Abstract
Molecular assays might improve the identification of causes of acute diarrheal disease but might lead to more frequent detection of asymptomatic infections. In the present study, real-time PCR targeting 14 pathogens was applied to rectal swabs from 330 children aged 2 to 59 months in Zanzibar, including 165 patients with acute diarrhea and 165 asymptomatic control subjects. At least one pathogen was detected for 94% of the patients and 84% of the controls, with higher rates among patients for norovirus genogroup II (20% versus 2.4%; P < 0.0001), rotavirus (10% versus 1.8%; P = 0.003), and Cryptosporidium (30% versus 11%; P < 0.0001). Detection rates did not differ significantly for enterotoxigenic Escherichia coli (ETEC)-estA (33% versus 24%), ETEC-eltB (44% versus 46%), Shigella (35% versus 33%), and Campylobacter (35% versus 33%), but for these agents threshold cycle (CT) values were lower (pathogen loads were higher) in sick children than in controls. In a multivariate analysis, CT values for norovirus genogroup II, rotavirus, Cryptosporidium, ETEC-estA, and Shigella were independently associated with diarrhea. We conclude that this real-time PCR allows convenient detection of essentially all diarrheagenic agents and provides CT values that may be critical for the interpretation of results for pathogens with similar detection rates in patients and controls. The results indicate that the assessment of pathogen loads may improve the identification of agents causing gastroenteritis in children.
INTRODUCTION
Acute diarrheal disease is the second most common cause of death worldwide in children younger than 5 years (1). Most of these deaths occur in low-income countries, where the etiologies of diarrheal infections have been incompletely understood because there are few comprehensive studies (2, 3). Such studies often used traditional diagnostic methods, such as culture, microscopy, or antigen detection, or focused on only one or a few diarrheal pathogens.
New multitargeting molecular PCR methods allow detection of diarrheal pathogens with high specificity and sensitivity (4–7), and their application may lead to improved understanding of diarrheal disease epidemiology. These methods provide better identification of viruses that cannot be cultured (e.g., Caliciviridae) or that previously have been diagnosed with methods with relatively low sensitivity (e.g., antigen testing for rotavirus) (8–10). They also have been shown to improve the detection of bacteria because of their higher sensitivity than culture (11–15). However, the mere presence of a pathogen in a fecal sample does not necessarily imply that it is the cause of disease, since high detection rates have been reported also for asymptomatic individuals with both conventional (2, 16) and molecular (12) methods. This is of particular importance in low-income countries, where children may be exposed to multiple enteric pathogens due to poor sanitary conditions. Thus, understanding the causes of diarrheal disease and how test results should be interpreted requires knowledge of the presence of pathogens in feces from both ill and healthy individuals.
In the present study, we used a broad real-time PCR assay to analyze pathogens in children, with or without diarrhea, in Zanzibar. In addition to comparing detection rates, we aimed at evaluating the potential utility of pathogen loads, in terms of real-time PCR threshold cycle (CT) values, to separate symptomatic from asymptomatic infections, as suggested by studies on norovirus, rotavirus, and Shigella infections (17–19).
MATERIALS AND METHODS
Study participants. (i) Patients.
Children 2 to 59 months of age who presented to the Kivunge Primary Health Care Centre (PHCC) in rural Zanzibar (North A district) with fever (measured axillary temperature of ≥37.5°C or a history of fever during the preceding 24 h, according to the accompanying guardian) and diarrhea (history of loose stools during the preceding 24 h) were eligible for study inclusion. Children with signs of severe disease according to Integrated Management of Childhood Illness (IMCI) guidelines (http://www.who.int/child_adolescent_health/documents/IMCI_chartbooklet/en/index.html) were excluded. Recruitment was performed in April to July 2011, corresponding to the end of the rainy season and the beginning of the dry season.
(ii) Asymptomatic control subjects.
Control subjects matched for living area and sampling time period, i.e., asymptomatic children 2 to 59 months of age, were recruited once a week during the entire study period, together with local representatives from 8 villages in the study area. No more than 2 children per household were recruited. An asymptomatic child was defined as having no history of diarrhea, cough, running nose, or fever in the preceding 10 days.
The study was approved in Zanzibar by the Zanzibar Medical Research Ethics Committee and in Sweden by the regional ethical review boards in Stockholm and Gothenburg. Written informed proxy consent was obtained from a guardian of all enrolled patients and asymptomatic control subjects. No financial incentives were given.
Samples.
Rectal swab samples were collected in a standardized manner with flocked swabs (Copan regular flocked swab 502CS01; Copan Italia Spa, Brescia, Italy) introduced 2 to 3 cm into the rectum and rotated. Directly after sampling, the swabs were placed in sterile vials containing 1 ml of 0.9% NaCl. Directly after rectal swab collection from asymptomatic community controls, the vials were placed in a vaccine carrier with a controlled temperature of 2 to 8°C. All swabs from controls and patients were transferred to microtubes, using disposable transfer pipettes, within 2 h after collection and were stored at a controlled temperature of −70°C. After completion of the field trial, all samples were transported to Sweden, on dry ice, for molecular analyses.
Extraction of nucleic acids and real-time PCR.
