Abstract
Thirty-two carbapenem-resistant Klebsiella pneumoniae isolates, representative of different resistance mechanisms and clonal lineages, were analyzed with the Pathogenica HAI BioDetection system, based on targeted next-generation sequencing (NGS) technology. With most strains, the system simultaneously yielded comprehensive information on relevant β-lactam resistance determinants and accurate discrimination of clonal lineages, in a shorter time frame and in a less labor-intensive manner than currently available methods for molecular epidemiology analysis. Results supported the usefulness of targeted NGS-based technologies for similar applications.
TEXT
The dissemination of carbapenem-resistant Enterobacteriaceae (CRE) has become a major public health problem of global dimensions, although with notable geographical variability (1, 2). Carbapenem-resistant Klebsiella pneumoniae (CRKP) strains constitute the majority of CRE in clinical settings (3, 4), and their epidemic diffusion is often sustained by the expansion of successful “high-risk” carbapenemase-producing clones, such as K. pneumoniae strains of sequence type (ST) 258 producing KPC-type enzymes (5, 6).
Surveillance and efficient infection control practices are of paramount importance to limit the spread of CRE within health care structures (7, 8), their crucial role being emphasized by the dearth of available therapeutic options (9) and by the high mortality rates observed in infected patients (2, 10–12). To this purpose, rapid identification of resistance mechanisms and of high-risk clonal lineages of CRE are essential steps. However, the two reference methods for CRE typing, i.e., multilocus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE), are time-consuming, expensive, and technically demanding procedures (13), and the same is partially true for the conventional methods for molecular detection and identification of resistance determinants (14).
Next-generation sequencing (NGS) technologies can simultaneously provide comprehensive information on the presence of resistance genes and on the clonal lineage of different isolates (15–18) and are promising for similar applications.
The Pathogenica HAI BioDetection system (Pathogenica Inc., Boston, MA, USA) is a commercial in vitro diagnostic (IVD) CE marked system, which yields simultaneous information on species identification, relevant resistance determinants, and clonal profiling of several bacterial pathogens. The system includes a set of probes targeted to bind specific genes. Each probe enriches by PCR the abundance of these loci and appends adaptor sequences to enable sequencing of this library of amplicons using an NGS platform. The product software performs multiple alignments across these sequenced amplicons and matches the contigs obtained with an internal database. Results are automatically incorporated in an electronic format, enabling comparison with new tested isolates, and also includes analytical software able to automatically and quickly process the sequence output into simple reports of species and resistance genes present.
In this work, we investigated the performance of this system for rapid characterization of the β-lactam resistome and clonal profiling of a collection of CRKP representative of different β-lactam resistance mechanisms and clonal lineages.
Thirty-two CRKP strains collected between 2009 and 2012, from various health care centers distributed across Italy, were investigated. Four strains of other Gram-negative species (Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa, and Acinetobacter baumannii), previously identified by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Vitek MS, bioMérieux, Marcy l'Etoile, France) and/or amplification and sequencing of 16S rRNA, were also included as a control for identification of common Gram-negative species other than K. pneumoniae by the system.
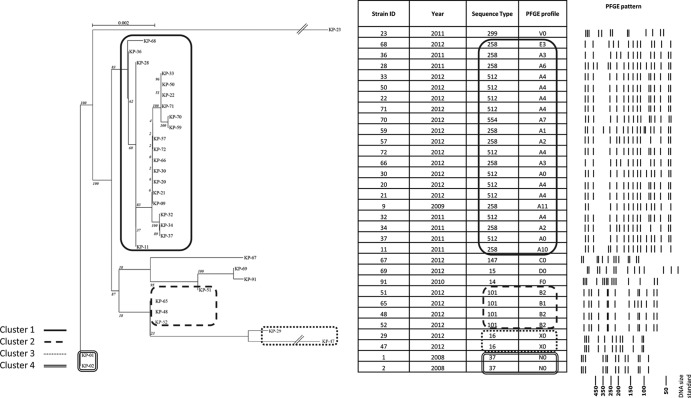
All CRKP strains had been previously characterized in the Microbiology Laboratory of the Department of Medical Biotechnologies, University of Siena, by MLST (19) and PFGE (20) and for carbapenem resistance mechanisms and had been selected as being representative of different resistance mechanisms and clonal lineages (Fig. 1). Interpretation of PFGE results had been made according to the work of van Belkum et al. (21). The presence of carbapenemases and other relevant ß-lactamase genes (including blaIMP, blaVIM, blaOXA-48, blaNDM, blaKPC, and blaFOX) had been investigated using primers and methods previously described (5, 22). Alleles encoding the outer membrane proteins OmpK35 and OmpK36 had been characterized as previously described (23).
