Abstract
Brucella species include important zoonotic pathogens that have a substantial impact on both agriculture and human health throughout the world. Brucellae are thought of as “stealth pathogens” that escape recognition by the host innate immune response, modulate the acquired immune response, and evade intracellular destruction. We analyzed the genome sequences of members of the family Brucellaceae to assess its evolutionary history from likely free-living soil-based progenitors into highly successful intracellular pathogens. Phylogenetic analysis split the genus into two groups: recently identified and early-dividing “atypical” strains and a highly conserved “classical” core clade containing the major pathogenic species. Lateral gene transfer events brought unique genomic regions into Brucella that differentiated them from Ochrobactrum and allowed the stepwise acquisition of virulence factors that include a type IV secretion system, a perosamine-based O antigen, and systems for sequestering metal ions that are absent in progenitors. Subsequent radiation within the core Brucella resulted in lineages that appear to have evolved within their preferred mammalian hosts, restricting their virulence to become stealth pathogens capable of causing long-term chronic infections.
INTRODUCTION
The Alphaproteobacteria are an ecologically diverse group of Gram-negative bacteria among which several lineages evolved from niches in the environment toward obligate intracellular parasitism of diverse eukaryotic hosts. The adaptation of certain Alphaproteobacteria to intracellular life within a host has been associated with genome reduction, resulting in the loss of genes no longer necessary in this specialized environment (1, 2). Free-living bacteria in water or soil must exploit diverse conditions and compete with other organisms in these environments, while bacteria that reside within host cells encounter less competition but are exposed to different stresses (3, 4). As facultative intracellular pathogens, Brucella species establish long-term, often chronic, interactions with higher eukaryotes (1) but also must survive outside the host. This genus includes species considered among the world's most important zoonotic pathogens (5) with a major impact in the poorer, rural areas of the world that lack the resources to establish surveillance and eradication programs for livestock. Brucellae use virulence factors, including a type IV secretion system (T4SS), to modulate host cell biology to create a novel intracellular replication niche in both professional and nonprofessional phagocytes (6), causing infectious abortion and sterility in infected animals and a debilitating disease known as Malta fever in humans.
For many years the genus Brucella comprised six “classical” species differentiated by a preferential mammalian host and a set of antigenic and metabolic phenotypes. Since the early 1990s, new Brucella strains have been isolated from marine mammals, rodents, and atypical human infections, raising the number of recognized species to 10 (5), with additional species likely to be described. A recent study has reported the isolation of Brucella-like strains from frogs, pushing the boundaries of natural hosts into amphibians (7). Brucellae grow poorly in the environment, and each species infects a preferential host. It has been proposed that each species represents a clonal lineage selected by this restricted virulence and evolution within the vertebrate host (8–10). The Brucellae provide a unique opportunity to examine a facultative pathogen, exploring the potential parallel genome reduction and genomic divergence resulting in unique species. The genomes of prominent strains have recently been sequenced, and here we detail the comparative genomics of 40 Brucella genomes with reference to their closest relative, Ochrobactrum, a soil bacterium.
MATERIALS AND METHODS
Isolate preparation.
Brucella isolates were revived from freeze-dried stocks in the Animal Health & Veterinary Laboratories Agency (AHVLA) collection by subculture onto serum dextrose agar (SDA) containing 10% equine serum and incubation at 37°C for 3 days in the presence of additional 10% CO2. DNA was extracted by standard phenol-chloroform procedures using growth from two spread plates, as described previously (11). After extraction, DNA was resuspended in an average of 400 μl Tris-EDTA buffer, resulting in an average DNA concentration of ∼3,000 μg/ml (minimum, 300 μg/ml), which was stored at 4°C prior to shipping to the Broad Institute.
Sequencing, assembly, and annotation.
A list of all genomes used in the present study is provided in Table S1 in the supplemental material. Genome sequencing and assembly for 25 of the genomes were performed at the Broad Institute. These genomes were sequenced to at least 15-fold coverage using 454 FLX pyrosequencing (Roche) with DNA fragment libraries and 3-kb paired-end reads according to the manufacturer's recommendations and assembled using Newbler. Before assembly, the quality of the 454 sequencing data was analyzed, and suspect libraries were removed. The runAssembly script was then used to assemble reads into contigs and scaffolds using the parameters −ar −rip −g. Final assemblies were used in BLAST searches of the NCBI nonredundant (NR) database, UniVecCore, and a reasonable mitochondrial database to remove any contaminating sequence. The other 17 genomes were sequenced and annotated by different sources and were downloaded from the Pathosystems Resource Integration Center (PATRIC) (www.patricbrc.org) (12, 13). All genomes used in this analysis were annotated consistently at PATRIC using RAST (14). In addition, PATRIC's Protein Family Sorter tool (13) was used to visualize shared homology and recognize areas of horizontal transfer.
A comparison of metabolic functionality across the genomes relied on FIGfams, a collection of over 100,000 protein families that are isofunctional homologs (15) and are available at PATRIC. Although all genomes were compared in the functional analysis, for simplicity only differences between Brucella suis 1330 and Ochrobactrum anthropi were displayed.
OGs.
OrthoMCL (16) was used to delineate groups of orthologous proteins. To create a representative set of ortholog groups (OGs) for Brucella and its closest relatives, genomes from Ochrobactrum anthropi, O. intermedium, Bartonella quintana, Mesorhizobium loti, and Agrobacterium tumefaciens were included. Multiple-sequence alignments were generated for each OG and were visually inspected.
SNP phylogeny.
Single nucleotide polymorphism (SNP) discovery was performed using MUMmer (17, 18) and In Silico Genotyper (TGen; unpublished program). MUMmer was run on the complete genomes to produce pairwise sequence alignments against the reference (B. suis 1330) utilizing a sliding window with each potential SNP flanked by 100 bases on each side. SNPs from the resulting binary alignment map (BAM) alignments were determined using SolSNP (http://sourceforge.net/projects/solsnp/). Taxa were then grouped by SNP loci shared across all taxa using In Silico Genotyper. Loci missing from one or more genomes (no read or sequence coverage in the alignment) were excluded from the analysis, as were repeated regions and paralogous genes. We defined homologous SNPs as those found in all genomes and paralogous SNPs as those that came from a region that had been duplicated. Orthologous SNPs were those homologous SNPs that remained after the paralogous SNPs were removed.
