Abstract
The rapid accurate detection of drug resistance mutations in Mycobacterium tuberculosis is essential for optimizing the treatment of tuberculosis and limiting the emergence and spread of drug-resistant strains. The TB Resistance line probe assay from Autoimmun Diagnostika GmbH (AID) (Strassburg, Germany) was designed to detect the most prevalent mutations that confer resistance to isoniazid, rifampin, streptomycin, amikacin, capreomycin, fluoroquinolones, and ethambutol. This assay detected resistance mutations in clinical M. tuberculosis isolates from areas with low and high levels of endemicity (Switzerland, n = 104; South Africa, n = 52) and in selected Mycobacterium bovis BCG 1721 mutant strains (n = 5) with 100% accuracy. Subsequently, the line probe assay was shown to be capable of rapid genetic assessment of drug resistance in MGIT broth cultures, the results of which were in 100% agreement with those of DNA sequencing and phenotypic drug susceptibility testing. Finally, the line probe assay was assessed for direct screening of smear-positive clinical specimens. Screening of 98 clinical specimens demonstrated that the test gave interpretable results for >95% of them. Antibiotic resistance mutations detected in the clinical samples were confirmed by DNA sequencing. We conclude that the AID TB Resistance line probe assay is an accurate tool for the rapid detection of resistance mutations in cultured isolates and in smear-positive clinical specimens.
INTRODUCTION
The diagnosis and treatment of multidrug-resistant (MDR) tuberculosis (TB), resistant to at least isoniazid and rifampin, represent a major challenge for tuberculosis control programs worldwide. In 2010, there were an estimated 650,000 cases of MDR-TB among the world's 12.0 million prevalent cases of TB (1). Extensively drug-resistant (XDR) Mycobacterium tuberculosis strains are defined as showing resistance to at least isoniazid and rifampin, to any fluoroquinolone, and to any of the 3 second-line injectable drugs (amikacin, capreomycin, and kanamycin) (2). Early rapid detection of resistance to both first- and second-line drugs is essential to ensure appropriate chemotherapy and to prevent the spread and amplification of resistance.
Antibiotic resistance in M. tuberculosis is mediated mostly through genetic changes in the chromosome, such as point mutations and deletions. The involvement of different mutational alterations and genes associated with drug resistance (3), resulting in different levels of resistance, complicates culture-based drug susceptibility testing (DST). Molecular methods offer fast accurate detection of resistance mutations by line probe assays, DNA sequencing, or multiplex (real-time) PCR assays. One of the few commercially available tests is the Genotype MTBDRplus assay (Hain Lifescience GmbH, Germany), which was endorsed by the World Health Organization (WHO) in 2008 and was recently extended for the detection of second-line drug resistance mutations (Genotype MTBDRsl) (4–8). The more recently endorsed Xpert MTB/RIF assay (Cepheid, USA) facilitates rapid detection of the M. tuberculosis complex (9) but is limited to detection of rifampin resistance mutations in rpoB (10, 11). Similarly, the INNO-LiPA RIF.TB line probe assay (Fujirebio Inc., Ghent, Belgium) is limited to the detection of rpoB mutations associated with rifampin resistance but also is able to detect the emergence of rifampin-resistant populations due to the presence of wild-type (wt) and mutant probes (12). Despite seminal improvements in PCR and DNA sequencing technologies (13, 14), line probe assays will remain the method of choice for simultaneous detection of multiple drug resistance mutations in M. tuberculosis, at least in the short term. Line probe assays are fast, efficient (testing for multiple chromosomal targets), accurate, and easy to handle in the laboratory. Results are usually obtained within 1 day. Performance of line probe assays does not require expensive laboratory equipment, although certain equipment to perform DNA extraction, PCR amplification, and DNA hybridization and the expertise to prevent amplicon cross-contamination are required.
Given the importance of line probe assays in clinical diagnostic testing, Autoimmun Diagnostika GmbH (AID) (Strassburg, Germany) has developed a line probe assay for the detection of chromosomal mutations associated with resistance to first-line (isoniazid and rifampin) and second-line (streptomycin, amikacin, capreomycin, fluoroquinolones, and ethambutol; ethambutol is considered a first-line drug in the United States) TB drugs. The aim of this study was to evaluate the specificity and sensitivity of the AID TB Resistance line probe assay for the detection of resistance mutations in cultured isolates and in smear-positive patient specimens.
MATERIALS AND METHODS
Clinical specimens, decontamination, microscopy, and culture.
Clinical specimens were decontaminated using the N-acetyl-l-cysteine-sodium hydroxide method (15). Auramine-rhodamine fluorochrome staining was used for acid-fast bacteria (AFB) microscopic examination; AFB-positive results were confirmed for specificity using Ziehl-Neelsen staining (16). Mycobacteria were recovered on standard culture media (7H11 plates and BBL MGIT [Becton, Dickinson and Company]) by incubation at 37°C for a maximum of 7 weeks.
Clinical isolates.
Clinical strains were obtained from the Institut für Medizinische Mikrobiologie (IMM), Universität Zürich (Zürich, Switzerland), and the DST/NRF Centre of Excellence for Biomedical TB Research/MRC Centre for Molecular and Cellular Biology, Stellenbosch University (Cape Town, South Africa).