Following defrosting and brief vortex-mixing, 250 μl of the suspension was mixed with 2 ml of lysis buffer. Nucleic acids were then extracted into 110 μl of elution buffer with a NucliSENS easyMAG robot (bioMérieux, Marcy l'Etoile, France). By diluting samples and extracting nucleic acids with an easyMAG instrument, inhibition of PCR was effectively prevented (20).
Amplification was carried out in an ABI 7900 instrument (Applied Biosystems, Foster City, CA). After a reverse transcription step, 45 cycles of two-step PCR (95°C for 15 s and 56°C for 60 s) were performed in 10 parallel reactions, targeting a broad range of diarrheagenic agents as described in Table 1. The result for each agent was recorded as the CT value, which is inversely related to the pathogen load in each specimen. The potential utility of this quantitative information was evaluated by comparing CT values for patients and controls, as discussed below.
TABLE 1.
Primers and probes targeting RNA or DNA of diarrheagenic agents
| Pathogen | Mixture | Forward primer | Reverse primer | Probe | Fluorophorea | Target gene/region | Reference or source |
|---|---|---|---|---|---|---|---|
| Norovirus GII | 1 | TGGAYTTTTAYGTGCCCAG | CGACGCCATCTTCATTCAC | AGCCAGATTGCGATCGCCC | VIC-TAMRA | Pol-capsid junction | 22 |
| Rotavirus | 1 | AACCATCTACACATGACCCTCTATGA | GGTCACATAACGCCCCTATAGC | CAATAGTTAAAAGCTAACACTGTCAAA | FAM-MGB | Nonstructural protein 3 | 23 |
| 1 | AACCATCTTCACGTAACCCTCTATGA | ||||||
| Astrovirus | 2 | GACTGCWAAGCAGCTTCGTGA | GCTAGCCATCACACTTCTTTGGTCCT | TCACAGAAGAGCAACTCCATCGCATTTG | FAM-BQ1 | Pol-capsid junction | 24 |
| Sapovirus | 2 | TTGGCCCTCGCCACCTAC | CCCTCCATYTCAAACACTA | CCRCCTATRAACCA | VIC-MGB | Pol-capsid junction | 25 |
| 2 | GAYCASGCTCTCGCYACCTAC | ||||||
| Norovirus GI | 3 | TGGCAGGCCATGTTCCGCT | TTTGKTGGGGCGTCCTTAGAC | ATTGCGATCTCCTGTCCA | VIC-MGB | Pol-capsid junction | 22 |
| 3 | CGCTTGATGTAGCGTCCTTAGAC | ||||||
| Campylobacter | 4 | ATGCAAACCATAATTGGGTTTCAAC | CGAGTATCAGCAACTTCTTCTACAGCT | TTGCCACCAAAACCAAAACT | NED-MGB | Fibronectin-binding protein | 26b |
| Yersinia | 5 | GCTKGATTGTCAGGAGTTGGTC | ATCCCCCGCAGTTGGCAT | ACCCGCTAATGAAGCA | VIC-MGB | Enterotoxin Yst precursor | 33b |
| Vibrio cholerae | 6 | CCACTTAGTGGGTCAAACTATATTGTC | ATGCCCCTAATACATCATTAACGTT | AGCCACTGCACCCAA | FAM-MGB | Cholera toxin A subunit | 34b |
| Salmonella | 7 | CGGGTTGCGTTATAGGTCTGA | TGAAATACGATGCGAACAACATC | AATACTGCGCTGCCAGAT | VIC-MGB | Outer membrane protein C | 35b |
| ETEC-estA | 7 | AAGCATGAATAGTAGCAATTACTGCT | TTAATAGCACCCGGTACAAGCA | AACAACACAATTCAC | NED-MGB | Heat-stable enterotoxin | 27b |
| ETEC-eltB | 8 | TCCGGCAGAGGATGGTTACA | CCAGGGTTCTTCTCTCCAAGC | AGCAGGTTTCCCACCGGATCACC | FAM-BQ1 | Heat-labile enterotoxin | 28b |
| Shigella-ipaH | 8 | ACCGGCGCTCTGCTCTC | GCAATGTCCTCCAGAATTTCG | CTGGGCAGGGAAATGTTCCGCC | JOE-BQ1 | Invasion plasmid antigen H | 31b |
| Cryptosporidium | 9 | CAAATTGATACCGTTTGTCCTTCTG | TGGTGCCATACATTGTTGTCCT | TGTCCTCCTGGATTCA | NED-MGB | Oocyst wall protein | 32b |
| Adenovirus | 10 | GCCACGGTGGGGTTTCTAAACTT | GCCCCAGTGGTCTTACATGCACATC | TGCACCAGACCCGGGCTCAGGTACTCCGA | FAM-BQ1 | Hexon | 21 |
| Adenovirus 40/41 | 11c | TGCCCGCGCCACCGAT | GAGCCACAGTGGGGTTTCTG | CCAGGCTGAAGTACG | FAM-BQ1 | Hexon | This study |
FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine; MGB, minor groove binding; BQ1, black hole quencher 1; JOE, 4,5-dichloro-dimethoxy-fluorescein; VIC, 4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein. The chemical structure for NED fluorescent dye (ABI) is not publicly available.
