Abstract
Microorganisms may colonize needleless connectors (NCs) on intravascular catheters, forming biofilms and predisposing patients to catheter-associated infection (CAI). Standard and silver-coated NCs were collected from catheterized intensive care unit patients to characterize biofilm formation using culture-dependent and culture-independent methods and to investigate the associations between NC usage and biofilm characteristics. Viable microorganisms were detected by plate counts from 46% of standard NCs and 59% of silver-coated NCs (P = 0.11). There were no significant associations (P > 0.05, chi-square test) between catheter type, side of catheter placement, number of catheter lumens, site of catheter placement, or NC placement duration and positive NC findings. There was an association (P = 0.04, chi-square test) between infusion type and positive findings for standard NCs. Viable microorganisms exhibiting intracellular esterase activity were detected on >90% of both NC types (P = 0.751), suggesting that a large percentage of organisms were not culturable using the conditions provided in this study. Amplification of the 16S rRNA gene from selected NCs provided a substantially larger number of operational taxonomic units per NC than did plate counts (26 to 43 versus 1 to 4 operational taxonomic units/NC, respectively), suggesting that culture-dependent methods may substantially underestimate microbial diversity on NCs. NC bacterial communities were clustered by patient and venous access type and may reflect the composition of the patient's local microbiome but also may contain organisms from the health care environment. NCs provide a portal of entry for a wide diversity of opportunistic pathogens to colonize the catheter lumen, forming a biofilm and increasing the potential for CAI, highlighting the importance of catheter maintenance practices to reduce microbial contamination.
INTRODUCTION
Most hospitalized patients have an intravenous catheter, and up to 30% have a central venous catheter for hemodynamic support, for infusion of medications that require instillation into a central vein, or for long-term venous access (1). These catheters are a major source of health care-associated infections. The Centers for Disease Control and Prevention estimated that 18,000 central line-associated bloodstream infections occurred among patients in U.S. intensive care units and 23,000 among patients in inpatient wards in 2009 (2). The estimate of central line-associated bloodstream infections in hemodialysis patients in 2008 was 37,000 (2). There are estimates of up to 250,000 cases per year when data from the entire hospital are included (3).
Needleless infusion systems were introduced to reduce the incidence of needle injuries and to prevent the transmission of blood-borne pathogens to health care workers. However, needleless connectors (NCs) are susceptible to microbial colonization resulting in the formation of biofilms on the catheter (4) through intraluminal spread, which can predispose catheterized patients to bloodstream infections (5–8).
The goals of this study were to (i) perform an in-depth analysis of biofilms on NCs harvested from intravenous catheters used for patients in intensive care units, (ii) compare biofilm formation on standard and antimicrobial-coated NCs, and (iii) investigate the associations between needleless connector usage and biofilm characteristics. The NCs analyzed in this study were mechanically identical valves, one type of which contained an antimicrobial (nano-silver) coating (V-Link; Baxter Healthcare, Round Lake, IL). The untreated/uncoated (standard) NC type analyzed in this study was the Clearlink device (Baxter Healthcare, Round Lake, IL). Our hypothesis was that the silver antimicrobial coating would decrease the attachment of microorganisms and biofilm formation on the luminal surfaces of NCs, compared with the standard (untreated) NCs.
(Portions of this work were presented at the 2008 Infectious Diseases Society of America annual meeting and the 2010 General Meeting of the American Society for Microbiology.)
MATERIALS AND METHODS
Study design.
Needleless connectors (NCs) were collected from central venous catheters used for patients hospitalized in the intensive care unit of a university hospital that was participating in a crossover study comparing standard and silver-coated NCs for their effects on bloodstream infection rates. This study protocol was approved by the institutional review boards of Emory University and the Centers for Disease Control and Prevention. A convenience sample of NCs removed as part of routine clinical care (normally removed from catheters approximately 7 days after placement) was subjected to biofilm analysis; no devices were removed solely for the purposes of this study. NCs examined for biofilm analysis were labeled with a study number, without patient identifiers on the devices, and with limited patient information. NCs attached to catheters were routinely disinfected by scrubbing the septum and threads with an alcohol wipe before the device was accessed.
Catheter characteristics.
A total of 151 NCs were collected from 62 patients between April 2010 and March 2011. The following information was provided for each NC that was collected: patient number, central line placement date, NC placement date, NC collection date, duration of NC placement (days), line type (peripherally inserted central catheter or tunneled or nontunneled central venous catheter), side of the patient for catheter placement (right or left), number of lumens (2 to 4 lumens), site of placement (internal jugular, basilic, subclavian, or femoral vein), infusion description (most recent prior to NC collection), infusion type (continuous, intermittent, or unknown), and NC type (standard or silver coated). The frequency of NC access was not measured, and clinical outcomes, such as the development of catheter-associated bloodstream infections, were not included in this study.
Collection of NCs from patients.
At the patient's bedside, a nurse wearing gloves grasped the proximal end of the NC with an alcohol wipe, removed the NC from the catheter hub, and placed the NC in a sterile petri dish. All manipulations of the NC at the hospital following removal from the catheter and prior to processing for biofilm recovery were performed by a single study coordinator. A 5-ml syringe containing 5 ml phosphate-buffered saline with 1 g/liter sodium thioglycolate (PBSST), designated the injecting syringe, was connected to the proximal end of the NC, and the other syringe, designated the receiving syringe, was connected to the distal end of the NC (4). The PBSST was added to hydrate the NC luminal surfaces and to neutralize any residual silver released from the NCs during collection and processing. For consistency, PBSST was added to both silver-coated and standard NCs during processing. Approximately 2 ml of PBSST was gently infused through the NC into the receiving syringe, in order to completely fill the NC with PBSST. The NC (connected to the syringes) was placed in a separate zipper-top bag and in a shipping box containing ice packs for transit to the CDC Biofilm Laboratory for analysis. Up to three NCs were collected for analysis on any sampling day. Samples were received and processed to recover and to characterize biofilms within 24 h after collection from the patient.
Protocol for recovering and characterizing biofilms on NCs.
