Abstract
Porcine contagious pleuropneumonia, caused by Actinobacillus pleuropneumoniae, has a major impact on economics, ecology, and animal welfare in the pig-rearing industry. Propionibacterium acnes, a facultative anaerobic Gram-positive corynebacterium, exists widely in normal healthy adult animals. We have shown previously that P. acnes can prevent A. pleuropneumoniae infections in mice and pigs. To elucidate the mechanism of this effect and to identify novel A. pleuropneumoniae vaccines, the role of anti-P. acnes antibodies in preventing infection was analyzed by indirect immunofluorescence and opsonophagocytosis assays in vitro. The role of the specific humoral immune response induced by P. acnes was confirmed in a B cell depletion mouse model. The survival rates of mice challenged with A. pleuropneumoniae exhibited a highly significant positive rank correlation with the levels of anti-P. acnes antibodies. The specific antibodies induced by P. acnes had the ability to combine with A. pleuropneumoniae and increase opsonization of A. pleuropneumoniae for phagocytosis. Furthermore, analysis in the murine B cell depletion model confirmed that the humoral immune response induced by P. acnes played an important role in resistance to A. pleuropneumoniae infection. In this study, we further elucidated the reasons that P. acnes can prevent A. pleuropneumoniae infection, which provides useful evidence for the development of heterologous vaccines for the control of porcine contagious pleuropneumonia.
INTRODUCTION
Porcine contagious pleuropneumonia is a highly contagious and fatal respiratory infectious disease, and the mortality of the most acute cases often reaches 80 to 100% (1). It was first reported in Britain in 1957 and has become widespread in many countries around the world since the 1980s. This disease, which is caused by Actinobacillus pleuropneumoniae, affects swine of all ages and has a major impact on economics, ecology, and animal welfare in the pig-rearing industry (2, 3). However, A. pleuropneumoniae has been divided into 15 serotypes (4), with the lack of cross-protection between the main serotypes (5, 6) resulting in slow progress in the development of vaccines. To date, many studies have been reported and several vaccines have been commercialized, but complete satisfaction has not been obtained in the protection of pigs against A. pleuropneumoniae infection (7, 8). Therefore, the development of new vaccines is urgently required.
In 2007, during an analysis of the genomic differences between A. pleuropneumoniae serotypes 1 and 5, we made the serendipitous discovery of a strong cross-reaction between the antisera against Propionibacterium acnes and A. pleuropneumoniae (9). Furthermore, through immunization and challenge studies in mice and pigs, we found that P. acnes can prevent A. pleuropneumoniae infection (9).
P. acnes bacteria, which are relatively avirulent organisms, are Gram-positive pleomorphic rods that grow under anaerobic conditions. They are the most frequent inhabitants of the sebaceous glands of normal skin, hair follicles, the mouth, and the upper respiratory tract and a frequent contaminant in laboratory cultures (10). Moreover, P. acnes is rarely identified as a cause of significant infection (11). In the present study, P. acnes induced biological effects that modulate the innate and acquired immune responses, causing increased phagocytic and tumoricidal activities in macrophages (12–14), increased antibody responses (15, 16), and increased resistance to different pathogens (15, 17). Therefore, the use of P. acnes and its components as immune enhancers and antineoplastic agents has become a focus in clinical and basic research.
The use of P. acnes to prevent A. pleuropneumoniae infection represents a novel and effective strategy for the prevention and control of porcine contagious pleuropneumonia and potentially other infections in the future. However, the effective applications for P. acnes are limited by the lack of information regarding the responses induced by P. acnes and how this organism can prevent A. pleuropneumoniae infection. In this study, we aimed to confirm that the humoral immune response induced by P. acnes plays an important role in resistance to A. pleuropneumoniae infection.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
A. pleuropneumoniae reference serotype 1 strain Shope 4074 (A. pleuropneumoniae S 4074), donated by the Shanghai Entry-Exit Inspection and Quarantine Bureau (Shanghai, China), was cultured in brain heart infusion (BHI; Difco Laboratories, Detroit, MI, USA) supplemented with NAD (10 μg/ml; Sigma) at 37°C for 6 h, while shaking at 150 rpm. Propionibacterium acnes strain 14 (P. acnes 14), which was separated and identified by our laboratory, was streaked on brucella broth agar (BD, Sparks, MD, USA), supplemented with 5% (vol/vol) defibrinated sheep blood (Lampire Biological Laboratories, Pipersville, PA, USA), vitamin K (5 μg/ml; Remel, Lenexa, KS, USA), and hemin (50 μg/ml; Remel), under anaerobic conditions using an anaerobic chamber (Mac500; Down Whitley Scientific, West Yorkshire, United Kingdom; 10% carbon dioxide, 10% hydrogen, 80% nitrogen) at 37°C. A single colony was inoculated in reinforced clostridium medium (Oxford, Hampshire, England) at 37°C under anaerobic conditions.
Immunogen preparation.
The preparation of viable P. acnes 14 antigen was performed as follows: P. acnes 14 was cultivated for 72 h to an optical density at 600 nm (OD600) of 1.0 to 3.0 (logarithmic growth phase). Bacteria were harvested by centrifugation at 5,000 × g for 10 min, washed with phosphate-buffered saline (PBS) three times, finally suspended in PBS, and adjusted to an OD600 of 1.0. The concentration of this suspension was approximately 1 × 109 CFU/ml, according to the relationship between OD600 and the P. acnes colony count. The suspension was concentrated or diluted 10 times to give suspensions of viable P. acnes 14 antigen of 1 × 108 CFU/ml, 1 × 109 CFU/ml, and 1 × 1010 CFU/ml for use in immunizations.
Inactivated P. acnes 14 antigen was prepared by heat inactivation of viable P. acnes 14 at 60°C for 30 min. After inactivation, P. acnes was unable to grow on an agar plate (data not shown). Inactivated P. acnes was harvested by centrifugation at 5,000 × g for 5 min and resuspended to an appropriate concentration in PBS.
P. acnes 14 antigen in adjuvant was prepared as follows: 10 g astragalus polysaccharide powder (adjuvant) was dissolved in distilled water (4% aqueous solution) and filtered (0.22 μm) to remove residual bacteria. Equal amounts of astragalus polysaccharide aqueous solution were mixed with viable or inactivated P. acnes 14 antigen under aseptic conditions. The resulting emulsions were vortexed and stored at 2 to 8°C for later use.
Animal immunizations and challenge.
Female BALB/c mice (aged 6 to 8 weeks; 18 to 22 g) were purchased from the Animal Experiment Center of Jilin University. Female Japanese rabbits (obtained from the same source) were used for generation of rabbit hyperimmune antiserum. BALB/c mice were randomly assigned to seven groups: the experimental groups (2 to 7) are shown in Table 1. Each group comprised 15 mice (10 mice for toxicity evaluation and 5 for T cell detection), and mice were immunized subcutaneously (s.c.) with viable P. acnes 14 (2 × 107 CFU, 2 × 108 CFU, and 2 × 109 CFU) and inactivated P. acnes 14 (2 × 107 CFU, 2 × 108 CFU, and 2 × 109 CFU). The control group (group 1) received 0.2 ml PBS only. One week later, all mice received the same dose of the respective immunogens. Thirty-five days after the first immunization, blood was drawn from the tail to collect serum for enzyme-linked immunosorbent assays (ELISAs). For the generation of antiserum to P. acnes or A. pleuropneumoniae serotype 1, rabbits were immunized s.c. on days 0, 14, and 35 with 1 × 109 CFU of the viable P. acnes 14 or A. pleuropneumoniae serotype 1, respectively. All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee, and all animals were cared for in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International.
