Abstract
Live oral Salmonella enterica serovar Typhi vaccine Ty21a induces specific antibodies that cross-react against Salmonella enterica serovar Paratyphi A and Salmonella enterica serovar Paratyphi B, although their functional role in clearance remains unknown. We utilized an in vitro assay with THP-1 macrophages to compare the phagocytosis and survival of Salmonella opsonized with heat-inactivated human sera obtained before and after vaccination with Ty21a or a live oral S. Typhi vaccine, CVD 909. Opsonization with postvaccination sera predominantly increased the phagocytosis of S. Typhi relative to the corresponding prevaccination sera, and increases were also observed with S. Paratyphi A and S. Paratyphi B, albeit of lower magnitudes. Relative to prevaccination sera, opsonization with the postvaccination sera reduced the survival inside macrophages of S. Typhi but not of S. Paratyphi A or S. Paratyphi B. Higher anti-S. Typhi O antigen (lipopolysaccharide [LPS]) IgG, but not IgA, antibody titers correlated significantly with postvaccination increases in opsonophagocytosis. No differences were observed between immunization with four doses of Ty21a or one dose of CVD 909. Ty21a and CVD 909 induced cross-reactive functional antibodies, predominantly against S. Typhi. IgG anti-LPS antibodies may be important in phagocytic clearance of these organisms. Therefore, measurement of functional antibodies might be important in assessing the immunogenicity of a new generation of typhoid and paratyphoid A vaccines. (The CVD 909 study has been registered at ClinicalTrials.gov under registration no. NCT00326443.)
INTRODUCTION
Salmonella enterica serovar Typhi (S. Typhi), a human-restricted pathogen, is the causative agent of typhoid fever, the most prevalent form of enteric fever, resulting in an estimated 21.7 million cases and 200,000 deaths per year worldwide (1). However, infection with Salmonella enterica serovar Paratyphi A (paratyphoid A fever) or Salmonella enterica serovar Paratyphi B (paratyphoid B fever) causes similar clinical manifestations (2). While less prevalent than typhoid fever, paratyphoid fever occurs in an estimated 5.4 million cases each year (3–6). Recent reports indicate that paratyphoid A fever is on the rise in areas of endemicity (e.g., South and Southeast Asia, China) and among travelers returning from those areas (3–6). The emergence of multiple antibiotic-resistant Salmonella strains has increased the health risks posed by these enteric fever infections (7).
Currently, two licensed vaccines against typhoid fever are available worldwide, parenteral Vi polysaccharide (Vi) and oral live attenuated Ty21a. Nevertheless, each of these vaccines exhibits limitations that have fostered the development of a new generation of vaccines, including both of the engineered live oral typhoid vaccines (e.g., CVD 908-htrA [ΔaroC ΔaroD ΔhtrA] and CVD 909 [CVD 908-htrA further engineered to constitutively express Vi]) (8) and the M01ZH09 (ΔaroC ΔssaV) (9) and Vi carrier protein conjugate vaccines (10). There are, however, no available licensed vaccines against S. Paratyphi A or B.
Whole-genome comparative analysis has revealed a high degree of homology among S. Typhi, S. Paratyphi A, and S. Paratyphi B at the DNA level, suggesting the theoretical potential for vaccines against S. Typhi to offer some degree of cross-protection against S. Paratyphi A and S. Paratyphi B (11, 12). Vi-based vaccines cannot confer cross-protection, since neither S. Paratyphi A nor S. Paratyphi B expresses Vi antigen. In contrast, large-scale field trials of the efficacy of the oral Ty21a vaccine have documented a moderate level of cross-protection against S. Paratyphi B but not against S. Paratyphi A (13–15). Therefore, the development of an effective vaccine against S. Paratyphi A has emerged as a public health priority (16).
We and others have extensively studied the induction of S. Typhi-specific humoral and cellular immune responses (cell-mediated immunity [CMI]) following immunization with Ty21a and other live oral typhoid vaccines in humans (9, 17–28) and have also begun to explore the cross-reactive immune responses elicited by the S. Typhi live oral vaccines against S. Paratyphi A and S. Paratyphi B that might explain the observed cross-protection and its serovar limitations (29, 30).
The capacity of oral typhoid vaccines to induce humoral responses has been well documented, but information on the functional capacity of the induced antibodies has been sparse except for a few reports on specific antibody-enhanced phagocytosis and intracellular killing. In the 1980s, Tagliabue et al. reported that the IgA antibodies induced following oral immunization with Ty21a mediate a CD4+ T cell-dependent antibody-dependent cellular cytotoxicity (ADCC) against S. Typhi and against S. Paratyphi A and S. Paratyphi B, but not against S. Paratyphi C (31). Levine et al. described the presence of plasma-dependent mononuclear cell killing of S. Typhi in subjects immunized orally with attenuated S. Typhi vaccine strains (32). More recently, antibody-mediated enhanced phagocytosis and intracellular killing of S. Typhi by macrophages was described following immunization with live oral S. Typhi strain M01ZH09 (33). However, the cross-reactivity of typhoid vaccine-induced antibodies in opsonin-mediated intake (opsonophagocytosis) and intracellular killing of S. Paratyphi A and S. Paratyphi B has not been reported. The aim of the present study was to investigate whether Ty21a or CVD 909 induce antibodies which mediate enhanced opsonophagocytosis and/or intracellular killing of S. Typhi and their cross-reactive activity against S. Paratyphi A and S. Paratyphi B. We further investigated the correlation of vaccine-induced antilipopolysaccharide (anti-LPS) antibody titers with the observed opsonin-mediated phagocytosis and intracellular killing.
MATERIALS AND METHODS
Subjects, immunizations, and serum samples.
We used serum samples from 15 healthy adult volunteers who received a live oral typhoid Ty21a vaccine or candidate vaccine strain CVD 909 (34). Ty21a vaccine recipients (n = 8 [2 male and 6 female]; age range, 22 to 28 years) received four doses of licensed Ty21a vaccine at 48-hour intervals (35). CVD 909 recipients (n = 7 [3 male and 4 female]; age range, 28 to 37 years) received a single oral dose (5 × 109 CFU) preceded by sodium bicarbonate buffer (36). Serum samples were collected prevaccination (day 0) and postvaccination (day 10 and/or 14) and stored at −70°C until used. Healthy volunteers who had no history of typhoid fever or immunization against typhoid fever and who were from the Baltimore, MD/Washington, DC, area and the University of Maryland Baltimore (UMB) community were recruited for these studies, which were approved by the UMB Institutional Review Board. The CVD 909 study has been registered at ClinicalTrials.gov under registration no. NCT00326443.
