Abstract
Under Korean field conditions, coinfection with porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) is most commonly observed in porcine respiratory disease complex (PRDC). Despite the wide use of PCV2 vaccination, PRDC remains a serious respiratory problem. Thus, the objective of this study was to determine and compare the efficacy of 4 one-dose PCV2 vaccines on 3-week-old pigs with an experimental PCV2-PRRSV challenge at 17 weeks postvaccination. Regardless of which commercial PCV2 vaccine was used, the vaccination of piglets at 3 weeks of age was efficacious against cochallenge of PCV2 and PRRSV, on the basis of growth performance and PCV2-associated lesions. However, the inactivated chimeric PCV1-2 and the PCV2 vaccines induced higher PCV2-specific neutralizing antibody (NA) titers and PCV2-specific gamma interferon-secreting cells and lower PCV2 viremia levels than the two PCV2 subunit vaccines. The vaccination of piglets against PCV2 at 3 weeks of age was effective in reducing PCV2 viremia and PCV2-associated lesions during the finishing period, which is an age at which pigs are frequently affected by PRDC caused by coinfection with PCV2 and PRRSV under Korean field conditions.
INTRODUCTION
Porcine circovirus type 2 (PCV2) is associated with a number of diseases and syndromes that are collectively referred to as porcine circovirus-associated diseases (PCVAD). Among them, postweaning multisystemic wasting syndrome (PMWS) and porcine respiratory disease complex (PRDC) are the most important (1, 2). Porcine reproductive and respiratory syndrome virus (PRRSV) causes reproductive failure in gilts and sows and severe respiratory disease in nursery and growing-finishing pigs (3).
In current Korean fields, PRDC is an important economic problem in growing and finishing pigs (typically around 16 to 22 weeks of age). Coinfection with PCV2 and PRRSV is most commonly observed in field cases (4). PCV2b and North American PRRSV are the most common circulating genotypes in the herds (5, 6). Despite the wide use of PCV2 vaccination, the incidence of PRDC remains high. In a European field study, vaccination against PCV2 alone can significantly improve the overall growth performance in herds that are suffering from PRDC caused by a coinfection with PCV2 and PRRSV (7). Hence, it is necessary to determine whether vaccination against PCV2 alone can control PRDC, which is caused by coinfection with PCV2 and PRRSV in the finishing period. This is important because PCV2 vaccination was administered to approximately 95.5% of all piglets farrowed in the past 3 years after implementation of the Korean government's subsidiary program (8).
Currently, 4 commercial one-dose PCV2 vaccines are available in the Korean market (8). As these vaccines differ in their antigens (whole PCV2, chimeric PCV1-2, and a baculovirus-expressed subunit based on open reading frame 2 of PCV2 [9]), the objective of this study was to determine and compare the efficacy of 4 one-dose PCV2 vaccines for pigs with an experimental PCV2-PRRSV challenge at 17 weeks postvaccination to mimic Korean field conditions.
MATERIALS AND METHODS
Experimental design.
A total of 60 colostrum-fed cross-bred conventional piglets were purchased at 14 days of age from a PRRSV-free commercial farm, which was positive for PCV2 and Mycoplasma hyopneumoniae. Selected piglets were negative for PCV2, PRRSV, and M. hyopneumoniae by routine serological testing and for PCV1-2 and PCV2 by real-time PCR, as previously described (10, 11). This study used a randomized, blinded, weight- and sex-matched, and controlled design. Sixty pigs were randomly assigned into 1 of 6 groups (10 pigs per group; Table 1). Four commercial PCV2 vaccines were administered intramuscularly in the right side of the neck at 3 weeks of age at different dosages according to the manufacturer's instructions: 2.0 ml of inactivated chimeric PCV1-2 vaccine (Fostera PCV; Zoetis, Madison, NJ) (group 1), 0.5 ml of inactivated PCV2 vaccine (Circovac; Merial, Lyon, France) (group 2), and 1.0 ml each of PCV2 subunit A (Circoflex; Boehringer Ingelheim Vetmedica Inc., St. Joseph, MO) (group 3) and B vaccine (Porcilis PCV; MSD Animal Health, Boxmeer, The Netherlands) (group 4). Phosphate-buffered saline (PBS) was also given in a 2.0-ml dose at 3 weeks of age to the positive (group 5) and negative (group 6) control groups.
TABLE 1.
