Abstract
Leptospirosis is a reemerging infectious disease that is underdiagnosed and under-recognized due to low-sensitivity and cumbersome serological tests. Rapid reliable alternative tests are needed for early diagnosis of the disease. Considering the importance of the pathogenesis-associated leptospiral LigA protein expressed in vivo, we have evaluated its application in the diagnosis of the acute form of leptospirosis. The C-terminal coding sequence of ligA (ligA-C) was cloned into pET15b and expressed in Escherichia coli. Furthermore, the B-cell-specific epitopes were predicted and were synthesized as peptides for evaluation along with recombinant LigA-C. Epitope 1 (VVIENTPGK), with a VaxiJen score of 1.3782, and epitope 2 (TALSVGSSK), with a score of 1.2767, were utilized. A total of 140 serum samples collected from leptospirosis cases during the acute stage of the disease and 138 serum samples collected from normal healthy controls were utilized for evaluation. The sensitivity, specificity, positive predictive value, and negative predictive value were calculated for the recombinant LigA-C-specific IgM enzyme-linked immunosorbent assay (ELISA) and were found to be 92.1%, 97.7%, 92.8%, and 97.5%, respectively. Epitopes 1 and 2 used in the study showed 5.1 to 5.8% increased sensitivity over recombinant LigA-C in single and combination assays for IgM antibody detection. These findings suggest that these peptides may be potential candidates for the early diagnosis of leptospirosis.
INTRODUCTION
Leptospirosis is a worldwide zoonotic disease affecting humans in rural and urban settings and in industrialized and developing countries. It is also an important public health problem in many parts of India and causes epidemics, especially after natural disasters such as floods and heavy monsoons (1). It is caused by spirochetes of the genus Leptospira, a group of bacteria that are morphologically, physiologically, metabolically, antigenically, and genetically distinct from other microorganisms. Clinical manifestations of acute leptospirosis range from mild febrile illness to more severe icteric Weil's disease, which is characterized by renal and liver failure. Globally, 300,000 to 500,000 humans are affected by leptospirosis annually, according to International Leptospirosis Society global data collection (2). Leptospirosis is underdiagnosed for many reasons, including difficulty in distinguishing clinical signs from those of other endemic diseases and a lack of appropriate diagnostic laboratory services (3). Misdiagnosis of leptospirosis leads to severe complications including kidney damage, liver failure, respiratory distress, and meningitis leading to death.
The diagnosis of leptospirosis by the microscopic agglutination test (MAT) and Leptospira culture can be performed only by reference laboratories and requires specially trained personnel to interpret the results. Moreover, the MAT requires paired serum samples to achieve sufficient sensitivity. The sensitivity of other rapid and less complicated serological techniques, such as the Lepto dipstick assay, the Lepto Dri Dot test, and immunofluorescence assays, is insignificant, especially during the early phase of the disease (4). Most of these assays employ antigens from nonpathogenic Leptospira biflexa strain Patoc I and detection by cross-reactivity with repeating disaccharides of lipopolysaccharide (5). Molecular methods, including conventional and real-time PCR assays, were used to overcome the aforementioned diagnostic difficulties. However, the molecular methods are unaffordable due to the need for specialized equipment and expensive reagents that are not available during outbreak situations in the field and for routine diagnosis.
Recombinant antigen-based serological tests are being developed for spirochetal infections such as Lyme disease and syphilis (6, 7). Recently, recombinant proteins of Leptospira, including LipL32, LigA, LigA-C, and Hsp60, were widely characterized for their diagnostic and immunogenic potential (3, 8–11). Several studies have emphasized surface-exposed or secreted leptospiral proteins to induce better humoral immune responses in humans (12, 13). Such surface-exposed immunoreactive proteins can thus play a major role in the diagnosis of leptospirosis.
After leptospiral infection, the host produces antibodies to a myriad of bacterial antigens expressed constitutively or expressed in vivo. Antibodies to the latter antigens are the diagnostic targets of antibody-based assays for early diagnosis, especially during the acute phase of the illness. The antigens that are expressed in vivo interact with the immune system and prime an immune response to eliminate leptospires. Antibody responses peak during the acute phase of infection. In contrast, antibodies to whole-cell lysates and constitutively expressed proteins continue to be present at high titers. Similar results have been observed with the p35 and p37 proteins of Borrelia burgdorferi, which are expressed in vivo (14). Among various Leptospira antigens expressed in vivo, the immunoglobulin-like proteins LigA and LigB are found only on the surface of freshly or recently isolated pathogenic Leptospira species (9, 10). The free-living nonpathogenic Leptospira species strains do not have LigA, and pathogenic counterparts that have been maintained in long-term culture in Ellinghausen-McCullough-Johnson-Harris (EMJH) medium may lose the ability to express Lig proteins (9, 10, 15).