DNA extraction.
DNA was extracted from decontaminated samples (0.5 ml) using a respiratory specimen preparation kit (Roche Diagnostics, Rotkreuz, Switzerland), according to the manufacturer's instructions (17). Chromosomal DNA of cultured isolates was obtained using InstaGene matrix (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer's instructions. Mycobacteria were identified by 16S rRNA gene sequence analysis, as previously described (18) and analyzed using SmartGene IDNS software (SmartGene GmbH, Zug, Switzerland).
Genotypic resistance testing.
Mycobacterium tuberculosis clinical isolates were analyzed for antibiotic resistance mutations by DNA sequencing. For sequencing, the corresponding chromosomal loci were amplified by PCR (see Table S1 in the supplemental material). Automated DNA sequence determinations were performed using the amplification primers and the BigDye Terminator v1.1 cycle sequencing kit, on an ABI Genetic Analyzer 3100 system (Applied Biosystems, Rotkreuz, Switzerland). Sequences were analyzed using DNASTAR Lasergene version 8 software (DNASTAR, Inc., Madison, WI USA).
For isoniazid resistance, mutations in katG at amino acid position 315 (19) and in the fabG1-inhA-hemZ operon promoter at positions −16, −15, and −8 (20) were determined. For rifampin resistance, mutations in rpoB at amino acid positions 516, 526, and 531 (corresponding to Escherichia coli RpoB positions) were analyzed (21). For streptomycin resistance, mutations in rpsL (K43R, K88R, and K88Q) (22, 23) and rrs (C513T, A514C, G515C, and C517T, corresponding to E. coli rrs positions 522, 523, 524, and 526, respectively) (18) were examined. Amikacin or capreomycin resistance was determined by screening the rrs gene for the mutations A1401G, C1402T, and G1484C/T (corresponding to E. coli rrs positions 1408, 1409, and 1491, respectively) (22, 24, 25). For fluoroquinolone resistance, GyrA amino acid mutations A90V, S91P, D94A, D94N, D94Y, and D94G were determined. The presence of the EmbB mutations M306V and M306I were regarded as indicative of ethambutol resistance (26).
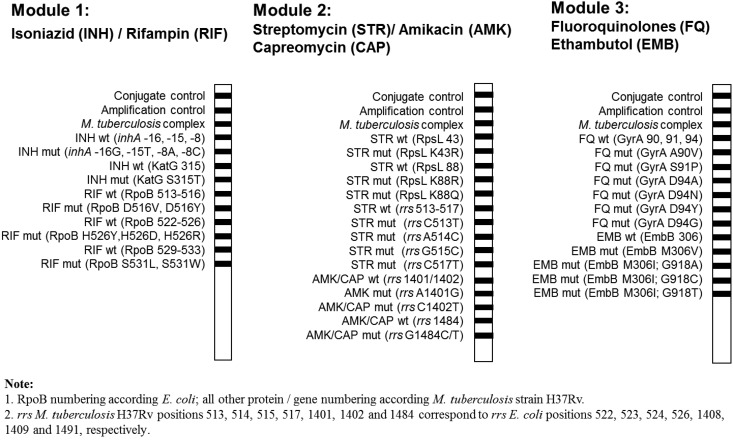
AID TB Resistance line probe testing using module 1 (isoniazid and rifampin), module 2 (streptomycin, amikacin, and capreomycin), and module 3 (fluoroquinolones and ethambutol) (Fig. 1) was performed following the instructions of the manufacturer (Autoimmun Diagnostika GmbH). For testing of clinical specimens with the AID TB Resistance line probe assay, DNA was extracted with a Roche respiratory specimen preparation kit. Mycobacterium tuberculosis strain H37Rv was used as a positive control for gene amplification.
FIG 1.
Schematic representation of the three-module Mycobacterium tuberculosis antibiotic resistance line probe assay (AID, Strassburg, Germany) for the detection of resistance mutations in Mycobacterium tuberculosis.
In vitro generation and selection of Mycobacterium bovis BCG rrs mutant strains resistant to aminoglycosides.
Selection of Mycobacterium bovis strain BCG 1721 (RpsL K43R) (27) for rrs mutations conferring resistance to kanamycin, amikacin, and capreomycin was done by plating M. bovis BCG 1721 (1 × 108 or 1 × 107 CFU) on 7H10 agar plates supplemented with amikacin (4 or 20 mg/liter), capreomycin (10 or 50 mg/liter), or a combination of capreomycin (10 mg/liter) and kanamycin (5 mg/liter). Plates were incubated at 37°C for 3 to 6 weeks. Single colonies were analyzed for the presence of mutations by rrs sequence analysis, as described above.
Drug susceptibility testing.
Conventional culture-based drug susceptibility testing (DST) (isoniazid, rifampin, streptomycin, amikacin, capreomycin, moxifloxacin, and ethambutol) was done using the automated MGIT 960 platform, in conjunction with EpiCenter software equipped with the TB eXiST module (Becton, Dickinson and Company, Sparks, MD, USA), and methods described previously (28). In general, DST was performed for one culture per clinical specimen.
RESULTS
Analytical assessment of the various detectable mutations of the AID TB resistance kit.
