Abstract
Magnesium is the most abundant divalent metal in cells and is required for many structural and enzymatic functions. For bacteria, at least three families of proteins function as magnesium transporters. In recent years, it has been shown that a subset of these transport proteins is regulated by magnesium-responsive genetic control elements. In this study, we investigated the cellular requirements for magnesium homeostasis in the model microorganism Bacillus subtilis. Putative magnesium transporter genes were mutationally disrupted, singly and in combination, in order to assess their general importance. Mutation of only one of these genes resulted in strong dependency on supplemental extracellular magnesium. Notably, this transporter gene, mgtE, is known to be under magnesium-responsive genetic regulatory control. This suggests that the identification of magnesium-responsive genetic mechanisms may generally denote primary transport proteins for bacteria. To investigate whether B. subtilis encodes yet additional classes of transport mechanisms, suppressor strains that permitted the growth of a transporter-defective mutant were identified. Several of these strains were sequenced to determine the genetic basis of the suppressor phenotypes. None of these mutations occurred in transport protein homologues; instead, they affected housekeeping functions, such as signal recognition particle components and ATP synthase machinery. From these aggregate data, we speculate that the mgtE protein provides the primary route of magnesium import in B. subtilis and that the other putative transport proteins are likely to be utilized for more-specialized growth conditions.
INTRODUCTION
Metal ions are a requirement for life due to their function as key cofactors in numerous cellular processes. However, these essential ions become an intracellular threat when present in excess. Their intracellular levels must therefore be carefully maintained. Many different studies have revealed how metal homeostasis mechanisms can be controlled (1–3). Typically, microbes sense intracellular metal concentrations through the action of metal-sensing regulatory (metalloregulatory) proteins. However, additional studies have identified at least two examples of RNA elements that also regulate the expression of metal ion transporter genes (4–6). In general, these RNA- and protein-based regulatory factors act together to maintain the correct balance of intracellular metals by controlling the expression of genes responsible for the uptake, efflux, and sequestration of target ions.
In general, divalent ions are maintained at relatively low concentrations in the cytoplasm, in part through the combined action of import and export proteins. However, magnesium transport appears to be unique in several respects. In particular, the intracellular concentration of free magnesium is maintained at a relatively high level (∼0.5 to 1.0 mM) (7–9). Additionally, there do not appear to be any significant efflux mechanisms that factor into the maintenance of proper intracellular magnesium concentrations (8). In contrast, studies have identified three major families of magnesium importers (CorA, MgtE, and MgtA/MgtB [MgtA/B] P-type ATPase proteins), which maintain the relatively high levels of cytoplasmic magnesium (8, 10–15). The genome sequence of Bacillus subtilis is predicted to encode at least one copy of all three of the major magnesium transporter families. Indeed, B. subtilis may encode as many as four dedicated potential magnesium uptake mechanisms, including two CorA homologues (YfjQ and YqxL), an MgtE homologue (YkoK), and an MgtA homologue (YloB) (see Fig. 1).
FIG 1.
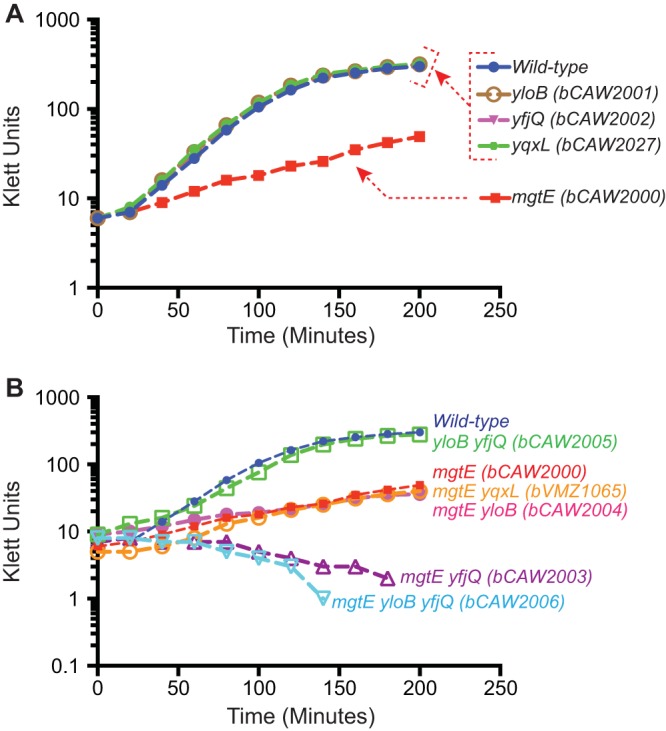
Growth in rich medium for B. subtilis strains containing deletions in putative magnesium transport proteins. (A) A total of four putative magnesium transporters were identified and were then individually deleted from the B. subtilis genome. Only the ΔmgtE mutant exhibited a measurable decrease in growth rate or yield under these conditions. (B) Combinations of deletion mutants were tested, revealing that mutation of a second putative magnesium transporter (yfjQ) further reduced growth yield when combined with the ΔmgtE mutation. The growth of cultures was measured by a Klett meter.
CorA family transporters are the most prevalent magnesium transport system in eubacterial organisms and therefore have been studied more extensively than other magnesium transport systems (11, 16–18). These proteins have no known homology to any other family of transporters (18). The cytosolic domain of CorA is predicted to possess a magnesium-sensing function. Under high levels of intracellular magnesium, this domain associates with magnesium, resulting in the formation of a closed conformation within the ion channel. In response to low intracellular magnesium concentrations, these magnesium ions are displaced, allowing extracellular magnesium to enter through the CorA pore (19–21). However, not all CorA homologues are actually utilized for magnesium transport (8). For example, a CorA homologue in Salmonella enterica serovar Typhimurium mediates the transport of Zn2+ (22). While no previous studies have uncovered any alternative functions for either of the two B. subtilis CorA homologues, YqxL has been shown to be upregulated in a σB-dependent manner under various stress conditions (23, 24), and YfjQ is upregulated by high salinity (25).
MgtE family magnesium transporters are the second most abundant magnesium transport system in bacteria (8). The MgtE magnesium transporter was originally discovered in Bacillus firmus through a genetic complementation screen that was designed to identify additional members of the CorA family (14). However, upon sequencing of this locus, it was determined that MgtE had no sequence similarity to CorA or any other known family of proteins. Like CorA, MgtE family transporters are believed to have magnesium-sensing functions in their cytoplasmic domains that act as gating mechanisms for metal import (26, 27). The B. subtilis homologue was previously referred to as YkoK but will be referred to as MgtE here. While no magnesium transport function has been directly demonstrated for endogenous B. subtilis MgtE, there are strong indications that this protein functions as an important magnesium transporter. First, while the expression of most of the other potential magnesium importers of B. subtilis is maintained at relatively low levels, MgtE is significantly upregulated during growth under low-magnesium conditions through the action of a magnesium-responsive regulatory RNA element (5, 6). Additionally, B. subtilis MgtE is capable of complementing a magnesium transport defect when expressed in Salmonella enterica (28, 29).
The third major family of magnesium importers, MgtA/B, belongs to a broader family of P-type ATPase divalent transporters. However, unlike most other P-type ATPases, which utilize the energy of ATP to transport cations against their electrochemical gradients, MgtA/B ATPases appear to import magnesium along its gradient (8). MgtA homologues are similar to the sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) and the Ca2+-transporting ATPases of the Golgi apparatus (8, 30). In fact, the B. subtilis MgtA homologue, YloB, appears to be particularly similar to these eukaryotic Ca2+ transporters, having 9 out of 10 conserved residues critical for the formation of Ca2+-binding sites (30). While some data indicate that this protein responds to Ca2+, YloB-deficient strains appear to accumulate as much Ca2+ as wild-type B. subtilis, indicating that there are additional mechanisms for Ca2+ import or that the actual substrate for YloB may be a different cation (30).