Bacterial strains.
Salmonella strains were obtained from the Center for Vaccine Development, University of Maryland, reference stocks. The S. Typhi (ISP-1820, Vi+) and S. Paratyphi B (CV 23) clinical isolates used were from Chile. The S. Paratyphi A (CV 223) strain was purchased from the American Type Culture Collection (ATCC, Rockville, MD) (catalog number 9150). The strains were grown from frozen stocks by an overnight incubation in Luria broth (LB) with vigorous shaking at 37°C. On the following day, the bacterial cultures were diluted 1:50 in LB and grown to log phase (optical density at 600 nm [OD600[, ∼0.4 to 0.6). The bacterial cultures were washed once with sterile phosphate-buffered saline (PBS) and further diluted to reach an OD600 of 0.2 (∼1 × 108 bacteria/ml).
Cell culture.
THP-1 macrophages-monocytes (ATCC catalog number TIB-202) were grown in complete medium (RPMI 1640 [Gibco Invitrogen, Carlsbad, CA] supplemented with 10% heat-inactivated fetal bovine serum [BioWhittaker, Walkersville, MD], 2 mM l-glutamine [HyClone, Logan, UT], 2.5 mM sodium pyruvate [Gibco], and 10 mM HEPES [Gibco] with or without 100 U/ml penicillin [Sigma-Aldrich, St. Louis, MO], 100 μg/ml streptomycin [Sigma-Aldrich], and 50 μg/ml gentamicin [Gibco]).
Opsonophagocytosis and bacterial killing assays.
The assays were performed as originally described by Lindow et al. with some modifications (33). In brief, THP-1 monocytes were seeded (5 × 105/well) onto 24-well plates and differentiated into macrophages by adding 50 ng/ml of phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) for 48 h. Cells were then washed twice with PBS and incubated at 37°C in 5% CO2 and in 0.5 ml RPMI complete medium without antibiotics for 2 to 4 h. Serum samples were preheated at 56°C for 30 min to destroy complement. Five-percent heat-inactivated serum samples were added to bacteria and were incubated for 30 min at 37°C to allow for antibody binding (opsonization).
The opsonized bacteria were added to the PMA-differentiated THP-1 macrophage (henceforth, macrophage) monolayers cultured in antibiotic-free complete medium at a multiplicity of infection of 1:10 (cell/bacteria) and spun immediately (200 × g) for 5 min to allow adhesion. The plates were incubated for 30 min at 37°C in 5% CO2. Identical plates were set up for opsonophagocytosis assays (30 min) and survival assays (24 h). Following incubation, both sets of plates were washed three times with cold PBS. Extracellular bacteria were killed by adding complete medium containing gentamicin (200 μg/ml) to the wells for an additional 90 min, followed by 2 washes with PBS. For the opsonophagocytosis assays, plates containing differentiated macrophages exposed to opsonized bacteria were immediately lysed with 1% Triton X-100 for 10 min at 37°C. The lysate was diluted in PBS and plated onto LB plates, which were incubated at 37°C overnight before enumeration of the CFU.
For the survival assays, plates were incubated overnight with 1 ml of complete medium containing gentamicin (10 μg/ml) and washed twice with cold PBS the following day. The surviving bacteria were counted by lysing and plating the cell lysates onto LB plates as described above.
In some experiments, macrophages were treated with 2 μM cytochalasin D (Sigma) for 1 h before adding the opsonized organisms.
Calculation of postvaccination fold changes.
The postvaccination fold increases in phagocytosis were calculated by dividing the number of phagocytosed bacteria (CFU/ml) following opsonization with postvaccination sera (day 10 or 14) by the CFU/ml of phagocytosed bacteria opsonized with the corresponding 5% prevaccination sera (day 0). The highest postvaccination fold increase at either day 10 or day 14 was considered the peak response.
The survival of opsonized bacteria that were phagocytosed by macrophages was quantified after 24 h in culture from the second set of identical plates described above. The rate of survival was calculated as (CFU/ml of bacteria recovered after 24 h)/(corresponding CFU/ml of phagocytosed bacteria) × 100. The postvaccination fold change in survival rate was calculated by dividing the survival rates with postvaccination sera (day 10 or 14) by that of the prevaccination (day 0) sera. As lower survival rates are indicative of increased killing, the lowest postvaccination fold changes (at day 10 or 14) in survival rates were considered the peak bacterial killing.
Serum antibody titer assay.
As described above, the serum samples used in this study were obtained from two different clinical studies previously described and reported in the literature (35, 36), which included the measurement of serum IgA and IgG antibody titers to S. Typhi LPS by an enzyme-linked immunosorbent assay (ELISA) using purified LPS from S. Typhi (Sigma, St. Louis, MO). We used those historical serological data for the correlation analyses performed in this study.
Statistical analysis.
Statistical analyses were performed using GraphPad Prism version 5.03 (GraphPad Software). The two-tailed Mann-Whitney U and Wilcoxon matched-pair tests were used to evaluate the statistical differences as indicated in the text. The significance of the correlation coefficients (nonparametric) was calculated using Spearman tests. P values of <0.05 were considered significant.
RESULTS
Normal human sera enhance antibody-mediated opsonophagocytosis of Salmonella strains.
Internalization of S. Typhi by differentiated THP-1 macrophages (macrophages) is mediated mainly by phagocytosis (33). We therefore evaluated whether a similar process of phagocytosis is involved in the internalization of S. Paratyphi A and S. Paratyphi B and whether opsonization with complement-inactivated normal human sera increases the internalization of bacteria. When nonopsonized bacterial strains (i.e., S. Typhi, S. Paratyphi A, and S. Paratyphi B) were added to macrophages, all three strains were internalized equally into the macrophages. This internalization was markedly reduced when the macrophages were pretreated with the phagocytosis-inhibiting agent cytochalasin D (Fig. 1A). Note that opsonization of bacteria by preincubation with normal (prevaccination) human sera increased phagocytosis of all three strains by at least 2.5-fold compared with that of nonopsonized bacteria (Fig. 1B).