Adaptation of traditional PCR to real-time PCR.
Run separately as a complementary analysis.
Microbial agents and target sequences.
The targets for real-time PCR are presented in Table 1. The amplified regions of rotavirus, norovirus, sapovirus, astrovirus, and adenovirus were located in conserved genomic regions (21–25), and these assays have been used in our diagnostic laboratory for several years. Bacterial PCRs were developed with guidance from available publications with respect to suitable target regions (26–35), usually by adapting a traditional PCR method to real-time PCR (when this study was planned, suitable real-time PCR assays were lacking for most nonviral targets). Thus, established target regions were used, and primers and probes were designed with the aim of obtaining similar melting temperatures (∼58 to 60°C for primers and ∼68 to 70°C for probes). For enterotoxigenic Escherichia coli (ETEC), both heat-labile toxin (eltB) and heat-stable toxin (estA) coding regions were targeted. Shigella was identified by amplification of the invasion plasmid antigen H (ipaH) gene (which also may be present in enteroinvasive E. coli [EIEC]), Campylobacter jejuni by the fibronectin-binding protein (cadF) gene, and Cryptosporidium parvum/hominis by the oocyst wall protein (OWP) gene.
Sufficient amplification efficiencies were documented for each real-time PCR by analyzing serial dilutions of pUC57 plasmids carrying synthetic target inserts. By comparing CT values for each target amplified alone or in duplex or triplex reactions, it was confirmed that performance was not compromised by multiplexing. In addition to optimization, which focused on analytical sensitivity, diagnostic accuracy was evaluated by analyzing well-characterized bacterial strains from the Culture Collection, University of Gothenburg (Campylobacter jejuni, Salmonella enteritidis, Shigella flexneri, Vibrio cholerae, and Yersinia enterocolitica), and the Department of Bacteriology at Sahlgrenska University Hospital (ETEC producing heat-labile [eltB] or heat-stable [estA] toxin). Cryptosporidium PCR was tested by analyzing Cryptosporidium parvum DNA purchased from the American Type Culture Collection (ATCC PRA-67D). The specificity of PCR for adenovirus types 40 and 41 was confirmed by analyzing DNA extracted from cultures of adenoviruses representing different genogroups.
Identification of CT cutoff values.
For pathogens with significant differences in CT values between patients and controls (Mann-Whitney U test), a cutoff value was identified as the CT value that gave the highest balanced accuracy, defined as 0.5 × (sensitivity + specificity). This cutoff value also produced the lowest P values with the χ2 test. Agents classified as more likely causes of diarrhea were those with marked differences (odds ratio [OR] of >4) in either crude detection rates or proportions with CT values below the defined cutoff value.
Statistics.
Statistical analysis was performed using Statview (SAS Institute), JMP (SAS), and Stata (StataCorp LP) software. Fisher's exact test was used for group comparisons of proportions of samples that were positive or negative by PCR assays or that were below or above the CT cutoff values that were set for some agents. CT values were compared with the Mann-Whitney U test. Multivariate analysis was performed as logistic regression with patient/control as the dependent variable and age (continuous), gender, and CT values (continuous) as independent variables. This analysis first included CT values for all agents (with negative results given a CT value of 45) and then omitted, in a stepwise manner, factors not independently associated with symptoms.
RESULTS
Patients and samples.
In total, fecal samples from 165 children with acute diarrhea and 165 asymptomatic controls were included in the analysis. The gender distributions were similar for the two groups, with 50% female subjects. The mean age was lower for patients than for controls (13.7 versus 26.3 months; P < 0.0001). For patients, the reported median duration of diarrhea was 3 days, and the reported median frequency of stools was 3 per day. At least one pathogen was detected for 94% of patients and 84% of controls.
Detection rates and median CT values.
Crude detection rates for each agent by real-time PCR are presented in Table 2. Cryptosporidium, rotavirus, and norovirus genogroup II (GII) were significantly more common in patients, whereas ETEC carrying an eltB gene (ETEC-eltB) (overall the most frequent finding), Campylobacter, Shigella, and adenovirus were detected at similar rates in the two groups. Adenovirus was detected in around 30% of samples in both patients and controls, but only a relatively small proportion of these (∼5% of all samples) were adenovirus 40 or 41, which are considered the diarrheagenic adenovirus types.
TABLE 2.