It remains possible that other mechanisms, in addition to these putative classes of transport proteins, contribute to magnesium homeostasis. For example, the citrate transporter CitM is known to transport divalent ions with citrate (31, 32). Therefore, to examine the functionally significant routes of magnesium transport, we investigated the effects of the mutational disruption of all known putative B. subtilis magnesium transport proteins, including MgtE, YqxL, YfjQ, YloB, and CitM.
MATERIALS AND METHODS
Construction of deletion strains.
The B. subtilis strains used in this study are listed in Table 1. For the construction of deletion strains, two PCR fragments approximately 500 bp long, corresponding to both the 5′ and 3′ regions of the targeted gene, were generated using Phusion polymerase (New England Biolabs). For each deleted gene, at least 200 nucleotides in the middle of the gene were deleted and an in-frame stop codon was inserted using appropriate oligonucleotides (IDT). The forward oligonucleotide for the 5′ region was designed to contain a restriction enzyme site for insertion into the pMAD plasmid (33). The reverse oligonucleotide for the 5′ region contained a stop codon (TAA) followed by 21 nucleotides complementary to the upstream portion of the 3′ region; therefore, the forward oligonucleotide for the 3′ region was the reverse complement of the reverse oligonucleotide for the 5′ region. Finally, the reverse oligonucleotide for the 3′ end of the gene contained a restriction site for subcloning into pMAD. These two PCR products were sewn together to produce a single DNA product that could be subcloned into pMAD. The protocol for making markerless mutations using pMAD has been described elsewhere (33). Briefly, natural competence was induced in Bacillus subtilis strains (34) by supplementing the growth medium with 10 mM MgCl2. During the transformation of the pMAD plasmid derivatives, cells were cultured at 30°C for 3 h to permit replication of the temperature-sensitive pMAD plasmid. Next, an outgrowth step was performed at 30°C for 2 h in a rich medium (2× YT; Sigma-Aldrich) containing 0.1 μg/ml erythromycin and 50 mM MgCl2. The transformed cells were plated on a rich medium (tryptose blood agar base [TBAB]) containing 1 μg/ml erythromycin, 25 μg/ml lincomycin, 80 μg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal), and 50 mM MgCl2 and were cultured for 2 to 3 days at 30°C. A blue colony was chosen for the inoculation of 5 ml of 2× YT containing 1 μg/ml erythromycin, 25 μg/ml lincomycin, and 50 mM MgCl2, and the culture was then incubated at 37°C overnight. At this nonpermissive temperature, the plasmid will not replicate, thus promoting the integration of the plasmid into the targeted genetic locus. The cells were then serially diluted and were plated onto TBAB containing 1 μg/ml erythromycin, 25 μg/ml lincomycin, 80 μg/ml X-gal, and 50 mM MgCl2 in order to obtain individual colonies. Upon recombination and loss of the plasmid, a subset of cells will revert to the wild-type sequence, and a different subset will retain the desired truncated version of the target gene. Representative white colonies were chosen, since they indicated the loss of pMAD plasmids, and the resulting cells were patched onto TBAB containing 80 μg/ml X-gal and 50 mM MgCl2 with or without 1 μg/ml erythromycin and 25 μg/ml lincomycin. White colonies that were also erythromycin sensitive were indicative of bacteria that had lost the plasmid. The target genomic locus of these bacteria was then PCR amplified and sequenced.
TABLE 1.
Bacillus subtilis strains used in this study
| Straina | Description | Growth requirement(s) |
|---|---|---|
| bCAW2000 | 168 ΔmgtE | >25 mM magnesium |
| bCAW2001 | 168 ΔyloB | |
| bCAW2002 | 168 ΔyfjQ | |
| bCAW2026 | 168 ΔcitM | |
| bCAW2027 | 168 ΔyqxL | |
| bCAW2003 | 168 ΔmgtE ΔyfjQ | >25 mM magnesium |
| bCAW2004 | 168 ΔmgtE ΔyloB | >25 mM magnesium |
| bCAW2005 | 168 ΔyloB ΔyfjQ | |
| VMZ1065 | 168 ΔmgtE ΔyqxL | >25 mM magnesium |
| bCAW2006 | 168 ΔmgtE ΔyloB ΔyfjQ | >25 mM magnesium |
| VMZ1066 | 168 ΔmgtE ΔyloB ΔyfjQ ΔyqxL | >25 mM magnesium |
| bCAW2095 | 168 ΔmgtE amyE::Plac-mgtE | >25 mM magnesium; Spcr |
| bCAW2089 | 168 ΔmgtE amyE::Plac-yqxL | >25 mM magnesium; Spcr |
| bCAW2091 | 168 ΔmgtE amyE::Plac-yloB | >25 mM magnesium; Spcr |
| bCAW2112 | 168 ΔmgtE amyE::Plac-citM | >25 mM magnesium; Spcr |
| bCAW2088 | 168 ΔmgtE amyE::Plac-yfjQ | >25 mM magnesium; Spcr |
| VMZ1063 | 168 ΔmgtE ΔcitM | >25 mM magnesium |
| bCAW2116 | 168 ΔmgtE ΔcitM amyE::Plac | >25 mM magnesium; Spcr |
| bCAW2117 | 168 ΔmgtE ΔcitM amyE::Plac-citM | >25 mM magnesium; Spcr |
| bCAW2118 | 168 ΔmgtE ΔyloB ΔyfjQ ΔcitM amyE::Plac | >25 mM magnesium; Spcr |
| bCAW2119 | 168 ΔmgtE ΔyloB ΔyfjQ ΔcitM amyE::Plac-citM | >25 mM magnesium; Spcr |
| bCAW2047 | 168 ΔmgtE ΔyloB ΔyfjQ yflB(S129F) scr(C172U) | |
| bCAW2051 | 168 ΔmgtE ΔyloB ΔyfjQ Δ1362nt (ywzB ywmA atpC atpD) | |
| bCAW2053 | 168 ΔmgtE ΔyloB ΔyfjQ ffh(R399W) ybbR(16nt insertion) cydC(Δ8nt) | |
| bCAW2056 | 168 amyE::cat | |
| bCAW2080 | 168 ΔmgtE ΔyloB ΔyfjQ ydhC(Y171D) yaaK (insertion of A at position 25 relative to translation start site) Δ21kb (ydcL-yddM) | |
| bCAW2084 | 168 ΔmgtE ΔyloB ΔyfjQ Δ1362nt (ywzB ywmA atpC atpD) | |
| bCAW2085 | 168 ΔmgtE ΔyloB ΔyfjQ Δ1362nt (ywzB ywmA atpC atpD) |
All strains were newly created in this study.
Growth conditions in rich media.
Bacillus subtilis strains were inoculated onto solid TBAB medium supplemented with 50 mM MgCl2 and, when appropriate, spectinomycin (100 μg/ml) or chloramphenicol (10 μg/ml) and were cultured overnight at 37°C. A 25-ml volume of 2× YT supplemented with 50 mM MgCl2 and no antibiotic was then inoculated from the freshly streaked bacterial growth. These cultures were incubated with shaking at 37°C until they reached an optical density at 600 nm (OD600) of ∼0.5, at which point they were pelleted, washed twice with 10 ml 2× YT, and resuspended in 10 ml 2× YT. OD600 readings were recorded, and cells were diluted to an OD600 of ∼0.05. At this point, 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the appropriate cultures. For some cultures, supplemental MgCl2 was added at concentrations indicated below (see Fig. 2). A 25-ml volume of culture was incubated in 250-ml Klett flasks, and Klett readings were taken every 20 min.
