Abstract
Cholera is a waterborne diarrheal disease caused by Vibrio cholerae strains of serogroups O1 and O139. Expression of the general stress response regulator RpoS and formation of biofilm communities enhance the capacity of V. cholerae to persist in aquatic environments. The transition of V. cholerae between free-swimming (planktonic) and biofilm life-styles is regulated by the second messenger cyclic di-GMP (c-di-GMP). We previously reported that increasing the c-di-GMP pool by overexpression of a diguanylate cyclase diminished RpoS expression. Here we show that c-di-GMP repression of RpoS expression is eliminated by deletion of the genes vpsR and vpsT, encoding positive regulators of biofilm development. To determine the mechanism of this regulation, we constructed a strain expressing a vpsT-FLAG allele from native transcription and translation signals. Increasing the c-di-GMP pool induced vpsT-FLAG expression. The interaction between VpsT-FLAG and the rpoS promoter was demonstrated by chromatin immunoprecipitation. Furthermore, purified VpsT interacted with the rpoS promoter in a c-di-GMP-dependent manner. Primer extension analysis identified two rpoS transcription initiation sites located 43 bp (P1) and 63 bp (P2) upstream of the rpoS start codon. DNase I footprinting showed that the VpsT binding site at the rpoS promoter overlaps the primary P1 transcriptional start site. Deletion of vpsT significantly enhanced rpoS expression in V. cholerae biofilms that do not make HapR. This result suggests that VpsT and c-di-GMP contribute to the transcriptional silencing of rpoS in biofilms prior to cells entering the quorum-sensing mode.
INTRODUCTION
Cholera is an acute waterborne diarrheal disease caused by strains of the Gram-negative bacterium Vibrio cholerae of serogroup O1 of the classical and El Tor biotypes and serogroup O139. The capacity of V. cholerae to survive and persist in fresh and estuarine waters is a major obstacle to the eradication of this illness. In nature, vibrios are subjected to numerous physical and chemical environmental stresses, which include nutrient limitation, extreme temperatures, high salinity, and oxidative stress. The rpoS gene encodes the RNA polymerase σS subunit, which initiates transcription of numerous genes involved in the environmental stress response (1). Expression of rpoS in Escherichia coli is controlled at the levels of transcription, translation, and protein stability (1). The regulation of rpoS expression in V. cholerae appears to be similarly complex but less understood. Similar to E. coli, V. cholerae rpoS mutants are more sensitive to carbon starvation, high osmolarity, and oxidative stresses (2).
The persistence of vibrios in the environment is also facilitated by the formation of sessile biofilm communities (3–6). Biofilm formation and adoption of a rugose colonial morphology correlate with the production of V. cholerae exopolysaccharide (Vps) (7). The V. cholerae rugose colonial variant described previously by White (8) is more resistant to chlorinated water (9, 10) and to osmotic and oxidative stresses (7, 11).
The transition between motile and biofilm life-styles is regulated by the second messenger cyclic di-GMP (c-di-GMP), which activates biofilm formation and inhibits motility (12–15). Expression of the vps genes encoding the biofilm matrix exopolysaccharide is enhanced at high intracellular c-di-GMP levels due to the induction and increased activity of two c-di-GMP binding regulators, VpsR and VpsT (16, 17). VpsR is a homologue of the NtrC subclass of response regulators which binds to the vpsT promoter to activate its transcription (18). VpsT is a LuxR-type regulator that senses c-di-GMP to directly activate vps gene transcription and diminish motility (7, 19). At a high cell density, the quorum-sensing regulator HapR inhibits biofilm formation by repressing vpsT and decreasing the c-di-GMP pool (20). In parallel, quorum sensing (HapR) positively influences the expression of rpoS (21, 22). These findings suggest that biofilm development and the general stress response are inversely regulated. Consistent with this interpretation, we found that elevated levels of intracellular c-di-GMP, a condition which favors biofilm development, diminished the expression of RpoS (22). Furthermore, we showed that the negative regulation of RpoS expression by c-di-GMP was apparently counteracted by HapR (22).
Although expression of RpoS and biofilm development contribute to environmental stress survival, the interrelationship between both stress response strategies has not been investigated. In this study, we show that elevated c-di-GMP levels repress rpoS transcription by inducing VpsT, which binds to the rpoS promoter in a c-di-GMP-dependent manner at a location overlapping rpoS transcription initiation sites. We show that in planktonic cells grown in LB medium, deletion of vpsT does not affect RpoS expression and that HapR enhances rpoS independent of VpsT. In biofilms, deletion of vpsT significantly enhanced RpoS expression when hapR was absent. Furthermore, our results suggest that HapR could have a negative effect on RpoS expression in biofilms that is overridden by repression of the negative regulator VpsT. We propose a model for the interplay between HapR and VpsT in the regulation of RpoS.
MATERIALS AND METHODS
Strains and media.
V. cholerae mutants used in this study were derived from El Tor biotype strain C7258 and are described in Table 1. Escherichia coli TOP10 (Invitrogen, Life Technologies) and S17-1λpir (23) were used for cloning purposes. E. coli strain ER2566 (New England BioLabs) was used for purification of VpsT. V. cholerae strains were grown in tryptic soy broth (TSB) or LB medium at 37°C with agitation (225 rpm). For assays of stress survival under conditions of nutrient limitation, V. cholerae was grown in morpholinepropanesulfonic acid (MOPS) minimal medium. When necessary, culture media were supplemented with ampicillin (Amp) (100 μg/ml), chloramphenicol (Cm) (10 μg/ml), kanamycin (Km) (50 μg/ml), tetracycline (Tet) (5 μg/ml), rifampin (Rf) (150 μg/ml), polymyxin B (PolB) (100 units/ml), isopropyl-β-d-thiogalactopyranoside (IPTG) (20 μg/ml), or 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) (20 μg/ml). Plasmid DNA was introduced into V. cholerae by electroporation (24). Plasmids and oligonucleotide primers used throughout this work are described in Tables 1 and 2, respectively.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| E. coli S17-1λpir | F− recA hsdR RP4-2 (Tc::Mu) (Km::Tn7) lysogenized with λpir | 23 |
| V. cholerae | ||
| C7258 | Wild-type O1, El Tor biotype, Ogawa | Peru, 1991 |
| C7258ΔlacZ | C7258 lacZ deletion mutant | 48 |
| AJB50 | C7258 ΔrpoS | 52 |
| AJB51 | C7258 ΔhapR | 52 |
| AJB51ΔlacZ | AJB51 ΔlacZ deletion mutant | 48 |
| HX11 | C7258 ΔlacZ ΔrpoS::rpoS-lacZ | This study |
| HX12 | C7258 ΔlacZ ΔhapR ΔrpoS::rpoS-lacZ | This study |
| HX13 | C7258 ΔvpsR | This study |
| HX14 | C7258 ΔhapR ΔvpsR | This study |
| HX15 | C7258 ΔlacZ ΔvpsR ΔrpoS::rpoS-lacZ | This study |
| HX16 | C7258 ΔlacZ ΔhapR ΔvpsR ΔrpoS::rpoS-lacZ | This study |
| HX17 | C7258 ΔvpsT | This study |
| HX18 | C7258 ΔvpsR ΔvpsT | This study |
| HX19 | C7258 ΔlacZ ΔvpsT ΔrpoS::rpoS-lacZ | This study |
| HX20 | C7258 ΔlacZ ΔhapR ΔvpsT ΔrpoS::rpoS-lacZ | This study |
| HX21 | C7258 ΔlacZ ΔvpsR ΔvpsT ΔrpoS::rpoS-lacZ | This study |
| HX03 | C7258 ΔhapR ΔvpsT | 22 |
| HX22 | C7258 vpsT::pCVDVpsT-FLAG | This study |
| HX23 | C7258 ΔvpsR vpsT::pCVDVpsT-FLAG | This study |
| Plasmids | ||
| pVIK111 | Suicide vector with promoterless lacZ; oriR6K Kmr | 27 |
| pCR-RpoS | 0.34-kb DNA fragment containing the rpoS promoter in pCR2.1 | This study |
| pRpoS-Up | 0.8-kb SmaI-SalI DNA fragment encoding the rpoS promoter and the N-terminal 223 amino acids of RpoS in pUC19 | This study |
| pRpoS-Down | 0.45-kb SphI-XbaI DNA fragment containing the 3′ terminus of the rpoS ORF in pUC19 | This study |
| pLacZ | 3.2-kb SalI-SphI DNA fragment encoding the lacZ ORF from pVIK111 in pUC19 | This study |
| pRpoS-Up-lacZ | 3.2-kb SalI-SphI DNA fragment from pLacZ in pRpoS-Up | This study |
| pCVDRpoS-Up-lacZ | 4-kb SmaI-SphI DNA fragment from pRpoS-Up-lacZ in pCVD442 | This study |
| pCVDΔRpoS-lacZ | 0.45-kb SphI-XbaI DNA fragment from pRpoS-Down in pCVDRpoS-Up-lacZ | This study |
| pUC-VpsR-UP | 0.5-kb XbaI-SalI DNA fragment containing the 5′ terminus of vpsR in pUC19 | This study |
| pUC-VpsR-Down | 0.5-kb SalI-SphI DNA fragment containing the 3′ terminus of vpsR in pUC19 | This study |
| pUCΔvpsR | XbaI-SalI and SalI-SphI DNA fragments flanking the vpsR ORF sequentially cloned into pUC19 | This study |
| pCVDΔvpsR | XbaI-SphI fragment harboring a vpsR deletion in pCVD442 | This study |
| pAT1662 | DGC VCA0956 cloned into pBAD33 | 25 |
| pCR2.1 | Bacterial expression vector for TA cloning (Ampr) | Invitrogen |
| pCR-VpsT1 | 0.67-kb XhoI-KpnI DNA fragment encoding the vpsT ORF in pCR2.1 | This study |
| pCR-VpsT2 | 0.67-kb NdeI-SapI PCR fragment encoding the vpsT ORF in pCR2.1 | This study |
| pTXB1 | Expression vector for construction of in-frame fusions with the intein/chitin binding domain | New England BioLabs |
| pTXB1-VpsT | Vector pTXB1 containing a VpsT-intein fusion | This study |
| pFLAG-CTC | Vector for cytoplasmic expression of C-terminal FLAG fusions | Sigma-Aldrich |
| pFLAG-CTC* | pFLAG-CTC with modified multicloning site lacking the SalI restriction site | This study |
| pTT3 | rrnB T1T2 transcription terminator in pUC19 | 28 |
| pTT7 | 0.43-kb SalI-BamHI DNA fragment encoding the rrnB T1T2 transcription terminator in pUC19 | This study |
| pTT7VpsT-FLAG | 0.73-kb BamHI-SmaI DNA fragment containing the vpsT ORF and FLAG epitope coding region in pTT7 | This study |
| pCVDVpsT-FLAG | SalI-SmaI vpsT-FLAG and rrnB T1T2 fragment in pCVD442 | This study |
| pRpoSLac5 | An SphI-HindIII DNA fragment containing the rpoS promoter region cloned between the rrnB T1T2 transcription terminator and a promoterless lacZ gene in pKRZ1a | 29 |
| pACTluz-RpoS | rrnB T1T2-rpoS promoter fragment from pRpoSLac5, promoterless luxCDABE operon, and OriT in pACYC184 | This study |
| pCVDΔvpsT-Km | pCVD442-based vector with vpsT deletion and Km insertion | 22 |
| pCMW75 | V. harveyi IPTG-inducible DGC QrgB expression vector | 20 |
See reference 53.