The protocol for processing NCs was based on a method described by Donlan et al. (4). Briefly, NCs connected to twin syringes were placed in a class II biological safety cabinet, disinfected on the outside with sodium hypochlorite, neutralized with sodium thiosulfate, and air dried. The remaining PBSST was gently instilled into the NC and collected in the receiving syringe, which was then disconnected from the NC. The entire volume of PBSST was expelled into a 50-ml centrifuge tube. The injecting syringe was removed from the NC and replaced with a flame-sterilized, custom-built, Luer lock handle. Flame-sterilized cutting tools were used to cut the NC housing and valve components to expose all luminal surfaces. All NC components were rinsed gently in 10 ml of PBSST and placed in the centrifuge tube containing 5 ml of PBSST. After the addition of an additional 5 ml PBSST to the tube, the contents were processed to recover biofilm cells with three 30-s alternating cycles of sonication at a frequency of 42 kHz (model 2510; Branson Co., Danbury, CT) and vortex-mixing. The liquid was transferred to a fresh tube and homogenized for 1 min at approximately 16,000 rpm, using a tissue homogenizer (model K-120; Polysciences, Niles, IL). The resulting biofilm suspension was then processed to quantify and to characterize biofilm microorganisms.
Biofilm quantification.
Serial dilutions (in Butterfield buffer) of the biofilm suspension were spread-plated onto tryptic soy agar containing 5% defibrinated sheep blood (Becton, Dickinson and Co., Sparks, MD), anaerobic blood agar (PathCon Laboratories, Norcross, GA), CHROMagar Candida (VWR, Batavia, IL), MacConkey agar (Becton, Dickinson and Co.), and R2A agar (Becton, Dickinson and Co.). All spread plates except anaerobic blood agar and R2A plates were incubated at 37°C for 48 h. Anaerobic blood agar plates were incubated at 35°C for 48 h in an anaerobic chamber. R2A plates were incubated at 37°C for 48 h and then at room temperature for an additional 5 days. One-milliliter portions of each biofilm suspension were also filtered through a 0.22-μm, 47-mm, nitrocellulose membrane filter (EMD Millipore, Billerica, MA). Filters were rolled onto the plates of each medium and incubated at 37°C for 48 h. All spread plates and filters were examined after 48 h, and colonies were counted to provide the heterotrophic plate count per NC. R2A plates were examined and colonies were counted at 48 h and again after 5 days of incubation at room temperature. The detection limit for the plate count procedure was 10 CFU/NC.
Viable microbial cell counts were also determined by solid-phase cytometry using the ChemScan RDI system (Chemunex, Ivry-Sur-Seine, France). Aliquots of the biofilm suspension were filtered through black 0.2-μm membrane filters, and background fluorescence was quenched with ChemScan reagent CSE/2. Each filter was then placed on a pad saturated with the proprietary nutrient solution Chemsol A4 and was incubated for 1 h at 37°C, to encourage metabolic activity. The filter was transferred to a pad saturated with the labeling solution ChemChrome V6 and was incubated for 30 min at 30°C. Viable cells produce esterase, which cleaves an ester group from the ChemChrome V6 molecule, producing a fluorescent signal. The filter was then placed in the scan module, the fluorescent events were counted by the cytometer, and each event was manually validated using the system software and an epifluorescence microscope.
Characterization of biofilm isolates.
Isolates from 17 standard and 29 silver-coated NC plates were characterized as follows. A single colony of each morphological type from the countable plates for each medium was subcultured twice onto the medium of initial growth and stored at −70°C. Each isolate was subcultured from the frozen stock, Gram stained, and identified using standard microbiological methods, with the Siemens MicroScan WalkAway plus system (Siemens Healthcare Diagnostics, Deerfield, IL) for Gram-positive cocci or the Vitek 2 Rapid Identification system (bioMérieux, Durham, NC) for Gram-negative bacteria and yeasts. Certain isolates were also characterized by using the ID 32 STAPH system (staphylococci) (bioMérieux, Durham, NC) or by 16S rRNA gene sequencing (bacteria). Yeasts were identified by amplifying a region of the internal transcribed spacer near the 18S rRNA gene primers.
Biofilm bacterial community analysis.
The remaining biofilm suspension (approximately 3 ml) was filtered through a 0.2-μm polycarbonate filter (Osmonics, Westboro, MA). The filter was placed in a sterile microcentrifuge tube and stored at −70°C for later processing. Nucleic acids were extracted from filters with the Ultraclean Soil DNA extraction kit (MoBio Laboratories, Carlsbad, CA). Each filter was aseptically placed in a bead tube from the kit and processed according to the manufacturer's instructions for optimal yield. Extracts were stored frozen at −30°C until PCR amplification. A known quantity of bacteria was added to control filters, which were extracted and amplified with the PCR conditions listed below. The lower limit of detection for recovering nucleic acids from the filters was 1 × 104 CFU/filter.
The V1 and V2 hypervariable regions of the 16S rRNA gene were targeted for amplification with bar-coded primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 338R (5′-TGCTGCCTCCCGTAGGAGT-3′) (9). The expected amplicon size was 350 bp. Reactions were performed with the Fast Start High Fidelity PCR system (Roche Applied Science, Indianapolis, IN), in 25-μl reaction mixtures containing 1.8 mM MgCl2, 200 μM deoxynucleoside triphosphates (dNTPs), 0.4 μM primers, 1.25 U enzyme blend, 2 μl dimethyl sulfoxide (DMSO), and 2 μl template. Amplification was performed in triplicate in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA), with the following conditions: 95°C for 2 min, 33 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s, and a final extension at 72°C for 7 min. Products were confirmed by agarose gel electrophoresis.