Average daily weight gains, proportions of viremic pigs and nasal shedders at different days postchallenge, histopathological lymphoid and pulmonary lesion scores, and immunohistochemical PCV2 and PRRSV antigen scores among the groups
| Data typea | Values for pigs in the indicated group |
|||||
|---|---|---|---|---|---|---|
| Fostera PCV | Circovac | Circoflex | Porcilis PCV | Positive control | Negative control | |
| ADWG (mean ± SD) (g/pig/day) at: | ||||||
| 3–20 wk | 609 ± 48 | 609 ± 44 | 604 ± 52 | 609 ± 43 | 601 ± 21 | 610 ± 24 |
| 20–25 wk | 709 ± 26 bb | 707 ± 38 b | 676 ± 28 b | 683 ± 37 b | 633 ± 28 a | 718 ± 31 b |
| 3–25 wk | 632 ± 38 | 631 ± 33 | 621 ± 43 | 626 ± 30 | 608 ± 21 | 634 ± 23 |
| No. of PCV2 viremic pigs (total n = 10) at: | ||||||
| 7 dpc | 3 a | 3 a | 4 a,b | 3 a | 8 b | 0 a |
| 14 dpc | 3 a | 3 a | 8 b,c | 4 a,b | 10 c | 0 a |
| 21 dpc | 2 a,b | 2 a,b | 6 b,c | 3 a,b | 10 c | 0 a |
| 35 dpc | 2 a | 2 a | 2 a | 2 a | 9 b | 0 a |
| Lymphoid score (mean ± SD) | 0.9 ± 0.99 a | 1 ± 0.94 a | 1.8 ± 0.91 a | 1.1 ± 0.99 a | 3.3 ± 0.82 b | 0.4 ± 0.51 a |
| Pulmonary score (mean ± SD) | 0.7 ± 0.67 a | 0.9 ± 0.87 a | 1.1 ± 0.99 a | 1.2 ± 1.13 a | 2.9 ± 0.99 b | 0.4 ± 0.64 a |
| PCV2 antigen score (mean ± SD) | ||||||
| LN | 10.5 ± 4.85 b | 11.4 ± 5.23 b | 19.6 ± 8.04 b | 10.5 ± 4.85 b | 39.5 ± 16.25 c | 0 a |
| Lung | 4.4 ± 3.62 b | 5.5 ± 3.59 b | 6.1 ± 3.47 b | 6.1 ± 3.47 b | 16.9 ± 6.29 c | 0 a |
| PRRS antigen score (mean ± SD) | ||||||
| LN | 1.9 ± 2.18 b | 1.6 ± 1.71 b | 2.4 ± 2.27 b | 2.3 ± 1.7 b | 2.7 ± 2.21 b | 0 a |
| Lung | 9.4 ± 6.02 b | 11.1 ± 5.87 b | 10.9 ± 7.72 b | 10.6 ± 4.5 b | 14 ± 8.7 b | 0 a |
ADWG, average daily weight gain; dpc, days postchallenge; PCV2, porcine circovirus type 2; LN, lymph nodes; PRRSV, porcine reproductive and respiratory syndrome virus.
Different lowercase letters indicate groups that are significantly (P < 0.05) different from each other.
At 17 weeks postvaccination (0 days postchallenge [dpc]), the pigs in the vaccinated (groups 1, 2, 3, and 4) and positive-control (group 5) groups were inoculated intranasally with 3 ml each of PCV2b (strain SNUVR000463, 5th passage, 1.0 × 105 50% tissue culture infective dose [TCID50]/ml) and North American PRRSV (strain SNUVR090851, 5th passage, 1.0 × 105 TCID50/ml) (Table 1).
The pigs in each group were housed separately within the facility as previously described (12). Clinical respiratory scores and rectal body temperatures were recorded daily from 0 to 35 dpc as previously described (13). Blood samples from each pig were collected by jugular venipuncture at −119, −98, −77, −56, −28, 0, 7, 14, 21, and 35 dpc. The pigs were tranquilized by intravenous injection of azaperone (Stresnil, Janssen Pharmaceutica, Beerse, Belgium) and then euthanized by electrocution for necropsy at 35 dpc. All methods were approved by the Seoul National University Institutional Animal Care and Use Committee.
Growth performance.
The live weight of each pig was measured at 3, 20, and 25 weeks of age. The average daily weight gain (ADWG) (gram/pig/day) was analyzed over two time periods, (i) between 3 and 20 weeks of age and (ii) between 20 and 25 weeks of age. The ADWG during the different production stages was calculated as the difference between the starting and final weights divided by the duration of the stage.
Quantification of PCV1-2, PCV2, and PRRSV in blood.
DNA extraction from serum samples was performed using the QIAamp DNA minikit (Qiagen Inc., Valencia, CA, USA). DNA extracts were used to quantify PCV1-2 and PCV2 genomic DNA copy numbers by real-time PCR as previously described (10, 11). RNA was extracted from serum samples from all pigs used in this study as previously described (14). Real-time PCR for PRRSV was used to quantify PRRSV genomic RNA copy numbers using RNA extracted from serum samples as previously described (14).
Serology.
Serum samples were tested using a commercial PCV2 IgG enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Synbiotics, Lyon, France), and they were considered positive for PCV2 antibody if the titer was >550. Serum virus neutralization tests were performed using a challenging PCV2b strain as previously described (15). Neutralizing antibody (NA) titers were expressed as the reciprocal of the highest serum dilution that completely blocked the infection in PK15 cells compared with the virus control.
Enzyme-linked immunospot assay.
The numbers of PCV2-specific gamma interferon-secreting cells (IFN-γ-SCs) were determined in peripheral blood mononuclear cells (PBMCs) by an enzyme-linked immunospot (ELISPOT) assay as previously described (16). Briefly, 96-well plates were coated with 0.5 μg/ml of mouse anti-porcine IFN-γ monoclonal antibody (5 μg/ml; Mabtech, Mariemont, OH, USA) diluted in PBS and stored at 4°C. The plates were washed five times with 200 μl of PBS. After plates were washed, 100 μl of RPMI 1640 medium supplemented with 10% fetal bovine serum that contained 2 × 106 PBMCs (HyClone Laboratories, Inc., SelectScience, Bath, United Kingdom) was dispensed per well and stimulated with either 100 μl of PCV2 antigen (20 μg/ml), phytohemagglutinin (10 μg/ml; Roche Diagnostics GmbH, Mannheim, Germany) (as a positive control), or PBS (as a negative control) for 40 h at 37°C in a 5% humidified CO2 atmosphere. Then, the wells were washed five times with PBS (200 μl per well). Thereafter, we followed the procedures in the manufacturer's instructions for the commercial ELISPOT assay kit (Mabtech, Mariemont, OH). The spots on the membranes were read by an AID automated ELISpot reader (AID GmbH, Strassberg, Germany). The results were expressed as the number of responding cells per million PBMCs.