In this regard, testing based on such surface-expressed proteins provides an alternative method for the diagnosis of leptospirosis. Several attempts to standardize the serological tests for leptospirosis based on recombinant Lig proteins have been made (3, 16), but considerable variations in sensitivity and specificity have been obtained. To overcome this disadvantage, peptide-based enzyme-linked immunosorbent assays (ELISAs) would be ideal for the serodiagnosis of leptospirosis. The highly conserved peptides of immunogenic proteins provide the advantage of enhanced specificity and can be easily implemented in a simple, rapid, sensitive, relatively inexpensive diagnostic kit.
The purpose of the present study was to compare the performance of five different ELISAs for the serological diagnosis of leptospirosis. ELISAs based on recombinant LigA-C (rLigA-C), a whole-cell lysate of Leptospira interrogans serovar Australis, synthetic epitope 1, epitope 2, and a combination of epitopes 1 and 2 were evaluated. This study was performed with a panel of human serum samples collected from patients with clinical diagnoses of leptospirosis.
MATERIALS AND METHODS
Leptospira cultures and microscopic agglutination test.
A panel of 12 reference strains were used for the MAT, including the following serogroups: Australis (serovar Australis, strain Ballico), Autumnalis (serovar Autumnalis, strain Akiyami A), Ballum (serovar Ballum, strain Mus 127), Bataviae (serovar Bataviae, strain Swart), Canicola (serovar Canicola, strain Hond Utrecht IV), Icterohaemorrhagiae (serovar Icterohaemorrhagiae, strain RGA), Grippotyphosa (serovar Grippotyphosa, strain Moskva V), Hebdomadis (serovar Hebdomadis, strain Hebdomadis), Javanica (serovar Poi, strain Poi), Pomona (serovar Pomona, strain Pomona), Sejroe (serovar Hardjo, strain hardjoprajitno), and Pyrogenes (serovar Pyrogenes, strain Salinem). In addition to the reference strains, the clinical isolates and the nonpathogenic strain Patoc I of serovar Semaranga were maintained. Clinical isolates included serovar Canicola of serogroup Canicola, serovar Icterohaemorrhagiae of serogroup Icterohaemorrhagiae, and serovar Javanica of serogroup Javanica. Leptospiral reference strains and isolates were maintained in EMJH medium at the Medical Microbiology Laboratory, Bharathidasan University (Tiruchirappalli, India). The MAT was performed using 7-day-old cultures grown at 30°C in EMJH medium (17).
Patients, case definitions, and ethics.
In total, 140 serum samples with MAT titers of ≥1:160 (see Table S1 in the supplemental material) were selected from a bank of 476 laboratory-confirmed samples (positive IgM ELISA results, isolation of leptospires from the blood, seroconversion, or 4-fold titer increases) collected during the early phase of illness (0 to 10 days after the onset of disease) through an active hospital-based surveillance program in Tiruchirappalli. A total of 138 seronegative healthy controls selected from a group of cases matched with respect to age (±5 years) and sex and 775 patients with diseases other than leptospirosis were also included to study the efficiency of the ELISAs (Table 1). Patients' sera were analyzed from the following control groups: patients with typhoid (n = 107), patients with malaria (n = 96), patients with hepatitis (n = 110), patients with dengue (n = 86), and patients who were hospitalized with clinical suspicion of leptospirosis and subsequently were diagnosed as having another illness based on laboratory and radiological evidence (n = 376). Written informed consent was obtained from both case and control subjects before blood sampling. The study protocol was approved by the institutional ethics committee of Bharathidasan University (DM/2007/101/373/Project 2) as well as permitted by the Directorate of Health Services (ref. no. 5796/TV 1/07), Tamil Nadu. The serum samples obtained were stored at −80°C until use.
TABLE 1.
Case definitions and groupings of the patients included in the study
| Group | Description | No. of cases |
|---|---|---|
| a | Clinically suspected laboratory-confirmed leptospirosisa | 140 |
| b | Clinically suspected laboratory-negative leptospirosisb | 439 |
| c | Seronegative healthy controls | 138 |
| d | Typhoid | 107 |
| e | Malaria | 96 |
| f | Hepatitis | 110 |
| g | Dengue fever | 86 |
Confirmed cases of leptospirosis.