Triplicate PCR products were pooled, 30 μl was mixed with 6 μl loading dye (Qiagen, Valencia, CA), at least 20 μl was loaded onto a 0.8% agarose gel in 1× Tris-borate-EDTA (TBE) with 1× GelRed (10,000× stock solution; Biotium Inc., Hayward, CA), and electrophoresis was performed at 70 V for 90 min. Bands were cut from the gel, and product was extracted with the QIAquick gel extraction kit (Qiagen), following a modified protocol; the agarose was dissolved at room temperature (10), and the elution buffer was heated to 65°C and held on the column for 3 min before elution of the sample. Samples were dried in a SpeedVac concentrator (ThermoScientific, Waltham, MA) and reconstituted in PCR-grade water for 454 pyrosequencing (Roche Applied Science).
Sequence analysis.
Sequence data for 16S rRNA generated by the 454 GS FLX titanium sequencer were demultiplexed, associated with the metadata, and analyzed separately. The samples were processed with the Quantitative Insights into Microbial Ecology (QIIME) tool (11). QIIME is a script-based package used to compare and to analyze microbial communities, primarily on the basis of high-throughput amplicon sequencing data. QIIME protocols (11) were used to create metadata.
A custom wrapper was coded to make calls to the required scripts within the QIIME tool and automated pipeline. The pipeline consists of data preprocessing, including the demultiplexing step in which the sequences of a run are split on the basis of the sample name, bar code, and linker primer. This was followed by BLAST-based operational taxonomic unit (OTU) picking and taxonomic assignments (12). Comparisons of the samples on the basis of the microbial communities assigned, including principal coordinate analysis (PCoA), were performed through QIIME.
Statistical analysis.
Chi-square tests were used to evaluate the associations between categorical variables. The nonparametric Wilcoxon test was used to compare median CFU/NC values for standard and silver-coated NCs, and the z test was used to compare viable microbial cell counts per NC for standard and silver-coated NCs.
Quality control.
Three sterile, silver-coated NCs were processed through the collection and biofilm recovery process and the samples were plated on each of the five media used for NC samples, to test for contamination during sample processing. All three NC samples were negative (<10 CFU/NC) on all media tested. Each PCR amplification included a negative control in which water was added instead of DNA template.
RESULTS
Microorganisms were recovered from 46% of standard NCs and 59% (P = 0.11) of silver-coated NCs, as determined by plate counts (Table 1). NC samples 54, 55, 62, 63 (silver-coated), and 100 (standard) contained plate counts of >104 CFU/NC (data not shown). Most standard and silver-coated NCs had CFU/NC values of <1,000 CFU/NC. Only 4/76 standard NCs and 7/71 silver-coated NCs had values of >1,000 CFU/NC (data not shown). Silver-coated and standard NCs had similar numbers of devices containing >1,000 CFU/NC (P = 0.456). Multiple species were isolated from 29% (5/17) and 48% (14/29) of standard and silver-coated NCs, respectively, that were characterized by culture-dependent methods, but these differences were not significant (P = 0.592). There were no significant associations (P > 0.05, chi-square test) between catheter type (peripherally inserted central catheter or tunneled or nontunneled central venous catheter), side of catheter placement (left or right side of the patient), number of catheter lumens (2 to 4 lumens), site of catheter placement (internal jugular, basilic, subclavian, or femoral vein), or NC placement duration (days) and positive NC findings for either standard or silver-coated NCs (data not shown). There was an association (P = 0.04, chi-square test) between infusion type (continuous, intermittent, or unknown) and positive NC findings for standard NCs but not silver-coated NCs (data not shown). The median NC placement durations for standard and silver-coated NCs were 7 days (range, 6 to 14 days) and 7 days (range, 1 to 15 days), respectively. Viable microbial cells were detected on >90% of both NC types (P = 0.751) using the viable microbial cell count method, demonstrating that a substantial percentage of microbial cells were not culturable using the medium and incubation conditions provided in this study (Table 2).
TABLE 1.
Comparison of heterotrophic plate counts for standard and silver-coated needleless connectors
| Sample parameter | Standard NCs | Silver-coated NCs | P value |
|---|---|---|---|
| Total no. of samples collected | 76 | 75 | |
| No. of samples with deviation from collection protocol | 0 | 4 | |
| No. of samples for analysis | 76 | 71 | |
| No. (%) of positive samples | 35 (46.1) | 42 (59.2) | 0.11a |
| No. of samples with >1,000 CFU/NC | 4 | 7 | 0.456b |
| No. of NC samples containing ≥2 species/no. of NC samples characterized | 5/17 | 14/29 | 0.592c |
Determined using the nonparametric Wilcoxon test.
Determined using the chi-square test.
Determined using the chi-square test.
TABLE 2.
Comparison of total viable microbial cell counts on standard and silver-coated needleless connectors
| Sample parameter | Standard NCs | Silver-coated NCs | P value |
|---|---|---|---|
| Total no. of samples collected | 76 | 75 | |
| No. of samples not analyzed | 12 | 4 | |
| No. of samples with deviation from collection protocol | 0 | 4 | |
| No. of samples used for analysis | 64 | 67 | |
| No. (%) of positive samples | 60 (93.8) | 63 (94.0) | 0.751a |
| Median viable cell count (no. of cells/NC) | 245 | 780 | |
| No. of samples with >1,000 cells/NC | 16 | 30 | 0.029b |
Determined using the z test.
Determined using the chi-square test.
Microorganisms isolated from both NC types were diverse (Table 3) and included known health care-associated pathogens (Table 4). To our knowledge, many of the organisms isolated from both devices had not been recovered and identified on needleless connectors or central venous catheters and reported in the published literature prior to this study. The most commonly isolated species for both devices was Staphylococcus epidermidis (Table 4). It was also noteworthy that several species (Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii) commonly associated with health care-associated infections were isolated only from silver-coated NCs.
TABLE 3.
