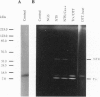
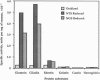
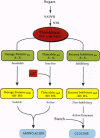
Abstract
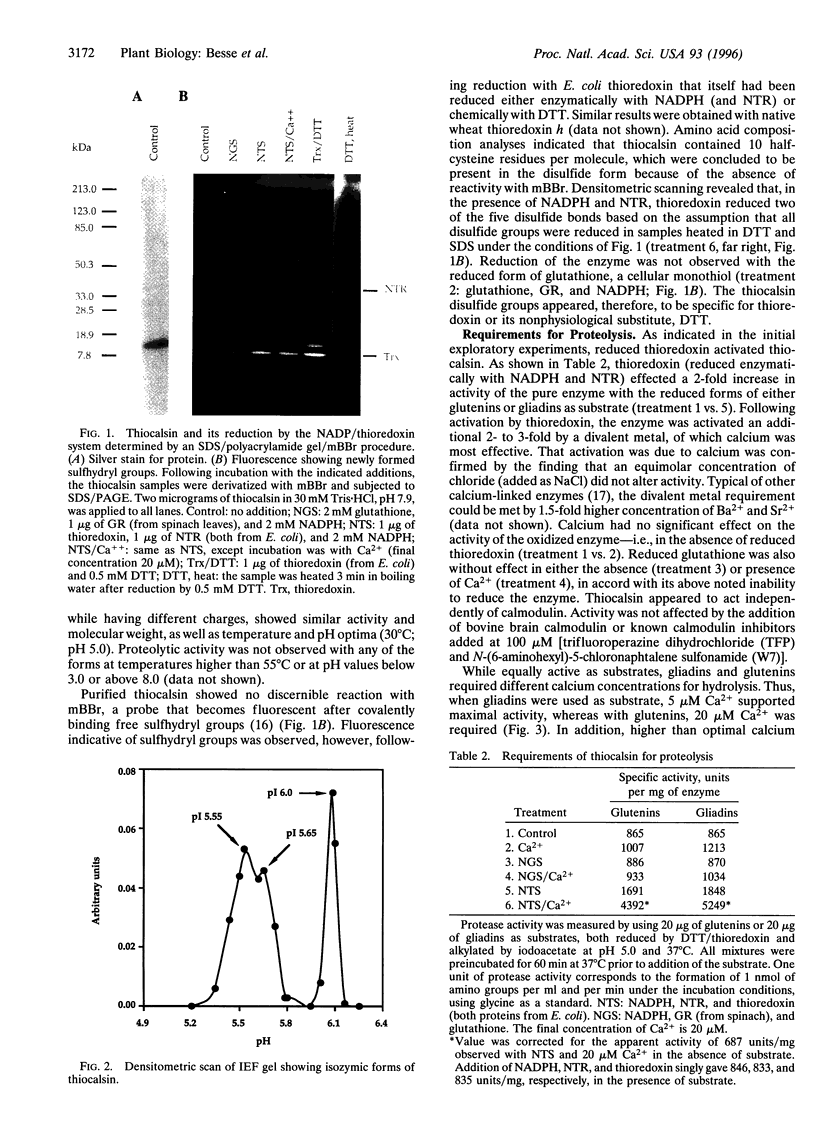
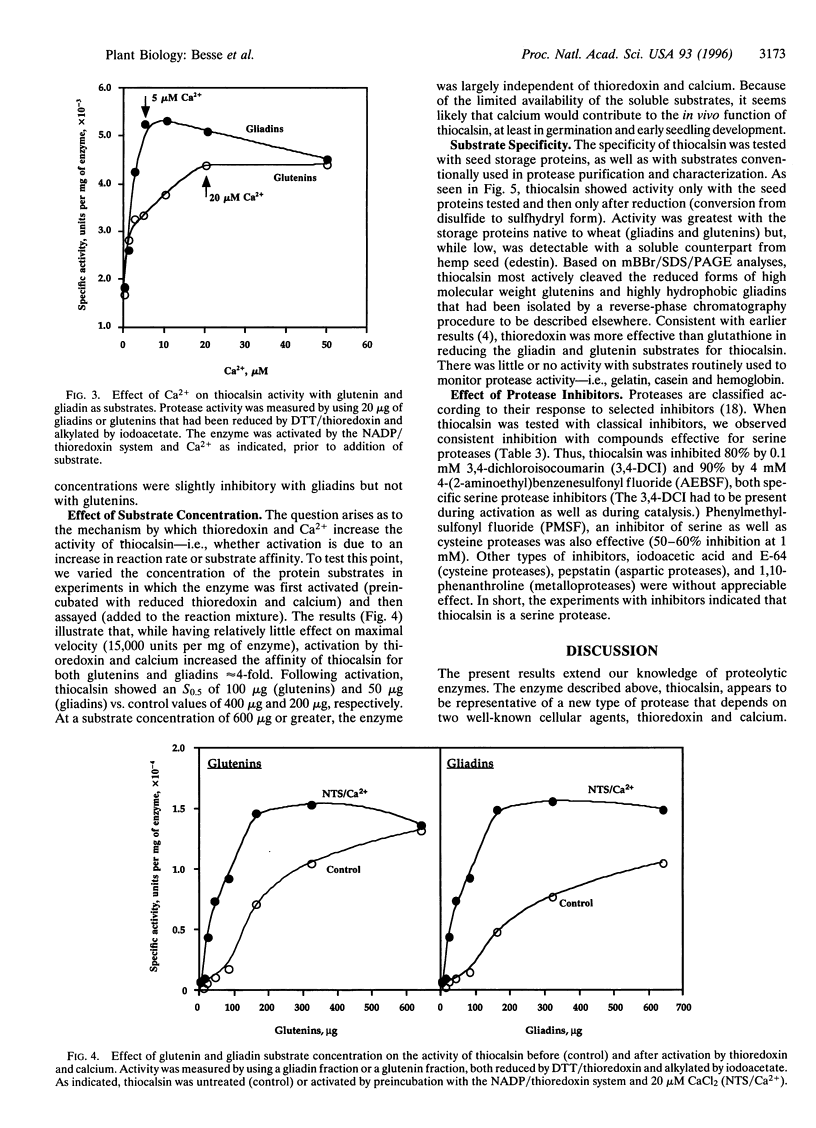
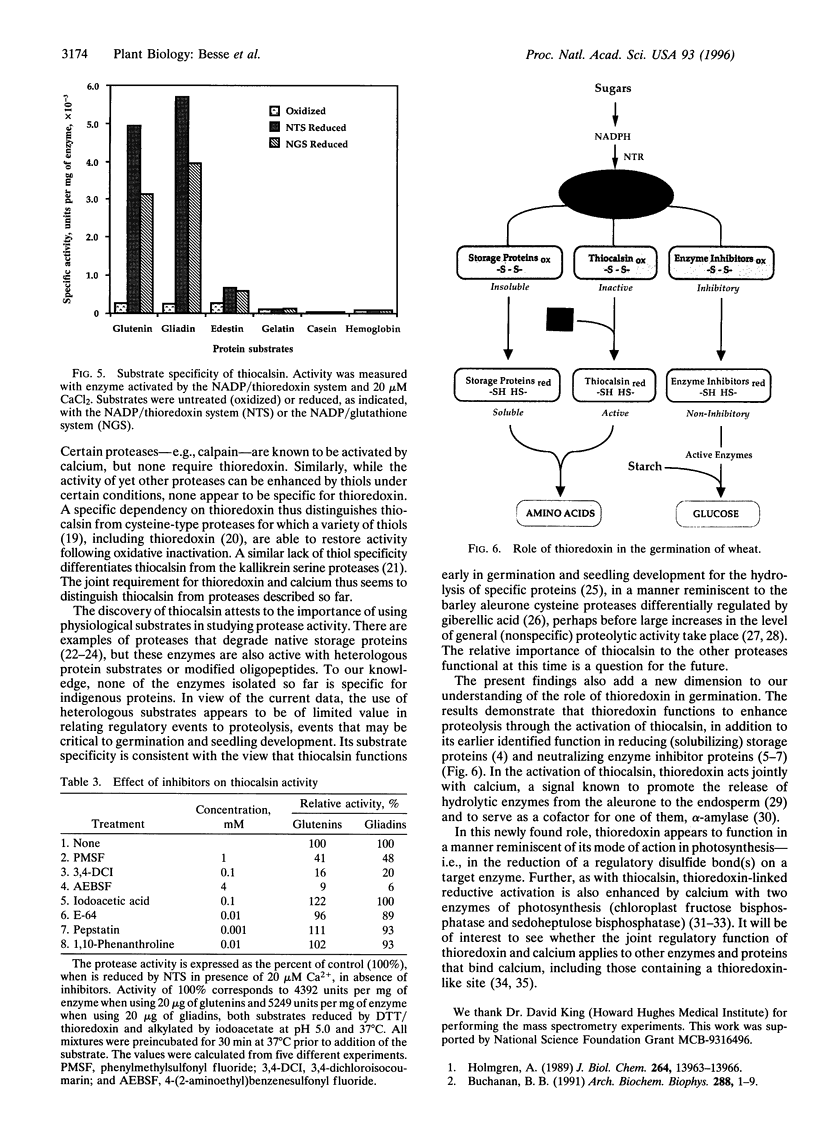
We describe a protease, named "thiocalsin," that is activated by calcium but only after reductive activation by thioredoxin, a small protein with a redox-active disulfide group that functions widely in regulation. Thiocalsin appeared to be a 14-kDa serine protease that functions independently of calmodulin. The enzyme, purified from germinating wheat grain, specifically cleaved the major indigenous storage proteins, gliadins and glutenins, after they too had been reduced, preferentially by thioredoxin. The disulfide groups of the enzyme, as well as its protein substrates, were reduced by thioredoxin via NADPH and the associated enzyme, NADP-thioredoxin reductase. The results broaden the roles of thioredoxin and calcium and suggest a joint function in activating thiocalsin, thereby providing amino acids for germination and seedling development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J Agric Food Chem. 1979 Nov-Dec;27(6):1256–1262. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B. Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch Biochem Biophys. 1991 Jul;288(1):1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Decottignies P., Lozano R. M. Thioredoxin: a multifunctional regulatory protein with a bright future in technology and medicine. Arch Biochem Biophys. 1994 Nov 1;314(2):257–260. doi: 10.1006/abbi.1994.1439. [DOI] [PubMed] [Google Scholar]
- Bush D. S., Sticher L., van Huystee R., Wagner D., Jones R. L. The calcium requirement for stability and enzymatic activity of two isoforms of barley aleurone alpha-amylase. J Biol Chem. 1989 Nov 15;264(32):19392–19398. [PubMed] [Google Scholar]
