Abstract
Coprological examination based on egg detection in stool samples is currently used as the gold standard for the diagnosis of human fascioliasis. However, this method is not effective during the acute phase of the disease and has poor sensitivity during the chronic phase. Serodiagnosis has become an excellent alternative to coprological examination in efforts to combat the effects of fascioliasis on human and animal health. Two novel recombinant Fasciola hepatica proteins, i.e., a ferritin (FhFtn-1) and a tegument-associated protein (FhTP16.5), were used as antigens to develop in-house enzyme-linked immunosorbent assay (ELISA) methods. The assays were optimized and validated using 152 serum samples from humans with a known infection status, including healthy subjects, patients with chronic fascioliasis, and patients with other parasitic diseases. The FhFtn-1 ELISA was shown to be 96.6% sensitive and 95.7% specific; the respective parameters for the FhTP16.5 ELISA were 91.4% and 92.4%. The performances of the FhFtn-1 and FhTP16.5 ELISAs were compared with that of an available commercial test (the DRG test) using a subset of serum samples. Our in-house tests were slightly more sensitive than the DRG test in detecting antibodies against F. hepatica, but the differences were not statistically significant. In conclusion, the present study provides evidence for the potential of the FhFtn-1 and FhTP16.5 ELISAs as diagnostic tools for human fascioliasis, as might be implemented in conjunction with standard assays for large-scale screenings in areas where the disease is endemic and for the detection of occasional cases in clinical laboratories.
INTRODUCTION
Fascioliasis is a disease caused by liver flukes of the genus Fasciola, of which Fasciola hepatica and Fasciola gigantica are the most common representatives. The disease causes significant economic losses worldwide, approaching 2 billion dollars per year due to ruminant livestock infection alone (1). Human fascioliasis cases have been steadily rising since the 1970s and this is now considered a reemerging parasitic disease in humans, a phenomenon that has been partly attributed to climate change (1–3). The World Health Organization recognized fascioliasis as an important infectious disease, with an estimated 17 million people affected worldwide (1). Humans become infected after ingestion of water or aquatic vegetation contaminated with F. hepatica metacercariae. F. hepatica predominates in temperate climates, and F. gigantica overlaps with F. hepatica and also is found in the tropical regions of Asia and Africa (4). Fascioliasis has historically been severely neglected by the medical and scientific communities; however, the disease has recently been recognized as a global human concern.
Confirmatory diagnosis of F. hepatica infection is based on the identification of eggs in feces or bile drainage. However, there is a consensus that this method is not wholly reliable, for several reasons. In regions in which the disease is not endemic, infections with immature flukes are not detected. Diagnosis (detection of eggs) often occurs during the chronic phase and, when eggs are detected, much of the liver damage has already occurred (5). The eggs are released intermittently from the bile ducts, so that stool samples from infected patients may not contain eggs (2). This makes it necessary to perform serial analyses of samples using concentration techniques, which makes coprological examination (CE) a labor-intensive method of diagnosis. Often, the number of eggs shed is so low that it is necessary to analyze up to six stool samples (6), which can lead to unreliable results in epidemiological studies and overburden clinical laboratories. Due to these limitations of coprological diagnosis, other standardized tests are urgently needed for both individual patient diagnoses and epidemiological surveys in areas in which human fascioliasis is endemic. To date, various diagnostic techniques have been developed, including molecular techniques such as PCR, facilitating the identification and discrimination of Fasciola spp. in areas in which F. gigantica and F. hepatica coexist (7, 8). Recently, evaluation of field-collected stools samples from ruminants and humans by duplex PCR revealed that this method is sensitive and is able to identify Fasciola spp. (8). Other serological techniques in which specific antibodies are detected, including a dot blot assay (9), lateral flow immunoassay (10), or indirect enzyme-linked immunosorbent assay (ELISA) (11–13), have been studied. Detection of antibodies in serum by ELISA is a frequently used diagnostic tool, is considered a sensitive and reliable means of diagnosing acute infections, and can be used as an adjunct to fecal analysis for the diagnosis of latent and chronic infections (14).
The antigens traditionally used in serological tests are crude extracts or excretory-secretory products (ESPs) of F. hepatica (11, 15, 16). Several F. hepatica purified antigens (17, 18) and recombinant antigens have been employed to enhance the specificity of diagnostic assays (11, 19, 20). The most notable are cathepsin L, the major protease involved in F. hepatica virulence (21), fatty acid-binding proteins (FABPs) (22–24), and saposin-like proteins (FhSAP2) (25), which have been documented as useful immunodiagnostic antigens for serological detection of fascioliasis. Although many serological methods have been published, only a few have been commercialized. One of these assays (Ildana Biotech) uses recombinant forms of cathepsin L1 as antigens and has been optimized for detection of antibodies in the serum and milk of cattle (26). Others methods, as the AccuDiag Fasciola IgG ELISA (Diagnostic Automation/Cortez Diagnostic, Inc.), Bio-X, and DRG (DRG Instruments GmbH, Germany) kits, have been optimized for detection of antibodies in the sera of cattle (27) and humans (28). These assays use ESPs as antigens, which could limit their usefulness due to cross-reactions with other parasites (27, 28).
Our research group recently reported the molecular cloning, purification, and characterization of two novel F. hepatica antigens. One of these antigens is a 16.5-kDa tegument-associated protein of unknown function, termed FhTP16.5 (29), and the other is a protein with ferroxidase activity classified as a member of the F. hepatica ferritin protein family (FhFtn-1) (30). Both molecules are differentially expressed during parasite development and have been shown to be highly reactive with sera from experimental animals with acute or chronic infections. The present study aimed to examine the potential of FhTP16.5 and FhFtn-1 as antigens for detection of antibodies in humans with chronic fascioliasis and to compare the suitability of the FhFtn-1 and FhTP16.5 ELISAs with a commercial ELISA method that uses crude excretory-secretory antigens.
MATERIALS AND METHODS
Human sera.