Clinically suspected but serologically negative.
Cloning and expression of LigA-C.
Primers ligA-C-F (5′-CCG CTC GAG ACA GAG CAA GTC ACC TGG A-3′) and ligA-C-R (5′-CGC GGA TCC TAT GGC TCC GTT TTA ATA GAG GC-3′), specific for the ligA C-terminal region (positions 708 to 1,225), were designed using Primer 2 software (Scientific and Educational Software). Following PCR amplification of chromosomal DNA from L. interrogans serovar Australis, strain Ballico, amplicons of 1.5 kb were digested with XhoI and BamHI and inserted into the pET15b plasmid (Novagen, Madison, WI), predigested with the same restriction endonucleases, to generate the plasmid pET15b-ligA-C. Successful cloning of the gene was confirmed by restriction endonuclease analysis, colony PCR testing, and sequencing.
Recombinant plasmids were transformed into Escherichia coli BL21(DE3) (Novagen, Madison, WI). Expression of His6-LigA-C was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) when cultures reached an optical density at 600 nm of 0.6, and cells were harvested after 2 to 3 h. Recombinant His-tagged proteins were isolated using Talon metal affinity resin (Clontech Laboratories, Inc.) in buffer containing 8 M urea, according to the manufacturer's recommendations. His6-LigA-C was dialyzed against 10 mM Tris buffer (pH 7.5) containing 50 mM NaCl, and the purity of the recombinant protein was confirmed by SDS-PAGE.
SDS-PAGE and immunoblotting.
SDS-PAGE was performed on 10% polyacrylamide gels using a discontinuous buffer system, as described elsewhere (18). The affinity-purified proteins were mixed with 2× SDS-PAGE sample loading buffer (125 mM Tris-HCl, 4% SDS, 2% glycerol, 1% β-mercaptoethanol, 0.5% bromphenol blue) and boiled for 5 min before loading. Electrophoresis was carried out in a vertical electrophoretic mini-cell unit (Bio-Rad, Hercules, CA), in Tris-glycine running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS [pH 8.3]), for 2 h at 120 V. Proteins were transferred to nitrocellulose membranes (pore size, 0.2 μm; Schleicher and Schuell, Keene, NH) and blocked with 4% nonfat dry milk in Tris-buffered saline (20 mM Tris, 150 mM NaCl, 0.05% Tween 20 [pH 7.5]). Membranes were incubated with serum samples from patients or hyperimmune sera raised in rabbits, followed by incubation with secondary antibody (anti-human IgG or anti-rabbit IgG conjugated with horseradish peroxidase [HRP]; Sigma, St. Louis, MO), and bands were visualized by using 4-chloro-α-naphthol (Sigma, St. Louis, MO).
Prediction of LigA-C-specific B-cell epitopes.
Protein sequences of cloned ligA from the L. interrogans serovar Australis, strain Ballico, were retrieved from NCBI and subjected to BCPreds analysis (19). BCPreds identifies common B-cell epitopes. Epitopes with BCPreds scores of >0.8 and VaxiJen scores of >0.4 were predicted to be highly immunogenic epitopes.
Peptide synthesis and purification.
The peptides listed were synthesized by the solid-phase method using 9-fluorenylmethoxy carbonyl (Fmoc) chemistry on a solid support of rink amide 4-methyl-benzhydrylamine resin. Then, 0.1 M N-hydroxybenzotriazole (HOBt) and 0.45 M 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluroniumhexafluorophosphate (HBTU) in dimethylformamide (DMF) and 2 M N,N-diisopropylethylamine (DIEA) in N-methylpyrrolidone (NMP) were used as coupling reagents, and 10-fold excess Fmoc-amino acid was added during every coupling cycle. Following a final deprotection step with a solution of 20% piperidine in DMF and cleavage with a mixture of trifluoroacetic acid (TFA), water, and triisopropylsilane (90:5:5) for 2 h at room temperature (20), the crude peptides were repeatedly extracted with diethyl ether and purified using reverse-phase preparative high-performance liquid chromatography (HPLC) on a C18 column (Capcell Pak C18 ACR, 30 by 250 mm; Shiseido, Japan). The molecular masses of the peptides were confirmed by using matrix-assisted laser desorption ionization mass spectrometry (see Fig. S1 in the supplemental material). The purity of all peptides was found to be >95%. Peptides were synthesized with biotin at the N terminus, linked to the peptide sequence through a spacer sequence of SGSG, to improve the solubility of the peptide and the ease of performing ELISAs.