The TB Resistance line probe assay was evaluated against the susceptible model strain M. tuberculosis H47Rv and a series of cultured clinical M. tuberculosis isolates (n = 156), which had been well characterized for drug resistance by phenotypic drug susceptibility testing and DNA sequence analyses. For M. tuberculosis strain H47Rv, only wt signals were observed for all three modules.
Module 1 (isoniazid and rifampin) was tested against 70 clinical M. tuberculosis strains isolated in Switzerland (Table 1). The set of strains allowed testing of all probes included in module 1. Mutations in the inhA promoter were detected at position −15 (11/70 strains) or −8 (1/70 strains). A katG 315 mutation was detected in 39/70 strains. A total of 42/70 strains were shown to harbor mutations in rpoB conferring resistance to rifampin; rpoB mutations in amino acid positions 516, 526, and 531 were observed in 4/70, 11/70, and 29/70 strains, respectively. Two strains showed double mutations in rpoB (Table 1); one strain contained mut516 and mut531 mutations, and the other strain contained mut516 and mut526 mutations. All mutations revealed by module 1 testing were confirmed by targeted DNA sequencing. Cross-reactivity signals resulting in false-positive results were not observed.
TABLE 1.
Evaluation of module 1 (isoniazid and rifampin) of the AID TB Resistance line probe assay using clinical M. tuberculosis strains (n = 70, Switzerland)
| Isoniazida |
Rifampin, RpoB |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
inhA |
KatG |
||||||||||||
| No. of isolates | AID module 1 |
DNA sequencing | AID module 1 |
DNA sequencing | AID module 1 |
DNA sequencing | |||||||
| wt (−16, −15, −8) | Mutant (−16, −15, −8) | wt 315 | mut315 | wt 513–516 | mut516 | wt 522–526 | mut526 | wt 529–533 | mut531 | ||||
| 1 | − | + | C-15T | − | + | S315T | + | − | − | + | + | − | H526D |
| 1 | − | + | C-15T | − | + | S315T | + | − | + | − | − | + | S531L |
| 1 | − | + | C-15T | + | − | wt | − | + | + | − | − | + | D516Y/S531L |
| 3 | − | + | C-15T | + | − | wt | + | − | + | − | − | + | S531L |
| 5 | − | + | C-15T | + | − | wt | + | − | + | − | + | − | wt |
| 1 | − | + | T-8C | − | + | S315T | + | − | − | + | + | − | H526Y |
| 10 | + | − | wt | − | + | S315T | + | − | + | − | + | − | wt |
| 1 | + | − | wt | − | + | S315T | − | + | + | − | + | − | D516Y |
| 1 | + | − | wt | − | + | S315T | − | + | − | + | + | − | D516Y/H526D |
| 6 | + | − | wt | − | + | S315T | + | − | − | + | + | − | H526D |
| 18 | + | − | wt | − | + | S315T | + | − | + | − | − | + | S531L |
| 1 | + | − | wt | + | − | wt | − | + | + | − | + | − | D516V |
| 2 | + | − | wt | + | − | wt | + | − | − | + | + | − | H526Y |
| 6 | + | − | wt | + | − | wt | + | − | + | − | − | + | S531L |
| 13 | + | − | wt | + | − | wt | + | − | + | − | + | − | wt |
−, no signal (band); +, signal (band); wt, wild type.
Module 2 (streptomycin, amikacin, and capreomycin) was tested against 22 clinical M. tuberculosis strains isolated in Switzerland (n = 4) or South Africa (n = 18). The RpsL K43R mutation conferring streptomycin resistance was detected in 7/22 strains, and the RpsL K88R mutation was detected in 1/22 strains. A strain with the RpsL K88Q mutation was not available. The rrs mutations A514C and C517T, which also confer resistance to streptomycin, were detected in 7/22 strains and 1/22 strains, respectively. The rrs mutation A1401G, which confers resistance to amikacin and capreomycin, was detected in 11/22 strains. Five BCG 1721 derivative strains with rrs point mutations (C1402T or G1484T) were also included for testing with module 2, as none of the clinical isolates harbored these mutations (Table 2). These strains were derived from Mycobacterium bovis strain BCG 1721, which contains the RpsL K43R mutation. All mutations identified by module 2 testing were confirmed by DNA sequencing. Cross-reactivity resulting in false-positive signals was not observed for module 2.
TABLE 2.