Histopathology and immunohistochemistry.
For the morphometric analysis of histopathological lesion scores in lymph nodes and lungs, three sections of each of the superficial inguinal lymph nodes and lungs were examined in a blinded manner as previously described (12, 13, 17). Superficial inguinal lymph node sections were also examined blindly, and their scores ranged from 0 (normal, i.e., no lymphoid depletion or granulomatous replacement) to 5 (severe lymphoid depletion and granulomatous replacement) (17). Lung sections were also scored for interstitial pneumonia, ranging from 0 (normal) to 6 (severe diffuse) as previously described (13).
For morphometric analyses to determine the PCV2 and PRRSV antigen scores, 3 sections were cut from each of three blocks of tissue from the lung and inguinal lymph nodes of each pig. The slides were analyzed using the NIH Image J 1.43m program (see http://rsb.info.nih.gov/ij) to obtain the quantitative data. In each slide, 10 fields were randomly selected, and the number of PCV2-positive cells per unit area (0.25 mm2) was determined as previously described (13, 17).
Statistical analysis.
Prior to the statistical analysis, real-time PCR and NA data were transformed to log10 and log2 values, respectively. Normality of the distribution of the examined variables was evaluated by the Shapiro-Wilk test. Continuous data (rectal body temperatures, PCV2 DNA, PCV2 serologies, number of PCV2-specific IFN-γ-SCs, and PCV2 antigen score) were subjected to a one-way analysis of variance (ANOVA). If the one-way ANOVA was significant (P < 0.05), pairwise testing using Tukey's adjustment was then performed. Discrete data (the clinical respiratory score and the lymphoid and pulmonary lesion scores) were analyzed using the chi-square and Fisher's exact tests. Pearson's correlation coefficient was used to assess the relationship between PCV2 viremia and PCV2-specific NAs and IFN-γ-SCs at certain time points. A P value of <0.05 was considered significant.
RESULTS
Growth performance.
No significant differences were observed in the ADWG between the vaccinated and negative-control groups during the 3- to 20-week period. The ADWG of the vaccinated and negative-control groups was significantly higher (P < 0.05) than that of the positive-control group during the 20- to 25-week period. The overall growth rates (from 3 to 25 weeks of age) were not significantly different among the 6 groups (Table 1).
Clinical signs.
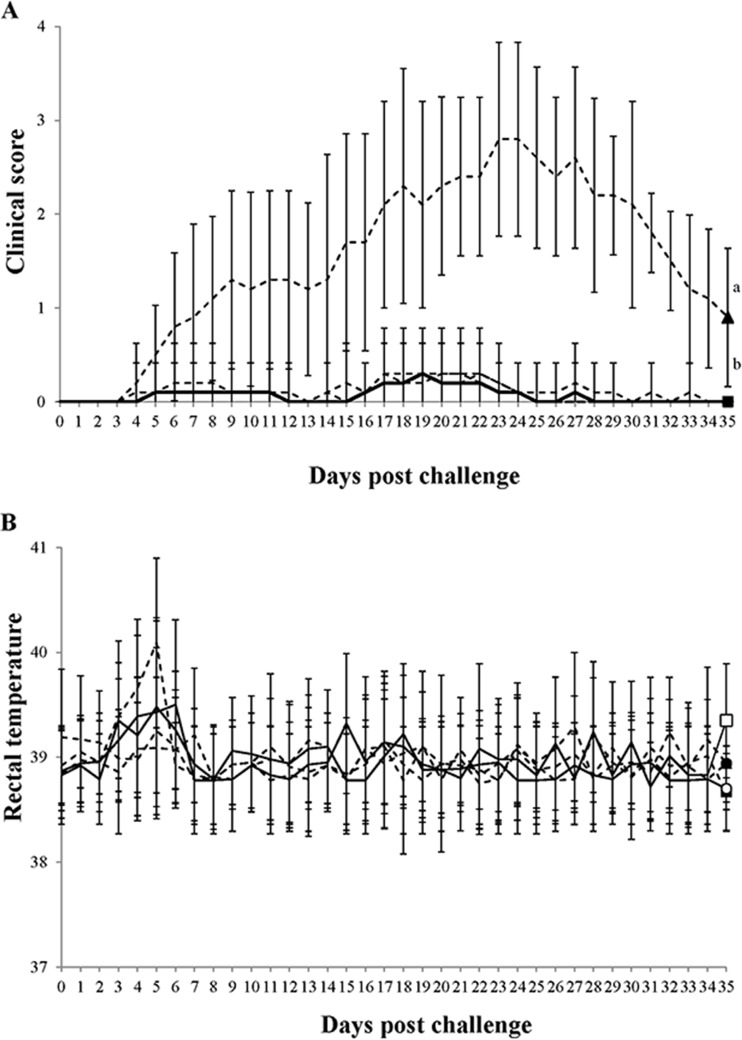
Clinical respiratory disease was not observed in the 4 vaccinated groups after challenge with PCV2 and PRRSV, whereas moderate to severe respiratory disease, characterized by sneezing and increased respiratory rates, was observed in the positive-control group. In the positive-control group, tachypnea and pronounced abdominal breathing were first observed at 5 dpc in 5 of 10 pigs. In addition, 7 pigs developed severe dyspnea and depression between 7 and 10 dpc. Mean clinical respiratory scores increased steadily from 8 to 35 dpc in the pigs in the positive-control group and were significantly higher (P < 0.05) than those in the vaccinated and negative-control groups (Fig. 1A). Although pigs from the positive-control group had slightly elevated rectal temperatures (ranging from 39.5 to 40°C at 4 and 5 dpc), no significant differences in mean rectal temperatures were observed in pigs among the vaccinated, positive-control, and negative-control groups throughout the experiment (Fig. 1B). No dead pigs were found in any of the groups.