When using only shared orthologous SNPs in a phylogeny, the number of SNPs will vary depending on which taxa are included in the comparisons. For example, when Ochrobactrum species are included in SNP discovery, fewer SNPs are found within a clade such as Brucella abortus due to the requirement that the locus containing the SNP occur in all genomes, including even more distantly related ones with higher levels of genomic changes. We resolved this issue by rerunning SNP discovery in a stepwise fashion down through the phylogeny. First we included both Ochrobactrum species and all Brucella species, then just the Brucella species, then just the core Brucella species, and finally strains within each species containing more than one taxon. Within-species comparisons used the most complete genome sequence of that species as the reference. None of these analyses changed the overall tree topology, but they did provide for more-detailed depictions of the SNPs relevant to each clade. Trees were constructed using maximum parsimony in the program PAUP* 4.0b10 using a matrix of SNP calls (19). We employed a full heuristic search and tested nodal support with 1,000 bootstrap repetitions.
Protein family phylogeny.
Of the 6,991 protein families (OGs) for the 40 Brucella and 5 outgroup genomes, 1,844 were found to have one and only one representative of each Brucella genome, and these were used for the phylogenetic analysis (20, 21). At each of these loci, outgroup sequences were included if present as a single locus but excluded if paralogous. Of the 1,844 protein families used in the analysis, O. anthropi was represented in 1,695 protein families, O. intermedium in 1,670, B. quintana in 794, M. loti in 1,484, and A. tumefaciens in 1,462. The protein sequences of each family were aligned using MUSCLE (22), and ambiguous portions of the alignment were removed using Gblocks (23). The concatenation of these trimmed alignments contained 521,801 amino acid positions. RAxML 7.2.3 (24) was used with the PROTGAMMALG model to prepare a maximum-likelihood tree and in its quick mode to prepare 100 bootstrap trees.
RESULTS AND DISCUSSION
Sequencing and annotation.
Twenty-five strains largely representing type strains or groups not previously characterized by whole-genome sequencing were chosen for sequencing and were compared with 17 previously sequenced genomes (see Table S1 in the supplemental material). The new genomes were finished but not closed (25), ranging from 11 (Brucella neotomae 5K33) to 89 (Brucella pinnipedialis M163/99/10) contigs. The Brucella genomes have an average of 3,463 genes annotated, ranging from 3,167 (Brucella sp. strains 83/13 and NF2653) to 3,610 genes (Brucella ceti Cudo).
Molecular phylogeny of the Brucellaceae.
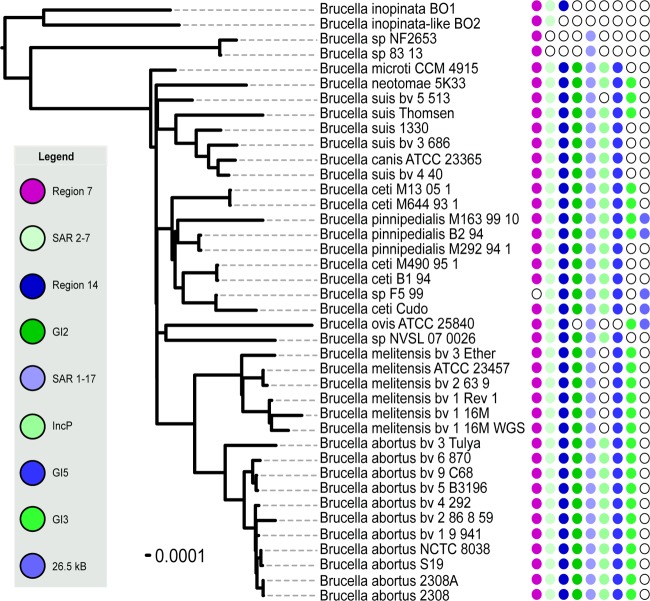
Phylogenetic trees were built from over 190,000 SNPs and separately from over 1,800 concatenated protein sequences (20, 21). These robust phylogenetic analyses of the Brucella and outgroup genomes shows that all Brucella species are monophyletic and distinct from their Ochrobactrum relatives (Fig. 1), confirming earlier studies (26). There is also a clear separation between the “classical” Brucella species that are united in a core clade and a second group that diverged earlier. This early diverging basal group contains strains from Australian rodents (83/13 and NF2653) and two recent atypical human isolates, BO1 and BO2, where the natural host is unknown (27, 28). Of the 193,760 shared SNPs within the overall phylogeny, Brucella species are separated from the two Ochrobactrum species by 158,016 SNPs. The basal Brucella species are clearly differentiated from the core Brucella species, with 2,672 SNPs uniting the core Brucella species. The relative diversity is substantially higher in the early-diverging Brucella species than in the classical species, suggesting that this group is undersampled.
FIG 1.
Phylogenetic analysis of 42 Brucellaceae genomes. The maximum-parsimony tree is based on 193,760 SNPs. The early-diverging Brucella strains are clearly differentiated from the classic Brucella strains, with 2,672 SNPs unique to the classic Brucella strains and 1,172 SNPs unique to the outer clade (strains NF2653, 83/13, BO1, and BO2). The tree was rooted with Ochrobactrum spp. as outgroups. All branches have 100% support unless otherwise noted.
The core clade experienced a radiation that resulted in six to eight lineages, with Brucella microti being the most basal and the remaining clades (Brucella neotomae, Brucella canis-Brucella suis, Brucella ceti-Brucella pinnipedialis, Brucella ovis, Brucella sp. strain NVSL07-0026, and Brucella abortus-Brucella melitensis) separated by such short internal branch lengths that they appear to have diverged nearly simultaneously (Fig. 2). In the phylogeny comparing all Brucella species to Ochrobactrum and in the core clade phylogeny, B. ovis and Brucella sp. strain NVSL07-0026 are united by an extremely shallow branch, indicating a shared common ancestor, but the lengths of the branches that separate these two indicate significant divergence since that time. This is also seen in the phylogeny based on protein families (Fig. 3). The phylogenetic placement of B. suis bv. 5 in the tree containing only the core Brucella species (Fig. 2) is within the B. suis-B. canis clade with an extremely shallow branch, but in the phylogeny of the whole genus it falls in its own separate lineage (Fig. 1).
FIG 2.
Phylogenetic analysis, based on maximum parsimony of the core Brucella genomes, showing a rapid radiation following the divergence of B. microti, resulting in six separate clades, with Brucella sp. strain NVSL07-0026 and B. suis bv. 5 as possible separate clades. The tree was rooted with B. microti as the outgroup based on results from Fig. 1. All branches have 100% support unless otherwise noted.
FIG 3.
Phylogenetic analysis based on shared protein families in Brucella, showing the presence (colored circles) or absence (open circles) of specific genomic islands that are not universally shared across all 40 genomes (as in Table 1).
Changes at the genus level: emergence of Brucella as a pathogen.
The virulence of Brucella is dependent on its ability to survive and multiply within host cells, including macrophages (6, 29, 30). Its closest known relatives, O. anthropi and O. intermedium, are soil bacteria that can cause opportunistic infections in immunocompromised hosts but are not known to replicate intracellularly within them. Specific events must have led to the evolution of the ability to survive and multiply within a vertebrate cell.
