Abstract
We have previously shown that an assay based on detection of anti-Salmonella enterica serotype Typhi antibodies in supernatant of lymphocytes harvested from patients presenting with typhoid fever (antibody in lymphocyte supernatant [ALS] assay) can identify 100% of patients with blood culture-confirmed typhoid fever in Bangladesh. In order to define immunodominant proteins within the S. Typhi membrane preparation used as antigen in these prior studies and to identify potential biomarkers unique to S. Typhi bacteremic patients, we probed microarrays containing 2,724 S. Typhi proteins with ALS collected at the time of clinical presentation from 10 Bangladeshis with acute typhoid fever. We identified 62 immunoreactive antigens when evaluating both the IgG and IgA responses. Immune responses to 10 of these antigens discriminated between individuals with acute typhoid infection and healthy control individuals from areas where typhoid infection is endemic, as well as Bangladeshi patients presenting with fever who were subsequently confirmed to have a nontyphoid illness. Using an ALS enzyme-linked immunosorbent assay (ELISA) format and purified antigen, we then confirmed that immune responses against the antigen with the highest immunoreactivity (hemolysin E [HlyE]) correctly identified individuals with acute typhoid or paratyphoid fever in Dhaka, Bangladesh. These observations suggest that purified antigens could be used with ALS and corresponding acute-phase activated B lymphocytes in diagnostic platforms to identify acutely infected patients, even in areas where enteric fever is endemic.
INTRODUCTION
Enteric fever is caused by the human-restricted pathogens Salmonella enterica serotype Typhi, serotype Paratyphi A, and rarely by serotype Paratyphi B. Enteric fever is endemic throughout the African and Asian continents, with typhoid fever causing an estimated 21.7 million cases per year and over 200,000 deaths annually and paratyphoid fever causing over 5 million illnesses per year (1). Clinical symptoms of enteric fever correlate with bacteremia and include fevers, malaise, and abdominal pain, with potential complications, including encephalopathy and intestinal perforation. Accurate diagnosis requires laboratory confirmation; unfortunately, there are currently no reliable diagnostic assays for enteric fever (2).
A reliable assay for enteric fever is needed not only for the diagnosis of acute infection but also for use in surveillance programs to assess disease burden within a community and evaluate prevention programs. Currently available diagnostics for enteric fever include blood culture that is positive in only 40 to 60% of presumptive cases. Bone marrow culture, the gold standard for diagnosis, has improved sensitivity, but its use is limited due to technical challenges and its invasiveness (2, 3). Antibody detection assays such as the Widal assay, which detects agglutinating antibody responses to S. Typhi lipopolysaccharide (LPS) (O) antigen and flagellar (H) antigen, are often nonspecifically cross-reactive in areas where enteric fever is endemic and ideally require comparison of responses in acute-phase versus convalescent-phase serum samples (2, 4, 5). Other commercially available antibody-based serological assays have similar limitations with sensitivities and specificities in areas where typhoid fever is endemic (2).
Typhoidal Salmonella bacteria cause minimal intestinal inflammation but survive within professional phagocytic cells, circulate systemically, and lead to a systemic state of inflammation. Activated lymphocytes, induced by a range of pathogens, are detectable in peripheral blood early in infection (6) and can be evaluated for antigen-specific responses (7–9). Alternatively, these cells can be cultured ex vivo without specific antigenic stimulation (10). During culturing, these already activated lymphocytes secrete antigen-specific antibodies into the culture supernatant that can then be detected via an enzyme-linked immunosorbent assay (ELISA) (11–13). Such liquid-based assays have been referred to as ALS (antibody in lymphocyte supernatant)-based assays, and their use has been described after infection or vaccination for a number of pathogens, including the pathogens causing cholera (11, 13), tuberculosis (14), typhoid fever (10, 15, 16), and influenza (17).
We have previously shown that an ALS assay based on detection of anti-S. Typhi antibodies in the supernatant of activated lymphocytes harvested from patients with acute-phase typhoid fever can identify 100% of patients with blood culture-confirmed typhoid fever and paratyphoid A fever in Bangladesh (10, 16). This initial assay was developed using a crude membrane preparation of S. Typhi as the target antigen. Therefore, we aimed to define immunodominant antigens within this membrane preparation and to identify potential biomarkers unique to S. Typhi bacteremic patients that can be used in an ALS-based assay.
MATERIALS AND METHODS
Study subject selection and sample collection.