FIG 1.
(A) Mean clinical respiratory scores; (B) mean rectal body temperatures. □, pigs which received the inactivated chimeric PCV1-2 vaccine followed by dual challenge; ■, pigs which received the inactivated PCV2 vaccine followed by dual challenge; ○, pigs which received the PCV2 subunit A vaccine followed by dual challenge; ●, pigs which received the PCV2 subunit B vaccine followed by dual challenge; ▲, pigs which were challenged with PCV2 and PRRSV. Different letters (a and b) indicate that the groups were significantly (P < 0.05) different from each other on the indicated day.
PCV2 DNA in sera.
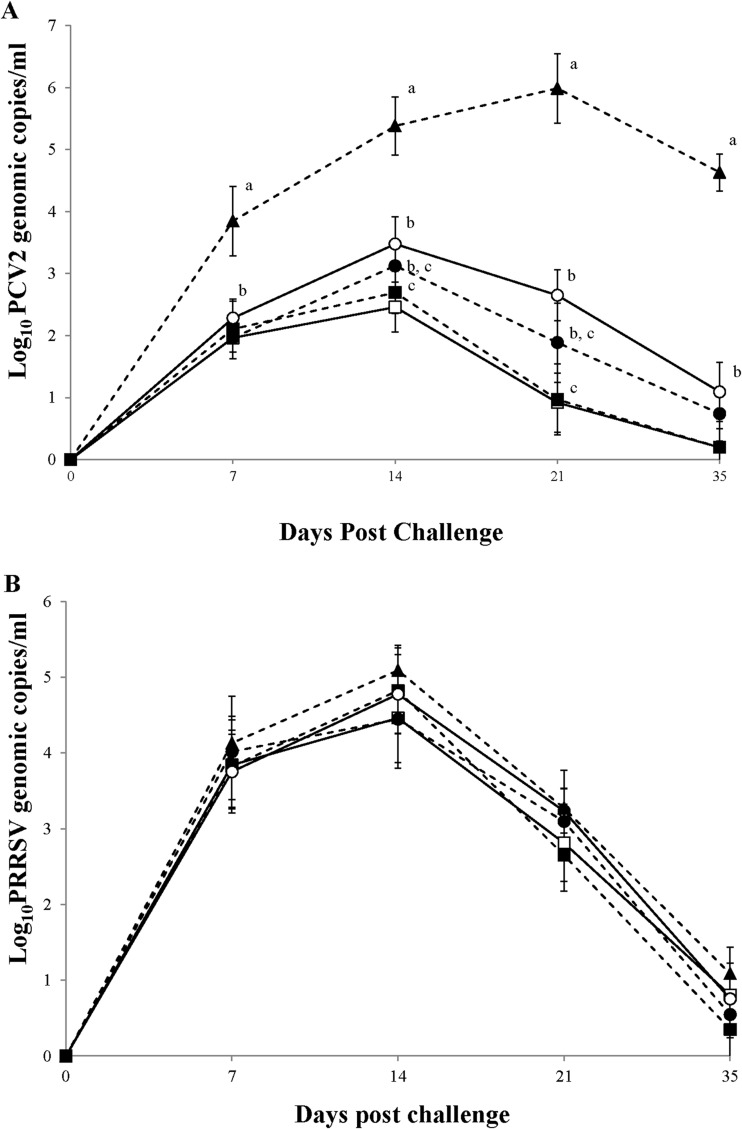
No PCV2b DNA was detected in the blood of pigs in the vaccinated or positive-control groups on the day of challenge. There was a significant (P < 0.001) difference in log-transformed PCV2b DNA amounts in the blood between the vaccinated and positive-control groups throughout the experiment, and significant (P < 0.05) differences were also noted among the vaccinated groups (Fig. 2A). At 14 and 21 dpc, the pigs vaccinated with the inactivated chimeric PCV1-2 and PCV2 vaccines had significantly lower numbers of genomic copies of PCV2b (P < 0.05) in their blood than the pigs vaccinated with the PCV2 subunit A vaccine (Fig. 2A). No PCV2b DNA was detected in the blood of pigs in the negative-control group throughout the experiment. No PCV1-2 or PCV2a was detected in the blood of any of the 6 groups throughout the experiment.
FIG 2.
(A) Mean genomic copy numbers of PCV2 DNA; (B) mean genomic copy numbers of PRRSV RNA. □, pigs which received the inactivated chimeric PCV1-2 vaccine followed by dual challenge; ■, pigs which received the inactivated PCV2 vaccine followed by dual challenge; ○, pigs which received the PCV2 subunit A vaccine followed by dual challenge; ●, pigs which received the PCV2 subunit B vaccine followed by dual challenge; ▲, pigs which were challenged with PCV2 and PRRSV. Different letters (a, b, and c) indicate that the groups were significantly (P < 0.05) different from each other on the indicated day.
PRRSV RNA in sera.
