Abstract
The nucleoprotein of respiratory syncytial virus (RSV-N) is immunogenic and elicits an IgG response following infection. The RSV-N gene was cloned into a mammalian expression vector, pREN2, and the expressed luciferase-tagged protein (Ruc-N) detected anti-RSV-N-specific IgG antibodies using a high-throughput immunoprecipitation method (the luciferase immunoprecipitation system [LIPS]-NRSV assay). The specificity of the assay was evaluated using monoclonal antibodies (MAbs) and monospecific pre- and postimmunization rabbit antisera. Blood serum samples from chimpanzees and humans with proven/probable RSV infection were also tested. The pre- and postimmunization serum samples from rabbits given human metapneumovirus (HMPV) or measles virus were negative when tested by the LIPS-NRSV assay, while antisera obtained after immunization with either the RSV-A or RSV-B strain gave positive signals in a dose-dependent manner. RSV-N MAb 858-3 gave a positive signal in the LIPS-NRSV assay, while MAbs against other paramyxovirus nucleoproteins or RSV-F or RSV-G did not. Serum samples from chimpanzees simultaneously immunized with vaccinia-RSV-F and vaccinia-RSV-G recombinant viruses were negative in the LIPS-NRSV assay; however, anti-RSV-N IgG responses were detected following subsequent RSV challenge. Seven of the 12 infants who were seronegative at 9 months of age had detectable anti-RSV-N antibodies when they were retested at 15 to 18 months of age. The LIPS-NRSV assay detects specific anti-RSV-N IgG responses that may be used as a biomarker of RSV infection.
INTRODUCTION
Respiratory syncytial virus (RSV) is an important pathogen for vaccine development since morbidity is high among infants and the elderly (1). Two distinct antigenic subtypes, RSV-A and -B, may either cocirculate or independently dominate during yearly epidemics (2, 3). The RSV genome encodes 11 viral proteins, and the two major envelope proteins, fusion (F) and attachment (G) glycoproteins, induce neutralizing antibody responses associated with long-term protection (1). Ideally, a successful RSV vaccine will elicit a robust neutralizing antibody response and significantly decrease the attack rate among vaccinated subjects compared with the attack rate seen in an unvaccinated group during clinical testing. Unfortunately, the subjects enrolled in these studies sometimes fail to be evaluated during the early period of an RSV illness when virus shedding is optimal, and as a result, infections may be missed. Intermittent shedding of RSV can also confound the ability to identify infections if sampling is sporadic or limited to culture, antigen detection, or a nucleic acid test. As a result, RSV attack rates may be underestimated. We believe that a serological assay capable of detecting a virus-specific response to a nonvaccine antigen can complement other virus detection methods when assessing attack rates during clinical trials. Since many of the current candidate RSV vaccines contain the F and/or G antigens only, we have focused our attention on the antibody responses to the nucleoprotein of RSV (RSV-N). RSV-N is an important structural protein required for encapsidating virion RNA (1). Although antibodies reactive to RSV-N are not associated with protection, they might serve as a biomarker of infection (2, 4, 5). In this study, we use the luciferase immunoprecipitation system (LIPS) to develop an assay to detect RSV-N-specific IgG antibodies using the recombinant N protein expressed in COS-1 cells. This high-throughput method provides an unlimited supply of antigen, eliminates the time and effort required to produce purified protein, and obviates the need for many of the optimization steps used to standardize enzyme-linked immunosorbent assays (ELISAs) (6).
MATERIALS AND METHODS
Cells and viruses.
COS-1 cells were grown in Dulbecco's modified Eagle's minimal (DMEM) (Gibco, Grand Island, NY) medium, while Vero and HEp-2 cells were grown in Eagle's medium containing Earl's salts (EMEM) (Cellgro, Manassas, VA), as previously described (7, 8). RSV strain A2 was obtained and prepared as described previously (9), and RSV-B1 was similarly amplified once in Vero cells. Cold-passaged RSV-cp52 was amplified twice in Vero cells at 32°C and purified for animal immunizations as described previously (9). The monoclonal antibody-resistant mutant 1142 (MARM-1142) was selected, plaque purified, amplified, and the sequence of the fusion glycoprotein was deduced as previously described (10).
Sera and antibodies.
The rabbits were immunized with purified UV-inactivated RSV-A2 administered subcutaneously using 106 50% tissue culture infective dose (TCID50) equivalents, first in Freund's complete adjuvant at 6 sites and then using Freund's incomplete adjuvant every 3 weeks, for a total of 3 injections. Blood serum samples were collected before the first immunization and 4 weeks after the last boost. The same protocol was used to obtain rabbit anti-RSV-cp52, anti-RSV-B1, and anti-measles virus sera. The rabbits were similarly immunized with affinity-purified RSV-F (10 μg/dose) or RSV-G (10 μg/dose) administered by subcutaneous injection using affinity-purified RSV-F and RSV-G proteins obtained from the WHO Reagent Bank for RSV and parainfluenza virus 3 (PIV3). The animal immunizations were performed under protocols approved by the Center for Biological Evaluation and Research (CBER) Institutional Animal Care and Use Committee.
Anti-N-specific IgG murine monoclonal antibodies were tested, including anti-RSV-N 858-3 (Chemicon International, Temecula, CA), anti-HMPV-N (Novus Biological, Littleton, CO) (11), anti-measles-N (12), anti-influenza-nucleoprotein (NP) (Millipore, USA), and anti-mumps-N (13). Monoclonal anti-RSV-F (1200 and 1243) and anti-RSV-G (1197) IgG antibodies were obtained and characterized as previously described (7).