Organisms isolated from biofilms of standard and silver-coated needleless connectors
| Organism type | Standard NCs | Silver-coated NCs |
|---|---|---|
| Gram positive | Corynebacterium amycolatum/freneyi/xerosisa | Bacillus cereus/thuringiensisa |
| Corynebacterium pseudogenitalium/tuberculostearicuma | Bacillus flexus/pumilis/megateriuma | |
| Enterococcus faeciuma | Bacillus licheniformisa | |
| Lactobacillus rhamnosus/caseia | Blautia coccoidesa | |
| Micrococcus lylae/luteusa | Clostridium aldenensea | |
| Staphylococcus capitisa | Brevibacterium massiliense/ravenspurgensea | |
| Staphylococcus cohnii subsp. cohniia | Corynebacterium coyleae/mucifaciens/ureicelerivoransa | |
| Staphylococcus epidermidis | Micrococcus luteus | |
| Staphylococcus hominis subsp. hominis | Paenibacillus lautus/ginsengagria | |
| Staphylococcus warneria | Paenibacillus pabuli/taichungensisa | |
| Thermoactinomyces spp.a | Paenibacillus urinalis/provincensis/xylanilyticusa | |
| Paenibacillus xylanilyticus/pabuli/taichungensisa | ||
| Sporosarcina luteola/koreensisa | ||
| Staphylococcus aureus | ||
| Staphylococcus auricularisa | ||
| Staphylococcus capitisa | ||
| Staphylococcus epidermidis | ||
| Staphylococcus haemolyticus | ||
| Staphylococcus hominis subsp. hominis | ||
| Gram negative | Ralstonia pickettiia | Acinetobacter baumannii complexa |
| Escherichia coli | ||
| Pseudomonas aeruginosa | ||
| Yeast | Candida albicans | Candida albicans |
| Candida glabrata | Candida lusitaniae | |
| Candida parapsilosis | ||
| Candida tropicalis | ||
| Candida spp. | ||
| Saccharomyces cerevisiaea |
Organisms that had not been recovered and identified for central venous catheters or needleless connectors and reported prior to this study.
TABLE 4.
Distribution of selected microorganisms isolated from standard and silver-coated needleless connectors
| Organism | No. (%) of devices colonizeda |
|
|---|---|---|
| Standard NCs (n = 17) | Silver-coated NCs (n = 29) | |
| CONSb | 12 (71) | 16 (55) |
| Staphylococcus epidermidis | 8 (47) | 14 (48) |
| Staphylococcus hominis | 2 (12) | 5 (17) |
| Staphylococcus aureus | 0 | 3 (10) |
| Enterococcus faecium | 1 (5.9) | 0 |
| Corynebacterium spp. | 2 (12) | 3 (10) |
| Escherichia coli | 0 | 2 (6.9) |
| Pseudomonas aeruginosa | 0 | 2 (6.9) |
| Acinetobacter baumannii | 0 | 1 (3.4) |
| Ralstonia pickettii | 3 (18) | 0 |
| Candida albicans | 2 (12) | 3 (10) |
| Candida spp. | 3 (18) | 6 (21) |
The values represent the numbers of devices for which the organisms recovered were isolated and identified.
CONS, coagulase-negative staphylococci.
Biofilm samples from 35 of 76 standard NCs were tested using the biofilm bacterial community analysis method. Only 10 (28%) yielded detectable PCR products, and none contained adequate product amounts to carry forward for gel purification and 454 sequencing. Biofilm samples from 51 of 71 silver-coated NCs were tested. Twenty-three samples (45%) yielded a detectable PCR product of the gene for 16S rRNA, and 7 (13.7%) provided usable sequence data. By comparison, of 71 silver-coated NCs characterized by culture, 42 (59.2%) yielded positive results. Therefore, all biofilm bacterial community analyses were based on results obtained from seven silver-coated NCs, i.e., NCs 25, 40, 54, 55, 56, 62, and 63 (Table 5). NCs 54, 55, and 56 were from the same patient, as were NCs 62 and 63. Results of these analyses provided a substantially greater list of microorganisms (26 to 43 OTUs per NC) than the 1 to 4 isolates per NC determined using culture-based methods for the seven NCs that were tested using both methods, which demonstrated that culture-dependent methods grossly underestimate the composition of microbial communities on these devices (data not shown). The data show that all organisms detected by culture were also detected by pyrosequencing. In some cases, the sequence was not resolved to the genus or species level. In NC 40, for example, Firmicutes sequences were detected and Bacillus and Paenibacillus were cultured.
TABLE 5.
Bacteria detected on NCs 25, 40, 54, 55, 56, 62, and 63, as determined by 454 pyrosequencing of bacterial DNA
| Phylum or class | Family or genus | No. of OTUsa |
|---|---|---|
| Actinobacteria | Actinomycetaceae | 1 |
| Coriobacteriaceae | 2 | |
| Corynebacteriaceae | 4 | |
| Dermabacteraceae | 1 | |
| Intrasporangiaceae | 1 | |
| Microbacteriaceae | 3 | |
| Micrococcaceae | 3 | |
| Nocardiaceae | 1 | |
| Nocardioidaceae | 4 | |
| Propionibacteriaceae | 2 | |
| Pseudonocardiaceae | 1 | |
| Sanguibacteraceae | 1 | |
| Solirubrobacteraceae | 1 | |
| Bacteroidetes | Bacteroidaceae | 2 |
| Flavobacteriaceae | 1 | |
| Porphyromonadaceae | 1 | |
| Prevotellaceae | 3 | |
| Sphingobacteriaceae | 2 | |
| Firmicutes | Alicyclobacillaceae | 1 |
| Bacillaceae | 1 | |
| Clostridiales family XIII incertae sedis | 3 | |
| Enterococcaceae | 4 | |
| Eubacteriaceae | 1 | |
| Lachnospiraceae | 9 | |
| Lactobacillaceae | 5 | |
| Paenibacillaceae | 1 | |
| Ruminococcaceae | 3 | |
| Staphylococcaceae | 4 | |
| Streptococcaceae | 4 | |
| Veillonellaceae | 4 | |
| Fusobacteria | Fusobacteriaceae | 1 |
| Gemmatimonadetes | Gemmatimonadaceae | 2 |
| Alphaproteobacteria | Bradyrhizobiaceae | 1 |
| Brucellaceae | 1 | |
| Caulobacteraceae | 3 | |
| Rhizobiaceae | 1 | |
| Rhodobacteraceae | 2 | |
| Sphingomonadaceae | 6 | |
| Betaproteobacteria | Alcaligenaceae | 1 |
| Aquabacterium | 1 | |
| Comamonadaceae | 5 | |
| Neisseriaceae | 1 | |
| Oxalobacteraceae | 3 | |
| Gammaproteobacteria | Enterobacteriaceae | 4 |
| Moraxellaceae | 3 | |
| Pseudomonadaceae | 4 | |
| Xanthomonadaceae | 3 | |
| Synergistetes | Dethiosulfovibrionaceae | 1 |
| Tenericutes | Erysipelotrichaceae | 3 |
| Verrucomicrobia | Verrucomicrobiaceae | 1 |
OTU, operational taxonomic unit.