FIG 1.
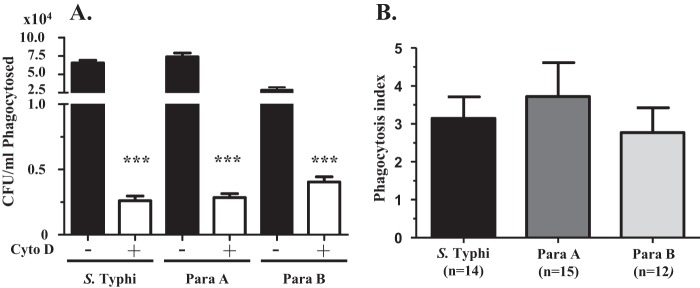
Internalization of Salmonella strains into differentiated THP-1 macrophages is mediated by phagocytosis and is enhanced by opsonization with human sera. (A) Differentiated THP-1 macrophages were infected with nonopsonized S. Typhi, S. Paratyphi A (Para A), or S. Paratyphi B (Para B) at a multiplicity of infection of 10:1 (bacteria/cell) (filled bars). Parallel cultures were pretreated with cytochalasin D (Cyto D) to prevent phagocytosis. Numbers of internalized bacteria after 30 min of infection were quantified as CFU/ml as described in Materials and Methods. (B) Fold increases of phagocytosis in differentiated THP-1 macrophages following opsonization with 5% prevaccination sera (phagocytosis index) were calculated relative to the corresponding values for nonopsonized bacteria. ***, P < 0.001 compared to the corresponding untreated group (two-tailed nonparametric Mann-Whitney test).
Opsonophagocytic activity is enhanced by vaccine-induced antibodies.
The two major opsonins present in human sera are antibodies and complement components. Heating human serum at 56°C for 30 min inactivates complement but does not impact the functionality of antibodies. We investigated whether oral typhoid vaccine-induced antibodies could enhance the opsonophagocytosis of S. Typhi, S. Paratyphi A, and/or S. Paratyphi B using archived pre- and postvaccination (day 10 and/or 14) sera from 15 volunteers who were immunized with either 4 doses of Ty21a (n = 8) or a single dose of CVD 909 (n = 7). We calculated the mean peak postvaccination fold increases in phagocytosis (compared to the prevaccination fold increases) with sera from Ty21a or CVD 909 vaccinees. The mean peak postvaccination fold increases in phagocytosis were similar for both groups (Fig. 2A). Therefore, we analyzed the data from both groups of vaccinees together (n = 15). When bacteria were opsonized with either pre- or postvaccination sera, increases in phagocytosis were observed compared to when nonopsonized (no-sera) bacteria were used (Fig. 1B and data not shown). Interestingly, postvaccination sera opsonized S. Paratyphi A, S. Paratyphi B, and S. Typhi; however, as shown in Fig. 2B, the postvaccination peak fold increases in phagocytosis (compared to the corresponding prevaccination levels [mean ± standard error (SE)]) for S. Typhi (1.9 ± 0.27 fold) were greater than those observed for S. Paratyphi A (1.38 ± 0.21) and S. Paratyphi B (1.40 ± 0.18). Although these differences did not reach statistical significance, there was a strong trend (P = 0.07) toward higher postvaccination opsonophagocytosis (Fig. 2A and B) for S. Typhi than for S. Paratyphi A and S. Paratyphi B.
FIG 2.
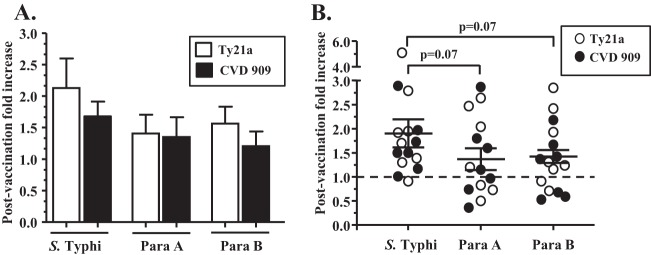
Fold increases in phagocytosis of bacteria following opsonization with postvaccination sera. Phagocytosis assays were performed in differentiated THP-1 macrophages with Salmonella strains opsonized with 5% prevaccination (day 0) or postvaccination (day 10 and/or 14) sera (see Materials and Methods). Fold increases in phagocytosis were calculated as the peak of phagocytosed bacteria (CFU/ml) opsonized with day 10 or day 14 sera/phagocytosed bacteria (CFU/ml) opsonized with corresponding day 0 sera. No changes with regard to preimmunization levels are denoted by the dotted line (1-fold). Data are presented as the mean fold increases ± the standard errors (SE) of volunteers who were immunized with either four doses of Ty21a (n = 8) or a single dose of CVD 909 (n = 7) separately (A) or together (B). The P values were determined by two-tailed Wilcoxon matched-pair tests.
Postvaccination sera decreased intracellular bacterial survival.
Macrophages play a major role in innate immune responses to intracellular organisms by capturing and killing engulfed bacteria. However, Salmonella is also known to survive within human macrophages (37). Since postvaccination sera increased macrophage bacterial phagocytosis, we further investigated whether opsonizing antibodies also influenced their intracellular survival. When preincubated with heat-inactivated prevaccination sera, the survival rates for phagocytosed S. Typhi (3.9 ± 0.65%) and S. Paratyphi A (2.7 ± 0.52%) were similar. In contrast, the survival rate for S. Paratyphi B (14.2 ± 2.1%) was significantly higher (P < 0.001) (Fig. 3). We then compared the survival rates of Salmonella (opsonized with pre- and postvaccination sera) within THP-1 macrophages by using samples from volunteers who were immunized with either Ty21a (n = 7) or CVD 909 (n = 7). Since no differences were observed between these two vaccination groups in regard to postvaccination changes in survival rates (data not shown), data from all the volunteers (n = 14) are presented together (Fig. 4).
FIG 3.