Individuals presenting to the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b) hospital or the Kamalapur field site of the icddr,b with possible enteric fever were eligible for enrollment if they met the following criteria at presentation: age of 1 to 59 years, fever duration of 3 to 7 days (≥39°C), no obvious focus of infection, and no alternate diagnosis. We collected 5 to 10 ml of venous blood from participants when they were enrolled in the study (day 0). Three to five milliliters of blood was used for microbiologic analysis using a BacT/Alert automated system. We subcultured bottles flagged as positive on MacConkey agar and identified Salmonella enterica serotype Typhi isolates in blood samples from 25 individuals using standard biochemical tests and reaction with Salmonella-specific antisera (18). After we collected the initial blood samples, we treated patients with parenteral ceftriaxone or oral cefixime for up to 14 days at the discretion of the attending physician. We also collected venous blood samples from 9 individuals with S. Paratyphi A bacteremia, 5 individuals with other febrile illnesses based on clinical findings and laboratory tests (i.e., 2 patients with visceral leishmaniasis confirmed by PCR and 3 patients with tuberculosis confirmed by a positive acid-fast bacillus sputum smear), and 10 healthy Bangladeshis. These studies were approved by the human studies committees of the icddr,b, Massachusetts General Hospital, and the University of California, Irvine.
Collection of antibody in lymphocyte supernatants.
We generated ALS from Bangladeshi patients as previously described (10). Briefly, we diluted heparinized venous blood 1:2 in phosphate-buffered saline and used density gradient centrifugation on Ficoll-Isopaque (Pharmacia, Uppsala, Sweden) to isolate peripheral blood mononuclear cells (PBMCs). We resuspended cells to a concentration of 107 cells/ml in RPMI 1640 medium (Gibco, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Ogden, UT), 200 mM l-glutamine, 100 mM pyruvate, 100 U of penicillin/ml, and 100 μg of streptomycin/ml and incubated PBMCs at 37°C in 5% CO2. After 48 h of incubation, we collected the culture supernatants, added a protease inhibitor, and stored samples frozen at −80°C.
Microarray construction and probing.
Protein arrays containing 2,724 S. Typhi antigens (>63% of proteome) were constructed as previously described (19). Proteins included in arrays were selected based on features enriched in seroreactive antigens in other bacterial species (e.g., proteins with signal peptide motifs and/or motifs characteristic of lipoproteins or proteins associated with the outer membrane or periplasm, as well as heat shock, chaperone, transport, or virulence proteins). Proteins that were nonhomologous (<50% identity at the amino acid level) to Escherichia coli were also included. The arrays were probed with ALS of 10 patients with confirmed S. Typhi bacteremia, 5 healthy Bangladeshis, and 5 patients with other febrile illnesses. The arrays were probed with ALS fluid diluted 1:2 with protein array blocking buffer (Schleicher & Schuell, Keene, NH) supplemented with E. coli (DH5α) lysate (McLab, San Francisco, CA) at a final concentration of 1 mg/ml protein to block anti-E. coli antibodies. Bound antibody was detected with biotin-conjugated anti-human IgG or IgA secondary antibody (Jackson ImmunoResearch, West Grove, PA) diluted 1:400 in blocking buffer, followed by streptavidin conjugated with SureLightHP-3 (Columbia Biosciences, Frederick, MD). The slides were scanned and analyzed using PerkinElmer ScanArray Express (Waltham, MA), and signal intensities were quantified using QuantArray software (PerkinElmer, Waltham, MA).
Data analysis.
Analysis of the protein microarray data was accomplished according to our previously published computational methods (19, 20). Microarray spot intensities were quantified using QuantArray software utilizing automatic local background subtraction for each spot. “No-DNA” negative controls consisted of in vitro transcription/translation reaction without the addition of plasmid template (19). “No-DNA” spots on each array were averaged, and this negative-control background value was subtracted from every other spot on the array. Variance stabilization and normalization (VSN) was applied to quantified array intensities. VSN normalization was performed using the R statistical programming language and implemented as part of the Bioconductor suite (www.bioconductor.org). In addition to removing heteroscedasticity, this procedure corrects for nonspecific noise effects by finding maximum likelihood shifting and scaling parameters for each array such that control probe variance is minimized (21, 22). Proteins were considered immunoreactive if signal intensity was greater than the average signal intensity plus 2 times the standard deviation of all negative-control “no-DNA” spots. Differentially reactive proteins between infected and uninfected groups were determined using a Bayes regularized t test adapted from Cyber-T for protein arrays (23), which has been shown to be more effective than other differential expression techniques. A P value smaller than 0.05 was considered significant.
Detection of anti-HlyE IgA responses in ALS by ELISA.
To validate the results of our microarray immunoscreen, we selected the antigen with the most prominent immunoreactivity, hemolysin E (HlyE, t1477). Using an ELISA format, we analyzed the immunoreactivity of HlyE in ALS at the time of presentation of 15 individuals with confirmed S. Typhi bacteremia (not included in our above immunoscreen), 9 individuals with confirmed S. Paratyphi A bacteremia, and 10 healthy Bangladeshi controls. We coated plates with 100 ng/well of purified HlyE prepared as previously described (24) and detected antigen-specific responses by adding 100 μl of ALS (diluted 1:2), detected with anti-human IgA conjugated with horseradish peroxidase at a 1:1,000 dilution, and we measured peroxidase activity with ortho-phenylene diamine (Sigma Chemical Co., St. Louis, MO) as previously described (10). We assessed differences between groups using a Mann-Whitney U test, and a P value smaller than 0.05 was considered significant.