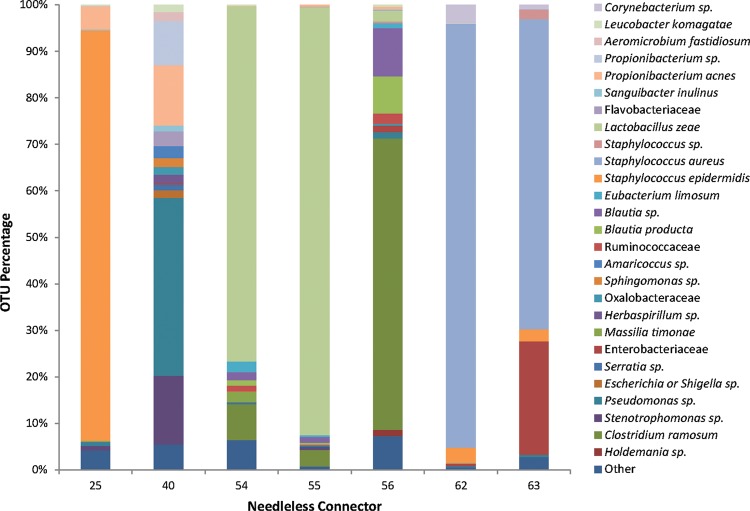
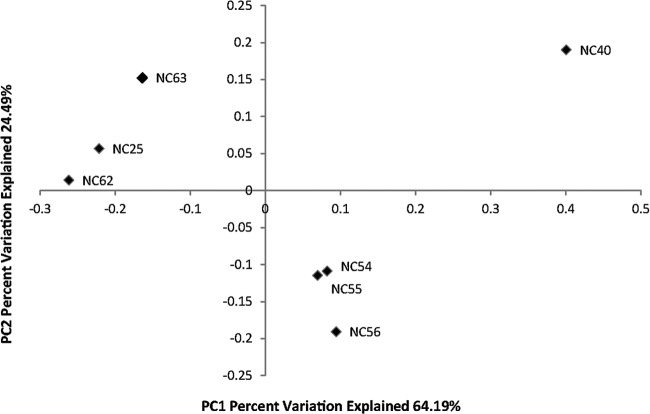
The total number of raw sequence reads from the seven silver-coated NCs was 31,075 reads. After filtering, 29,042 sequence reads were included in the analysis. The mean and median sequence read sizes were both 340 bp. In NCs 25, 62, and 63, Staphylococcus was the dominant sequence observed, specifically, S. epidermidis in NC 25 and S. aureus in NCs 62 and 63 (Fig. 1). Sequences matching these species were not observed in the other NCs. Other dominant sequences included Propionibacterium acnes (NCs 25 and 40), Pseudomonas sp. (NC 40), Stenotrophomonas sp. (NC 40), Lactobacillus zeae (NCs 54 and 55), Blautia sp. (NC 56), Clostridium ramosum (NC 56), and Enterobacteriaceae (NC 63). The NCs from the same patient contained similar OTUs, especially NCs 54 and 55 and NCs 62 and 63. NC 56 contained many of the same OTUs as NCs 54 and 55 but in very different proportions. This similarity is seen in the weighted PCoA plot (Fig. 2), in which NCs from the same patient lines are grouped together.
FIG 1.
Biofilm bacterial community compositions for selected needleless connectors. Each bar represents all taxonomic units that constituted ≥1% of the total number of sequences for each needleless connector sample. Sequence data <1% of the total were grouped together in the category “other.” Operational taxonomic units were defined to the species level if possible and to the family or genus level in other cases.
FIG 2.
Weighted principal coordinate analysis (PCoA) plot of biofilm bacterial communities on selected needleless connector samples, as determined using QIIME. NCs 25 and 40 were from different patients. NCs 54, 55, and 56 were from different lumens of the same catheter in a single patient. NCs 62 and 63 were also from different lumens of the same catheter in a single patient. PC, principal coordinate.
DISCUSSION
Silver is toxic to microorganisms and has been used topically, in germicide formulations, for water disinfection, and on medical devices, including Foley catheters, intravascular catheters, and collagen cuffs of intravascular catheters (13). The silver-coated NC type evaluated in this study contains a novel nano-silver coating on all surfaces of the fluid pathway except the septum, and the working hypothesis of this study was that silver released from the device would reduce microbial colonization and biofilm formation on luminal surfaces, in comparison with a similar NC that did not contain the antimicrobial coating.
Several of our results were unexpected. There were no significant differences in the numbers of viable microorganisms recovered from untreated and silver-coated NCs, as determined by plate count or total viable microbial cell count (non-culture-based) methods. The silver-coated NC type investigated in the present study has been evaluated in both laboratory (14, 15) and clinical (16) studies. Both laboratory studies reported substantial reductions in the numbers of challenge organisms following exposure in the NC lumen, but neither study quantified attached organisms. The NCs evaluated in the clinical study by Casey et al. (16) were removed from patients 4 days after placement, and organisms on the external surface of the septum and in the NC effluent following flushing were quantified and identified. The percentage of NCs containing microorganisms in the NC effluent was significantly smaller for the silver-coated NC devices, in contrast to our results. Recent studies performed in our laboratory (17) suggest that substantial numbers of viable microbial cells may not be recovered from NC luminal surfaces solely by flushing but may require more-vigorous mechanical forces such as sonication and vortex-mixing treatment for removal. Differences in quantification methods may at least partly explain differences in the results of these studies. Silver ions released into the NC lumen may bind to albumin (18) or other serum proteins in human blood (19–21), mitigating biocidal activity. Biofilm formation on the silver-coated NCs also may have been promoted by the presence of blood. The opaque brown color of these devices may make it difficult to clearly observe blood that may have contaminated the fluid pathway during use. Murga et al. (22) demonstrated enhanced biofilm formation on NCs contaminated with human blood in an in vitro study.