The serum samples used in this study were kindly donated by collaborators from the National University of Cajamarca (Cajamarca, Peru) (approved by the ethics committee of the Regional Hospital of Health from Cajamarca and the General Direction of Zoonosis from the Ministry of Health [MINSA], Lima, Peru), the Department of Sanitary Parasitology, ANLIS Dr. Carlos G. Malbrán (Buenos Aires, Argentina), the Tropical Medicine Institute (IMT) of the Central University of Venezuela (Caracas, Venezuela) (approved by the ethical scientific committee of the IMT), and the Infectious Diseases Division, Washington University School of Medicine (St. Louis, MO) (approved by the institutional review boards at Washington University School of Medicine). Serum samples all originated from adult individuals and were collected after written informed consent was obtained from all subjects. All serum samples had been previously sent to the respective laboratories and therefore were not collected specifically for this study. No personal identifiers were retained. Samples were stored frozen at −70°C for up to 6 months until use, and the aliquots tested in this study had not been thawed prior to testing. In total, 152 serum samples were analyzed. Sixty serum samples were from patients with confirmed diagnoses of fascioliasis based on detection of F. hepatica eggs in their stools and an absence of other parasitic infections (true-positive cases). Of these, 42 samples were from subjects who lived in the Bolivian Altiplano, and 18 were from subjects who lived in Cajamarca, Peru, where fascioliasis is highly endemic (12, 31). Serum samples from 51 healthy subjects who lived in areas in which trematode infections were not endemic (Puerto Rico and Argentina) during the sampling period were also obtained. The stool samples from these subjects were negative for F. hepatica and other parasites by coprological examination (CE), and these cases were used as negative controls (true-negative cases). To evaluate potential cross-reactivity, serum samples were also obtained from parasitologically and/or serologically confirmed cases of paragonimiasis (n = 5), schistosomiasis (n = 15), filariasis (n = 5), visceral larva migrans (VLM) caused by Toxocara canis (n = 4), trichinellosis (n = 4), toxoplasmosis (n = 4), and hydatidosis (n = 4).
Preparation of recombinant FhFtn-1 and FhTP16.5 proteins.
cDNA encoding FhFtn-1 (GenBank accession no. HQ316639.1) was cloned into the pRSET A plasmid (Invitrogen, Carlsbad, CA) and used to transform Escherichia coli BL21(DE3) cells (Stratagene, Santa Clara, CA). The recombinant polypeptide was overexpressed as a fusion protein with a His tag and was purified using a HisTrap FF crude affinity column (GE Healthcare Biosciences, Pittsburgh, PA), as described previously (30). cDNA encoding FhTP16.5 (GenBank accession no. AY851159) was cloned into the pGEX4T-1 plasmid and expressed in the same bacterial system. The glutathione S-transferase (GST)-tagged recombinant protein was purified by affinity chromatography using a GST-Trap HR 5/5 column (GE Healthcare) and was cleaved from the GST tag as described by Gaudier et al. (29).
Indirect enzyme-linked immunosorbent assay.
ELISA was performed following a basic protocol that was reported previously (15) and was optimized by checkerboard titration to maximize the sensitivity of the assay. Briefly, polystyrene ELISA plates (Costar, Cambridge, MA) were coated overnight at 4°C with 100 μl/well of recombinant FhFtn-1 (5 μg/ml) or FhTP16.5 (10 μg/ml) in 0.1 ml of 0.05 M carbonate buffer (pH 9.6). After three washes with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBST), the plates were blocked with 1% bovine serum albumin (BSA) in PBST (300 μl/well) at 37°C for 1 h. The sera were diluted 1:800 (for FhFtn-1 ELISA) or 1:1,600 (for FhTP16.5 ELISA) in PBST (100 μl/well) and incubated at 37°C for 1 h. Excess antibody was washed off with PBST, horseradish peroxidase (HRP)-anti-human IgG conjugate (Bio-Rad, Hercules, CA) diluted 1:5,000 was added to the wells (100 μl/well), and the wells were incubated at 37°C for 1 h. After another washing step, the substrate solution (25 ml of 0.1 M citrate-phosphate buffer [pH 5.0] containing 20 mg o-phenylenediamine dihydrochloride and 30% H2O2) was added (100 μl/well), and the plates were incubated in the dark at room temperature for 30 min. The reaction was stopped with 50 μl/well of 12.5% sulfuric acid, and the absorbance at 490 nm (A490) was measured using an ELISA reader (Bio-Rad). Positive and negative controls were included on each plate. Each ELISA determination was performed in duplicate, and the results were expressed as the mean A490 value for each determination.
DRG Fasciola hepatica IgG ELISA.
FhFtn-1 and FhTP16.5 ELISAs were compared with an ELISA (DRG test; DRG Instruments GmbH, Germany) that is based on excretory-secretory products (ESPs) from adult F. hepatica, mainly containing cysteine proteases (27, 28). This test was used with a subset of 86 serum samples selected among those that had been used previously to validate the FhFtn-1 and FhTP16.5 ELISAs. To avoid any bias, the sera used in the subset were selected blindly and included serum samples that were misdiagnosed with FhFtn-1 or FhTP16.5 ELISAs. The subset of sera included 10 negative-control serum samples, 41 serum samples from patients infected with F. hepatica, and 35 serum samples from subjects infected with other parasitic diseases. All procedures, data analysis, and validation were carried out according to the manufacturer's instructions. Human serum samples, negative- and positive-control samples, and a cutoff (CO)-control sample (1:100 dilution) were added to duplicate wells. Plates were read at 450 nm using a Bio-Rad ELISA reader. The test run was considered valid if the substrate blank had an absorbance value below 0.1, the negative-control sample below 0.2, the CO-control sample between 0.25 and 0.75, and the positive-control sample above 0.6. A serum sample was considered positive when its absorbance value was above 10% of the CO-control value. The results in DRG units (DUs) were calculated according to the following formula: DU = (sample mean A490 × 10)/CO-control value. The results were negative at DU values of <9 and positive at DU values of >11.
Data analysis.
The optimal cutoff value for each ELISA method, with 95% confidence interval (CI), was established by receiver operating characteristic (ROC) curve analysis using the EpiTools epidemiological calculator (http://epitools.ausvet.com.au). According to arbitrary guidelines, the area under the curve (AUC) values were considered as follows: noninformative, AUC = 0.5; low accurate, 0.5 < AUC ≤ 0.7; moderately accurate, 0.7 < AUC ≤ 0.9; highly accurate, 0.9 < AUC < 1; perfect, AUC = 1 (32). Intraplate repeatability was evaluated for both in-house tests by measuring the coefficient of variation (CV) of 96 repeats of 10 serum samples. Reproducibility was evaluated by performing 5 independent assays for each recombinant protein, using 5 positive-control and 5 negative-control serum samples in separate runs. The robustness of the ELISAs was also evaluated when different operators performed the test using two batches of each antigen. Results obtained with FhFtn-1 and FhTP16.5 ELISAs were compared to those obtained with the commercial test (DRG test) using a subset of serum samples. Correlations between the in-house ELISA and DRG test results were evaluated using the Pearson correlation coefficient (with the 95% CI). To evaluate the agreements among the ELISA methods or with CE, inter-rater agreement (kappa) was calculated according to the method described by Thrusfield (33). The kappa values were considered as follows: poor agreement, κ < 0.2; fair agreement, κ = 0.21 to 0.4; moderate agreement, κ = 0.41 to 0.6; good agreement, κ = 0.61 to 0.8; very good agreement, κ = 0.81 to 1.0.
