri 2a 2457T (34). Increases in protective efficacy against an ocular challenge were achieved when LTR192G was administered either orally or intranasally with EcSf2a-3, but there was no increase in the serum IgG response directed to LPS. Similar efficacy results were achieved with heat-killed S. flexneri, 2457T delivered orally with LTR192G but not when the vaccine was administered intranasally. These observations combined with results achieved in other laboratories suggest there may be an optimal dose of the LT mutants, dependent on the model and an antigen capable of providing an adjuvant effect (John Clements, personal communication). The studies presented here only investigated a high dose of dmLT in the mouse (5 μg) and guinea pig (10 μg) models. Alternatively, like the Shanchol cholera vaccine, the SWC vaccine may be sufficiently immunogenic without an adjuvant.
In the present study, intranasal immunization with dmLT alone nonspecifically protected mice (64% protection, P = 0.0002) from death after a challenge with S. flexneri 2a (Table 1), although many of the surviving mice exhibited a significant weight loss after the challenge. Similar levels of nonspecific protection were achieved in other studies when the challenge was extended to 3 and 4 weeks after the final immunization and lower doses of dmLT were used (R. W. Kaminski and E. V. Oaks, unpublished results). Others have indicated a lack of nonspecific protection with dmLT in mice, but weight loss was used as a surrogate for death (35). The immunological mechanism responsible for dmLT-induced protection is unknown, but it may be due to stimulation of the innate immune system and a non-antigen-specific response. None of the dmLT-immunized mice produced antibodies to Shigella antigens by ELISA. Regardless, caution may be justified when interpreting protection results derived from studies evaluating dmLT or similar toxin-based adjuvants in the Shigella lethal pulmonary mouse model. The mouse model is an acceptable tool for the preliminary evaluation of Shigella vaccine candidates, but it is unclear if this model is predictive of outcomes in larger animals or humans.
To date, protection against multiple Shigella serotypes with most vaccine approaches has required the use of a multivalent vaccine to achieve broad protection across several serotypes. Bivalent vaccines against S. flexneri 2a and S. sonnei have been successfully developed (36). It has been proposed that a vaccine consisting of S. flexneri 2a, 3a, and 6 would provide broad coverage against the S. flexneri group (12) based on common type antigens. The addition of S. sonnei would improve coverage to the large majority of prevalent Shigella serotypes found worldwide. The inclusion of a broad repertoire of antigens in SWC vaccines, although many are uncharacterized, provides an opportunity to elicit an immune response that possesses activity across multiple Shigella serotypes. In the present work with a monovalent vaccine approach, protection against a challenge with heterologous Shigella serotypes was not observed. This is consistent with numerous other studies with guinea pigs, monkeys, and humans (29, 37, 38). Rather, protection in the guinea pig Sereny model required the use of a multivalent vaccine to induce protective, serotype-specific immune responses, confirming previous observations (12). In our studies, however, immune responses directed to conserved antigens, such as the IpaB, IpaC, and IpaD proteins, were low to undetectable in guinea pigs. The possibility remains that robust immune responses directed to key conserved antigens, such as the Ipa proteins, may provide a broad-based protective immune response (35, 39) even though humans and monkeys with antibodies to the Ipa proteins are susceptible to reinfection (15, 37).