FIG 1.
Cluster groups of isolates obtained from different outbreak episodes. Isolates in cluster 1 are representative of the recent nationwide oligoclonal diffusion of KPC-producing isolates in Italy (5). Isolates in clusters 2, 3, and 4 were obtained from different earlier outbreaks of CRKP infection in Italian hospitals (22; also this work). The phylogenetic tree was generated using RAxML software. After tree construction, each tree's leaves were ordered using the optimal leaf-ordering algorithm of Bar-Joseph et al. (28). Numbers at nodes represent the probability of each node, based on the bootstrap method used for resampling.
To verify the performance of Pathogenica HAI BioDetection system in the detection of other β-lactamases, when revealed, the presence of blaCTX-M, blaSHV, and blaTEM was confirmed by PCR and sequencing (24, 25). Amplicons were sequenced on both strands at an external facility (Macrogen Inc., Seoul, South Korea), and sequences were compared with those available in GenBank using BLAST.
For analysis with the Pathogenica HAI BioDetection system, genomic DNA of each strain was extracted, processing 400 μl of a 0.5 McFarland suspension in normal saline using the Complex400_V3_DSP protocol with the DSP virus/pathogen midikit on a QIAsymphony SP (Qiagen, Valencia, CA). Each sample was diluted to a working DNA concentration of 0.5 ng/μl and prepared with the Pathogenica HAI BioDetection kit according to the manufacturer's instructions. Template preparation was carried out with the Ion PGM template OT2 200 kit (Life Technologies, Gaithersburg, MD, USA), and sequencing was performed using the Ion PGM sequencing 200 kit v2 with a PGM sequencer (Life Technologies) according to the manufacturer's instructions. Data were analyzed using an internal version of the Pathogenica HAI BioDetection software v1.2. The RAxML software was used to generate a phylogenetic tree for the samples with a GTR+Γ model (26).
The analysis was run under blinded conditions. The time to perform the analysis of each strain, from purified DNA sample to results provided by the system, was 12.5 h.
All strains tested were correctly identified to the species level by the Pathogenica system. Concerning analysis of the β-lactam resistance mechanisms, the system correctly detected the presence of all NDM-1, KPC-type, and VIM-type ß-lactamase genes. All TEM-type, SHV-type, and CTX-M-type variants detected by the system were confirmed (Table 1). On the other hand, the genes encoding the OXA-48 and FOX-7 ß-lactamases were not detected, since the current version of the system does not include probes targeting those genes. The system was also unable to detect genetic alterations of porin genes leading to permeability defects that can cause decreased carbapenem susceptibility in strains producing extended-spectrum β-lactamases (ESBLs).
TABLE 1.