TABLE 1.
Frequencies of CD3, CD4, CD8, and CD19 cells determined by flow cytometry
| Group | Immunogen | Immunization dose (CFU/ml in 0.2-ml suspension) | Frequency (%) |
Ratio |
||||
|---|---|---|---|---|---|---|---|---|
| CD3+ | CD3+ CD4+ | CD3+ CD8+ | CD3− CD19+ | CD3+/CD19+ | CD4+/CD8+ | |||
| 1 | PBS | 0.2 ml | 39.41 ± 0.66 | 25.36 ± 0.33 | 11.71 ± 0.38 | 45.38 ± 0.85 | 0.87 ± 0.01 | 2.17 ± 0.06 |
| 2 | Viable P. acnes | 108 | 39.95 ± 0.33 | 27.27 ± 0.21a | 9.86 ± 0.16a | 48.57 ± 0.67a | 0.82 ± 0.01a | 2.77 ± 0.07a |
| 3 | Viable P. acnes | 109 | 40.76 ± 0.40a | 28.12 ± 0.23a | 9.81 ± 0.20a | 49.33 ± 0.43a | 0.83 ± 0.01a | 2.87 ± 0.03a |
| 4 | Viable P. acnes | 1010 | 32.5 ± 0.65a | 22.63 ± 0.48a | 6.95 ± 0.63a | 55.07 ± 0.50a | 0.59 ± 0.01a | 3.27 ± 0.23a |
| 5 | Inactivated P. acnes | 108 | 49.76 ± 0.71a | 35.29 ± 0.36a | 14.28 ± 0.33a | 42.46 ± 0.91a | 1.17 ± 0.03a | 2.47 ± 0.04a |
| 6 | Inactivated P. acnes | 109 | 51.28 ± 0.71a | 39.01 ± 0.31a | 12.49 ± 0.44a | 45.04 ± 0.49 | 1.14 ± 0.01a | 3.13 ± 0.10a |
| 7 | Inactivated P. acnes | 1010 | 52.74 ± 0.59a | 38.46 ± 0.38a | 9.64 ± 0.20a | 40.35 ± 0.75a | 1.31 ± 0.01a | 4.02 ± 0.05a |
The mean difference between the immunization group and the control group is significant at the 0.05 level.
A. pleuropneumoniae serotype 1 was cultured in BHI medium for 6 h at 37°C. The bacteria were washed three times with PBS and then resuspended. At 35 days after the first immunization, the mice in all groups were challenged intraperitoneally (i.p.) with 0.2 ml of A. pleuropneumoniae bacterial suspension containing 4.0 × 107 CFU (equivalent to 5× 50% lethal dose [LD50]). The survival rates of all groups were calculated at day 7 postchallenge.
For evaluating the dynamic relationship between levels of anti-P. acnes antibodies and prevention of A. pleuropneumoniae infection, BALB/c mice were immunized with the optimal immunization condition of P. acnes. Then, mice were divided into various groups based on the different IgG titers at different time points. The control groups were the PBS control group and the astragalus polysaccharide control group. The mice in each group were challenged with A. pleuropneumoniae as soon as they were selected on different IgG titers.
ELISAs.
Serum antibody responses to P. acnes or A. pleuropneumoniae were assessed by ELISA as previously described (18). Wells of microtiter plates (Thermo Labsystems) were coated overnight at 4°C with P. acnes 14 or A. pleuropneumoniae serotype 1 bacteria (1 × 106 CFU per well) lysed by ultrasonic treatment in 0.05 M carbonate buffer (pH 9.5). The wells were then washed three times with PBS containing 0.05% Tween 20 (PBST). Blocking for nonspecific interactions was carried out by the addition of 200 μl of 5% skim milk in PBST per well, and plates were incubated at 37°C for 1 h. Serial 2-fold dilutions of individual mouse or rabbit sera were analyzed using horseradish peroxidase (HRP)-conjugated secondary antibodies specific for either rabbit IgG or mouse IgG (Tianjin Sungene Biotech Co., Ltd., Tianjin, China) at a 1:4,000 dilution. Plates were washed five times with PBST, and 3,3′,5,5′-tetramethylbenzidine (Sigma) was used as the substrate. Absorbance (OD450) was determined using the Labsystems Multiskan Plus plate reader. Absorbance was plotted against dilution, and the lower limit of detection of the ELISA was the reciprocal dilution of 100. The titer obtained was the reciprocal of maximum dilution when the OD450 value of serum was greater than 2.1 times that of the preimmune serum.
The concentrations of IgA and IgM at different IgG levels of mouse serum were determined using a mouse enzyme-linked immunosorbent assay kit for immunoglobulin A (IgA) (Uscn Life Science Inc.) and mouse enzyme-linked immunosorbent assay kit for immunoglobulin M (IgM) (Uscn Life Science Inc.), respectively, according to the manufacturer's instructions.
Flow cytometry.
Erythrocyte-depleted single-cell suspensions of individual mouse spleens were stained on ice using optimal concentrations (0.5 μg per million cells) of rat anti-mouse CD16/CD32 (Fc block; BD Biosciences, San Diego, CA, USA). For detection of lymphocytes (including CD3+ CD4+ T cells, CD3+ CD8+ T cells, and CD3− CD19+ B cells) in mouse spleens, the single-cell suspensions were stained with optimal concentrations of antibodies specific for mouse CD3 (1.0 μg fluorescein isothiocyanate [FITC] anti-mouse CD3 per million cells in a 100-μl volume), CD4 (0.25 μg phycoerythrin [PE] anti-mouse CD4 per million cells in a 100-μl volume), CD8 (0.25 μg PE anti-mouse CD8a per million cells in a 100-μl volume), and CD19 (0.25 μg PE anti-mouse CD19 per million cells in a 100-μl volume) (Biolegend, Inc., San Diego, CA, USA). For depletion experiments, the single-cell suspensions were incubated with directly conjugated antibodies specific for mouse CD19 and mouse CD3.
Cells with light scattering properties of lymphocytes were then assessed by immunofluorescence on a FACScan cytometer using Cellquest software (BD Biosciences, San Diego, CA, USA). A total of 10,000 gated events were acquired for each sample.
Indirect immunofluorescence.
For indirect immunofluorescence, A. pleuropneumoniae serotype 1 bacteria were cultured, washed, and resuspended in PBS. Then, bacteria were fixed with 1% paraformaldehyde for 20 min at room temperature. Following a 5-min centrifugation at 8,000 × g, bacteria were washed three times with PBS and resuspended in PBS containing 10% fetal bovine serum (FBS) and 1% NaN3. P. acnes or A. pleuropneumoniae hyperimmune rabbit serum was added, and tubes were incubated at 37°C for 1 h. There was no P. acnes or A. pleuropneumoniae hyperimmune rabbit serum in the negative control and blank control, and the blank control also did not include A. pleuropneumoniae serotype 1 bacteria. Bacteria were washed three times with PBS, and bound antibodies were detected using FITC-labeled goat anti-rabbit IgG (Tianjin Sungene Biotech Co., Ltd.) antibodies. Then, bacteria were washed three times with PBS and resuspended in PBS containing 3% bovine serum albumin (BSA), 1% NaN3 in darkness. The samples were observed by fluorescence microscopy (Lavision Biotec, Germany), and photographs were taken as soon as possible.