Anonymous serum samples were previously obtained from subjects 6 to 18 months of age, following parental informed consent, and were stored frozen; the FDA Research in Human Subjects Committee gave approval for the testing. Human immunoglobulin, RSV IgG Lot 1 (RSV Lot 1), a pooled human reference immunoglobulin, was obtained from BEI Resources and used at a starting concentration of 1% IgG, except in tests used to determine the limit of detection, in which case RSV Lot 1 was tested in triplicate at concentrations ranging from 10% to 0.000001% IgG in parallel with IgG-depleted human immunoglobulin (IgGΔ) (Sunny Lab, United Kingdom). The serum and immunoglobulin samples were also tested using a plaque reduction neutralization (PRN) assay versus RSV-A2, as previously described (14, 15). The endpoint titers were calculated using the Spearman-Kärber method (16).
Chimpanzee serum samples were previously generated under approved protocols at the NIH. Briefly, chimpanzees (2 per group) were given RSV-MARM-1142 or recombinant vaccinia viruses expressing the RSV-F or RSV-G proteins (17). Chimpanzees no. 329 and no. 338 (group 1) were infected by combined intratracheal (i.t.) and intranasal (i.n.) inoculation on day 0 with MARM-1142. Chimpanzees no. 1,475 and no. 1,479 (group 2) were simultaneously inoculated subcutaneously on day 0 with vaccinia-RSV-F and vaccinia-RSV-G recombinant viruses. All chimps were challenged with RSV-A2 administered i.n. and i.t. on day 28 using 106 TCID50 per site, as reported previously. The serum samples were obtained preimmunization (day 0), prechallenge (day 28), and postchallenge on day 42 (group 1) or day 56 (group 2).
Generation of Renilla luciferase-tagged RSV-A2 nucleoprotein construct.
A mammalian expression vector, pREN2, was used to generate Renilla luciferase (Ruc)-tagged RSV-N protein (Ruc-N) (6). The RSV-N gene was inserted into the pREN2 polycloning site downstream of the sequences for the FLAG epitope and Renilla luciferase, such that the expressed fusion protein was tagged at its amino terminus (8). HEp-2 cell monolayers were infected with RSV-A2 and mRNA extracted from the cell lysates using an RNeasy minikit (Qiagen, Valencia, CA). RSV strain A2 (GenBank accession no. M11486.1) was used to design primers for cDNA synthesis and PCR amplification of the N gene insert. An EcoRI restriction site at the 5′ end and an XhoI site at the 3′ end facilitated cloning. The primers were 5′-GGGCTCGAGAACTCAAAGCTCTACATCATTATC (reverse) and 5′-AAGGAATTCAAGATGGCTCTTAGCAAAGTCAAG (forward). Real-time PCR (RT-PCR) (ProtoScript AMV first strand cDNA synthesis kit; New England BioLabs, Ipswich, MA) was used to synthesize and amplify RSV-N cDNA, followed by gel electrophoresis and cloning into pCRII-TOPO prior to amplification in Escherichia coli (Invitrogen, Carlsbad, CA). The resulting TOPO-insert vector was prepared using the Qiagen maxi kit and digested using EcoRI and XhoI restriction enzymes. The insert was extracted and purified using QIAquick (Qiagen), ligated into EcoRI-XhoI-cut pREN2 vector, and amplified in E. coli. The resulting pREN2+RSV-N was prepared using a Maxiprep kit (Qiagen). The sequence and integrity of the DNA construct were confirmed using automated DNA sequencing.
Renilla luciferase-nucleoprotein expression and characterization.
COS-1 cell monolayers were transfected with pREN2 or with pREN2+RSV-N, as previously described (8). Renilla light units (RLU) per 20 μl of harvested lysates were determined using a luminometer (SpectraMax L; Molecular Devices, Sunnyvale, CA). To confirm the expression of Ruc-N fusion protein, COS-1 cell lysates transfected with plasmid pREN2 or pREN2+RSV-N were harvested and the tagged proteins precipitated using anti-FLAG-coated Sepharose beads (Clontech, Mountain View, CA); the proteins were separated by SDS-PAGE using a 4 to 12% polyacrylamide gel and then transferred to nitrocellulose using iBlot (Invitrogen). The blots were probed with rabbit anti-FLAG IgG (Sigma-Aldrich, St. Louis, MO) and with rabbit anti-RSV-A2 antisera, and specific bands were detected using peroxidase-conjugated goat anti-rabbit IgG antibody (KPL, Gaithersburg, MD) and LumiGLO substrate (KPL).
LIPS assay to detect RSV-N-specific IgG antibodies.
COS-1 cell lysates containing Ruc-tagged RSV-N protein (Ruc-N) or Ruc only (without insert) were obtained and the LIPS assay performed as previously described (8). Briefly, a master plate was prepared by diluting serum samples 1:10 in assay buffer A (50 mM Tris [pH 7.5], 100 mM NaCl, 5 mM MgCl2, 1% Triton X-100) in a 96-deep-well polypropylene plate. Next, the following were added in triplicate to each well of a round-bottom 96-shallow-well polypropylene plate: 40 μl of buffer A, 10 μl of diluted serum from the master plate, and 50 μl of Ruc-RSV-N antigen from crude COS-1 lysate diluted in buffer A, such that 1 × 107 RLU were added per well, followed by 1 h incubation at room temperature on a rotary shaker. The antigen-antibody mixtures (100 μl/well) were then transferred to 96-well high-throughput sequencing (HTS)-filter plates containing 5 μl of a 30% suspension of UltraLink protein A/G beads and further incubated for 1 h at room temperature on a rotary shaker. The filter plates were washed with buffer A (8×) and phosphate-buffered saline (PBS) (2×) using a vacuum manifold and dried. The RLU/well content was determined following the addition of coelenterazine (100 μl/well; Promega, Madison, WI) in a SpectraMax L luminometer. The RLU values from the wells containing beads, antigen, and buffer A but no serum served as buffer blanks and were averaged and subtracted from the values from all other wells. The data from the triplicate wells were then averaged to generate an RLU value for each sample. A positive response was defined as a value of >5 standard deviations above the mean value obtained with control serum tested against Ruc-N antigen, as recommended (8). The antisera were tested in at least two independent assays and in parallel against Ruc antigen without insert to confirm that the reactivity was specific for the RSV-N antigen.