Our results clearly show that a trivalent Shigella vaccine is effective in a robust challenge model. However, it is also noted that immunogenicity and efficacy were lower in the trivalent formulation groups than in the monovalent formulation groups. This was particularly evident with S. flexneri 3a and the immune response to S. flexneri 3a LPS in trivalent-vaccine-immunized guinea pigs. One of the major challenges in the development of multivalent vaccines is ensuring that the immunogenicity elicited is comparable to the response induced by the protective monovalent formulation. These issues have arisen during the development of live-attenuated (40) and formalin-inactivated polio vaccines (41) and more recently with dengue vaccines (42). For example, seroconversion rates that exceeded 90% for monovalent polio vaccines were reduced to 68% for type 1, 82% for type 2, and 43% for type 3 in trivalent formulations (40). The generation of potent antibody responses after immunization with multivalent vaccines has been problematic because of immunological, viral, or bacterial interference between different strains or serotypes. A significant hurdle with Shigella vaccines that rely on LPS antigens for immunogenicity and protection is the diversity of O antigens expressed by differing serotypes. Previous studies have shown that the addition of two LPS serotypes in a bivalent formulation did not result in competition or interference (36, 40, 43). In the studies presented here, immunization with a combination of three SWC vaccines resulted in lower LPS-specific immunogenicity than the immune responses induced with the monovalent counterpart that resulted in reduced efficacy. Therefore, significant efforts are required to ensure that multivalent Shigella vaccines are properly formulated to achieve optimal immune responses to each component.
The optimal manufacture of inactivated whole-cell vaccines should yield a product that has been fully inactivated while maintaining key antigenic components to facilitate appropriate immunogenicity. Growth conditions to enhance antigen expression, such as growth in the presence of bile salts, are used to increase the expression of Ipa proteins prior to the inactivation of whole cells with formalin (17). Although deoxycholate did not appear to enhance Ipa expression in the study by McKenzie et al., S. sonnei whole-cell preparations induced significantly higher anti-IpaC serum IgA titers after oral vaccination than prevaccination did in a phase 1 trial (17). The SsWC used by McKenzie et al. was inactivated with 1% formaldehyde for 72 h at room temperature. In the present study, a lower level of formalin (0.2 or 0.6%) and a shorter incubation time (48 h) were found to be sufficient for complete inactivation of the bacteria. Increases in the temperature, formalin concentration, or incubation time resulted in a loss of the antigenicity of IpaB, IpaC, and IpaD in a Western blot analysis. Despite these efforts, Ipa-specific immune responses in guinea pigs immunized with SsWC, Sfl2aWC, or Sfl3aWC were low to undetectable. Responses to IpaB and IpaC were present in mice. Responses in humans are under evaluation in an ongoing phase 1 trial with the Sfl2aWC vaccine manufactured under conditions described in this work (Clayton Harro, personal communication). Although, not evaluated in this study, the low-titer response of guinea pigs to IpaB, IpaC, and IpaD suggests that the proteins were not properly presented to the mucosal immune system. This may have been a function of unstable linkages (fixation) to the whole cells. Future analysis should include the evaluation of free versus bound Ipa proteins and LPS as part of a comprehensive vaccine stability program.
Immune correlates of vaccine efficacy against diarrhea and severe shigellosis have been associated with LPS-specific IgA (44). Recent studies suggest that IpaB and IpaD might be involved in protective immunity (35), but no true correlation has been identified for any of the Ipa proteins in humans. Even so, the involvement of the Ipa proteins in the invasion event suggests that they are attractive targets for protective immunity. Other studies have shown that after oral immunization with a live-attenuated S. flexneri 2a vaccine strain, SC602, human volunteers with the highest levels of LPS-specific IgA antibody-secreting cells were protected. The protection afforded after oral immunization with SC602 suggests that local production of LPS-specific IgA may play a role in vaccine efficacy in the human model. Intranasal immunization of guinea pigs with SWC vaccines generated ocular IgA specific for LPS and Invaplex antigens from the same Shigella serotype used for vaccination. Protection afforded by the SWC vaccines correlated well with IgA titers in ocular wash samples collected 1 week prior to a challenge. Similar to protection in the human challenge model, local production of mucosal IgA in ocular wash samples correlated with protection from disease in the guinea pig model. The correlation between ocular IgA levels and protection in the Sereny model has been extended to animals immunized with other Shigella vaccines such as Invaplex and live-attenuated vaccines (Kaminski and Oaks, unpublished) and therefore may provide a useful tool for Shigella vaccine development. It is possible that measurement of the mucosal response in the human intestinal tract will also correlate with protection.