Opsonophagocytosis assay.
The murine macrophage cell line J774A.1 was cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% FBS (HyClone) and 4 mM l-glutamine (Invitrogen). Approximately 2 × 105 cells were seeded into a 24-well cell culture plate (Corning) and incubated overnight at 37°C under 5% CO2. For the opsonophagocytosis assay (19), P. acnes hyperimmune rabbit serum, A. pleuropneumoniae hyperimmune rabbit serum, preimmune rabbit serum, and unrelated glutathione S-transferase(GST) hyperimmune rabbit serum were heat inactivated by incubation at 55°C for 15 min. A. pleuropneumoniae serotype 1 was grown to an OD600 of 1, and approximately 2 × 106 cells were incubated with heat-inactivated P. acnes hyperimmune rabbit serum (at 1% or 10%), A. pleuropneumoniae hyperimmune rabbit serum (10%), preimmune rabbit serum (10%), unrelated (GST) hyperimmune rabbit serum (10%), or PBS in 100 μl at 37°C for 30 min. The assay was performed by incubating macrophage cells with the previously mentioned mixture of serum or PBS containing bacteria for 1 h. This was followed by gentamicin (100 μg/ml) (Invitrogen) treatment for 1 h to kill the extracellular bacteria, washing twice with PBS to remove adherent bacteria, and lysing with 0.1% Triton X-100 (Invitrogen). Different dilutions were plated to enumerate the number of phagocytosed bacteria. The fold change in CFU for each treatment was calculated by dividing the intracellular CFU obtained from different serum groups by the CFU obtained from the PBS control. The assay was performed in triplicate and repeated two to three times.
Humoral immune response analysis in B cell depletion model mice vaccinated with P. acnes.
Referring to studies of Oscherwitz et al. (20), we constructed and expressed a recombinant immunogen which contains six copies of the 22-amino-acid peptide sequence from the C terminus of the murine CD20 extracellular domain (ECD), covalently linked to the C terminus of maltose-binding protein (MBP) (henceforth referred to as CD20ECD-6). Figure 1 is a schematic representation of CD20ECD-6. The recombinant immunogen is capable of eliciting antibodies that recognize the folded CD20 ECD sequence and effectively binds cell surface CD20, leading to the pronounced depletion of splenic B cells to generate a murine B cell depletion model. In brief, the process is as follows: six copies of the DNA sequence (5′-CGGCTACGTGGACATCTACGACTGCGAACCGTCCAACTCCTCTGAGAAGAACTCTCCGTCCACCCAGTA-3′) which encodes a 22-amino-acid peptide sequence (amino acids 155 to 176; GYVDIYDCEPSNSSEKNSPSTQY) from the C terminus of the murine CD20 ECD (GenBank accession no. P19437) were synthesized by ShengGong Biological Engineering (Shanghai, China). The construction, expression in Escherichia coli, and purification of the fusion proteins using amylose agarose were as described previously (21). Two groups of BALB/c mice (13/group) were immunized s.c. five times at 2-week intervals with 40 μg CD20ECD-6 immunogen in complete Freund's adjuvant (CFA) for priming immunizations and incomplete Freund's adjuvant (IFA) for boosting immunizations. Mice in the normal control group were immunized with the same dose of adjuvant alone by the same protocol. Ten days after the final immunization, erythrocyte-depleted single-cell suspensions of individual mouse spleens were assessed using flow cytometry to enumerate CD19+ B cells as previously described.
FIG 1.
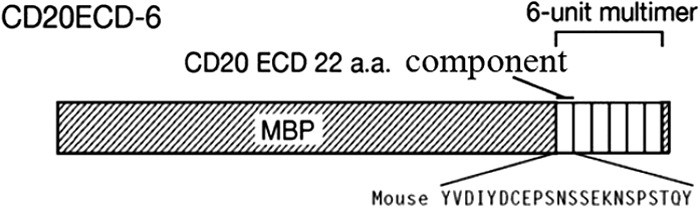
Schematic representation of CD20ECD-6. Each of the six CD20 components represents amino acids 155 to 176 of the murine CD20 ECD. A single glycine spacer is interposed between the CD20 component sequences, and the repeats are covalently linked to the C terminus of maltose-binding protein. Shown below the diagram is the single-letter amino acid sequence for mouse CD20 in the ECD region. The diagram is not to scale.
Mice in one B cell depletion model group and one normal control group were immunized s.c. with viable P. acnes (2 × 108 CFU) on two occasions at 1-week intervals. Mice in the other B cell depletion model group were immunized with PBS. At day 35 after the first immunization, blood was drawn from the tail to collect serum for ELISAs. The mice in all groups were challenged i.p. with 0.2 ml of A. pleuropneumoniae serotype 1 bacterial suspension containing 4.0 × 107 CFU (equivalent to 5× LD50). The survival rates of all groups were calculated on day 7 postchallenge.
Statistical analysis.
Student's t test was used for comparison of the differences between different groups. Spearman rank correlation analysis was used for analysis of the dynamic relationship between levels of anti-P. acnes antibody and prevention of A. pleuropneumoniae infection. The survival rates of mice challenged with A. pleuropneumoniae were analyzed by the Kaplan-Meier method. All statistical analysis was performed using SPSS software (IBM Corporation, USA).
RESULTS
Analysis of humoral immune responses induced by P. acnes.
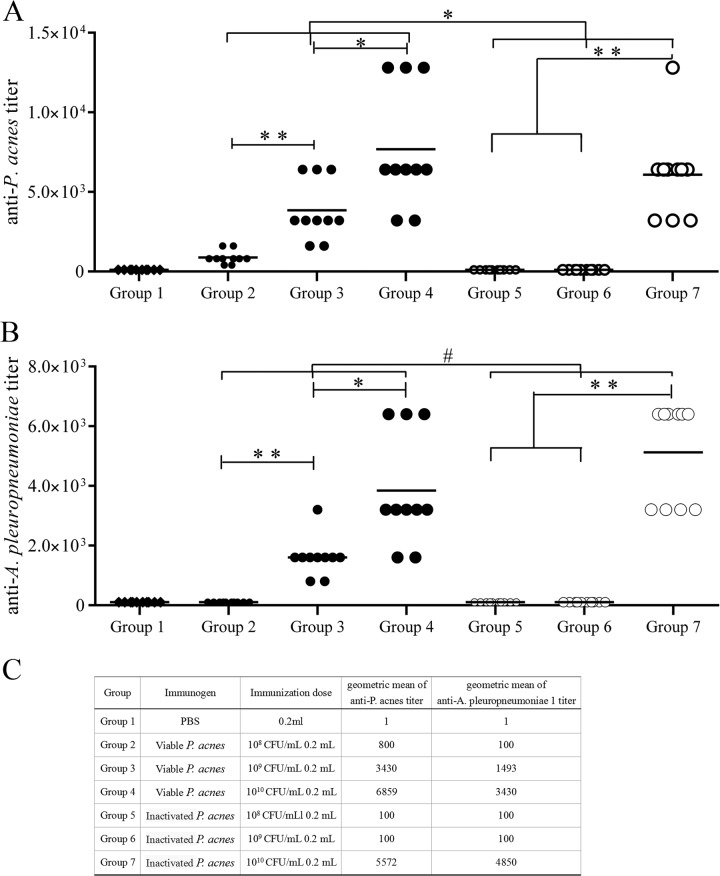
At day 35 after immunization with P. acnes, IgG antibodies specific for P. acnes and A. pleuropneumoniae serotype 1 were detected in the peripheral blood in all groups by ELISA (Fig. 2). The antibody titers of mice vaccinated with viable P. acnes were significantly higher than those of mice vaccinated with inactivated P. acnes (P < 0.05). The serum antibody titers were found to increase in a dose-dependent manner, especially in the viable P. acnes groups. However, in the inactivated P. acnes groups, high immunization doses were required to induce high levels of antibodies (Fig. 2). It was worth noting that the serum antibody titers of mice vaccinated with P. acnes were also high when detected for A. pleuropneumoniae serotype 1 (Fig. 2B).