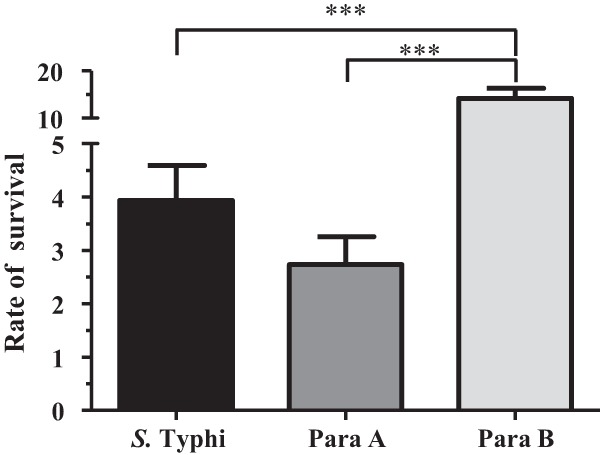
Survival of bacteria incubated with prevaccination sera within differentiated THP-1 macrophages. The 3 Salmonella strains were opsonized with prevaccination (day 0) sera (n = 14 [7 Ty21a and 7 CVD 909]), and survival assays were performed as described in Materials and Methods. Rate of survival was calculated as (bacteria recovered [CFU/ml] after 24 h/corresponding opsonophagocytosed bacteria [CFU/ml] after 30 min of infection) × 100. The P values were determined by a two-tailed Mann-Whitney test. ***, P < 0.001.
FIG 4.
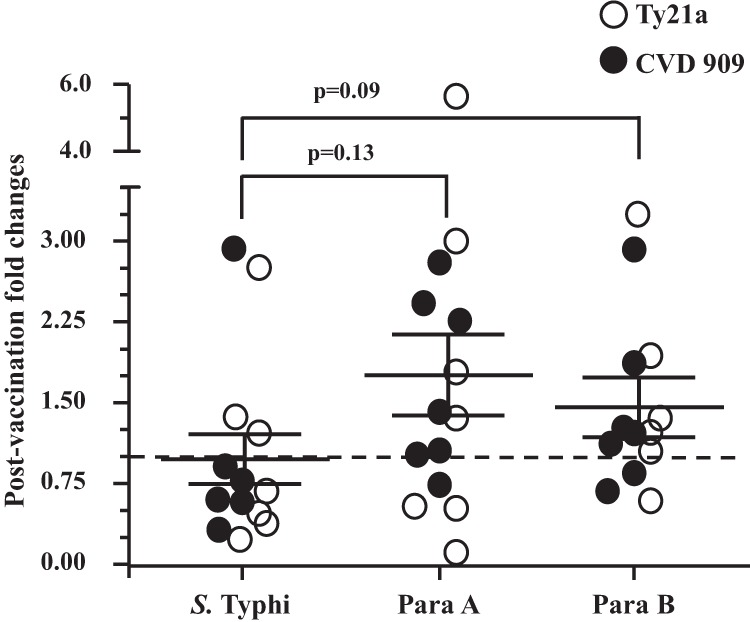
Fold changes in survival of opsonophagocytosed bacteria within differentiated THP-1 macrophages. The 3 strains of Salmonella were opsonized with prevaccination (day 0) and postvaccination (day 10 and/or 14) sera, and survival assays were performed as described in Materials and Methods. The rates of survival were calculated as (bacteria recovered [CFU/ml] after 24 h/corresponding opsonophagocytosed bacteria [CFU/ml] after 30 min of infection] × 100. Postvaccination fold changes in rate of survival were determined by comparing postvaccination rates of survival with their corresponding day 0 values. No changes with regard to preimmunization levels are denoted by the dotted line (1-fold). Data are presented as means ± SE of the peak postvaccination changes in survival (lowest fold changes at either day 10 or 14) in volunteers (n = 14) vaccinated with either four doses of Ty21a (n = 7) or a single dose of CVD 909 (n = 7). The P values were determined by a two-tailed Wilcoxon matched-pair test.
Decreases in the rates of survival (compared to prevaccination level) were observed in most volunteers for S. Typhi only when the bacteria were preincubated with postvaccination sera (Fig. 4). The differences in mean fold changes among the Salmonella strains did not reach statistical significance (Fig. 4).
Correlation of opsonophagocytosis activity and bacterial survival rates with serum anti-S. Typhi LPS antibody titers.
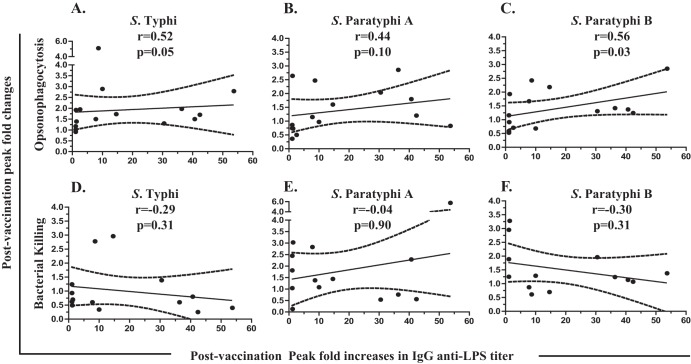
The levels of antibodies to LPS have been shown to rise within the first 2 weeks following immunization with live oral typhoid vaccines (25, 29, 36–42). Therefore, we explored whether the functional activity of antibodies described above correlated with anti-LPS titers. As shown in Fig. 5 and Table 1, postvaccination fold increases in IgG anti-S. Typhi LPS antibody titers correlated significantly with the corresponding increases in the opsonophagocytosis of S. Typhi (P = 0.05) and S. Paratyphi B (P = 0.03), while there was a trend toward correlation for S. Paratyphi A (P = 0.1). Note that the positive correlation observed in Fig. 5A was significant (rho = 0.52, P < 0.05) when all the subjects were included in the calculations. Removal of the outlier (defined as >mean + 3 SE) showing the highest postvaccination increase in opsonophagocytosis activity (5.1-fold) increased the significance of the observed correlations (rho = 0.57, P = 0.03).
FIG 5.