Production and purification of recombinant RSV-N protein in E. coli.
Maxiprep plasmid for pCRII-TOPO-RSV-N was digested using EcoRI and XhoI restriction enzymes to extract the RSV-N cDNA insert, which was purified by agarose gel electrophoresis and cloned into pET 28a(+) vector (Novagen, Madison, WI) containing the 6×His tag downstream of the T7 promoter. This vector was transformed into E. coli strain BL21 cells (DE3) (Novagen) and the expression of recombinant RSV-N protein induced by adding 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Novagen). The cells were centrifuged at 4,500 rpm for 30 min at 4°C, the pellets resuspended in lysis buffer, and RSV-N protein purified using nickel-nitrilotriacetic acid (NTA) agarose (Qiagen). RSV-N protein production was confirmed by Western blotting using monoclonal anti-RSV-N IgG antibody.
Statistical analysis.
A two-tailed t test was performed to compare the mean RLU values obtained for the assays using pre- and postinfection/immunization sera obtained from animal infections/immunizations.
RESULTS
Characterization of Ruc-N protein in COS-1 lysates.
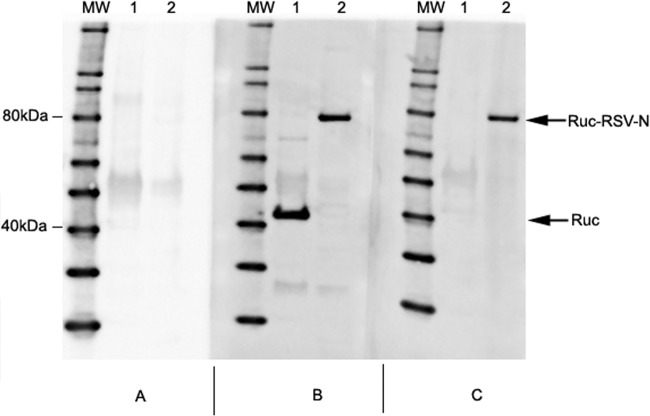
Rabbit anti-FLAG antibody detected the FLAG epitope present at the 5′end of expressed Ruc antigen at ∼40 kDa in the COS-1 lysate transfected with pREN2 plasmid, as expected (Fig. 1), as well as a specific band at ∼80 kDa in the COS-1 lysates transfected with pREN2+RSV-N (Fig. 1). Rabbit anti-RSV polyclonal serum similarly revealed a specific band at ∼80 kDa, consistent with the estimated molecular weight of Ruc-tagged RSV-N.
FIG 1.
Western blot assay confirms the expression of Ruc and Ruc-N antigen in COS-1 lysates. COS-1 cell monolayers were transfected with pREN2 or pREN2+RSV-N and cell lysates harvested. FLAG-tagged proteins were precipitated using anti-FLAG-coated Sepharose beads, and precipitated proteins were separated by SDS-PAGE using 4 to 12% polyacrylamide gels prior to transfer to nitrocellulose using iBlot. The blots were probed with normal preimmune rabbit serum (A), rabbit anti-FLAG IgG (B), or with rabbit anti-RSV-A2 antiserum (C). Specific bands were detected using peroxidase-conjugated goat anti-rabbit IgG antibody and LumiGLO substrate. The arrows designate Ruc in pREN2-transfected COS-1 lysate (lane 1) and Ruc-tagged RSV-N protein in pREN2+RSV-N transfected COS-1 lysate (lane 2).
Assay specificity.
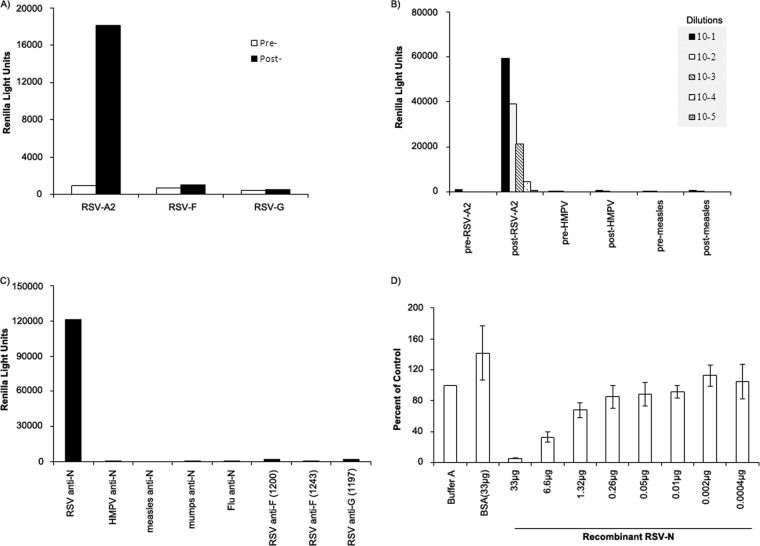
Serum samples from rabbits immunized with purified UV-inactivated RSV-A2 were positive for anti-RSV-N IgG antibodies, while serum samples obtained prior to immunization and from rabbits immunized with affinity-purified RSV-F or RSV-G were negative in the LIPS-NRSV assay (P < 0.05, Fig. 2A).
FIG 2.