FIG 2.
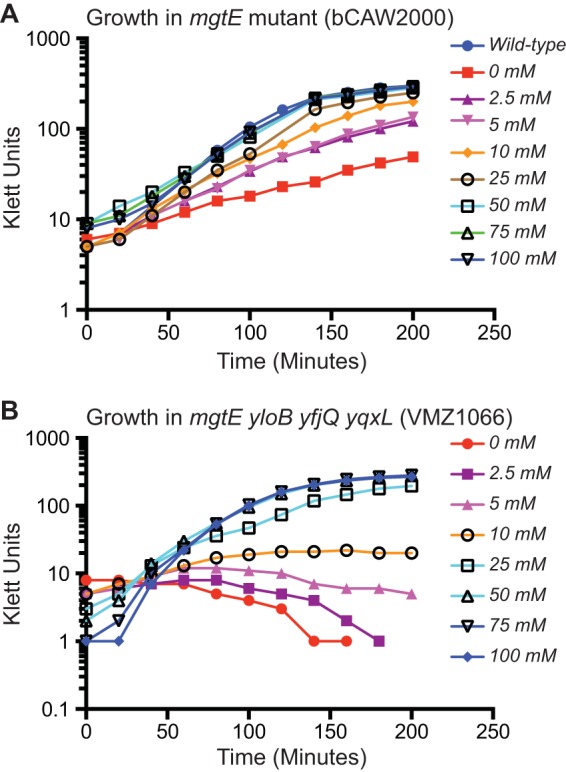
Supplemental magnesium can restore growth to the transporter mutant strains. A range of magnesium concentrations was added to cultures of bCAW2000 (ΔmgtE) and VMZ1066 (ΔmgtE ΔyloB ΔyfjQ ΔyqxL), and the growth of the cultures was measured by Klett readings.
For all Salmonella enterica strains, 5 ml of LB broth, containing 50 μg/ml ampicillin and supplemented with 100 mM MgCl2, was incubated with shaking overnight at 37°C. The next morning, this culture was diluted 1:100 in 20 ml of LB broth containing 50 μg/ml ampicillin and 100 mM MgCl2 and was cultured to an OD600 of ∼0.5. The cells were pelleted, washed twice with 10 ml of LB broth, and resuspended in LB broth containing 50 μg/ml ampicillin. OD600 readings were taken, and the culture was diluted to an OD600 of ∼0.05. A 25-ml volume of culture was grown in 250-ml Klett flasks, and Klett readings were taken every 20 min.
Growth conditions in minimal media.
Bacillus subtilis cultures were streaked onto TBAB that was supplemented with 50 mM MgCl2 and contained either no antibiotic, spectinomycin (100 μg/ml), or chloramphenicol (10 μg/ml), depending on the strain. These cultures were incubated overnight at 37°C. The next day, 25 ml glucose minimal medium [0.5% glucose, 0.5 mM CaCl2, 5 μM MnCl2, 15 mM (NH4)2SO4, 80 mM K2HPO4, 44 mM KH2PO4, 3.9 mM sodium citrate, and 50 μg/ml tryptophan], supplemented with 10 mM MgCl2, was inoculated from the fresh TBAB streak. This culture was incubated without shaking at 37°C overnight before being transferred to a 37°C shaker the next morning. Once the shaking culture reached an OD600 of ∼0.5, the cells were pelleted, washed twice in 10 ml glucose minimal medium with no added MgCl2, and resuspended in 10 ml of the same medium. OD600 readings were recorded, and cells were diluted to an OD600 of ∼0.1. At this point, 2.5 mM MgCl2 was added to all cultures, and 0.5 mM IPTG was added to the appropriate cultures. A 12.5-ml volume of culture was cultured in 125-ml Klett flasks, and Klett readings were taken every 1 h.
Complementation studies.
For the complementation studies in B. subtilis, targeted genes were subcloned and were expressed in the amyE integration vector pHyper-SPANK under the control of the IPTG-inducible promoter Pspac (a gift from David Rudner, Harvard University). Depending on the experiment, the growth conditions described above for either rich media or the minimal medium were used. Induction was accomplished using 0.5 mM IPTG, except for MgtE, which was induced by 5 μM IPTG, because concentrations higher than 5 μM proved toxic (as indicated by a decreased growth rate).
Generation of suppressor mutations.
The triple knockout strain (bCAW2006) that contained deletions of MgtE, YfjQ, and YloB was grown with shaking overnight at 37°C in 2× YT supplemented with 50 mM MgCl2. This culture was pelleted, washed twice in 10 ml 2× YT with no added MgCl2, and resuspended in 5 ml 2× YT. Four 1-ml aliquots were pelleted and were spread over TBAB plates. A serial dilution was performed using the remaining cells, and the diluted cultures were plated on TBAB supplemented with 50 mM MgCl2 so that the rate of spontaneous suppressor mutations could be quantified. After incubation at 37°C for 2 days, several colonies exhibiting different morphologies were observed. The rate of spontaneous mutation measured was ∼1.7 × 10−8 mutant/CFU. Representative strains were retained for further analysis.
Preparation for Illumina sequencing.
Cells were incubated with shaking overnight at 37°C in 5 ml 2× YT supplemented with 50 mM MgCl2. Cultures were then pelleted and were resuspended in 360 μl saline-EDTA (0.15 M NaCl, 0.01 M EDTA [pH 8.0]). A 40-μl volume of lysozyme (8 mg/ml in saline-EDTA) was added, and the samples were incubated at 37°C for 1 h. In order to separate the nucleic acids from protein, 400 μl buffered phenol was added, and the samples were vortexed briefly. The samples were then centrifuged at 15,000 × g for 10 min, and the upper aqueous layer was retained. A 400-μl volume of phenol-chloroform-isoamyl alcohol (25:24:1) was added to the aqueous layer, and the vortex and centrifugation steps were repeated. A 400-μl volume of chloroform was then added to the new aqueous layer, and the extraction steps were repeated again. Finally, the purified aqueous layer was removed, and 1 ml ethanol was added. Samples were centrifuged at 15,000 × g for 10 min. The DNA pellet was washed once in 200 μl 70% ethanol, air dried for 5 min, and resuspended in 100 μl H2O.
Prior to Illumina genomic DNA library preparation, the DNA must be sheared into fragments with an average size of 200 bp. Five micrograms of DNA was added to 130 μl of TE buffer (10 mM Tris [pH 7.4], 1 mM EDTA [pH 8.0]) in Covaris Snap-Cap microTUBEs and was fragmented in a Covaris S220 system, using the settings suggested by the manufacturer. One microgram of fragmented DNA was used as input to the Illumina TruSeq DNA sample preparation kit. Paired-end libraries were prepared according to Illumina's suggested protocol for whole-genome resequencing. Library sizes and concentrations were validated on an Agilent Technologies 2100 Bioanalyzer using the high-sensitivity DNA chip, followed by quantification of DNA by quantitative PCR (qPCR). Samples were then pooled and sequenced on an Illumina HiSeq 1000 system in a single lane. Mutations were identified using the BRESEQ computational pipeline with Bowtie 2 for sequence alignment and were confirmed by manual examination of the Bowtie 2 alignment using SAMtools. The BRESEQ gdtools utility program was used to compare the mutations present in the wild-type strain, bCAW2006, and the five suppressor strains.
