Abstract
This phase II study evaluated the effect of chloroquine on the specific CD8+ T-cell responses to and the safety of a booster dose of investigational human immunodeficiency virus type 1 (HIV-1) F4/AS01B vaccine containing 10 μg of recombinant fusion protein (F4) adjuvanted with the AS01B adjuvant system. Healthy adults aged 21 to 41 years, primed 3 years before with two F4/AS01B doses containing 10 or 30 μg of F4 (ClinicalTrials.gov registration number NCT00434512), were randomized (1:1) to receive the F4/AS01B booster administered alone or 2 days after chloroquine (300 mg). F4-specific CD8+/CD4+ T-cell responses were characterized by intracellular cytokine staining and lymphoproliferation assays and anti-F4 antibodies by enzyme-linked immunosorbent assays (ELISAs). No effect of chloroquine on CD4+/CD8+ T-cell and antibody responses and no vaccine effect on CD8+ T-cell responses (cytokine secretion or proliferation) were detected following F4/AS01B booster administration. In vitro, chloroquine had a direct inhibitory effect on AS01B adjuvant properties; AS01-induced cytokine production decreased upon coincubation of cells with chloroquine. In the pooled group of participants primed with F4/AS01B containing 10 μg of F4, CD4+ T-cell and antibody responses induced by primary vaccination persisted for at least 3 years. The F4/AS01B booster induced strong F4-specific CD4+ T-cell responses, which persisted for at least 6 months with similar frequencies and polyfunctional phenotypes as following primary vaccination, and high anti-F4 antibody concentrations, reaching higher levels than those following primary vaccination. The F4/AS01B booster had a clinically acceptable safety and reactogenicity profile. An F4/AS01B booster dose, administered alone or after chloroquine, induced robust antibody and F4-specific CD4+ T-cell responses but no significant CD8+ T-cell responses (cytokine secretion or proliferation) in healthy adults. (This study has been registered at ClinicalTrials.gov under registration number NCT00972725).
INTRODUCTION
The development of a safe and effective vaccine against human immunodeficiency virus type 1 (HIV-1) is a global health priority (1). Disease-modifying vaccines inducing strong T-cell-mediated immune responses against HIV are currently under development. These vaccine interventions were primarily aimed to control HIV load, to delay disease progression, and to reduce the transmission rate in high-incidence populations. There is now a renewed interest in using these vaccines in combined strategies to allow durable control, if not eradication, of the virus in patients treated with highly active antiretroviral therapy (2–4). While CD8+ T cells are known to be an important immune effector mechanism for the elimination of HIV-infected cells, it is increasingly apparent that CD4+ T cells provide essential help to other components of the adaptive immune response and are also crucial for the induction of effective HIV immunity (5–7). In particular, CD4+ T cells appear to play an important role in maintaining functional CD8+ T-cell responses (8–15).
A novel HIV-1 investigational vaccine consisting of a recombinant fusion protein (F4) containing four HIV-1 clade B antigens (p17, p24, reverse transcriptase [RT], and Nef) adjuvanted with the AS01B adjuvant system (F4/AS01B vaccine) has recently been developed. The F4/AS01B investigational vaccine has been shown to have an acceptable safety and reactogenicity profile and to induce high frequencies of long-lasting, F4-specific, polyfunctional CD4+ T-cell responses, but no F4-specific CD8+ T-cell responses, in healthy seronegative adults and in HIV-1-infected subjects (16, 17). The efficacy of this vaccine in reducing viral load was specifically assessed in a phase IIb, proof-of-concept trial (ClinicalTrials.gov registration number NCT01218113), but results are not available yet. An earlier formulation of this vaccine, composed of three HIV-1 viral antigens (gp120, Nef, and Tat) and the AS02A adjuvant system, was previously shown to induce a transient increase of gp120-specific CD8+ T-cell proliferation in healthy adults and in adults with well-controlled chronic HIV-1 infection treated with highly active antiretroviral therapy (2, 18, 19).
The immune response to the F4/AS01B investigational vaccine could be improved by enhancing the antigen-specific CD8+ T-cell response. In mice, a short course of treatment with chloroquine during primary immunization with soluble antigens improved the priming of naive CD8+ T-cell responses (20, 21). In humans, the recall of specific CD8+ T cells was enhanced by oral administration of chloroquine 2 days before a booster dose of a hepatitis B vaccine (22). The proposed mechanism of chloroquine action is to improve antigen cross-presentation by dendritic cells (DCs). Upon in vitro incubation of human DCs with chloroquine, the alkalization of the acidic intracellular compartments and the permeabilization of the lysosomal membranes induce an increased availability of nondegraded peptides in the cytosol for export into the class I processing pathway and cross-presentation to CD8+ T cells (21, 22). However, chloroquine may also have an inhibitory effect on the innate immune system as it has been shown to decrease the activation of human primary cells, including monocytes, by Toll-like receptor (TLR) agonists (23–25).
In the study presented in the manuscript, healthy adults who had previously received two primary doses of the investigational F4/AS01B vaccine approximately 3 years before (16) were recruited to assess whether chloroquine had an effect on the F4-specific CD8+ T-cell response induced by a booster dose of this vaccine. This study also evaluated the safety and immunogenicity of the F4/AS01B vaccine before and after booster dose administration. Additionally, the impact of chloroquine on the adjuvant properties of AS01B was evaluated in vitro.
MATERIALS AND METHODS
Study design and participants.
This phase II, self-contained, parallel, open, randomized study (registered at ClinicalTrials.gov under registration number NCT00972725) was conducted in a single center in Belgium between December 2009 and October 2010. Study participants were healthy adults who were between 18 and 40 years of age at the time of the first vaccination in the initial study (ClinicalTrials.gov registration number NCT00434512), which was conducted approximately 3 years earlier (16). In the initial study, two primary doses of the F4/AS01B investigational vaccine, containing 10 or 30 μg of F4 recombinant protein per dose, were administered according to a 0- and 1-month vaccination schedule. Eligible participants had received both primary doses of the F4/AS01B investigational vaccine and were CD4+ T-cell responders at 14 days after the second dose. Participants were considered to be CD4+ T-cell responders if they had a frequency of CD4+ T cells specific to at least one component of the F4 antigen, producing at least two markers among interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and CD40-ligand (CD40L) higher than 0.03% (Fig. 1).
FIG 1.
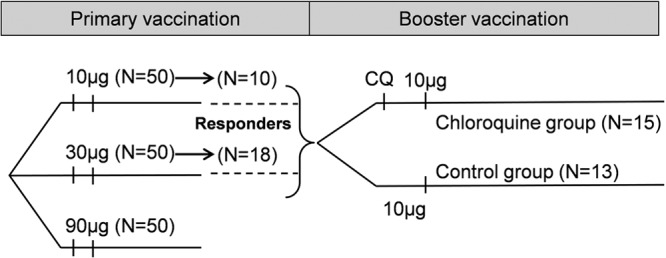
Study design. Study participants received two doses of the F4/AS01B investigational vaccine (10, 30, or 90 μg of F4 recombinant protein per dose) in a previous study (primary vaccination; study NCT00434512) and a third dose (10 μg of F4 recombinant protein per dose) in the current study (booster vaccination). Responders, participants with a frequency of antigen-specific CD4+ T cells of >0.03% at 44 days after the first dose of the F4/AS01B vaccine in the previous study; N, number of participants; CQ, chloroquine.