Our study incorporated plating on multiple nonselective and selective microbiological media to enhance the recovery of viable organisms from NC luminal surfaces. Differences in viable count estimates provided by the plate count and total viable microbial cell count assays for both standard and silver-coated NCs demonstrated that microbial communities on NCs, similar to the microbial communities of soil and aquatic systems, contain large percentages of organisms that are not culturable using standard microbiological medium and incubation conditions. The viable microbial cell count assay, which is based on the activity of esterase enzymes within cells with intact cell membranes (23), indicated that more than 90% of untreated and silver-coated NCs contained viable cells. Our results are consistent with other published studies (24, 25), indicating that solid-phase cytometry using ChemChrome will detect substantially higher numbers of viable bacteria in environmental samples than will culture-dependent methods. These results suggest that the culture-based methods used in this study select for organisms that can best compete under the growth conditions provided by the medium used for isolation, and the results may not represent the predominant organisms in the NC microbial communities.
There have been a limited number of studies of the microbial communities on indwelling medical devices using culture-independent methods. In one study, Frank et al. (26) performed sequence analysis of DNA from urinary catheter biofilms and detected OTUs of a number of organisms not normally observed on these devices using culture-dependent methods. Most of the catheters examined contained multiple species. In another study, Larsen et al. (27) developed clonal libraries of the 16S rRNA gene products of biofilms recovered from central venous catheters and compared the results with data obtained using culture-dependent methods. While organisms were recovered from 11 of 18 catheters by culture and all isolates belonged to the Gammaproteobacteria or Firmicutes phylum, OTUs were detected in all catheters characterized using the culture-independent method. Representative species from seven different phyla were detected, and approximately two-thirds of the catheters contained OTUs from two or more phyla. Finally, Zhang et al. (28) characterized bacterial communities on the outer and inner surfaces of peripheral venous catheters (PVCs) using culture-dependent and culture-independent methods. Catheters from all 15 patients examined were negative by culture (using the roll-plate method), but bacterial DNA was recovered from all catheter samples and PCR products were sequenced using 454 pyrosequencing. Microbial communities on PVCs were diverse, with 14 different bacterial phyla being detected.
In agreement with all three of the reports described above, the culture-independent biofilm bacterial community analysis method detected a vastly larger number of OTUs than did the culture-dependent approach in the present study. The biofilm community analysis method detected 12 phyla representing 52 families and 123 species; the culture-dependent approach detected 3 phyla representing 12 families and 22 species. Despite the small sample size of NCs in this study evaluated with 454 pyrosequencing (7 NCs), there was a tendency of NC microbial communities to cluster according to patient and venous access, as indicated in Fig. 2 and Table 6. The principal coordinate analysis (PCoA) in Fig. 2 is a method to demonstrate the relatedness of samples that have many sources of variation (29). This method reduces the dimensionality of the data while retaining as much variation as possible. The transformed variables are not correlated with the former variables of the data set (e.g., type of catheter and location of the catheter in the patient), so the axes do not have traditional scales or definitions. PCoA assigns variation so that the first few components contain the majority of variation between the samples. When plotted, this may result in the grouping of similar samples. The data in Fig. 2 were derived from phylogenetic analysis of the sequence data for the seven NC samples. NCs 62 and 63 and NCs 54 to 56 were collected from different lumens of the same catheters, and this was reflected in similar community diversity, as might be expected. However, venous access also appeared to be associated with community diversity. The predominant bacterial species in samples 54 to 56 (femoral catheter access) were Lactobacillus zeae, Clostridium ramosum, and Blautia spp., all reported as common inhabitants of the gastrointestinal tract (30). NC 40 had a distinctly different microbial community, in comparison with the other NCs sampled, and many of the bacteria found are normally isolated from environmental sources, although primary skin (P. acnes) and gut (Enterobacteriaceae, E. coli, or Shigella sp.) bacteria were also found. Grice and Segre (31) reported the predominance of Proteobacteria on the forearm and noted that these skin sites have among the greatest microbial diversity. Our findings support this observation; the majority of organisms detected were Proteobacteria, and NC 40 was from a peripheral intravenous central catheter placed in the antecubital fossa. In general, our results suggest that NC communities may reflect the community composition of the local microbiome but, because NCs do not contain resident flora that can regulate microbial colonization dynamics (32), there may also be an abundance of environmental organisms due to the exposure of patients to microbes in the health care environment.
TABLE 6.
Metadata for NCs that were characterized using culture-independent methods
| Patient | NC no. | Line typea | No. of lumens | Venous access siteb | Infusion typec | Line duration (days) | NC duration (days) |
|---|---|---|---|---|---|---|---|
| A | 25 | TLC | 3 | IJ | INT | 7 | 7 |
| B | 40 | PICC | 3 | Basilic | CONT | 7 | 7 |
| C | 54 | TLC | 3 | Femoral | INT | 27 | 6 |
| C | 55 | TLC | 3 | Femoral | INT | 27 | 6 |
| C | 56 | TLC | 3 | Femoral | CONT | 27 | 6 |
| D | 62 | TLC | 3 | IJ | UNK | 13 | 9 |
| D | 63 | TLC | 3 | IJ | UNK | 13 | 9 |
TLC, triple-lumen central venous catheter; PICC, peripherally inserted central catheter.
IJ, intrajugular.
INT, intermittent; CONT, continuous; UNK, unknown
Some sequences obtained through 454 sequencing were resolved only to the level of the phylum or another high taxonomic level. This is attributed to the short sequence length (340 bp) and to the limitations of the 16S rRNA gene in differentiating all species. For example, a Bacillus species and a Paenibacillus species were cultured from NC40 and identified to the genus level by sequence analysis of the entire 16S rRNA gene. However, the 454 sequence for the same NC was resolved to the Firmicutes phylum only.