RESULTS
ALS IgG and IgA profiles.
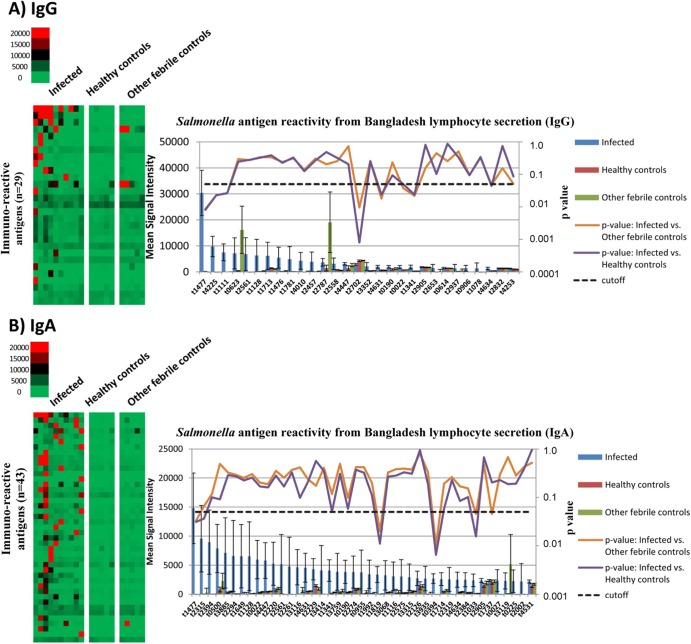
We probed protein arrays containing 2,724 S. Typhi antigens with ALS from 10 individuals with confirmed S. Typhi bacteremia. In total, we identified 62 immunoreactive S. Typhi proteins as defined as having a mean reactivity greater than 2 times the standard deviation above the mean of the no-DNA controls. Nineteen antigens were specific to IgG, 33 antigens were specific to IgA, and 10 were common to both antibody subclasses (Fig. 1). These antigens included a number of membrane-associated proteins, including a hemolysin, lipoproteins, fimbrial proteins, and transport proteins (see Table S1 in the supplemental material).
FIG 1.
ALS IgG and IgA profiles. (A and B) S. Typhi arrays probed for IgG (A) and IgA (B) responses with ALS from individuals with acute typhoid infection, healthy Bangladeshis, and Bangladeshi patients presenting with fever who were subsequently confirmed to have a nontyphoid illness. On the heat map, antigens are listed on the vertical axis, and individual ALS samples by cohort are listed along the top. The mean IgG or IgA reactivity to the antigens for each sample cohort is plotted on the graph, and the Cyber-T P values for comparison of acute typhoid patients to healthy controls and other febrile controls are plotted on the right-hand y axis. A cutoff P value of <0.05 was considered significant.
Ten antigens (listed below using the S. Typhi Ty2 open reading frame [ORF] label) were identified in our differential immunoscreen using ALS from individuals with acute typhoid infection, healthy Bangladeshis, and Bangladeshi patients presenting with fever who were subsequently confirmed to have a nontyphoid illness. Of the 10 antigens, 7 antigens were found to have significantly higher IgG immunoreactivity compared to healthy controls and individuals with other febrile illnesses (Cyber-T P value < 0.05): hemolysin E, HlyE (t1477); nonspecific acid phosphatase precursor, PhoN (t4225); toxin-like protein, CdtB (t1111); fimbrial subunits SthD (t4631), SthA (t4634), and BcfA (t0022); and an outer membrane protein, OmpS2 (t1341). HlyE, homoprotocatechuate degradative operon repressor (HpcR, t1819), a putative ethanolamine utilization protein, EutN (t0394), and a tail-specific protease, Prc (t1033), had statistically significantly higher IgA immunoreactivity when typhoid patients were compared to healthy controls and those with other febrile illnesses.
Anti-HlyE IgA responses in ALS.
To further characterize HlyE immunoreactivity in ALS, we evaluated immunoreactivity to purified HlyE using an ELISA-based format and IgA detection. We assessed anti-HlyE responses in ALS at the time of presentation of 15 individuals with confirmed S. Typhi bacteremia (individuals not included in our immunoscreen), 9 individuals with confirmed S. Paratyphi A bacteremia, and 10 healthy controls. We found significantly higher IgA immunoreactivity to HlyE in patients with S. Typhi (P < 0.0001) or S. Paratyphi A fever (P = 0.001) compared to healthy Bangladeshis (Fig. 2). All 15 patients with acute typhoid fever (100%) and 8 out of 9 patients with paratyphoid A fever (88.9%) had a detectable anti-HlyE IgA response (ELISA unit [EU] > 1) (Fig. 2). None of 10 healthy Bangladeshis (0%) had an anti-HlyE IgA response.