FIG 2.
Specific antibody responses in mice elicited through immunization with P. acnes. BALB/c mice were randomly assigned to seven groups (n = 15 per group). Mice in groups 2 to 7 were immunized subcutaneously (s.c.) with viable P. acnes 14 (2 × 107 CFU, 2 × 108 CFU, and 2 × 109 CFU) and inactivated P. acnes 14 (2 × 107 CFU, 2 × 108 CFU, and 2 × 109 CFU). The control group (group 1) received 0.2 ml PBS only. One week later, all mice received the same dose of the respective immunogens. Thirty-five days after the first immunization, blood was drawn from the tail to collect serum for enzyme-linked immunosorbent assays (ELISAs). (A) Serum anti-P. acnes antibody titers. (B) Serum anti-A. pleuropneumoniae antibody titers. (C) Geometric mean of anti-P. acnes titers and anti-A. pleuropneumoniae serotype 1 titers. *, P < 0.05; **, P < 0.01; #, P > 0.05.
The types and proportions of lymphocytes in mouse spleen cell suspensions prepared from all groups on day 35 postimmunization were analyzed by flow cytometry (Table 1). The percentage of CD3+ and CD4+ T cells in the inactivated P. acnes immunization groups increased significantly compared with that of the control group (P < 0.05); the ratio increased in a dose-dependent manner. Also, the ratio of CD4+/CD8+ T cells in all immunization groups increased significantly compared with that of the control group (P < 0.05), which showed that the CD4+ T cell population was predominant after vaccination with P. acnes.
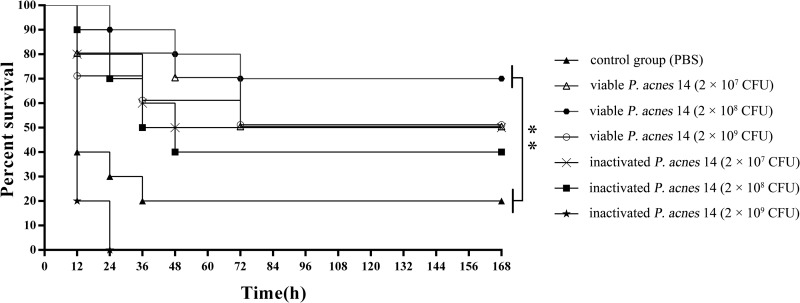
On day 35 postimmunization, all groups were challenged with A. pleuropneumoniae serotype 1 (5 times the LD). The survival rates varied with the immunization dose (Fig. 3). The survival rates of groups vaccinated with viable P. acnes were higher than those of groups vaccinated with inactivated P. acnes and the control group. Also, the protective effects in group 3, vaccinated with viable P. acnes (2 × 108 CFU/mouse), were significantly greater than those in the control group (P < 0.01). It is important to note that the mice of group 7 all died. It accorded with the literature reporting that injection with high doses of heat–killed P. acnes and then challenge with lipopolysaccharide (also existing in A. pleuropneumoniae) can induce fulminant hepatitis, which causes acute death, in many kinds of animals, including mice (22–24).
FIG 3.
Survival rates of mice challenged with A. pleuropneumoniae after immunization with P. acnes. BALB/c mice were randomly assigned to seven groups (n = 10 per group). Mice in groups 2 to 7 were immunized subcutaneously (s.c.) with viable P. acnes 14 (2 × 107 CFU, 2 × 108 CFU, and 2 × 109 CFU) and inactivated P. acnes 14 (2 × 107 CFU, 2 × 108 CFU, and 2 × 109 CFU). The control group (group 1) received 0.2 ml PBS only. At 35 days after the first immunization, the mice in all groups were challenged intraperitoneally (i.p.) with 0.2 ml of A. pleuropneumoniae bacterial suspension containing 4.0 × 107 CFU (equivalent to 5× LD50). The survival rates of all groups were calculated at day 7 postchallenge. Group 1, control group, solid triangles; group 2, viable P. acnes 14 (2 × 107 CFU), open triangles; group 3, viable P. acnes 14 (2 × 108 CFU), hexagons; group 4, viable P. acnes 14 (2 × 109 CFU), circles; group 5, inactivated P. acnes 14 (2 × 107 CFU), crosses; group 6, inactivated P. acnes 14 (2 × 108 CFU), squares; group 7, inactivated P. acnes 14 (2 × 109 CFU), stars. **, P < 0.01.
Based on the results of these experiments, 2 × 108 CFU/mouse was selected as the optimal immunization dose of viable P. acnes. This dose was used in all subsequent experiments.
Dynamic relationship between levels of anti-P. acnes antibodies and prevention of A. pleuropneumoniae infection.
After immunization with P. acnes emulsified with astragalus polysaccharide adjuvant, P. acnes-specific IgG antibodies were detected by ELISA in the peripheral blood of all mice at the indicated time points. Based on the different IgG titers, all mice were divided into eight groups (groups 1 to 8: PBS control group, astragalus polysaccharide control group, group with titer of <100 at day 35, and groups with titers of 400, 800, 1,600, 3,200, and 6,400, respectively). At the same time the concentrations of IgA and IgM in each group were examined (Table 2). The concentrations of IgA in groups 7 and 8 (IgG titers of approximately 3,200 and 6,400, respectively) were significantly higher than those in the two control groups (P < 0.01). However, there were no significant differences in the concentrations of IgM between the groups with different IgG titers.
TABLE 2.
IgA and IgM titers at different IgG titers
| Group | Immunogen | Anti-P. acnes IgG titer | Mean concn (μg/ml) |
|
|---|---|---|---|---|
| IgA | IgM | |||
| 1 | PBS | 0 | 139.142 ± 2.558 | 6.434 ± 0.150 |
| 2 | Astragalus polysaccharides | 0 | 140.482 ± 1.258 | 6.276 ± 0.492 |
| 3 | P. acnes plus astragalus polysaccharides | <100 at 35 days | 141.887 ± 4.104 | 6.205 ± 0.260 |
| 4 | P. acnes plus astragalus polysaccharides | 400 | 135.798 ± 0.547 | 5.761 ± 0.126 |
| 5 | P. acnes plus astragalus polysaccharides | 800 | 139.091 ± 1.531 | 5.671 ± 0.233 |
| 6 | P. acnes plus astragalus polysaccharides | 1,600 | 142.240 ± 2.912 | 6.391 ± 0.458 |
| 7 | P. acnes plus astragalus polysaccharides | 3,200 | 146.418 ± 2.900a | 6.350 ± 0.138 |
| 8 | P. acnes plus astragalus polysaccharides | 6,400 | 146.597 ± 0.785a | 6.338 ± 0.443 |
The concentrations of IgA in groups 7 and 8 were significantly higher than those in the two control groups (P < 0.01).