Correlation of opsonophagocytosis activity and bacterial survival rates with serum IgG anti-S. Typhi lipopolysaccharide (LPS) antibody titers. Peak postvaccination fold changes (day 10 and/or 14) were determined by comparison to the corresponding prevaccination levels. Peak postvaccination increases in serum IgG anti-LPS antibody titers in volunteers (n = 14) following immunization with either Ty21a (n = 7) or CVD 909 (n = 7) were correlated with the corresponding peak postvaccination fold increases in opsonophagocytosis (A, B, C) or peak bacterial killing (D, E, F) for S. Typhi (A, D), S. Paratyphi A (B, E), and S. Paratyphi B (C, F). The broken lines represent 95% confidence intervals. p, P value; r, Spearman's rho.
TABLE 1.
Correlation of opsonophagocytosis activity and bacterial survival rates of Salmonella strains with serum antibody titers against S. Typhi LPSa
| Serum anti-LPS isotype and Salmonella serovar | Opsonophagocytosis P value (r)b | Bacterial survival P value (r)c |
|---|---|---|
| IgG | ||
| S. Typhi | 0.05d (0.52) | 0.31 (−0.30) |
| S. Paratyphi A | 0.10 (0.44) | 0.90 (−0.04) |
| S. Paratyphi B | 0.03d (0.56) | 0.31 (−0.30) |
| IgA | ||
| S. Typhi | 0.14 (0.40) | 0.40 (0.23) |
| S. Paratyphi A | 0.27 (0.31) | 0.19 (0.38) |
| S. Paratyphi B | <0.01e (0.71) | 0.38 (−0.26) |
LPS, lipopolysaccharide (S. Typhi).
Peak fold increase on postvaccination day 10 or 14 compared to that on corresponding day 0 (prevaccination). r, Spearman's rho.
Lowest fold changes in survival rates on postvaccination day 10 or 14 compared to that on corresponding day 0 (prevaccination).
P < 0.05.
P < 0.01 (two-tailed Spearman test).
For IgA anti-LPS, a significant positive correlation with increases in opsonophagocytosis was observed only with S. Paratyphi B (P < 0.01). Overall, increased opsonophagocytosis of all three Salmonella strains showed greater association with IgG anti-LPS than with IgA. The survival of phagocytosed bacteria opsonized with postvaccination sera did not show significant correlations with either IgG or IgA anti-LPS antibody titers (Fig. 5, Table 1).
DISCUSSION
Currently available licensed vaccines against typhoid fever offer no effective cross-protection against paratyphoid A fever, and the recent emergence of S. Paratyphi A infection has emphasized the need for a vaccine against this pathogen (5, 14, 16). The measurement of strain-specific humoral immune responses, including antibody-secreting cells (ASC) and/or serum antibodies, is a fundamental part of assessing the immunogenicity of live oral Salmonella vaccines. Ty21a and other candidate typhoid vaccines (including CVD 909) are capable of eliciting S. Typhi-specific antibodies (e.g., against O and H antigens) (25, 29, 36, 39, 41, 43, 44), as well as cross-reactive antibodies against S. Paratyphi A and S. Paratyphi B (29). Nevertheless, the role of such antibodies, if any, in protection from disease or in the elimination of intracellular bacteria is not well understood.
In the 1980s, Tagliabue et al. reported that following oral immunization with Ty21a, a type of antibody-dependent cellular killing of S. Typhi was detected in which serum postvaccination IgA antibodies provided the specificity and CD4 lymphocytes implemented the killing (31). Levine et al. (32) corroborated that postvaccination plasma plus peripheral blood mononuclear cells (PBMC) from subjects immunized with two otherwise poorly immunogenic candidate live oral vaccines achieved intracellular killing of wild-type S. Typhi. A recent study with another candidate oral typhoid vaccine, strain M01ZH09, showed that, independent of complement, immunoglobulins from early (within 2 weeks) postvaccination sera enhanced the phagocytosis and killing of S. Typhi by THP-1 macrophages (33).
In the current study, we extended these observations to yet another novel live oral candidate vaccine, strain CVD 909, and revisited the responses following immunization with Ty21a. Furthermore, we have described the cross-reactivity of opsonophagocytic antibodies with S. Paratyphi A and S. Paratyphi B. The volunteers who participated in this study were healthy adults without any history of typhoid disease or vaccination. Despite a lack of previous S. Typhi exposure, complement-inactivated preimmune sera from these volunteers contained opsonins that were able to enhance the opsonophagocytosis of S. Typhi, S. Paratyphi A, and S. Paratyphi B by THP-1 macrophages. This observation is not surprising since it is known that healthy human adult serum contains components that can aid in the elimination of Salmonella pathogens (45, 46). These components include natural antibodies, cross-reacting antibodies resulting from clinical or subclinical infection with nontyphoidal Salmonella serovars, such as Salmonella enterica serovar Enteritidis (group D) or Salmonella enterica serovar Typhimurium (group B), that are common causes of food-borne gastroenteritis, and nonspecific serum binding proteins, such as mannose binding proteins. Moreover, given the considerable homology of Salmonella strains with other common Gram-negative pathogens found in the gut, cross-reactive functional antibodies are likely to be present. These cross-reactive antibodies are usually considered nonspecific.
In our study, following immunization with either Ty21a or CVD 909, we observed increases in opsonophagocytosis (compared to prevaccination opsonophagocytosis levels). Increased opsonophagocytosis of S. Typhi was greater in magnitude than that observed for S. Paratyphi A and S. Paratyphi B. This increase in opsonophagocytosis correlated with anti-LPS IgG antibodies directed against S. Typhi. It is possible that these functional antibodies contribute to protection. Indeed, IgG-opsonized bacteria can be phagocytosed and degraded inside phagocytic cells (i.e., dendritic cells) via binding to low-affinity FcγRs (47). Additionally, they may initiate a cascade of responses that are critical for bacterial elimination through the activation of the complement system, cell degranulation, production of reactive oxygen species, or ADCC and subsequent presentation of pathogen-derived antigens to T cells (31, 48–50). In a previous study, we showed that serum antibody levels against the LPS antigens of S. Typhi, S. Paratyphi A, and S. Paratyphi B elicited by Ty21a vaccination were of similar magnitudes (29). In contrast, the antibody functionality data presented in this study show that Ty21a and CVD 909 induce antibodies that enhance opsonophagocytosis and intracellular killing to a larger extent for S. Typhi than for S. Paratyphi A or S. Paratyphi B. It is probable that the magnitude of vaccine-induced antibody titers is not always indicative of differences in antibody functional abilities. Thus, if these humoral immune responses play a key role in protection, it is likely that S. Paratyphi A and/or S. Paratyphi B vaccines will be needed to optimally combat paratyphoid fevers.