- Callis J. Regulation of Protein Degradation. Plant Cell. 1995 Jul;7(7):845–857. doi: 10.1105/tpc.7.7.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. A., Droux M., Kosower N. S., Buchanan B. B. Evidence for function of the ferredoxin/thioredoxin system in the reductive activation of target enzymes of isolated intact chloroplasts. Arch Biochem Biophys. 1989 May 15;271(1):223–239. doi: 10.1016/0003-9861(89)90273-7. [DOI] [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991 Jul;71(3):813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Florencio F. J., Yee B. C., Johnson T. C., Buchanan B. B. An NADP/thioredoxin system in leaves: purification and characterization of NADP-thioredoxin reductase and thioredoxin h from spinach. Arch Biochem Biophys. 1988 Nov 1;266(2):496–507. doi: 10.1016/0003-9861(88)90282-2. [DOI] [PubMed] [Google Scholar]
- Füllekrug J., Sönnichsen B., Wünsch U., Arseven K., Nguyen Van P., Söling H. D., Mieskes G. CaBP1, a calcium binding protein of the thioredoxin family, is a resident KDEL protein of the ER and not of the intermediate compartment. J Cell Sci. 1994 Oct;107(Pt 10):2719–2727. doi: 10.1242/jcs.107.10.2719. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hatala M. A., DiPippo V. A., Powers C. A. Biological thiols elicit prolactin proteolysis by glandular kallikrein and permit regulation by biochemical pathways linked to redox control. Biochemistry. 1991 Aug 6;30(31):7666–7672. doi: 10.1021/bi00245a002. [DOI] [PubMed] [Google Scholar]
- Hertig C. M., Wolosiuk R. A. Studies on the hysteretic properties of chloroplast fructose-1,6-bisphosphatase. J Biol Chem. 1983 Jan 25;258(2):984–989. [PubMed] [Google Scholar]
- Hertig C., Wolosiuk R. A. A dual effect of Ca2+ on chloroplast fructose-1,6-bisphosphatase. Biochem Biophys Res Commun. 1980 Nov 17;97(1):325–333. doi: 10.1016/s0006-291x(80)80171-9. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Kobrehel K., Wong J. H., Balogh A., Kiss F., Yee B. C., Buchanan B. B. Specific reduction of wheat storage proteins by thioredoxin h. Plant Physiol. 