TABLE 2.
Oligonucleotide primers
Manipulation of c-di-GMP pools.
To artificially increase the c-di-GMP pool, V. cholerae strains were transformed with plasmid pAT1662 expressing the diguanylate cyclase (DGC) VCA0956 from an arabinose promoter (25) or with plasmid pCMW75 expressing the V. harveyi DGC QrgB from an IPTG-inducible promoter (20). DGC expression was induced by the addition of 0.2% l-arabinose or 0.5 mM IPTG, as previously described (22).
Construction of rpoS-lacZ fusions.
For strain construction, we used the suicide vector pCVD442 (26) and sucrose selection as previously described (22). To construct strains expressing a chromosomally integrated rpoS-lacZ fusion from native transcription and translation initiation signals, we amplified a DNA fragment spanning the rpoS promoter and two-thirds of its open reading frame (ORF) N terminus by using primers RpoS-F3 and RpoS-R3. The resulting PCR fragment was cloned as an SmaI-SalI fragment into pUC19 to generate plasmid pRpoS-Up. The 3′ end of the rpoS ORF was also amplified by using primers RpoS-F4 and RpoS-R4 and cloned as an SphI-XbaI fragment into pUC19 to generate pRpoS-Down. A promoterless lacZ gene was amplified from plasmid pVIK111 (27) by using primers LacZ-F1 and LacZ-R1 and Pfu DNA polymerase AD (Agilent Technologies) and cloned as an SalI-SphI fragment into pUC19 to generate pLacZ. Next, the SalI-SphI lacZ fragment was inserted into pRpoS-Up to yield pRpoS-Up-LacZ. Finally, the 5′ rpoS-lacZ and 3′ rpoS fragments were sequentially cloned into pCVD442 to generate the final suicide vector, pCVDΔRpoS-LacZ. This plasmid was transferred from S17-1λpir to strains C7258ΔlacZ and AJB51ΔlacZ. Exconjugants were selected in LB agar containing Amp and PolB, and strains HX11 and HX12, containing the rpoS-lacZ fusion in place of the wild-type rpoS allele, were obtained by sucrose selection.
Construction of vpsR and vpsT mutants.
To construct vpsR deletion mutants, we amplified DNA fragments flanking the vpsR locus from C7258 genomic DNA using primer sets VpsR-UF/VpsR-UR and VpsR-DF/VpsR-DR and the Advantage 2 PCR kit (BD Biosciences Clontech). The PCR products were sequentially cloned into pUC19 to yield pUCΔvpsR and were confirmed by DNA sequencing. Next, the chromosomal DNA fragment harboring the vpsR deletion was transferred to the suicide vector pCVD442 to yield pCVDΔvpsR. Finally, pCVDΔvpsR was transferred by conjugation from E. coli S17-1λpir to strains C7258, AJB51, HX11, and HX12 (Table 1). V. cholerae exconjugants were selected in LB agar containing Amp and PolB, and segregant strains HX13, HX14, HX15, and HX16 (Table 1) were isolated by sucrose selection. To construct vpsT deletion mutants, the suicide vector pCVDΔVpsT-Km (22) was transferred from S17-1λpir to C7258, HX11, HX12, HX13, and HX15. vpsT deletion strains HX17, HX19, HX20, HX18, and HX21 (Table 1) were obtained as described above.
Construction of strains expressing VpsT-FLAG.
To construct a strain expressing a VpsT-FLAG protein from native transcription and translation initiation signals, we amplified the vpsT ORF lacking its stop codon from C7258 genomic DNA using primers VpsT-F1 and VpsT-R2. The resulting PCR fragment was cloned into linearized plasmid pCR2.1 by using the TOPO TA cloning kit (Invitrogen, Life Technologies) to yield pCR-VpsT1. The SalI site in the polylinker of pFLAG-CTC (Sigma-Aldrich) was inactivated by using the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies). A C→G mutation was introduced into this site by using primers SalI-F and SalI-R to yield pFLAG-CTC*. An XhoI-KpnI DNA fragment encoding the VpsT ORF was then inserted into pFLAG-CTC* in frame with the FLAG epitope. To construct plasmid pTT7, a SalI-BamHI DNA fragment containing the rrnB T1T2 transcription terminator was amplified from pTT3 (28) by using primers RrnB-F1 and RrnB-R1 and cloned into pUC19. The VpsT-FLAG fusion was then retrieved by PCR with primers VpsT-FLAG-F and VpsT-FLAG-R and inserted into plasmid pTT7 upstream of the rrnB T1T2 transcription terminator to yield pTT7VpsT-FLAG. Finally, the vpsT-FLAG-rrnB T1T2 cassette was transferred to the suicide vector pCVD442 to yield pCVDVpsT-FLAG. The resulting suicide vector was transferred from S17-1λpir to V. cholerae strains C7258 and HX13 by conjugation, and the exconjugants HX22 and HX23 were selected on LB plates containing Amp and PolB. Integration by homologous recombination within the vpsT locus was confirmed by PCR using primer VpsT-P, which anneals to the vpsT promoter region (not present in pCVDVpsT-FLAG), and primer VpsT-FLAG-R, which anneals to the FLAG epitope. The resulting PCR products were further confirmed by DNA sequencing, and the expression of VpsT-FLAG was established by Western blotting.
Construction of rpoS-luxCDABE fusions to determine rpoS expression in intact biofilms.
To determine rpoS expression in intact V. cholerae biofilms, we constructed a plasmid expressing an rpoS-luxCDABE promoter fusion. To this end, a fragment containing the rrnB T1T2 transcription terminator and the rpoS promoter region was amplified from pRpoSLac5 (29) by using primers RpoS-XhoI-F and RpoS-XhoI-R. Next, the luxCDABE operon lacking its promoter was amplified from cosmid pBB1 (30) by using primers Lux-5 and Lux-3. Finally, the rrnB T1T2-rpoS promoter fragment, the promoterless luxCDABE operon, and a fragment encoding OriT derived from pCVD442 were sequentially cloned into pACYC184 (New England BioLabs) to yield pACTluz-RpoS. This plasmid was introduced into C7258, AJB51, HX13, HX14, HX17, HX03, and HX18 by conjugation using strain S17-1λpir as a donor. Cultures of V. cholerae strains containing the rpoS-luxCDABE reporter plasmid grown overnight were inoculated at a 1:50 dilution into LB containing 5 μg/ml Tet and incubated in 96-well black-wall clear-bottom microtiter plates (Costar) for 24 h at 37°C. Planktonic cells were discarded, and the plates were rinsed 3 times with sterile phosphate-buffered saline (PBS) (pH 7.4). Light production was measured with a Synergy 2 BioTek plate reader. Biofilm mass was then determined by using the standard crystal violet method, as previously described (31). Finally, the amount of rpoS-dependent light produced in the biofilm was normalized by the crystal violet OD570 (optical density at 570 nm) reading.
Chromatin immunoprecipitation.