FIG 2.
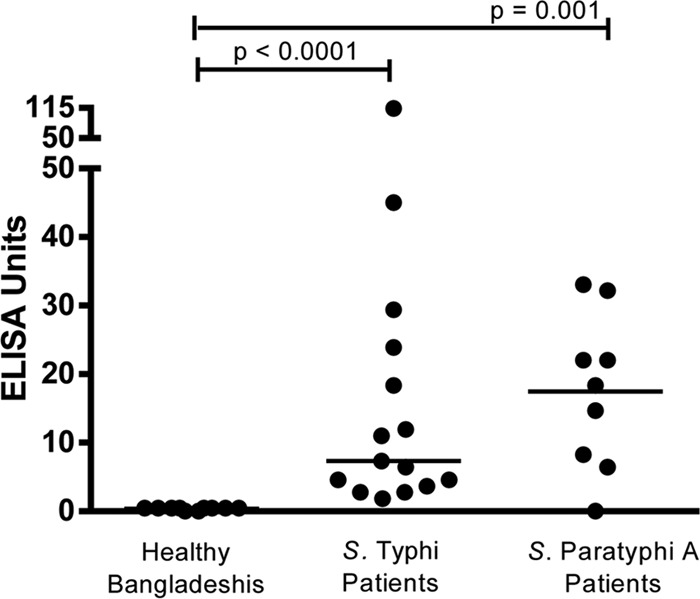
Anti-HlyE IgA responses in ALS. Anti-HlyE IgA responses in healthy Bangladeshis and patients at the time of presentation (day 0) of acute enteric fever with confirmed S. Typhi or S. Paratyphi A bacteremia. Each symbol represents the value for one individual, and the short horizontal line is the median for the group. Differences between groups were assessed using a Mann-Whitney U test, and a P value smaller than 0.05 was considered significant.
DISCUSSION
There is currently no optimal assay for diagnosing patients with acute typhoid or paratyphoid fever or for assessing enteric fever disease burden within a community. Detection of S. Typhi or S. Paratyphi A by microbiologic culture or nucleic acid amplification techniques is limited due to low bacterial burden present in peripheral blood, and serodiagnostic assays in areas where these bacteria are endemic are hampered by the high likelihood of prior exposure to these organisms (2). New alternative approaches for typhoid diagnostic assays and surveillance tools are needed. In this analysis, we took advantage of the rapid expansion of activated antigen-specific antibody-secreting cells (ASC) that occurs following infection or vaccination. These activated lymphocytes can act as an early biomarker that can be used in diagnostic assays, and given their transient nature, they could prove particularly useful in developing assays for use in areas where enteric fever is endemic.
We have previously shown that an assay based on detection of anti-S. Typhi antibodies in supernatant of lymphocytes (ALS) harvested from patients with typhoid fever can identify 100% of patients with blood culture-confirmed typhoid fever and paratyphoid A fever in Bangladesh (10, 16). This initial assay was developed using a crude membrane preparation of S. Typhi as the target antigen (10), and at the present time, it is used as a diagnostic assay at the icddr,b clinical facilities for diagnosis of enteric fever (16). Here we extend these observations by probing S. Typhi protein arrays with ALS of patients with confirmed S. Typhi bacteremia to assess the antigenic profile of antibodies in ALS. We were able to identify 62 immunoreactive antigens in total when evaluating both IgG and IgA. Of these responses, 10 antigens discriminated between individuals with acute typhoid infection and healthy Bangladeshi controls, as well as Bangladeshi patients presenting with fever who were subsequently confirmed to have a nontyphoid illness. These antigens included HlyE, CdtB, PhoN, SthD, SthA, BcfA, HpcR, Prc, EutN, and OmpS2. Prior studies have shown that individuals with typhoid fever develop strong anti-HlyE, anti-CdtB, and anti-PhoN serum responses (19, 25, 26).
Hemolysin E (HlyE), also referred to as ClyA, had the highest IgG and IgA immunoreactivity in our screen. Hemolysin E is a pore-forming toxin that contributes to the cytotoxicity and invasion of epithelial cells and also affects bacterial growth within human macrophages (27–29). HlyE shares >90% amino acid identity with ClyA in E. coli K-12 (29), and in S. Typhi, it has been shown to be expressed under the control of the PhoP regulon (27, 30) that regulates gene expression important in intracellular survival of S. enterica. Within the genus Salmonella, HlyE was originally found only in the typhoidal serotypes S. Typhi and S. Paratyphi A (29), but an analysis of a larger spectrum of salmonellae (by genome sequencing, comparative genomic hybridization, and PCR analysis) recently revealed that the hlyE gene has a wider distribution and that it is also present in some of the nontyphoidal Salmonella serotypes (including, but not limited to, invasive isolates of Salmonella serotypes Schwarzengrund, Montevideo, Bredeney, and others) (28, 31, 32).