Comparison between patients and controls with respect to crude PCR detection rates and CT values
| Pathogen | No. (%) of patients | No. (%) of controls | OR | Pa | Median CT for patients | Median CT for controls | Pb |
|---|---|---|---|---|---|---|---|
| Viruses | |||||||
| Adenovirus (any) | 45 (27) | 53 (32) | 0.79 | 0.40 | 38.2 | 39.3 | 0.05 |
| Adenovirus 40/41 | 10 (6.1) | 6 (3.6) | 1.71 | 0.44 | 36.6 | 35.0 | 0.66 |
| Astrovirus | 4 (2.4) | 1 (0.6) | 4.07 | 0.37 | 19.9 | 31.5 | |
| Norovirus GI | 1 (0.6) | 1 (0.6) | 1.00 | ||||
| Norovirus GII | 33 (20) | 4 (2.4) | 10.1 | <0.0001 | 25.1 | 26.9 | 0.28 |
| Rotavirus | 16 (10) | 3 (1.8) | 5.80 | 0.003 | 24.4 | 26.0 | 0.50 |
| Sapovirus | 14 (8.5) | 7 (4.2) | 2.09 | 0.18 | 25.6 | 28.3 | 0.50 |
| Bacteria | |||||||
| Campylobacter | 58 (35) | 54 (33) | 1.11 | 0.73 | 31.8 | 33.3 | 0.12 |
| Vibrio cholerae | 1 (0.6) | 0 (0) | |||||
| ETEC-eltB | 72 (44) | 76 (46) | 0.91 | 0.74 | 31.3 | 34.6 | 0.002 |
| ETEC-estA | 55 (33) | 39 (24) | 1.62 | 0.07 | 32.6 | 37.3 | 0.0001 |
| Salmonella | 9 (5.5) | 4 (2.5) | 2.32 | 0.26 | 42.2 | 40.6 | 0.22 |
| Shigella | 57 (35) | 54 (33) | 1.08 | 0.82 | 29.2 | 34.5 | <0.0001 |
| Yersinia | 0 (0) | 0 (0) | |||||
| Protozoa | |||||||
| Cryptosporidium | 49 (30) | 18 (11) | 3.45 | <0.0001 | 32.1 | 36.8 | 0.0009 |
| Negative | 10 (6) | 27 (16) |
Fisher's exact test.
Mann-Whitney U test for agents with a total of >10 positive samples.
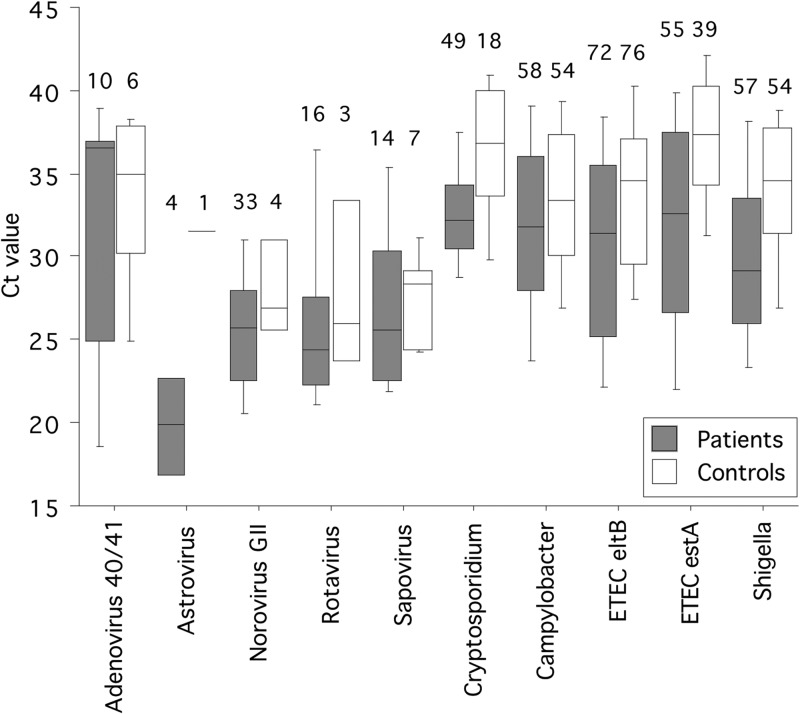
The CT values were significantly lower in patients than controls for Shigella and ETEC-eltB, which had similar crude detection rates (Table 2 and Fig. 1). CT values were also lower for Cryptosporidium and ETEC-estA, which both were more common among patients.
FIG 1.
Box plot showing CT values for agents targeted by real-time PCR among patients and controls. Boxes show the median (midline) and the 25th and 75th percentiles, and bars show the 10th and 90th percentiles. Numbers indicate the number of positive subjects for the respective agent.
CT values were further compared by multivariate logistic regression analysis that also included age and gender. For agents that were independently associated with disease in this analysis, a CT cutoff value was established. Comparing patients and controls with CT values below or above this cutoff value resulted in better distinction in terms of odds ratios, compared with crude detection rates, for Cryptosporidium, ETEC-estA, and Shigella, as shown in Table 3. The most important diarrheagenic agents, when CT cutoff values were considered (as described in Materials and Methods), were Cryptosporidium (25% with CT values of <35), Shigella (20% with CT values of <30), norovirus GII (19% with any CT value), ETEC-estA (16% with CT values of <31), and rotavirus (9.1% with any CT value).
TABLE 3.
Multivariate logistic regression analysis and discrimination by CT cutoff values
| Pathogen | Logistic regression analysis |
CT cutoff value analysisa |
|||
|---|---|---|---|---|---|
| Coefficient (SE) | P | CT value | OR (CI) | Pb | |
| Norovirus GII | 0.18 (0.04) | <0.0001 | 45 | 10.1 (3.5–29.1) | <0.0001 |
| Rotavirus | 0.12 (0.04) | 0.002 | 45 | 5.8 (1.7–20.3) | 0.003 |
| Cryptosporidium | 0.16 (0.04) | <0.0001 | 35 | 8.5 (3.5–20.6) | <0.0001 |
| ETEC-estA | 0.08 (0.03) | 0.008 | 31 | 10.1 (3.0–34.1) | <0.0001 |
| Shigella | 0.16 (0.03) | <0.0001 | 30 | 4.3 (2.0–9.4) | <0.0001 |
Patient/control was the dependent variable, and CT values, gender, and age were independent variables. Only agents with P values of <0.05 were included in the final analysis and are shown here. CI, confidence interval.