Participants were negative for anti-hepatitis B core protein and anti-hepatitis C virus antibodies at screening, had provided written informed consent, and had agreed to receive the HIV test results planned during the whole study duration (26). Women of childbearing potential were enrolled if they had practiced adequate contraception for 30 days prior to vaccination, had a negative pregnancy test on the day of vaccination, and agreed to continue adequate contraception during the study. Standard eligibility criteria were used for enrollment, as detailed in the ClinicalTrials.gov registry.
In the present study, participants were randomized (1:1) to receive either a single dose of chloroquine 2 days before the booster dose of the F4/AS01B vaccine containing 10 μg of F4 (chloroquine group) or only the booster dose of the F4/AS01B vaccine (control group). Blood samples were collected from the participants just before the booster dose administration (day 0) and on days 7, 14, 30, and 180.
The randomization was performed at GlaxoSmithKline (GSK) Vaccines (Rixensart, Belgium) using a standard SAS (SAS Institute Inc., Cary, NC) program. The randomization list was generated using a blocking scheme (block size, 2), and the treatments were distributed to the study centers in accordance with the randomization block size. The randomization used a minimization procedure accounting for the CD4+ T-cell category values of the participants, which were based on both their CD4+ T-cell response frequencies (compared to the median value of their group) and the F4/AS01B vaccine dosage (10 μg or 30 μg) that they had received in the initial study. The study was open due to the different treatment schedules between the two groups, but the laboratory personnel responsible for immunogenicity testing were blinded.
The study was conducted in accordance with the Good Clinical Practice Guidelines and the Declaration of Helsinki, and the protocol and associated documents were reviewed and approved by the investigational independent ethics committee of the University Hospital of Ghent, Belgium. Written informed consent was obtained from each participant prior to the performance of any study-related procedure. A summary of the protocol is available at http://www.gsk-clinicalstudyregister.com (GSK study 113165).
Study vaccines and products.
The freeze-dried fraction of the F4/AS01B investigational vaccine contained 10 μg of the recombinant fusion protein F4 comprising four HIV-1 clade B antigens (p24 [BH10], RT [HXB2], Nef [Bru-Lai], and p17 [BH10]), 20 mg of sucrose, and 630 μg of sodium sulfite. The freeze-dried fraction was reconstituted with the AS01B adjuvant system containing 50 μg of 3-O-desacyl-4′-monophosphoryl lipid A (MPL) and 50 μg of QS-21 (Quillaja saponaria Molina, fraction 21; Antigenics, Inc., Lexington, MA, USA) in a suspension of liposomes in phosphate-buffered saline. The reconstituted vaccine solution (0.5 ml) was injected into the deltoid muscle of the participant's nondominant arm on day 0.
In the chloroquine group, one tablet of 300 mg of chloroquine (Nivaquine; Sanofi-Aventis, France) was administered orally 2 days before the booster dose of the F4/AS01B vaccine. The same conditions in terms of chloroquine dose and timing of administration were used in the previous study, in which an effect of chloroquine on vaccine-induced CD8+ T-cell responses was observed following administration of a booster dose of hepatitis B vaccine (22).
Study objectives.
The first coprimary objective of this study was to evaluate the effect of chloroquine on the specific CD8+ T-cell response to a booster dose of the F4/AS01B investigational vaccine at day 14. The second coprimary objective was to evaluate the reactogenicity and safety of the booster dose of the F4/AS01B vaccine. The secondary objectives of this study included the evaluation of the F4-specific CD8+/CD4+ T-cell and antibody responses induced by the F4/AS01B vaccine with or without chloroquine.
Post hoc exploratory analyses were also performed to assess the CD8+/CD4+ T-cell proliferation and the ability of proliferating CD8+/CD4+ T cells to produce F4-specific cytokines following administration of the F4/AS01B vaccine. In the manuscript, we also present the results of in vitro experiments evaluating the effect of chloroquine on the MPL- and QS-21-dependent activation of human primary cells.
CD4+ and CD8+ T-cell responses. (i) Intracellular cytokine staining.
The frequencies of CD4+ and CD8+ T cells expressing specific markers (IL-2, TNF-α, IFN-γ, or CD40L [BD Biosciences]) upon in vitro stimulation of peripheral blood mononuclear cells (PBMCs) with pools of peptides covering the sequences of the p17, p24, RT, and Nef antigens were determined by flow cytometry (LSRII cytometer; Becton Dickinson) using intracellular cytokine staining (ICS), as previously described (16). Flow cytometry analyses were performed using FlowJo, version 9 (Tree Star), software. The ICS results were expressed as percentages of CD4+ or CD8+ T cells expressing specific markers after background subtraction (net frequencies). Frequencies of CD4+ or CD8+ T cells expressing specific markers in response to F4 were determined by addition of the individual frequencies of CD4+ or CD8+ T-cell responses to each of the four individual antigens.
CD8+ T-cell responder rates were defined as percentages of individuals who exhibited frequencies of CD8+ T cells expressing at least one cytokine among IL-2, TNF-α, and IFN-γ equal to or above prespecified cutoffs upon stimulation with at least one, two, three, or all four antigens and with each individual antigen. The prespecified cutoffs, which were based on the 95th percentile of the specific CD8+ T-cell responses at the prebooster time point, were 0.0439% for RT, 0.0464% for Nef, 0.0240% for p17, 0.0296% for p24, and 0.0711% for F4. CD4+ T-cell responder rates were defined as percentages of individuals who exhibited frequencies of CD4+ T cells expressing at least two cytokines, including IL-2, equal to or above 0.03% upon stimulation with at least one, two, three, or all four antigens and with each individual antigen (16). Cytokine coexpression profiles for CD4+ T-cell responses were defined as frequencies of p17-, p24-, Nef-, and RT-specific CD4+ T cells expressing IL-2 and/or TNF-α and/or IFN-γ and/or CD40L.
(ii) Lymphoproliferation assay.
In a post hoc analysis, the specificity of the CD4+ and CD8+ T-cell proliferation and the potential impact of an in vitro bystander effect linked to the IL-2 produced by the F4-stimulated CD4+ T cells were assessed at prevaccination and at 44 and 360 days postvaccination in the initial study and at days 0, 14, and 180 in the present study by combining a 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) proliferation assay with a short in vitro ICS.
Thawed PBMCs were labeled with the fluorescent dye CFSE and cultured for 5 days in medium containing a pool of peptides covering the sequence of the F4 antigen (1.25 μg/peptide/ml) or in medium alone. Five days later, the cells of these primary cultures were harvested, washed, and split in three fractions that were restimulated during 18 h in medium that did not contain peptides or that contained 1.25 μg/peptide/ml of irrelevant peptides (thrombospondin-related anonymous protein [TRAP]) or 1.25 μg/peptide/ml of the pool of peptides covering the sequence of the F4 antigen (27). After the two first hours, brefeldin A was added to all cultures to block the secretion of cytokines and induce their accumulation in the cells. After this second culture lasting 18 h, extracellular staining (CD4 and CD8 [BD Biosciences] and Live/Dead [L&D; Invitrogen]) was performed, followed by intracellular staining (CD3, IL-2, IFN-γ, and TNF-α [BD Biosciences]). Proliferating cells showed a reduction in CFSE fluorescence intensity (CFSElow T cells). The lymphoproliferation results were expressed, on the one hand, as frequencies of F4-specific CD4+ or CD8+ CFSElow T cells among all CD4+ or CD8+ T cells (expressed as stimulation index) and, on the other hand, as frequencies of F4- and TRAP-specific CD4+ or CD8+ CFSElow T cells expressing a combination of cytokines (IL-2, TNF-α, or IFN-γ).
Antibody responses.