Our results show that postvaccination sera not only enhanced the opsonophagocytosis of S. Typhi into macrophages but also decreased their intracellular survival. These observations with Ty21a and CVD 909 vaccinees are similar to those reported with postvaccination sera from M01ZH09-immunized volunteers (33). Interestingly, we noted that the survival of S. Paratyphi A or S. Paratyphi B inside macrophages was not inhibited by opsonization with antibodies. Although we do not have a clear explanation for this discrepancy, it is possible that either the typhoid vaccines do not induce sufficient functional cross-reactive antibodies against these organisms above a necessary threshold or, as our system was devoid of complement, the degradation or elimination of S. Paratyphi A or S. Paratyphi B may be dependent on complement. It is also conceivable that, in addition to the presence of cross-reactive functional antibodies, the differences seen between the functional activities against the three different Salmonella species are also related, at least in part, to inherent resistance to opsonophagocytosis/intracellular killing.
The historical data available for the samples used in these studies showed minimal, if any, postvaccination increases in serum IgG and IgA antibody titers measured against an S. Typhi H:d flagellum antigen (data not shown). The anti-Vi antibody titers were also measured in CVD 909, which constitutively expresses Vi (36). Although antibody-secreting cells and memory B cells against Vi were elicited following immunization with CVD 909, we did not observe the induction of anti-Vi serum antibody titers in postvaccination samples (36). Anti-Vi serology was not performed on the samples obtained from Ty21a-immunized subjects, since Ty21a does not constitutively express Vi antigen (51). From the above discussion, it is reasonable to conclude that it is unlikely that antibodies against these two key S. Typhi antigens (flagella and Vi) play a key role in the opsonophagocytosis and bactericidal cross-reactive activities in Ty21a and CVD 909 vaccinees described in the current studies. However, the role of vaccine-induced antibodies against another relevant protein antigen(s) common to all three serovars of Salmonella (e.g., type III secreting system proteins or outer membrane proteins) requires further investigation.
In this study, we used purified S. Typhi-specific LPS preparations for ELISA. The most immunogenic portion of the LPS molecule, the O antigen, differs between S. Typhi (O:9), S. Paratyphi A (O:2), and S. Paratyphi B (O:4); however, O:12, the trisaccharide (mannose-rhamnose-galactose) repeating unit that comprises the backbone, is common to Salmonella groups A, B, and D and is likely responsible, at least in part, for the observed cross-reactivity. Further detailed studies are needed to identify whether the observed induction of the cross-reactive functional antibodies following immunization with Ty21a and CVD 909 are indeed directed against the shared O:12 antigen.
A limitation of this study was our relatively small sample size. Additional studies with a larger number of volunteers and exploration of other humoral responses (including IgM isotypes) involved in the elimination of Salmonella (e.g., antibody and complement-mediated bactericidal ability, ADCC [31, 33]) are required for a better understanding of the role of antibodies in cross-protection from enteric fevers.
Our results suggest that antibodies alone are not sufficient to clear Salmonella infections, especially when they become intracellular. Therefore, to better understand the complex immunological mechanisms required to confer protection against S. Typhi and cross-protection against S. Paratyphi A or S. Paratyphi B, the present observations must be evaluated in conjunction with CMI induced by live oral S. Typhi vaccines, which is believed to play a key role in protection (9, 17–29, 36).
In summary, the results of antibody functional assays presented herein further support our previously published observations that, although the predominant postvaccination responses following Ty21a (and CVD 909) are directed against S. Typhi, cross-reactive responses of lower magnitudes were also observed against S. Paratyphi A and S. Paratyphi B. Moreover, the similarity of the functional antibody responses elicited following immunization with one dose of CVD 909 or after four doses of Ty21a provides further impetus for the continued development of CVD 909 as a candidate new-generation single-dose live vaccine against S. Typhi.
ACKNOWLEDGMENTS
We are indebted to the volunteers who allowed us to perform this study.
This paper includes work funded, in part, by NIAID, NIH, DHHS grants R01-AI036525 (to M.B.S.), U19 AI082655 (Cooperative Center for Translational Research in Human Immunology and Biodefense [CCHI] [to M.B.S.]), and U54-AI057168 (Regional Center for Excellence for Biodefense and Emerging Infectious Diseases Research Mid-Atlantic Region [MARCE] [to M.M.L. and M.B.S.]).
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
M.M.L. and M.B.S. are coinventors in a patent for the development of typhoid and paratyphoid vaccines licensed to Bharat Biotech International Limited.