Fisher's exact test.
Infections with multiple pathogens.
Infection with more than one pathogen was a common finding for both patients and asymptomatic controls, as shown in Table 4. Thus, 2 or 3 pathogens were detected for approximately 50% of both patients and controls, and a single pathogen was found in only 22% of patient samples.
TABLE 4.
Number of pathogens detected by PCR in patients and controls
| No. of pathogens detected | No. (%) of healthy controlsa | No. (%) of patients witha: |
|
|---|---|---|---|
| All agents | Selected agentsb | ||
| None | 27 (16) | 10 (6.1) | 11 (6.7) |
| 1 | 46 (28) | 37 (22) | 91 (55) |
| 2 | 55 (33) | 50 (30) | 26 (16) |
| 3 | 31 (18) | 37 (22) | 1 (0.6) |
| 4 | 5 (3.0) | 23 (14) | 0 (0) |
| 5 | 1 (0.6) | 6 (3.6) | 0 (0) |
| 6 | 0 (0) | 2 (1.2) | 0 (0) |
Adenoviruses other than 40/41 were excluded from this analysis.
Cryptosporidium with CT values of <35, ETEC-estA with CT values of <31, Shigella with CT values of <30, norovirus GII, and rotavirus.
Age and pathogen detection.
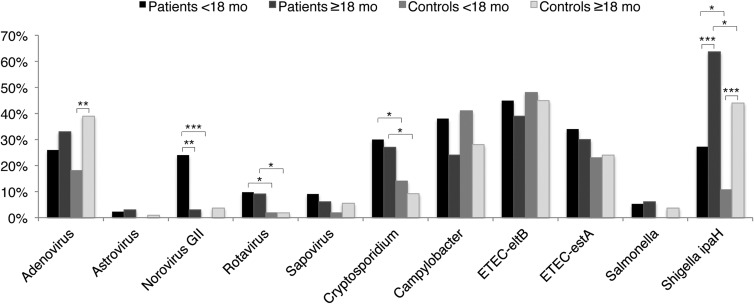
Both patients and controls who were PCR positive for Shigella or adenovirus were older than those who were PCR negative for these agents, whereas detection of norovirus GII was associated with younger age among patients and older age among controls (Table 5). Accordingly, detection rates were related to age for some agents. As shown in Fig. 2, norovirus GII was found mainly in children younger than 1.5 years, who all had symptomatic infections. Detection rates for rotavirus, Cryptosporidium, and Shigella were (or tended to be) higher for patients than controls, for children with ages below (P = 0.07, P = 0.03, and P = 0.01, respectively) or above (P = 0.08, P = 0.02, and P = 0.07, respectively) 18 months.
TABLE 5.
Median ages of patients and controls who tested positive or negative by real-time PCR
| Pathogen | Patients |
Controls |
||||
|---|---|---|---|---|---|---|
| Median age (mo [n]) |
Pa | Median age (mo [n]) |
Pa | |||
| Positive | Negative | Positive | Negative | |||
| Adenovirus (all) | 14 (45) | 11 (120) | 0.003 | 30 (53) | 21.5 (112) | 0.01 |
| Norovirus GII | 9 (33) | 12 (132) | 0.0002 | 40 (4) | 24 (161) | 0.03 |
| Rotavirus | 12 (16) | 12 (149) | 0.74 | 24 (3) | 24 (162) | 0.64 |
| Cryptosporidium | 12 (49) | 12 (116) | 0.36 | 21.5 (18) | 24 (147) | 0.23 |
| ETEC-estA | 12 (55) | 12 (110) | 0.49 | 24 (39) | 24 (126) | 0.73 |
| Salmonella | 14 (9) | 12 (156) | 0.24 | 48 (4) | 24 (161) | 0.01 |
| Shigella ipaH | 13 (57) | 11 (108) | 0.0007 | 36 (54) | 18 (111) | <0.0001 |
Mann-Whitney U test. Data for agents showing associations with age are presented. The median age was 12 months for all 165 patients, in comparison with 24 months for all 165 controls.
FIG 2.
Proportions of patients and asymptomatic controls younger or older than 18 months who tested positive by PCR. *, P < 0.10; **, P < 0.01; ***, P < 0.001.
DISCUSSION
This study demonstrates the utility of a real-time PCR assay that targets essentially all pathogens that may cause acute infectious diarrhea in children. Previous studies have shown that molecular methods have higher analytical sensitivities than conventional techniques (12–15), but data are lacking from clinical studies applying PCR assays targeting the whole spectrum of causative agents.