(i) Genome reduction.
Adaptation to eukaryotic hosts in the Alphaproteobacteria has been associated with genome reduction (1), and the typical Brucella genome (∼3.3 Mb) is at least 30% smaller than that of Ochrobactrum (4.77 Mb). Further evidence of genome reduction can be seen in the analysis of protein families defined by OrthoMCL. The two Ochrobactrum genomes share more than 900 OGs that are not found in any Brucella genome (see Table S2 in the supplemental material). Our data cannot rule out the possibility that Ochrobactrum species have larger genomes due to acquisition by lateral gene transfer, but only 24 of these OGs were found on the plasmids associated with the O. anthropi genome, which would rule out plasmids as the source of lateral transfer.
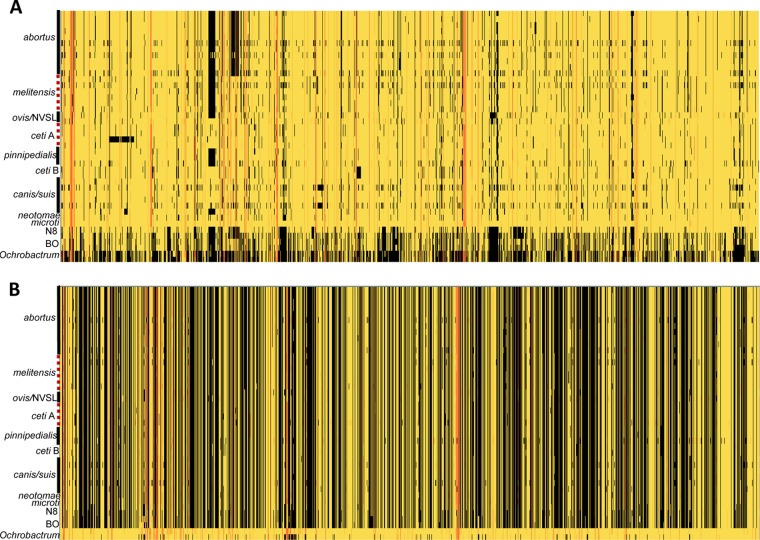
We calculated a pan-genome for Brucella, defined as the superset of proteins encoded across all 40 genomes. There were 5,920 OGs in the pan-genome and 2,285 OGs in the core genome. A representation of the differences between Brucella and Ochrobactrum showing the presence or absence of protein families is depicted in Fig. 4, organized in the order that the genes occur in either B. microti (Fig. 4A) or O. anthropi (Fig. 4B). Black areas indicate regions that are missing, and the areas of loss are especially prevalent when Brucella is oriented according to Ochrobactrum (Fig. 4B). The Brucella orientation confirms what previous studies across fewer strains have found: there is high conservation of genes across Brucella (31) and extensive similarity in genetic content and gene order (32).
FIG 4.
The first half of the Brucellaceae pan-proteome with protein families oriented by either B. microti (A) or O. anthropi (B), generated using the Protein Family Sorter tool at PATRIC. Black cells (columns, protein families; rows, genomes) indicate no annotated proteins, yellow indicates a single protein, and orange to red indicate increasing numbers of proteins annotated in that specific genome.
A comparison of metabolic function between Brucella and Ochrobactrum using the subsystem approach (33) was performed and highlights the likely process of genome reduction (see Fig. S1 in the supplemental material). In almost all categories, Brucella has fewer genes than Ochrobactrum; marked losses are seen in the number of genes encoding proteins involved in metabolism and in utilization, degradation, or biosynthesis of both carbohydrates and amino acids. There are two exceptions. Brucella has seven genes involved in aromatic amino acid degradation that Ochrobactrum lacks, and it also has more genes involved in membrane transport, specifically in the genes used to transport nickel. All genes included in the functional analysis are listed in Table S3 in the supplemental material.
(ii) Gene acquisition.
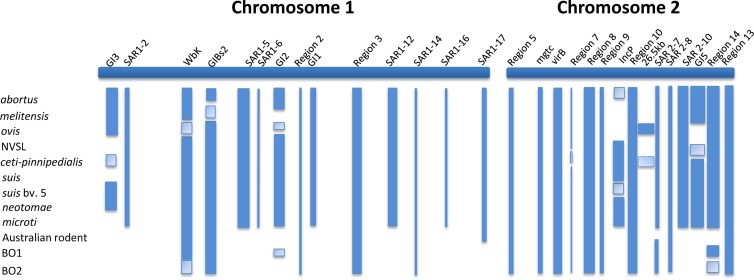
In addition to the nickel transport genes, Brucella also has a small number of genes that are not found Ochrobactrum, many of which are found clustered in 15 genomic regions that are depicted visually in Fig. 3 and detailed in Table S4 in the supplemental material. Many of these regions have been previously identified as genomic islands and in most cases are completely missing in Ochrobactrum (Table 1). In wbk and region 14, Ochrobactrum has a small number of the total genes, indicating that these could have been present in the common ancestor and have since been lost in Ochrobactrum. The presence or absence of these regions, showing their locations on the two chromosomes of a typical Brucella genome, is shown in Fig. 5.
TABLE 1.
Presence and absence of genomic regions across the genus Brucella and close relatives
| Region | Other identifier | Presencea in: |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. abortus | B. melitensis | B. ovis | Brucella sp. strain NVSL | B. ceti A | B. pinnipedialis | B. ceti B | B. canis-B. suis | B. suis bv. 5 | B. neotomae | B. microti | Brucella sp. strain N8 | Brucella sp. strain BO1 | Brucella sp. strain BO2 | O. anthropi | O. intermedium | B. quintana | M. loti | A. tumefaciens | ||
| 1 | GIBs2 | ✓ | ✓b | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 |
| 2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | |
| 3 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | |
| 4 | SAR 1–14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 |
| 5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | |
| 6 | mgtC | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 |
| 7 | ✓ | ✓ | ✓ | ✓ | ✓c | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | |
| 8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | |
| 9 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | |
| 10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | |
| 11 | SAR 2–7 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0k | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 |
| 12 | SAR 2–8 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 |
| 13 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0l | 0 | 0 | 0 | 0 | |
| 14 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | ✓ | ✓d | 0 | 0 | 0 | 0 | 0 | |
| 15 | virB | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | # | # | # | # | # |
| 16 | wbk | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0m | 0n | ✓e | 0 | 0 | 0 |
| Classic 1 | SAR 1–2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 2 | SAR 1–5 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 3 | SAR 1–6 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 4 | GI2 | ✓ | ✓ | 0o | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0p | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 5 | GI1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 6 | SAR 1–12 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 7 | SAR 1–16 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 8 | SAR 1–17 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓f | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 9 | IncP | ✓g | 0 | 0 | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 10 | SAR 2–10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 11 | GI5 | ✓ | ✓ | 0 | ✓h | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 12 | GI3 | ✓ | ✓ | ✓ | 0 | 0 | ✓i | ✓ | 0q | ✓ | ✓ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Classic 13 | 26.5 kb | 0 | 0 | ✓ | 0 | ✓j | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
✓, region is present; 0, region is absent; #, genes of this type are present but are not homologs to those found in Brucella.