To determine rpoS promoter occupancy by VpsT in the cell, strain HX22 was transformed with plasmid pAT1662 (25). The transformant was grown in TSB to an OD600 of 2.0. At this time, cultures were divided in halves; one half was induced with l-arabinose to increase the c-di-GMP pool, and the second half was used as a control. Cultures were incubated at 37°C with shaking for 4 h. Chromatin immunoprecipitation (ChIP) was conducted with the anti-FLAG M2 monoclonal antibody (MAb) (Sigma-Aldrich) as previously described (31, 32). Briefly, cultures were sequentially treated with rifampin (20 min at 37°C), 1% formaldehyde (cross-linking) (10 min at 30°C), and 227 mM glycine (30 min at 4°C). The cells were collected by centrifugation, washed with PBS, and lysed by incubation in Tris-HCl buffer containing RNase A and Ready-Lyse lysozyme (Epicentre Biotechnologies). The lysate was mixed with 1 volume of double-strength immunoprecipitation (IP) buffer (31, 32), and DNA was broken down by sonication to a molecular weight range of 150 to 1,000 bp. The cell debris was removed, and the lysate was diluted 10-fold in IP buffer. At this stage, a 10-μl input sample was saved as a reference and PCR efficacy control. Protein-DNA complexes were immunoprecipitated by overnight incubation with anti-FLAG M2 monoclonal antibody or the unrelated mouse monoclonal antibody G3A1 IgG1 isotype control (Cell Signaling Technology) for a mock ChIP. The antibody-protein-DNA complexes were pulled down with protein A/G agarose beads (Imgenex, San Diego, CA). The beads were washed with Tris-HCl buffer containing 2% Triton X-100, collected in Spin-X centrifuge tube filters, and washed sequentially with IP buffer containing 600 mM NaCl, IP buffer, and Tris-EDTA (TE) buffer. The immunoprecipitated complexes were eluted from the beads by incubation at 65°C in TE buffer containing 1% SDS. After the reversal of cross-linking, proteins were removed by treatment with proteinase K, and immunoprecipitated DNA was purified by using the MiniElute PCR purification kit (Qiagen). Immunoprecipitated DNA was qualitatively detected by PCR and agarose gel electrophoresis using primers RpoS-P4 and RpoS-P2 to amplify the rpoS promoter region. Real-time quantitative PCR (qPCR) was used to quantitate promoter occupancy by VpsT-FLAG as described previously (31, 32). The quantity of immunoprecipitated DNA was calculated as a percentage of the input DNA by using the formula , where CT is the fractional threshold cycle of the input and IP DNAs. The relative IP was calculated by standardizing the IP of each sample by the IP of a mock ChIP. The rpsL promoter and a region of the aphA promoter, which is not regulated by VpsT (18), were used as negative controls. These DNAs were amplified with primer sets RpsL-F/RpsL-R and AphA-50/AphA+150, respectively. A region within the vpsL promoter to which VpsT binds to enhance transcription (19) was used as a positive control and amplified with primers VpsL-P1 and VpsL-P2.
Purification of VpsT.
The vpsT ORF was amplified by using primers VpsT-F and VpsT-R (Table 2). The amplified PCR fragment was cloned into the pCR2.1 vector by using the TA cloning kit (Invitrogen, Life Technologies) to generate pCR-VpsT2. Subsequently, a 673-bp NdeI-SapI restriction fragment encoding the vpsT ORF was transferred from pCR-VpsT2 into similarly digested pTXB1 (New England BioLabs) to generate pTXB1-VpsT. The vpsT-intein fusion in pTXB1-VpsT was confirmed by DNA sequencing using T7 universal and Mxe Intein II reverse primers (New England BioLabs). VpsT was then expressed from the T7 promoter in E. coli ER2566 and purified by using the Impact-CN protein purification system (New England BioLabs) according to the kit's accompanying protocol. Briefly, cells of E. coli ER2566 containing plasmid pTXB1-VpsT were grown in shaken flasks (220 rpm) containing 100 ml LB (pH 7.5) supplemented with Amp at 37°C to an OD600 of 0.5 to 0.7. At this point, the expression of VpsT was induced with IPTG (0.05 mM), and the culture was incubated for 18 h at 14°C with agitation (220 rpm). The cells were collected by centrifugation; resuspended in a solution containing 20 mM Tris-HCl (pH 8.0), 0.5 M NaCl, and 1 mM EDTA; and disrupted by sonication. The cell debris was removed by centrifugation, and VpsT was purified by affinity chromatography using a gravity flow column (Qiagen) packed with chitin resin. VpsT-intein cleavage was accomplished by incubation with 100 mM dithiothreitol (DTT) for 26 h at 6°C. VpsT-containing fractions were combined and concentrated by using a centrifugal filter unit (Millipore). Finally, the protein preparation was exchanged into a buffer containing 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 60 mM NaCl, and 0.1 mM DTT by using PD-10 columns (Amersham Biosciences). Protein purity was estimated to be >95% by densitometry analysis of Coomassie-stained gels with the aid of ImageQuant TL software (GE Healthcare).
Electrophoresis mobility shift assays.
Electrophoresis mobility shift assays (EMSAs) were conducted by using a second-generation digoxigenin (DIG) gel shift kit (Roche Applied Sciences), as previously described (31, 32). Reaction mixtures, consisting of 20 mM HEPES (pH 7.6), 1 mM EDTA, 10 mM (NH4)2SO4, 1 mM DTT, 0.2% Tween 20, 30 mM KCl, 75 mM NaCl, 25 ng of calf thymus DNA (Sigma-Aldrich), 5.4 fmol of DIG-labeled target DNA, purified VpsT (150 to 1,000 nM), and (when indicated) 50 μM c-di-GMP (Biolog, Bremen, Germany) in a volume of 20 μl, were incubated for 25 min at 30°C. Protein-DNA complexes were separated by electrophoresis in 5% Mini-Protean Tris-borate-EDTA (TBE) precast gels (Bio-Rad) and transferred onto nylon membranes (Roche). DIG-labeled DNA was detected with an antidigoxigenin Fab fragment-alkaline phosphatase (AP) conjugate (Roche) and chemiluminescence. Primers RpoS-P4 and RpoS-P2 were used to amplify the rpoS promoter region under study. DNA binding was quantitated by densitometry analysis of the gels using ImageQuant TL software. The DNA-VpsT equilibrium dissociation constant (KD) was estimated by nonlinear regression, as described previously (33), using GraphPad Prism 5.0 (GraphPad, San Diego, CA).
DNase I footprinting.
DNase footprinting was conducted as described previously (34). An rpoS promoter fragment was amplified from C7258 chromosomal DNA with primer pair RpoS-P4 and RpoS-P2. The fragment was ligated into pCR2.1 to generate pCR-RpoS and sequenced. Plasmid pCR-RpoS was used as a template to generate a 334-bp probe that encompasses bases −307 to +27 of rpoS with reference to the start codon. Fluorescently labeled DNA was generated by PCR with primers RpoS-FAM (6-carboxyfluorescein) and RpoS-HEX (6-carboxy-2′,4,4′,5′,7,7′-hexachlorofluorescein). Binding reactions were performed in a solution of final volume 100 μl containing 200 ng of fluorescently labeled DNA, 15 μg of purified VpsT protein, 20 mM HEPES (pH 7.6), 1 mM EDTA, 10 mM (NH4)2SO4, 1 mM DTT, 0.2% (wt/vol) Tween 20, 30 mM KCl, 37.5 mM NaCl, 125 ng of calf thymus DNA, and 100 μM c-di-GMP. After incubation at 30°C for 25 min, protein-DNA complexes were digested with 10 μl of 0.067 U/μl DNase I (Promega) for 5 min at 30°C. Reactions were stopped with 2 mM EGTA, followed by heat inactivation at 68°C for 10 min. Control reactions were performed with 15 μg of bovine serum albumin (BSA) instead of VpsT. The DNA fragments were purified with the MiniElute PCR purification kit (Qiagen) and eluted in 20 μl H2O. The digested DNAs (0.5 μl) were mixed with 10 μl HiDi formamide (Applied Biosystems) and 0.1 μl GeneScan-500 LIZ size standards (Applied Biosystems) and analyzed with the 3730 DNA analyzer running in the default genotyping module. All DNA patterns were analyzed with GeneMapper software v. 4.0 (Applied Biosystems) and were horizontally aligned in the sample plot window (microsatellite default plot) through the use of the size standards.
Primer extension analysis.
The rpoS transcriptional start sites were identified by primer extension using a 5′-fluorescently labeled primer and analysis on an automated capillary electrophoresis instrument, as described previously (35), with modifications. Briefly, strain C7258 was grown in LB at 37°C to an OD600 of 2.0, and 1 ml of the culture was resuspended in 200 μl of RNAlater solution (Ambion). DNA-free total RNA was isolated by using the RNeasy Plus Minikit (Qiagen), treated with DNase I (Qiagen RNase-free DNase set), and concentrated with the Qiagen RNeasy MiniElute Cleanup kit. RNA was reverse transcribed into cDNA with avian myeloblastosis virus reverse transcriptase (AMV RT; Promega) by using the RpoS-HEX primer in two steps. For the annealing step, the reaction mixture, consisting of 30 μg total RNA and 20 pmol primers in AMV RT reaction buffer, was incubated at 90°C (3 min), 53°C (60 min), and 25°C (10 min). For primer extension, the reaction mixture was supplemented with 1 mM each deoxynucleoside triphosphate (dNTP), 40 U RNase inhibitor (Ambion), 2.8 mM sodium pyrophosphate, and 10 U AMV RT and further incubated for 60 min at 42°C. Primer extension products were purified by using the MiniElute PCR purification kit (Qiagen) and resuspended in 15 μl H2O. One microliter of the primer extension sample was combined with 10 μl HiDi and 0.1 μl GeneScan 500 LIZ size standard (Applied Biosystems) and detected with a 3730 capillary DNA analyzer (Applied Biosystems) running in the default genotyping module. The length and abundance (height and area below the peaks) of the HEX-labeled cDNA primer extension products were analyzed by using GeneMapper software v. 4.0.