In this study of children younger than 5 years, detection rates were high, identifying at least one agent for 94% of patients. Two or more agents were observed for 72% of patients and for 52% of children without diarrhea. Whereas some agents (norovirus GII and rotavirus) were found mainly among patients, others were found at high rates also among asymptomatic controls. The latter group included ETEC, Shigella, and Campylobacter, which previously have been found at significant rates among children without diarrhea (3, 10, 16, 36–38). These findings suggest substantial exposure to enteric pathogens in the study population, probably due to poor sanitation. This underlines the importance of including asymptomatic controls in diarrheal studies conducted in low-income countries. Even if a control group is included, however, it may be difficult to assess the pathogenic importance of some agents if they appear at similar rates among patients and controls, as observed in this study. Therefore, we explored the potential utility of CT values as markers for pathogen loads in the interpretation of results. This was done first by including CT values when analyzing the associations between PCR results and disease and then by setting and applying CT cutoff values for some agents, in an effort to improve the distinction of causative agents.
Thus, when CT values were analyzed, ETEC, Shigella, Campylobacter, and sapovirus (all with similar detection frequencies among patients and controls) were found to be associated with symptomatic infections. Multivariate analysis confirmed independent associations with diarrhea for norovirus GII, rotavirus, Cryptosporidium, ETEC-estA, and Shigella. For the latter three agents, CT cutoff values improved the distinctions between symptomatic and asymptomatic children. When CT cutoff values were considered, the 5 most important causative agents among patients were Cryptosporidium (25%), Shigella (20%), ETEC-estA (16%), norovirus GII (19%), and rotavirus (9.1%). This is in agreement with the findings of the Global Enteric Multicenter Study (GEMS), with the exception that norovirus GII was more common than rotavirus, which was classified as the causative agent for only 9% of our patients. An explanation for this low rate may be that the study was conducted during the rainy season, when rotavirus might be less common (39).
ETEC carrying estA or eltB genes was found at high rates among both patients and asymptomatic children. Whereas ETEC-estA tended to be more common among patients (33% versus 24%), ETEC-eltB was detected at equal rates for patients and controls (44% and 46%, respectively). When CT values were included in the comparison, both ETEC-estA and ETEC-eltB were associated with diarrhea, but this association remained significant only for ETEC-estA in the multivariate analysis. A stronger association with diarrhea for ETEC-estA than ETEC-eltB has been observed by others (40). The crude detection rates for ETEC among our patients were higher than those observed in most previous studies, which typically reported rates of 10 to 20% (40–43).
Shigella infections were identified by PCR targeting the ipaH gene, a multicopy gene that is present in all Shigella species but also may be found in enteroinvasive E. coli (EIEC) (44). In this study, Shigella was detected, with CT values below 30, in 20% of children with diarrhea. Our results confirm that Shigella is an important cause of childhood diarrhea (43) and they indicate that PCR may improve detection, compared with bacterial culture (13, 15, 19).
Previous studies have shown that Cryptosporidium is an important cause of gastroenteritis in children in developing countries (45–47); in the recent GEMS, around 10% of diarrhea cases in children younger than 2 years were attributed to Cryptosporidium (3). In our study, Cryptosporidium was detected in 30% of children with diarrhea versus 11% of asymptomatic children, and 25% of patients had Cryptosporidium detected with CT values below the cutoff of 35, indicating a causative role. This is a remarkably large proportion, which might reflect the seasonality of this infection or contamination of drinking water (48).
Campylobacter was the second most common pathogen but was found as frequently among healthy children (33%) as among sick children (35%). There was a weak association between CT values and disease (P = 0.03), but an independent association could not be confirmed in the multivariate analysis. Previous studies in sub-Saharan Africa also reported similar detection rates for children with and without diarrhea, but in general the rates were lower than we observed (37, 38, 49), a difference that might be explained by the lower sensitivity of culture versus PCR (11).
Norovirus GII was common (19%) and was strongly associated with symptomatic infections and with younger age, as recently observed by others (50), with a remarkably high rate (24%) among sick children younger than 1.5 years. Both rotavirus and norovirus GII were rare among asymptomatic children; therefore, an association between the viral load (CT value) and symptomatic infection, as reported by others (17, 18), could not be properly investigated.
The younger age of the patients versus the controls is a limitation of the study that might bias the analysis of associations between pathogens and disease. When age was included in the multivariate analysis, however, the main associations between pathogen detection and diarrhea remained or were stronger. We did not use bacterial culture as a comparative method, since such cultures could not be performed at the site of the study. As a result, we cannot evaluate whether and how much molecular analysis improved the sensitivity of detection. However, it is likely that the rates of detection were greater than would have been observed with nonmolecular methods, particularly among asymptomatic subjects, for whom the pathogen loads were generally lower.
Our results suggest that pathogen quantification in feces may be clinically useful. In order to compare different studies and to implement quantification in diagnostic testing, standardization would be important but also difficult. Whereas real-time PCR quantification in blood can be accurately performed as copies/ml, a corresponding unit is neither available nor relevant for feces, mainly because 1 volume unit of watery feces is not comparable to 1 volume unit of feces with normal consistence. An option for standardization might be to relate the CT value for each bacterial target to the CT value determined by PCR targeting a conserved part of the gene coding for 16S rRNA (51). We plan to evaluate this type of normalization with respect to the total bacterial content in the sample, which would be relevant also when rectal swabs are used for molecular testing. Even if such standardization is desirable, it should be kept in mind that the differences in fecal contents between samples (which differ by a factor of 10 or less in most cases, corresponding to 3 cycles in CT values) are considerably smaller than the differences in pathogen loads between samples (which may correspond to 20 cycles or more in CT values). We realize that CT cutoff values may differ between laboratories. However, the key finding was not the cutoff values but rather the finding that pathogen loads were relevant and that the application of cutoff values increased specificity. These observations encourage further studies on the importance of pathogen loads in feces and how to standardize quantification.