Brucella melitensis 16M, 16M WGS, and Rev1 are missing 5 of the 9 genes in this region.
Brucella sp. strain F5/99 is missing all genes in this region.
Brucella inopinata BO2 is missing 5 of the total 24 genes.
Of the 12 genes in this area important for forming the LPS in Brucella, Ochrobactrum intermedium has 7.
Brucella sp. strains 83/13 and NF2653 have all genes in this region.
Brucella abortus bv. 5 strain B3196 has 9 of the 16 genes in this region.
Brucella sp. strain NVSL has 22 of the 37 genes in this region.
Present in both Brucella pinnipedialis B2/94 and M163/99/10, but all 27 genes are missing in M292/94/1.
All genes missing Brucella ceti M490/95/1 and B1/94, but all are present in B. ceti Cudo and Brucella sp. strain F5/99. Similarly, all genes are missing in B. pinnipedialis M163/99/10, and all present in strains B2/94 and M292/94/1. B. ceti A includes B. ceti strains Cudo, F5/99, M490/95/1, and B1/94, while B. ceti B includes strains M13/05/1 and M644/93/1.
Brucella sp. strains 83/13 and NF2653 have 2 of the 8 genes in this region.
Ochrobactrum anthropi has only 2 of the 11 genes in this region.
Brucella inopinata BO2 has only 2 of the 12 genes in this region that other brucellae have. It has four genes in the rml operon in the same place.
Ochrobactrum anthropi has only 2 of the 12 genes in this region that other brucellae have. It has four genes in the rml operon in the same place.
Brucella ovis is missing 21 of the 22 genes in this region.
Brucella inopinata BO1 has only 5 of the 22 genes in this region.
Brucella suis Thomsen (ATCC 23445) is the only member of this clade that has any of these genes, and it has all 28 of them.
FIG 5.
Location of genomic regions of interest across a “typical” Brucella strain of two chromosomes. Dark blue bands indicate that a genome or clade has all the genes in the region present. A lighter band indicates that a genome or clade is missing some or all of the genes in a region. Absence of a band shows that the genome or clade does not have this region. These data should be cross-referenced with column 2 of Table 1.
(iii) Acquisition of virulence determinants.
These 15 regions have many genes that encode virulence factors. Region 15 contains the VirB type 4 secretion system (T4SS), a key virulence factor for Brucella (6, 34). Ochrobactrum has a T4SS of completely different origin that contains a relaxosome component and a coupling protein, both essential for DNA translocation, which are absent in Brucella. The T4SS most similar to that of Brucella is found on a broad-host-range plasmid isolated from an unidentified bacterium in the rhizosphere of alfalfa (35).
Three other regions unique to Brucella could encode virulence factors. Region 4 (shared anomalous region 1–14 [SAR 1–14]) contains genes encoding enzymes involved in the synthesis of polysaccharides in other bacteria (36) and also the omp31 gene, encoding a phospholipase with homology to eukaryotic patatin. Some animal and plant pathogens contain a high number of genes with patatin domains, and it has been suggested that these proteins may help them in competition, adaptation to new environments, and interactions with a host (37). Region 7 contains two proteins with homology to eukaryotic proteins (BRA0135 and BRA0136) and a protein with homology to a phospholipase secreted by Legionella (BRA0131). Region 14 carries several genes found in pathogenicity islands described in plant pathogens, including hpaE (BRA1161) (38).
Brucella encounters acid stress during the infection process: the strong acid in the stomach if transmitted via ingestion and the acid conditions in the phagosome (39), which are known to induce expression of several virulence factors (40). Genes involved in acid resistance are located on region 9, which encodes the Hfq-regulated gene hdeA that has been shown to be required for resistance to mild acid shock (41). The gad operon, which produces the nonproteinogenic amino acid γ-aminobutyric acid (GABA), conferring resistance to strong acid (<pH 3) in B. microti (42), is also encoded here (VBIBruSui107850_2552 and VBIBruSui107850_2553). This region seems to be undergoing degradation in the classical species, because several genes are nonfunctional due to frameshifts or stop codons.
Most brucellae produce a smooth lipopolysaccharide (LPS) with an O antigen composed as a homopolymer of N-formyl-perosamine with α(1,3) and α(1,2) linkages. The majority of the genes involved in O-antigen biosynthesis are found in the wbk region (region 16 [see Table S4 in the supplemental material]) and have been hypothesized to have been acquired by horizontal transfer (43). Two of the “classical” Brucella species, B. canis and B. ovis, are naturally rough, reflecting distinct mutations. In B. canis this is due to truncations in both wbkD and wbkF (44, 45), while B. ovis has a deletion of the wbo region (45–47), as well as a mutation that truncates wzt (45). Our analysis has also identified additional mutations that truncate per and wbkE in B. ovis (data not shown). Other than these mutations, the LPS biosynthesis genes in “classical” Brucella strains are very conserved (44). Examination of the genes encoding the LPS of the atypical Brucella strains show considerable diversity (48), although all have a smooth phenotype. The major difference is seen in BO2, which produces a smooth LPS with an O antigen different from that of all other brucellae, using four genes encoding enzymes that synthesize a rhamnose-based LPS that it shares with Ochrobactrum (48). Recent data suggest that unlike BO1, strain BO2 is unable to replicate in mammalian cells (B. Saadeh and D. O'Callaghan, unpublished data). This suggests that the loss of the ancestral rhamnose-based LPS and acquisition of the genes encoding the perosamine-based LPS were key steps in the path to intracellular parasitism.
(iv) Adaptation to a limited-metal environment.
Bacteria require metal ions for crucial enzymatic functions, including virulence, and intracellular bacteria live in an environment where there is limited availability (48). Many of the regions that are unique to Brucella carry genes involved in the transport or acquisition of metal ions, specifically, iron, magnesium, and nickel. Details on these genes are provided in Table S4 in the supplemental material.
Brucellae produce the monocatechol siderophore 2,3-dihydroxybenzoic acid (2,3-DHBA) in response to iron limitation, and the genes that encode this siderophore (49) are in an operon (BRA0013 to BRA0016) found in region 5. Production of this siderophore appears to be an adaptation to the environment found in the ruminant placenta (50–52). Another gene involved in iron regulation is dhbR. This gene, BRA1192 (in region 13), encodes an AraC-type transcriptional regulator that provides a second level of regulation of siderophore biosynthesis genes in response to the availability of Fe3+ encountered in the external environment (53).