Enzyme-linked immunosorbent assays.
Whole-cell lysates were prepared by heat extraction, as described previously (21). Checkerboard titrations were performed to determine the optimal concentrations of whole-cell lysate, recombinant LigA-C, and LigA-C-specific peptides (22). A total of 0.2 μg of whole-cell lysate or recombinant protein per well was coated on flat-bottom polystyrene microtiter plates at 4°C overnight, using carbonate coating buffer (pH 9.6), followed by blocking with 4% nonfat dry milk. Serum samples (1:200) in triplicate were added and incubated for 1 h at 37°C. Bound IgM was detected using HRP-conjugated rabbit anti-human IgM (Sigma-Aldrich, St. Louis, MO) at a dilution of 1:8,000. Plates were developed with o–phenylenediamine (Sigma-Aldrich, St. Louis, MO). The reaction was stopped with the addition of 50 μl of 1 N H2SO4, and the optical density was measured at 490 nm (Bio-Rad).
The peptides were screened for reactivity as described previously (23). The wells of plates were coated with streptavidin (0.5 μg in 100 μl H2O), washed, and blocked with 4% milk powder. Triplicate aliquots (100 μl) of each peptide diluted 1:1,000 in phosphate-buffered saline (PBS) (pH 7.4) with 0.1% Tween 20 (PBS-T) were added to the wells. Following a PBS-T wash, 100 μl of serum diluted (1:200) in PBS in triplicate was incubated with each peptide for 2 h, and the wells were washed again and incubated with HRP-conjugated rabbit anti-human IgM (1:8,000; Sigma-Aldrich, St. Louis, MO).
Polyclonal antisera.
Polyclonal antisera were raised in New Zealand White rabbits by subcutaneous administration of 1 μl of N-acetylmuramyl-l-alanyl-d-isoglutamine (Sigma, St. Louis, MO) and 100 μg of recombinant protein (rLigA-C) adsorbed to aluminum hydroxide (Alhydrogel; Accurate Chemical & Scientific Corp., Westbury, NY). Booster injections containing 100 μg of the antigen were administered 14 and 28 days after the primary immunization. Serum was obtained 35 days after the primary immunization (24).
Data analysis.
Data were analyzed with SPSS and GraphPad Prism version 5.0 (GraphPad, San Diego, CA) software. Cutoff values for ELISAs were determined to be the mean + 2 SD. LigA and peptide sequences were analyzed using CLC Main Workbench (version 6.8.1).
Nucleotide sequence accession number.
The nucleotide sequence obtained from the plasmid pET15b-ligA-C was submitted to GenBank with accession number JQ317187.
RESULTS
Sequence analysis of LigA-C.
Sequencing and BLASTN analysis of the amplified ligA-C fragments revealed a 1,557-bp sequence between bp 2214 and 3771 of the full-length ligA sequence (GenBank accession number AF368236). The sequence showed 100% similarity with Leptospira interrogans serovar Pomona (GenBank accession numbers EU700270, U95056, FJ030917, and AF368236) and L. interrogans strain Kito (GenBank accession number EU700267); ligA-C encodes a protein of 517 amino acids with a predicted molecular mass of 58 kDa that shares sequence identity with LigA of other leptospires, including L. interrogans serovars Pomona (GenBank accession number ACH89909) (99.6%), Copenhageni (GenBank accession number AAS69086) (60%), and Lai (GenBank accession number ACK58260) (100%).
Overexpression, purification, and immunoblot analysis of recombinant LigA-C.
Figure S2A in the supplemental material shows the SDS-PAGE profile of recombinant LigA-C with expression induced by 1.0 mM IPTG. The apparent molecular mass (58 kDa) of the purified recombinant protein, as determined by SDS-PAGE, was consistent with the molecular mass predicted from the amino acid composition (see Fig. S2B in the supplemental material). In an immunoblot analysis, polyclonal rabbit antibodies against recombinant LigA-C reacted with a protein of 58 kDa. Immunoblots developed with pooled MAT-positive patient sera showed reactivity for recombinant LigA-C (see Fig. S2C in the supplemental material). No reactivity was observed on immunoblots probed with MAT-negative patient sera.
Prediction of B-cell epitopes.