Generation of DNA sequence ladders.
To accurately assign a nucleotide base to the peaks detected in the primer extension and DNase I footprint assays, a sequence ladder was generated by using the Thermo Sequenase Dye Primer Manual cycle sequencing kit (USB Corporation). Briefly, a DNA template was generated by PCR using primers RpoS-P3 and RpoS-P2. Sequencing reactions were conducted with 200 fmol of template DNA and 2 pmol of either primer RpoS-FAM (coding strand) or RpoS-HEX (template strand) according to the manufacturer's instructions. Each reaction mixture (G, A, T, and C) was diluted 5-fold in water, and 1 μl was loaded onto the 3730 DNA analyzer. The electropherograms of the sequencing reactions were horizontally aligned with those generated in the primer extension and DNase I footprint analyses by using GeneMapper 4.0.
Western blot analysis.
To detect expression of VpsT-FLAG, a volume of culture (μl) containing 0.5 OD600 units of cells was centrifuged, and the pellet was resuspended in 0.1 ml of Laemmli sample buffer (Bio-Rad Laboratories). The cell suspension was placed into a boiling-water bath for 10 min, and the cell debris was removed by centrifugation. Ten microliters of each protein sample was separated by using a Mini-Protean TGX precast gel (Bio-Rad) and subsequently transferred onto polyvinylidene difluoride membranes. The expression level of VpsT-FLAG was determined with monoclonal anti-FLAG M2-peroxidase (Sigma-Aldrich) and the BM Bioluminescence Western blotting kit (Roche Applied Science). To detect RpoS protein in V. cholerae biofilms, cultures of wild-type and mutant strains grown overnight were diluted in fresh LB broth to an OD600 of 0.05, and 300-μl aliquots of the diluted cultures were dispensed in quadruplicate onto 24-well cell culture plates (Corning). Biofilms were allowed to develop for 22 h at 30°C. Next, planktonic cells were discarded, biofilms were washed twice with PBS, and the cells were lysed and recovered in 100 μl of Laemmli sample buffer. Samples (30 μl) were placed into a boiling-water bath for 10 min and loaded onto Mini-Protean TGX gels for Western blot detection. RpoS was detected by using a rabbit polyclonal antibody raised against purified RpoS protein, as previously described (32).
Enzyme assays.
β-Galactosidase activity was measured as described previously (36), using the substrate o-nitrophenyl-β-d-galactopyranoside (ONPG). Specific activities are given in Miller units {1,000[OD420/(t × v × OD600)]}, where t is the reaction time and v is the volume of enzyme extract per reaction.
RESULTS
Repression of rpoS expression by c-di-GMP requires vpsR and vpsT.
In a previous study, we showed that artificially increasing the c-di-GMP pool diminished the expression of an rpoS-FLAG allele from native transcription and translation signals in Western blots and that this effect was more pronounced in the absence of HapR (22). To quantify the strength of this regulation, we constructed strains containing chromosomally integrated rpoS-lacZ translational fusions. Consistent with previous results (21, 22), the expression level of rpoS was significantly lower in the hapR mutants (Fig. 1). Increasing the c-di-GMP pool by overexpression of the DGC VCA0956 diminished the expression of rpoS-lacZ in both the wild-type and ΔhapR genetic backgrounds (Fig. 1). We hypothesized that the effect of c-di-GMP on rpoS expression could involve the regulators VpsR and/or VpsT, which directly sense c-di-GMP (18, 19). To test this possibility, we introduced ΔvpsR and/or ΔvpsT mutations into our wild-type and ΔhapR reporter strains and examined the effect of increasing the c-di-GMP pool on rpoS expression. As shown in Fig. 1, increasing the c-di-GMP pool did not diminish rpoS expression in the vpsR and vpsT mutants. We conclude that increasing the c-di-GMP pool negatively affects rpoS expression by inducing and augmenting the activity of these regulators. This result suggests a novel regulatory connection between quorum sensing (HapR), VpsR/VpsT, and the general stress response. We note, however, that deletion of vpsR and/or vpsT did not affect rpoS expression in planktonic cells grown in rich bacteriological media without artificially enhancing the c-di-GMP pool (Fig. 1).
FIG 1.
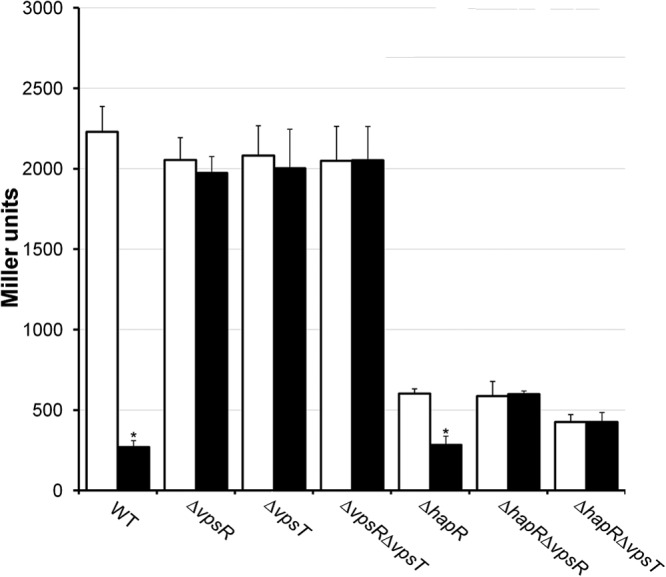
Repression of rpoS by c-di-GMP requires VpsR and VpsT. Strain HX11, containing a chromosomally integrated rpoS-lacZ fusion (wild type [WT]), and isogenic mutant strains HX15 (ΔvpsR), HX19 (ΔvpsT), HX21 (ΔvpsR ΔvpsT), HX12 (ΔhapR), HX16 (ΔhapR ΔvpsR), and HX20 (ΔhapR ΔvpsT) were transformed with plasmid pAT1662 expressing the DGC VCA0956. Expression of VCA095 was induced as described in Materials and Methods, and β-galactosidase activity was measured as an indicator of rpoS expression. Each value represents the mean for six independent cultures, and error bars indicate standard deviations (*, significantly different from uninduced cultures [P < 0.01]). Open bars, uninduced cultures; filled bars, induced cultures.
Repression of rpoS expression by c-di-GMP renders V. cholerae more susceptible to stress.
V. cholerae rpoS mutants are more sensitive to oxidative, osmotic, and nutrient limitation stresses (2). We predicted that the negative effect of enhancing the c-di-GMP pool on rpoS expression should render V. cholerae more susceptible to the above-mentioned environmental stressors. To examine this possibility, we transformed strain C7258 with plasmid pCMW75 expressing the V. harveyi DGC QrgB from an IPTG-inducible promoter (20). Overexpression of this DGC does not require induction by a usable carbon source and results in a 10-fold increase in c-di-GMP content (20). As shown in Fig. 2, enhancing the c-di-GMP pool rendered strain C7258 more susceptible to hydrogen peroxide stress, 2 M NaCl, and carbon starvation. In all cases, the percent survival in IPTG-induced cultures was significantly lower than that in the controls (P < 0.05) at the final time point. To confirm that the reduction in survival was due to negative regulation of rpoS by c-di-GMP, we introduced pCMW75 into strain AJB50 (C7258ΔrpoS). This transformant was very sensitive to the above-described stresses without enhancing the c-di-GMP pool, precluding its comparison to the wild type under equivalent conditions. We noticed that incubation of C7258 with 2 M NaCl (40 min) diminished viability to 5 and 0.25% in the control and c-di-GMP-enhanced cultures, respectively (Fig. 2A). However, in AJB50, this stress diminished viability to 3.3% (control) and 3.0% (c-di-GMP-enhanced) in 15 min. Incubation of C7258 with hydrogen peroxide (20 min) diminished viability to 3% (control) and 0.06% (c-di-GMP-enhanced) (Fig. 2B). With AJB50 subjected to the same stress for 10 min, viability dropped to <0.01%, and increasing the c-di-GMP pool did not result in an additional loss. Finally, incubation of C7258 in MOPS medium lacking a carbon source (96 h) diminished viability to 3.5% and 1.5% in the control and c-di-GMP-enhanced cultures, respectively (Fig. 2C). Under identical conditions, the viability of AJB50 dropped to 0.56% (control) and 0.43% (c-di-GMP enhanced). No statistical differences could be demonstrated (n = 3) between controls and c-di-GMP-enhanced cultures in strain AJB50. These results strongly suggest that the diminished stress survival observed when the c-di-GMP pool is enhanced is due to lower expression levels of RpoS.