Footnotes
Published ahead of print 15 January 2014
REFERENCES
- 1.Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull. World Health Organ. 82:346–353 [PMC free article] [PubMed] [Google Scholar]
- 2.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. 2002. Typhoid fever. N. Engl. J. Med. 347:1770–1782. 10.1056/NEJMra020201 [DOI] [PubMed] [Google Scholar]
- 3.Crump JA, Mintz ED. 2010. Global trends in typhoid and paratyphoid fever. Clin. Infect. Dis. 50:241–246. 10.1086/649541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fangtham M, Wilde H. 2008. Emergence of Salmonella Paratyphi A as a major cause of enteric fever: need for early detection, preventive measures, and effective vaccines. J. Travel Med. 15:344–350. 10.1111/j.1708-8305.2008.00237.x [DOI] [PubMed] [Google Scholar]
- 5.Meltzer E, Schwartz E. 2010. Enteric fever: a travel medicine oriented view. Curr. Opin. Infect. Dis. 23:432–437. 10.1097/QCO.0b013e32833c7ca1 [DOI] [PubMed] [Google Scholar]
- 6.Ochiai RL, Wang X, von Seidlein L, Yang J, Bhutta ZA, Bhattacharya SK, Agtini M, Deen JL, Wain J, Kim DR, Ali M, Acosta CJ, Jodar L, Clemens JD. 2005. Salmonella Paratyphi A rates, Asia. Emerg. Infect. Dis. 11:1764–1766. 10.3201/eid1111.050168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parry CM, Threlfall EJ. 2008. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr. Opin. Infect. Dis. 21:531–538. 10.1097/QCO.0b013e32830f453a [DOI] [PubMed] [Google Scholar]
- 8.Tacket CO, Levine MM. 2007. CVD 908, CVD 908-htrA, and CVD 909 live oral typhoid vaccines: a logical progression. Clin. Infect. Dis. 45(Suppl 1):S20–S23. 10.1086/518135 [DOI] [PubMed] [Google Scholar]
- 9.Kirkpatrick BD, McKenzie R, O'Neill JP, Larsson CJ, Bourgeois AL, Shimko J, Bentley M, Makin J, Chatfield S, Hindle Z, Fidler C, Robinson BE, Ventrone CH, Bansal N, Carpenter CM, Kutzko D, Hamlet S, LaPointe C, Taylor DN. 2006. Evaluation of Salmonella enterica serovar Typhi (Ty2 aroC-ssaV-) M01ZH09, with a defined mutation in the Salmonella pathogenicity island 2, as a live, oral typhoid vaccine in human volunteers. Vaccine 24:116–123. 10.1016/j.vaccine.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 10.Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, Schneerson R, Szu SC. 2001. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N. Engl. J. Med. 344:1263–1269. 10.1056/NEJM200104263441701 [DOI] [PubMed] [Google Scholar]
- 11.Holt KE, Thomson NR, Wain J, Langridge GC, Hasan R, Bhutta ZA, Quail MA, Norbertczak H, Walker D, Simmonds M, White B, Bason N, Mungall K, Dougan G, Parkhill J. 2009. Pseudogene accumulation in the evolutionary histories of Salmonella enterica serovars Paratyphi A and Typhi. BMC Genomics 10:36. 10.1186/1471-2164-10-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, Sabo A, Meyer R, Bieri T, Ozersky P, McLellan M, Harkins CR, Wang C, Nguyen C, Berghoff A, Elliott G, Kohlberg S, Strong C, Du F, Carter J, Kremizki C, Layman D, Leonard S, Sun H, Fulton L, Nash W, Miner T, Minx P, Delehaunty K, Fronick C, Magrini V, Nhan M, Warren W, Florea L, Spieth J, Wilson RK. 2004. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat. Genet. 36:1268–1274. 10.1038/ng1470 [DOI] [PubMed] [Google Scholar]
- 13.Black RE, Levine MM, Ferreccio C, Clements ML, Lanata C, Rooney J, Germanier R. 1990. Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Chilean Typhoid Committee. Vaccine 8:81–84 [DOI] [PubMed] [Google Scholar]
- 14.Levine MM, Ferreccio C, Black RE, Lagos R, San MO, Blackwelder WC. 2007. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica Serovar Paratyphi B. Clin. Infect. Dis. 45(Suppl 1):S24–S28. 10.1086/518141 [DOI] [PubMed] [Google Scholar]
- 15.Simanjuntak CH, Paleologo FP, Punjabi NH, Darmowigoto R, Soeprawoto , Totosudirjo H, Haryanto P, Suprijanto E, Witham ND, Hoffman SL. 1991. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet 338:1055–1059. 10.1016/0140-6736(91)91910-M [DOI] [PubMed] [Google Scholar]
- 16.Gupta SK, Medalla F, Omondi MW, Whichard JM, Fields PI, Gerner-Smidt P, Patel NJ, Cooper KL, Chiller TM, Mintz ED. 2008. Laboratory-based surveillance of paratyphoid fever in the United States: travel and antimicrobial resistance. Clin. Infect. Dis. 46:1656–1663. 10.1086/587894 [DOI] [PubMed] [Google Scholar]
- 17.Sztein MB, Tanner MK, Polotsky Y, Orenstein JM, Levine MM. 1995. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J. Immunol. 155:3987–3993 [PubMed] [Google Scholar]
- 18.Salerno-Goncalves R, Pasetti MF, Sztein MB. 2002. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J. Immunol. 169:2196–2203 [DOI] [PubMed] [Google Scholar]
- 19.Salerno-Goncalves R, Wyant TL, Pasetti MF, Fernandez-Vina M, Tacket CO, Levine MM, Sztein MB. 2003. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar typhi strain CVD 908-htrA. J. Immunol. 170:2734–2741 [DOI] [PubMed] [Google Scholar]
- 20.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. 2004. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J. Immunol. 173:5852–5862 [DOI] [PubMed] [Google Scholar]
- 21.Salerno-Goncalves R, Wahid R, Sztein MB. 2005. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect. Immun. 73:3521–3530. 10.1128/IAI.73.6.3521-3530.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salerno-Goncalves R, Sztein MB. 2006. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 14:536–542. 10.1016/j.tim.2006.10.004 [DOI] [PubMed] [Google Scholar]
- 23.Salerno-Goncalves R, Wahid R, Sztein MB. 2010. Ex vivo kinetics of early and long-term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin. Vaccine Immunol. 17:1305–1314. 10.1128/CVI.00234-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sztein MB, Wasserman SS, Tacket CO, Edelman R, Hone D, Lindberg AA, Levine MM. 1994. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J. Infect. Dis. 170:1508–1517. 10.1093/infdis/170.6.1508 [DOI] [PubMed] [Google Scholar]
- 25.Sztein MB. 2007. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica Serovar Typhi strains used as live oral vaccines in humans. Clin. Infect. Dis. 45(Suppl 1):S15–S19. 10.1086/518140 [DOI] [PubMed] [Google Scholar]