The ability of the LIPS-NRSV assay to specifically detect anti-RSV-N IgG antibodies was shown using rabbit serum samples and murine monoclonal antibodies. (A) LIPS-NRSV assay detected a specific anti-RSV IgG antibody response in serum from rabbits immunized with UV-inactivated RSV-A2, while preimmune serum and serum from rabbits immunized with affinity-purified RSV-F or RSV-G proteins did not react in this assay. (B) Pre-and postimmunization serum samples from rabbits given UV-inactivated viruses, including RSV-A2, HMPV or measles virus were tested. All preimmune and post-HMPV and postmeasles samples were negative, while rabbit anti-RSV-A2 gave positive signals. (C) Murine MAbs against RSV-N, RSV-F, and RSV-G were tested in parallel with MAbs reactive with nucleoproteins from HMPV, measles, mumps, or influenza virus. The LIPS-NRSV assay detected an anti-N specific IgG antibody response with the murine anti-RSV-N MAb only, while the tests using anti-HMPV, anti-measles, anti-mumps, and anti-influenza N MAbs were negative, as were the tests using anti-RSV-F and anti-RSV-G MAbs. (D) The specificity of the assay was also shown using soluble purified recombinant RSV-N protein to block the binding of anti-RSV-N IgG antibodies to Ruc-RSV-N protein as follows: 10 μl of rabbit anti-RSV-A2 antiserum (diluted 1:10) was first incubated with either 40 μl of buffer A or 40 μl buffer A containing bovine serum albumin (BSA) or recombinant RSV-N (rRSV-N) protein over a wide range of concentrations and then tested using the LIPS-NRSV assay. As the concentration of rRSV-N decreased, the RLU values rose, demonstrating a specific increase in anti-RSV-N IgG available to form immune complexes with Ruc-N antigen (the data are only shown for buffer A containing BSA at the highest concentration [33 μg]).
RSV-N-specific IgG antibody responses were tested using serial dilutions of rabbit antisera obtained from animals before and after immunization with RSV-A2, HMPV, or measles virus (Fig. 2B). The signals obtained using all preimmune, anti-HMPV, and anti-measles antisera were at background levels at all dilutions tested (range, 300 to 900 RLU). In contrast, rabbit anti-RSV-A2 antiserum gave positive signals in a dose-dependent manner at all dilutions tested up to and including 10−4 (4,557 RLU); these values were significantly above the cutoff set using all preimmune rabbit antisera (mean + 5 standard deviations [SD] = 1,397 RLU); rabbit anti-RSV-A2 did not react when tested against Ruc antigen alone.
Similarly, a specific IgG response was detected when RSV-N-specific MAb 858-3 was tested using the LIPS-NRSV assay (∼120,000 RLU), while MAbs specific for RSV-F, RSV-G, or that were directed against other myxovirus and paramyxovirus N proteins were negative in this assay (all readings < 2,000 RLU) (Fig. 2C).
To further demonstrate the specificity of the assay, rabbit anti-RSV-A2 antiserum was first incubated with serial dilutions of purified recombinant RSV-N prior to testing in the LIPS-NRSV assay. As shown in Fig. 2D, the RLU signal obtained following incubation of the serum with the highest concentration of recombinant RSV-N tested was decreased by ∼95% compared with the value obtained in the absence of soluble recombinant RSV-N protein (mean ± SD RLU = 203,243 ± 53,630 RLU), while no inhibition was seen following incubation with bovine serum albumin; the RLU signals for rabbit anti-RSV-A2 were inversely related to the concentration of soluble recombinant RSV-N protein used for the preincubation.
LIPS-NRSV assay detects RSV-N-specific IgG antibodies elicited by either subtype A or B RSV strains.
Since the pREN2+RSV-N construct was obtained using the RSV-N gene derived from a subtype A strain, we next compared the ability of Ruc-N antigen to bind IgG antibodies using serum samples from rabbits immunized with either subtype A (RSV-A2) or subtype B viruses (RSV-B1 or cp52) (Fig. 3). As expected, preimmune serum samples contained no detectable RSV-N-specific IgG antibodies, whereas antiserum samples obtained following immunization with RSV-A2 gave a positive response in the LIPS-NRSV assay at dilutions up to and including 10−5, and antisera obtained from rabbits given RSV-B1 or RSV-cp52 were positive at dilutions up to and including 10−4 (pre- versus postimmunization samples, P < 0.05). Preimmunization serum samples obtained from rabbits given RSV-A2, RSV-B1, or RSV-cp52 had no reactivity when tested against the control Ruc antigen.
FIG 3.
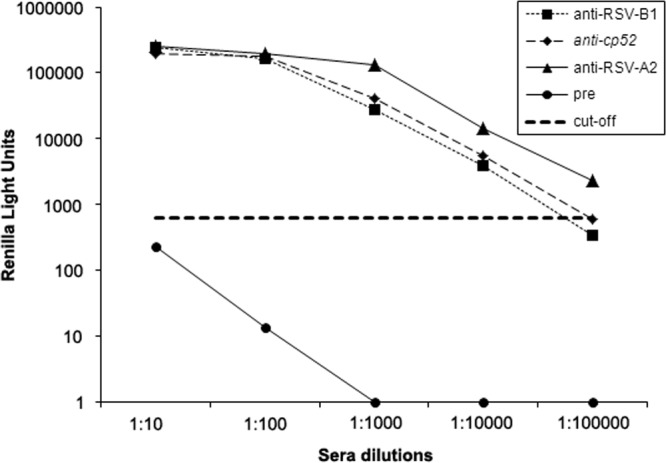
LIPS-NRSV assay detects IgG antibodies elicited following exposure to either subtype A (RSV-A2) or subtype B (RSV-cp52, RSV-B1) RSV strains. Rabbit pre- and postimmunization serum samples were evaluated using the LIPS-NRSV assay. Specific anti-N IgG antibody responses were detected in antisera from rabbits immunized with UV-inactivated RSV-A2 (at dilutions as high as 10−5), or with RSV-cp52 or RSV-B1 (at dilutions as high as 10−4). The bold dashed line represents the cutoff for a positive result and is equal to the mean + 5 SD above the value obtained for the preimmune sera diluted 1:10.
LIPS-NRSV detects antibodies in human serum elicited by natural infection.