PRRSV RNA was not detected in the blood of the pigs in the vaccinated or positive-control groups on the day of challenge, but it was detected in the blood of the same pigs at 7 dpc. The numbers of genomic copies of PRRSV were gradually increased until 14 dpc and thereafter were decreased until 35 dpc. However, no significant differences in log-transformed PRRSV RNA amounts were observed in the blood between the vaccinated and positive-control groups throughout the experiment (Fig. 2B). No PRRSV RNA was detected in the blood of pigs in the negative-control group throughout the experiment.
Anti-PCV2 IgG antibodies.
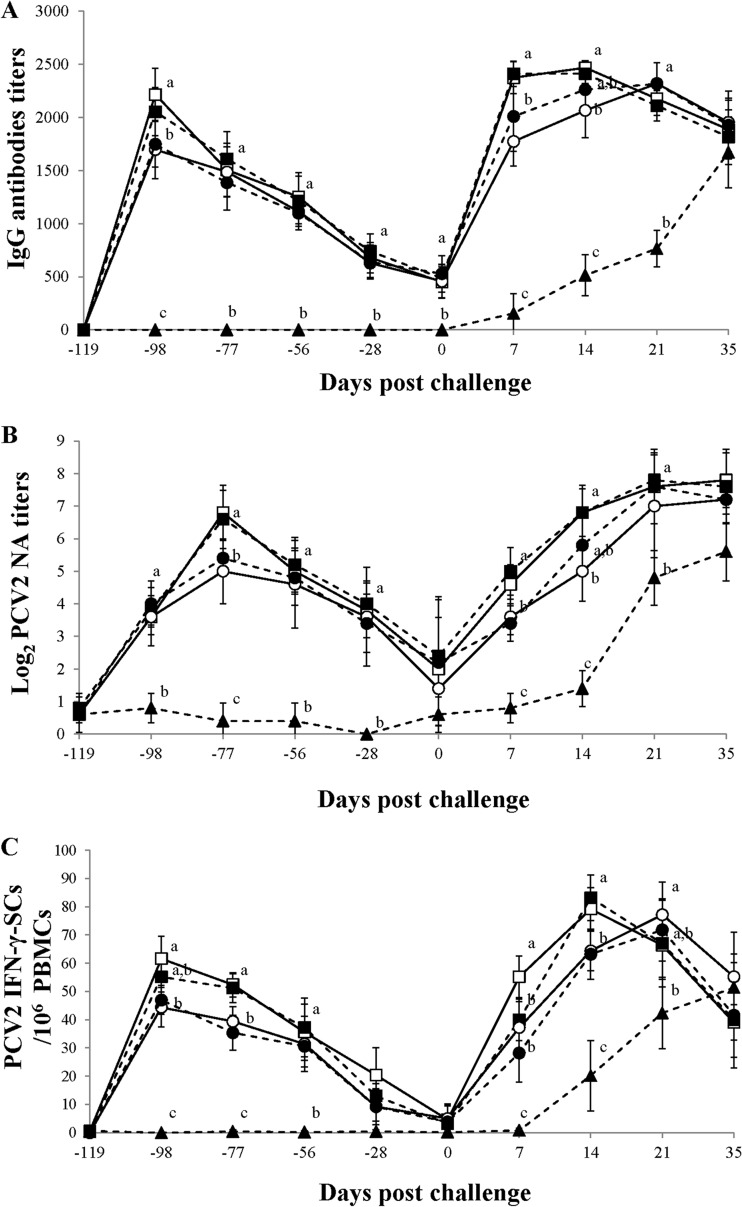
At 21 days postvaccination, pigs vaccinated with the inactivated PCV1-2 and PCV2 vaccines exhibited significantly higher levels of anti-PCV2 IgG antibodies than pigs receiving the PCV2 subunit A and B vaccines (P < 0.05). At 7 and 14 dpc, pigs vaccinated with the inactivated chimeric PCV1-2 and PCV2 vaccines exhibited significantly higher levels of anti-PCV2 IgG antibodies than pigs receiving the PCV2 subunit A vaccine (P < 0.045). There was a significant (P < 0.001) difference in the levels of anti-PCV2 IgG antibodies between the vaccinated and positive-control groups from −119 to 21 dpc (Fig. 3A). No anti-PCV2 IgG antibodies were detected in the negative-control group throughout the experiment.
FIG 3.
(A) Mean anti PCV2 IgG antibodies titers; (B) mean serum NA titers against PCV2 in serum samples; (C) mean numbers of PCV2-specific IFN-γ-SCs in PBMCs. □, pigs which received the inactivated chimeric PCV1-2 vaccine followed by dual challenge; ■, pigs which received the inactivated PCV2 vaccine followed by dual challenge; ○, pigs which received the PCV2 subunit A vaccine followed by dual challenge; ●, pigs which received the PCV2 subunit B vaccine followed by dual challenge; ▲, pigs which were challenged with PCV2 and PRRSV. Different letters (a, b, c) indicate that the groups were significantly (P < 0.05) different from each other on the indicated day.
PCV2-specific neutralizing antibodies.