Standard in-house enzyme-linked immunosorbent assays (ELISA) were used to measure immunoglobulin G (IgG) antibody concentrations in ELISA units (EU) for p17, p24, RT, Nef, and F4, as previously described (16). The cutoffs for seropositivity were 187 mEU/ml for p17, 119 mEU/ml for p24, 125 mEU/ml for RT, 232 mEU/ml for Nef, and 42 mEU/ml for F4.
In vitro impact of chloroquine on MPL- and QS-21-dependent activation of PBMCs.
PBMCs were isolated from the buffy coat fraction obtained from routine blood donor donations. Mononuclear cells were obtained by centrifugation over Ficoll-Hypaque gradients. PBMCs were cultured in complete medium containing RPMI 1640 medium supplemented with 50 μM 2-mercaptoethanol (2-ME), 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 1% nonessential amino acids (Life Technologies), and 10% fetal calf serum (FCS; PAA Laboratories). A total of 1 × 106 PBMCs were preincubated in 96-well plates for 1 h with chloroquine (Sigma-Aldrich) at the indicated concentration. MPL (1 μg/ml) or QS-21 (5 μg/ml) was added to the cell culture, and the cells were incubated overnight. Supernatants were then collected in triplicates, and the levels of IL-6, TNF-α, and IL-1-β were assessed by cytokine bead array (CBA; BD Bioscience).
Safety.
The occurrence, intensity, and relationship to vaccination of local (injection site pain, redness, and swelling) and general (fever, fatigue, headache, and gastrointestinal symptoms) solicited adverse events (AEs) were recorded for 7 days after vaccination and of unsolicited AEs for 30 days after vaccination. The severity of AEs was graded on a scale of 1 to 3, with grade 3 symptoms defined as redness or swelling of >50 mm, fever of >39.0°C, and any other symptom preventing normal daily activities.
The occurrence and relationship to vaccination of serious adverse events (SAEs) and AEs of specific interest, including immune-mediated diseases (IMDs), were reported during the entire study period.
Routine hematology and biochemistry tests were performed on days 0, 7, 30, and 180.
Statistical methods. (i) Study cohorts.
The effect of chloroquine on the CD8+/CD4+ T-cell and antibody responses was evaluated on the according-to-protocol (ATP) immunogenicity cohort, which included all evaluable participants meeting all eligibility criteria and complying with the procedures defined in the protocol and for whom data concerning immunogenicity endpoints were available.
As the 10-μg F4 dosage of the F4/AS01B investigational vaccine was selected to progress to further clinical development, analyses of safety were done on the pooled total vaccinated cohort (TVC), which included all vaccinated participants who had received this formulation of the vaccine in the initial study (pooled TVC for 10-μg-primed participants, i.e., no distinction between participants having received chloroquine or not).
The secondary immunogenicity objectives were evaluated for the pooled group of participants from the ATP immunogenicity cohort, who had received the 10-μg F4 dosage of the F4/AS01B investigational vaccine in the initial study (pooled ATP immunogenicity cohort for 10-μg-primed participants).
(ii) Statistical analyses.
To evaluate the first coprimary objective, two groups were considered significantly different if the standardized asymptotic 95% confidence interval (CI) for the difference in rates between groups did not contain the value 0.
Descriptive statistics were used to present the demographic characteristics, the CD4+ and CD8+ T-cell responses, and the results of the lymphoproliferation assays.
The stimulation index was obtained by dividing the geometric mean of the percentage of CD4+ or CD8+ T cells that were F4-specific CFSElow CD4+ or CD8+ T cells (obtained from cells cultured in medium containing the F4 pool of peptides and restimulated in medium containing no peptides, irrelevant peptides, or the F4 pool of peptides) by the geometric mean of the background results (obtained from cells cultured and restimulated in medium alone).
Seropositivity rates and geometric mean antibody concentrations (GMCs) for anti-F4 antibodies were calculated with 95% CIs, using the exact method for binomial variables for seropositivity rates and the antilogs of the 95% CIs of the mean log10-transformed antibody concentrations for GMCs. Antibody concentrations below the assay cutoff were given an arbitrary value of half the cutoff for the GMC calculation.
The percentages of participants reporting each solicited and unsolicited symptom were tabulated with 95% CIs. SAEs were described in detail.
Analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC, United States), and Proc StatXact, version 8.1.
(iii) Sample size and study power.
With a total of 40 evaluable participants, the study would have 99% power to detect a difference in frequencies of F4-specific CD8+ T-cell responder rates between the chloroquine and the control groups. Considering that up to 10% of the participants might not be evaluable for immunogenicity, enrollment was planned at 44 participants.
RESULTS
Demographics.
Of the 34 participants who agreed to attend a screening for this study, two did not fulfill the eligibility criteria, one was not eligible due to time schedule, one was not willing to participate, and two withdrew before vaccination (loss of follow-up and consent withdrawal not due to an AE). Therefore, 28 participants were included in the TVC: 10 and 18 participants who received the 10-μg and the 30-μg F4 doses of the F4/AS01B investigational vaccine in the initial study, respectively (Fig. 1). These participants were randomized: 13 were included in the chloroquine group, and 15 were in the control group. All participants were included in the ATP immunogenicity cohort except for one in the control group who was excluded due to noncompliance with the blood sampling schedule. The pooled ATP immunogenicity cohort for 10-μg-primed participants included 10 participants.
In the TVC, the mean (standard deviation) age of the participants was 24.6 (4.5) years at vaccination, 60.7% of participants were women, and 92.9% were White-Caucasian (Table 1).
TABLE 1.
Demographic characteristics
| Characteristic | Value for TVC |
|
|---|---|---|
| Chloroquine group (n = 13) | Control group (n = 15) | |
| Age | ||
| Mean age ± SD (yr) | 25.1 ± 5.3 | 24.3 ± 3.7 |
| Age range (yr) | 21–41 | 21–34 |
| Sex (no. [%]) | ||
| Female | 7 (53.8) | 10 (66.7) |
| Male | 6 (46.2) | 5 (33.3) |
| Race (no. [%]) | ||
| White, Caucasian/European heritage | 13 (100) | 13 (86.7) |
| White, Arabic/North African heritage | 0 (0.0) | 1 (6.7) |
| Other | 0 (0.0) | 1 (6.7) |
Chloroquine effect on the immune response. (i) CD8+ T-cell response.
In the ATP immunogenicity cohort, only 7.7% (1/13) of participants in the chloroquine group and 23.1% (3/13) in the control group responded to at least one antigen in terms of CD8+ T cells expressing at least one cytokine among IL-2, TNF-α, and IFN-γ at day 14. No participant responded to more than one antigen. The group difference, which was −15.4% (95% CI, −45.1 to 15.39), did not reach the predefined statistical significance. Of note, the planned number of 20 evaluable participants per group was not reached.
At day 14, the median frequency of F4-specific CD8+ T cells was 0.0478% and 0.0450% of CD8+ T cells in the chloroquine and control groups, respectively (Fig. 2). The median frequency of CD8+ T cells specific to each individual antigen stayed below the predefined cutoffs at all time points in both groups (data not shown). However, at day 7, one participant in the control group had a relatively high frequency of p17- and p24-specific CD8+ T cells (0.303% and 0.187% of CD8+ T cells, respectively), and one participant in the chloroquine group had a relatively high frequency of RT-specific CD8+ T cells (0.1539% of CD8+ T cells) compared to the other participants in their groups (third quartile [Q3], 0.01% for p17 and p24 and 0.02% for RT).
FIG 2.