FIG 2.
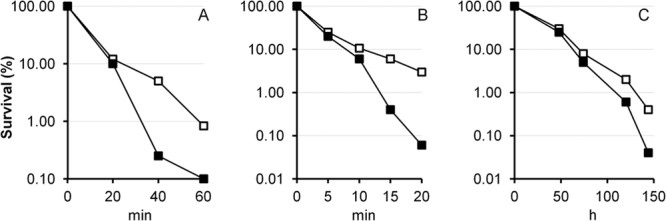
Repression of rpoS transcription by c-di-GMP results in enhanced susceptibility to environmental stressors. V. cholerae C7258 was transformed with plasmid pCMW75 encoding an IPTG-inducible DGC from V. harveyi. Survival in the presence of 2 M NaCl (A), 1 mM H2O2 (B), and carbon starvation in MOPS minimal medium (C) was determined at different time points by dilution plating on LB agar. Symbols: □, control; ■, induced.
VpsT binds to the rpoS promoter in a c-di-GMP-dependent manner.
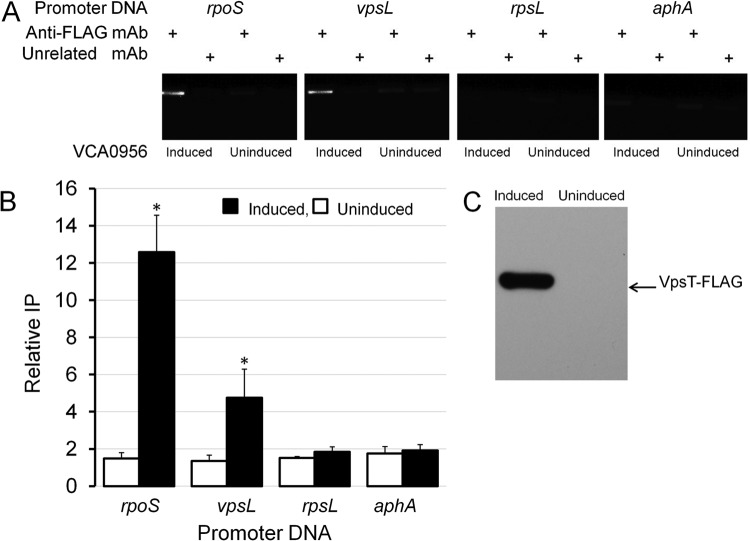
It was recently shown that VpsR directly binds c-di-GMP to induce the transcription of vpsT (18). Based on this report, we considered that VpsT could act as the downstream regulator repressing rpoS expression. To characterize the interaction between VpsT and the rpoS promoter, we cloned, expressed, and purified VpsT. In Fig. 3, we show that VpsT binds to the rpoS promoter in vitro and that binding requires c-di-GMP. In the presence of c-di-GMP, most of the rpoS promoter DNA was shifted at a concentration of VpsT of 600 nM. Based on densitometry quantification of EMSA data, we estimated the DNA-VpsT equilibrium dissociation constant to be 72.2 ± 11 nM. We constructed a strain expressing a chromosomally integrated vpsT-FLAG allele from its native transcription and translation signals and used ChIP to determine if VpsT-FLAG interacts with the rpoS promoter in vivo. In Fig. 4, we show that enhancing the c-di-GMP pool results in the expression of VpsT-FLAG (Fig. 4C) and that VpsT-FLAG occupies the rpoS promoter (Fig. 4AB). Taken together, these results suggest that VpsT can bind the rpoS promoter under conditions in which the cellular c-di-GMP pool is increased.
FIG 3.
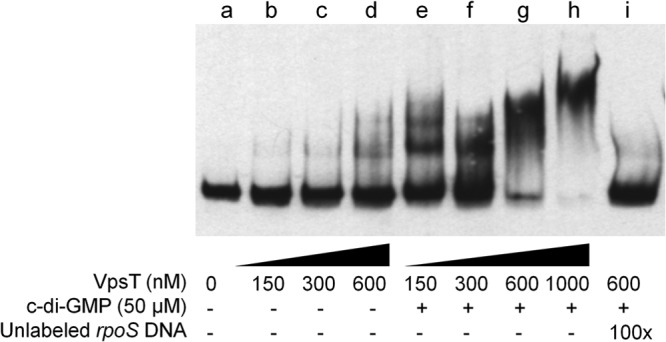
VpsT binds to the rpoS promoter in a c-di-GMP-dependent manner. A DIG-labeled DNA fragment containing the rpoS promoter was incubated with increasing concentrations of purified VpsT with or without c-di-GMP. Lane i was incubated with a 100-fold excess of unlabeled competitor DNA.
FIG 4.
VpsT is induced when the c-di-GMP pool is increased and interacts with the rpoS promoter in the cell. Strain HX22 expressing VpsT-FLAG was transformed with plasmid pAT1662 expressing the DGC VCA0956 from the arabinose promoter. Expression of VCA0956 was induced by addition of l-arabinose to the culture medium, as described in Materials and Methods. (A) Agarose gel electrophoresis of the PCR products obtained after ChIP using an anti-FLAG MAb and the mouse MAb G3A1 IgG1 isotype control as an unrelated antibody (mock reaction). (B) Quantification of VpsT occupancy at the corresponding promoter by qPCR. Promoter occupancies are expressed as relative immunoprecipitation (IP), the amount of promoter DNA immunoprecipitated by the anti-FLAG MAb normalized by the amount immunoprecipitated in the mock reaction. Each value represents the mean of three experiments, and error bars indicate the standard deviations (*, significantly different from the uninduced culture [P < 0.05]). (C) Western blot showing that VpsT-FLAG is induced when the c-di-GMP pool is enhanced.
Binding of VpsT to the rpoS promoter overlaps rpoS transcription initiation sites.
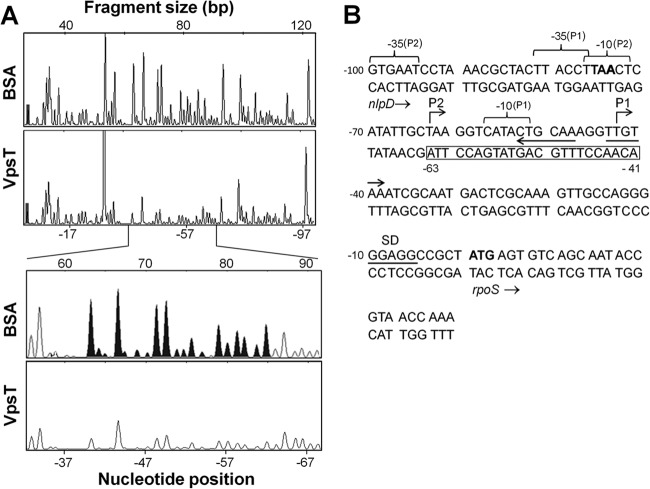
To determine the mechanism of transcriptional repression, we conducted primer extension and DNase I footprinting analyses. Primer extension analysis identified two rpoS transcription initiation sites located 43 bp (P1) and 63 bp (P2) upstream of the rpoS start codon (Fig. 5). The upstream transcription initiation site (P2) is preceded by −10 and −35 elements separated by a 17-bp spacer and located within the nlpD gene. The P1 initiation site was preceded by a −10 promoter element in which 4 bases matched the TATAAT σ70 consensus sequence. The 19-bp spacer separating the −10 element from the upstream −35 hexamer is less common but within the permissible range (17 ± 2 bp) (Fig. 6B). DNase I footprinting (Fig. 6A) showed that VpsT protects a DNA region from DNase I digestion that overlaps the P1 and P2 transcription initiation sites (Fig. 6B). We used EMBOSS explorer software (http://emboss.bioinformatics.nl/cgi-bin/emboss/palindrome) to search for Lux-box-like structures. The search revealed two inverted repeats of partial symmetry overlapping the VpsT-protected promoter region and the P1 transcriptional start site (Fig. 6B). Taken together, we conclude that VpsT is a repressor of rpoS expression.
FIG 5.
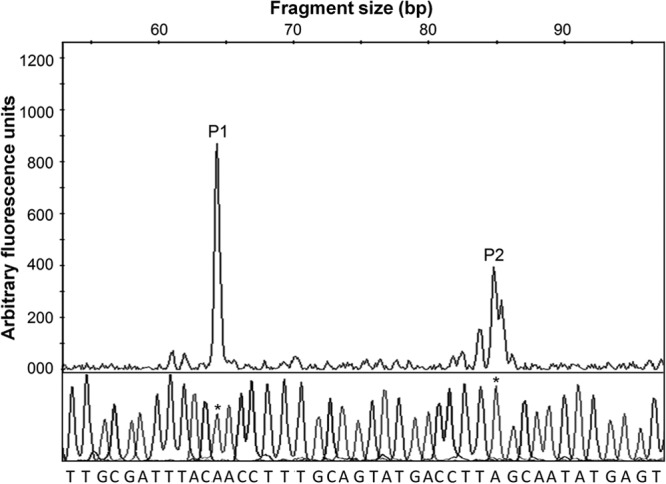
Mapping of transcriptional start sites in the rpoS promoter by primer extension. Shown are electropherograms of fluorescently (HEX) labeled primer extension products and a sequence ladder indicating the presence of two transcriptional start sites (P1 and P2). The nucleotides shown below the sequence ladder correspond to the template strand. Bases corresponding to the start sites in the ladder are marked with asterisks.