Cytolethal distending toxin (CdtB) is one of the A components of typhoid toxin, a unique AB-type toxin made up of 2 A subunits (CdtB and pertussis-like toxin A [PltA]), and 5 B subunits (PltB, a homolog of one of the heterologous B subunits of pertussis toxin). CdtB is a DNase that is upregulated intracellularly and induces cell cycle arrest of host cells by causing DNA damage (33). Typhoid toxin, through its CdtB subunit, is responsible for many of the symptoms associated with acute typhoid infection (34). Like HlyE, CdtB is present in S. Typhi and S. Paratyphi A but is rarely found in other Salmonella serovars (35).
We also identified fimbrial subunits (SthD, SthA, and BcfA) that are unique to Salmonella. The sth and bcf fimbrial operons play a role in colonization and long-term intestinal persistence of S. Typhimurium in a mouse model (36), and antibody responses to BcfA and SthA have been demonstrated after S. Typhimurium infection in mice (37). In a comparative genomic hybridization analysis of invasive nontyphoidal Salmonella isolates from bacteremic human patients, the sth and bcf fimbrial operons were found to be part of a core set of 5 fimbrial operons that are conserved across isolates of invasive nontyphoidal Salmonella (31).
OmpS2 is a porin found in Salmonella spp.; along with major porins such as OmpC and OmpF, it plays a role in virulence in S. Typhimurium (38). In a mouse model, OmpS2 was also found to be a potent inducer of the innate immune response acting as both a Toll-like receptor 2 (TLR2) and TLR4 agonist (39). In addition, OmpS2 has adjuvant capability, boosting antibody responses when coimmunized with other antigens (39).
In our analysis, we also identified PhoP-regulated nonspecific acid phosphatase, PhoN (30); homoprotocatechuate degradative operon repressor, HpcR, a negative regulator of the hpc cluster involved in catabolism of aromatic amino acids (40); and EutN, an ethanolamine utilization protein in the eut operon involved in use of alternative carbon sources. We have previously demonstrated upregulation of the eut operon in individuals bacteremic with S. Typhi and S. Paratyphi A (41, 42). These antigens are common to all Salmonella enterica serotypes and are also found in E. coli and other Gram-negative organisms.
To validate our findings, we assessed IgA ALS responses to purified HlyE in a standard ELISA format. We focused on IgA responses since we had previously shown that ALS IgA responses are transient markers of recent infection (10). Using such an approach, we confirmed both a high specificity (100%) and a high sensitivity of 100% and 89% in identifying patients bacteremic with either S. Typhi or S. Paratyphi A, respectively.
Measurement of responses in ALS fluid may have several advantages over assessing responses in serum. We have previously shown that 84% of positive ALS responses become negative by day 21 (the last time point we have evaluated) (10, 16), thus supporting the idea that the ALS response is due to recent acute infection and would be a useful diagnostic in areas where there is a high prevalence of prior exposure to S. Typhi or S. Paratyphi A. In addition, we observed marked differences in immunoreactivity in microarrays using ALS from patients with acute S. Typhi infections compared to ALS from healthy individuals in areas where enteric fever is endemic and febrile Bangladeshi controls. These observations suggest that ALS and acute-phase lymphocytes could be used to identify acutely infected patients, even in areas where enteric fever is endemic.
A limitation of this analysis is that we included only individuals from Bangladesh and had a low number and variety of other febrile illnesses included in our analysis; thus, further testing will be needed to further investigate the specificity of our assay and the generalizability of our findings to other geographic areas where typhoid and paratyphoid fever are endemic. In addition, we performed our microarray immunoprobe using ALS from patients infected with S. Typhi, and it may be useful to identify antigens that would distinguish S. Typhi from S. Paratyphi A infection. Although the ALS assay at this time is not a point-of-care test, we have previously shown that the analysis can be performed in areas with limited laboratory setups and requires minimal training of personnel (16), suggesting it might have particular utility as a surveillance tool. Our identification of a core set of target antigens, and confirmation of assay utility using a purified antigen (HlyE), should also facilitate development of a sensitive, specific, and reproducible assay.