The results of these studies highlight the importance of optimizing the dose of each serotype used in a multivalent vaccine to ensure optimal immunogenicity and protective efficacy. Serum IgG responses directed to S. flexneri 2a and 3a LPS were significantly higher in groups administered a monovalent SWC vaccine compared to groups immunized with a trivalent vaccine. Additionally, ocular IgA titers were significantly higher in groups administered a monovalent SWC vaccine compared to a multivalent vaccine. Consequently, protection against disease was lower in groups immunized with a multivalent vaccine than in groups immunized with a monovalent vaccine, with 28/60 guinea pigs being Sereny positive on day 3 postinfection in groups immunized with multivalent vaccine compared to 6/60 guinea pigs Sereny positive administered a monovalent vaccine and challenged with a homologous Shigella serotype. It is therefore essential to evaluate multivalent vaccines and adjust the dose of each component to ensure optimal immunogenicity and efficacy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by PATH through a Cooperative Research and Development Agreement (W81WWH:10-0121) with the Walter Reed Army Institute of Research.
We are grateful to K. Chen, J. Bosiacki, N. Bryant, M. Marll, and S. Rasamalle for providing excellent technical assistance.
The views expressed in this article are ours and do not necessarily reflect the official policy or position of the Department of the Army, the Department of Defense, or the U.S. Government. The mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government.
Footnotes
Published ahead of print 8 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00683-13.
REFERENCES
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382(9888):209–222. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 77:651–666 [PMC free article] [PubMed] [Google Scholar]
- 3.Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Han SH, Attridge S, Donner A, Ganguly NK, Bhattacharya SK, Nair GB, Clemens JD, Lopez AL. 2011. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl. Trop. Dis. 5:e1289. 10.1371/journal.pntd.0001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Donner A, Ganguly NK, Nair GB, Bhattacharya SK, Clemens JD. 2009. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 374:1694–1702. 10.1016/S0140-6736(09)61297-6 [DOI] [PubMed] [Google Scholar]
- 5.Saha A, Chowdhury MI, Khanam F, Bhuiyan MS, Chowdhury F, Khan AI, Khan IA, Clemens J, Ali M, Cravioto A, Qadri F. 2011. Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine 29:8285–8292. 10.1016/j.vaccine.2011.08.108 [DOI] [PubMed] [Google Scholar]
- 6.Barry EM, Pasetti MF, Sztein MB, Fasano A, Kotloff KL, Levine MM. 2013. Progress and pitfalls in Shigella vaccine research. Nat. Rev. Gastroenterol. Hepatol. 10:245–255. 10.1038/nrgastro.2013.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camacho AI, Irache JM, Gamazo C. 2013. Recent progress towards development of a Shigella vaccine. Expert Rev. Vaccines 12:43–55. 10.1586/erv.12.135 [DOI] [PubMed] [Google Scholar]
- 8.Kaminski RW, Oaks EV. 2009. Inactivated and subunit vaccines to prevent shigellosis. Expert Rev. Vaccines 8:1693–1704. 10.1586/erv.09.127 [DOI] [PubMed] [Google Scholar]
- 9.Venkatesan MM, Ranallo RT. 2006. Live-attenuated Shigella vaccines. Expert Rev. Vaccines 5:669–686. 10.1586/14760584.5.5.669 [DOI] [PubMed] [Google Scholar]
- 10.Walker RI. 2005. Considerations for development of whole cell bacterial vaccines to prevent diarrheal diseases in children in developing countries. Vaccine 23:3369–3385. 10.1016/j.vaccine.2004.12.029 [DOI] [PubMed] [Google Scholar]
- 11.Robbins JB, Chu C, Schneerson R. 1992. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. Clin. Infect. Dis. 15:346–361. 10.1093/clinids/15.2.346 [DOI] [PubMed] [Google Scholar]