In total, six peptides were predicted to be immunogenic, of which two peptides, with sequences of VVIENTPGK (epitope 1) and TALSVGSSK (epitope 2) and VaxiJen scores of 1.3782 and 1.2767, respectively, were selected for synthesis and ELISA validation. The epitopes were predicted to lie in the C-terminal region at positions 1084 to 1092 (epitope 1) and 1189 to 1197 (epitope 2). The conservation of the epitopes was determined by multiple sequence alignment. The LigA sequences of L. interrogans serovar Copenhageni strain Fiocruz L1-130 (GenBank accession number AAS69086), L. interrogans serovar Pomona (GenBank accession number ACH89909), L. interrogans serovar Icterohaemorrhagiae (GenBank accession number ACU87695), L. interrogans serovar Australis (GenBank accession number AFG28560), L. interrogans serovar Lai strain Lai (GenBank accession number ACK58260), L. interrogans serovar Kennewicki (GenBank accession number ACH98097), and Leptospira kirschneri serovar Grippotyphosa (GenBank accession number AAP04735) were retrieved from GenBank and analyzed with the BioEdit sequence alignment editor (version 7.1.3.0). The two epitopes were found to be highly conserved among different serovars of L. interrogans and between L. interrogans and L. kirschneri species (Fig. 1). Antibodies to these epitopes were readily detected in human sera in the format of epitope-blocking ELISA.
FIG 1.

Conservation of the peptides among different serovars of Leptospira. The conservation of the epitopes was determined by multiple sequence alignment. The LigA sequences of L. interrogans serovar Copenhageni strain Fiocruz L1-130 (GenBank accession number AAS69086), L. interrogans serovar Pomona (GenBank accession number ACH89909), L. interrogans serovar Icterohaemorrhagiae (GenBank accession number ACU87695), L. interrogans serovar Australis (GenBank accession number AFG28560), L. interrogans serovar Lai strain Lai (GenBank accession number ACK58260), L. interrogans serovar Kennewicki (GenBank accession number ACH98097), and L. kirschneri serovar Grippotyphosa (GenBank accession number AAP04735) were analyzed with the BioEdit sequence alignment editor. *, epitope conservation.
LigA-C-, epitope 1-, and epitope 2-based IgM ELISAs.
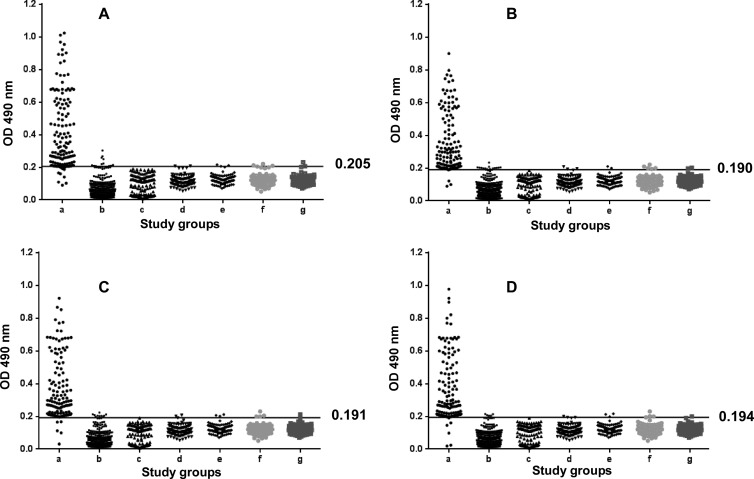
The overall results of the recombinant LigA-C-, epitope 1-, and epitope 2-based ELISAs are shown in Table 2 and Fig. 2A, B, C, and D. The mean + 2 SD absorbance values for seronegative healthy individuals were defined as the cutoff values to achieve diagnostic specificity of the ELISAs in comparison with the MAT. The cutoff values were determined to be 0.209 for whole-cell lysate, 0.205 for rLigA-C, 0.190 for epitope 1, 0.191 for epitope 2, and 0.194 for the combination of epitope 1 and epitope 2. The ELISAs demonstrated sensitivity and specificity values of 85.2% and 85.7% for whole-cell lysate, 92.1% and 97.7% for rLigA-C, 97.1% and 98.2% for epitope 1, 97.9% and 98.4% for epitope 2, and 97.9% and 99.1% for epitopes 1 and 2, respectively, in serum samples from confirmed cases of leptospirosis.
TABLE 2.