Because NCs provide a portal for the microbial colonization of intravascular catheters, the presence of normally nonpathogenic organisms in NC lumens may take on greater significance, particularly for critically ill patients, as in the present study. Many of the organisms isolated in this study or detected using culture-independent methods have not been previously reported for NCs or intravascular catheters. For example, Staphylococcus capitis, Staphylococcus auricularis, Staphylococcus cohnii, Staphylococcus warneri, Corynebacterium species, Brevibacterium species, Bacillus species, and Clostridium aldenense isolated from NCs can be considered opportunistic pathogens (30). A larger unpublished study determined that none of the patients supplying NCs for the current study met the criteria for central line-associated bloodstream infection, as defined by the National Healthcare Safety Network (http://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf). This may be due to the relatively low frequency of these events and the modest sample size in this study. Alternatively, these results may suggest that there are organisms that may colonize a NC, and ultimately the catheter, without causing a bloodstream infection.
This study was limited to the analysis of NCs. Catheters were not collected and processed to recover and to characterize biofilms, which limited our ability to determine associations between the presence of organisms in the NC lumen and catheter colonization and biofilm formation. The frequency of NC accessing also could not be measured. However, it is unlikely that many clinical events occurred in this small sample of patients and their catheters. The low number of sequence reads, the small surface area from which to obtain biofilm, and an extraction detection limit of approximately 104 cells per sample for the culture-independent methods may have limited these findings, since the majority of NCs had plate counts of <104 CFU/NC. Culture-independent methods may also detect DNA from nonviable organisms, overestimating microbial diversity. Nevertheless, our findings suggest that most NCs, when placed on intravascular catheters of similar patient populations, are likely to be rapidly colonized with a diverse microbial community.
More than 90% of standard and silver-coated NCs were colonized by viable microorganisms, as measured by a total viable microbial cell count assay, and approximately 50% of each NC type contained organisms that were recovered by plate counting. The substantial difference in viable counts between the two methods suggests that media, incubation conditions, or biofilm recovery procedures may have selected for a subset of organisms. The absence of a significant difference between NC types suggests that the silver antimicrobial coating did not decrease attachment and biofilm formation on NC luminal surfaces. In general, there were no significant associations between the device usage characteristics captured and the number of positive samples for either device type, as determined by plate counts. A wide diversity of organisms, including known health care-associated pathogens, were isolated from standard and silver-coated NCs. These organisms originate primarily from human skin and gastrointestinal microbiome communities, as well as environmental sources. Most of the organisms isolated have the potential to become opportunistic pathogens. Many have not been reported for intravascular catheters or NCs. The most commonly isolated organisms on both devices were coagulase-negative staphylococci. Amplification of the 16S rRNA gene from biofilm DNA samples from silver-coated NCs provided a substantially wider list of microorganisms on NCs and demonstrated that culture-dependent methods grossly underestimate the composition of the microbial communities on these devices. Our results suggest that NCs can provide entry portals for a wide diversity of opportunistic pathogens to colonize the catheter lumen, leading to the formation of biofilms and increasing the potential for catheter-related bloodstream infections, which highlights the importance of central line maintenance practices to reduce microbial contamination of NCs.
ACKNOWLEDGMENTS
This research was funded by Baxter Healthcare Corporation as an investigator-initiated project and was performed under a research collaboration agreement between Emory University and the CDC. J. T. Jacob, M. D. Reyes, S. Chernetsky Tejedor, and J. P. Steinberg were supported in part by the investigator-initiated project.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
We acknowledge Kirk Easley and Jeffrey Switchenko for statistical analysis and Catherine Armbruster, Sarah Gilbert, Lois Wiggs, David Lonsway, Karen Anderson, Bette Jensen, Scott Sammons, Mike Frace, Duncan MacCannell, Judith Noble-Wang, and Matthew Arduino for laboratory assistance or helpful suggestions.
Footnotes
Published ahead of print 26 December 2013
REFERENCES
- 1.Climo M, Diekema D, Warren DK, Herwaldt LA, Perl TM, Peterson L, Plaskett T, Price C, Sepkowitz K, Soloman S, Tokars J, Fraser V, Wong E. 2003. Prevalence of the use of central venous access devices within and outside of the intensive care unit: results of a survey among hospitals in the Prevention Epicenter Program of the Centers for Disease Control and Prevention. Infect. Control Hosp. Epidemiol. 24:942–945. 10.1086/502163 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention 2011. Vital signs: central line-associated bloodstream infections—United States, 2001, 2008, 2009. MMWR Morb. Mortal. Wkly. Rep. 60:243–248 [PubMed] [Google Scholar]
- 3.Maki DG, Kluger DM, Crnich CJ. 2006. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin. Proc. 81:1159–1171. 10.4065/81.9.1159 [DOI] [PubMed] [Google Scholar]
- 4.Donlan RM, Murga R, Bell M, Toscano CM, Carr JH, Novicki TJ, Zuckerman C, Corey LC, Miller JM. 2001. Protocol for the detection of biofilms on needleless connectors attached to central venous catheters. J. Clin. Microbiol. 39:750–753. 10.1128/JCM.39.2.750-753.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donlan RM. 2011. Biofilm elimination on intravascular catheters: important considerations for the infectious disease practitioner. Clin. Infect. Dis. 52:1038–1045. 10.1093/cid/cir077 [DOI] [PubMed] [Google Scholar]