- 12.Noriega FR, Liao FM, Maneval DR, Ren S, Formal SB, Levine MM. 1999. Strategy for cross-protection among Shigella flexneri serotypes. Infect. Immun. 67:782–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills JA, Buysse JM, Oaks EV. 1988. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect. Immun. 56:2933–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oaks EV, Hale TL, Formal SB. 1986. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect. Immun. 53:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oberhelman RA, Kopecko DJ, Salazar-Lindo E, Gotuzzo E, Buysse JM, Venkatesan MM, Yi A, Fernandez-Prada C, Guzman M, Leon-Barua R, Sack RB. 1991. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect. Immun. 59:2341–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osorio M, Bray MD, Walker RI. 2007. Vaccine potential for inactivated shigellae. Vaccine 25:1581–1592. 10.1016/j.vaccine.2006.11.012 [DOI] [PubMed] [Google Scholar]
- 17.McKenzie R, Walker RI, Nabors GS, Van De Verg LL, Carpenter C, Gomes G, Forbes E, Tian JH, Yang HH, Pace JL, Jackson WJ, Bourgeois AL. 2006. Safety and immunogenicity of an oral, inactivated, whole-cell vaccine for Shigella sonnei: preclinical studies and a phase I trial. Vaccine 24:3735–3745. 10.1016/j.vaccine.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 18.Turbyfill KR, Mertz JA, Mallett CP, Oaks EV. 1998. Identification of epitope and surface-exposed domains of Shigella flexneri invasion plasmid antigen D (IpaD). Infect. Immun. 66:1999–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turbyfill KR, Hartman AB, Oaks EV. 2000. Isolation and characterization of a Shigella flexneri invasin complex subunit vaccine. Infect. Immun. 68:6624–6632. 10.1128/IAI.68.12.6624-6632.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norton EB, Lawson LB, Freytag LC, Clements JD. 2011. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 18:546–551. 10.1128/CVI.00538-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallett CP, VanDeVerg L, Collins HH, Hale TL. 1993. Evaluation of Shigella vaccine safety and efficacy in an intranasally challenged mouse model. Vaccine 11:190–196. 10.1016/0264-410X(93)90016-Q [DOI] [PubMed] [Google Scholar]
- 22.Hartman AB, Powell CJ, Schultz CL, Oaks EV, Eckels KH. 1991. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect. Immun. 59:4075–4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaminski RW, Turbyfill KR, Oaks EV. 2006. Mucosal adjuvant properties of the Shigella invasin complex. Infect. Immun. 74:2856–2866. 10.1128/IAI.74.5.2856-2866.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westphal O, Jann K. 1965. Bacterial lipopolysaccharides. Extraction with phenolwater and further applications of the procedure, p 83–91 In Whistler RL, Wolfan ML. (ed), Methods in carbohydrate chemistry. Academic Press, New York, NY [Google Scholar]
- 25.Picking WL, Mertz JA, Marquart ME, Picking WD. 1996. Cloning, expression, and affinity purification of recombinant Shigella flexneri invasion plasmid antigens IpaB and IpaC. Protein Expr. Purif. 8:401–408. 10.1006/prep.1996.0117 [DOI] [PubMed] [Google Scholar]
- 26.Picking WL, Nishioka H, Hearn PD, Baxter MA, Harrington AT, Blocker A, Picking WD. 2005. IpaD of Shigella flexneri is independently required for regulation of Ipa protein secretion and efficient insertion of IpaB and IpaC into host membranes. Infect. Immun. 73:1432–1440. 10.1128/IAI.73.3.1432-1440.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pore D, Mahata N, Pal A, Chakrabarti MK. 2011. Outer membrane protein A (OmpA) of Shigella flexneri 2a, induces protective immune response in a mouse model. PLoS One 6:e22663. 10.1371/journal.pone.0022663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakrabarti MK, Bhattacharya J, Bhattacharya MK, Nair GB, Bhattacharya SK, Mahalanabis D. 1999. Killed oral Shigella vaccine made from Shigella flexneri 2a protects against challenge in the rabbit model of shigellosis. Acta Paediatr. 88:161–165. 10.1111/j.1651-2227.1999.tb01075.x [DOI] [PubMed] [Google Scholar]