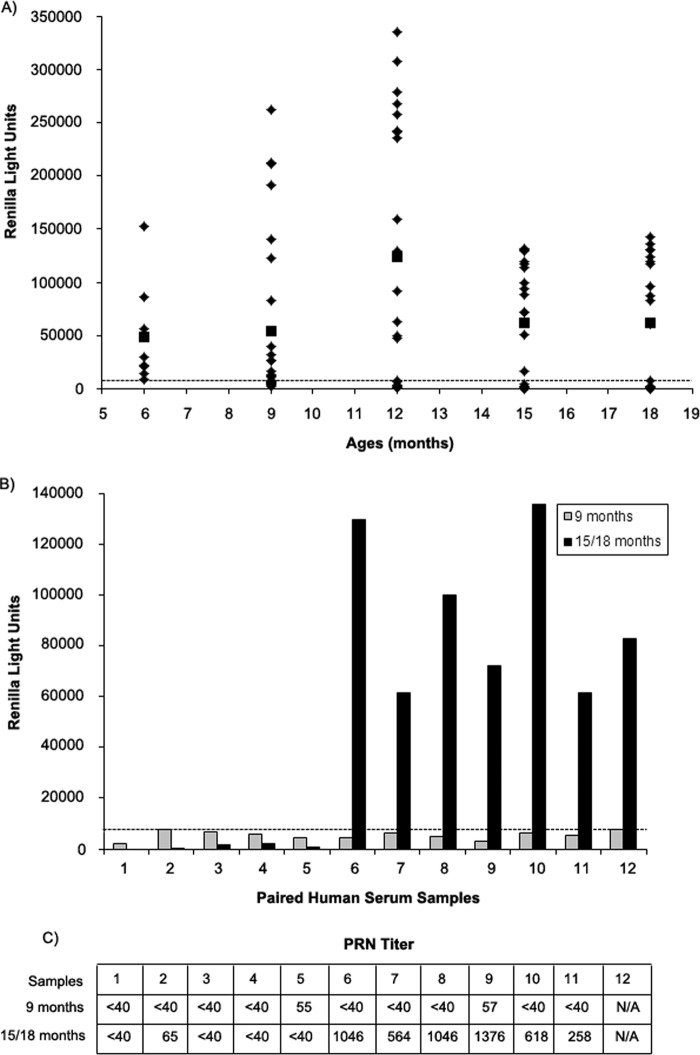
Human serum (100 samples) obtained from subjects 6 to 18 months of age were tested using the LIPS-NRSV assay to determine if the test could discriminate between positive and negative samples from infants and toddlers and to see if we could detect seroconversion among RSV-naive subjects using paired samples. In this assay, the cutoff for a positive result was 8,000 RLU (mean + 5 SD) for the test on IgGΔ. Of the samples tested, 36/100 had signals below the assay cutoff, while the remaining samples were positive by the LIPS-NRSV assay (range, 10,000 to 335,214 RLU; Fig. 4A). Among the seronegative samples, 12 from infants 9 months of age had a paired sample obtained 6 or 9 months thereafter. Seven of the 12 paired samples (58%) had detectable RSV-N IgG antibodies in samples collected at 15 or 18 months of age (Fig. 4B), while 5 of 12 paired samples remained negative at the later time point. Eleven of the 12 paired samples had sufficient quantities remaining for PRN testing. When the samples collected at 9 months of age were tested, 9/11 subjects had PRN titers of <1:40, and two infants had 50% neutralization dose (ND50) titers of 1:55 and 1:57. Likewise, in the samples collected at 15 to 18 months of age, the PRN ND50 titers were negative (<1:40) or low (1:65) for the 5 subjects with no detectable anti-RSV-N IgG by the LIPS-NRSV assay, while the 6 subjects with a positive LIPS-NRSV assay result had ND50 titers of >1:248 (range, 1:258 to 1:1,376) (Fig. 4C).
FIG 4.
One hundred serum samples from children 6 to 18 months of age were tested for anti-RSV-N-specific IgG antibodies. (A) RLU values for each sample (◊) tested are plotted versus the age at which the sample was collected; ■, mean for the group. Of the 100 samples tested, 36 samples were found to be negative for anti-RSV-N IgG antibodies using a cutoff value of 8,000 RLU obtained from testing IgGΔ (mean + 5 SD), as shown by the horizontal dashed line. (B) Of the 36 seronegative samples, 12 were obtained from subjects at 9 months of age, and these subjects had paired samples obtained at 15 or 18 months of age. Seven of the 12 paired samples tested positive at follow-up, while 5 of 12 remained negative. (C) ND50 titers are shown for 11 of the 12 paired samples tested using a PRN assay versus RSV-A2.
RSV Lot 1 was also tested over a wide range of IgG concentrations using both the LIPS-NRSV assay and a traditional PRN test in order to compare the sensitivities in detecting RSV-specific responses. As shown in Fig. 5, an anti-RSV-N-specific IgG response was detected at concentrations as low as 0.001% IgG, while no reactivity was seen at any dilution when tested against Ruc antigen alone. In contrast, PRN antibodies were detected at concentrations as low as 0.01% IgG. IgGΔ did not inhibit RSV in the PRN test (data not shown).
FIG 5.
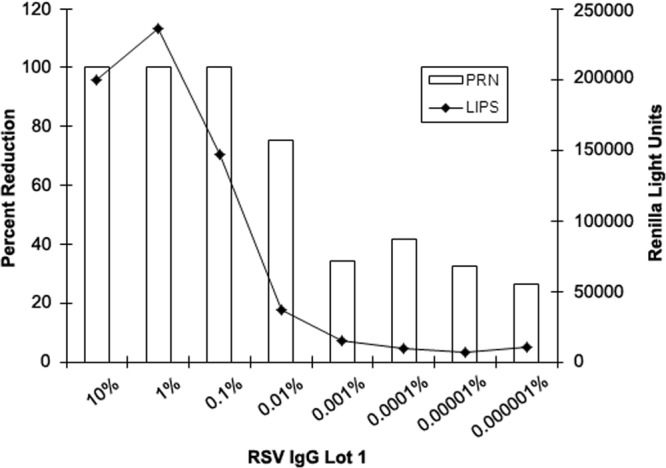
Pooled human IgG immunoglobulin (RSV Lot 1, 10% IgG) was tested neat or serially diluted 10-fold into IgGΔ from 10% to 0.000001% IgG and tested using the LIPS-NRSV assay and using a PRN assay versus RSV-A2. Anti-N-specific IgG antibodies were detected at dilutions up to and including 0.001% IgG in the LIPS-NRSV assay, while plaque counts were reduced by 50% or more at dilutions up to and including 0.01% IgG. IgGΔ (unspiked) inhibited plaque counts by ∼20 to 40% when tested in the PRN assay.