Sensitivities, specificities, positive predictive values, and negative predictive values for IgM ELISAs using whole-cell lysate, LigA-C, epitope 1, epitope 2, and epitopes 1 and 2
| Antigen | Sensitivity (%) | Specificity (%) | PPVa (%) | NPVb (%) |
|---|---|---|---|---|
| Whole-cell lysate | 85.2 | 85.7 | 82.3 | 83.4 |
| LigA-C | 92.1 | 97.7 | 92.8 | 97.5 |
| Epitope 1 | 97.1 | 98.2 | 94.4 | 99.1 |
| Epitope 2 | 97.9 | 98.4 | 95.1 | 99.3 |
| Epitopes 1 and 2 | 97.9 | 99.1 | 97.2 | 99.3 |
PPV, positive predictive value.
NPV, negative predictive value.
FIG 2.
Evaluation of different ELISAs with sera from patients with different clinical manifestations. Groups with different clinical manifestations are indicated on the x axis and the optical density (OD) at 490 nm on the y axis. IgM responses to recombinant LigA-C (A), epitope 1 (B), epitope 2 (C), and the combination of epitopes 1 and 2 (D) are shown. Study groups were as described in Table 1. The horizontal line shows the cutoff value for each ELISA.
DISCUSSION
Leptospirosis is recognized as a globally reemerging public health problem; in humans, this disease may be fatal due to the potential damage to multiple organs such as liver, lung, kidney, and brain (25). The search for new tools for the diagnosis and treatment of leptospirosis, especially an early sensitive and reliable diagnostic test, would improve patients' quality of life (26), as L. interrogans can rapidly disseminate to multiple organs and cause multiorgan system complications, including jaundice, meningitis, pulmonary hemorrhage, hepatic and renal dysfunction, and cardiovascular collapse. Therefore, it is important to identify novel candidate antigens to improve diagnostic methods to assist in early treatment.
Recently, the improved serological diagnosis of leptospirosis has been targeted with recombinant proteins. This may achieve high sensitivity and specificity because of the high concentrations of immunogenic antigens and specific antigenic moieties in the purified fractions (27). The identification and characterization of a new family of Big domain proteins (bacterial immunoglobulin-like proteins), referred to as Lig proteins, in pathogenic Leptospira have been reported (8, 9). Previously published articles demonstrated that LigA is unique to pathogenic Leptospira species and that Leptospira-infected hosts produced antibodies to LigA (8, 9). Furthermore, these proteins have been proved to be potential vaccine candidates for immunoprotection in infected hamster models (28). However, the diagnostic potential of these proteins in human cases, especially during the acute stage of the illness, is unconvincing. Previous reports on N-terminal recombinant Lig-based Western blot analysis gave a specificity of 93%, owing to the cross-reactivity of sera from patients with dengue fever, hepatitis, Lyme disease, or positive VDRL test results (16). Similarly, Srimanote et al. (3) reported the diagnosis of human leptospirosis using recombinant C-terminal LigA and described achievable specificity values of 100% and 98% for IgG and IgM ELISAs, respectively. In this regard, recombinant LigA-C is considered a good antigen for detecting antibodies in the sera of patients with suspected leptospirosis during the acute phase of the illness.
In this study, five different ELISAs were carried out to study the sensitivity and specificity for samples collected during the acute stage of leptospirosis. The C-terminal portion of ligA (bp 2214 to 3771) was cloned into pET15b and expressed in E. coli BL21(DE3). The purified recombinant proteins were assayed for the detection of IgM-specific antibodies, and the results were compared with those for the whole-cell lysate and the predicted B-cell-specific peptides alone and in combination. The IgM ELISAs specific for the whole-cell lysate and recombinant LigA-C revealed sensitivity values of 85.2% and 92.1%, respectively. Interestingly, the B-cell-specific peptide-based ELISA showed increased sensitivity of ∼98%, i.e., ∼13% and ∼6% higher than the values for the whole-cell lysate- and recombinant LigA-C-specific ELISAs, respectively, confirming the peptides as promising diagnostic candidates.
The whole-cell lysate-based ELISA offers reasonable sensitivity and the possibility of handling many samples at one time; the major drawback of this test system is the need for the maintenance of live leptospires for antigen preparation. Moreover, the antigenic preparations are generally crude in nature and from a single serovar, with lipopolysaccharide as the major antigenic component. Since lipopolysaccharide is serovar specific, the antigens may not detect antibodies produced against serovars other than that used for antigen preparation, thus limiting the widespread use of the assay (11). The full-length recombinant LigA-C can serve as an effective reagent for immunodiagnostic testing with multiple epitopes. However, the highly antigenic epitopes are buried in the complex structures of proteins and hence are unavailable for the induction of immunogenic responses, consequently yielding reduced immune responses and decreased sensitivity in ELISAs.