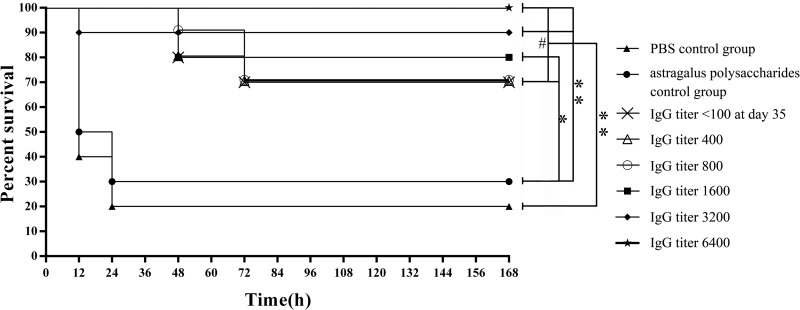
Selected mice in each group were challenged with A. pleuropneumoniae serotype 1 (5× LD50). The survival rates of all groups are shown in Fig. 4. The presence of anti-P. acnes antibodies had protective effects against A. pleuropneumoniae infection. Spearman rank correlation analysis demonstrated a significantly positive rank correlation between the survival rates of mice challenged with A. pleuropneumoniae serotype 1 and the levels of anti-P. acnes antibodies (P < 0.01). Partial protection against A. pleuropneumoniae infection was observed in mice with low levels of anti-P. acnes antibodies (<100, 400, or 800) at day 35 after immunization with P. acnes.
FIG 4.
Dynamic relationship between level of anti-P. acnes antibody and survival rates. Based on the different IgG titers at the indicated time points, all mice immunized with P. acnes with astragalus polysaccharide adjuvant were divided into eight groups (group 1, PBS control group, solid triangles; group 2, astragalus polysaccharide control group, solid circles; group 3, IgG titer of <100 at day 35, crosses; group 4, IgG titer of 400, open triangles; group 5, IgG titer of 800, open circles; group 6, IgG titer of 1,600, squares; group 7, IgG titer of 3,200, diamonds; group 8, IgG titer of 6,400, stars). Selected mice in each group were challenged with A. pleuropneumoniae serotype 1 (5× LD50). The survival rates of all groups were calculated at day 7 postchallenge. There were no significant differences among groups 3 to 8 (P > 0.05). Also, the survival rates of groups 3 to 8 were significantly higher than those of control groups 1 and 2. *, P < 0.05; **, P < 0.01; #, P > 0.05.
Cross-reaction between anti-P. acnes serum and A. pleuropneumoniae.
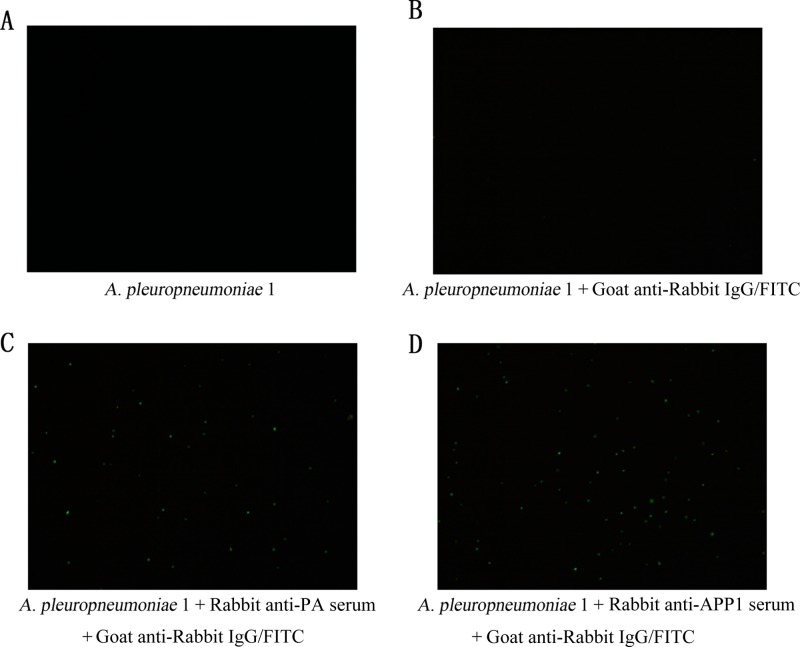
Rabbits were immunized with P. acnes, and anti-P. acnes hyperimmune serum was prepared (titer of ≈40,000). No immunofluorescence was detected in the absence of anti-P. acnes hyperimmune serum (Fig. 5A and B). Positive indirect immunofluorescence signals indicated binding of anti-P. acnes hyperimmune serum with A. pleuropneumoniae serotype 1 (Fig. 5C). This was consistent with the positive control (Fig. 5D). These data suggest that the antibodies induced by P. acnes had the ability to combine with A. pleuropneumoniae, which was consistent with the results of ELISA shown in Fig. 2B. These was strong cross-reaction between anti-P. acnes serum and A. pleuropneumoniae.
FIG 5.
Indirect immunofluorescence. Rabbit anti-P. acnes hyperimmune serum was prepared (titer, ≈1:40,000). No immunofluorescence was detected in the absence of anti-P. acnes hyperimmune serum (A and B). Positive indirect immunofluorescence signals indicated binding of anti-P. acnes hyperimmune serum with A. pleuropneumoniae serotype 1 (C). This was consistent with the positive control (D).
Anti-P. acnes sera were opsonic.
The presence of IgG in the immune serum has been correlated with high opsonic activity (25). We examined the efficiency of P. acnes hyperimmune rabbit serum in promoting opsonization and phagocytosis of A. pleuropneumoniae by J774A.1 murine macrophage cells (Fig. 6). These cells are frequently utilized to study pathogenic bacteria and for phagocytic assays (26, 27). Preimmune rabbit serum, PBS, and unrelated (GST) hyperimmune rabbit serum were used as negative controls, and titers of all the hyperimmune rabbit sera were approximately 40,000. Opsonization with P. acnes hyperimmune rabbit serum increased the efficiency of uptake of A. pleuropneumoniae by J774A.1 macrophages compared to incubation with preimmune rabbit serum, PBS, and unrelated (GST) hyperimmune rabbit serum (Fig. 6). In contrast, there were no significant differences in the phagocytosis of A. pleuropneumoniae following opsonization with either P. acnes hyperimmune rabbit serum or A. pleuropneumoniae hyperimmune rabbit serum. These observations confirm the specificity of anti-P. acnes antibody-mediated opsonization. These results therefore suggest that one of the mechanisms for the observed P. acnes-mediated protection is increased opsonization and phagocytosis of A. pleuropneumoniae.
FIG 6.