RESULTS
Using the CE method as a gold standard, ROC curves were built on the basis of the absorbance values obtained with serum samples from the two reference populations, i.e., subjects infected with F. hepatica (positive in CE) and healthy subjects (negative controls). In the FhFtn-1 ELISA, absorbance values for the negative-control group ranged between 0.07 and 0.31, with a mean ± standard deviation A490 value of 0.216 ± 0.065; in the FhTP16.5 ELISA, the absorbance values for the negative-control group ranged between 0.05 and 0.46, with a mean value of 0.182 ± 0.094. The AUC values were 0.999 for the FhFtn-1 ELISA and 0.981 for the FhTP16.5 ELISA, indicating that the two assays provide equally accurate results (Fig. 1). The ROC-optimized cutoff values were 0.48 and 0.57 for the FhFtn-1 and FhTP16.5 ELISAs, respectively. When serum samples from patients with confirmed fascioliasis were tested in the FhFtn-1 ELISA, the absorbance values ranged between 0.37 and 1.7, with a mean absorbance value of 0.97 ± 0.36, whereas the absorbance in the FhTP16.5 ELISA ranged between 0.22 and 2.45, with a mean value of 1.1 ± 0.47. Based on the established cutoff values, no seropositive samples were found in the true-negative population, irrespective of the antigen used. However, 2 seronegative serum samples were detected in the true-positive population using the FhFtn-1 ELISA, and 5 seronegative serum samples were found with the FhTP16.5 ELISA. Thus, the FhFtn-1 and FhTP16.5 ELISAs reached sensitivity values of 96.6% (95% CI, 88.3% to 99.0%) and 91.4% (95% CI, 81.4% to 96.3%), respectively, and yielded specificity values of 100% (95% CI, 93% to 100%). The false-negative serum samples found with the FhFtn-1 ELISA were from Peruvian patients, whereas the false-negative serum samples found with the FhTP16.5 ELISA were from Bolivian patients. Therefore, when the results for the two antigens were combined, the assay rendered 100% sensitivity. No statistical differences were found in the absorbance values of the CE-positive samples based on their geographic origins, with either of the antigens.
FIG 1.
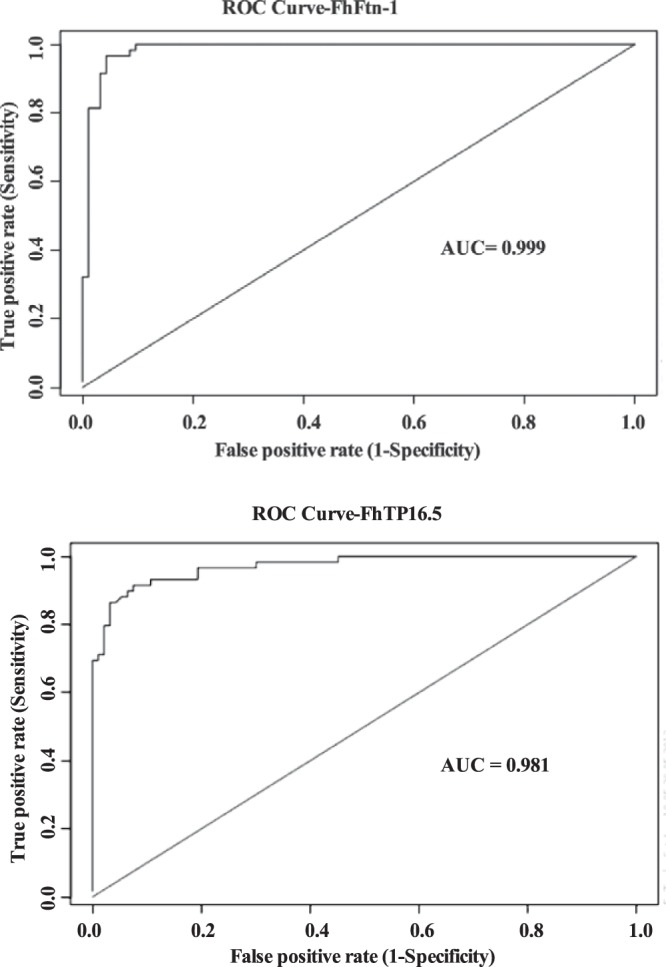
Receiver-operator characteristic (ROC) curves. The ROC curves were constructed with 51 serum samples from healthy subjects and 60 serum samples from patients with confirmed diagnoses of fascioliasis (F. hepatica eggs found in their stool samples). The values for the area under the ROC curve (accuracy) were 0.999 for the FhFtn-1 ELISA and 0.981 for the FhTP16.5 ELISA.
We assessed the reproducibility of the ELISAs by calculating the CV of data from 5 different assays and 96 repeats of 10 serum samples. The intra-assay and interassay reproducibility values were 4.9% and 11.8% for the FhFtn-1 ELISA and 6% and 15% for the FhTP16.5 ELISA, respectively.
When serum samples from subjects with parasitic infections other than fascioliasis were tested, 4 seropositive serum samples were found with the FhFtn-1 ELISA and 7 were found with the FhTP16.5 ELISA (Fig. 2). Therefore, when the cross-reactions were analyzed, the specificity dropped to 95.7% (95% CI, 89.3% to 98.3%) for the FhFtn-1 ELISA and to 92.4% for the FhTP16.5 ELISA (95% CI, 85.0% to 96.3%), as summarized in Table 1. There was good agreement between the two ELISA methods (κ = 0.84) and between the FhFtn-1 or FhTP16.5 ELISA and CE (κ values of 0.92 and 0.84, respectively); a kappa value of >0.81 indicates very good agreement between two tests (33).
FIG 2.