Results of analysis of β-lactamase content of the 32 CRKP strains by the Pathogenica HAI BioDetection system
| Strain | Beta-lactam resistance determinants detected with: |
|
|---|---|---|
| PCR and sequencing | Pathogenica | |
| KP-01 | SHV-12, CTX-M-15, TEM-1 plus OmpK35 porin loss | SHV, CTX-M, TEM |
| KP-02 | SHV-12, CTX-M-15, TEM-1 plus OmpK35 porin loss | SHV, CTX-M, TEM |
| KP-09 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-11 | KPC-2, SHV-12, TEM-1 | KPC, SHV, TEM |
| KP-20 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-21 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-22 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-23 | VIM-1, SHV-12 | VIM, SHV |
| KP-28 | KPC-2, SHV-12, TEM-1 | KPC, SHV, TEM |
| KP-29 | OXA-48, CTX-M-15, SHV-1, TEM-1 | CTX-M, SHV, TEM |
| KP-30 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-32 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-33 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-34 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-36 | KPC-3, SHV-12 | KPC, SHV |
| KP-37 | KPC-2, SHV-12, TEM-1 | KPC, SHV, TEM |
| KP-47 | OXA-48, CTX-M-15, SHV-1, TEM-1 | CTX-M, SHV, TEM |
| KP-48 | KPC-2, SHV-1, TEM-1 | KPC, SHV, TEM |
| KP-50 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-51 | KPC-2, SHV-1, TEM-1 | KPC, SHV, TEM |
| KP-52 | KPC-2, SHV-1, TEM-1 | KPC, SHV, TEM |
| KP-57 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-59 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-65 | KPC-2, SHV-1, TEM-1 | KPC, SHV, TEM |
| KP-66 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-67 | KPC-3, SHV-1, TEM-1 | KPC, SHV, TEM |
| KP-68 | KPC-2, SHV-1 | KPC, SHV |
| KP-69 | KPC-2, SHV-1, TEM-1 | KPC, SHV, TEM |
| KP-70 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-71 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-72 | KPC-3, SHV-11, TEM-1 | KPC, SHV, TEM |
| KP-91 | FOX-7, SHV-1, TEM-1 plus OmpK35 porin loss | SHV, TEM |
Results on ß-lactamase genotypes are provided in terms of ß-lactamase types (e.g., TEM, SHV, CTX-M, KPC, VIM, and NDM). With TEM and SHV types, the possibility of an ESBL genotype is also reported in the presence of mutations known to be associated with ESBL activity (Table 1). However, since the sequences targeted for amplification and sequencing do not cover the entire coding sequences of the blaTEM and blaSHV genes, some mutations associated with ESBL activity (e.g., that leading to G238S substitution in TEM-type enzymes) can remain undetected with the current version of the kit.
Additional sequence analysis of the contigs, automatically generated by the system with ß-lactamase genes, could provide additional information about the nature of their groups/allelic variants. For instance, with blaCTX-M-type genes, the sequence analysis allowed distinction between group 1 and group 9 CTX-M-type lineages, although they cannot discriminate the entire repertoire of known allelic variants of each group. Within blaKPC-type genes, it was possible to identify some point mutations associated with KPC allelic variability (for instance enabling distinction between blaKPC-2 and blaKPC-3). With blaVIM-type it was possible to distinguish between members of the VIM-1 and VIM-2 lineages.
Concerning the analysis of clonal relatedness, 17 distinct lineages were recognized by the Pathogenica system, with an overall high concordance with the reference typing methods (Fig. 1). In terms of discriminatory power, the Pathogenica system showed superiority over the other typing techniques, with a Simpson's index of discrimination (27) of 94.8%, compared to 82.1% and 92.7% for MLST and PFGE, respectively.
The largest barriers to the implementation of NGS technologies in clinical microbiology are represented by the relatively high cost of DNA sequencing platforms and sequencing reactions and by the difficulties encountered in translating the magnitude of data obtained with these technologies into information that will be useful to microbiologists in a timely manner. Altogether, the Pathogenica system was able to provide meaningful information on resistance determinants and clonal lineages with most of the tested CRKP strains, significantly reducing the bioinformatic burden and the costs (approximately $100 [U.S. dollars] per isolates) compared to a conventional whole-genome sequencing approach. The major advantage of the Pathogenica system is the ability to simultaneously provide identification to the species level, a repertoire of relevant resistance genes, and fine-tuned clonal profiling of bacterial isolates, in a less technically demanding and labor-intensive procedure and within a shorter time frame (approximately 25 min of technician's hands-on time for library preparation and 30 min of sample and data processing time) than the standard methods currently being used in clinical microbiology laboratories for characterization of resistance genes (e.g., conventional PCR and sequencing) and clonal profiling (e.g., PFGE and MLST).