Magnesium is another metal found in limiting quantities in mammalian hosts. Brucella appears to only have one system, the MgtBC transporter, to acquire magnesium from the environment. MgtC has been shown to be a key virulence factor required for the survival and growth in low concentrations of Mg2+ and within macrophages of several bacterial pathogens, including Brucella (44, 45). In Brucella, mgtC (BRA0040) is found in region 6 (54).
Many bacteria, including Ochrobactrum, use a single protein (HupE/UreJ) to acquire nickel. Brucellae are unusual in that they have two distinct operons (nikABCDE and nikKMLQO) for nickel transport. They also have two known metalloenzymes that require nickel (carbon monoxide dehydrogenase and urease) and two urease operons. The transporter encoded by nikKLMQO (region 3) is the most common transporter found in both Archaea and Bacteria (55) and has been identified as the primary transporter of nickel in B. abortus 2308 (56). One of the urease operons, also in region 3, is located upstream of this transporter. The nikABCDE operon (region 12) is not common and is sporadically found across the known bacterial genomes (55). The genes in this operon are among the first to be upregulated during intracellular growth of Brucella, suggesting that its niche in the host cell is poor in nickel but that nickel is required by the bacterium, necessitating expression of high-affinity uptake systems (57). Yet the role in virulence of the nikABCDE operon is not clear. Mutation of the nikA gene in B. suis drastically reduces urease activity but does not affect virulence in macrophage infection models (57). Further, many virulent strains of B. abortus have a frameshift in nikA (56), which may explain the low levels of urease activity reported for many field isolates (58).
It is tempting to speculate that the urease system is undergoing the early events of genome reduction. This is suggested by the lack of urease activity in B. ovis and the frequent mutations seen in nikA in B. abortus. It could be hypothesized that the first pathogenic Brucella strains were transmitted by the oral route (perhaps via infected milk), but this has now shifted to include other mucosal routes. Oral infection is still possible, especially when animals consume heavily contaminated infected placentas or abortion material, which can contain up to 1010 viable bacteria per gram. It is interesting to note that NVSL07-0026, the smooth strain most closely related to B. ovis and recently isolated from a baboon stillbirth, also shares the mutations in the urease system (data not shown). It may be that this strain, like B. ovis, is transmitted by sexual contact.
Changes within the genus: differentiation within the Brucella clades.
Brucella has undergone differentiation following its divergence from Ochrobactrum. The core Brucella clade is distinct from the branch with the two Australian rodent strains and the genomes from the recent atypical human infections (Fig. 1). These genomic comparisons and the genetic relationships among isolates seen here have a relative consistency with a variety of tree topologies from early restriction mapping and multilocus sequence analysis through recent whole-genome analyses (10, 28, 45, 59–63). Phenotypically, the Australian rodent strains are very similar to the core Brucella strains, with slow growth and phage sensitivity. B. inopinata strain BO1 and B. inopinata-like strain BO2 are different in that they have very fast growth on bacteriological media, are not sensitive to the known Brucella phage, and have very different antigenic characteristics (27). However, fast growth is not a distinguishing criterion for the “atypical” strains, since B. microti (basal to the divergence of the strains in the core clade) also shows rapid growth. Audic et al. (64) have suggested that the rapid growth of B. microti is linked to the presence of an unusual spacer region in the 23S rRNA gene; this spacer is also found in BO1 and BO2.
There are 13 regions that are present in the core strains that are absent in the atypical strains, and a list of genes in these regions is provided in Table S5 in the supplemental material. Most appear to encode proteins that have been acquired horizontally (phage/plasmid or flanked by tRNA genes), and only a few contain proteins with known function. A more detailed analysis of the genomes of the early-diverging strains is addressed in a separate study (48). There are two proteins found in the core clade, BR0735 (GI13, region 2) and BAB1_0279 (region 12), which have eukaryotic Toll interleukin receptor (TIR) domains and have been shown to play a role in the modulation of host innate immune responses (65–71). It is unclear exactly how the acquisition of these TIR domain proteins affected the core Brucella, but it is tempting to speculate that this ability to modulate host immunity was a key step in the dramatic spread of Brucella through a wide range of mammalian hosts.
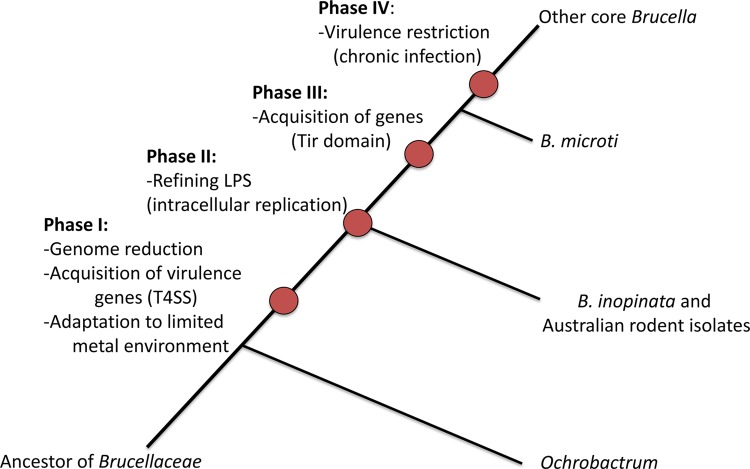
A path to the evolution of virulence.
Our analysis has identified certain steps that we hypothesize as important developments in the path to pathogenesis (Fig. 6). The first crucial step was the acquisition of the VirB T4SS, a key element that has allowed Brucella to adapt to a pathogenic niche. This was accompanied by the adaptation of a small set of other factors that have allowed Brucella to survive within host cells, and these include genes encoding systems important for sequestering metal ions. A second stage involved the change to a perosamine-based O antigen that seems to be associated with intracellular replication. There is a third step in the path toward virulence; development of the ability to modulate the host immune system using the TIR domain proteins could have been a key step in Brucella becoming a stealth pathogen before the radiation to the currently recognized species. As with many intracellular Alphaproteobacteria, another key factor appears to be loss or restriction of virulence, allowing the pathogen to avoid a rapid fatal infection and instead keeping the host alive and establishing a chronic infection (1). As more atypical brucellae, such as the recently described strains from frogs (7), are identified and analyzed, we will test our scenario of evolution toward intracellular pathogenicity with more precision.
FIG 6.
A model for the evolution of virulence in the genus Brucella. The phylogenetic tree is not drawn to scale.