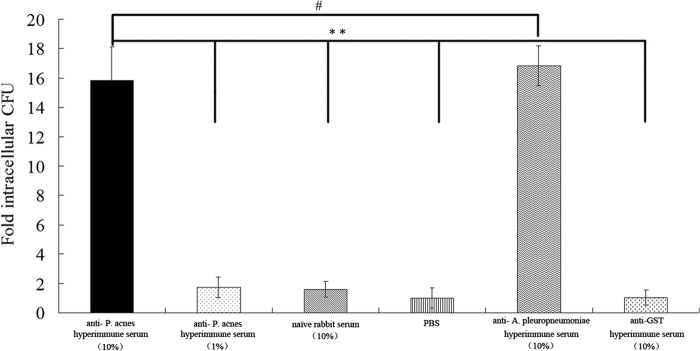
Opsonization with anti-P. acnes serum enhances the phagocytosis of A. pleuropneumoniae by J774 murine macrophages. Opsonization with P. acnes hyperimmune rabbit serum increased the efficiency of uptake of A. pleuropneumoniae by J774 macrophages compared to control serum. In contrast, the fold intracellular CFU of group anti-P. acnes hyperimmune rabbit serum (10%) was significantly more than that of negative-control groups, including the preimmune rabbit serum, PBS, and unrelated (GST) hyperimmune rabbit serum groups (P < 0.01). Also, there were no significant differences in the uptake of A. pleuropneumoniae following opsonization with either P. acnes hyperimmune rabbit serum or A. pleuropneumoniae hyperimmune rabbit serum. Results are expressed as fold CFU of intracellular bacteria over the PBS treatment and are representative of three independent experiments performed in triplicate. Bars represent means ± standard deviations. Statistical analysis was carried out using the unpaired two-tailed Student t test. **, P < 0.01; #, P > 0.05.
Humoral immune response analysis in B cell depletion model mice vaccinated with P. acnes.
BALB/c mice were immunized five times at 2-week intervals with the CD20ECD-6 immunogen using CFA for priming immunizations and IFA for boosting immunizations. At the conclusion of the immunization protocol, individual mouse spleens were assessed for determination of B cell numbers by flow cytometry as described in Materials and Methods. Immunization with CD20ECD-6 led to significant levels of B cell depletion compared to mice in the control group, with a 40% reduction in splenic B cells (Fig. 7). This was consistent with previous reports (20) which showed that a murine B cell depletion model was successfully established.
FIG 7.
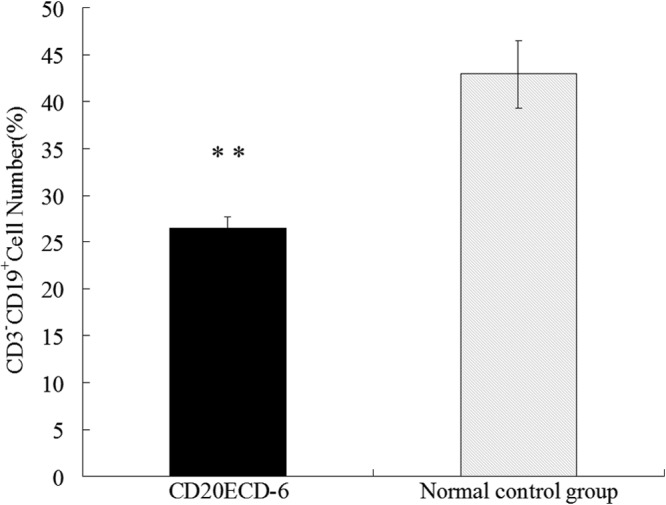
Splenic B cell depletion in BALB/c mice. Two groups of BALB/c mice (13/group) were immunized s.c. five times at 2-week intervals with 40 μg CD20ECD-6 immunogen in complete Freund's adjuvant (CFA) for priming immunizations and incomplete Freund's adjuvant (IFA) for boosting immunizations. Mice in the normal control group were immunized with the same dose of adjuvant alone by the same protocol. Ten days after the final immunization, erythrocyte-depleted single-cell suspensions of individual mouse spleens were assessed using flow cytometry to enumerate CD19+ B cell numbers as described in Materials and Methods. Bar graphs represent group means ± standard deviations of B cell numbers (%). **, P < 0.01.
At day 35 after vaccination of B cell depletion model mice with P. acnes, P. acnes-specific IgG antibodies were detected by ELISA in the peripheral blood in all groups (Fig. 8). However, the serum antibody titer of B cell depletion model mice vaccinated with P. acnes was significantly lower than that of control mice (800 versus >6,400) (P < 0.01). This suggested that the titer of mice vaccinated with P. acnes is markedly reduced due to the depletion of B cells.
FIG 8.
Antibody responses in B cell depletion mice elicited through immunization with P. acnes. Mice in one B cell depletion model group and one normal control group (n = 10 per group) were immunized s.c. with viable P. acnes (2 × 108 CFU) on two occasions at 1-week intervals. Mice in the other B cell depletion model group were immunized with PBS. At day 35 after the first immunization, blood was drawn from the tail to collect serum for ELISAs. Immune serum reactivity was compared with that of preimmune serum of normal mice. The anti-P. acnes serum titers of B cell depletion mice vaccinated with P. acnes were significantly lower than those of normal mice vaccinated with P. acnes (P < 0.01). **, P < 0.01.
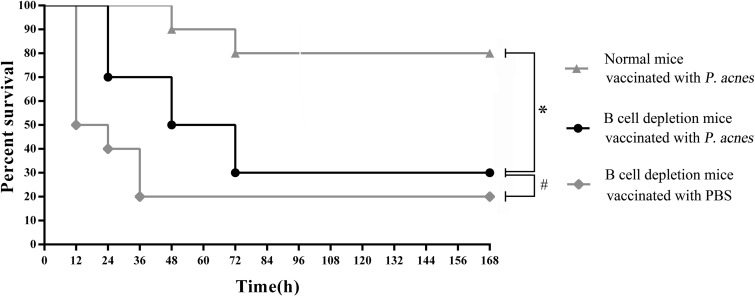
At day 35 postimmunization, the mice in all groups were challenged with A. pleuropneumoniae serotype 1 (5× LD50) (Fig. 9). The survival rate of B cell depletion model mice vaccinated with P. acnes was significantly lower than that of the normal control group, and no difference was observed from the rate observed in B cell depletion model mice vaccinated with PBS. This implied that there were significant reductions both in the level of antibodies induced by P. acnes and in the ability to resist A. pleuropneumoniae infection.
FIG 9.
Survival rates of B cell depletion mice challenged with A. pleuropneumoniae after immunization with P. acnes. Mice in one B cell depletion model group and one normal control group were immunized s.c. with viable P. acnes (2 × 108 CFU) on two occasions at 1-week intervals. Mice in the other B cell depletion model group were immunized with PBS. At day 35 after the first immunization, the mice in all groups were challenged i.p. with 0.2 ml of A. pleuropneumoniae bacterial suspension containing 4.0 × 107 CFU (equivalent to 5× LD50). The survival rates of all groups were calculated on day 7 postchallenge. *, P < 0.05; #, P > 0.05.