LIPS-NRSV detects anti-N antibodies in immunized animals following experimental challenge with live virus.
Serum samples from 4 chimpanzees were tested for anti-RSV-N-specific antibody responses using the LIPS-NRSV assay, and the results are shown in Fig. 6. All preimmunization sera were negative for RSV-neutralizing antibodies, as reported previously (17), and signals detected using the LIPS-NRSV assay were <500 RLU. Accordingly, the assay cutoff for a positive result was set at 1,115 RLU (mean + 5 SD) using data obtained from the tests on the preimmune sera from all 4 chimpanzees. In contrast, serum samples obtained on study day 28 from chimpanzees infected i.n./i.t. with RSV-MARM-1142 had detectable anti-RSV-N IgG antibody responses (26,000 and 28,000 RLU), whereas day 28 serum samples from chimpanzees concomitantly immunized with vaccinia-RSV-F and vaccinia-RSV-G recombinant viruses had no detectable anti-RSV-N antibodies, as expected. Following challenge with live RSV-A2, all 4 chimpanzees had high levels of anti-RSV-N-specific IgG antibodies (with values of 41,000, 52,000, 17,000, and 40,000 RLUs in chimpanzees no. 329, 338, 1,475, and 1,479, respectively), and the anti-RSV-N IgG antibody responses in chimps previously inoculated with RSV-MARM-1142 were boosted ∼1.5- to 1.8-fold after rechallenge with RSV-A2 virus.
FIG 6.
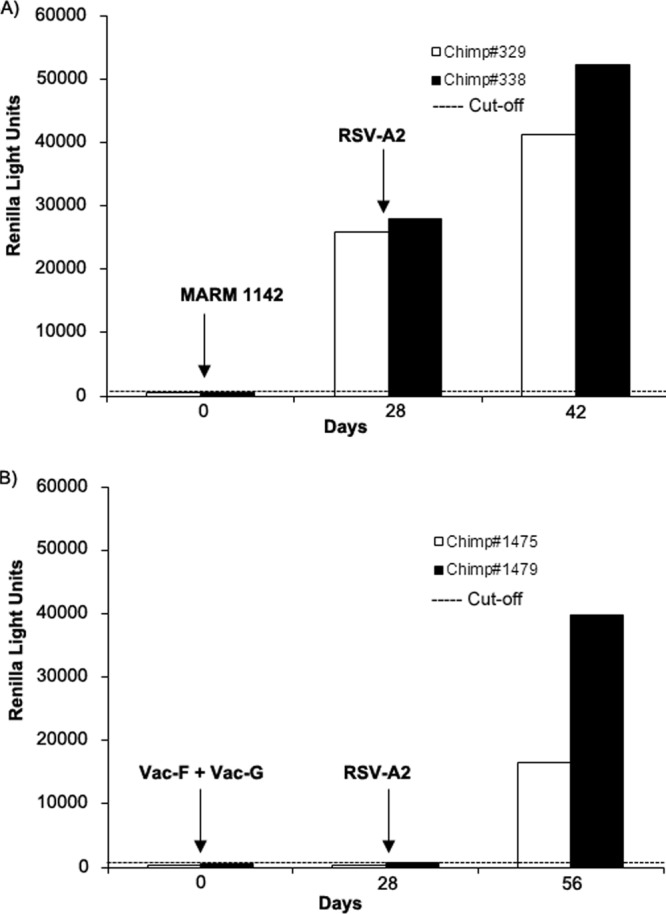
Two groups of chimpanzees (group 1, chimps no. 329 and 338; group 2, chimps no. 1,475 and 1,479) were immunized with live RSV-MARM-1142 by combined intranasal/intratracheal installation or inoculated with vaccinia-RSV-F and vaccinia-RSV-G viruses, respectively, and then challenged with live RSV-A2 using combined intranasal/intratracheal installation of the virus. Preimmune, postimmunization, and postchallenge antisera were tested for anti-RSV-N-specific IgG antibodies using the LIPS-NRSV assay. (A) Group 1 chimps were seronegative prior to immunization but showed a positive anti-RSV-N IgG response following administration of RSV-MARM-1142 and had a boost in the IgG response following infection with RSV-A2. (B) Group 2 chimps were seronegative prior to immunization and showed no anti-RSV-N-specific IgG antibody response following inoculation with vaccinia-RSV-F and vaccinia-RSV-G viruses. However, anti-RSV-N IgG responses were detected following challenge with RSV-A2.
DISCUSSION
We developed a simple, rapid, and specific LIPS assay to detect IgG antibody responses against RSV-N that may be used as markers of infection or exposure. Many studies have demonstrated that RSV-F protein elicits both neutralizing antibody responses and cytotoxic T lymphocytes that are protective against subsequent infection (4, 5, 18). In addition, a passively administered monoclonal antibody that binds to a single epitope on the RSV-F glycoprotein can protect high-risk infants from severe disease (19). RSV-G protein also elicits a neutralizing antibody response that is protective, and for these reasons, many recent RSV candidate vaccines contain or express RSV-F only or a combination of RSV-F and RSV-G protein antigens (4, 20). Since these candidate vaccines lack RSV-N, monitoring the anti-RSV-N IgG response among subjects 6 months to 2 years of age can help to identify an RSV-naive population prior to vaccine administration and potentially confirm natural infections and exposures that occur during the RSV season following immunization.