Evaluation of module 2 (aminoglycosides) of the AID TB Resistance assay using clinical M. tuberculosis strains (Switzerland, n = 4; South Africa, n = 18) and in vitro-selected M. bovis BCG mutant strains (n = 5)
| No. of isolates from: |
Streptomycina |
Amikacin-capreomycin, rrsb |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RpsL |
rrs |
||||||||||||||||||
| AID module 2 |
DNA sequencing | AID module 2 |
DNA sequencing | AID module 2 |
DNA sequencing | ||||||||||||||
| Switzerland | South Africa | wt 43 | K43R | wt 88 | K88R | K88Q | wt 513–517 | C513T | A514C | G515C | C517T | wt 1401/1402 | A1401G | C1402T | wt 1484 | G1484C/T | |||
| 2 | 3 | − | + | + | − | − | K43R | + | − | − | − | − | ND | − | + | − | + | − | A1401G |
| 2 | − | + | + | − | − | K43R | + | − | − | − | − | wt | + | − | − | + | − | wt | |
| 3b | − | + | + | − | − | K43R | + | − | − | − | − | ND | − | − | + | + | − | C1402T | |
| 2b | − | + | + | − | − | K43R | + | − | − | − | − | ND | + | − | − | − | + | G1484T | |
| 1 | + | − | − | + | − | K88R | + | − | − | − | − | ND | + | − | − | + | − | ND | |
| 4 | + | − | + | − | − | ND | − | − | + | − | − | A514C | − | + | − | + | − | A1401G | |
| 2 | + | − | + | − | − | wt | − | − | + | − | − | A514C | − | + | − | + | − | A1401G | |
| 1 | + | − | + | − | − | wt | − | − | + | − | − | A514C | + | − | − | + | − | wt | |
| 1 | + | − | + | − | − | wt | − | − | − | − | + | C517T | + | − | − | + | − | ND | |
| 6 | + | − | + | − | − | wt | + | − | − | − | − | wt | + | − | − | + | − | wt | |
−, no signal (band); +, signal (band); wt, wild-type; ND, not determined.
Mycobacterium bovis BCG 1721 (RpsL K43R) derivatives selected for kanamycin-capreomycin resistance.
Module 3 (fluoroquinolones and ethambutol) was tested against 64 clinical M. tuberculosis isolates (Switzerland, n = 30; South Africa, n = 34) (Table 3). A total of 35/64 M. tuberculosis strains were gyrA wt and 29/64 had gyrA mutations. Among these, the line probe assay detected gyrA mutations in 27/29 of the fluoroquinolone-resistant strains (Table 3). Sequencing data revealed that the two strains that showed no gyrA wt or mutant signals had GyrA D94V (GAC to GTC) and GyrA D94H (GAC to CAC) mutations, for which probes were not included in the assay. A total of 23/64 M. tuberculosis strains were embB wt and 41/64 had embB mutations; 40/41 of the embB resistance mutations were detected by the line probe assay. One ethambutol-resistant strain was not identified due to the presence of the EmbB M306L (ATG to CTG) mutation, which was not covered by the assay. An M. tuberculosis strain containing the EmbB M306I (ATG to ATT) mutation was not available to validate the corresponding probe in the module 3 line probe. Module 3 showed 100% accuracy for detection of resistance mutations, as confirmed by DNA sequencing (Table 3). No cross-reactivity resulting in false-positive results was observed for module 3.
TABLE 3.
Evaluation of module 3 (fluoroquinolones and ethambutol) of the AID TB Resistance line probe assay using clinical M. tuberculosis strains (Switzerland, n = 30; South Africa, n = 34)
| No. of isolates from: |
Fluoroquinolones, GyrAa |
Ethambutol, EmbB |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AID module 3 |
DNA sequencing | AID module 3 |
DNA sequencing | ||||||||||||
| Switzerland | South Africa | wt 90, 91, 94 | A90V | S91P | D94A | D94N | D94Y | D94G | wt306 | M306V, ATG to GTG | M306I, ATG to ATA | M306I, ATG to ATC | M306I, ATG to ATT | ||
| 15 | 3 | + | − | − | − | − | − | − | wt | + | − | − | − | − | wt |
| 8 | 1 | + | − | − | − | − | − | − | wt | − | + | − | − | − | M306V, ATG to GTG |
| 3 | + | − | − | − | − | − | − | wt | − | − | + | − | − | M306I, ATG to ATA | |
| 1 | 3 | + | − | − | − | − | − | − | wt | − | − | − | + | − | M306I, ATG to ATC |
| 1 | + | − | − | − | − | − | − | wt | − | − | − | − | − | M306L, ATG to CTG | |
| 2 | − | + | − | − | − | − | − | A90V | − | + | − | − | − | M306V, ATG to GTG | |
| 3 | − | + | − | − | − | − | − | A90V | − | − | + | − | − | M306I, ATG to ATA | |
| 1 | − | + | − | − | − | − | − | A90V | − | − | − | + | − | M306I, ATG to ATC | |
| 2 | − | − | + | − | − | − | − | S91P | − | + | − | − | − | M306V, ATG to GTG | |
| 1 | − | − | + | − | − | − | − | S91P | − | − | + | − | − | M306I, ATG to ATA | |
| 2 | − | − | + | − | − | − | − | S91P | − | − | − | + | − | M306I, ATG to ATC | |
| 2 | − | − | − | + | − | − | − | D94A | − | + | − | − | − | M306V, ATG to GTG | |
| 2 | − | − | − | − | + | − | − | D94N | − | + | − | − | − | M306V, ATG to GTG | |
| 1 | − | − | − | − | + | − | − | D94N | − | − | − | + | − | M306I, ATG to ATC | |
| 1 | − | − | − | − | − | + | − | D94Y | + | − | − | − | − | wt | |
| 3 | − | − | − | − | − | + | − | D94Y | − | − | + | − | − | M306I, ATG to ATA | |
| 1 | 2 | − | − | − | − | − | − | + | D94G | + | − | − | − | − | wt |
| 1 | − | − | − | − | − | − | + | D94G | − | + | − | − | − | M306V, ATG to GTG | |
| 2 | − | − | − | − | − | − | + | D94G | − | − | + | − | − | M306I, ATG to ATA | |
| 1 | − | − | − | − | − | − | + | D94G | − | − | − | + | − | M306I, ATG to ATC | |
| 1 | − | − | − | − | − | − | − | D94V (GTC) | + | − | − | − | − | wt | |
| 1 | − | − | − | − | − | − | − | D94H (CAC) | − | + | − | − | − | M306V, ATG to GTG | |
−, no signal (band); +, signal (band); wt, wild-type.