FIG 6.
Binding of VpsT to the rpoS promoter can inhibit transcription initiation. (A) DNase I footprinting. Electropherograms show the protection pattern of a fluorescently (HEX) labeled rpoS promoter template strand digested with DNase I after incubation with bovine serum albumin (BSA) (negative control) or VpsT. The bottom panel shows the VpsT protected region (filled peaks in the BSA control) expanded from the top electropherogram. Nucleotides are numbered with reference to the first base (+1) of the rpoS start codon. (B) Sequence of the rpoS promoter region located downstream of the nlpD gene. The nlpD stop and rpoS start codons are shown in boldface type; the rpoS Shine-Dalgarno sequence (SD) is underlined, and putative −10 and −35 promoter elements are indicated by braces above the sequence. The region protected by VpsT in the template strand is boxed, and inverted repeats are indicated with arrows beneath the codon strand.
VpsT negatively regulates rpoS transcription in V. cholerae biofilms.
The results shown in Fig. 1 suggested that the intracellular level of c-di-GMP in planktonic cells growing in LB medium might not be sufficiently elevated for VpsT to bind the rpoS promoter and repress its transcription. However, it is well established that elevated cellular c-di-GMP levels promote bacteria to adopt the biofilm life-style (37–39). Therefore, we decided to study the role of HapR, VpsR, and VpsT in the expression of rpoS in intact V. cholerae biofilms. As a first and qualitative approach, we used an anti-RpoS polyclonal antibody developed in our laboratory to detect RpoS expression in wild-type, hapR, vpsR, and vpsT biofilms. As shown in Fig. 7A, no RpoS protein was detected in the hapR mutant in spite of this mutant exhibiting the highest biofilm mass (OD570). However, RpoS could be detected in the vpsT and vpsR mutants, which made the lowest biofilm mass. We note that the small amount of RpoS antigen detected in the ΔvpsT, ΔvpsR, and ΔvpsR ΔvpsT wells is due to the low biofilm biomass contained in these samples, as indicated by the amount of crystal violet-stained material (OD570) (Fig. 7A). Significant RpoS expression could be detected in the ΔhapR ΔvpsR and ΔhapR ΔvpsT double mutants (Fig. 7A). To quantify this regulation, we constructed RpoS reporter plasmid pACTluz-RpoS, which contains the rrnB T1T2 transcription terminator, to prevent spurious transcription from vector sequences, followed by an rpoS-luxCDABE promoter fusion (Table 1). The plasmid was introduced into the wild type and the hapR, vpsR, and vpsT mutants, and the amount of RpoS-dependent light production was normalized by biofilm mass (OD570). As shown in Fig. 7B, the hapR mutant expressed the lowest RpoS level, consistent with the Western blot data. Deletion of vpsT and vpsR did not impact RpoS expression in the hapR-positive genetic background, consistent with HapR silencing vpsT expression in a 24-h biofilm. Interestingly, the ΔhapR ΔvpsR and ΔhapR ΔvpsT mutants exhibited elevated RpoS levels compared to the vpsR and vpsT single mutants (Fig. 6B). This result suggests that in the biofilm, HapR or a HapR-dependent factor could have a negative effect on rpoS expression.
FIG 7.
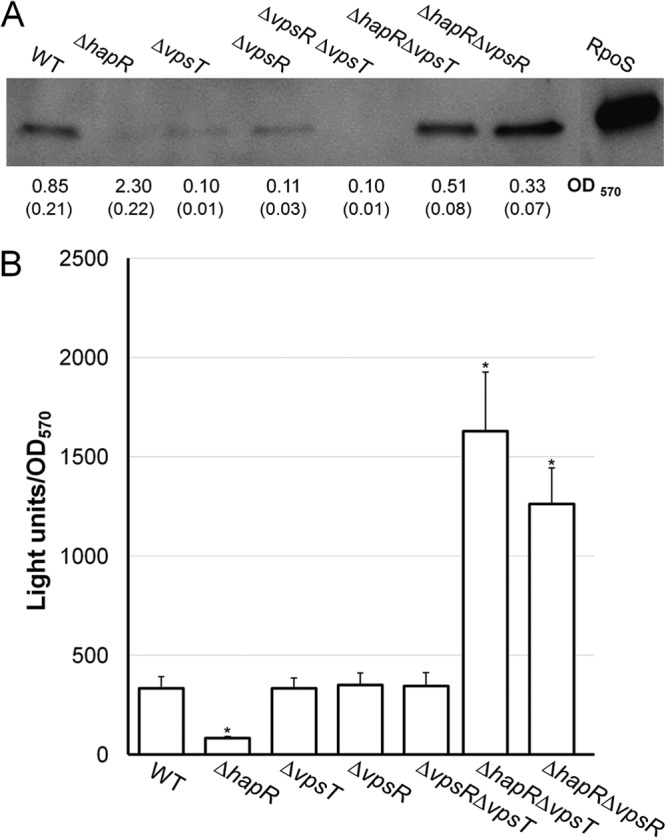
Regulation of rpoS expression in intact biofilms by HapR, VpsR, and VpsT. (A) Western blot detection of RpoS in intact V. cholerae biofilms. The wild type and mutants were grown as indicated in Materials and Methods, and RpoS was detected by using a polyclonal antibody as previously described (32). Biofilm mass was measured by using a crystal violet assay (OD570), and standard deviations are shown in parentheses. (B) Quantitative measurement of RpoS expression in V. cholerae biofilms. V. cholerae C7258 (wild type) and isogenic mutant strains AJB51 (ΔhapR), HX17 (ΔvpsT), HX13 (ΔvpsR), HX18 (ΔvpsR ΔvpsT), HX03 (ΔhapR ΔvpsT), and HX14 (ΔhapR ΔvpsR) containing an rpoS-luxCDABE promoter fusion were allowed to develop static biofilms in 96-well plates for 24 h at 30°C. The planktonic cells were removed, and light was measured. Light production was normalized by biofilm mass after staining of adherent cells with crystal violet. Each value represents the mean for six independent cultures. Error bars indicate standard deviations (*, significantly different from the wild type [P < 0.01]).
DISCUSSION
Expression of the general stress response regulator RpoS and formation of biofilms are strategies used by bacteria to overcome stressful conditions. Our finding that elevated levels of c-di-GMP, which favor biofilm formation, diminish RpoS expression suggests that these stress resistance mechanisms are inversely regulated and could play distinct roles in the V. cholerae life cycle. The finding that artificially increasing the c-di-GMP pool rendered V. cholerae more susceptible to environmental stressors supports this interpretation. We note that the expression system used to enhance the c-di-GMP content prior to exposure to environmental stresses results in a 10-fold increase in the c-di-GMP content (20). This enhanced level of c-di-GMP was recently shown to lie within the physiological range of this metabolite when V. cholerae is grown under different culture conditions (40).
In this study, we show that repression of rpoS expression by c-di-GMP requires VpsR and VpsT. Based on previous studies showing that VpsR activates vpsT transcription (18), we hypothesized that VpsT could act as the downstream regulator to directly repress rpoS transcription. Consistently, artificially increasing the c-di-GMP pool did not induce the expression of a vpsT-FLAG allele from its native promoter in vpsR mutant strain HX23 (data not shown). Moreover, both EMSAs and ChIP assays showed that VpsT can bind to the rpoS promoter in a c-di-GMP-dependent manner. The amount of VpsT protein required to completely shift the rpoS promoter DNA was similar to that required to shift the vpsL promoter (19), but we have not detected significant sequence similarity between these promoters. VpsT proteins, however, differ in their roles in these promoters by acting as an activator at vpsL and a repressor at rpoS. Primer extension analysis identified two transcription start sites upstream of the rpoS start codon. The downstream P1 site appeared to be stronger based on peak height and was preceded by −10 and −35 promoter elements. The −10 promoter element matched 4 bases of the TATAAT consensus sequence, with one mismatch being the same nucleotide in the −10 element of the E. coli rpoS promoter. The putative −35 element was weaker, matching 3 out of 6 bases to the TTGACA consensus, but exhibited more sequence similarity to the corresponding E. coli promoter element. The P2 transcription initiation site appears to be controlled by a promoter within the upstream nlpD gene similarly to E. coli (41). Distinct from E. coli, however, no primer extension products were found originating upstream of nlpD.
The finding that VpsT protected a DNA region partially overlapping the major P1 transcription initiation site is consistent with this regulator acting as a transcriptional repressor of rpoS. Binding of c-di-GMP to VpsT has been shown to promote dimerization (19). Inspection of the rpoS promoter region protected by VpsT with EMBOSS explorer palindrome software revealed potential sites to which VpsT could bind as a dimer. An interaction between a LuxR-type transcription factor and the rpoS promoter has been reported for Pseudomonas aeruginosa (42). In this case, the LuxR-type regulator RhIR enhanced rpoS transcription (42). However, it has been reported that a LuxR-type regulator can also function as a repressor when its binding site overlaps and interferes with the positioning of RNA polymerase at the promoter, as we show in this study (43).