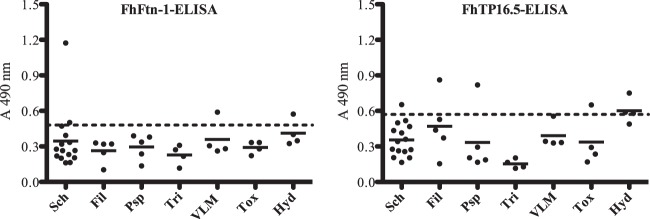
Analysis of serum samples obtained from subjects with other parasitoses tested with the FhFtn-1 and FhTP16.5 ELISAs. Serum samples from individuals carrying parasitic infections other than F. hepatica were studied. The study included 15 serum samples from patients with Schistosomiasis mansoni (Sch), 5 with lymphatic filariasis (Fil), 5 with Paragonimiasis westermani/kellicotti (Psp), 4 with trichinellosis (Tri), 4 with visceral larva migrans (VLM), 4 with toxoplasmosis (Tox), and 4 with hydatidosis (Hyd). Dashed lines, cutoff points that give the best balance between sensitivity and specificity according to the ROC curves.
TABLE 1.
Results for serum samples from patients with a diagnosis of Fasciola hepatica infection (confirmed by detection of F. hepatica eggs in patient stools), serum samples from healthy subjects, and serum samples from persons with other parasitic infections
| ELISA result | Infected |
Uninfected |
Total no. | ||
|---|---|---|---|---|---|
| Finding | No. | Finding | No. | ||
| FhFtn-1a | |||||
| Positive | True-positive | 58 | False-positive | 4 | 62 |
| Negative | False-negative | 2 | True-negative | 88 | 90 |
| Total | 60 | 92 | 152 | ||
| FhTP16.5b | |||||
| Positive | True-positive | 55 | False-positive | 7 | 62 |
| Negative | False-negative | 5 | True-negative | 85 | 90 |
| Total | 60 | 92 | 152 | ||
Sensitivity = 96.6% (95% CI, 88.5% to 99.1%), calculated as true-positive number/(true-positive number + false-negative number) × 100; specificity = 95.7% (95% CI, 89.5% to 98.3%), calculated as true-negative number/(true-negative number + false-positive number) × 100. True-positive samples were positive in CE, true-negative samples were negative in CE, false-negative samples were positive in CE but negative in serological assays, and false-positive samples were negative in CE but positive in serological assays. CE was used as the gold standard.
Sensitivity = 91.4% (95% CI, 81.4% to 96.3%), calculated as true-positive number/(true-positive number + false-negative number) × 100; specificity = 92.4% (95% CI, 85.3% to 96.3%), calculated as true-negative number/(true-negative number + false-negative number) × 100.
Table 2 shows the comparison of the FhFtn-1 and FhTP16.5 ELISAs with the DRG test using a subset of the serum samples. No seropositive samples were found in the true-negative population tested, irrespective of the test used; however, using the DRG test, 6 positive-control serum samples had readings below the cutoff value (DU value of 10) and 4 serum samples from patients with other parasitic infections, namely, schistosomiasis (n = 1) and paragonimiasis (n = 3), were recorded as seropositive. Thus, the DRG test had a sensitivity of 87.2%, a specificity of 91.8%, and good agreement with CE (κ = 0.77). The FhFtn-1 ELISA detected 39 of the 41 fascioliasis serum samples, compared with the 35 detected by the DRG test. Thus, the FhFtn-1 ELISA was slightly more sensitive than the DRG test, but the differences were not statistically significant (P = 0.26). Moreover, in terms of specificity, the performances of the two assays were similar. Although the sensitivity of the FhTP16.5 ELISA was slightly greater than that of the DRG test, the FhTP16.5 ELISA also recorded a larger number of false-positive results, compared with the DRG test or the FhFtn-1 ELISA. We found a moderate positive correlation (r = 0.68) and good agreement (κ = 0.79) when the DRG test and the FhFtn-1 ELISA were compared. The results of the comparison between the DRG test and the FhTP16.5 ELISA also demonstrated a moderate positive correlation (r = 0.61) and good agreement (κ = 0.79), using a 95% CI (Fig. 3).
TABLE 2.
Testing of serum samples from patients with F. hepatica or other parasite infections and healthy donors with the DRG test, FhFtn-1 ELISA, or FhTP16.5 ELISA
| Sample | No. of serum samples | No. (%) with positive detection by: |
||
|---|---|---|---|---|
| DRG test | FhFtn-1 ELISA | FhTP16.5 ELISA | ||
| Fascioliasisa | 41 | 35 (85.4) | 39 (95.12) | 36 (87.8) |
| Schistosomiasis | 10 | 1 (10) | 2 (20) | 1 (10) |
| Paragonimiasis | 5 | 3 (60) | 0 | 1 (20) |
| Hydatidosis | 4 | 0 | 1(25) | 3 (75) |
| Other parasitesb | 16 | 0 | 1 (6.2) | 2 (12.5) |
| Healthy | 10 | 0 | 0 | 0 |
Cases diagnosed by coprological examination (100% positive).
Including filariasis (n = 4), visceral larva migrans (n = 4), trichinellosis (n = 4), and toxoplasmosis (n = 4).
FIG 3.
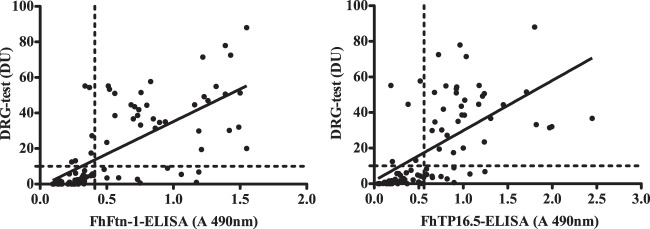
Correlations between the results of the FhFtn-1 and FhTP16.5 ELISAs and the DRG commercial test. A subset of 86 serum samples was blindly selected from the panel used to validate the FhFtn-1 and FhTP16.5 ELISAs, and the samples were tested with the DRG test for detection of antibodies. The Pearson correlation coefficient for the correlation of the DRG test and FhFtn-1 ELISA results was 0.68, and the kappa value was 0.79. The correlation coefficient for the correlation of the DRG test and FhTP16.5 ELISA results was 0.61, and the kappa value was 0.79. The results of both ELISAs are in good agreement with those of the DRG test.