Supplementary Material
ACKNOWLEDGMENTS
We thank Claire Dawson for technical assistance with DNA preparation and Annette Vergunst, Marty Roop, and Tom Ficht for critical review of the manuscript.
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272200900040C. Work carried out at AHVLA was supported by the United Kingdom Department of the Environment, Food and Rural Affairs (Defra). INSERM U1047 was supported by institutional grants from INSERM and the Université Montpellier 1, the Agence Nationale de la Recherche (ANR), the Region Languedoc-Roussillon, and the Ville de Nîmes.
Footnotes
Published ahead of print 13 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01091-13.
REFERENCES
- 1.Batut J, Andersson SG, O'Callaghan D. 2004. The evolution of chronic infection strategies in the alpha-proteobacteria. Nat. Rev. Microbiol. 2:933–945. 10.1038/nrmicro1044 [DOI] [PubMed] [Google Scholar]
- 2.Andersson SG, Kurland CG. 1998. Reductive evolution of resident genomes. Trends Microbiol. 6:263–268. 10.1016/S0966-842X(98)01312-2 [DOI] [PubMed] [Google Scholar]
- 3.Losada L, Ronning CM, DeShazer D, Woods D, Fedorova N, Stanley Kim H, Shabalina SA, Pearson TR, Brinkac L, Tan Nandi PT, Crabtree J, Badger J, Beckstrom-Sternberg S, Saqib M, Schutzer SE, Keim P, Nierman WC. 2010. Continuing evolution of Burkholderia mallei through genome reduction and large-scale rearrangements. Genome Biol. Evol. 2:102–116. 10.1093/gbe/evq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song H, Hwang J, Yi H, Ulrich RL, Yu Y, Nierman WC, Kim HS. 2010. The early stage of bacterial genome-reductive evolution in the host. PLoS Pathog. 6:e1000922. 10.1371/journal.ppat.1000922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Callaghan D, Whatmore AM. 2011. Brucella genomics as we enter the multi-genome era. Brief. Funct. Genomics 10:334–341. 10.1093/bfgp/elr026 [DOI] [PubMed] [Google Scholar]
- 6.de Jong MF, Tsolis RM. 2012. Brucellosis and type IV secretion. Future Microbiol. 7:47–58. 10.2217/fmb.11.136 [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg T, Hamann HP, Kaim U, Schlez K, Seeger H, Schauerte N, Melzer F, Tomaso H, Scholz HC, Koylass MS, Whatmore AM, Zschöck M. 2012. Isolation of potentially novel Brucella spp. from frogs. Appl. Environ. Microbiol. 78:3753–3755. 10.1128/AEM.07509-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michaux-Charachon S, Bourg G, Jumas-Bilak E, Guigue-Talet P, Allardet-Servent A, O'Callaghan D, Ramuz M. 1997. Genome structure and phylogeny in the genus Brucella. J. Bacteriol. 179:3244–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno E, Cloeckaert A, Moriyon I. 2002. Brucella evolution and taxonomy. Vet. Microbiol., 90:209–227. 10.1016/S0378-1135(02)00210-9 [DOI] [PubMed] [Google Scholar]
- 10.Bourg G, O'Callaghan D, Boschiroli ML. 2007. The genomic structure of Brucella strains isolated from marine mammals gives clues to evolutionary history within the genus. Vet. Microbiol. 125:375–380. 10.1016/j.vetmic.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 11.Whatmore AM, Murphy TJ, Shankster S, Young E, Cutler SJ, MacMillan AP. 2005. Use of amplified fragment length polymorphism to identify and type Brucella isolates of medical and veterinary interest. J. Clin. Microbiol. 43:761–769. 10.1128/JCM.43.2.761-769.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillespie JJ, Wattam AR, Cammer SA, Gabbard JL, Shukla MP, Dalay O, Driscoll T, Hix D, Mane SP, Mao Nordberg CEK, Scott M, Schulman JR, Snyder EE, Sullivan DE, Wang C, Warren A, Williams KP, Xue T, Yoo HS, Zhang C, Zhang Y, Will R, Kenyon RW, Sobral BW. 2011. PATRIC: the comprehensive bacterial bioinformatics resource with a focus on human pathogenic species. Infect. Immun. 79:4286–4298. 10.1128/IAI.00207-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, Gillespie JJ, Gough R, Hix D, Kenyon R, Machi D, Mao C, Nordberg EK, Olson R, Overbeek R, Pusch GD, Shukla M, Schulman J, Stevens RL, Sullivan DE, Vonstein V, Warren A, Will R, Wilson MJ, Yoo HS, Zhang C, Zhang Y, Sobral BW. 2014. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 42:D581–D591. 10.1093/nar/gkt1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 42:D206–D214. 10.1093/nar/gkt1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer F, Overbeek R, Rodriguez A. 2009. FIGfams: yet another set of protein families. Nucleic Acids Res. 37:6643–6654. 10.1093/nar/gkp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Stoeckert CJ, Jr, Roos DS. 2003. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13:2178–2189. 10.1101/gr.1224503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delcher AL, Phillippy A, Carlton J, Salzberg SL. 2002. Fast algorithms for large-scale genome alignment and comparison. Nucleic Acids Res. 30:2478–2483. 10.1093/nar/30.11.2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5:R12. 10.1186/gb-2004-5-2-r12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilgenbusch JC, Swofford D. 2003. Inferring evolutionary trees with PAUP*. Curr.Protoc. Bioinformatics Chapter 6:Unit 6 4 [DOI] [PubMed] [Google Scholar]
- 20.Williams KP, Gillespie JJ, Sobral BW, Nordberg EK, Snyder EE, Shallom JM, Dickerman AW. 2010. Phylogeny of gammaproteobacteria. J. Bacteriol. 192:2305–2314. 10.1128/JB.01480-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams KP, Sobral BW, Dickerman AW. 2007. A robust species tree for the alphaproteobacteria. J. Bacteriol. 189:4578–4586. 10.1128/JB.00269-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 5:113. 10.1186/1471-2105-5-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17:540–552. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 24.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 25.Mardis E, McPherson J, Martienssen R, Wilson RK, McCombie WR. 2002. What is finished, and why does it matter. Genome Res. 12:669–671. 10.1101/gr.032102 [DOI] [PubMed] [Google Scholar]
- 26.De Ley J, Mannheim W, Segers P, Lievens A, Denijn M, Vanhoucke M, Gillis M. 1987. Ribosomal ribonucleic acid cistron similarities and taxonomic neighborhood of Brucella and CDC group Vd. Int. J. Syst. Bacteriol. 37:35–42. 10.1099/00207713-37-1-35 [DOI] [Google Scholar]