Implementation of the AID TB resistance kit in routine diagnostic testing.
To determine the feasibility of implementing the AID TB line probe resistance assay in a diagnostic workflow, we evaluated the suitability of the assay for the rapid detection of resistance in early positive MGIT cultures. All positive clinical M. tuberculosis cultures (n = 50) obtained in January through April 2012 were subjected to the AID TB Resistance line probe assay. The 50 cultures were from sputum (n = 18), tracheal bronchial secretion (n = 12), aspirate (n = 7), tissue (n = 5), gastric fluid (n = 4), bronchoalveolar lavage fluid (n = 2), lymph node (n = 1), and urine (n = 1) specimens.
The AID line probe assay indicated that 47 of the 50 isolates lacked mutations in the inhA, katG, and rpoB regions (Table 4). These results correlated with phenotypic drug susceptibility testing results for rifampin and isoniazid with the MGIT 960 system (see Table S2 in the supplemental material). The remaining 3 isolates showed mutations in inhA, katG, and rpoB (Table 4) and were subsequently subjected to module 2 screening. Two of the 3 strains had a mutation in rspL (K43R), while a mutation in rrs (A514C) was detected in the third strain (Table 5). Mutations in rrs that are associated with amikacin and capreomycin resistance were not detected. Module 3 of the line probe assay did not show gyrA or embB mutations (Table 6). Complete agreement was observed between the AID TB line probe results and the results of subsequent DNA sequence analyses. Phenotypic DST of the cultured isolates showed 100% congruence with the genetic predictions (see Table S2 in the supplemental material).
TABLE 4.
Prospective analysis of clinical M. tuberculosis strains (n = 50) using module 1 of the AID TB resistance assay
| No. of isolates | Isoniazida |
Rifampin, RpoB |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
inhA |
KatG |
|||||||||
| wt (−16, −15, −8) | Mutant (−16, −15, −8) | wt 315 | mut315 | wt 513–516 | mut516 | wt 522–526 | mut526 | wt 529–533 | mut531 | |
| 47 | + | − | + | − | + | − | + | − | + | − |
| 1b | + | − | − | + | + | − | + | − | − | + |
| 1c | + | − | − | + | + | − | + | − | − | + |
| 1d | − | + | − | + | − | + | + | − | + | − |
All detected signals were confirmed by DNA sequencing. The three strains showing resistance mutations with module 1 were tested using modules 2 and 3. −, no signal (band); +, signal (band); wt, wild-type.
MDR isolate 1.
MDR isolate 2.
MDR isolate 3.
TABLE 5.
Prospective analysis of clinical M. tuberculosis strains using module 2 of the AID TB resistance assay
| No. of isolates | Streptomycina |
Amikacin-capreomycin, rrs |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RpsL |
rrs |
wt 1401/1402 | A1401G | C1402T | wt 1484 | G1484C/T | |||||||||
| wt 43 | K43R | wt 88 | K88R | K88Q | wt 513–517 | C513T | A514C | G515C | C517T | ||||||
| 1b | − | + | + | − | − | + | − | − | − | − | + | − | − | + | − |
| 1c | − | + | + | − | − | + | − | − | − | − | + | − | − | + | − |
| 1d | + | − | + | − | − | − | − | + | − | − | + | − | − | + | − |
All detected signals were confirmed by DNA sequencing. −, no signal (band); +, signal (band); wt, wild-type.
MDR isolate 1.
MDR isolate 2.
MDR isolate 3.
TABLE 6.
Prospective analysis of clinical M. tuberculosis strains using module 3 of the AID TB resistance assay
| No. of isolates | Fluoroquinolones, GyrAa |
Ethambutol, EmbB |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt 90, 91, 94 | A90V | S91P | D94A | D94N | D94Y | D94G | wt 306 | M306V, ATG to GTG | M306I, ATG to ATA | M306I, ATG to ATC | M306I, ATG to ATT | |
| 3b | + | − | − | − | − | − | − | + | − | − | − | − |
All detected signals were confirmed by DNA sequencing. −, no signal (band); +, signal (band).
MDR isolates 1, 2, and 3.
Direct detection of antibiotic resistance mutations in patient specimens.
Clinical specimens were used to evaluate the performance of the AID TB Resistance line probe assay for direct detection of resistance mutations. As the modules were sequentially developed over time, different sets of samples were used to exclude the possibility of quality loss of extracted DNA through repeated freezing and thawing and prolonged storage.