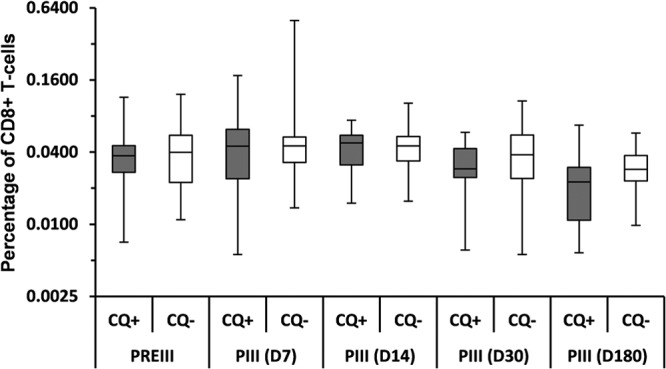
Frequency of CD8+ T cells expressing at least one cytokine (IL-2, TNF-α, or IFN-γ) in response to the F4 fusion protein following a booster dose of the F4/AS01B vaccine (ATP immunogenicity cohort). CQ+, participants in the chloroquine group, who received a chloroquine dose 2 days before the booster dose of F4/AS01B; CQ−, participants in the control group, who only received the booster dose of F4/AS01B; PREIII, prebooster dose given approximately 3 years post-primary vaccination; PIII(D7), PIII(D14), PIII(D30), PIII(D180), 7, 14, 30, and 180 days after the booster dose, respectively. The percentage of CD8+ T cells expressing cytokines in response to the fusion protein F4 was determined by adding the individual frequencies of the CD8+ T-cell response to each of the four individual antigens. In the box plot, the central box shows the interquartile range (Q1 to Q3), with the thick horizontal line representing the median (Q2) and the whiskers (above and below the box) representing the maximum and the minimum, respectively.
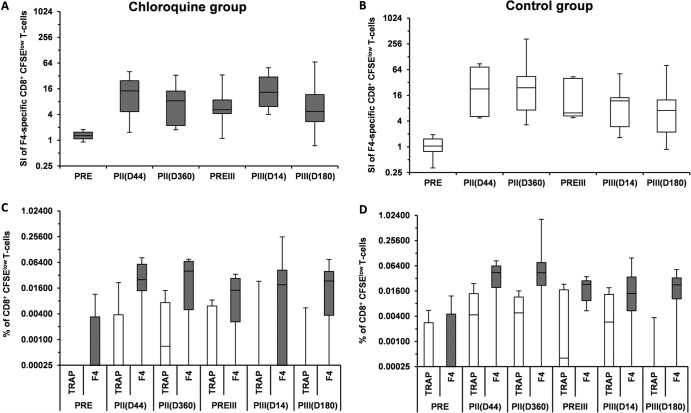
To further identify a potential chloroquine effect on the CD8+ T-cell response induced by the F4/AS01B vaccine, the ability of CD8+ T cells to divide upon in vitro stimulation with the F4 pool of peptides was evaluated in post hoc analyses. In the ATP immunogenicity cohort, the median stimulation index values of CD8+ CFSElow T cells stimulated with F4 peptides at day 14 were 13.31 and 11.85 in the chloroquine (n = 8) and the control (n = 9) groups, respectively (Fig. 3A and B). In order to take into account a potential in vitro bystander cell proliferation, all cells were restimulated with a pool of peptides covering the sequence of the F4 antigen or with irrelevant peptides (TRAP, as a negative control) and analyzed by ICS. The median frequencies of F4-specific CD8+ CFSElow T cells expressing at least one cytokine at day 14 were 0.019% and 0.014% in the chloroquine (n = 11) and control (n = 11) groups, respectively (Fig. 3C and D). The median frequencies of TRAP-specific CD8+ CFSElow T cells expressing at least one cytokine at day 14 were 0.0001% and 0.0029% in the chloroquine (n = 10) and the control (n = 7) groups, respectively. There was a minor observed difference between frequencies of F4-specific and TRAP-specific CD8+ CFSElow T cells although the frequencies were low, and no statistical comparison was performed.
FIG 3.
Stimulation index of CD8+ CFSElow T cells stimulated with F4 peptide pools (A and B) and frequency of F4- and TRAP-specific CD8+ CFSElow T cells expressing at least one cytokine following primary and booster doses of the F4/AS01B investigational vaccine (C and D) in the chloroquine (A and C) and the control (B and D) groups (ATP immunogenicity cohort). CFSE, 5,6-carboxyfluorescein diacetate succinimidyl ester; F4, pool of peptides covering the sequence of F4 antigens; PRE, prevaccination in the initial study; PII(D44), 44 days after primary vaccination; PII(D360), 360 days after primary vaccination; PREIII, prebooster dose given approximately 3 years post-primary vaccination; PIII(D14), 14 days after the booster dose; PIII(D180), 180 days after the booster dose; SI, stimulation index; TRAP, tartrate-resistant acid phosphatase (irrelevant peptides). In the box plot, the central box shows the interquartile range (Q1 to Q3), with the thick horizontal line representing the median (Q2) and the whiskers (above and below the box) representing the maximum and the minimum, respectively.
The median stimulation index and frequency of F4 specific CD8+ CFSElow T cells observed after F4/AS01 booster dose were similar to those observed following the two primary doses.
(ii) CD4+ T-cell response.
At day 14, the observed CD4+ T-cell responder rates to at least one antigen were similar in the chloroquine and control groups (92.3% [12/13] of participants in each group). The median frequencies of F4-specific CD4+ T cells expressing at least two cytokines, including IL-2, were also similar in the chloroquine (0.5272%) and control (0.6035%) groups (see Fig. S1A in the supplemental material).
The median stimulation index values of CD4+ CFSElow T cells stimulated with F4 peptides at day 14 were 76.57 and 30.23 in the chloroquine (n = 10) and control (n = 10) groups, respectively (see Fig. S2A and B in the supplemental material). The median frequencies of F4-specific CD4+ CFSElow T cells expressing at least one cytokine were 0.851% and 0.5332% in the chloroquine (n = 11) and control (n = 11) groups, respectively. The median frequency of TRAP-specific CD4+ CFSElow T cells was 0.0001% in both the chloroquine (n = 10) and the control (n = 7) groups (see Fig. S2C and D).
(iii) Antibody response.
The anti-F4 antibody seropositivity rates and GMCs observed in both groups were comparable although no statistical analyses were performed to detect differences between groups. All participants were seropositive for anti-F4 antibodies throughout the follow-up period. The anti-F4 antibody GMCs peaked at day 14 in the chloroquine group (83,863.4 mEU/ml; 95% CI, 56,912.6 to 123,576.8 mEU/ml) and at day 30 in the control group (65,633.5 mEU/ml; 95% CI, 42,838.2 to 100,558.8 mEU/ml) (see Fig. S1B in the supplemental material).
(iv) In vitro impact of chloroquine on MPL- and QS-21-dependent activation of PBMCs.
The adjuvant AS01B is thought to play a key role in the response to the F4/AS01B investigational vaccine by activating the innate immune system due to its immunostimulatory components MPL, a TLR4 agonist, and the saponin QS-21 (28, 29). Here, the potential impact of chloroquine on the response induced by the adjuvant was assessed by measuring the ability of PBMCs to secrete IL-6 upon incubation with MPL and QS-21.
In the in vitro experiments, chloroquine decreased both MPL- and QS-21-dependent IL-6 production by stimulated PBMCs from healthy donors in a dose-dependent manner, suggesting that chloroquine decreased the ability of primary human cells to respond to the immunostimulatory components of AS01B (Fig. 4). A similar dose-dependent decrease was observed in TNF-α and IL-1-β production (data not shown).