- 26.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. 2007. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine 25:1416–1425. 10.1016/j.vaccine.2006.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. 2008. Generation of specific effector and memory T cells with gut- and secondary lymphoid tissue-homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal Immunol. 1:389–398. 10.1038/mi.2008.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McArthur MA, Sztein MB. 2012. Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PLoS One 7:e38408. 10.1371/journal.pone.0038408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahid R, Simon R, Zafar SJ, Levine MM, Sztein MB. 2012. Live oral typhoid vaccine Ty21a induces cross-reactive humoral immune responses against Salmonella enterica serovar Paratyphi A and S. Paratyphi B in humans. Clin. Vaccine Immunol. 19:825–834. 10.1128/CVI.00058-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pakkanen SH, Kantele JM, Kantele A. 2012. Cross-reactive gut-directed immune response against Salmonella enterica serovar Paratyphi A and B in typhoid fever and after oral Ty21a typhoid vaccination. Vaccine 30:6047–6053. 10.1016/j.vaccine.2012.07.051 [DOI] [PubMed] [Google Scholar]
- 31.Tagliabue A, Villa L, De Magistris MT, Romano M, Silvestri S, Boraschi D, Nencioni L. 1986. IgA-driven T cell-mediated anti-bacterial immunity in man after live oral Ty21a vaccine. J. Immunol. 137:1504–1510 [PubMed] [Google Scholar]
- 32.Levine MM, Herrington D, Murphy JR, Morris JG, Losonsky G, Tall B, Lindberg AA, Svenson S, Baqar S, Edwards MF. 1987. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J. Clin. Invest. 79:888–902. 10.1172/JCI112899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindow JC, Fimlaid KA, Bunn JY, Kirkpatrick BD. 2011. Antibodies in action: role of human opsonins in killing Salmonella enterica serovar Typhi. Infect. Immun. 79:3188–3194. 10.1128/IAI.05081-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JY, Noriega FR, Galen JE, Barry E, Levine MM. 2000. Constitutive expression of the Vi polysaccharide capsular antigen in attenuated Salmonella enterica serovar typhi oral vaccine strain CVD 909. Infect. Immun. 68:4647–4652. 10.1128/IAI.68.8.4647-4652.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eloe-Fadrosh EA, McArthur MA, Seekatz AM, Drabek EF, Rasko DA, Sztein MB, Fraser CM. 2013. Impact of oral typhoid vaccination on the human gut microbiota and correlations with S. Typhi-specific immunological responses. PLoS One 8:e62026. 10.1371/journal.pone.0062026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahid R, Pasetti MF, Maciel M, Jr, Simon JK, Tacket CO, Levine MM, Sztein MB. 2011. Oral priming with Salmonella Typhi vaccine strain CVD 909 followed by parenteral boost with the S. Typhi Vi capsular polysaccharide vaccine induces CD27+IgD-S. Typhi-specific IgA and IgG B memory cells in humans. Clin. Immunol. 138:187–200. 10.1016/j.clim.2010.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishibashi Y, Arai T. 1996. A possible mechanism for host-specific pathogenesis of Salmonella serovars. Microb. Pathog. 21:435–446. 10.1006/mpat.1996.0074 [DOI] [PubMed] [Google Scholar]
- 38.Forrest BD, LaBrooy JT, Beyer L, Dearlove CE, Shearman DJ. 1991. The human humoral immune response to Salmonella typhi Ty21a. J. Infect. Dis. 163:336–345. 10.1093/infdis/163.2.336 [DOI] [PubMed] [Google Scholar]
- 39.Kirkpatrick BD, Tenney KM, Larsson CJ, O'Neill JP, Ventrone C, Bentley M, Upton A, Hindle Z, Fidler C, Kutzko D, Holdridge R, LaPointe C, Hamlet S, Chatfield SN. 2005. The novel oral typhoid vaccine M01ZH09 is well tolerated and highly immunogenic in 2 vaccine presentations. J. Infect. Dis. 192:360–366. 10.1086/431605 [DOI] [PubMed] [Google Scholar]
- 40.Kollaritsch H, Cryz SJ, Jr, Lang AB, Herzog C, Que JU, Wiedermann G. 2000. Local and systemic immune responses to combined Vibrio cholerae CVD103-HgR and Salmonella typhi ty21a live oral vaccines after primary immunization and reimmunization. Vaccine 18:3031–3039. 10.1016/S0264-410X(00)00101-8 [DOI] [PubMed] [Google Scholar]
- 41.Tacket CO, Pasetti MF, Sztein MB, Livio S, Levine MM. 2004. Immune responses to an oral typhoid vaccine strain that is modified to constitutively express Vi capsular polysaccharide. J. Infect. Dis. 190:565–570. 10.1086/421469 [DOI] [PubMed] [Google Scholar]
- 42.Viret JF, Favre D, Wegmuller B, Herzog C, Que JU, Cryz SJ, Jr, Lang AB. 1999. Mucosal and systemic immune responses in humans after primary and booster immunizations with orally administered invasive and noninvasive live attenuated bacteria. Infect. Immun. 67:3680–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tacket CO, Sztein MB, Wasserman SS, Losonsky G, Kotloff KL, Wyant TL, Nataro JP, Edelman R, Perry J, Bedford P, Brown D, Chatfield S, Dougan G, Levine MM. 2000. Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect. Immun. 68:1196–1201. 10.1128/IAI.68.3.1196-1201.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forrest BD, LaBrooy JT, Robinson P, Dearlove CE, Shearman DJ. 1991. Specific immune response in the human respiratory tract following oral immunization with live typhoid vaccine. Infect. Immun. 59:1206–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jack DL, Dodds AW, Anwar N, Ison CA, Law A, Frosch M, Turner MW, Klein NJ. 1998. Activation of complement by mannose-binding lectin on isogenic mutants of Neisseria meningitidis serogroup B. J. Immunol. 160:1346–1353 [PubMed] [Google Scholar]
- 46.Ochsenbein AF, Zinkernagel RM. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 21:624–630. 10.1016/S0167-5699(00)01754-0 [DOI] [PubMed] [Google Scholar]
- 47.Daeron M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203–234. 10.1146/annurev.immunol.15.1.203 [DOI] [PubMed] [Google Scholar]
- 48.Nimmerjahn F, Ravetch JV. 2007. Fc-receptors as regulators of immunity. Adv. Immunol. 96:179–204. 10.1016/S0065-2776(07)96005-8 [DOI] [PubMed] [Google Scholar]
- 49.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. 1999. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371–380. 10.1084/jem.189.2.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricklin D, Hajishengallis G, Yang K, Lambris JD. 2010. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11:785–797. 10.1038/ni.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Germanier R, Furer E. 1975. Isolation and characterization of gale E mutant Ty21a of Salmonella typhi: a candidate strain for a live oral typhoid vaccine. J. Infect. Dis. 141:553–558 [DOI] [PubMed] [Google Scholar]