DISCUSSION
Several ELISA techniques have been described for the serodiagnosis of fascioliasis, and most rely on excretory-secretory products (11, 15) or recombinant proteases (11, 34) that are the major components of ESPs. In the present study, we demonstrated that a cytosolic ferritin-like protein (FhFtn-1) and a tegument-associated protein (FhTP16.5) are also excellent antigens for the serodiagnosis of chronic fascioliasis. Although the observation of parasite eggs in stools by microscopic examination has multiple drawbacks, it is still used for confirmatory diagnosis of fascioliasis. In an effort to improve the methods of fascioliasis diagnosis, we validated the FhFtn-1 and FhTP16.5 ELISAs using a panel of serum samples obtained from human subjects with known infection status, as confirmed by stool analysis. A new diagnostic test can be evaluated with a number of different parameters, including sensitivity, specificity, accuracy, efficiency, and positive and negative predictive values (35). A critical point for such evaluations is how the cutoff point is established; therefore, in the validation of our assays we employed two well-characterized sets of serum samples with sample sizes large enough to minimize the stochastic uncertainty in cutoff selection (http://epitools.ausvet.com.au). By using ROC curve analysis, we were able to select the cutoff values giving the best balance of sensitivity and specificity for the developed ELISAs (36).
The high sensitivity values shown by the FhFtn-1 and FhTP16.5 ELISAs (96.6% and 91.4%, respectively) demonstrate that, during active infections, humans have high levels of antibodies against these proteins, which indicates that these molecules are exposed to the host immune system. This finding supports our previous studies using experimentally infected rabbits, in which antibodies were detected between 2 to 4 weeks after infection using recombinant FhFtn-1 and FhTP16.5 proteins (29, 30). Unfortunately, in the present study we were not able to obtain serum samples from human subjects with acute fascioliasis. It is worth mentioning that proper diagnosis of acute fascioliasis remains a challenge because infections are often asymptomatic and evolve to chronic disease without early treatment. However, based on the performance of the recombinant proteins using sera from animals with acute infections, it is likely that the in-house ELISAs will be similarly useful for diagnosis at early stages of human infections. Moreover, very interesting studies have identified ferritin-like and tegument-associated proteins in the vomitus and exosome-like vesicles of trematodes (37–40). These extracellular vesicles account for 52% of the F. hepatica secretomes (39–42), providing another explanation for the secretion of atypical proteins in helminths and their exposure to the host interface.
In our study, only 2 (3.3%) true-positive cases (positive in CE) fell below the cutoff value with FhFtn-1, whereas 5 (8.3%) true-positive cases were recorded as negative with FhTP16.5. Since all serum samples were properly preserved at −70°C from their collection until use, it is possible to speculate that the sensitivities of the two assays were influenced by factors such as the intensity of infection, the persistence of antibodies postinfection, and parasite senescence (21). Northern Peru and Bolivian Altiplano are two regions in which human fascioliasis is highly endemic (3, 6, 43–46). Therefore, it is also possible that the size of the dose of infectious metacercariae could affect the development of a detectable antibody response to a particular antigen (47), as human subjects exposed to F. hepatica infections are likely to ingest low doses of metacercariae over long periods.
When sera from persons with other parasites were tested, the specificity of both ELISAs dropped, since 4 sera from persons carrying other infections were positive with FhFtn-1 and 7 were positive with FhTP16.5, most with absorbance values very close to the cutoff points. Nevertheless, the diagnostic sensitivity and specificity of the FhFtn-1 and FhTP16.5 ELISAs are similar to the results obtained with other in-house ELISAs, i.e., 92.4% and 83.6% (12), 97.2% and 100% (48), and 100% and 98.9% (18, 49, 50), respectively. The cross-reactions observed in this study could be due to the existence of common epitopes between FhFtn-1 and FhTP16.5 and proteins of other parasites (51). Cross-reactivity is a significant problem, mainly for helminths, because there are many molecules (such as cathepsins, hormones, and receptors) that have been conserved during evolution and share common epitopes (51). However, other possible explanations also could be considered, i.e., (i) the persistence of low antibody levels as these persons could have been previously exposed to F. hepatica, although at the time of sample collection they did not have eggs in their feces (we do not know whether these persons lived in regions with endemic fascioliasis prior to sample collection), or (ii) cases of resolved fascioliasis infections (we recorded no history of possible previous treatment with triclabendazole). We further evaluated the false-positive serum samples with two other ELISA methods that were previously validated in our laboratory, utilizing as antigens recombinant F. hepatica saposin-2 (25) and F. hepatica tegumental proteins (52), which showed diagnostic specificity values greater than 95%. The results from these two additional ELISAs indicated that 5 of the 11 false-positive serum samples (2 samples with schistosomiasis, 1 with paragonimiasis, and 2 with hydatidosis) could be true-positive cases that were misdiagnosed in the microscopic examinations. It should be also noted that the numbers of serum samples with some of the parasitic infections tested in this study were relatively small and serum samples from patients infected with other food-borne trematodes (e.g., Clonorchis sinensis and Opisthorchis viverrini) were not available for study, which prevented us from establishing whether these pathogens may show cross-reactions. Therefore, it will be necessary to conduct a larger-scale validation study with a larger panel of serum samples with other helminthiases, particularly schistosomiasis, paragonimiasis, and clonorchiasis, which are major causes of cross-reactivity (53).
Both in-house ELISAs were compared with the DRG test, an ELISA for antibody detection based on ESPs. Our results demonstrated that the FhFtn-1 ELISA was slightly more sensitive than the FhTP16.5 ELISA or the DRG test and similar sensitivities were found for the FhTP16.5 ELISA and the DRG test. The low sensitivity obtained here with the DRG test is in contrast to the results reported by Valero et al. (28), who validated the DRG test in populations of different epidemiological statuses, obtaining sensitivity and specificity values of >95% in comparison with CE. Since the DRG test uses ESPs presumably obtained from European isolates, we could speculate that the sensitivity of the DRG test was affected by using serum samples from individuals exposed to South American isolates of F. hepatica. This presumption is supported by the fact that most of the fascioliasis serum samples analyzed here had been evaluated in a previous study employing locally produced ESPs, which yielded 100% positivity (25). However, other authors who validated the Bio-X ELISA (a cattle version of the commercial test) in animals from farms in the Cajamarca region of northern Peru, obtained 98% sensitivity and 96% specificity (27). Based on these observations, we suggest that the differences in the performance of this assay could be related to differences in the quality of the ESP preparations, rather than to problems associated with the genotypic diversity of various isolates of F. hepatica. Thus, different laboratories that have obtained ESPs from American or European isolates to test serum samples from persons or animals exposed to the same isolates report sensitivities and specificities ranging from 52% to 100% (11, 15, 25, 47, 54, 55). Despite these differences, both the FhFtn-1 and FhTP16.5 ELISAs showed good agreement with the DRG test. However, we believe that ELISAs employing recombinant proteins instead of crude ESPs could improve the possibilities for standardization of a test for fascioliasis. Also, the use of recombinant proteins will be an advantage in scaling up production for mass screening. Comparisons of results between different laboratories might be easier if the same antigen is used in all laboratories. The precision of an immunoassay is defined as the reproducibility of results within and between assays. The low CV values (<20%) obtained for both intra-assay and interassay precision of the in-house ELISAs ensure that the results obtained will be reproducible, instilling confidence about assay performance.