In conclusion, we have performed an immunoscreen of anti-S. Typhi responses present in the supernatant of naturally activated lymphocytes recovered from individuals acutely bacteremic with S. Typhi. Our analysis has revealed a subset of antigens, including HlyE, and immunoreactivity against these antigens was able to distinguish patients with acute typhoid fever from healthy controls and febrile patients with other illnesses. The screening of protein arrays with ALS is a method that could be applied to a number of pathogens to identify candidate antigens that could be used in diagnostic assay development.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the icddr,b and grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (AI100023 and AI077883 [E.T.R. and F.Q.], AI058935 [S.B.C., E.T.R., and F.Q.], AI073672 [L.L. and P.L.F.], AI078213 [L.L. and P.L.F.], Sida [F.Q. and F.K.], Training Grant in Vaccine Development and Public Health (D43 TW005572 [F.K., E.T.R., and F.Q.]), Career Development Awards (K08 AI089721 [R.C.C.], K08 AI100923 [D.T.L.]), a Massachusetts General Hospital Physician Scientist Development Award (R.C.C.), a Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene Postdoctoral Fellowship in Tropical Infectious Diseases (D.T.L.), and grants from the Deutsche Forschungsgemeinschaft (LU 842/1-1 [A.L.]).
Footnotes
Published ahead of print 26 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00661-13.
REFERENCES
- 1.Crump JA, Mintz ED. 2010. Global trends in typhoid and paratyphoid fever. Clin. Infect. Dis. 50:241–246. 10.1086/649541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parry CM, Wijedoru L, Arjyal A, Baker S. 2011. The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev. Anti Infect. Ther. 9:711–725. 10.1586/eri.11.47 [DOI] [PubMed] [Google Scholar]
- 3.Gilman RH, Terminel M, Levine MM, Hernandez-Mendoza P, Hornick RB. 1975. Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella typhi in typhoid fever. Lancet i:1211–1213 [DOI] [PubMed] [Google Scholar]
- 4.Levine MM, Grados O, Gilman RH, Woodward WE, Solis-Plaza R, Waldman W. 1978. Diagnostic value of the Widal test in areas endemic for typhoid fever. Am. J. Trop. Med. Hyg. 27:795–800 [DOI] [PubMed] [Google Scholar]
- 5.Espersen F, Hoiby N, Hertz JB. 1980. Cross-reactions between Salmonella typhi and 24 other bacterial species. Acta Pathol. Microbiol. Scand. B 88:243–248 [DOI] [PubMed] [Google Scholar]
- 6.Czerkinsky C, Prince SJ, Michalek SM, Jackson S, Moldoveanu Z, Russell MW, McGhee JR, Mestecky J. 1987. Oral immunization with bacterial antigen induces IgA-secreting cells in peripheral blood in humans. Adv. Exp. Med. Biol. 216B:1709–1719 [PubMed] [Google Scholar]
- 7.Czerkinsky C, Prince SJ, Michalek SM, Jackson S, Russell MW, Moldoveanu Z, McGhee JR, Mestecky J. 1987. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc. Natl. Acad. Sci. U. S. A. 84:2449–2453. 10.1073/pnas.84.8.2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czerkinsky C, Svennerholm AM, Quiding M, Jonsson R, Holmgren J. 1991. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans. Infect. Immun. 59:996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saletti G, Cuburu N, Yang JS, Dey A, Czerkinsky C. 2013. Enzyme-linked immunospot assays for direct ex vivo measurement of vaccine-induced human humoral immune responses in blood. Nat. Protoc. 8:1073–1087. 10.1038/nprot.2013.058 [DOI] [PubMed] [Google Scholar]
- 10.Sheikh A, Bhuiyan MS, Khanam F, Chowdhury F, Saha A, Ahmed D, Jamil KM, LaRocque RC, Harris JB, Ahmad MM, Charles R, Brooks WA, Calderwood SB, Cravioto A, Ryan ET, Qadri F. 2009. Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin. Vaccine Immunol. 16:1587–1594. 10.1128/CVI.00311-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang HS, Sack DA. 2001. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin. Diagn. Lab. Immunol. 8:482–488. 10.1128/CDLI.8.3.482-488.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forrest BD. 1988. Identification of an intestinal immune response using peripheral blood lymphocytes. Lancet i:81–83 [DOI] [PubMed] [Google Scholar]
- 13.Qadri F, Ryan ET, Faruque AS, Ahmed F, Khan AI, Islam MM, Akramuzzaman SM, Sack DA, Calderwood SB. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 71:4808–4814. 10.1128/IAI.71.8.4808-4814.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raqib R, Rahman J, Kamaluddin AK, Kamal SM, Banu FA, Ahmed S, Rahim Z, Bardhan PK, Andersson J, Sack DA. 2003. Rapid diagnosis of active tuberculosis by detecting antibodies from lymphocyte secretions. J. Infect. Dis. 188:364–370. 10.1086/376511 [DOI] [PubMed] [Google Scholar]