As a result, immunodiagnostic tests utilizing peptides offer several advantages over diagnostic tests that rely on more-complex biological materials. For instance, synthetic peptides represent chemically defined antigens and, because they are not derived from biological material, assay standardization and validation are often greatly simplified. Peptide reagents also offer flexibility in terms of antigen specificity, including species-specific diagnostic testing. Furthermore, by screening overlapping peptides within an immunogenic protein, the highly specific epitopes can be maintained while peptide epitopes that are cross-reactive or that demonstrate poor specificity are purged.
In conclusion, our results showed increased sensitivity and specificity for peptide-based ELISAs for the diagnosis of human leptospirosis during the early stage of the disease. Although diagnostic tests based on a single peptide may lack sensitivity in certain cases due to the dependence on a single antibody epitope, the use of elongated peptides or multiple peptides may reduce or eliminate this potential problem in the near future. Thus, LigA-C peptide-based ELISAs should be considered MAT alternatives in primary and secondary health care centers, not only because of their simplicity and rapid performance but also because of their affordability in developing countries where leptospirosis has been established as an endemic disease.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India, for the research and development grant (BT/PR6872/MED/14/892/2005) that it provided to carry out this study.
We are grateful to Noriko Fujii, Research Reactor Institute, Kyoto University (Osaka, Japan), for the synthesis of peptides.
Footnotes
Published ahead of print 8 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00456-13.
REFERENCES
- 1.Ratnam S, Sundararaj T, Subramaniana S. 1983. Serological evidence of leptospirosis in human population following an outbreak of the disease in cattle. Trans. R. Soc. Trop. Med. Hyg. 77:94–98 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization 1999. Leptospirosis worldwide, 1999. Wkly. Epidemiol. Rec. 74:237–242 [PubMed] [Google Scholar]
- 3.Srimanote P, Wongdeethai N, Jieanampunkul P, Samonkiert S, Leepiyasakulchai C, Kalambaheti T, Prachayasittikul V. 2008. Recombinant ligA for leptospirosis diagnosis and ligA among the Leptospira spp. clinical isolates. J. Microbiol. Methods 72:73–81. 10.1016/j.mimet.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 4.Cumberland P, Everard CO, Levett PN. 1999. Assessment of the efficacy of an IgM-ELISA and microscopic agglutination test (MAT) in the diagnosis of acute leptospirosis. Am. J. Trop. Med. Hyg. 61:731–734 [DOI] [PubMed] [Google Scholar]
- 5.Toyokawa T, Ohnishi M, Koizumi N. 2011. Diagnosis of acute leptospirosis. Expert Rev. Anti Infect. Ther. 9:111–121. 10.1586/eri.10.151 [DOI] [PubMed] [Google Scholar]
- 6.Magnarelli LA, Ijdo JW, Padula SJ, Flavelli RA, Fikrig E. 2000. Serologic diagnosis of Lyme borreliosis by using enzyme linked immunosorbent assays with recombinant antigens. J. Clin. Microbiol. 38:1735–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noordhoek GT, Cockayne A, Schouls LM, Meloen RH, Stolz E, van Embden JD. 1990. A new attempt to distinguish serologically the subspecies of Treponema pallidum causing syphilis and yaws. J. Clin. Microbiol. 28:1600–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haake DA, Chao G, Zuerner RL, Barnett JK, Barnett D, Mazel M, Matsunaga J, Levett PN, Bolin CA. 2000. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 68:2276–2285. 10.1128/IAI.68.4.2276-2285.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palaniappan RU, Chang YF, Jusuf SS, Artiushin S, Timoney JF, McDonough SP, Barr SC, Divers TJ, Simpson KW, McDonough PL, Mohammed HO. 2002. Cloning and molecular characterization of an immunogenic LigA protein of Leptospira interrogans. Infect. Immun. 70:5924–5930. 10.1128/IAI.70.11.5924-5930.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsunaga J, Barocchi MA, Croda J, Young TA, Sanchez Y, Siqueira I, Bolin CA, Reis MG, Riley LW, Haake DA, Ko AI. 2003. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol. Microbiol. 49:929–945. 10.1046/j.1365-2958.2003.03619.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natarajaseenivasan K, Shanmughapriya S, Velineni S, Artiushin SC, Timoney JF. 2011. Cloning, expression, and homology modeling of GroEL protein from Leptospira interrogans serovar autumnalis strain N2. Genomics Proteomics Bioinformatics 9:151–157. 10.1016/S1672-0229(11)60018-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natarajaseenivasan K, Vijayachari P, Sugunan AP, Sharma S, Sehgal SC. 2004. Leptospiral proteins expressed during acute & convalescent phases of human leptospirosis. Indian J. Med. Res. 120:151–159 [PubMed] [Google Scholar]