- 6.Jarvis WR, Murphy C, Hall KK, Fogle PJ, Karchmer TB, Harrington G, Salgado C, Giannetta ET, Cameron C, Sherertz RJ. 2009. Health care-associated bloodstream infections associated with negative- or positive-pressure or displacement mechanical valve needleless connectors. Clin. Infect. Dis. 49:1821–1827. 10.1086/648418 [DOI] [PubMed] [Google Scholar]
- 7.Rupp ME, Sholtz LA, Jourdan DR, Marion ND, Tyner LK, Fey PD, Iwen PC, Anderson JR. 2007. Outbreak of bloodstream infection temporally associated with the use of an intravascular needleless valve. Clin. Infect. Dis. 44:1408–1414. 10.1086/517538 [DOI] [PubMed] [Google Scholar]
- 8.Salgado CD, Chinnes L, Paczesney TH, Cantey JR. 2007. Increased rate of catheter-related bloodstream infection associated with use of a needleless mechanical valve at a long-term acute care hospital. Infect. Control Hosp. Epidemiol. 28:684–688. 10.1086/516800 [DOI] [PubMed] [Google Scholar]
- 9.Donia MS, Frick WF, Partensky F, Cox J, Elshahawi SI, White JR, Phillippy AM, Schatz MC, Piel J, Haygood MG, Ravel J, Schmidt EW. 2011. Complex microbiome underlying secondary and primary metabolism in the tunicate-Prochloron symbiosis. Proc. Natl. Acad. Sci. U. S. A. 108:E1423–E1432. 10.1073/pnas.1111712108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quail MA, Kozarewa I, Smith F, Scally A, Stephens PJ, Durbin R, Swerdlow H, Turner DJ. 2008. A large genome center's improvements to the Illumina sequencing system. Nat. Methods 5:1005–1010. 10.1038/nmeth.1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Informatics 10:421. 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber DJ, Rutala WA. 2001. Use of metals as microbicides in preventing infections in healthcare, p 415–430 In Block SS. (ed), Disinfection, sterilization, and preservation, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 14.Edmiston CE, Jr, Markina V. 2010. Reducing the risk of infection in vascular access patients: an in vitro evaluation of an antimicrobial silver nanotechnology Luer activated device. Am. J. Infect. Control 38:421–423. 10.1016/j.ajic.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 15.Maki DG. 2010. In vitro studies of a novel antimicrobial Luer-activated needleless connector for prevention of catheter-related bloodstream infection. Clin. Infect. Dis. 50:1580–1587. 10.1086/652764 [DOI] [PubMed] [Google Scholar]
- 16.Casey AL, Karpanen TJ, Nightingale P, Cook M, Elliott TSJ. 2012. Microbiological comparison of a silver-coated and a non-coated needleless intravascular connector in clinical use. J. Hosp. Infect. 80:299–303. 10.1016/j.jhin.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 17.Mazher MA, Kallen A, Edwards JR, Donlan RM. 2013. An in vitro evaluation of disinfection protocols used for needleless connectors of central venous catheters. Lett. Appl. Microbiol. 57:282–287. 10.1111/lam.12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams RL, Williams DF. 1988. Albumin adsorption on metal surfaces. Biomaterials 9:206–212. 10.1016/0142-9612(88)90085-3 [DOI] [PubMed] [Google Scholar]
- 19.Clement JL, Jarrett PS. 1994. Antibacterial silver. Metal Based Drugs 1:467–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox CL, Jr, Modak SM. 1974. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob. Agents Chemother. 5:582–588. 10.1128/AAC.5.6.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD. 1997. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett. Appl. Microbiol. 25:279–283. 10.1046/j.1472-765X.1997.00219.x [DOI] [PubMed] [Google Scholar]
- 22.Murga R, Miller JM, Donlan RM. 2001. Biofilm formation by Gram-negative bacteria on central venous catheter connectors: effect of conditioning films in a laboratory model. J. Clin. Microbiol. 39:2294–2297. 10.1128/JCM.39.6.2294-2297.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costanzo SP, Borazjani RN, McCormick PJ. 2002. Validation of the Scan RDI for routine microbiological analysis of process water. PDA J. Pharm. Sci. Technol. 56:206–219 [PubMed] [Google Scholar]
- 24.Reynolds DT, Fricker CR. 1999. Application of laser scanning for the rapid and automated detection of bacteria in water samples. J. Appl. Microbiol. 86:785–795. 10.1046/j.1365-2672.1999.00721.x [DOI] [PubMed] [Google Scholar]
- 25.Parthuisot N, Catala P, Lemarchand K, Baudart J, Lebaron P. 2000. Evaluation of ChemChrome V6 for bacterial viability assessment in waters. J. Appl. Microbiol. 89:370–380. 10.1046/j.1365-2672.2000.01126.x [DOI] [PubMed] [Google Scholar]
- 26.Frank DN, Wilson SS, St Armand AL, Pace NR. 2009. Culture-independent microbiological analysis of Foley urinary catheter biofilms. PLoS One 4:e7811. 10.1371/journal.pone.0007811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen MKS, Thomsen TR, Moser C, Hoiby N, Nielsen PH. 2008. Use of culture-dependent and -independent techniques to assess contamination of central venous catheters: a pilot study. BMC Clin. Pathol. 8:10. 10.1186/1472-6890-8-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Morrison M, Nimmo GR, Sriprakash KS, Mondot S, Gwardman JR, George N, Marsh N, Rickard CM. 2013. Molecular investigation of bacterial communities on the inner and outer surfaces of peripheral venous catheters. Eur. J. Clin. Microbial. Infect. Dis. 32:1083–1090. 10.1007/s10096-013-1854-4 [DOI] [PubMed] [Google Scholar]
- 29.Jolliffe IT. 2002. Principal component analysis, 2nd ed. Springer, New York, NY [Google Scholar]
- 30.Schleifer K-H. 2001. Phylum XIII: Firmicutes, p 19–1317 In Garrity GM. (ed), Bergey's manual of systematic bacteriology, 2nd ed. Springer-Verlag, New York, NY [Google Scholar]
- 31.Grice EA, Segre JA. 2011. The skin microbiome. Nat. Rev. Microbiol. 9:244–253. 10.1038/nrmicro2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth RR, James WD. 1988. Microbial ecology of the skin. Annu. Rev. Microbiol. 42:441–464. 10.1146/annurev.mi.42.100188.002301 [DOI] [PubMed] [Google Scholar]