- 27.De BK, Stauffer L, Koylass MS, Sharp SE, Gee JE, Helsel LO, Steigerwalt AG, Vega R, Clark TA, Daneshvar MI, Wilkins PP, Whatmore AM. 2008. Novel Brucella strain (BO1) associated with a prosthetic breast implant infection. J. Clin. Microbiol. 46:43–49. 10.1128/JCM.01494-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiller RV, Gee JE, Lonsway DR, Gribble S, Bell SC, Jennison AV, Bates J, Coulter C, Hoffmaster AR, De BK. 2010. Identification of an unusual Brucella strain (BO2) from a lung biopsy in a 52 year-old patient with chronic destructive pneumonia. BMC Microbiol. 10:23. 10.1186/1471-2180-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Celli J. 2006. Surviving inside a macrophage: the many ways of Brucella. Res. Microbiol. 157:93–98. 10.1016/j.resmic.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 30.Barbier T, Nicolas C, Letesson JJ. 2011. Brucella adaptation and survival at the crossroad of metabolism and virulence. FEBS Lett. 585:2929–2934. 10.1016/j.febslet.2011.08.011 [DOI] [PubMed] [Google Scholar]
- 31.Verger JM, Grimont F, Grimont PAD, Grayon M. 1985. Brucella, a monospecific genus as shown by deoxyribonucleic acid hybridization. Int. J. Syst. Bacteriol. 35:292–295. 10.1099/00207713-35-3-292 [DOI] [Google Scholar]
- 32.Paulsen IT, Seshadri R, Nelson KE, Eisen JA, Heidelberg JF, Read TD, Dodson RJ, Umayam L, Brinkac LM, Beanan MJ, Daugherty SC, Deboy RT, Durkin AS, Kolonay JF, Madupu R, Nelson WC, Ayodeji B, Kraul M, Shetty J, Malek J, Van Aken SE, Riedmuller S, Tettelin H, Gill SR, White O, Salzberg SL, Hoover DL, Lindler LE, Halling SM, Boyle SM, Fraser CM. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Nat. Acad. Sci. U. S. A. 99:13148–13153. 10.1073/pnas.192319099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overbeek R, Larsen N, Pusch GD, D'Souza M, Selkov E, Jr, Kyrpides N, Fonstein M, Maltsev N, Selkov E. 2000. WIT: integrated system for high-throughput genome sequence analysis and metabolic reconstruction. Nucleic Acids Res. 28:123–125. 10.1093/nar/28.1.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210–1220 [DOI] [PubMed] [Google Scholar]
- 35.Schneiker S, Keller M, Droge M, Lanka E, Puhler A, Selbitschka W. 2001. The genetic organization and evolution of the broad host range mercury resistance plasmid pSB102 isolated from a microbial population residing in the rhizosphere of alfalfa. Nucleic Acids Res. 29:5169–5181. 10.1093/nar/29.24.5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vizcaino N, Cloeckaert A, Zygmunt MS, Fernandez-Lago L. 2001. Characterization of a Brucella species 25-kilobase DNA fragment deleted from Brucella abortus reveals a large gene cluster related to the synthesis of a polysaccharide. Infect. Immun. 69:6738–6748. 10.1128/IAI.69.11.6738-6748.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerji S, Flieger A. 2004. Patatin-like proteins: a new family of lipolytic enzymes present in bacteria? Microbiology 150:522–525. 10.1099/mic.0.26957-0 [DOI] [PubMed] [Google Scholar]
- 38.Buttner D, Noel L, Stuttmann J, Bonas U. 2007. Characterization of the nonconserved hpaB-hrpF region in the hrp pathogenicity island from Xanthomonas campestris pv. vesicatoria. Mol. Plant Microbe Interact. 20:1063–1074. 10.1094/MPMI-20-9-1063 [DOI] [PubMed] [Google Scholar]
- 39.Porte F, Liautard JP, Kohler S. 1999. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 67:4041–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boschiroli ML, Ouahrani-Bettache S, Foulongne V, Michaux-Charachon S, Bourg G, Allardet-Servent A, Cazevieille C, Liautard JP, Ramuz M, O'Callaghan D. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Nat. Acad. Sci. U. S. A. 99:1544–1549. 10.1073/pnas.032514299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valderas MW, Alcantara RB, Baumgartner JE, Bellaire BH, Robertson GT, Ng WL, Richardson JM, Winkler ME, Roop RM., II 2005. Role of HdeA in acid resistance and virulence in Brucella abortus 2308. Vet. Microbiol. 107:307–312. 10.1016/j.vetmic.2005.01.018 [DOI] [PubMed] [Google Scholar]
- 42.Occhialini A, Jimenez de Bagues MP, Saadeh B, Bastianelli D, Hanna N, De Biase D, Kohler S. 2012. The glutamic acid decarboxylase system of the new species Brucella microti contributes to its acid resistance and to oral infection of mice. J. Infect. Dis. 206:1424–1432. 10.1093/infdis/jis522 [DOI] [PubMed] [Google Scholar]
- 43.Godfroid F, Cloeckaert A, Taminiau B, Danese I, Tibor A, de Bolle X, Mertens P, Letesson JJ. 2000. Genetic organisation of the lipopolysaccharide O-antigen biosynthesis region of Brucella melitensis 16M (wbk). Res. Microbiol. 151:655–668. 10.1016/S0923-2508(00)90130-X [DOI] [PubMed] [Google Scholar]
- 44.Zygmunt MS, Blasco JM, Letesson JJ, Cloeckaert A, Moriyon I. 2009. DNA polymorphism analysis of Brucella lipopolysaccharide genes reveals marked differences in O-polysaccharide biosynthetic genes between smooth and rough Brucella species and novel species-specific markers. BMC Microbiol. 9:92. 10.1186/1471-2180-9-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wattam AR, Williams KP, Snyder EE, Almeida NF, Jr, Shukla M, Dickerman AW, Crasta OR, Kenyon R, Lu J, Shallom JM, Yoo H, Ficht TA, Tsolis RM, Munk C, Tapia R, Han CS, Detter JC, Bruce D, Brettin TS, Sobral BW, Boyle SM, Setubal JC. 2009. Analysis of ten Brucella genomes reveals evidence for horizontal gene transfer despite a preferred intracellular lifestyle. J. Bacteriol. 191:3569–3579. 10.1128/JB.01767-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rajashekara G, Glasner JD, Glover DA, Splitter GA. 2004. Comparative whole-genome hybridization reveals genomic islands in Brucella species. J. Bacteriol. 186:5040–5051. 10.1128/JB.186.15.5040-5051.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vizcaino N, Caro-Hernandez P, Cloeckaert A, Fernandez-Lago L. 2004. DNA polymorphism in the omp25/omp31 family of Brucella spp.: identification of a 1.7-kb inversion in Brucella cetaceae and of a 15.1-kb genomic island, absent from Brucella ovis, related to the synthesis of smooth lipopolysaccharide. Microbes Infect. 6:821–834. 10.1016/j.micinf.2004.04.009 [DOI] [PubMed] [Google Scholar]