- 29.Mukhopadhaya A, Mahalanabis D, Khanam J, Chakrabarti MK. 2003. Protective efficacy of oral immunization with heat-killed Shigella flexneri 2a in animal model: study of cross protection, immune response and antigenic recognition. Vaccine 21:3043–3050. 10.1016/S0264-410X(03)00111-7 [DOI] [PubMed] [Google Scholar]
- 30.Formal SB, Maenza RM, Austin S, LaBrec EH. 1967. Failure of parenteral vaccines to protect monkeys against experimental shigellosis. Proc. Soc. Exp. Biol. Med. 125:347–349. 10.3181/00379727-125-32087 [DOI] [PubMed] [Google Scholar]
- 31.Camacho GI, Souza-Reboucas G, Irache GM, Gamazo G. 2013. Towards a non-living vaccine against Shigella flexneri: from the inactivation procedure to protection studies. Methods 60:264–268. 10.1016/j.ymeth.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 32.Svennerholm AM. 2011. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J. Med. Res. 133:188–196 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3089050/ [PMC free article] [PubMed] [Google Scholar]
- 33.Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, Nygren E, Tobias J, Walker R, Svennerholm AM. 2013. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 31:2457–2464. 10.1016/j.vaccine.2013.03.027 [DOI] [PubMed] [Google Scholar]
- 34.Hartman AB, Van De Verg LL, Venkatesan MM. 1999. Native and mutant forms of cholera toxin and heat-labile enterotoxin effectively enhance protective efficacy of live attenuated and heat-killed Shigella vaccines. Infect. Immun. 67:5841–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, Clements JD, Pasetti MF, Picking WL. 2012. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect. Immun. 80:1222–1231. 10.1128/IAI.06174-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oaks EV, Turbyfill KR. 2006. Development and evaluation of a Shigella flexneri 2a and S. sonnei bivalent invasin complex (Invaplex) vaccine. Vaccine 24:2290–2301. 10.1016/j.vaccine.2005.11.040 [DOI] [PubMed] [Google Scholar]
- 37.Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. 1991. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 164:533–537. 10.1093/infdis/164.3.533 [DOI] [PubMed] [Google Scholar]
- 38.Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, Levine MM. 1991. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am. J. Epidemiol. 134:614–627 [DOI] [PubMed] [Google Scholar]
- 39.Szijártó V, Hunyadi-Gulyas E, Emody L, Pal T, Nagy G. 2013. Cross-protection provided by live Shigella mutants lacking major antigens. Int. J. Med. Microbiol. 303:167–175. 10.1016/j.ijmm.2013.02.017 [DOI] [PubMed] [Google Scholar]
- 40.Payne AM. 1960. Oral immunization against poliomyelitis. Bull. World Health Organ. 23:695–703 [PMC free article] [PubMed] [Google Scholar]
- 41.Katz SL. 2006. Polio—new challenges in 2006. J. Clin. Virol. 36:163–165. 10.1016/j.jcv.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 42.Anderson KB, Gibbons RV, Edelman R, Eckels KH, Putnak RJ, Innis BL, Sun W. 2011. Interference and facilitation between dengue serotypes in a tetravalent live dengue virus vaccine candidate. J. Infect. Dis. 204:442–450. 10.1093/infdis/jir279 [DOI] [PubMed] [Google Scholar]
- 43.Klee SR, Tzschaschel BD, Singh M, Falt I, Lindberg AA, Timmis KN, Guzman CA. 1997. Construction and characterization of genetically-marked bivalent anti-Shigella dysenteriae 1 and anti-Shigella flexneri Y live vaccine candidates. Microb. Pathog. 22:363–376. 10.1006/mpat.1996.0127 [DOI] [PubMed] [Google Scholar]
- 44.Coster TS, Hoge CW, VanDeVerg LL, Hartman AB, Oaks EV, Venkatesan MM, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti PJ, Hale TL. 1999. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect. Immun. 67:3437–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.