- 15.Sztein MB. 2007. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica serovar Typhi strains used as live oral vaccines in humans. Clin. Infect. Dis. 45(Suppl 1):S15–S19. 10.1086/518140 [DOI] [PubMed] [Google Scholar]
- 16.Khanam F, Sheikh A, Sayeed MA, Bhuiyan MS, Choudhury FK, Salma U, Pervin S, Sultana T, Ahmed D, Goswami D, Hossain ML, Mamun KZ, Charles RC, Brooks WA, Calderwood SB, Cravioto A, Ryan ET, Qadri F. 2013. Evaluation of a typhoid/paratyphoid diagnostic assay (TPTest) detecting anti-Salmonella IgA in secretions of peripheral blood lymphocytes in patients in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 7:e2316. 10.1371/journal.pntd.0002316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halliley JL, Kyu S, Kobie JJ, Walsh EE, Falsey AR, Randall TD, Treanor J, Feng C, Sanz I, Lee FE. 2010. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine 28:3582–3587. 10.1016/j.vaccine.2010.02.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruickshank R, Duguid JP, Marmion BP, Swain RHA. 1975. The Enterobacteriaceae: Salmonella, p 403–419 In Medical microbiology: the practice of medical microbiology, 12th ed, vol 11 Churchill Livingstone, Edinburgh, Scotland [Google Scholar]
- 19.Liang L, Juarez S, Nga TV, Dunstan S, Nakajima-Sasaki R, Davies DH, McSorley S, Baker S, Felgner PL. 2013. Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoid. Sci. Rep. 3:1043. 10.1038/srep01043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK. 2010. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc. Natl. Acad. Sci. U. S. A. 107:6958–6963. 10.1073/pnas.1001323107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durbin BP, Hardin JS, Hawkins DM, Rocke DM. 2002. A variance-stabilizing transformation for gene-expression microarray data. Bioinformatics 18(Suppl 1):S105–S110. 10.1093/bioinformatics/18.suppl_1.S105 [DOI] [PubMed] [Google Scholar]
- 22.Huber W, von Heydebreck A, Sultmann H, Poustka A, Vingron M. 2002. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18(Suppl 1):S96–S104. 10.1093/bioinformatics/18.suppl_1.S96 [DOI] [PubMed] [Google Scholar]
- 23.Long AD, Mangalam HJ, Chan BY, Tolleri L, Hatfield GW, Baldi P. 2001. Improved statistical inference from DNA microarray data using analysis of variance and a Bayesian statistical framework. Analysis of global gene expression in Escherichia coli K12. J. Biol. Chem. 276:19937–19944 [DOI] [PubMed] [Google Scholar]
- 24.von Rhein C, Bauer S, Lopez Sanjurjo EJ, Benz R, Goebel W, Ludwig A. 2009. ClyA cytolysin from Salmonella: distribution within the genus, regulation of expression by SlyA, and pore-forming characteristics. Int. J. Med. Microbiol. 299:21–35. 10.1016/j.ijmm.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 25.Charles RC, Sheikh A, Krastins B, Harris JB, Bhuiyan MS, LaRocque RC, Logvinenko T, Sarracino DA, Kudva IT, Eisenstein J, Podolsky MJ, Kalsy A, Brooks WA, Ludwig A, John M, Calderwood SB, Qadri F, Ryan ET. 2010. Characterization of anti-Salmonella enterica serotype Typhi antibody responses in bacteremic Bangladeshi patients by an immunoaffinity proteomics-based technology. Clin. Vaccine Immunol. 17:1188–1195. 10.1128/CVI.00104-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Rhein C, Hunfeld KP, Ludwig A. 2006. Serologic evidence for effective production of cytolysin A in Salmonella enterica serovars Typhi and Paratyphi A during human infection. Infect. Immun. 74:6505–6508. 10.1128/IAI.00779-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faucher SP, Forest C, Beland M, Daigle F. 2009. A novel PhoP-regulated locus encoding the cytolysin ClyA and the secreted invasin TaiA of Salmonella enterica serovar Typhi is involved in virulence. Microbiology 155:477–488. 10.1099/mic.0.022988-0 [DOI] [PubMed] [Google Scholar]
- 28.Fuentes JA, Villagra N, Castillo-Ruiz M, Mora GC. 2008. The Salmonella Typhi hlyE gene plays a role in invasion of cultured epithelial cells and its functional transfer to S. Typhimurium promotes deep organ infection in mice. Res. Microbiol. 159:279–287 [DOI] [PubMed] [Google Scholar]