- 13.Guerreiro H, Croda J, Flannery B, Mazel M, Matsunaga J, Galvao Reis M. 2001. Leptospiral proteins recognized during the humoral immune response to leptospirosis in humans. Infect. Immun. 69:4958–4968. 10.1128/IAI.69.8.4958-4968.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fikrig E, Barthold SW, Sun W, Feng W, Telford SR, Flavell RA. 1997. Borrelia burgdorferi p35 and p37 proteins, expressed in vivo, elicit protective immunity. Immunity 6:531–539. 10.1016/S1074-7613(00)80341-6 [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga J, Sanchez Y, Xu X, Haake DA. 2005. Osmolarity, a key environmental signal controlling expression of leptospiral proteins LigA and LigB and the extracellular release of LigA. Infect. Immun. 73:70–78. 10.1128/IAI.73.1.70-78.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croda J, Ramos JGR, Matsunaga J, Queiroz A, Homma A, Riley LW, Haake DA, Reis MG, Ko AI. 2007. Leptospira immunoglobulin-like proteins as a serodiagnostic marker for acute leptospirosis. J. Clin. Microbiol. 45:1528–1534. 10.1128/JCM.02344-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faine S, Adler B, Perolat P, Bolin CA. 1999. Leptospira and leptospirosis, 2nd ed. MediSci, Melbourne, Australia [Google Scholar]
- 18.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 19.El-Manzalawy Y, Dobbs D, Honavar V. 2008. Predicting linear B-cell epitopes using string kernels. J. Mol. Recognit. 21:243–255. 10.1002/jmr.893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrifield RB. 1963. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 85:2149–2154 [Google Scholar]
- 21.Terpstra WJ, Lighthart GS, Schoone CJ. 1985. ELISA for the detection of specific IgM and IgG in human leptospirosis. J. Gen. Microbiol. 131:377–385 [DOI] [PubMed] [Google Scholar]
- 22.Verma A, Artiushin S, Matsunaga J, Haake DA, Timoney JF. 2005. LruA and LruB, novel lipoproteins of pathogenic Leptospira interrogans associated with equine recurrent uveitis. Infect. Immun. 73:7259–7266. 10.1128/IAI.73.11.7259-7266.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timoney JF, DeNegri R, Sheoran A, Forster N. 2010. Affects of N-terminal variation in the SeM protein of Streptococcus equi on antibody and fibrinogen binding. Vaccine 28:1522–1527. 10.1016/j.vaccine.2009.11.064 [DOI] [PubMed] [Google Scholar]
- 24.Harlow E, Lane D. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 25.Dutta TK, Christopher M. 2005. Leptospirosis: an overview. J. Assoc. Physicians India 53:545–551 [PubMed] [Google Scholar]
- 26.Lin YP, McDonough SP, Sharma Y, Chang YF. 2010. The terminal immunoglobulin-like repeats of LigA and LigB of Leptospira enhance their binding to gelatin binding domain of fibronectin and host cells. PLoS One 5:e11301. 10.1371/journal.pone.0011301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flannery B, Costa D, Carvalho FP, Guerreiro H, Matsunaga J, Da Silva ED, Ferreira AG, Riley LW, Reis MG, Haake DA, Ko AI. 2001. Evaluation of recombinant Leptospira antigen-based enzyme-linked immunosorbent assays for the serodiagnosis of leptospirosis. J. Clin. Microbiol. 39:3303–3310. 10.1128/JCM.39.9.3303-3310.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva EF, Medeiros MA, McBride AJ, Matsunaga J, Esteves GS, Ramos JG, Santos CS, Croda J, Homma A, Dellagostin OA, Haake DA, Reis MG, Ko AI. 2007. The terminal portion of leptospiral immunoglobulin-like protein LigA confers protective immunity against lethal infection in the hamster model of leptospirosis. Vaccine 25:6277–6286. 10.1016/j.vaccine.2007.05.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.