In conclusion, we have developed two sensitive and specific ELISAs, employing a recombinant ferritin and a tegument-associated protein, for the detection of F. hepatica antibodies in humans and validated the ELISAs using serum samples from humans of known infection status. These assays are part of the arsenal of immunodiagnostic tools that our laboratory is developing to improve the serodiagnosis of human fascioliasis. Due to the concern that positive results in antibody detection tests do not necessarily indicate current infections but may indicate a history of exposure, we are working on alternatives such as an antigen detection ELISA with monoclonal antibodies. Also, further studies are in progress to adapt our in-house ELISA methods to simpler and more-reliable formats such as immunochromatography or dot ELISA, to facilitate their possible commercialization and validation in areas in which the disease is endemic.
ACKNOWLEDGMENTS
This work was supported by SCORE grant 1SC1AI096108-01A2 and MBRS-RISE grant R25GM061838-13 from the U.S. National Institutes of Health.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
We thank Pedro Ortiz (University of Cajamarca, Faculty of Veterinary Sciences, Cajamarca, Peru), Silvana Carnevale (Infectious Diseases Hospital Dr. Carlos G. Malbrán, Buenos Aires, Argentina), Oscar Noya (Tropical Medicine Institute, Central University of Venezuela), and Gary J. Weil (Infectious Diseases Division, Washington University School of Medicine) for kindly providing the serum samples used in this study. We thank Daryl Henderson for proofreading.
Footnotes
Published ahead of print 18 December 2013
REFERENCES
- 1.Mas-Coma S. 2005. Epidemiology of fascioliasis in human endemic areas. J. Helminthol. 79:207–216. 10.1079/JOH2005296 [DOI] [PubMed] [Google Scholar]
- 2.Mas-Coma MS, Esteban JG, Bargues MD. 1999. Epidemiology of human fascioliasis: a review and proposed new classification. Bull. World Health Organ. 77:340–346 [PMC free article] [PubMed] [Google Scholar]
- 3.Mas-Coma S, Esteban JG, Bargues MD. 1999. The Northern Bolivian Altiplano: a region highly endemic for human fascioliasis. Trop. Med. Int. Health 4:454–467. 10.1046/j.1365-3156.1999.00418.x [DOI] [PubMed] [Google Scholar]
- 4.Andrews SJ, McGonigle S, Smith AM, Dalton JP, Clery D, Mulcahy G. 1995. A bolus for the administration to cattle of metacercariae of the liver fluke Fasciola hepatica. J. Helminthol. 69:165–167. 10.1017/S0022149X00014061 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Perez J, Rodriguez-Medina JR, Garcia-Blanco MA, Hillyer GV. 1992. Fasciola hepatica: molecular cloning, nucleotide sequence, and expression of a gene encoding a polypeptide homologous to a Schistosoma mansoni fatty acid-binding protein. Exp. Parasitol. 74:400–407. 10.1016/0014-4894(92)90202-L [DOI] [PubMed] [Google Scholar]
- 6.Espino AM, Diaz A, Perez A, Finlay CM. 1998. Dynamics of antigenemia and coproantigens during a human Fasciola hepatica outbreak. J. Clin. Microbiol. 36:2723–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalifa RM, El-Hady HA, Omran EK, Ahmed NS. 2013. Genetically confirmed Fasciola hepatogigantica n.sp. J. Egypt. Soc. Parasitol. 43:23–32 [DOI] [PubMed] [Google Scholar]
- 8.Le TH, Nguyen KT, Nguyen NT, Doan HT, Le XT, Hoang CT, De NV. 2012. Development and evaluation of a single-step duplex PCR for simultaneous detection of Fasciola hepatica and Fasciola gigantica (family Fasciolidae, class Trematoda, phylum Platyhelminthes). J. Clin. Microbiol. 50:2720–2726. 10.1128/JCM.00662-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamel HH, Saad GA, Sarhan RM. 2013. Dot-blot immunoassay of Fasciola gigantica infection using 27 kDa and adult worm regurge antigens in Egyptian patients. Korean J. Parasitol. 51:177–182. 10.3347/kjp.2013.51.2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Sernandez V, Muino L, Perteguer MJ, Garate T, Mezo M, Gonzalez-Warleta M, Muro A, Correia da Costa JM, Romaris F, Ubeira FM. 2011. Development and evaluation of a new lateral flow immunoassay for serodiagnosis of human fasciolosis. PLoS Negl. Trop. Dis. 5:e1376. 10.1371/journal.pntd.0001376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carnevale S, Rodriguez MI, Santillan G, Labbe JH, Cabrera MG, Bellegarde EJ, Velasquez JN, Trgovcic JE, Guarnera EA. 2001. Immunodiagnosis of human fascioliasis by an enzyme-linked immunosorbent assay (ELISA) and a micro-ELISA. Clin. Diagn. Lab. Immunol. 8:174–177. 10.1128/CDLI.8.1.174-177.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinoza JR, Maco V, Marcos L, Saez S, Neyra V, Terashima A, Samalvides F, Gotuzzo E, Chavarry E, Huaman MC, Bargues MD, Valero MA, Mas-Coma S. 2007. Evaluation of Fas2-ELISA for the serological detection of Fasciola hepatica infection in humans. Am. J. Trop. Med. Hyg. 76:977–982 [PubMed] [Google Scholar]
- 13.Nguyen TG, Le TH, De NV, Doan TT, Dao TH, Vercruysse J, Dorny P. 2010. Assessment of a 27-kDa antigen in enzyme-linked immunosorbent assay for the diagnosis of fasciolosis in Vietnamese patients. Trop. Med. Int. Health 15:462–467. 10.1111/j.1365-3156.2010.02468.x [DOI] [PubMed] [Google Scholar]
- 14.Hillyer GV, Soler de Galanes M, Rodriguez-Perez J, Bjorland J, Silva de Lagrava M, Ramirez Guzman S, Bryan RT. 1992. Use of the Falcon assay screening test–enzyme-linked immunosorbent assay (FAST-ELISA) and the enzyme-linked immunoelectrotransfer blot (EITB) to determine the prevalence of human fascioliasis in the Bolivian Altiplano. Am. J. Trop. Med. Hyg. 46:603–609 [DOI] [PubMed] [Google Scholar]