FIG 4.
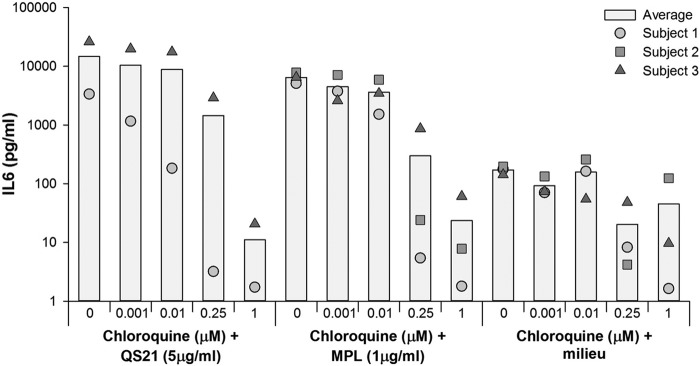
In vivo impact of chloroquine on the MPL- and QS-21-dependent IL-6 production by stimulated human peripheral blood mononuclear cells. MPL (1 μg/ml), 3-O-desacyl-4′-monophosphoryl lipid A; QS-21 (5 μg/ml), Quillaja saponaria Molina, fraction 21.
Persistence and booster effect of the F4/AS01B investigational vaccine.
Primary comparisons did not reveal any effect of chloroquine on the CD4+/CD8+ T-cell or antibody responses. Thus, further immunogenicity and safety analyses were carried out on the pooled group of participants, who had received the F4/AS01B investigational formulation selected to progress to further clinical development (containing 10 μg of F4) in the initial study. Moreover, as no significant vaccine effects on CD8+ T-cell response and proliferation were detected, the persistence and booster effect of the F4/AS01B vaccine were not further described in terms of CD8+ T-cell response.
(i) CD4+ T-cell response.
Before the administration of the booster dose of the F4/AS01B vaccine, approximately 3 years after the primary vaccination course, the CD4+ T-cell responder rate was 60% (6/10) to at least one or two antigens, 30% (3/10) to at least three antigens, and 20% (2/10) to all four antigens (see Table S3 in the supplemental material). Most of the participants displayed a specific CD4+ T-cell response upon in vitro stimulation with p24 and RT, but the frequencies of specific CD4+ T cells expressing at least two cytokines, including IL-2, stayed below the predefined cutoff after stimulation with Nef and p17.
After the administration of the booster dose of the F4/AS01B vaccine, a high percentage of participants developed a specific CD4+ T-cell response to all four antigens at all time points (see Table S3 in the supplemental material). At day 14, the CD4+ T-cell responder rate was 100% (9/9) to at least one antigen, 88.9% (8/9) to at least two or three antigens, and 66.7% (6/9) to all four antigens. The frequency of F4-specific CD4+ T cells peaked between day 7 and day 14 and was comparable to that measured after the second primary dose (Fig. 5).
FIG 5.
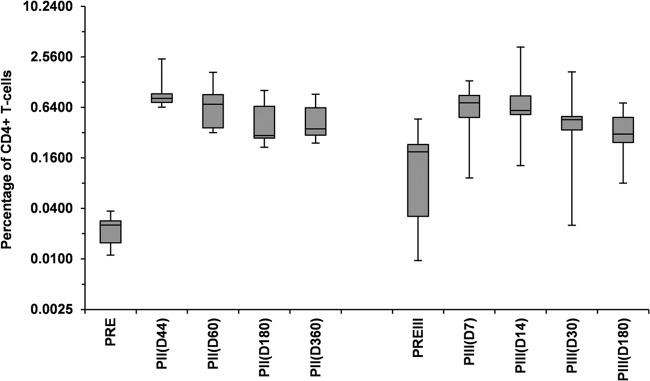
Frequency of CD4+ T cells expressing IL-2 and at least one other cytokine in response to the F4 fusion protein following primary and booster doses of the F4/AS01B investigational vaccine (pooled ATP immunogenicity cohort for 10-μg-primed participants). PRE, prevaccination in the initial study; PII(D44), PII(D60), PII(D180), PII(D360), 44, 60, 180, and 360 days after primary vaccination; PREIII, prebooster dose given approximately 3 years post-primary vaccination; PIII(D7), PIII(D14), PIII(D30), PIII(D180), 7, 14, 30, and 180 days after the booster dose. The percentage of CD4+ T cells expressing cytokines in response to the fusion protein F4 was determined by adding the individual frequencies of the CD4+ T-cell response to each of the four individual antigens. In the box plot, the central box shows the interquartile range (Q1 to Q3), with the thick horizontal line representing the median (Q2) and the whiskers (above and below the box) representing the maximum and the minimum, respectively.
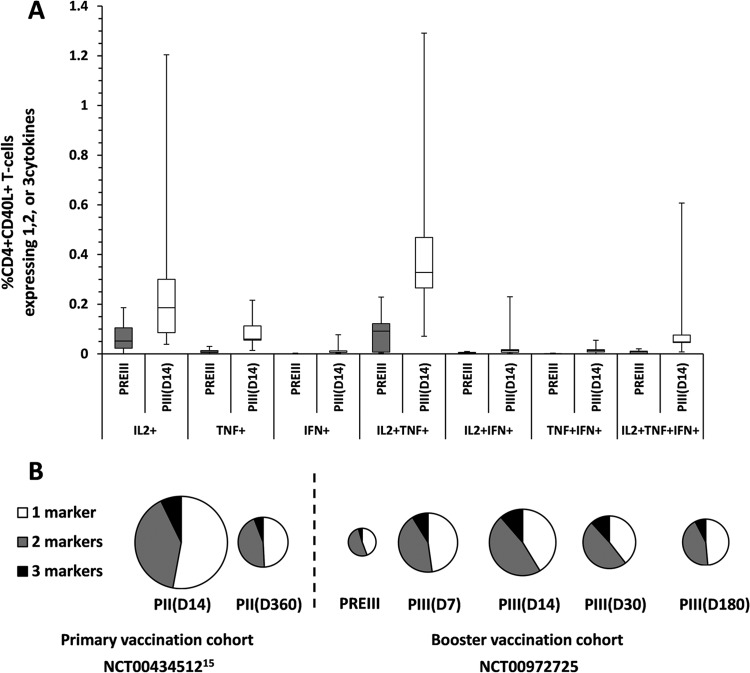
The booster dose of the F4/AS01B investigational vaccine induced F4-specific CD4+ T cells that exhibited a polyfunctional phenotype, expressing mainly CD40L and IL-2 in combination or not with TNF-α (Fig. 6A). This cytokine coexpression profile, in which approximately 50% of the F4-specific CD4+ CD40L+ T cells secreted at least two cytokines, was maintained during at least 6 months and was similar to that observed following primary vaccination (Fig. 6B).
FIG 6.
(A) Cytokine coexpression profile of F4-specific CD4+ CD40L+ T cells before and following a booster dose of the F4/AS01B investigational vaccine (pooled ATP immunogenicity cohort for 10-μg-primed participants). (B) Pie chart of F4-specific CD4+ CD40L+ T-cell responses after primary vaccination in the initial study (NCT00434512 [15]) (ATP immunogenicity cohort for 10-μg F4/AS01 group) and before and after the booster dose in the present study (pooled ATP immunogenicity cohort for 10-μg-primed participants). PREII, prevaccination in the initial study; PII(D14) and PII (360), 14 and 360 days after primary vaccination; PREIII, prebooster dose given approximately 3 years post-primary vaccination; PIII(D7), PIII(D14), PIII(D30), and PIII(D180), 7, 14, 30, and 180 days after the booster dose. The percentage of CD4+ CD40L+ T cells expressing cytokines in response to the fusion protein F4 was determined by adding the individual frequencies of the CD4+ CD40L+ T-cell response to each of the four individual antigens. In the box plot, the central box shows the interquartile range (Q1 to Q3), with the thick horizontal line representing the median (Q2) and the whiskers (above and below the box) representing the maximum and the minimum, respectively. Pie charts represent percentages as proportions.