Currently, commercially available RSV ELISA kits using whole virus or lysates from infected cells contain the entire repertoire of RSV antigens; these assays are not designed to detect a protein-specific response. An ELISA that detects anti-N responses using vector-expressed antigen has been developed; however, this assay is not commercially available (21). A serological assay capable of detecting a virus-specific response to a nonvaccine antigen might serve as a complement to other virus detection methods for determining RSV attack rates during clinical trials, since subjects enrolled in these studies sometimes fail to be evaluated during the early period of an illness and intermittent shedding of RSV can also complicate the ability to identify infection even if sensitive methods are used to detect virus, antigen, or genome.
As a proof of concept, tests on preimmune, postimmune, and postchallenge chimpanzee antisera demonstrate that the LIPS-NRSV assay can distinguish serum samples from animals with documented RSV exposure from samples obtained from RSV-naive animals; they also show that anti-RSV-N IgG antibodies may develop following experimental infection in spite of high levels of serum-neutralizing antibodies elicited after immunization with RSV-F and RSV-G antigens.
The LIPS-NRSV assay specifically detects anti-RSV-N antibodies induced in response to either RSV infection or immunization with whole-virus antigens. Monospecific rabbit polyclonal antibodies raised against RSV were positive in the LIPS-NRSV assay while preimmune sera and antibodies elicited in response to HMPV and measles virus were not. Likewise, an anti-RSV-N monoclonal antibody was positive in this assay, while monoclonal antibodies specific for RSV-F, RSV-G, or the N proteins of other myxoviruses and paramyxoviruses did not react. Additionally, signals were significantly reduced by preincubating anti-RSV antiserum with soluble purified RSV-N protein prior to testing, providing further confirmation of the specificity of the assay. The LIPS-NRSV assay was also able to detect antibodies elicited by either subtype A or B RSV.
Although we were not able to test serum samples from infants before and after their first documented RSV infection, paired serum samples from infants and toddlers demonstrated that the LIPS-NRSV assay can identify RSV-naive infants and detect seroresponses due to subsequent probable RSV exposures. There was a good correlation between the results obtained using the LIPS-NRSV assay with those obtained using a traditional PRN test, with 6 of 9 samples positive by both the PRN and LIPS-NRSV assays, for a positive predictive value of 67%; there were no false positives when the PRN results were compared to those obtained using the LIPS-NRSV test.
Due to transcriptional attenuation, there is an abundance of nucleoprotein produced in RSV-infected cells, and RSV-N is highly conserved among different RSV strains; therefore, RSV-N protein seems to be a good candidate antigen for use in developing a serological assay to monitor RSV exposures (1, 3, 5, 21, 22). Nucleoprotein antigen has been used to develop serological assays for a variety of paramyxoviruses and pneumoviruses; anti-N responses are routinely used to assess exposure to measles virus in epidemiological studies (12, 23–27). A side-by-side comparison of a human immunoglobulin RSV Lot 1 using the LIPS-NRSV and PRN tests over a wide range of IgG concentrations suggests that the LIPS-NRSV assay may also be more sensitive than the PRN assay in detecting RSV antibodies.
There are several limitations to this study. We were not able to test sequential serum samples from infants before and after their first RSV infection, so we cannot be certain that the LIPS-NRSV assay detected a specific response within this cohort, since antibody responses in infants may be masked by the presence of maternal antibodies or blunted by immune system immaturity (28). Also, while passively acquired anti-RSV neutralizing maternal antibodies have mostly dwindled to undetectable levels by 3 to 6 months of age, it is not known if anti-RSV-N antibodies persist beyond this time point, and this needs to be investigated further. Although we show that the LIPS-NRSV assay specifically detects anti-RSV-N antibodies using a murine monoclonal antibody and by blocking antibody binding in the presence of soluble purified RSV-N, we have not demonstrated specificity using serum samples from animals immunized with purified RSV-N antigen. We were not able to compare the sensitivity of the LIPS-NRSV assay with that of an ELISA-RSV-N since these tests are not commercially available; however, others have suggested that the LIPS assay has better specificity and sensitivity over ELISA (29).
In conclusion, the LIPS-NRSV assay represents progress in using a serological assay to detect prior RSV exposure. This test is a rapid, sensitive, and specific high-throughput assay that may help to monitor infections among RSV-naive infants following the receipt of mono- or bivalent RSV-F or RSV-F+G vaccines.
ACKNOWLEDGMENTS
We thank Sonnie Kim Grossman (DMID, NIAID, NIH) for her support, and we thank the following investigators for sharing the pREN2 vector, viruses, and antibodies/antisera: Peter Burbelo, Ursula Buchholz, Jim Crowe, Brian Murphy, Steven Whitehead, William Bellini, Paul Rota, Biao He, Steven Rubin, and Hang Xie. We also thank Surender Khurana and Steven Rubin for their critical review of the manuscript.
The WHO Reagent Bank for RSV and PIV3 provided purified RSV-F and RSV-G proteins for rabbit immunizations.