Reversion of suppressor mutations.
Suppressor mutations were reverted to the wild-type sequence using congression. Briefly, natural competence was induced in the suppressor mutants by using the nitrogen-limiting conditions described by Jarmer et al. in 2002 (34). These cells were transformed with 20 μl of chromosomal DNA from strain bCAW2056 (bCAW2006 with a chloramphenicol resistance cassette integrated into the amyE locus) and were plated onto a solid rich medium (TBAB) supplemented with 50 mM MgCl2 and 10 μg/ml chloramphenicol. Transformants were patched onto TBAB chloramphenicol plates containing either 50 mM MgCl2 or no added MgCl2 to screen for colonies that were no longer able to grow under lower-magnesium conditions. Of the colonies that were patched onto these plates, 6.5% were unable to grow unless they were supplied with excess magnesium. The genomic loci that contained the suppressor-specific mutations were sequenced to determine which of these mutations had reverted to the wild-type sequence.
RESULTS AND DISCUSSION
Deletion of putative B. subtilis magnesium transporters.
The putative magnesium import system genes yfjQ, yqxL, mgtE, and yloB were mutationally disrupted using a markerless system for introducing genetic changes into B. subtilis (33). The individual deletion of each of these genes revealed that only the deletion of mgtE resulted in a significant growth defect under rich-medium growth conditions (Fig. 1A). All other single deletion mutants exhibited growth curves identical to that of the wild type. The observation that deletion of a single magnesium transporter in B. subtilis yields such a significant growth phenotype is intriguing, because individual deletion of S. Typhimurium magnesium transporters does not appear to result in a phenotypic change in either rich or minimal media; only combinations of gene deletions demonstrate a very strong phenotype (8). Therefore, under standard rich-medium conditions, B. subtilis is particularly dependent on the MgtE transport protein.
However, an additive growth defect became apparent when combinations of these gene deletions were analyzed. Specifically, combining the ΔmgtE mutation with the mutation of one of the CorA family proteins, YfjQ, resulted in a more severe growth defect (Fig. 1B). Both the ΔmgtE ΔyfjQ double mutant strain and theΔmgtE ΔyfjQ ΔyloB triple deletion strain were incapable of growth in rich media, whereas the growth of the ΔmgtE ΔyqxL and ΔmgtE ΔyloB mutants was similar to that of the ΔmgtE single deletion mutant. The ΔyfjQ mutation reduced growth only when it was combined with the ΔmgtE mutation, a finding exemplified by the ΔyloB ΔyfjQ double mutant, which grew similarly to the wild type. These data together suggest that the MgtE and YfjQ proteins are likely to serve redundant functions, although MgtE is the dominant transporter under rich-medium growth conditions. This result was somewhat surprising, because while many eubacteria possess both a CorA and an MgtE homologue, the well-characterized examples of MgtE family proteins appear to have slightly poorer affinity for magnesium than CorA proteins (8, 35).
To better quantify the magnesium dependency of these mutants, two strains exhibiting a growth defect in standard rich medium were chosen for further analysis. Specifically, bCAW2000, which contains a deletion of mgtE, was cultured in a rich medium where varying amounts of extracellular magnesium had been added to the culture (Fig. 2A). This revealed a requirement for more than 25 mM magnesium before growth resembled that of the wild type, although lower concentrations (e.g., 2.5 mM) still resulted in moderately improved growth. Similarly, analysis of VMZ1066, which contains ΔmgtE ΔyloB ΔyfjQ ΔyqxL mutations, also revealed restoration of wild-type growth with ∼25 mM magnesium (Fig. 2B). This strain contains deletions of all known magnesium transport candidates; therefore, the growth that is observed at >25 mM is likely to result from the import of magnesium through an unknown mechanism.
A subset of the putative magnesium transporters can complement magnesium transport-deficient phenotypes.
The growth experiments suggested a potential requirement for MgtE and YfjQ in magnesium transport. To measure magnesium transport activity for these proteins in a different context, and to investigate whether only MgtE and YfjQ provide magnesium transport activity, these proteins were expressed in the well-characterized magnesium transport-defective Salmonella enterica strain MM281 (a gift from Michael Maguire, Case Western Reserve University), which requires 100 mM extracellular magnesium for growth. It is possible that some of the putative magnesium transporters may actually provide magnesium transport activity but are simply not expressed in B. subtilis under the growth conditions investigated here. Therefore, each gene was subcloned into a multiple-copy vector and individually expressed in MM281 (Fig. 3A). Only MgtE and YfjQ were capable of rescuing growth in rich medium lacking excess magnesium.
FIG 3.
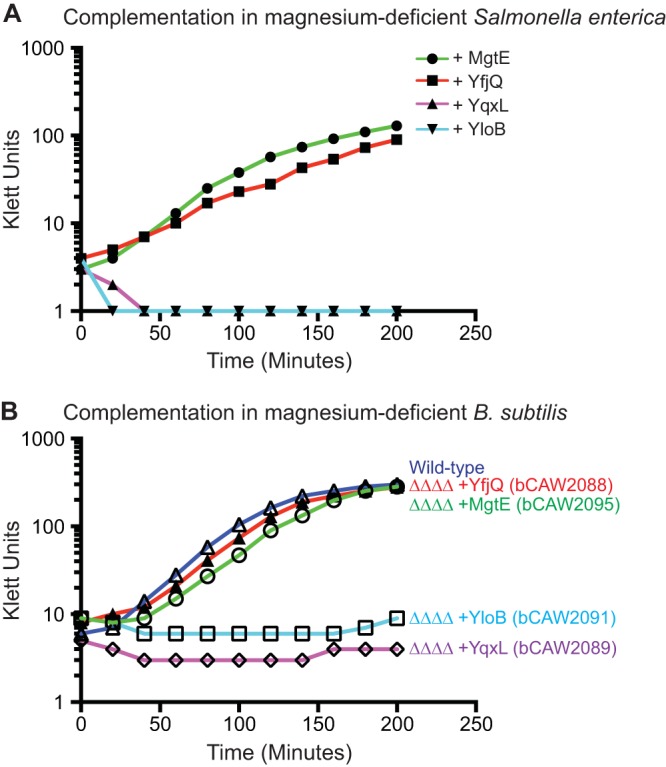
A subset of B. subtilis putative magnesium transporters restore growth to transporter-deficient strains. (A) Plasmids encoding MgtE, YfjQ, YqxL, and YloB were introduced into a magnesium transporter-deficient Salmonella enterica mutant, which requires ∼100 mM magnesium for growth and replication. Only expression of MgtE or YfjQ restored growth in rich medium to this strain. (B) Similarly, only expression of MgtE or YfjQ restored growth to VMZ1066 (ΔmgtE ΔyloB ΔyfjQ ΔyqxL), which requires ∼50 mM magnesium for growth and replication. For these experiments, the candidate transporter genes were ectopically integrated in a single copy within the nonessential amyE gene, under a controllable promoter. The growth of the cultures was measured by Klett readings.