In conclusion, this work describes the first application of an innovative commercial system based on a targeted sequencing approach using an NGS platform in the characterization of a collection of CRKP strains representative of many emerging resistance determinants and clones of clinical relevance.
ACKNOWLEDGMENTS
This work was partially supported by grants from FP7 projects TEMPOTEST-QC (no. HEALTH-2009-241742) and from EvoTAR (no. HEALTH-F3-2011-2011-282004) to G.M.R.
P.A.R., G.D., S.G., T.C., and Y.A. are employees of Pathogenica Inc., which produces the HAI BioDetection system.
Footnotes
Published ahead of print 8 January 2014
REFERENCES
- 1.Canton R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen Ø, Seifert H, Woodford N, Nordmann P, European Network on Carbapenemases 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 18:413–431. 10.1111/j.1469-0691.2012.03821.x [DOI] [PubMed] [Google Scholar]
- 2.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 13:785–796. 10.1016/S1473-3099(13)70190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798. 10.3201/eid1710.110655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microbiol. Rev. 25:682–707. 10.1128/CMR.05035-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giani T, Pini B, Arena F, Conte V, Bracco S, Migliavacca R, AMCLI-CRE Survey Participants, Pantosti A, Pagani L, Luzzaro F, Rossolini GM. 2013. Epidemic diffusion of KPC carbapenemase-producing Klebsiella pneumoniae in Italy: results of the first countrywide survey, 15 May to 30 June 2011. Euro Surveill. 18:20489 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20489 [PubMed] [Google Scholar]
- 6.Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35:736–755. 10.1111/j.1574-6976.2011.00268.x [DOI] [PubMed] [Google Scholar]
- 7.Nordmann P, Gniadkowski M, Giske CG, Poirel L, Woodford N, Miriagou V, European Network on Carbapenemases 2012. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Infect. 18:432–438. 10.1111/j.1469-0691.2012.03815.x [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin. Infect. Dis. 53:60–67. 10.1093/cid/cir202 [DOI] [PubMed] [Google Scholar]
- 9.Livermore DM, Warner M, Mushtaq S, Doumith M, Zhang J, Woodford N. 2011. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 37:415–419. 10.1016/j.ijantimicag.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 10.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 29:1099–1106. 10.1086/592412 [DOI] [PubMed] [Google Scholar]
- 11.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob. Agents Chemother. 52:1028–1033. 10.1128/AAC.01020-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro-San Francisco C, Mora-Rillo M, Romero-Gómez MP, Moreno-Ramos F, Rico-Nieto A, Ruiz-Carrascoso G, Gómez-Gil R, Arribas-López JR, Mingorance J, Paño-Pardo JR. 2013. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin. Microbiol. Infect. 19:E72–9. 10.1111/1469-0691.12091 [DOI] [PubMed] [Google Scholar]
- 13.Vimont S, Mnif B, Fevre C, Brisse S. 2008. Comparison of PFGE and multilocus sequence typing for analysis of Klebsiella pneumoniae isolates. J. Med. Microbiol. 57:1308–1310. 10.1099/jmm.0.2008/003798-0 [DOI] [PubMed] [Google Scholar]
- 14.Pulido MR, García-Quintanilla M, Martín-Peña R, Cisneros JM, McConnell MJ. 2013. Progress on the development of rapid methods for antimicrobial susceptibility testing. J. Antimicrob. Chemother. 68:270–2717. 10.1093/jac/dkt253 [DOI] [PubMed] [Google Scholar]