To analyze the performance of module 1 for direct detection of resistance mutations in clinical specimens, 98 smear-positive clinical samples were tested (Table 7). All smear-positive samples (100%) gave interpretable results. Nine samples showed mutation signals for inhA, katG, or rpoB, which were all confirmed by DNA sequencing. For one sample, which lacked hybridization signals for both rpoB wt 513 to 516 and the rpoB mutant probes, DNA sequencing revealed mutations resulting in L511P and D516G. All other samples showed wt signals, which were in complete agreement with the results of phenotypic DST of the corresponding cultures that were subsequently grown.
TABLE 7.
Evaluation of AID TB resistance line probe assay module 1 for detection of isoniazid and rifampin resistance in smear-positive clinical samples (n = 98)
| Smear result | No. of clinical samples | Isoniazida |
Rifampin, RpoB |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
inhA |
KatG |
|||||||||||||
| AID module 1 |
DNA sequencing | AID module 1 |
DNA sequencing | AID module 1 |
DNA sequencing | |||||||||
| wt (−16, −15, −8) | Mutant (−16, −15, −8) | wt 315 | mut315 | wt 513–516 | mut516 | wt 522–526 | mut526 | wt 529–533 | mut531 | |||||
| Positive | 1b | − | + | C15T | − | − | wt | + | − | + | − | − | + | S531L |
| Positive | 1c | − | + | C15T | + | − | ND | + | − | + | − | + | − | ND |
| Positive | 6d | + | − | wt | − | + | S315T | + | − | + | − | + | − | ND |
| Positive | 1e | + | − | wt | − | + | S315T | − | − | + | − | + | − | L511P, D516G |
| Positive | 89f | + | − | ND | + | − | ND | + | − | + | − | + | − | ND |
In cases in which no mutations for isoniazid and/or rifampin were detected, wt signals were confirmed by DST only. ND, not done; −, no signal (band); +, signal (band); wt: wild-type.
Respiratory specimen (n = 1).
Respiratory specimen (n = 1).
Respiratory (n = 4), biopsy (n = 1), and abscess (n = 1) specimens.
Respiratory specimen (n = 1).
Respiratory (n = 78), tissue (n = 3), wound (n = 1), lymph node (n = 3), abscess (n = 2), biopsy (n = 1), and unspecified (n = 1) specimens.
A set of 35 smear-positive clinical samples was used to evaluate modules 2 and 3. For module 2, 34/35 (97.1%) smear-positive samples (respiratory, n = 30; lymph node, n = 2; abscess, n = 1; aspirate, n = 1; tissue, n = 1) showed interpretable results. Only one tissue sample produced an uninterpretable result, due to high background signals. Only wild-type signals were observed for the 34 smear-positive samples, which were in complete agreement with the DST results for the corresponding cultures.
For module 3 (fluoroquinolones and ethambutol), 29/29 smear-positive samples (respiratory, n = 24; aspirate, n = 2; lymph node, n = 1; cerebrospinal fluid, n = 1; urine, n = 1) produced interpretable results. Only wild-type signals were detected for gyrA. One clinical sample showed the EmbB M306I mutation, and 28/29 samples showed wild-type signals for EmbB. The signals observed were in complete agreement with DNA sequencing results and the DST results for the corresponding cultures.
DISCUSSION
Rapid detection of M. tuberculosis resistance against first-line and second-line drugs is essential to identify monoresistant, MDR, or XDR M. tuberculosis strains. Based on epidemiological analyses of mutations associated with drug resistance in MDR-TB and XDR-TB strains, the TB Resistance line probe assay (Autoimmun Diagnostika GmbH, Strassburg, Germany) was designed to cover high-confidence resistance mutations for the following drugs: isoniazid (KatG 315 and inhA −16, −15, and −8), rifampin (RpoB 516, 526, and 531), streptomycin (rpsL K43R, K88R and K88Q and rrs C513T, A514C, G515C, and C517T), amikacin-capreomycin (rrs A1401G, C1402T, and G1484C/T), fluoroquinolones (GyrA A90V, S91P, D94A, D94N, D94Y, and D94G), and ethambutol (EmbB M306V and M306I) (Fig. 1). Notably, the AID line probe assay includes probes for detection of streptomycin resistance, i.e., rpsL A43G, A88G, and A88C and rrs C513T, A514C, G515C, and C517T. Mutations in pncA and ethA, which are associated with resistance to pyrazinamide and ethionamide, respectively, were not suitable for inclusion in the line probe assays. Resistance to these antibiotics is conferred by a diverse range of mutations scattered throughout the relatively large pncA and ethA genes (29, 30), which hampers the design of an appropriate line probe assay.
In this study, we have evaluated the performance of the TB Resistance line probe assay (AID) for the detection of resistance to first- and second-line drugs. Using clinical strains and laboratory-generated mutant strains, the specificity of the assay was shown to be excellent for all three modules (Tables 1, 2, and 3). No false-positive or false-negative results were observed. Less common mutations are indicated by the absence of signals for both wt and mutant probes. For example, two Mycobacterium tuberculosis strains lacking signals for gyrA were shown to contain mutations resulting in D94V and D94H (Table 3). A clinical culture lacking signals for embB was shown to harbor the EmbB M306L (ATG to CTG) mutation (Table 3). In addition, one of the clinical samples lacking a signal for rpoB was shown by sequencing to contain the RpoB L511P mutation (Table 7). A small prospective study, including 50 clinical isolates cultured in our diagnostic laboratory, confirmed the specificity shown by sequence analysis (Table 2) and the successful implementation of the line probe assay for routine diagnostic testing.