A similar complementation experiment was also performed with B. subtilis VMZ1066 (ΔmgtE ΔyloB ΔyfjQ ΔyqxL), where each gene was individually ectopically integrated into the genome under the control of an IPTG-responsive promoter (Fig. 3B). This revealed that, again, only MgtE and YfjQ were capable of complementing the loss of magnesium transport activity to levels that were sufficient for wild-type growth and replication. Indeed, only MgtE and YfjQ restored growth even when the strains had been incubated in the presence of 2.5 mM magnesium (data not shown). Interestingly, the leaky basal level of gene expression exhibited by the Pspac promoter was sufficient to fully restore the growth of VMZ1066 (data not shown). In fact, induction of mgtE with IPTG at levels greater than 5 μM proved moderately deleterious to growth. We speculate from these aggregate observations that MgtE is the primary magnesium transporter under standard conditions but that its expression levels must be appropriately maintained. Interestingly, the mgtE gene is the only putative magnesium transporter that is subjected to a known genetic regulatory mechanism. Specifically, a cis-acting regulatory RNA (i.e., riboswitch) located within the 5′ leader region controls an intrinsic transcription terminator in a magnesium-dependent manner (5, 6). Therefore, for B. subtilis, a magnesium-responsive genetic control element appears to coordinate closely with the transporter that is in greatest demand, and whose levels must be maintained within a certain expression range.
An alternate magnesium uptake route.
Early studies on prokaryotic magnesium transport revealed that rates of magnesium transport in B. subtilis differ significantly depending on the growth medium and cell differentiation status (36). While our mutant analysis has demonstrated a role for specific MgtE and CorA homologues, the ability to control magnesium transport may still involve other proteins in addition to the primary transport classes. For example, B. subtilis citrate transport is coupled to divalent ion import (37). While a number of divalent ions are capable of participating in citrate uptake, the B. subtilis CitM protein prefers the magnesium-citrate complex and may therefore serve as a potentially meaningful route of magnesium import (38). However, citM is not expressed constitutively. The expression of CitM is increased in the presence of extracellular citrate through the action of the CitST two-component system and is decreased in the presence of glucose by CcpA-mediated repression (39). Therefore, CitM is likely to serve as a source of magnesium import only under certain growth conditions. To test the phenotype of ΔcitM mutants under defined medium conditions, the various transporter mutant strains introduced here were examined in glucose minimal medium. In this medium, the ΔmgtE mutant strains grew similarly to a wild-type strain, exhibiting no obvious defect in the growth rate (Fig. 4A). Even the ΔmgtE ΔyloB ΔyfjQ ΔyqxL strain exhibited no obvious defect under these conditions. Similarly, the ΔcitM strain exhibited no defect in growth. However, the ΔmgtE ΔcitM double mutant strain exhibited significantly poorer growth (Fig. 4A), which could be complemented by ectopic, inducible expression of CitM (Fig. 4B). Together, these data suggest that mgtE and citM represent fully redundant routes of magnesium import and that the citrate transport protein alone is sufficient to satisfy the cellular demand for magnesium, under conditions where citM is expressed.
FIG 4.
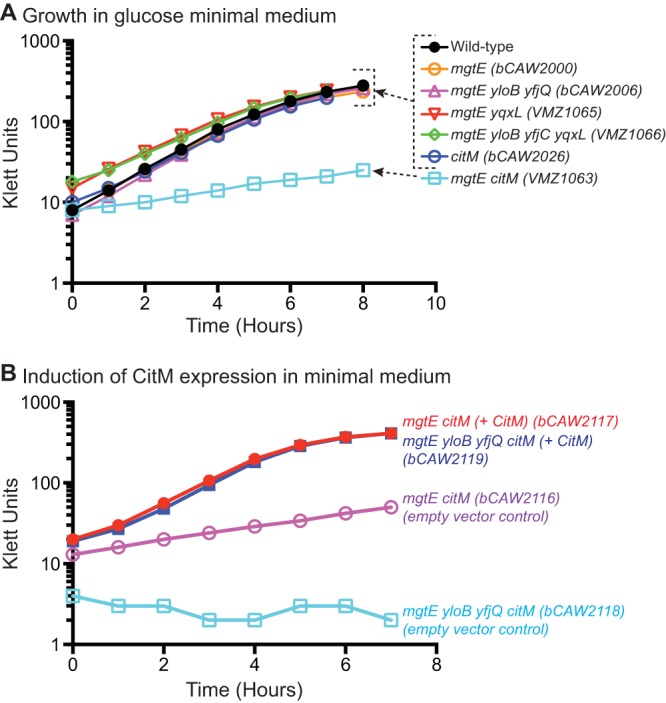
CitM can fully complement a deficiency in magnesium transport activity. (A) The growth of strains containing deletions in transporter genes was measured by Klett readings for cells cultured in glucose minimal medium. (B) An inducible version of the citM gene was ectopically integrated in a single copy into a nonessential gene (amyE) for several of the mutant strains, and then growth was measured with and without induction of CitM expression.
Suppressor analysis of a magnesium transporter-deficient strain.
In this study, we began with the assumption that magnesium transport activity is likely to be provided by one or more of the three primary classes of putative magnesium transport proteins, CorA, MgtE, and MgtA/B. However, it remains possible that additional routes of magnesium transport still remain to be discovered in bacteria. To investigate this for B. subtilis, bCAW2006 (ΔmgtE ΔyloB ΔyfjQ), which requires >25 mM magnesium for growth and replication, was cultured on a solid rich medium lacking magnesium supplementation. Colonies emerged on these plates at a frequency of 1.7 × 10−8 (standard deviation, 5.7 × 10−9) and were assumed to result from suppressor mutations. A small number of suppressor strains were randomly chosen for further characterization. Growth curve analyses of these strains revealed partial restoration of growth. However, these strains still required supplementation with 2.5 mM magnesium when grown in liquid medium in order for the suppressor phenotype to be observed (Fig. 5A); they correspondingly grew poorly in a rich medium lacking 2.5 mM magnesium (data not shown). This suggests that the suppressor mutations only moderately increased the level of magnesium import on the background of the transporter-deficient strain.
FIG 5.
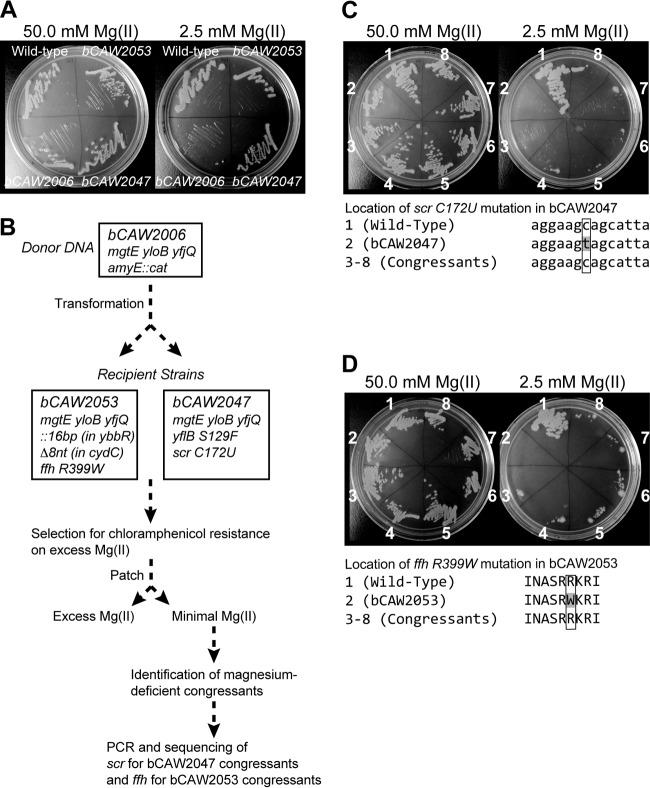
Selection of mutant strains that suppress a strong defect in magnesium transport activity. (A) A strain that was defective in mgtE yloB and yfjQ, and that required >25 mM magnesium for growth and replication, was incubated on solid medium in the absence of supplemental magnesium. A small number of suppressor mutants emerged from this selection experiment and were investigated further. In this experiment, four suppressors (bCAW2047, bCAW2051, bCAW2053, and bCAW2080) were cultured in a rich medium in the presence of 2.5 mM magnesium. (B) In an attempt to identify the basis of the suppressor phenotypes, these strains were subjected to genome sequencing analysis using Illumina sequencing. A small number of genomic changes were identified in the suppressor strains and are listed here.