DISCUSSION
Despite all the research and trials in A. pleuropneumoniae vaccination conducted in the past, a safe vaccine that offers complete protection against all serotypes has not yet reached the market (5). Our previous studies have demonstrated that vaccination with P. acnes provides protection against A. pleuropneumoniae infection in mice and pigs. In these models, P. acnes vaccination inhibits the proliferation of the pathogen upon infection with A. pleuropneumoniae and the animals usually remain asymptomatic. Furthermore, over time, complete clearance of A. pleuropneumoniae by the immune system can be achieved in vaccinated animals (9).
The innate immune system is the first line of defense against infection. In the absence of complete pathogen clearance, the adaptive immune response is stimulated, in which specific antibodies, including IgA and IgG, play a vital role in clearing extracellular bacteria such as A. pleuropneumoniae.
The predominance of the CD4+ T cell population after vaccination with P. acnes implied the importance of humoral immunity in the response to A. pleuropneumoniae. Anti-P. acnes polyclonal antibodies can bind to and promote phagocytosis of A. pleuropneumoniae by phagocytes through specific opsonization. Its effect is the same as that of the anti-A. pleuropneumoniae antibody. Mice were immunized with the immunogen CD20ECD-6, resulting in significant elimination of B cells in the spleen and successful establishment of the murine B cell depletion model. The main effect of the B cell response is to produce specific antibodies to clear pathogens through neutralizing toxins, activating complement, opsonization, and antibody-dependent cellular cytotoxicity (28). The production of specific antibodies is hindered by B cell depletion. Thereby, the significant reduction both in the level of antibodies induced by P. acnes and in the ability to resist A. pleuropneumoniae infection confirmed that the humoral immune response induced by P. acnes plays an important role in resisting infection by A. pleuropneumoniae.
The survival rates of mice challenged with A. pleuropneumoniae serotype 1 exhibited a significantly positive rank correlation with the antibody levels of mice immunized with P. acnes. It was noticeable that partial protection against A. pleuropneumoniae infection was observed in mice with low levels of anti-P. acnes antibodies (<100, 400, or 800) at day 35 after immunization with P. acnes. It is possible that the cytokines stimulated by P. acnes also play an important role in resistance to A. pleuropneumoniae infection. This speculation is consistent with our previous whole-genome screening of mice immunized with P. acnes for differentially expressed genes by high-throughput microarray. Significant differentially expressed cytokines included Epor, Retnlg, Selplg, Xcl1, interleukin 33 (IL-33), Pik3ip1, IL-17ra, IL-2, IL-7, IL-11, IL-15, and gamma interferon (IFN-γ). These cytokines are known to (i) regulate immune recognition (for instance, IFN-γ induces the expression of major histocompatibility complex II molecules by antigen-presenting cells to promote antigen presentation), (ii) participate in immune cell proliferation of immune cells (for example, IL-2, IL-4, IL-5, and IL-6 induced by P. acnes [29] promote T cell and B cell activation, proliferation, and differentiation), (iii) participate in immune effects (for example, IFN-γ released by CD8+ effector T lymphocytes has the ability to inhibit viral replication and resolve intracellular infection without killing cells; P. acnes enhances Th17 cells to secrete IL-17, IL-6, tumor necrosis factor [TNF], and CXCL1, which recruit neutrophils to infected sites at the onset of the infection [30]; P. acnes also has the ability to promote Th1 cells to produce cytokines such as TNF-α, IFN-γ, and granulocyte-macrophage colony-stimulating factor [GM-CSF], which may enhance phagocytosis and cytotoxicity mediated by macrophages [30]; and IL-3 and GM-CSF secreted by Th1 and Th2 cells stimulate the bone marrow to generate macrophages, granulocytes, and dendritic cells), and (iv) participate in immune regulation. Immune cells are capable of stimulating and inhibiting each other by secreting cytokines during the course of immune response, thereby mediating effective regulation.
P. acnes and A. pleuropneumoniae are Gram-positive and Gram-negative bacteria, respectively. Why could the Gram-positive bacteria provide cross protection against Gram-negative bacterial infection? From our experimental results, we speculated that the possible reasons are as follows: (i) there may be some Forssman antigens shared between P. acnes and A. pleuropneumoniae, which are unrelated to the species and are the common antigens among humans, animals, and microorganisms; (ii) Forssman antigen is a glycosphingolipid with antigenic specificity determined by extramembrane haptenic sugars similar to blood group antigens and antigens that are the main barriers to xenogeneic organ transplantation (31); and (iii) there may be some common B cell epitopes between P. acnes and A. pleuropneumoniae, which we have published elsewhere (32). It is possible that the cytokines stimulated by P. acnes also play an important role in resistance to A. pleuropneumoniae infection. However, these hypotheses need to be studied further.
In this study, we further elucidate the mechanism by which P. acnes prevents porcine contagious pleuropneumonia, which provides the basis of further investigations into the development of heterologous vaccines for the control of porcine pleuropneumonia.
ACKNOWLEDGMENT
This work was supported by a grant from the National Natural Science Foundation of China (31172351).
Footnotes
Published ahead of print 15 January 2014
REFERENCES
- 1.Macinnes JI, Rosendal S. 1988. Prevention and control of Actinobacillus (Haemophilus) pleuropneumoniae infection in swine: a review. Can. Vet. J. 29:572–574 [PMC free article] [PubMed] [Google Scholar]
- 2.Tobias TJ, Raymakers RJ, van Nes A, van Leengoed LA. 2009. Outbreak of respiratory distress resembling influenza caused by Actinobacillus pleuropneumoniae in pigs. Vet. Rec. 164:402–403. 10.1136/vr.164.13.402 [DOI] [PubMed] [Google Scholar]
- 3.Reiner G, Fresen C, Bronnert S, Haack I, Willems H. 2010. Prevalence of Actinobacillus pleuropneumoniae infection in hunted wild boars (Sus scrofa) in Germany. J. Wildl. Dis. 46:551–555. 10.7589/0090-3558-46.2.551 [DOI] [PubMed] [Google Scholar]
- 4.Blackall PJ, Klaasen HL, van den Bosch H, Kuhnert P, Frey J. 2002. Proposal of a new serovar of Actinobacillus pleuropneumoniae: serovar 15. Vet. Microbiol. 84:47–52. 10.1016/S0378-1135(01)00428-X [DOI] [PubMed] [Google Scholar]
- 5.Ramjeet M, Deslandes V, Goure J, Jacques M. 2008. Actinobacillus pleuropneumoniae vaccines: from bacterins to new insights into vaccination strategies. Anim. Health Res. Rev. 9:25–45. 10.1017/S1466252307001338 [DOI] [PubMed] [Google Scholar]