Evaluation of modules 1, 2, and 3 for direct screening of smear-positive respiratory and nonrespiratory specimens showed high percentages of interpretable results, i.e., 100%, 97.1%, and 100%, respectively. Mutations for isoniazid and/or rifampin resistance were detected in 9/98 samples (Table 7); these results were confirmed by DNA sequencing and were in agreement with the results of phenotypic testing of the corresponding cultured isolates. No mutations involved in aminoglycoside and fluoroquinolone resistance were found, reflecting their low prevalence in Switzerland. For all three modules, complete agreement (100%) between hybridization signals and drug susceptibility testing results for the corresponding cultured isolates was observed.
Our study addressing the suitability of the AID line probe assay for early positive MGIT cultures and smear-positive samples is biased since we had to focus on the samples available to us, i.e., specimens from an area with low disease prevalence and mostly drug-susceptible TB. We conclude that the TB Resistance line probe assay (AID) is a rapid method for accurate reliable detection of the most common mutations resulting in drug resistance in M. tuberculosis. The assay is easy to use, has a short time to results (producing results within 1 day), and can be readily implemented in a diagnostic laboratory for testing of cultured isolates as well as for direct testing of smear-positive clinical specimens. The usefulness of the assay in areas with high disease prevalence rates and large proportions of drug-resistant TB needs to be addressed in future studies.
Supplementary Material
ACKNOWLEDGMENTS
AID is acknowledged for providing line probe assays. We thank Akos Somoskövi for critical reading of the manuscript.
E.C.B. is a consultant for AID. The other authors have nothing to declare.
This work was supported in part by the University of Zurich and the BAG (Federal Office of Public Health, Switzerland).
Footnotes
Published ahead of print 8 January 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02597-13.
REFERENCES
- 1.World Health Organization 2011. WHO report 2011: global tuberculosis control. WHO/HTM/TB/2011.16. WHO, Geneva, Switzerland [Google Scholar]
- 2.World Health Organization 2006. Extensively drug-resistant tuberculosis (XDR-TB): recommendations for prevention and control. Wkly. Epidemiol. Rec. 81:430–432 [PubMed] [Google Scholar]
- 3.Böttger EC. 2011. The ins and outs of Mycobacterium tuberculosis drug susceptibility testing. Clin. Microbiol. Infect. 17:1128–1134. 10.1111/j.1469-0691.2011.03551.x [DOI] [PubMed] [Google Scholar]
- 4.Hillemann D, Weizenegger M, Kubica T, Richter E, Niemann S. 2005. Use of the genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 43:3699–3703. 10.1128/JCM.43.8.3699-3703.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hillemann D, Rüsch-Gerdes S, Richter E. 2009. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin-capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J. Clin. Microbiol. 47:1767–1772. 10.1128/JCM.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson KR, Theron D, Kendall EA, Franke MF, Barnard M, van Helden PD, Victor TC, Streicher EM, Murray MB, Warren RM. 2013. Implementation of genotype MTBDRplus reduces time to multidrug-resistant tuberculosis therapy initiation in South Africa. Clin. Infect. Dis. 56:503–508. 10.1093/cid/cis920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnard M, Warren R, Van Pittius NG, van Helden P, Bosman M, Streicher E, Coetzee G, O'Brien R. 2012. Genotype MTBDRsl line probe assay shortens time to diagnosis of extensively drug-resistant tuberculosis in a high-throughput diagnostic laboratory. Am. J. Respir. Crit. Care Med. 186:1298–1305. 10.1164/rccm.201205-0960OC [DOI] [PubMed] [Google Scholar]
- 8.Kontsevaya I, Ignatyeva O, Nikolayevskyy V, Balabanova Y, Kovalyov A, Kritsky A, Matskevich O, Drobniewski F. 2013. Diagnostic accuracy of the genotype MTBDRsl assay for rapid diagnosis of extensively drug-resistant tuberculosis in HIV-coinfected patients. J. Clin. Microbiol. 51:243–248. 10.1128/JCM.02513-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N. Engl. J. Med. 363:1005–1015. 10.1056/NEJMoa0907847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zbinden A, Keller PM, Bloemberg GV. 2011. Rapid molecular detection of tuberculosis. N. Engl. J. Med. 364:183. 10.1056/NEJMc1011919#SA3. [DOI] [PubMed] [Google Scholar]