Although VpsT can bind the rpoS promoter to repress its transcription, deletion of vpsT did not affect rpoS expression in planktonic cells grown in rich media without artificially increasing the c-di-GMP pool. It is likely that under these conditions, the c-di-GMP pool is not sufficiently increased for VpsT to bind the rpoS promoter and repress its transcription. In addition, it is well established that expression of the quorum-sensing regulator HapR represses VpsT transcription and diminishes the c-di-GMP pool (20, 22). Thus, the stress conditions required to induce RpoS expression in planktonic cells grown in rich media are not expected to occur until stationary phase. At this stage (high cell density), VpsT is expected to be repressed by quorum sensing and to play a minor role, if any, in rpoS expression. Following this argument, we propose that the lower expression level of rpoS in hapR planktonic cells should involve an additional mechanism(s) independent of VpsR and VpsT. Accordingly, enhancing the c-di-GMP pool diminished rpoS expression in the hapR mutant, but subsequent deletion of vpsT (encoding an rpoS repressor) did not restore wild-type levels of rpoS expression. However, we do not dismiss the possibility that VpsT could repress rpoS expression in planktonic cells under conditions other than laboratory cultures in LB medium. The mechanism by which HapR enhances rpoS expression in planktonic cells is not clear and could be indirect, since binding of HapR to the rpoS promoter has not been detected in vitro (21). The crystal structure of HapR revealed a binding pocket for an unknown ligand (44). Thus, attempts to demonstrate binding of HapR to the rpoS promoter in vitro might have been precluded by this lack of knowledge. Expression of rpoS in biofilms was also diminished in the hapR mutant. However, distinct from planktonic cells, deletion of vpsT in biofilm cells lacking hapR not only restored but significantly enhanced rpoS expression. The finding that ΔhapR ΔvpsR and ΔhapR ΔvpsT mutants expressed significantly elevated rpoS levels compared to the vpsR and vpsT single mutants suggests that HapR (or a HapR-dependent factor) could have a negative effect on rpoS expression in biofilm cells. This negative effect could be masked in the hapR mutant due to overexpression and activation of VpsT resulting in a net repression of rpoS transcription. Taken together, our data suggest that, in biofilms, VpsT could play a major role in silencing rpoS expression prior to cells entering that quorum-sensing mode and that HapR could have dual effects but mainly acts to enhance rpoS transcription by repressing VpsT and decreasing the c-di-GMP pool.
We suggest that differences in rpoS regulation by VpsT observed in planktonic and biofilm cells could result from multiple factors. Cells in biofilms could exhibit higher c-di-GMP contents than planktonic cells cultivated in rich bacteriological media, resulting in elevated VpsT protein and activity levels. Furthermore, nutrient accessibility, limited by diffusion in biofilms (45), could result in elevated c-di-GMP levels and earlier expression of rpoS prior to cells entering the quorum-sensing mode. Also, stress conditions contributing to early rpoS expression in biofilms could result from slower diffusion of metabolic waste products away from cells. Accordingly, DNA microarray analysis revealed that wild-type E. coli biofilms show a gene expression pattern similar to that of wild-type planktonic cells in stationary phase (46), and expression of an rpoS-gfp fusion was detected at an early stage of biofilm development (47). In summary, conditions favoring elevated c-di-GMP levels and the temporal coexpression of rpoS and vpsT in V. cholerae biofilms could result in strong VpsT repression of RpoS expression.
In Fig. 8, we propose a model for the interplay between HapR and VpsT in the regulation of RpoS expression in planktonic and biofilm cells grown in nutrient-rich media. We propose that in V. cholerae planktonic cells cultivated in rich media, the c-di-GMP pool is not sufficiently increased for VpsT to repress rpoS transcription. Furthermore, in nutrient-rich medium, the stress conditions required to induce RpoS occur in the stationary phase. At this stage (high cell density), expression of HapR further diminishes the c-di-GMP pool and silences vpsT transcription (20). Thus, we propose that VpsT plays a minor role in rpoS regulation in planktonic cells under these growth conditions (Fig. 8A). We suggest that under these conditions, HapR enhances RpoS expression by a mechanism independent of VpsT. We propose that cells in a biofilm could exhibit higher intracellular c-di-GMP levels and be subject to stress conditions resulting in earlier RpoS expression. As a result, VpsT could play a major negative role in rpoS expression by binding to the rpoS promoter and interfering with transcription initiation prior to expression of HapR (Fig. 8B). As the cell density in a biofilm increases, HapR represses vpsT transcription and relieves rpoS from VpsT repression.
FIG 8.
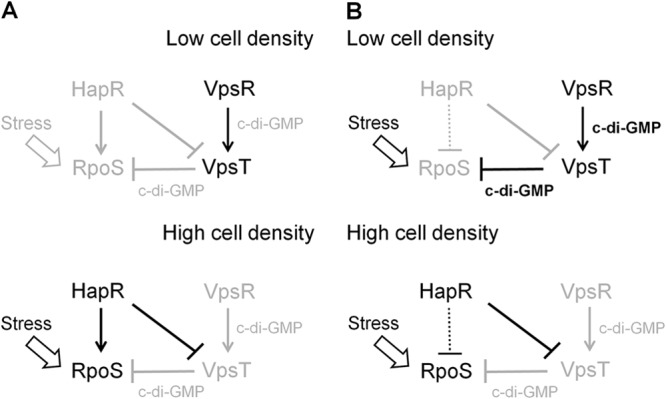
Model for the interplay between HapR and VpsT in the regulation of RpoS expression. Disabled regulations and low c-di-GMP content are represented by lines and fonts in light grayscale. Symbols: →, activation; ⊥, repression. (A) Planktonic cells. In nutrient-rich medium, the level of c-di-GMP is not sufficiently high for VpsT to repress rpoS transcription. HapR enhances rpoS in a VpsT-independent manner. (B) Biofilm cells. Biofilm cells can exhibit a higher starting intracellular c-di-GMP level than planktonic cells and experience stress conditions resulting in earlier expression of RpoS. At a low cell density, VpsT represses rpoS transcription. At a high cell density, HapR represses VpsT and diminishes the c-di-GMP pool to relieve rpoS from VpsT repression. We postulate that in biofilms, HapR could have a negative effect on rpoS expression (dotted line) that is masked in the hapR mutant due to the much stronger repressive effect of VpsT.
Expression of RpoS has been shown to enhance motility (32, 48, 49) and facilitate detachment of vibrios from intestinal tissue or mucosal escape (49). It is well documented that flagellar motility is downregulated when vibrios enter the monolayer stage (50) and that cells in a biofilm detach when conditions become unfavorable (51). Therefore, we suggest that derepression of rpoS from VpsT in biofilms enhances motility and promotes bacterial detachment so that vibrios can swim toward a more favorable environment.
ACKNOWLEDGMENTS
This study was supported by PHS grant AI104993-02 to A.J.S. and a Southern Research Institute endowment fund to J.A.B.
We are grateful to Michael Crowley (University of Alabama at Birmingham Heflin Center for Genomic Sciences) for assistance in DNase I footprinting and using the GeneMapper software. We are grateful to Javier Campos for his assistance in constructing an rpoS-luxCDABE reporter plasmid. Plasmids pAT1662 and pCMW75 were kindly provided by Andrew Camilli (Tufts University School of Medicine) and Christopher Waters (Michigan State University), respectively.