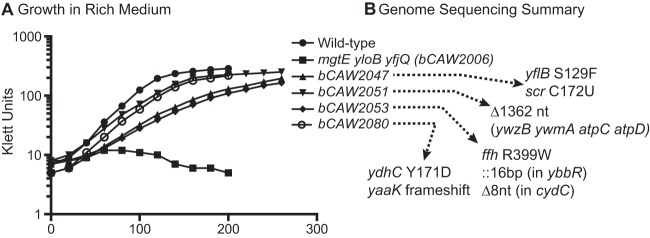
To begin to identify the basis of the suppressor phenotype, chromosomal DNA was extracted from four representative suppressor strains (bCAW2047, bCAW2051, bCAW2053, and bCAW2080) and was subjected to Illumina DNA sequencing, followed by genome resequencing analysis using the BRESEQ computational pipeline (40). Genomic DNA from the initial bCAW2006 strain was included alongside this analysis. As expected, cDNA reads were lacking for the internal portions of the mgtE, yloB, and yfjQ genes, providing further confirmation of the bCAW2006 genotype. The BRESEQ pipeline detected no additional mutations in bCAW2006. However, we noted several additional genomic differences exhibited by each of the suppressor strains.
The genomic analysis of the bCAW2047 suppressor strain revealed both an amino acid change in yflB (S129F), an unknown, uncharacterized gene, and a nucleotide change (C172U) in scr, which corresponds to the RNA subunit of the signal recognition particle (SRP) (Fig. 5B). Interestingly, another suppressor strain, bCAW2053, exhibited an amino acid change (R399W) within the protein subunit (ffh) rather than the RNA subunit (scr) of the signal recognition particle. bCAW2053 also contained several additional mutations, including a 16-nucleotide insertion in an uncharacterized gene, ybbR. Structural resolution of SRP complexes have revealed that ffh R399 and scr C172, and their immediately adjacent residues, associate directly with one another within the SRP ribonucleoprotein complex (41, 42). Specifically, the exact nucleotides surrounding position 172 are located within a conserved portion of 4.5S RNA helix 8, which is positioned only a few angstroms from the amino acid residues surrounding position 399, located within the “M” domain of ffh. Therefore, we speculated that the suppressor phenotype exhibited by both of these strains was somehow tied to functional alteration of the signal recognition particle. To test this hypothesis, chromosomal DNA from the initial bCAW2006 strain, containing a chloramphenicol resistance marker integrated at the amyE locus, was transformed back into the bCAW2047 and bCAW2053 suppressor strains in a congression experiment (Fig. 6A). In a typical congression experiment, a selectable marker is transformed into a recipient strain by using excessive amounts of donor DNA and the resulting transformants are screened for the presence of a second, unlinked mutation that recombines at a lower frequency. For our experiment, chloramphenicol-resistant transformants were patched onto a rich medium in the presence and absence of supplemental magnesium, and at a rate consistent with standard B. subtilis congression experiments, magnesium-dependent transformants were identified. The ffh and scr loci from these transformants were PCR amplified and sequenced in order to investigate whether changes at these loci correlated with the loss of the suppressor phenotype. Indeed, all of the magnesium-dependent congressants exhibited restoration of wild-type sequences at these loci (ffh for bCAW2053 and scr for bCAW2047), rather than the mutated sequences found in the original suppressor isolates (Fig. 6C and D). Moreover, the growth of these isolates closely resembled that of the initial, transporter-deficient strain bCAW2006 (Fig. 6A). This experiment strongly suggested that the ffh and scr single nucleotide polymorphisms (SNPs) identified in this study were responsible for the suppressor phenotype. Therefore, taken together, these data suggest that mutational alteration of signal recognition particle subunits can partially suppress a defect in magnesium transport, although the molecular basis for this phenotype is not intuitively obvious and will require further analysis.
FIG 6.
Isolation of revertant mutations for bCAW2047 and bCAW2053 suppressor strains. (A) Growth of the wild type, bCAW2006 (ΔmgtE ΔyloB ΔyfjQ), and the bCAW2047 and bCAW2053 suppressor strains on a rich medium containing either 50 mM or 2.5 mM magnesium. (B) Schematic for the method of isolating revertant mutants. Briefly, a congression experiment was performed whereby the suppressor strains (either bCAW2047 or bCAW2053) served as recipients for saturating levels of chromosomal DNA from a donor strain. The donor strain in this experiment was bCAW2006 (ΔmgtE ΔyloB ΔyfjQ), which is the same background strain used for the isolation of bCAW2047 and bCAW2053 and which contained a chloramphenicol resistance marker integrated into the nonessential amyE gene. Transformants were selected for transfer of the chloramphenicol resistance marker and were then patched onto rich medium plates that either contained or lacked supplemental magnesium (25 mM). (C and D) Of the congressants, 6.5% exhibited a magnesium-deficient phenotype, similar to bCAW2006. Sequencing of the scr and ffh loci, which are unlinked to the chloramphenicol resistance gene, revealed reversion to the wild-type sequence for all of the magnesium-deficient congressants. Therefore, the scr and ffh mutations in bCAW2047 and bCAW2053, respectively, are likely to be functionally responsible for the suppressor phenotype.
While bCAW2047 and bCAW2053 permitted growth in a rich medium supplemented with 2.5 mM magnesium, they still exhibited a lower rate of growth than the wild-type strain. In contrast, the bCAW2051 suppressor strain more closely resembled wild-type growth under these conditions (Fig. 5A). Sequencing of bCAW2051 revealed a large deletion (∼1,300 bp), located at the end of the operon that encodes ATP synthase (Fig. 7A). This deletion affects four genes. Two of these genes are unknown, uncharacterized genes (ywzB and ywmA), while the remaining genes, atpC and atpD, encode the epsilon and beta subunits of the F1 unit of ATP synthase, respectively. To test the importance of this observation, this region of the genome was deleted from the original transporter-deficient strain, bCAW2006. The newly created strain indeed exhibited enhanced growth in the absence of supplemental magnesium (Fig. 7B). This suggests strongly that the bCAW2051 suppressor phenotype is caused by the genomic deletion at this locus.
FIG 7.
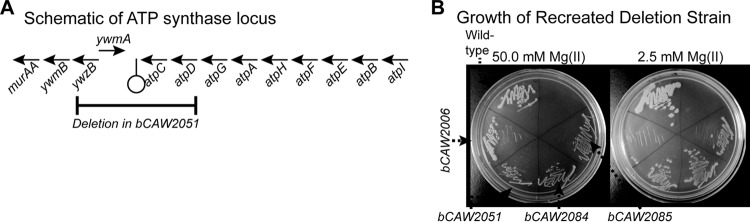
A genomic deletion near the ATP synthase locus is responsible for suppressing a defect in magnesium transport activity. (A) As shown in this schematic, the bCAW2051 suppressor mutant contains a large deletion that encompasses the last two genes of the atp operon as well as two neighboring genes, ywzB and ywmA. (B) To verify that the deletion at the atp locus was responsible for the bCAW2051 suppressor phenotype, the deletion was recreated in a clean bCAW2006 background strain, resulting in two identical strains, bCAW2084 and bCAW2085. Both of these mutants grew on media with lowered magnesium concentrations, in contrast to their background strain, bCAW2006, confirming a functional relationship between the deleted genes and the suppressor phenotype.