- 15.Espino AM, Dumenigo BE, Fernandez R, Finlay CM. 1987. Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay using excretory-secretory products. Am. J. Trop. Med. Hyg. 37:605–608 [DOI] [PubMed] [Google Scholar]
- 16.Rivera Marrero CA, Santiago N, Hillyer GV. 1988. Evaluation of immunodiagnostic antigens in the excretory-secretory products of Fasciola hepatica. J. Parasitol. 74:646–652. 10.2307/3282184 [DOI] [PubMed] [Google Scholar]
- 17.O'Neill SM, Parkinson M, Strauss W, Angles R, Dalton JP. 1998. Immunodiagnosis of Fasciola hepatica infection (fascioliasis) in a human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. Am. J. Trop. Med. Hyg. 58:417–423 [DOI] [PubMed] [Google Scholar]
- 18.Rokni MB, Massoud J, O'Neill SM, Parkinson M, Dalton JP. 2002. Diagnosis of human fasciolosis in the Gilan province of Northern Iran: application of cathepsin L-ELISA. Diagn. Microbiol. Infect. Dis. 44:175–179. 10.1016/S0732-8893(02)00431-5 [DOI] [PubMed] [Google Scholar]
- 19.Awad WS, Ibrahim AK, Salib FA. 2009. Using indirect ELISA to assess different antigens for the serodiagnosis of Fasciola gigantica infection in cattle, sheep and donkeys. Res. Vet. Sci. 86:466–471. 10.1016/j.rvsc.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 20.Ruiz A, Molina JM, Gonzalez J, Martinez-Moreno FJ, Gutierrez PN, Martinez-Moreno A. 2003. Humoral response (IgG) of goats experimentally infected with Fasciola hepatica against cysteine proteinases of adult fluke. Vet. Res. 34:435–443. 10.1051/vetres:2003016 [DOI] [PubMed] [Google Scholar]
- 21.Raina OK, Yadav SC, Sriveny D, Gupta SC. 2006. Immuno-diagnosis of bubaline fasciolosis with Fasciola gigantica cathepsin-L and recombinant cathepsin L 1-D proteases. Acta Trop. 98:145–151. 10.1016/j.actatropica.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 22.Allam G, Bauomy IR, Hemyeda ZM, Diab TM, Sakran TF. 2013. Diagnostic potential of Fasciola gigantica-derived 14.5 kDa fatty acid binding protein in the immunodiagnosis of bubaline fascioliasis. J. Helminthol. 87:147–153. 10.1017/S0022149X12000168 [DOI] [PubMed] [Google Scholar]
- 23.Allam G, Bauomy IR, Hemyeda ZM, Sakran TF. 2012. Evaluation of a 14.5 kDa-Fasciola gigantica fatty acid binding protein as a diagnostic antigen for human fascioliasis. Parasitol. Res. 110:1863–1871. 10.1007/s00436-011-2711-y [DOI] [PubMed] [Google Scholar]
- 24.Sobhon P, Anantavara S, Dangprasert T, Viyanant V, Krailas D, Upatham ES, Wanichanon C, Kusamran T. 1998. Fasciola gigantica: studies of the tegument as a basis for the developments of immunodiagnosis and vaccine. Southeast Asian J. Trop. Med. Public Health 29:387–400 [PubMed] [Google Scholar]
- 25.Figueroa-Santiago O, Delgado B, Espino AM. 2011. Fasciola hepatica saposin-like protein-2-based ELISA for the serodiagnosis of chronic human fascioliasis. Diagn. Microbiol. Infect. Dis. 70:355–361. 10.1016/j.diagmicrobio.2011.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins PR, Stack CM, O'Neill SM, Doyle S, Ryan T, Brennan GP, Mousley A, Stewart M, Maule AG, Dalton JP, Donnelly S. 2004. Cathepsin L1, the major protease involved in liver fluke (Fasciola hepatica) virulence: propeptide cleavage sites and autoactivation of the zymogen secreted from gastrodermal cells. J. Biol. Chem. 279:17038–17046. 10.1074/jbc.M308831200 [DOI] [PubMed] [Google Scholar]
- 27.Salimi-Bejestani MR, McGarry JW, Felstead S, Ortiz P, Akca A, Williams DJ. 2005. Development of an antibody-detection ELISA for Fasciola hepatica and its evaluation against a commercially available test. Res. Vet. Sci. 78:177–181. 10.1016/j.rvsc.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 28.Valero MA, Periago MV, Perez-Crespo I, Rodriguez E, Perteguer MJ, Garate T, Gonzalez-Barbera E, Mas-Coma S. 2012. Assessing the validity of an ELISA test for the serological diagnosis of human fascioliasis in different epidemiological situations. Trop. Med. Int. Health 17:630–636. 10.1111/j.1365-3156.2012.02964.x [DOI] [PubMed] [Google Scholar]
- 29.Gaudier JF, Caban-Hernandez K, Osuna A, Espino AM. 2012. Biochemical characterization and differential expression of a 16.5-kilodalton tegument-associated antigen from the liver fluke Fasciola hepatica. Clin. Vaccine Immunol. 19:325–333. 10.1128/CVI.05501-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caban-Hernandez K, Gaudier JF, Espino AM. 2012. Characterization and differential expression of a ferritin protein from Fasciola hepatica. Mol. Biochem. Parasitol. 182:54–61. 10.1016/j.molbiopara.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcos LA, Terashima A, Leguia G, Canales M, Espinoza JR, Gotuzzo E. 2007. Fasciola hepatica infection in Peru: an emergent disease. Rev. Gastroenterol. Peru 27:389–396 [PubMed] [Google Scholar]
- 32.Swets JA. 1988. Measuring the accuracy of diagnostic systems. Science 240:1285–1293 [DOI] [PubMed] [Google Scholar]
- 33.Thrusfield M. 1995. Veterinary epidemiology, 2nd ed. Blackwell Science, London, United Kingdom [Google Scholar]
- 34.Cordova M, Reategui L, Espinoza JR. 1999. Immunodiagnosis of human fascioliasis with Fasciola hepatica cysteine proteinases. Trans. R. Soc. Trop. Med. Hyg. 93:54–57. 10.1016/S0035-9203(99)90178-5 [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Lohr J, Greiner M. 1997. The selection of ELISA cut-off points for testing antibody to Newcastle disease by two-graph receiver operating characteristic (TG-ROC) analysis. J. Immunol. Methods 208:61–64. 10.1016/S0022-1759(97)00128-2 [DOI] [PubMed] [Google Scholar]