(ii) Antibody response.
Before the administration of the booster dose of the F4/AS01B vaccine, all participants in the pooled ATP immunogenicity cohort for 10-μg-primed participants were seropositive for anti-F4 antibodies. The anti-F4 antibody GMCs at 3 years after primary vaccination (316.7 mEU/ml; 95% CI, 154.6 to 648.6 mEU/ml) had decreased by 21.7-fold compared with those measured at 14 days after primary vaccination (6,874.0 mEU/ml; 95% CI, 5,621.3 to 8405.8 mEU/ml).
The booster dose of the F4/AS01B vaccine elicited a robust increase in anti-F4 antibody concentrations, with GMCs well above those observed after the primary vaccination course (Fig. 7). At day 14, anti-F4 antibody GMCs (91,109.3 mEU/ml; 95% CI, 54,647.1 to 151,900.2 mEU/ml) were 13.3- and 287.7-fold higher than anti-F4 antibody GMCs measured at 14 days and 3 years after the primary vaccination course, respectively.
FIG 7.
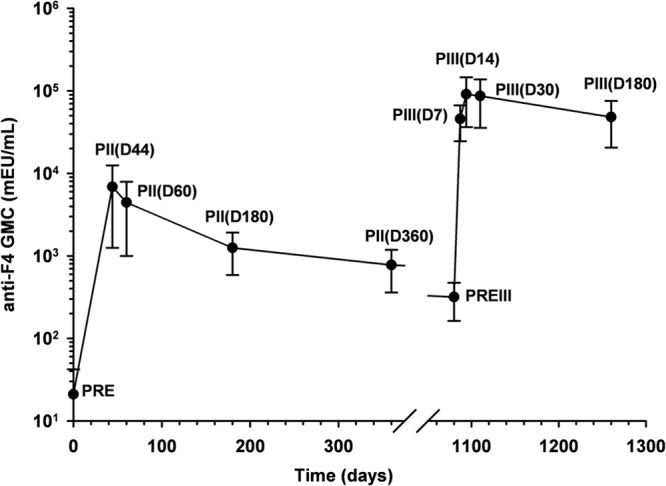
Geometric mean concentrations (GMCs) for anti-F4 IgG antibodies after administration of the primary and booster doses of the F4/AS01B investigational vaccine (pooled ATP immunogenicity cohort for 10-μg-primed participants). Dosing abbreviations are as defined in previous legends.
Safety.
No differences in reactogenicity and safety of the booster dose of the F4/AS01B vaccine were observed between the chloroquine and control groups (no formal statistical comparisons were performed, and data are not shown), allowing pooling of the safety results.
In the pooled TVC for 10-μg-primed participants (n = 10), all participants reported pain during the 7-day follow-up period after administration of the booster dose of the F4/AS01B vaccine (Fig. 8). Only two participants reported solicited local symptoms with grade 3 intensity; one participant had grade 3 pain, and the other had grade 3 swelling. The most frequently reported solicited general symptom was fatigue, which was reported by nine participants (90%). Grade 3 fatigue and headache were reported by two participants (20%). No grade 3 fever (temperature of >39°C) was reported, and solicited symptoms with grade 3 intensity lasted for a maximum of 2 days.
FIG 8.
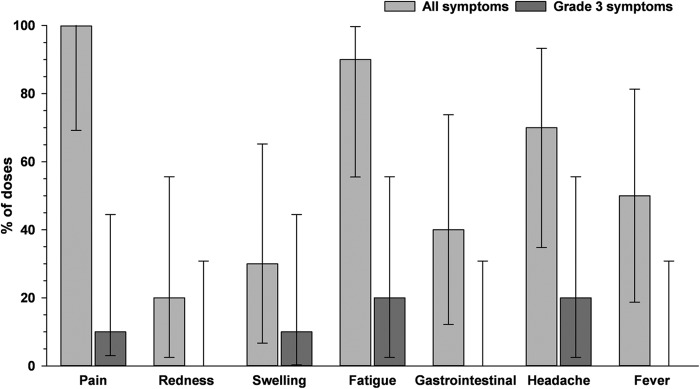
Solicited local and general symptoms during the 7-day postvaccination period following administration of a booster dose of the F4/AS01B investigational vaccine (pooled total vaccinated cohort for 10-μg-primed participants). Error bars represent 95% confidence intervals.
During the 30-day postvaccination period, 16 unsolicited AEs were reported by eight participants (80%), of which 10 AEs were considered causally related to vaccination: three participants had chills; two participants had myalgia; and lymphadenopathy, influenza-like illness, injection site reaction, arthralgia, and insomnia were each reported by one participant. Four grade 3 unsolicited AEs were reported by three participants (30%). Influenza-like illness was reported by two participants, and chills and insomnia were each reported by one participant. All grade 3 unsolicited AEs resolved without sequels. No IMDs or SAEs were reported in this study.
DISCUSSION
The present study was designed to evaluate the effect of previous administration of chloroquine on the specific CD8+ T-cell response induced by a booster dose of the F4/AS01B investigational vaccine in healthy adults. When ICS assays were used, F4-specific CD8+ T-cell responder rates and magnitudes remained low after the booster dose administration and were not affected by the previous administration of chloroquine. The lack of F4-specific CD8+ T-cell responses observed in both groups was in line with the results of the initial study, in which no F4-specific CD8+ T cells were detected in healthy HIV-uninfected adults following primary vaccination with the F4/AS01B vaccine (16). High levels of F4-specific CD8+ T cells were detected in HIV-1-infected patients using this assay (17), suggesting that the absence of detectable CD8+ T cells was more linked to the lack of vaccine response than to the ICS assay.
Since the ability to proliferate to recall antigens is an important feature of protective memory T cells, vaccine-induced CD8+ T-cell proliferation upon restimulation with F4 peptides was assessed in post hoc analyses. The F4/AS01B investigational vaccine, which induced high frequencies of F4-specific CD4+ T cells expressing IL-2, a growth factor that supports T-cell proliferation upon reactivation by cognate antigen recognition, could trigger in vitro bystander T-cell proliferation (30, 31). Therefore, the frequency of vaccine-specific proliferating CD8+ T cells was evaluated using an alternative lymphoproliferation assay, combining a CFSE proliferation assay followed by a short antigen stimulation and ICS. Following administration of the booster dose of the F4/AS01B investigational vaccine, no effect of previous administration of chloroquine on CD8+ T-cell proliferation was detected. CD8+ T-cell proliferation seemed higher after stimulation with F4-specific peptides than with irrelevant peptides although the frequencies were very low (<0.02%), and no formal statistical comparisons were performed. These results suggest either that the vast majority of CD8+ T cells detected by the initial ICS assay proliferated due to a bystander effect of the IL-2 produced by the stimulated CD4+ T cells or that the methods used in the present study were not optimal to detect vaccine-induced CD8+ T-cell proliferation.