Although it is possible that the bCAW2051 phenotype is due to the ΔymzB ΔywmA mutations, we speculate that deletion of B. subtilis atpCD is likely to be responsible. A recent publication has demonstrated a direct interaction between MgtC proteins and subunits of F1Fo ATP synthase, which results in a biologically important inhibition of ATP synthase function (43). Interestingly, the mgtC gene is also known to be under the control of magnesium-responsive regulation and assists survival under low-magnesium conditions (8, 44). Therefore, it is possible that the deletion in bCAW2051 may somehow affect the interaction site of a regulatory protein that is similar to MgtC. However, it is also possible that the deletion may directly affect ATP synthase enzymatic activity. For example, the epsilon subunit functions as an intrinsic inhibitor of ATP synthesis in vitro (45–47) and counteracts MgADP-mediated repression of ATP synthase activity in B. subtilis (48). Regardless, it is not obvious why deletion of these ATP synthase components would affect magnesium import, and further study will be required to determine the relationship between ATP synthesis and magnesium homeostasis.
A final suppressor strain, bCAW2080, was also analyzed in this study. Sequencing analysis of this strain revealed several putative mutations, including a frameshift mutation in the uncharacterized yaaK gene and a 21-kb deletion between ydcL and yddM; however, it is not obvious what connection might exist between magnesium transport and these particular mutations.
Concluding remarks.
Magnesium is an essential divalent metal for all cells. Therefore, all organisms must produce proteins that function as transporters of the ion. There are three known major classes of bacterial magnesium transport proteins: CorA, MgtE, and MgtA/B. B. subtilis, the primary model system for Gram-positive bacteria, appears to encode at least one homologue of the three transport categories. However, the functional importance of these transporters has yet to be systematically examined. While mgtE is regulated by a magnesium-responsive riboswitch (5, 6), the remaining genes have not been shown to be under magnesium-dependent regulation. In this study, we find that MgtE constitutes the primary route of magnesium import but that a CorA homologue (YfjQ) is also fully capable of complementing a magnesium transport defect, both in B. subtilis and in a heterologous host (S. enterica). Additionally, we find that the CitM citrate transporter is fully sufficient for magnesium import and can functionally replace all other magnesium transporters under conditions where it is expressed. Finally, to investigate whether other uncharacterized magnesium transporters might also be encoded in the B. subtilis genome, we selected for suppressor mutants that could restore growth to a strain that was defective in magnesium transport. We did not observe mutations in genes that would be predicted to function as dedicated divalent transporters. Instead, this genetic selection identified mutations in signal recognition particle and F1Fo ATP synthase components, which permitted moderately improved growth in a medium lacking supplemental magnesium. While it is not immediately obvious why these particular genetic changes might result in the magnesium suppressor phenotype, they reveal a curious relationship between SRP functional activity and divalent homeostasis. Moreover, they provide further support for a growing body of circumstantial evidence showing a regulatory relationship between magnesium homeostasis and ATP synthase function. Since metal ion homeostasis mechanisms are generally critical for microbial biology and pathogenesis, these data represent an important reference point for the bacterial transport of magnesium.
ACKNOWLEDGMENTS
We thank Michael Maguire for the magnesium transport-deficient Salmonella strain MM281.
This work was funded by NIH R01 GM081882.
Footnotes
Published ahead of print 10 January 2014
REFERENCES
- 1.Moore CM, Helmann JD. 2005. Metal ion homeostasis in Bacillus subtilis. Curr. Opin. Microbiol. 8:188–195. 10.1016/j.mib.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 2.Pennella MA, Giedroc DP. 2005. Structural determinants of metal selectivity in prokaryotic metal-responsive transcriptional regulators. Biometals 18:413–428. 10.1007/s10534-005-3716-8 [DOI] [PubMed] [Google Scholar]
- 3.Giedroc DP, Arunkumar AI. 2007. Metal sensor proteins: nature's metalloregulated allosteric switches. Dalton Trans. 2007(29):3107–3120. 10.1039/B706769K [DOI] [PubMed] [Google Scholar]
- 4.Cromie MJ, Shi Y, Latifi T, Groisman EA. 2006. An RNA sensor for intracellular Mg2+. Cell 125:71–84. 10.1016/j.cell.2006.01.043 [DOI] [PubMed] [Google Scholar]
- 5.Dann CE, Wakeman CA, Sieling CL, Baker SC, Irnov I, Winkler WC. 2007. Structure and mechanism of a metal-sensing regulatory RNA. Cell 130:878–892. 10.1016/j.cell.2007.06.051 [DOI] [PubMed] [Google Scholar]
- 6.Ramesh A, Winkler WC. 2010. Magnesium-sensing riboswitches in bacteria. RNA Biol. 7:77–83. 10.4161/rna.7.1.10490 [DOI] [PubMed] [Google Scholar]
- 7.Froschauer EM, Kolisek M, Dieterich F, Schweigel M, Schweyen RJ. 2004. Fluorescence measurements of free [Mg2+] by use of mag-fura 2 in Salmonella enterica. FEMS Microbiol. Lett. 237:49–55. 10.1111/j.1574-6968.2004.tb09677.x [DOI] [PubMed] [Google Scholar]
- 8.Maguire ME. 2006. Magnesium transporters: properties, regulation and structure. Front. Biosci. 11:3149–3163. 10.2741/2039 [DOI] [PubMed] [Google Scholar]
- 9.Moomaw AS, Maguire ME. 2008. The unique nature of Mg2+ channels. Physiology (Bethesda, Md.) 23:275–285. 10.1152/physiol.00019.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner RC. 2003. Genes for magnesium transport. Curr. Opin. Plant Biol. 6:263–267. 10.1016/S1369-5266(03)00032-3 [DOI] [PubMed] [Google Scholar]
- 11.Hmiel SP, Snavely MD, Miller CG, Maguire ME. 1986. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 168:1444–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehres DG, Maguire ME. 2002. Structure, properties and regulation of magnesium transport proteins. Biometals 15:261–270. 10.1023/A:1016078832697 [DOI] [PubMed] [Google Scholar]
- 13.MacDiarmid CW. 1998. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J. Biol. Chem. 273:1727–1732. 10.1074/jbc.273.3.1727 [DOI] [PubMed] [Google Scholar]
- 14.Smith RL, Maguire ME. 1995. Distribution of the CorA Mg2+ transport system in gram-negative bacteria. J. Bacteriol. 177:1638–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith RL, Maguire ME. 1998. Microbial magnesium transport: unusual transporters searching for identity. Mol. Microbiol. 28:217–226. 10.1046/j.1365-2958.1998.00810.x [DOI] [PubMed] [Google Scholar]
- 16.Knoop V, Groth-Malonek M, Gebert M, Eifler K, Weyand K. 2005. Transport of magnesium and other divalent cations: evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol. Genet. Genomics 274:205–216. 10.1007/s00438-005-0011-x [DOI] [PubMed] [Google Scholar]
- 17.Kehres DG, Lawyer CH, Maguire ME. 1998. The CorA magnesium transporter gene family. Microb. Comp. Genomics 3:151–169. 10.1089/omi.1.1998.3.151 [DOI] [PubMed] [Google Scholar]