- 6.Cruijsen T, van Leengoed LA, Ham-Hoffies M, Verheijden JH. 1995. Convalescent pigs are protected completely against infection with a homologous Actinobacillus pleuropneumoniae strain but incompletely against a heterologous-serotype strain. Infect. Immun. 63:2341–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backstrom L. 1999. Present uses of and experiences with swine vaccines. Adv. Vet. Med. 41:419–428. 10.1016/S0065-3519(99)80032-9 [DOI] [PubMed] [Google Scholar]
- 8.Haesebrouck F, Pasmans F, Chiers K, Maes D, Ducatelle R, Decostere A. 2004. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet. Microbiol. 100:255–268. 10.1016/j.vetmic.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 9.Lei L, Sun C, Lu S, Feng X, Wang J, Han W. 2008. Selection of serotype-specific vaccine candidate genes in Actinobacillus pleuropneumoniae and heterologous immunization with Propionibacterium acnes. Vaccine 26:6274–6280. 10.1016/j.vaccine.2008.09.039 [DOI] [PubMed] [Google Scholar]
- 10.Webster GF. 1990. Inflammatory acne. Int. J. Dermatol. 29:313–317. 10.1111/j.1365-4362.1990.tb04749.x [DOI] [PubMed] [Google Scholar]
- 11.Hensel A, Stockhofe-Zurwieden N, Petzoldt K, Lubitz W. 1995. Oral immunization of pigs with viable or inactivated Actinobacillus pleuropneumoniae serotype 9 induces pulmonary and systemic antibodies and protects against homologous aerosol challenge. Infect. Immun. 63:3048–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ananias RZ, Rodrigues EG, Braga EG, Squaiella CC, Mussalem JS, Longhini AL, Travassos LR, Longo-Maugeri IM. 2007. Modulatory effect of killed Propionibacterium acnes and its purified soluble polysaccharide on peritoneal exudate cells from C57Bl/6 mice: major NKT cell recruitment and increased cytotoxicity. Scand. J. Immunol. 65:538–548. 10.1111/j.1365-3083.2007.01939.x [DOI] [PubMed] [Google Scholar]
- 13.Woodruff MF, McBride WH, Dunbar N. 1974. Tumour growth, phagocytic activity and antibody response in Corynebacterium parvum-treated mice. Clin. Exp. Immunol. 17:509–518 [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuda K, Yamanaka K, Linan W, Miyahara Y, Akeda T, Nakanishi T, Kitagawa H, Kakeda M, Kurokawa I, Shiku H, Gabazza EC, Mizutani H. 2011. Intratumoral injection of Propionibacterium acnes suppresses malignant melanoma by enhancing Th1 immune responses. PLoS One 6:e29020. 10.1371/journal.pone.0029020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mussalem JS, Vasconcelos JR, Squaiella CC, Ananias RZ, Braga EG, Rodrigues MM, Longo-Maugeri IM. 2006. Adjuvant effect of the Propionibacterium acnes and its purified soluble polysaccharide on the immunization with plasmidial DNA containing a Trypanosoma cruzi gene. Microbiol. Immunol. 50:253–263. 10.1111/j.1348-0421.2006.tb03791.x [DOI] [PubMed] [Google Scholar]
- 16.Tchaptchet S, Gumenscheimer M, Kalis C, Freudenberg N, Holscher C, Kirschning CJ, Lamers M, Galanos C, Freudenberg MA. 2012. TLR9-dependent and independent pathways drive activation of the immune system by Propionibacterium acnes. PLoS One 7:e39155. 10.1371/journal.pone.0039155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung YS, Matsumoto SE, Yamashita M, Tomimatsu K, Teruya K, Katakura Y, Shirahata S. 2007. Propionibacterium acnes acts as an adjuvant in in vitro immunization of human peripheral blood mononuclear cells. Biosci. Biotechnol. Biochem. 71:1963–1969. 10.1271/bbb.70159 [DOI] [PubMed] [Google Scholar]
- 18.Levine M, Brumley RL., Jr 1989. Fast ELISA for measuring serum antibody responses. J. Immunol. Methods 119:211–215. 10.1016/0022-1759(89)90398-0 [DOI] [PubMed] [Google Scholar]
- 19.Sukumar N, Love CF, Conover MS, Kock ND, Dubey P, Deora R. 2009. Active and passive immunizations with Bordetella colonization factor A protect mice against respiratory challenge with Bordetella bronchiseptica. Infect. Immun. 77:885–895. 10.1128/IAI.01076-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oscherwitz J, Gribbin TE, Cease KB. 2010. A CD20 tandem-epitope immunogen elicits antibody in mice that binds murine cell surface CD20 and depletes splenic B cells in vivo. Mol. Immunol. 47:1484–1491. 10.1016/j.molimm.2010.01.026 [DOI] [PubMed] [Google Scholar]
- 21.Oscherwitz J, Zeigler ME, Gribbin TE, Cease KB. 1999. A V3 loop haptenic peptide sequence, when tandemly repeated, enhances immunogenicity by facilitating helper T-cell responses to a covalently linked carrier protein. Vaccine 17:2392–2399. 10.1016/S0264-410X(99)00030-4 [DOI] [PubMed] [Google Scholar]
- 22.Yoneyama H, Narumi S, Zhang Y, Murai M, Baggiolini M, Lanzavecchia A, Ichida T, Asakura H, Matsushima K. 2002. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J. Exp. Med. 195:1257–1266. 10.1084/jem.20011983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoneyama H, Matsuno K, Zhang Y, Murai M, Itakura M, Ishikawa S, Hasegawa G, Naito M, Asakura H, Matsushima K. 2001. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J. Exp. Med. 193:35–49. 10.1084/jem.193.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakayama Y, Shimizu Y, Hirano K, Ebata K, Minemura M, Watanabe A, Sugiyama T. 2005. CTLA-4Ig suppresses liver injury by inhibiting acquired immune responses in a mouse model of fulminant hepatitis. Hepatology 42:915–924. 10.1002/hep.20872 [DOI] [PubMed] [Google Scholar]
- 25.Schlageter AM, Kozel TR. 1990. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect. Immun. 58:1914–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewlett EL, Donato GM, Gray MC. 2006. Macrophage cytotoxicity produced by adenylate cyclase toxin from Bordetella pertussis: more than just making cyclic AMP! Mol. Microbiol. 59:447–459. 10.1111/j.1365-2958.2005.04958.x [DOI] [PubMed] [Google Scholar]
- 27.Ross PJ, Lavelle EC, Mills KH, Boyd AP. 2004. Adenylate cyclase toxin from Bordetella pertussis synergizes with lipopolysaccharide to promote innate interleukin-10 production and enhances the induction of Th2 and regulatory T cells. Infect. Immun. 72:1568–1579. 10.1128/IAI.72.3.1568-1579.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozakiewicz L, Phuah J, Flynn J, Chan J. 2013. The role of B cells and humoral immunity in Mycobacterium tuberculosis infection. Adv. Exp. Med. Biol. 783:225–250. 10.1007/978-1-4614-6111-1_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. 2011. Regulation of inflammation by short chain fatty acids. Nutrients 3:858–876. 10.3390/nu3100858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furusawa H, Suzuki Y, Miyazaki Y, Inase N, Eishi Y. 2012. Th1 and Th17 immune responses to viable Propionibacterium acnes in patients with sarcoidosis. Respir. Investig. 50:104–109. 10.1016/j.resinv.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 31.Yuzawa Y, Brett J, Fukatsu A, Matsuo S, Caldwell PR, Niesen N, Milgrom F, Godman G, Stern D, Andres G. 1995. Interaction of antibody with Forssman antigen in guinea pigs. A mechanism of adaptation to antibody- and complement-mediated injury. Am. J. Pathol. 146:1260–1272 [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Sun C, Yang F, Yang S, Feng X, Gu J, Han W, Langford PR, Lei L. 2013. Identification of proteins of Propionibacterium acnes for use as vaccine candidates to prevent infection by the pig pathogen Actinobacillus pleuropneumoniae. Vaccine 31:5269–5275. 10.1016/j.vaccine.2013.08.054 [DOI] [PubMed] [Google Scholar]