- 11.Somoskovi A, Deggim V, Ciardo D, Bloemberg GV. 2013. Diagnostic implications of inconsistent results obtained with the Xpert MTB/Rif assay in detection of Mycobacterium tuberculosis isolates with an rpoB mutation associated with low-level rifampin resistance. J. Clin. Microbiol. 51:3127–3129. 10.1128/JCM.01377-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossau R, Traore H, De Beenhouwer H, Mijs W, Jannes G, De Rijk P, Portaels F. 1997. Evaluation of the INNO-LiPA Rif.TB assay, a reverse hybridization assay for the simultaneous detection of Mycobacterium tuberculosis complex and its resistance to rifampin. Antimicrob. Agents Chemother. 41:2093–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engström A, Morcillo N, Imperiale B, Hoffner SE, Juréen P. 2012. Detection of first- and second-line drug resistance in Mycobacterium tuberculosis clinical isolates by pyrosequencing. J. Clin. Microbiol. 50:2026–2033. 10.1128/JCM.06664-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacoma A, García-Sierra N, Prat C, Maldonado J, Ruiz-Manzano J, Haba L, Gavin P, Samper S, Ausina V, Domínguez J. 2012. GenoType MTBDRsl for molecular detection of second-line-drug and ethambutol resistance in Mycobacterium tuberculosis strains and clinical samples. J. Clin. Microbiol. 50:30–36. 10.1128/JCM.05274-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kent PT, Kubica GP. 1985. Public health mycobacteriology: a guide for the level III laboratory. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 16.Isenberg HD. (ed). 1992. Clinical microbiology procedures handbook, vol 1 American Society for Microbiology, Washington, DC [Google Scholar]
- 17.Roche Diagnostics 2009. Cobas Taqman Mycobacterium tuberculosis test: instruction manual. Roche Diagnostics, Mannheim, Germany [Google Scholar]
- 18.Kirschner P, Rosenau J, Springer B, Teschner K, Feldmann K, Böttger EC. 1996. Diagnosis of mycobacterial infections by nucleic acid amplification: 18-month prospective study. J. Clin. Microbiol. 34:304–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipin MY, Stepanshina VN, Shemyakin IG, Shinnick TM. 2007. Association of specific mutations in katG, rpoB, rpsL and rrs genes with spoligotypes of multidrug-resistant Mycobacterium tuberculosis isolates in Russia. Clin. Microbiol. Infect. 13:620–626. 10.1111/j.1469-0691.2007.01711.x [DOI] [PubMed] [Google Scholar]
- 20.Leung ET, Ho PL, Yuen KY, Woo WL, Lam TH, Kao RY, Seto WH, Yam WC. 2006. Molecular characterization of isoniazid resistance in Mycobacterium tuberculosis: identification of a novel mutation in inhA. Antimicrob. Agents Chemother. 50:1075–1078. 10.1128/AAC.50.3.1075-1078.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue J, Shi W, Xie J, Li Y, Zeng E, Wang H. 2003. Mutations in the rpoB gene of multidrug-resistant Mycobacterium tuberculosis isolates from China. J. Clin. Microbiol. 41:2209–2212. 10.1128/JCM.41.5.2209-2212.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finken M, Kirschner P, Meier A, Wrede A, Böttger EC. 1993. Molecular basis of streptomycin resistance in Mycobacterium tuberculosis: alterations of the ribosomal protein S12 gene and point mutations within a functional 16S ribosomal RNA pseudoknot. Mol. Microbiol. 9:1239–1246. 10.1111/j.1365-2958.1993.tb01253.x [DOI] [PubMed] [Google Scholar]
- 23.Baker L, Brown T, Maiden MC, Drobniewski F. 2004. Silent nucleotide polymorphisms and a phylogeny for Mycobacterium tuberculosis. Emerg. Infect. Dis. 10:1568–1577. 10.3201/eid1009.040046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfister P, Hobbie S, Brüll C, Corti N, Vasella A, Westhof E, Böttger EC. 2005. Mutagenesis of 16S rRNA C1402-G1484 base-pair differentiates between 6′OH and 6′NH3+ aminoglycosides. J. Mol. Biol. 346:467–475. 10.1016/j.jmb.2004.11.073 [DOI] [PubMed] [Google Scholar]
- 25.Akbergenov R, Shcherbakov D, Matt T, Duscha S, Meyer M, Wilson DN, Böttger EC. 2011. Molecular basis for the selectivity of antituberculosis compounds capreomycin and viomycin. Antimicrob. Agents Chemother. 55:4712–4717. 10.1128/AAC.00628-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson R, Jordaan AM, Pretorius L, Engelke E, van der Spuy G, Kewley C, Bosman M, van Helden PD, Warren R, Victor TC. 2006. Ethambutol resistance testing by mutation detection. Int. J. Tuberc. Lung Dis. 10:68–73 [PubMed] [Google Scholar]
- 27.Weber W, Schoenmakers R, Keller B, Gitzinger M, Grau T, Daoud-El Baba M, Sander P, Fussenegger M. 2008. A synthetic mammalian gene circuit reveals antituberculosis compounds. Proc. Natl. Acad. Sci. U. S. A. 105:9994–9998. 10.1073/pnas.0800663105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Springer B, Lucke K, Calligaris-Maibach R, Ritter C, Böttger EC. 2009. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and EpiCenter instrumentation. J. Clin. Microbiol. 47:1773–1780. 10.1128/JCM.02501-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morlock GP, Metchock B, Sikes D, Crawford JT, Cooksey RC. 2003. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 47:3799–3805. 10.1128/AAC.47.12.3799-3805.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Böttger EC, Springer B. 2009. Mycobacterium tuberculosis: drug resistance and genetic mechanisms—facts, artifacts and fallacies, p 103–121 In Kaufmann SHE, Walker B. (ed), HIV and tuberculosis: a deadly liaison. Wiley-VCH, Weinheim, Germany [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.