- 29.Oscarsson J, Westermark M, Löfdahl S, Olsen B, Palmgren H, Mizunoe Y, Wai SN, Uhlin BE. 2002. Characterization of a pore-forming cytotoxin expressed by Salmonella enterica serovars Typhi and Paratyphi A. Infect. Immun. 70:5759–5769. 10.1128/IAI.70.10.5759-5769.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charles RC, Harris JB, Chase MR, Lebrun LM, Sheikh A, LaRocque RC, Logvinenko T, Rollins SM, Tarique A, Hohmann EL, Rosenberg I, Krastins B, Sarracino DA, Qadri F, Calderwood SB, Ryan ET. 2009. Comparative proteomic analysis of the PhoP regulon in Salmonella enterica serovar Typhi versus Typhimurium. PLoS One 4:e6994. 10.1371/journal.pone.0006994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suez J, Porwollik S, Dagan A, Marzel A, Schorr YI, Desai PT, Agmon V, McClelland M, Rahav G, Gal-Mor O. 2013. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS One 8:e58449. 10.1371/journal.pone.0058449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.den Bakker HC, Moreno Switt AI, Govoni G, Cummings CA, Ranieri ML, Degoricija L, Hoelzer K, Rodriguez-Rivera LD, Brown S, Bolchacova E, Furtado MR, Wiedmann M. 2011. Genome sequencing reveals diversification of virulence factor content and possible host adaptation in distinct subpopulations of Salmonella enterica. BMC Genomics 12:425. 10.1186/1471-2164-12-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haghjoo E, Galan JE. 2004. Salmonella Typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. U. S. A. 101:4614–4619. 10.1073/pnas.0400932101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song J, Gao X, Galan JE. 2013. Structure and function of the Salmonella Typhi chimaeric AB typhoid toxin. Nature 499:350–354. 10.1038/nature12377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClelland M, Sanderson KE, Clifton SW, Latreille P, Porwollik S, Sabo A, Meyer R, Bieri T, Ozersky P, McLellan M, Harkins CR, Wang C, Nguyen C, Berghoff A, Elliott G, Kohlberg S, Strong C, Du F, Carter J, Kremizki C, Layman D, Leonard S, Sun H, Fulton L, Nash W, Miner T, Minx P, Delehaunty K, Fronick C, Magrini V, Nhan M, Warren W, Florea L, Spieth J, Wilson RK. 2004. Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat. Genet. 36:1268–1274. 10.1038/ng1470 [DOI] [PubMed] [Google Scholar]
- 36.Weening EH, Barker JD, Laarakker MC, Humphries AD, Tsolis RM, Baumler AJ. 2005. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 73:3358–3366. 10.1128/IAI.73.6.3358-3366.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humphries A, Deridder S, Baumler AJ. 2005. Salmonella enterica serotype Typhimurium fimbrial proteins serve as antigens during infection of mice. Infect. Immun. 73:5329–5338. 10.1128/IAI.73.9.5329-5338.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Morales O, Fernandez-Mora M, Hernandez-Lucas I, Vazquez A, Puente JL, Calva E. 2006. Salmonella enterica serovar Typhimurium ompS1 and ompS2 mutants are attenuated for virulence in mice. Infect. Immun. 74:1398–1402. 10.1128/IAI.74.2.1398-1402.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moreno-Eutimio MA, Tenorio-Calvo A, Pastelin-Palacios R, Perez-Shibayama C, Gil-Cruz C, Lopez-Santiago R, Baeza I, Fernandez-Mora M, Bonifaz L, Isibasi A, Calva E, Lopez-Macias C. 2013. Salmonella Typhi OmpS1 and OmpS2 porins are potent protective immunogens with adjuvant properties. Immunology 139:459–471. 10.1111/imm.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenkins JR, Cooper RA. 1988. Molecular cloning, expression, and analysis of the genes of the homoprotocatechuate catabolic pathway of Escherichia coli C. J. Bacteriol. 170:5317–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheikh A, Charles RC, Rollins SM, Harris JB, Bhuiyan MS, Khanam F, Bukka A, Kalsy A, Porwollik S, Brooks WA, LaRocque RC, Hohmann EL, Cravioto A, Logvinenko T, Calderwood SB, McClelland M, Graham JE, Qadri F, Ryan ET. 2010. Analysis of Salmonella enterica serotype Paratyphi A gene expression in the blood of bacteremic patients in Bangladesh. PLoS Negl. Trop. Dis. 4:e908. 10.1371/journal.pntd.0000908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheikh A, Charles RC, Sharmeen N, Rollins SM, Harris JB, Bhuiyan MS, Arifuzzaman M, Khanam F, Bukka A, Kalsy A, Porwollik S, Leung DT, Brooks WA, LaRocque RC, Hohmann EL, Cravioto A, Logvinenko T, Calderwood SB, McClelland M, Graham JE, Qadri F, Ryan ET. 2011. In vivo expression of Salmonella enterica serotype Typhi genes in the blood of patients with typhoid fever in Bangladesh. PLoS Negl. Trop. Dis. 5:e1419. 10.1371/journal.pntd.0001419 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.