Footnotes
Published ahead of print 8 January 2014
REFERENCES
- 1.Collins PL, Crowe JE. 2007. Respiratory syncytial virus and metapneumovirus, p 1601–1636 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2.Hendry RM, Talis AL, Godfrey E, Anderson LJ, Fernie BF, McIntosh K. 1986. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. J. Infect. Dis. 153:291–297. 10.1093/infdis/153.2.291 [DOI] [PubMed] [Google Scholar]
- 3.Mufson MA, Orvell C, Rafnar B, Norrby E. 1985. Two distinct subtypes of human respiratory syncytial virus. J. Gen. Virol. 66(Pt 10):2111–2124 [DOI] [PubMed] [Google Scholar]
- 4.Connors M, Collins PL, Firestone CY, Murphy BR. 1991. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 65:1634–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward KA, Lambden PR, Ogilvie MM, Watt PJ. 1983. Antibodies to respiratory syncytial virus polypeptides and their significance in human infection. J. Gen. Virol. 64(Pt 9):1867–1876 [DOI] [PubMed] [Google Scholar]
- 6.Burbelo PD, Goldman R, Mattson TL. 2005. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 5:22. 10.1186/1472-6750-5-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeler JA, van Wyke Coelingh K. 1989. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J. Virol. 63:2941–2950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burbelo PD, Ching KH, Klimavicz CM, Iadarola MJ. 2009. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS). J. Vis. Exp.(32):1549. 10.3791/1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crim RL, Audet SA, Feldman SA, Mostowski HS, Beeler JA. 2007. Identification of linear heparin-binding peptides derived from human respiratory syncytial virus fusion glycoprotein that inhibit infectivity. J. Virol. 81:261–271. 10.1128/JVI.01226-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe JE, Firestone CY, Crim R, Beeler JA, Coelingh KL, Barbas CF, Burton DR, Chanock RM, Murphy BR. 1998. Monoclonal antibody-resistant mutants selected with a respiratory syncytial virus-neutralizing human antibody fab fragment (Fab 19) define a unique epitope on the fusion (F) glycoprotein. Virology 252:373–375. 10.1006/viro.1998.9462 [DOI] [PubMed] [Google Scholar]
- 11.Fenwick F, Young B, McGuckin R, Robinson MJ, Taha Y, Taylor CE, Toms GL. 2007. Diagnosis of human metapneumovirus by immunofluorescence staining with monoclonal antibodies in the North-East of England. J. Clin. Virol. 40:193–196. 10.1016/j.jcv.2007.07.018 [DOI] [PubMed] [Google Scholar]
- 12.Hummel KB, Erdman DD, Heath J, Bellini WJ. 1992. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J. Clin. Microbiol. 30:2874–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu P, Li Z, Sun D, Lin Y, Wu J, Rota PA, He B. 2011. Rescue of wild-type mumps virus from a strain associated with recent outbreaks helps to define the role of the SH ORF in the pathogenesis of mumps virus. Virology 417:126–136. 10.1016/j.virol.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coates HV, Alling DW, Chanock RM. 1966. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am. J. Epidemiol. 83:299–313 [DOI] [PubMed] [Google Scholar]
- 15.Varada JC, Teferedegne B, Crim RL, Mdluli T, Audet S, Peden K, Beeler J, Murata H. 2013. A neutralization assay for respiratory syncytial virus using a quantitative PCR-based endpoint assessment. Virol. J. 10:195. 10.1186/1743-422X-10-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen BJ, Audet S, Andrews N, Beeler J, WHO Working Group on Measles Plaque Reduction Neutralization Test 2007. Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine 26:59–66. 10.1016/j.vaccine.2007.10.046 [DOI] [PubMed] [Google Scholar]
- 17.Crowe JE, Jr, Collins PL, London WT, Chanock RM, Murphy BR. 1993. A comparison in chimpanzees of the immunogenicity and efficacy of live attenuated respiratory syncytial virus (RSV) temperature-sensitive mutant vaccines and vaccinia virus recombinants that express the surface glycoproteins of RSV. Vaccine 11:1395–1404. 10.1016/0264-410X(93)90168-W [DOI] [PubMed] [Google Scholar]
- 18.Cherrie AH, Anderson K, Wertz GW, Openshaw PJ. 1992. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J. Virol. 66:2102–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The IMpact-RSV Study Group 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102:531–537 [PubMed] [Google Scholar]
- 20.Schmidt AC. 2011. Progress in respiratory virus vaccine development. Semin. Respir. Crit. Care Med. 32:527–540. 10.1055/s-0031-1283289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buraphacheep W, Britt WJ, Sullender WM. 1997. Detection of antibodies to respiratory syncytial virus attachment and nucleocapsid proteins with recombinant baculovirus-expressed antigens. J. Clin. Microbiol. 35:354–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goto-Sugai K, Tsukagoshi H, Mizuta K, Matsuda S, Noda M, Sugai T, Saito Y, Okabe N, Tashiro M, Kozawa K, Tanaka R, Morita Y, Nishina A, Kimura H. 2010. Genotyping and phylogenetic analysis of the major genes in respiratory syncytial virus isolated from infants with bronchiolitis. Jpn. J. Infect. Dis. 63:393–400 [PubMed] [Google Scholar]
- 23.Gulati BR, Munir S, Patnayak DP, Goyal SM, Kapur V. 2001. Detection of antibodies to U.S. isolates of avian pneumovirus by a recombinant nucleocapsid protein-based sandwich enzyme-linked immunosorbent assay. J. Clin. Microbiol. 39:2967–2970. 10.1128/JCM.39.8.2967-2970.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamelin ME, Boivin G. 2005. Development and validation of an enzyme-linked immunosorbent assay for human metapneumovirus serology based on a recombinant viral protein. Clin. Diagn. Lab. Immunol. 12:249–253. 10.1128/CDLI.12.2.249-253.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samal SK, Pastey MK, McPhillips T, Carmel DK, Mohanty SB. 1993. Reliable confirmation of antibodies to bovine respiratory syncytial virus (BRSV) by enzyme-linked immunosorbent assay using BRSV nucleocapsid protein expressed in insect cells. J. Clin. Microbiol. 31:3147–3152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warnes A, Fooks AR, Stephenson JR. 1994. Production of measles nucleoprotein in different expression systems and its use as a diagnostic reagent. J. Virol. Methods 49:257–268. 10.1016/0166-0934(94)90141-4 [DOI] [PubMed] [Google Scholar]
- 27.Backhouse JL, Gidding HF, McIntyre PB, Gilbert GL. 2006. Evaluation of two enzyme immunoassays for detection of immunoglobulin G antibodies to mumps virus. Clin. Vaccine Immunol. 13:764–767. 10.1128/CVI.00199-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gans HA. 2012. The status of live viral vaccination in early life. Vaccine 10.1016/j.vaccine.2012.09.043 [DOI] [PubMed] [Google Scholar]
- 29.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. 2008. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J. Infect. Dis. 198:444–451. 10.1086/589718 [DOI] [PMC free article] [PubMed] [Google Scholar]