- 36.Zweig MH, Campbell G. 1993. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin. Chem. 39:561–577 [PubMed] [Google Scholar]
- 37.Delcroix M, Medzihradsky K, Caffrey CR, Fetter RD, McKerrow JH. 2007. Proteomic analysis of adult S. mansoni gut contents. Mol. Biochem. Parasitol. 154:95–97. 10.1016/j.molbiopara.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall SL, Braschi S, Truscott M, Mathieson W, Cesari IM, Wilson RA. 2011. Insights into blood feeding by schistosomes from a proteomic analysis of worm vomitus. Mol. Biochem. Parasitol. 179:18–29. 10.1016/j.molbiopara.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 39.Marcilla A, Trelis M, Cortes A, Sotillo J, Cantalapiedra F, Minguez MT, Valero ML, Sanchez del Pino MM, Munoz-Antoli C, Toledo R, Bernal D. 2012. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One 7:e45974. 10.1371/journal.pone.0045974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson RA, Wright JM, de Castro-Borges W, Parker-Manuel SJ, Dowle AA, Ashton PD, Young ND, Gasser RB, Spithill TW. 2011. Exploring the Fasciola hepatica tegument proteome. Int. J. Parasitol. 41:1347–1359. 10.1016/j.ijpara.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 41.Jarvius M, Paulsson J, Weibrecht I, Leuchowius KJ, Andersson AC, Wahlby C, Gulberg M, Botlin J, Sjoblom T, Markova B, Ostman A, Landergren U, Soderberg O. 2007. In situ detection of phosphorylated platelet-derived growth factor receptor beta using a generalized proximity ligation method. Mol. Cell Proteomics 6:1500–1509. 10.1074/mcp.M700166-MCP200 [DOI] [PubMed] [Google Scholar]
- 42.Morphew RM, Wright HA, LaCourse EJ, Woods DJ, Brophy PM. 2007. Comparative proteomics of excretory-secretory proteins released by the liver fluke Fasciola hepatica in sheep host bile and during in vitro culture ex host. Mol. Cell Proteomics 6:963–972. 10.1074/mcp.M600375-MCP200 [DOI] [PubMed] [Google Scholar]
- 43.Espinoza JR, Terashima A, Herrera-Velit P, Marcos LA. 2010. Human and animal fascioliasis in Peru: impact in the economy of endemic zones. Rev. Peru. Med. Exp. Salud Publica 27:604–612 (In Spanish.) 10.1590/S1726-46342010000400018 [DOI] [PubMed] [Google Scholar]
- 44.Esteban JG, Flores A, Angles R, Mas-Coma S. 1999. High endemicity of human fascioliasis between Lake Titicaca and La Paz valley, Bolivia. Trans. R. Soc. Trop. Med. Hyg. 93:151–156. 10.1016/S0035-9203(99)90289-4 [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez LC, Esteban JG, Bargues MD, Valero MA, Ortiz P, Naquira C, Mas-Coma S. 2011. Hyperendemic human fascioliasis in Andean valleys: an altitudinal transect analysis in children of Cajamarca province, Peru. Acta Trop. 120:119–129. 10.1016/j.actatropica.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 46.Millan JC, Mull R, Freise S, Richter J. 2000. The efficacy and tolerability of triclabendazole in Cuban patients with latent and chronic Fasciola hepatica infection. Am. J. Trop. Med. Hyg. 63:264–269 [DOI] [PubMed] [Google Scholar]
- 47.Cornelissen JB, Gaasenbeek CP, Boersma W, Borgsteede FH, van Milligen FJ. 1999. Use of a pre-selected epitope of cathepsin-L1 in a highly specific peptide-based immunoassay for the diagnosis of Fasciola hepatica infections in cattle. Int. J. Parasitol. 29:685–696. 10.1016/S0020-7519(99)00017-X [DOI] [PubMed] [Google Scholar]
- 48.Rahimi MT, Ashrafi K, Koosha S, Abdi J, Rokni MB. 2011. Evaluation of Fast-ELISA versus standard-ELISA to diagnose human fasciolosis. Arch. Iran. Med. 14:18–21 [PubMed] [Google Scholar]
- 49.Intapan PM, Tantrawatpan C, Maleewong W, Wongkham S, Wongkham C, Nakashima K. 2005. Potent epitopes derived from Fasciola gigantica cathepsin L1 in peptide-based immunoassay for the serodiagnosis of human fascioliasis. Diagn. Microbiol. Infect. Dis. 53:125–129. 10.1016/j.diagmicrobio.2005.05.010 [DOI] [PubMed] [Google Scholar]
- 50.Tantrawatpan C, Maleewong W, Wongkham C, Wongkham S, Intapan PM, Nakashima K. 2005. Serodiagnosis of human fascioliasis by a cystatin capture enzyme-linked immunosorbent assay with recombinant Fasciola gigantica cathepsin L antigen. Am. J. Trop. Med. Hyg. 72:82–86 [PubMed] [Google Scholar]
- 51.Losada S, Chacon N, Colmenares C, Bermudez H, Lorenzo A, Pointier JP, Theron A, Alarcon de Noya B, Noya O. 2005. Schistosoma: cross-reactivity and antigenic community among different species. Exp. Parasitol. 111:182–190. 10.1016/j.exppara.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 52.Morales A, Espino AM. 2012. Evaluation and characterization of Fasciola hepatica tegument protein extract for serodiagnosis of human fascioliasis. Clin. Vaccine Immunol. 19:1870–1878. 10.1128/CVI.00487-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikeda T, Oikawa Y, Nishiyama T. 1996. Enzyme-linked immunosorbent assay using cysteine proteinase antigens for immunodiagnosis of human paragonimiasis. Am. J. Trop. Med. Hyg. 55:435–437 [PubMed] [Google Scholar]
- 54.Arias M, Morrondo P, Hillyer GV, Sanchez-Andrade R, Suarez JL, Lomba C, Pedreira J, Diaz P, Diez-Banos P, Paz-Silva A. 2007. Immunodiagnosis of current fasciolosis in sheep naturally exposed to Fasciola hepatica by using a 2.9kDa recombinant protein. Vet. Parasitol. 146:46–49. 10.1016/j.vetpar.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 55.Parkinson M, O'Neill SM, Dalton JP. 2007. Endemic human fasciolosis in the Bolivian Altiplano. Epidemiol. Infect. 135:669–674. 10.1017/S095026880600728X [DOI] [PMC free article] [PubMed] [Google Scholar]