Footnotes
Published ahead of print 20 December 2013
REFERENCES
- 1.Hengge-Aronis R. 2002. Signal transduction and regulatory mechanisms involved in control of the sigma S (RpoS) subunit of RNA polymerase. Microbiol. Mol. Biol. Rev. 66:373–395. 10.1128/MMBR.66.3.373-395.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yildiz FH, Schoolnik GK. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faruque SM, Biswas K, Udden SM, Ahmad QS, Sack DA, Nair GB, Mekalanos JJ. 2006. Transmissibility of cholera: in vivo formed biofilms and their relationship to infectivity and persistence in the environment. Proc. Natl. Acad. Sci. U. S. A. 103:6350–6355. 10.1073/pnas.0601277103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joelsson A, Liu Z, Zhu J. 2006. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect. Immun. 74:1141–1147. 10.1128/IAI.74.2.1141-1147.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matz C, McDougald D, Moreno AM, Yung PY, Yildiz FH, Kjelleberg S. 2005. Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 102:16819–16824. 10.1073/pnas.0505350102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoolnik GK, Yildiz FH. 2000. The complete genome sequence of Vibrio cholerae: a tale of two chromosomes and two lifestyles. Genome Biol. 1:REVIEWS1016.1–REVIEWS1016.3. 10.1186/gb-2000-1-3-reviews1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yildiz FH, Schoolnik GK. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 96:4028–4033. 10.1073/pnas.96.7.4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White PB. 1938. The rugose variant of vibrios. J. Pathol. Bacteriol. 46:1–6. 10.1002/path.1700460102 [DOI] [Google Scholar]
- 9.Morris JG, Jr, Sztein MB, Rice EB, Nataro JP, Losonsky JA, Panigrahi P, Tacket CO, Johnson JA. 1996. Vibrio cholerae O1 can assume a chlorine-resistant rugose survival form that is virulent for humans. J. Infect. Dis. 174:1364–1368. 10.1093/infdis/174.6.1364 [DOI] [PubMed] [Google Scholar]
- 10.Rice EW, Johnson CJ, Clark RM, Fox KR, Reasoner DJ, Dunnigan ME, Panigrahi P, Johnson JA, Morris JG., Jr 1992. Chlorine and survival of “rugose” Vibrio cholerae. Lancet 340:740. [DOI] [PubMed] [Google Scholar]
- 11.Wai SN, Mizunoe Y, Takade A, Kawabata SI, Yoshida SI. 1998. Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl. Environ. Microbiol. 64:3648–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Argenio DA, Miller SI. 2004. Cyclic di-GMP as a bacterial second messenger. Microbiology 150:2497–2502. 10.1099/mic.0.27099-0 [DOI] [PubMed] [Google Scholar]
- 13.Hengge R. 2009. Principles of c-di-GMP signaling in bacteria. Nat. Rev. Microbiol. 7:263–273. 10.1038/nrmicro2109 [DOI] [PubMed] [Google Scholar]
- 14.Simm R, Morr M, Kader A, Nimtz M, Romling U. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123–1134. 10.1111/j.1365-2958.2004.04206.x [DOI] [PubMed] [Google Scholar]
- 15.Tamayo R, Pratt JT, Camilli A. 2007. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu. Rev. Microbiol. 61:131–148. 10.1146/annurev.micro.61.080706.093426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yildiz FH, Dolganov NA, Schoolnik GK. 2001. VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPS (ETr)-associated phenotypes in Vibrio cholerae O1 El Tor. J. Bacteriol. 183:1716–1726. 10.1128/JB.183.5.1716-1726.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casper-Lindley C, Yildiz FH. 2004. VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J. Bacteriol. 186:1574–1578. 10.1128/JB.186.5.1574-1578.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava D, Harris RC, Waters CM. 2011. Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. J. Bacteriol. 193:6331–6341. 10.1128/JB.05167-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasteva PV, Fong JCN, Shikuma NJ, Beyhan S, Navarro MV, Yildiz FH, Sondermann H. 2010. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science 327:866–868. 10.1126/science.1181185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waters CM, Lu W, Rabinowitz JD, Bassler BL. 2008. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J. Bacteriol. 190:2527–2536. 10.1128/JB.01756-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joelsson A, Kan B, Zhu J. 2007. Quorum sensing enhances the stress response in Vibrio cholerae. Appl. Environ. Microbiol. 73:3742–3746. 10.1128/AEM.02804-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Wu J-H, Ayala JC, Benitez JA, Silva AJ. 2011. Interplay among cyclic diguanylate, HapR, and the general stress response regulator (RpoS) in the regulation of Vibrio cholerae hemagglutinin/protease. J. Bacteriol. 193:6529–6538. 10.1128/JB.05166-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Lorenzo V, Eltis L, Kessler B, Timmis KN. 1993. Analysis of the Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene 123:17–24. 10.1016/0378-1119(93)90533-9 [DOI] [PubMed] [Google Scholar]
- 24.Marcus H, Ketley JM, Kaper JB, Holmes RK. 1990. Effect of DNase production, plasmid size and restriction barrier on transformation of Vibrio cholerae by electroporation and osmotic shock. FEMS Microbiol. Lett. 68:149–154. 10.1111/j.1574-6968.1990.tb04139.x [DOI] [PubMed] [Google Scholar]
- 25.Tischler AT, Camilli A. 2004. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 53:857–869. 10.1111/j.1365-2958.2004.04155.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnenberg MS, Kaper JB. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive selection suicide vector. Infect. Immun. 59:4310–4317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalogeraki VS, Winans SC. 1997. Suicide plasmids containing promoterless reporter genes can simultaneously disrupt and create fusions to target genes of diverse bacteria. Gene 188:69–75. 10.1016/S0378-1119(96)00778-0 [DOI] [PubMed] [Google Scholar]
- 28.Silva AJ, Pham K, Benitez JA. 2003. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149:1883–1891. 10.1099/mic.0.26086-0 [DOI] [PubMed] [Google Scholar]
- 29.Silva AJ, Benitez JA. 2004. Transcriptional regulation of Vibrio cholerae hemagglutinin/protease by the cyclic AMP receptor protein and RpoS. J. Bacteriol. 186:6374–6382. 10.1128/JB.186.19.6374-6382.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303–314. 10.1016/S0092-8674(02)00829-2 [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Ayala JC, Silva AJ, Benitez JA. 2012. The histone-like nucleoid structuring protein (H-NS) is a repressor of Vibrio cholerae exopolysaccharide biosynthesis (vps) genes. Appl. Environ. Microbiol. 78:2482–2488. 10.1128/AEM.07629-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Ayala JC, Benitez JA, Silva AJ. 2012. Interaction of the histone-like nucleoid structuring protein and the general stress response regulator RpoS at Vibrio cholerae promoters that regulate motility and hemagglutinin/protease expression. J. Bacteriol. 194:1205–1215. 10.1128/JB.05900-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heffler MA, Watters RD, Kugel JF. 2012. Using electrophoretic mobility shift assays to measure equilibrium dissociation constants: Gal4-p53 binding DNA as a model system. Biochem. Mol. Biol. Educ. 40:383–387. 10.1002/bmb.20649 [DOI] [PubMed] [Google Scholar]
- 34.Zianni M, Tessanne K, Merighi M, Laguna R, Tabita FR. 2006. Identification of the DNA bases of a DNase I footprint by the use of dye primer sequencing on an automated capillary DNA analysis instrument. J. Biomol. Tech. 17:103–113 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2291779/ [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd AL, Marshall BJ, Mee BJ. 2005. Identifying cloned Helicobacter pylori promoters by primer extension using a FAM-labelled primer and GeneScan analysis. J. Microbiol. Methods 60:291–298. 10.1016/j.mimet.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 36.Miller JH. 1971. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 37.Cotter PA, Stibitz S. 2007. c-di-GMP-mediated regulation of virulence and biofilm formation. Curr. Opin. Microbiol. 10:17–23. 10.1016/j.mib.2006.12.006 [DOI] [PubMed] [Google Scholar]
- 38.Yildiz FH, Visick KL. 2009. Vibrio biofilms: so much the same yet so different. Trends Microbiol. 17:109–118. 10.1016/j.tim.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sondermann H, Shikuma NJ, Yildiz FH. 2012. Mechanism of c-di-GMP signaling. Curr. Opin. Microbiol. 15:140–146. 10.1016/j.mib.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koestler BJ, Waters CM. 2013. Exploring environmental control of c-di-GMP signaling in Vibrio cholerae by using the ex vivo lysate cyclic di-GMP assay (TELCA). Appl. Environ. Microbiol. 79:5233–5241. 10.1128/AEM.01596-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65:189–213. 10.1146/annurev-micro-090110-102946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links transcriptional activators LasR and RhlR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137–1146. 10.1046/j.1365-2958.1996.00063.x [DOI] [PubMed] [Google Scholar]
- 43.Egland KA, Greenberg EP. 2000. Conversion of the Vibrio fischeri transcriptional activator, LuxR, to a repressor. J. Bacteriol. 182:805–811. 10.1128/JB.182.3.805-811.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Silva RS, Kovacikova G, Lin W, Taylor RK, Skorupski K, Kull FJ. 2007. Crystal structure of the Vibrio cholerae quorum-sensing regulatory protein HapR. J. Bacteriol. 189:5683–5691. 10.1128/JB.01807-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart PS. 2003. Diffusion in biofilms. J. Bacteriol. 185:1485–1491. 10.1128/JB.185.5.1485-1491.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito A, May T, Kawata K, Okabe S. 2008. Significance of rpoS during maturation of Escherichia coli biofilms. Biotechnol. Bioeng. 99:1462–1471. 10.1002/bit.21695 [DOI] [PubMed] [Google Scholar]
- 47.Ito A, May T, Taniuchi A, Kawata K, Okabe S. 2009. Localized expression profiles of rpoS in Escherichia coli biofilms. Biotechnol. Bioeng. 103:975–983. 10.1002/bit.22305 [DOI] [PubMed] [Google Scholar]
- 48.Silva AJ, Sultan SZ, Liang W, Benitez JA. 2008. Role of the histone-like nucleoid structuring protein (H-NS) in the regulation of RpoS and RpoS-dependent genes in Vibrio cholerae. J. Bacteriol. 190:7335–7345. 10.1128/JB.00360-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nielsen AT, Dolganov NA, Otto G, Miller MC, Wu CY, Schoolnik GK. 2006. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog. 2:e109. 10.1371/journal.ppat.0020109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moorthy S, Watnick PI. 2004. Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol. Microbiol. 52:573–587. 10.1111/j.1365-2958.2004.04000.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watnick P, Kolter R. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675–2679. 10.1128/JB.182.10.2675-2679.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang W, Pascual-Montano A, Silva AJ, Benitez JA. 2007. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae. Microbiology 153:2964–2975. 10.1099/mic.0.2007/006668-0 [DOI] [PubMed] [Google Scholar]
- 53.Rothmel RD, Shinabarger D, Parsek M, Aldrich T, Chakrabarty AM. 1991. Functional analysis of the Pseudomonas putida regulatory protein CatR: transcriptional studies and determination of the CatR DNA binding site by hydroxyl-radical footprinting. J. Bacteriol. 173:4717–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]