- 15.Mellmann A, Harmsen D, Cummings CA, Zentz EB, Leopold SR, Rico A, Prior K, Szczepanowski R, Ji Y, Zhang W, McLaughlin SF, Henkhaus JK, Leopold B, Bielaszewska M, Prager R, Brzoska PM, Moore RL, Guenther S, Rothberg JM, Karch H. 2011. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One 6:e22751. 10.1371/journal.pone.0022751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Köser CU, Holden MTG, Ellington MJ, Cartwright EJP, Brown NM, Ogilvy-Stuart AL, Hsu LY, Chewapreecha C, Croucher NJ, Harris SR, Sanders M, Enright MC, Dougan G, Bentley SD, Parkhill J, Fraser LJ, Betley JR, Schulz-Trieglaff OB, Smith GP, Peacock SJ. 2012. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N. Engl. J. Med. 366:2267–2275. 10.1056/NEJMoa1109910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, NISC Comparative Sequencing Program Group. Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci. Transl. Med. 4:148ra116. 10.1126/scitranslmed.3004129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reuter S, Ellington MJ, Cartwright EJP, Köser CU, Török ME, Gouliouris T, Harris SR, Brown NM, Holden MTG, Quail M, Parkhill J, Smith GP, Bentley SD, Peacock SJ. 2013. Rapid bacterial whole-genome sequencing to enhance diagnostic and public health microbiology. JAMA Intern. Med. 173:1397–1404. 10.1001/jamainternmed.2013.7734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diancourt L, Passet V, Verhoef J, Grimont PAD, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43:4178–4182. 10.1128/JCM.43.8.4178-4182.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan M. 1998. Epidemiological typing of Klebsiellae with extended-spectrum β-lactamases from European intensive care units. J. Antimicrob. Chemother. 41:527–539. 10.1093/jac/41.5.527 [DOI] [PubMed] [Google Scholar]
- 21.van Belkum A, Tassios PT, Dijkshoorn L, Haeggman S, Cookson B, Fry NK, Fussing V, Green J, Feil E, Gerner-Smidt P, Brisse S, Struelens M, European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group on Epidemiological Markers (ESGEM) 2007. Guidelines for the validation and application of typing methods for use in bacterial epidemiology. Clin. Microbiol. Infect. 13(Suppl 3):1–46. 10.1111/j.1469-0691.2007.01786.x [DOI] [PubMed] [Google Scholar]
- 22.Arena F, Giani T, Becucci E, Conte V, Zanelli G, D'Andrea MM, Buonocore G, Bagnoli F, Zanchi A, Montagnani F, Rossolini GM. 2013. Large oligoclonal outbreak due to Klebsiella pneumoniae ST14 and ST26 producing the FOX-7 AmpC β-lactamase in a neonatal intensive care unit. J. Clin. Microbiol. 51:4067–4072. 10.1128/JCM.01982-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaczmarek FM, Dib-Hajj F, Shang W, Gootz TD. 2006. High-level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of bla(ACT-1) beta-lactamase production, porin OmpK35/36 insertional inactivation, and down-regulation of the phosphate transport porin PhoE. Antimicrob. Agents Chemother. 50:3396–3406. 10.1128/AAC.00285-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pallecchi L, Malossi M, Mantella A, Gotuzzo E, Trigoso C, Bartoloni A, Paradisi F, Kronvall G, Rossolini GM. 2004. Detection of CTX-M-type beta-lactamase genes in fecal Escherichia coli isolates from healthy children in Bolivia and Peru. Antimicrob. Agents Chemother. 48:4556–4561. 10.1128/AAC.48.12.4556-4561.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perilli M, Dell'Amico E, Segatore B, de Massis MR, Bianchi C, Luzzaro F, Rossolini GM, Toniolo A, Nicoletti G, Amicosante G. 2002. Molecular characterization of extended-spectrum beta-lactamases produced by nosocomial isolates of Enterobacteriaceae from an Italian nationwide survey. J. Clin. Microbiol. 40:611–614. 10.1128/JCM.40.2.611-614.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 27.Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bar-Joseph Z, Demaine ED, Gifford DK, Srebro N, Hamel AM, Jaakkola TS. 2003. K-ary clustering with optimal leaf ordering for gene expression data. Bioinformatics 19:1070–1078 [DOI] [PubMed] [Google Scholar]