In conclusion, this investigation of acute diarrhea in children younger than 5 years in rural Zanzibar demonstrates the utility of broad molecular diagnostic testing for studying the etiology of acute gastroenteritis and underlines the necessity to include healthy controls in such studies. In addition to high analytical sensitivity and specificity, real-time PCR provided quantitative information that appears to be useful for data interpretation, at least for some agents. Thus, for Cryptosporidium, ETEC carrying an estA gene, and Shigella, CT cutoff values could be identified by comparing data from patients and controls, and the application of these cutoffs improved specificity and allowed stricter interpretation of the results, which we believe provided a more correct description of the diarrheal etiology, with a smaller proportion of cases classified as being caused by multiple pathogens. The most common causative agents were Shigella, Cryptosporidium, ETEC, and norovirus GII. In comparison with previous studies, rotavirus was rarer and Cryptosporidium more frequent, differences that may be related to the limited time period for this study and the seasonality of these infections.
ACKNOWLEDGMENTS
We thank the participants, their guardians, and the study team at the Kivunge PHCC in Zanzibar for their important contributions to this study.
This work was supported by the ACT Consortium through an award from the Bill and Melinda Gates Foundation to the London School of Hygiene and Tropical Medicine.
Footnotes
Published ahead of print 8 January 2014
REFERENCES
- 1.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. 2013. Global burden of childhood pneumonia and diarrhoea. Lancet 381:1405–1416. 10.1016/S0140-6736(13)60222-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huilan S, Zhen LG, Mathan MM, Mathew MM, Olarte J, Espejo R, Khin Maung U, Ghafoor MA, Khan MA, Sami Z, Sutton RG. 1991. Etiology of acute diarrhoea among children in developing countries: a multicentre study in five countries. Bull. World Health Organ. 69:549–555 [PMC free article] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382:209–222. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Gratz J, Amour C, Kibiki G, Becker S, Janaki L, Verweij JJ, Taniuchi M, Sobuz SU, Haque R, Haverstick DM, Houpt ER. 2013. A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J. Clin. Microbiol. 51:472–480. 10.1128/JCM.02658-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, Yao JD, Wengenack NL, Rosenblatt JE, Cockerill FR, III, Smith TF. 2006. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin. Microbiol. Rev. 19:165–256. 10.1128/CMR.19.1.165-256.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer RF, Ott A, Kesztyus B, Kooistra-Smid AM. 2010. Improved detection of five major gastrointestinal pathogens by use of a molecular screening approach. J. Clin. Microbiol. 48:4140–4146. 10.1128/JCM.01124-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolffs PFG, Bruggeman CA, van Well GTJ, van Loo IHM. 2011. Replacing traditional diagnostics of fecal viral pathogens by a comprehensive panel of real-time PCRs. J. Clin. Microbiol. 49:1926–1931. 10.1128/JCM.01925-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rackoff LA, Bok K, Green KY, Kapikian AZ. 2013. Epidemiology and evolution of rotaviruses and noroviruses from an archival WHO Global Study in Children (1976–79) with implications for vaccine design. PLoS One 8:e59394. 10.1371/journal.pone.0059394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitiema LW, Nordgren J, Ouermi D, Dianou D, Traore AS, Svensson L, Simpore J. 2011. Burden of rotavirus and other enteropathogens among children with diarrhea in Burkina Faso. Int. J. Infect. Dis. 15:e646–e652. 10.1016/j.ijid.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 10.Bodhidatta L, McDaniel P, Sornsakrin S, Srijan A, Serichantalergs O, Mason CJ. 2010. Case-control study of diarrheal disease etiology in a remote rural area in Western Thailand. Am. J. Trop. Med. Hyg. 83:1106–1109. 10.4269/ajtmh.2010.10-0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bessede E, Delcamp A, Sifre E, Buissonniere A, Megraud F. 2011. New methods for detection of campylobacters in stool samples in comparison to culture. J. Clin. Microbiol. 49:941–944. 10.1128/JCM.01489-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha A, Sengupta S, Guin S, Dutta S, Ghosh S, Mukherjee P, Mukhopadhyay AK, Ramamurthy T, Takeda Y, Kurakawa T, Nomoto K, Nair GB, Nandy RK. 2013. Culture-independent real-time PCR reveals extensive polymicrobial infections in hospitalized diarrhoea cases in Kolkata, India. Clin. Microbiol. Infect. 19:173–180. 10.1111/j.1469-0691.2011.03746.x [DOI] [PubMed] [Google Scholar]