There were significant (P < 0.001) differences in the log2-transformed group mean NA titers between the vaccinated and positive-control groups throughout the experiment, except at 0 dpc. Significant (P < 0.05) differences were also noted among the different PCV2 vaccines. At −77 and 7 dpc, the pigs vaccinated with inactivated PCV1-2 and PCV2 vaccines exhibited significantly higher log-transformed group mean NA titers than the pigs vaccinated with the PCV2 subunit A or B vaccine (P < 0.05). At 14 dpc, the inactivated chimeric PCV1-2 and PCV2 vaccines induced significantly higher log-transformed group mean NA titers (P < 0.05) than the PCV2 subunit A vaccine (Fig. 3B). In the vaccinated and control groups, the log-transformed group mean NA titers inversely correlated with the numbers of genomic copies of PCV2 in the blood (for the inactivated chimeric PCV1-2 vaccine, r = −0.945 and P = 0.015 [at 21 dpc] and r = −0.928 and P = 0.023 [at 35 dpc]; for the inactivated PCV2 vaccine, r = −0.944 and P = 0.016 [at 21 dpc] and r = −0.928 and P = 0.023 [35 dpc]; for the PCV2 subunit A vaccine, r = −0.894 and P = 0.041 [at 21 dpc] and r = −0.943 and P = 0.016 [at 35 dpc]; for the PCV2 subunit B vaccine, r = −0.924 and P = 0.025 [at 21 dpc] and r = −0.922 and P = 0.026 [at 35 dpc]; and for the positive control, r = −0.890 and P = 0.018 [at 35 dpc]).
PCV2-specific gamma-interferon-secreting cells.
There was a significant (P < 0.001) difference in the group mean numbers of PCV2-specific IFN-γ-SCs between the vaccinated and positive-control groups throughout the experiment except at −119, −28, 0, and 35 dpc. Significant (P < 0.05) differences were also noted among the PCV2 vaccines. At −98 dpc, the inactivated chimeric PCV1-2 vaccine induced significantly higher numbers of PCV2-specific IFN-γ-SCs than the PCV2 subunit A and B vaccines. At 7 dpc, the inactivated chimeric PCV1-2 vaccine induced significantly higher numbers of PCV2-specific IFN-γ-SCs than the other 3 vaccinated groups (P < 0.05). At −77 and 14 dpc, the inactivated chimeric PCV1-2 and PCV2 vaccines induced significantly higher numbers of PCV2-specific IFN-γ-SCs (P < 0.05) than the other 2 vaccines (Fig. 3C). In the vaccinated and control groups, the group mean numbers of PCV2-specific IFN-γ-SCs correlated inversely with the numbers of genomic copies of PCV2 in the blood (for the inactivated chimeric PCV1-2 vaccine, r = −0.633 and P = 0.050 at 21 dpc; for the inactivated PCV2 vaccine, r = −0.684 and P = 0.029; for the PCV2 subunit A vaccine, r = −0.826 and P = 0.003 at 21 dpc; for the PCV2 subunit B vaccine, r = −0.784 and P = 0.007 at 21 dpc; and for the positive control, r = −0.740 and P = 0.023 at 35 dpc).
Histopathology and immunohistochemistry.
The histopathological lymphoid and pulmonary lesion scores induced by coinfection with PCV2 and PRRSV were significantly lower (P < 0.05) in the vaccinated groups than in the positive-control group (Table 1). There were no histopathological lymphoid or pulmonary lesions in the negative-control pigs. PCV2 and PRRSV antigens were detected in macrophages in the lymph node and lung. There were significantly different PCV2 antigen scores (P < 0.001) between the vaccinated and positive-control groups (Table 1). There were no significantly different PRRSV antigen scores between the vaccinated and positive-control groups. No PCV2 or PRRSV antigens were detected in the lung and lymph nodes of pigs in the negative-control group.
DISCUSSION
Swine producers are interested in controlling PCV2-associated PRDC to reduce the fattening period from birth to slaughter for economical benefit. Additionally, the longer the fattening period, the greater the chance of developing PRDC in herds. Therefore, the most critical parameter for evaluating the efficacy of PCV2 vaccines under field conditions is the comparison of growth performances. Regardless of which commercial PCV2 vaccine was used, PCV2 vaccination of piglets at 3 weeks of age was effective in improving growth performance (as measured by ADWG) during the finishing period after cochallenge with PCV2 and PRRSV. We do not know why some swine herds still have PCV2-associated PRDC even when PCV2 vaccines have been administered to same-aged pigs. Since PRDC is a multifactorial disease, the efficacy of the PCV2 vaccine in the fields can be affected by many factors, including the environment, feed, the pig source, the farm facility, management, and the production system (i.e., all-in/all-out, continuous production, and multiple-site production).
Currently, all commercial PCV2 vaccines used worldwide are based on the PCV2a genotype (9). Vaccination with 4 single-dose commercial PCV2a-based vaccines used in this study reduced PCV2 viremia and PCV2-associated lymphoid lesions in experimental PCV2b challenge situations. These results are further supported by the cross-protection of PCV2a-based vaccines against a PCV2b challenge (10, 18–20). This cross-protection is clinically significant because PCV2b is the predominant genotype circulated in most Korean herds (5).
In the challenge model, coinfection with PCV2 and PRRSV induced prolonged and unusually severe clinical respiratory disease, which is similar to typical PRDC. The most striking and consistent microscopic lesions were severe interstitial pneumonia with some degree of peribronchial and peribronchiolar fibrosis, as previously described (4). PCV2 vaccination of piglets at 3 weeks of age was effective in reducing PCV2 viremia and PCV2-associated lesions during the finishing period, an age of frequent outbreaks of PRDC caused by coinfection with PCV2 and PRRSV under Korean field conditions. These results extend previous findings in which the administration of either the inactivated chimeric PCV1-2 or the PCV2 subunit A vaccine to pigs was effective in reducing PCV2 viremia during the growth period following a triple challenge with PCV2-PRRSV-porcine parvovirus or PCV2-PRRSV-swine influenza virus at 12 to 13 weeks postvaccination (10, 18). In contrast, vaccination against PCV2 alone did not reduce PRRSV viremia in dually challenged pigs in the present or previous studies (21). Although no significant differences in growth performance were observed among the 4 vaccinated groups, the present study demonstrated a quantitative difference in the reduction of PCV2 viremia among them. The inactivated chimeric PCV1-2 and PCV2 vaccines yielded significantly lower PCV2 viremia levels compared to those of the two PCV2 subunit vaccines.