The present study did not confirm the chloroquine effect on vaccine-induced CD8+ T-cell responses that was observed in the study conducted by Accapezzato et al. following administration of a booster dose of hepatitis B vaccine although the same conditions were used in terms of chloroquine dose and timing of administration in both trials (22). One explanation could be the antigen specificity of the vaccine-induced CD8+ T-cell responses. Although the peptide sequences included in F4 have CD8+ T-cell epitopes, this large synthetic polyantigenic protein might not have been delivered to the cytoplasm, processed via the major histocompatibility complex class I pathway, and presented to CD8+ T cells as efficiently as the particulate hepatitis B surface (HBs) antigens (22). Moreover, the chloroquine-mediated delivery of the HBs antigens to the cytoplasm might be increased by the aluminum hydroxide component of the hepatitis B vaccine since it is known that aluminum induces an increase in antigen uptake by the antigen-presenting cells (29). The F4 fusion protein could be more efficiently processed by the human major histocompatibility complex class I molecules to induce a more potent HIV-specific cytotoxic immune response if a recombinant vector was used to carry the HIV antigens as this has been previously shown for a similar HIV fusion antigen delivered by an attenuated recombinant poxvirus (32). A second potential explanation could be the negative interference between chloroquine and components of the AS01B adjuvant system. As observed for other innate signals, such as TLR4 and TLR9 (23–25), chloroquine decreased the immune-stimulatory properties of two adjuvant components (QS-21 and MPL) in terms of cytokine production by stimulated human primary cells. Although the measured response may not be directly linked to CD8+ T-cell induction, it suggests that the innate response to the adjuvant could be altered in vivo, thereby decreasing its ability to stimulate an antigen-specific response (33). However, this hypothesis is not supported by the results of the present study as the chloroquine did not affect the CD4+ T-cell or antibody responses induced by the booster dose of the F4/AS01B investigational vaccine, which were comparable or higher than after the two primary doses.
The high polyfunctional F4-specific CD4+ T-cell frequencies and responder rates and anti-F4 antibody concentrations induced by the two primary doses of the F4/AS01B investigational vaccine persisted for at least 3 years in healthy adults, in line with the results of the initial study (16). The booster dose of the F4/AS01B vaccine induced a strong F4-specific CD4+ T-cell recall response, which persisted for at least 6 months, had approximately the same frequency and exhibited a similar polyfunctional phenotype as that observed following primary vaccination. Strong and persistent CD4+ T-cell responses were also induced by the F4/AS01B vaccine (16) or by a previous HIV-1 vaccine candidate comprising gp120 and a Nef-Tat fusion protein in healthy adults and in subjects with HIV-1 infection (2, 17–19). These findings are important since the presence of polyfunctional and persistent HIV-specific CD4+ T cells in HIV-infected individuals is associated with long-term nonprogression (11, 34–36). Furthermore, the loss of HIV-1-specific CD8+ T-cell proliferation after acute HIV infection appears to be restored by vaccine-induced HIV-specific CD4+ T cells both in vitro and in vivo (11).
The booster dose of the F4/AS01B vaccine also induced a robust increase in anti-F4 antibody concentrations, which reached higher levels than those observed after primary vaccination, suggesting that the F4/AS01B investigational vaccine induced immune memory in healthy adults. The high level and persistence of antibodies could be explained by the concomitant induction of CD4+ T cells that could provide strong help in differentiating B cells into long-lived plasma cells or memory B cells. Further analysis would be needed to better characterize these B-cell responses.
The safety profile of the booster dose of the F4/AS01B investigational vaccine was clinically acceptable. No SAEs or IMDs were reported, nor were any additional safety concerns raised after the booster dose administration (16).
Although this study was limited by the low number of participants, it is unlikely that the conclusions on lack of chloroquine effect would have been different if the planned number of 20 evaluable participants per group was reached. Another potential limitation of this study was its open design, due to the different treatment schedules between the two groups, which is unlikely to have influenced the immunogenicity assessments since the laboratory personnel responsible for immunogenicity testing were blinded.
In conclusion, this study showed that a booster dose of the F4/AS01B investigational vaccine, administered alone or 2 days after chloroquine, induced a strong humoral immune response and a robust F4-specific CD4+ T-cell immune response exhibiting a polyfunctional phenotype but no significant CD8+ T-cell response. These results suggest that two primary doses of the F4/AS01B investigational vaccine induce long-lasting memory B-cell and CD4+ T-cell responses in healthy adults.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to all trial participants and acknowledge the contributions of the clinicians, nurses, and laboratory technicians at the Center for Vaccinology (CEVAC), Ghent University Hospital. We also thank Johanna Geyssens (GSK Vaccines) for study coordination, Isabelle Carletti (GSK Vaccines) for additional statistical support and review of the manuscript, Delphine Anthony (4Clinics, on behalf of GSK Vaccines) for the development of programs for the analyses, Joke Debruyn (XPE Pharma and Science, on behalf of GSK Vaccines) for writing of the clinical report, Hajar Larbi (GSK Vaccines) for technical help for the in vitro experiment, Wouter Houthoofd (XPE Pharma and Science, on behalf of GSK Vaccines) for publication management, and Claire Verbelen (XPE Pharma and Science, on behalf of GSK Vaccines) for initial drafting of the manuscript and incorporation of comments received from the authors.
G.L-R and F.C. received funding from GSK Biologicals SA (GSK) via their institute to cover study costs. G.L-R. received payments from GSK for consultancy on influenza vaccines and adjuvants and from Immune Targeting Systems (United Kingdom) for consultancy on influenza and hepatitis B vaccines. GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript.
P.B., M.J., A.D., L.F., F.R., and D.B. are employees of GSK; F.R., A.D., and D.B. own GSK stock and stock options. J.W. has no conflicts of interest to disclose.
Nivaquine is a registered trademark of Sanofi-Aventis, France.
Footnotes
Published ahead of print 3 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00617-13.