1992 Jul;99(3):919–924. doi: 10.1104/pp.99.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrehel K., Yee B. C., Buchanan B. B. Role of the NADP/thioredoxin system in the reduction of alpha-amylase and trypsin inhibitor proteins. J Biol Chem. 1991 Aug 25;266(24):16135–16140. [PubMed] [Google Scholar]
- Koehler S. M., Ho T. H. A major gibberellic Acid-induced barley aleurone cysteine proteinase which digests hordein : purification and characterization. Plant Physiol. 1990 Sep;94(1):251–258. doi: 10.1104/pp.94.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler S. M., Ho T. H. Hormonal regulation, processing, and secretion of cysteine proteinases in barley aleurone layers. Plant Cell. 1990 Aug;2(8):769–783. doi: 10.1105/tpc.2.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundström-Ljung J., Birnbach U., Rupp K., Söling H. D., Holmgren A. Two resident ER-proteins, CaBP1 and CaBP2, with thioredoxin domains, are substrates for thioredoxin reductase: comparison with protein disulfide isomerase. FEBS Lett. 1995 Jan 9;357(3):305–308. doi: 10.1016/0014-5793(94)01386-f. [DOI] [PubMed] [Google Scholar]
- Singh R., Blättler W. A., Collinson A. R. An amplified assay for thiols based on reactivation of papain. Anal Biochem. 1993 Aug 15;213(1):49–56. doi: 10.1006/abio.1993.1384. [DOI] [PubMed] [Google Scholar]
- Stephen A. G., Powls R., Beynon R. J. Activation of oxidized cysteine proteinases by thioredoxin-mediated reduction in vitro. Biochem J. 1993 Apr 15;291(Pt 2):345–347. doi: 10.1042/bj2910345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining S. S. Regulation of proteolytic activity in tissues. Crit Rev Biochem Mol Biol. 1994;29(5):315–383. doi: 10.3109/10409239409083484. [DOI] [PubMed] [Google Scholar]
- Wrobel R., Jones B. L. Appearance of Endoproteolytic Enzymes during the Germination of Barley. Plant Physiol. 1992 Nov;100(3):1508–1516. doi: 10.1104/pp.100.3.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]