In the present study, we show that the 4 commercial single-dose PCV2 vaccines provide enough prolonged active protective immunity to control PCV2-associated PRDC throughout the finishing period. The induction of PCV2-specific NAs and IFN-γ-SCs by the PCV2 vaccine is an important protective immune response that leads to the reduction of PCV2 viremia (22, 23). Induction of PCV2-specific NAs and IFN-γ-SCs was also observed in this study; however, the 4 commercial PCV2 vaccines elicited different levels of protective immunity. The inactivated chimeric PCV1-2 and PCV2 vaccines induced higher PCV2-specific NA titers and PCV2-specific IFN-γ-SCs than the two PCV2 subunit vaccines. We have no clear explanation for these differences in the induction of protective immunity and the reduction of PCV2 viremia; however, they may be due to the different types of antigens and adjuvants among the 4 PCV2 vaccines (9). Nevertheless, the 4 commercial PCV2 vaccines used in this study were shown to be efficacious in controlling PCV2 infection under experimental conditions (10, 19, 20, 22, 24) and PCVAD under field conditions (16, 23, 25, 26). Hence, further studies may be needed to determine the clinical significance of different levels of PCV2 viremia in pigs vaccinated with different commercial PCV2 vaccines in the finishing period.
To our knowledge, this is the first comparative study of 4 single-dose PCV2 vaccines based on clinical, virological, immunological, and pathological evaluations. The conditions in this experiment were designed to mirror those seen in the Korean fields. However, this experiment included only piglets that were negative for PCV2-specific antibodies and therefore did not represent the true field conditions under which PCV2 infection is common. In addition, seronegative piglets selected for the experiments were from a PCV2-positive farm, and these piglets had been fed with colostrum. Thus, some piglets may have had maternally derived antibodies (MDA) against PCV2 when immunized with the PCV2 vaccines, leading to potential interference from the MDA. However, piglets with high immunoperoxidase monolayer assay (>9 log2) or NA (>7 log2) titers seemed to only show interference with the development of the humoral immune response after PCV2 vaccination (16, 22). Hence, the results in this study were not likely to have been affected by the presence of MDA in the piglets. The economic impact of PRDC is important in growing-finishing pigs, and concurrent PCV2 and PRRSV infection is one of the most frequent combinations found in the field. The present data provide swine practitioners and producers with useful clinical information on how to select a proper PCV2 vaccine for the control of PCV2-associated PRDC.
ACKNOWLEDGMENTS
This research was supported by Zoetis, by contract research funds of the Research Institute for Veterinary Science (RIVS) from the College of Veterinary Medicine, and by the BK 21 Plus Program for Creative Veterinary Science Research in the Republic of Korea.
We thank Zoetis Korea personnel and Su-Jin Park.
Footnotes
Published ahead of print 8 January 2014
REFERENCES
- 1.Chae C. 2004. Postweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathology. Vet. J. 168:41–49. 10.1016/S1090-0233(03)00182-5, [DOI] [PubMed] [Google Scholar]
- 2.Chae C. 2005. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 169:326–336. 10.1016/j.tvjl.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorell M. 2012. Porcine reproductive and respiratory syndrome virus (porcine arterivirus), p 461–486 In Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW. (ed), Diseases of Swine, 10th ed, Wiley-Blackwell, West Sussex, UK [Google Scholar]
- 4.Kim J, Chung HK, Chae C. 2003. Association of porcine circovirus 2 with porcine respiratory disease complex. Vet. J. 166:251–256. 10.1016/S1090-0233(02)00257-5 [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Ha Y, Oh Y, Chae C. 2011. Prevalence of porcine circovirus types 2a and b in pigs with and without post-weaning multi-systemic wasting syndrome. Vet. J. 188:115–117. 10.1016/j.tvjl.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 6.Lee C, Kim H, Kang B, Yeom M, Han S, Moon H, Park S, Kim H, Song D, Park B. 2010. Prevalence and phylogenetic analysis of the isolated type I porcine reproductive and respiratory syndrome virus from 2007 to 2008 in Korea. Virus Genes 40:225–230. 10.1007/s11262-009-0433-3 [DOI] [PubMed] [Google Scholar]
- 7.Fachinger V, Bischoff R, Jedidia SB, Saalmuller A, Elbers K. 2008. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine 26:1488–1499. 10.1016/j.vaccine.2007.11.053 [DOI] [PubMed] [Google Scholar]