- 48.Wattam AR, Inzana TJ, Williams KP, Mane SP, Shukla M, Almeida NF, Dickerman AW, Mason S, Moriyon I, O'Callaghan D, Whatmore AM, Sobral BW, Tiller RV, Hoffmaster AR, Frace MA, De Castro C, Molinaro A, Boyle SM, De BK, Setubal JC. 2012. Comparative genomics of early-diverging Brucella strains reveals a novel lipopolysaccharide biosynthesis pathway. mBio. 3(5):e00246–12. 10.1128/mBio.00388-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellaire BH, Elzer PH, Baldwin CL, Roop RM., II 1999. The siderophore 2,3-dihydroxybenzoic acid is not required for virulence of Brucella abortus in BALB/c mice. Infect. Immun. 67:2615–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellaire BH, Elzer PH, Hagius S, Walker J, Baldwin CL, Roop RM., II 2003. Genetic organization and iron-responsive regulation of the Brucella abortus 2,3-dihydroxybenzoic acid biosynthesis operon, a cluster of genes required for wild-type virulence in pregnant cattle. Infect. Immun. 71:1794–1803. 10.1128/IAI.71.4.1794-1803.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellaire BH, Elzer PH, Baldwin CL, Roop RM., II 1999. The siderophore 2,3-dihydroxybenzoic acid is not required for virulence of Brucella abortus in BALB/c mice. Infect. Immun. 67:2615–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez Carrero MI, Sangari FJ, Aguero J, Garcia Lobo JM. 2002. Brucella abortus strain 2308 produces brucebactin, a highly efficient catecholic siderophore. Microbiology 148:353–360 [DOI] [PubMed] [Google Scholar]
- 53.Anderson ES, Paulley JT, Roop RM., II 2008. The AraC-like transcriptional regulator DhbR is required for maximum expression of the 2,3-dihydroxybenzoic acid biosynthesis genes in Brucella abortus 2308 in response to iron deprivation. J. Bacteriol. 190:1838–1842. 10.1128/JB.01551-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanc-Potard AB, Lafay B. 2003. MgtC as a horizontally-acquired virulence factor of intracellular bacterial pathogens: evidence from molecular phylogeny and comparative genomics. J. Mol. Evol. 57:479–486. 10.1007/s00239-003-2496-4 [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Rodionov DA, Gelfand MS, Gladyshev VN. 2009. Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genomics 10:78. 10.1186/1471-2164-10-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sangari FJ, Cayon AM, Seoane A, Garcia-Lobo JM. 2010. Brucella abortus ure2 region contains an acid-activated urea transporter and a nickel transport system. BMC Microbiol. 10:107. 10.1186/1471-2180-10-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jubier-Maurin V, Rodrigue A, Ouahrani-Bettache S, Layssac M, Mandrand-Berthelot MA, Kohler S, Liautard JP. 2001. Identification of the nik gene cluster of Brucella suis: regulation and contribution to urease activity. J. Bacteriol. 183:426–434. 10.1128/JB.183.2.426-434.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corbel MJ, Hendry DM. 1985. Urease activity of Brucella species. Res. Vet. Sci. 38:252–253 [PubMed] [Google Scholar]
- 59.Foster JT, Beckstrom-Sternberg SM, Pearson T, Beckstrom-Sternberg JS, Chain PS, Roberto FF, Hnath J, Brettin T, Keim P. 2009. Whole-genome-based phylogeny and divergence of the genus Brucella. J. Bacteriol. 191:2864–2870. 10.1128/JB.01581-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tiller RV, Gee JE, Frace MA, Taylor TK, Setubal JC, Hoffmaster AR, De BK. 2010. Characterization of novel Brucella strains originating from wild native rodent species in North Queensland, Australia. Appl. Environ. Microbiol. 76:5837–5845. 10.1128/AEM.00620-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whatmore AM. 2009. Current understanding of the genetic diversity of Brucella, an expanding genus of zoonotic pathogens. Infect. Genet. Evol. 9:1168–1184. 10.1016/j.meegid.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 62.Whatmore AM, Perrett LL, MacMillan AP. 2007. Characterisation of the genetic diversity of Brucella by multilocus sequencing. BMC Microbiol. 7:34. 10.1186/1471-2180-7-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Audic S, Lescot M, Claverie JM, Cloeckaert A, Zygmunt MS. 2011. The genome sequence of Brucella pinnipedialis B2/94 sheds light on the evolutionary history of the genus Brucella. BMC Evol. Biol. 11:200. 10.1186/1471-2148-11-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Audic S, Lescot M, Claverie JM, Scholz HC. 2009. Brucella microti: the genome sequence of an emerging pathogen. BMC Genomics 10:352. 10.1186/1471-2164-10-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chaudhary A, Ganguly K, Cabantous S, Waldo GS, Micheva-Viteva SN, Nag K, Hlavacek WS, Tung CS. 2012. The Brucella TIR-like protein TcpB interacts with the death domain of MyD88. Biochem. Biophys. Res. Commun. 417:299–304. 10.1016/j.bbrc.2011.11.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Radhakrishnan GK, Harms JS, Splitter GA. 2011. Modulation of microtubule dynamics by a TIR domain protein from the intracellular pathogen Brucella melitensis. Biochem. J. 439:79–83. 10.1042/BJ20110577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radhakrishnan GK, Splitter GA. 2010. Biochemical and functional analysis of TIR domain containing protein from Brucella melitensis. Biochem. Biophys. Res. Commun. 397:59–63. 10.1016/j.bbrc.2010.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radhakrishnan GK, Yu Q, Harms JS, Splitter GA. 2009. Brucella TIR domain-containing protein mimics properties of the Toll-like receptor adaptor protein TIRAP. J. Biol. Chem. 284:9892–9898. 10.1074/jbc.M805458200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sengupta D, Koblansky A, Gaines J, Brown T, West AP, Zhang D, Nishikawa T, Park SG, Roop RM, II, Ghosh S. 2010. Subversion of innate immune responses by Brucella through the targeted degradation of the TLR signaling adapter, MAL. J. Immunol. 184:956–964. 10.4049/jimmunol.0902008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexopoulou L, Comerci DJ, Ugalde RA, Pierre P, Gorvel JP. 2008. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathog. 4:e21. 10.1371/journal.ppat.0040021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, Stappert D, Wantia N, Rodriguez N, Wagner H, Svanborg C, Miethke T. 2008. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nat. Med. 14:399–406. 10.1038/nm1734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.