- 18.Dalmas O, Cuello L, Jogini V, Cortes D, Roux B, Perozo E. 2010. Structural dynamics of the magnesium-bound conformation of CorA in a lipid bilayer. Structure 18:868–878. 10.1016/j.str.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunin VV, Dobrovetsky E, Khutoreskaya G, Zhang R, Joachimiak A, Doyle DA, Bochkarev A, Maguire ME, Edwards AM, Koth CM. 2006. Crystal structure of the CorA Mg2+ transporter. Nature 440:833–837. 10.1038/nature04642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payandeh J, Pai EF. 2006. A structural basis for Mg2+ homeostasis and the CorA translocation cycle. EMBO J. 25:3762–3773. 10.1038/sj.emboj.7601269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eshaghi S, Niegowski D, Kohl A, Molina DM, Lesley SA, Nordlund P. 2006. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science 313:354–357. 10.1126/science.1127121 [DOI] [PubMed] [Google Scholar]
- 22.Worlock AJ, Smith RL. 2002. ZntB is a novel Zn2+ transporter in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:4369–4373. 10.1128/JB.184.16.4369-4373.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyyryläinen H-L, Sarvas M, Kontinen VP. 2005. Transcriptome analysis of the secretion stress response of Bacillus subtilis. Appl. Microbiol. Biotechnol. 67:389–396. 10.1007/s00253-005-1898-1 [DOI] [PubMed] [Google Scholar]
- 24.Höper D, Völker U, Hecker M. 2005. Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis. J. Bacteriol. 187:2810–2826. 10.1128/JB.187.8.2810-2826.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steil L, Hoffmann T, Budde I, Völker U, Bremer E. 2003. Genome-wide transcriptional profiling analysis of adaptation of Bacillus subtilis to high salinity. J. Bacteriol. 185:6358–6370. 10.1128/JB.185.21.6358-6370.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattori M, Tanaka Y, Fukai S, Ishitani R, Nureki O. 2007. Crystal structure of the MgtE Mg2+ transporter. Nature 448:1072–1075. 10.1038/nature06093 [DOI] [PubMed] [Google Scholar]
- 27.Ishitani R, Sugita Y, Dohmae N, Furuya N, Hattori M, Nureki O. 2008. Mg2+-sensing mechanism of Mg2+ transporter MgtE probed by molecular dynamics study. Proc. Natl. Acad. Sci. U. S. A. 105:15393–15398. 10.1073/pnas.0802991105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papp-Wallace KM, Maguire ME. 2008. Regulation of CorA Mg2+ channel function affects the virulence of Salmonella enterica serovar Typhimurium. J. Bacteriol. 190:6509–6516. 10.1128/JB.00144-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor K, Fletcher SA, Csonka LN. 2009. Increased expression of Mg2+ transport proteins enhances the survival of Salmonella enterica at high temperature. Proc. Natl. Acad. Sci. U. S. A. 106:17522–17527. 10.1073/pnas.0906160106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raeymaekers L, Wuytack E, Willems I, Michiels CW, Wuytack F. 2002. Expression of a P-type Ca2+-transport ATPase in Bacillus subtilis during sporulation. Cell Calcium 32:93. 10.1016/S0143-4160(02)00125-2 [DOI] [PubMed] [Google Scholar]
- 31.Warner JB, Krom BP, Magni C, Konings WN, Lolkema JS. 2000. Catabolite repression and induction of the Mg2+-citrate transporter CitM of Bacillus subtilis. J. Bacteriol. 182:6099–6105. 10.1128/JB.182.21.6099-6105.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Pajor AM. 2002. Functional characterization of CitM, the Mg2+-citrate transporter. J. Membr. Biol. 185:9–16. 10.1007/s00232-001-0106-1 [DOI] [PubMed] [Google Scholar]
- 33.Arnaud M, Chastanet A, Débarbouillé M. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl. Environ. Microbiol. 70:6887–6891. 10.1128/AEM.70.11.6887-6891.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarmer H, Berka R, Knudsen S, Saxild HH. 2002. Transcriptome analysis documents induced competence of Bacillus subtilis during nitrogen limiting conditions. FEMS Microbiol. Lett. 206:197–200. 10.1111/j.1574-6968.2002.tb11009.x [DOI] [PubMed] [Google Scholar]
- 35.Smith RL, Thompson LJ, Maguire ME. 1995. Cloning and characterization of MgtE, a putative new class of Mg2+ transporter from Bacillus firmus OF4. J. Bacteriol. 177:1233–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scribner H, Eisenstadt E, Silver S. 1974. Magnesium transport in Bacillus subtilis W23 during growth and sporulation. J. Bacteriol. 117:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willecke K, Gries EM, Oehr P. 1973. Coupled transport of citrate and magnesium in Bacillus subtilis. J. Biol. Chem. 248:807–814 [PubMed] [Google Scholar]
- 38.Boorsma A, Van Der Rest ME, Lolkema JS, Konings WN. 1996. Secondary transporters for citrate and the Mg2+-citrate complex in Bacillus subtilis are homologous proteins. J. Bacteriol. 178:6216–6222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto H, Murata M, Sekiguchi J. 2000. The CitST two-component system regulates the expression of the Mg-citrate transporter in Bacillus subtilis. Mol. Microbiol. 37:898–912. 10.1046/j.1365-2958.2000.02055.x [DOI] [PubMed] [Google Scholar]
- 40.Barrick JE, Yu DS, Yoon SH, Jeong H, Oh TK, Schneider D, Lenski RE, Kim JF. 2009. Genome evolution and adaptation in a long-term experiment with Escherichia coli. Nature 461:1243–1247. 10.1038/nature08480 [DOI] [PubMed] [Google Scholar]
- 41.Batey RT. 2000. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science 287:1232–1239. 10.1126/science.287.5456.1232 [DOI] [PubMed] [Google Scholar]
- 42.Ataide SF, Schmitz N, Shen K, Ke A, Shan S, Doudna JA, Ban N. 2011. The crystal structure of the signal recognition particle in complex with its receptor. Science 331:881–886. 10.1126/science.1196473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee E-J, Pontes MH, Groisman EA. 2013. A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium's own F1Fo ATP synthase. Cell 154:146–156. 10.1016/j.cell.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Retamal P, Castillo-Ruiz M, Mora GC. 2009. Characterization of MgtC, a virulence factor of Salmonella enterica serovar Typhi. PLoS One 4:e5551. 10.1371/journal.pone.0005551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JB, Sternweis PC, Heppel LA. 1975. Partial purification of active delta and epsilon subunits of the membrane ATPase from Escherichia coli. J. Supramol. Struct. 3:248–255. 10.1002/jss.400030307 [DOI] [PubMed] [Google Scholar]
- 46.Iino R, Hasegawa R, Tabata KV, Noji H. 2009. Mechanism of inhibition by C-terminal α-helices of the ε subunit of Escherichia coli FoF1-ATP synthase. J. Biol. Chem. 284:17457–17464. 10.1074/jbc.M109.003798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taniguchi N, Suzuki T, Berney M, Yoshida M, Cook GM. 2011. The regulatory C-terminal domain of subunit ε of FoF1 ATP synthase is dispensable for growth and survival of Escherichia coli. J. Bacteriol. 193:2046–2052. 10.1128/JB.01422-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizumoto J, Kikuchi Y, Nakanishi Y-H, Mouri N, Cai A, Ohta T, Haruyama T, Kato-Yamada Y. 2013. ε subunit of Bacillus subtilis F1-ATPase relieves MgADP inhibition. PLoS One 8:e73888. 10.1371/journal.pone.0073888 [DOI] [PMC free article] [PubMed] [Google Scholar]