- 8.Chae C. 2012. Porcine circovirus type 2 and its associated disease in Korea. Virus Res. 164:107–113. 10.1016/j.virusres.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 9.Chae C. 2012. Commercial porcine circovirus type 2 vaccines: efficacy and clinical application. Vet. J. 194:151–157. 10.1016/j.tvjl.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 10.Shen H, Beach NM, Huang YW, Halbur PG, Meng XJ, Opriessnig T. 2010. Comparison of commercial and experimental porcine circovirus type 2 (PCV2) vaccines using a triple challenge with PCV2, porcine reproductive and respiratory syndrome virus (PRRSV), and porcine parvovirus (PPV). Vaccine 28:5960–5966. 10.1016/j.vaccine.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 11.Gagnon CA, Del-Castillo JR, Music N, Fontaine G, Harel J, Tremblay D. 2008. Development and use of a multiplex real-time quantitative polymerase chain reaction assay for detection and differentiation of Porcine circovirus-2 genotypes 2a and 2b in an epidemiological survey. J. Vet. Diagn. Invest. 20:545–558. 10.1177/104063870802000503 [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Kim CH, Han K, Seo HW, Oh Y, Park C, Kang I, Chae C. 2011. Comparative efficacy of commercial Mycoplasma hyopneumoniae and porcine circovirus 2 (PCV2) vaccines in pigs experimentally infected with M. hyopneumoniae and PCV2. Vaccine 29:3206–3212. 10.1016/j.vaccine.2011.02.034 [DOI] [PubMed] [Google Scholar]
- 13.Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. 1995. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 32:648–660. 10.1177/030098589503200606 [DOI] [PubMed] [Google Scholar]
- 14.Wasilk A, Callahan JD, Christopher-Hennings J, Gay TA, Fang Y, Dammen M, Reos MF, Torremorell M, Polson D, Mellencamp M, Nelson E, Nelson WM. 2004. Detection of U.S., Lelystad, and European-like porcine reproductive and respiratory syndrome viruses and relative quantitation in boar semen and serum samples by real-time PCR. J. Clin. Microbiol. 42:4453–4461. 10.1128/JCM.42.10.4453-4461.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pogranichnyy RM, Yoon KJ, Harms PA, Swenson SL, Zimmerman JJ, Sorden SD. 2000. Characterization of immune response of young pigs to porcine circovirus type 2 infection. Viral Immunol. 13:143–153. 10.1089/vim.2000.13.143 [DOI] [PubMed] [Google Scholar]
- 16.Seo HW, Han K, Oh Y, Park C, Chae C. 2012. Efficacy of a reformulated inactivated chimeric PCV1-2 vaccine based on clinical, virological, pathological and immunological examination under field conditions. Vaccine 30:6671–6677. 10.1016/j.vaccine.2012.08.065 [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Chae C. 2004. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 in porcine circovirus 2-induced granulomatous inflammation. J. Comp. Pathol. 131:121–126. 10.1016/j.jcpa.2004.02.001 [DOI] [PubMed] [Google Scholar]
- 18.Opriessnig T, Patterson AR, Madson DM, Pal N, Halbur PG. 2009. Comparison of efficacy of commercial one dose and two dose PCV2 vaccines using a mixed PRRSV-PCV2-SIV clinical infection model 2-3-months post vaccination. Vaccine 27:1002–1007. 10.1016/j.vaccine.2008.11.105 [DOI] [PubMed] [Google Scholar]
- 19.Fort M, Sibila M, Allepuz A, Mateu E, Roerink F, Segalés J. 2008. Porcine circovirus type 2 (PCV2) vaccination of conventional pigs prevents viremia against PCV2 isolates of different genotypes and geographic origins. Vaccine 26:1063–1071. 10.1016/j.vaccine.2007.12.019 [DOI] [PubMed] [Google Scholar]
- 20.Seo HW, Oh Y, Han K, Park C, Chae C. 2012. Reduction of porcine circovirus type 2 (PCV2) viremia by a reformulated inactivated chimeric PCV1-2 vaccine-induced humoral and cellular immunity after experimental PCV2 challenge. BMC Vet. Res. 8:194. 10.1186/1746-6148-8-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park C, Oh Y, Seo HW, Han Km Chae C. 2013. Comparative effects of vaccination against porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) in a PCV2-PRRSV challenge model. Clin. Vaccine Immunol. 20:369–376. 10.1128/CVI.00497-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fort M, Sibila M, Perez-Martin E, Nofrarias M, Mateu E, Segales J. 2009. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 27:4031–4037. 10.1016/j.vaccine.2009.04.028 [DOI] [PubMed] [Google Scholar]
- 23.Martelli P, Ferrari L, Morganti M, Angelis DE, Bonilauri P, Guazzetti S, Caleffi A, Borghetti P. 2011. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet. Microbiol. 149:339–351. 10.1016/j.vetmic.2010.12.008 [DOI] [PubMed] [Google Scholar]
- 24.Opriessnig T, Patterson AR, Madson DM, Pal N, Ramamoorthy S, Meng XJ, Halbur PG. 2010. Comparison of the effectiveness of passive (dam) versus active (piglet) immunization against porcine circovirus type 2 (PCV2) and impact of passively derived PCV2 vaccine-induced immunity on vaccination. Vet. Microbiol. 142:177–183. 10.1016/j.vetmic.2009.09.056 [DOI] [PubMed] [Google Scholar]
- 25.Fraile L, Grau-Roma L, Sarasola P, Sinovas N, Nofrarias M, Lopez-Jimenez R, Lopez-Soria S, Silbila M, Segalés J. 2012. Inactivated PCV2 one shot vaccine applied in 3-week-old piglets: improvement of production parameters and interaction with maternally derived immunity. Vaccine 30:1986–1992. 10.1016/j.vaccine.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 26.Kixmoller M, Ritzmann M, Eddicks M, Saalmuller A, Elbers K, Fachinger V. 2008. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine 26:3443–3451. 10.1016/j.vaccine.2008.04.032 [DOI] [PubMed] [Google Scholar]