REFERENCES
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) 2013. Global fact sheet. UNAIDS, Geneva, Switzerland: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/201207_FactSheet_Global_en.pdf [Google Scholar]
- 2.Leroux-Roels I, Koutsoukos M, Clement F, Steyaert S, Janssens M, Bourguignon P, Cohen K, Altfeld M, Vandepapeliere P, Pedneault L, McNally L, Leroux-Roels G, Voss G. 2010. Strong and persistent CD4+ T-cell response in healthy adults immunized with a candidate HIV-1 vaccine containing gp120, Nef and Tat antigens formulated in three adjuvant systems. Vaccine 28:7016–7024. 10.1016/j.vaccine.2010.08.035 [DOI] [PubMed] [Google Scholar]
- 3.Berkley SF, Koff WC. 2007. Scientific and policy challenges to development of an AIDS vaccine. Lancet 370:94–101. 10.1016/S0140-6736(07)61054-X [DOI] [PubMed] [Google Scholar]
- 4.Carcelain G, Autran B. 2013. Immune interventions in HIV infection. Immunol. Rev. 254:355–371. 10.1111/imr.12083 [DOI] [PubMed] [Google Scholar]
- 5.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789. 10.1182/blood-2005-12-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Braeckel E, Leroux-Roels G. 2012. HIV vaccines: can CD4+ T cells be of help? Hum. Vaccin. Immunother. 8:1795–1798. 10.4161/hv.21760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streeck H, D'Souza MP, Littman DR, Crotty S. 2013. Harnessing CD4+ T cell responses in HIV vaccine development. Nat. Med. 19:143–149. 10.1038/nm.3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourgeois C, Veiga-Fernandes H, Joret AM, Rocha B, Tanchot C. 2002. CD8 lethargy in the absence of CD4 help. Eur. J. Immunol. 32:2199–2207. [DOI] [PubMed] [Google Scholar]
- 9.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852–856. 10.1038/nature01441 [DOI] [PubMed] [Google Scholar]
- 10.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530–1533. 10.1126/science.1124226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, Cohen D, Robbins GK, Pae E, Alter G, Wurcel A, Stone D, Rosenberg ES, Walker BD, Altfeld M. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200:701–712. 10.1084/jem.20041270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shedlock DJ, Shen H. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337–339. 10.1126/science.1082305 [DOI] [PubMed] [Google Scholar]
- 13.Sun JC, Bevan MJ. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339–342. 10.1126/science.1083317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun JC, Williams MA, Bevan MJ. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927–933. 10.1038/ni1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang TC, Millar J, Groves T, Zhou W, Grinshtein N, Parsons R, Evelegh C, Xing Z, Wan Y, Bramson J. 2007. On the role of CD4+ T cells in the CD8+ T-cell response elicited by recombinant adenovirus vaccines. Mol. Ther. 15:997–1006. 10.1038/sj.mt.6300130 [DOI] [PubMed] [Google Scholar]
- 16.Van Braeckel E, Bourguignon P, Koutsoukos M, Clement F, Janssens M, Carletti I, Collard A, Demoitie MA, Voss G, Leroux-Roels G, McNally L. 2011. An adjuvanted polyprotein HIV-1 vaccine induces polyfunctional cross-reactive CD4+ T cell responses in seronegative volunteers. Clin. Infect. Dis. 52:522–531. 10.1093/cid/ciq160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrer T, Plettenberg A, Arastéh K, Van Luzen J, Fätkenheuer G, Jaeger H, Janssens M, Burny W, Collard A, Roman F, Loeliger A, Koutsoukos M, Bourguignon P, Lavreys L, Voss G. 19 October 2013. Safety and immunogenicity of an adjuvanted protein therapeutic HIV-1 vaccine in subjects with HIV-1 infection: a randomised placebo-controlled study. Vaccine 10.1016/j.vaccine.2013.10.030 [DOI] [PubMed] [Google Scholar]
- 18.Goepfert PA, Tomaras GD, Horton H, Montefiori D, Ferrari G, Deers M, Voss G, Koutsoukos M, Pedneault L, Vandepapeliere P, McElrath MJ, Spearman P, Fuchs JD, Koblin BA, Blattner WA, Frey S, Baden LR, Harro C, Evans T, NIAID HIV Vaccine Trials Network 2007. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine 25:510–518. 10.1016/j.vaccine.2006.07.050 [DOI] [PubMed] [Google Scholar]
- 19.Lichterfeld M, Gandhi RT, Simmons RP, Flynn T, Sbrolla A, Yu XG, Basgoz N, Mui S, Williams K, Streeck H, Burgett-Yandow N, Roy G, Janssens M, Pedneault L, Vandepapeliere P, Koutsoukos M, Demoitie MA, Bourguignon P, McNally L, Voss G, Altfeld M. 2012. Induction of strong HIV-1-specific CD4+ T-cell responses using an HIV-1 gp120/NefTat vaccine adjuvanted with AS02A in antiretroviral-treated HIV-1-infected individuals. J. Acquir. Immune. Defic. Syndr. 59:1–9. 10.1097/QAI.0b013e3182373b77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garulli B, Di Mario G, Sciaraffia E, Accapezzato D, Barnaba V, Castrucci MR. 2013. Enhancement of T cell-mediated immune responses to whole inactivated influenza virus by chloroquine treatment in vivo. Vaccine 31:1717–1724. 10.1016/j.vaccine.2013.01.037 [DOI] [PubMed] [Google Scholar]
- 21.Garulli B, Stillitano MG, Barnaba V, Castrucci MR. 2008. Primary CD8+ T-cell response to soluble ovalbumin is improved by chloroquine treatment in vivo. Clin. Vaccine Immunol. 15:1497–1504. 10.1128/CVI.00166-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Accapezzato D, Visco V, Francavilla V, Molette C, Donato T, Paroli M, Mondelli MU, Doria M, Torrisi MR, Barnaba V. 2005. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J. Exp. Med. 202:817–828. 10.1084/jem.20051106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, Horii T, Akira S. 2005. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J. Exp. Med. 201:19–25. 10.1084/jem.20041836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong Z, Jiang Z, Liangxi W, Guofu D, Ping L, Yongling L, Wendong P, Minghai W. 2004. Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int. Immunopharmacol. 4:223–234. 10.1016/j.intimp.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Ding G, Liu W, Wang L, Lu Y, Cao H, Zheng J. 2004. Lipopolysaccharide could be internalized into human peripheral blood mononuclear cells and elicit TNF-alpha release, but not via the pathway of Toll-like receptor 4 on the cell surface. Cell Mol. Immunol. 1:373–377 [PubMed] [Google Scholar]
- 26.Van Braeckel E, Koutsoukos M, Bourguignon P, Clement F, McNally L, Leroux-Roels G. 2011. Vaccine-induced HIV seropositivity: a problem on the rise. J. Clin. Virol. 50:334–337. 10.1016/j.jcv.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 27.Quah BJ, Warren HS, Parish CR. 2007. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat. Protoc. 2:2049–2056. 10.1038/nprot.2007.296 [DOI] [PubMed] [Google Scholar]
- 28.Garcon N, Van Mechelen M. 2011. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev. Vaccines 10:471–486. 10.1586/erv.11.29 [DOI] [PubMed] [Google Scholar]
- 29.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garcon N. 2009. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 183:6186–6197. 10.4049/jimmunol.0901474 [DOI] [PubMed] [Google Scholar]
- 30.Anthony DD, Milkovich KA, Zhang W, Rodriguez B, Yonkers NL, Tary-Lehmann M, Lehman PV. 2012. Dissecting the T cell response: proliferation assays vs. cytokine signatures by ELISPOT. Cells 1:127–140. 10.3390/cells1020127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heath SL, Sabbaj S, Bansal A, Kilby JM, Goepfert PA. 2011. CD8 T-cell proliferative capacity is compromised in primary HIV-1 infection. J. Acquir. Immune. Defic. Syndr. 56:213–221. 10.1097/QAI.0b013e3181ff2aba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Didierlaurent A, Ramirez JC, Gherardi M, Zimmerli SC, Graf M, Orbea HA, Pantaleo G, Wagner R, Esteban M, Kraehenbuhl JP, Sirard JC. 2004. Attenuated poxviruses expressing a synthetic HIV protein stimulate HLA-A2-restricted cytotoxic T-cell responses. Vaccine 22:3395–3403. 10.1016/j.vaccine.2004.02.025 [DOI] [PubMed] [Google Scholar]
- 33.Levitz SM, Golenbock DT. 2012. Beyond empiricism: informing vaccine development through innate immunity research. Cell 148:1284–1292. 10.1016/j.cell.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kannanganat S, Kapogiannis BG, Ibegbu C, Chennareddi L, Goepfert P, Robinson HL, Lennox J, Amara RR. 2007. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J. Virol. 81:12071–12076. 10.1128/JVI.01261-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, Colle JH, Urrutia A, Scott-Algara D, Boufassa F, Delfraissy JS, Theze J, Venet A, Chakrabarti LA. 2007. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J. Virol. 81:13904–13915. 10.1128/JVI.01401-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Braeckel E, Desombere I, Clement F, Vandekerckhove L, Verhofstede C, Vogelaers D, Leroux-Roels G. 2013. Polyfunctional CD4+ T cell responses in HIV-1-infected viral controllers compared with those in healthy recipients of an adjuvanted polyprotein HIV-1 vaccine. Vaccine 31:3739–3746. 10.1016/j.vaccine.2013.05.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.