- 13.Wang S-M, Ma J-C, Hao Z-Y, Zhang Z-Y, Mason C, Sethabutr O, von Seidlein L, Wang X-Y, Xu Z-Y. 2010. Surveillance of shigellosis by real-time PCR suggests underestimation of shigellosis prevalence by culture-based methods in a population of rural China. J. Infect. 61:471–475. 10.1016/j.jinf.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 14.Wiemer D, Loderstaedt U, von Wulffen H, Priesnitz S, Fischer M, Tannich E, Hagen RM. 2011. Real-time multiplex PCR for simultaneous detection of Campylobacter jejuni, Salmonella, Shigella and Yersinia species in fecal samples. Int. J. Med. Microbiol. 301:577–584. 10.1016/j.ijmm.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 15.Vu DT, Sethabutr O, Von Seidlein L, Tran VT, Do GC, Bui TC, Le HT, Lee H, Houng HS, Hale TL, Clemens JD, Mason C, Dang DT. 2004. Detection of Shigella by a PCR assay targeting the ipaH gene suggests increased prevalence of shigellosis in Nha Trang, Vietnam. J. Clin. Microbiol. 42:2031–2035. 10.1128/JCM.42.5.2031-2035.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng CY, Smith BL, Bodhidatta L, Richard SA, Vansith K, Thy B, Srijan A, Serichantalergs O, Mason CJ. 2011. Etiology of diarrhea in young children and patterns of antibiotic resistance in Cambodia. Pediatr. Infect. Dis. J. 30:331–335. 10.1097/INF.0b013e3181fb6f82 [DOI] [PubMed] [Google Scholar]
- 17.Phillips G, Lopman B, Tam CC, Iturriza-Gomara M, Brown D, Gray J. 2009. Diagnosing norovirus-associated infectious intestinal disease using viral load. BMC Infect. Dis. 9:63. 10.1186/1471-2334-9-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dung TT, Phat VV, Nga TV, My PV, Duy PT, Campbell JI, Thuy CT, Hoang NV, Van Minh P, Le Phuc H, Tuyet PT, Vinh H, Kien DT, Huy Hle A, Vinh NT, Nga TT, Hau NT, Chinh NT, Thuong TC, Tuan HM, Simmons C, Farrar JJ, Baker S. 2013. The validation and utility of a quantitative one-step multiplex RT real-time PCR targeting rotavirus A and norovirus. J. Virol. Methods 187:138–143. 10.1016/j.jviromet.2012.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsay B, Ochieng JB, Ikumapayi UN, Toure A, Ahmed D, Li S, Panchalingam S, Levine MM, Kotloff K, Rasko DA, Morris CR, Juma J, Fields BS, Dione M, Malle D, Becker SM, Houpt ER, Nataro JP, Sommerfelt H, Pop M, Oundo J, Antonio M, Hossain A, Tamboura B, Stine OC. 2013. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J. Clin. Microbiol. 51:1740–1746. 10.1128/JCM.02713-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabayiza JC, Andersson ME, Welinder-Olsson C, Bergström T, Muhirwa G, Lindh M. 2013. Comparison of rectal swabs and faeces for real-time PCR detection of enteric agents in Rwandan children with gastroenteritis. BMC Infect. Dis. 13:447. 10.1186/1471-2334-13-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heim A, Ebnet C, Harste G, Pring-Akerblom P. 2003. Rapid and quantitative detection of human adenovirus DNA by real-time PCR. J. Med. Virol. 70:228–239. 10.1002/jmv.10382 [DOI] [PubMed] [Google Scholar]
- 22.Nenonen NP, Hannoun C, Olsson MB, Bergström T. 2009. Molecular analysis of an oyster-related norovirus outbreak. J. Clin. Virol. 45:105–108. 10.1016/j.jcv.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 23.Pang XL, Lee B, Boroumand N, Leblanc B, Preiksaitis JK, Yu Ip CC. 2004. Increased detection of rotavirus using a real time reverse transcription-polymerase chain reaction (RT-PCR) assay in stool specimens from children with diarrhea. J. Med. Virol. 72:496–501. 10.1002/jmv.20009 [DOI] [PubMed] [Google Scholar]
- 24.Gustavsson L, Westin J, Andersson LM, Lindh M. 2011. Rectal swabs can be used for diagnosis of viral gastroenteritis with a multiple real-time PCR assay. J. Clin. Virol. 51:279–282. 10.1016/j.jcv.2011.05.025 [DOI] [PubMed] [Google Scholar]
- 25.Oka T, Katayama K, Hansman GS, Kageyama T, Ogawa S, Wu FT, White PA, Takeda N. 2006. Detection of human sapovirus by real-time reverse transcription-polymerase chain reaction. J. Med. Virol. 78:1347–1353. 10.1002/jmv.20699 [DOI] [PubMed] [Google Scholar]
- 26.Konkel ME, Gray SA, Kim BJ, Garvis SG, Yoon J. 1999. Identification of the enteropathogens Campylobacter jejuni and Campylobacter coli based on the cadF virulence gene and its product. J. Clin. Microbiol. 37:510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stacy-Phipps S, Mecca JJ, Weiss JB. 1995. Multiplex PCR assay and simple preparation method for stool specimens detect enterotoxigenic Escherichia coli DNA during course of infection. J. Clin. Microbiol. 33:1054–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Victor T, du Toit R, van Zyl J, Bester AJ, van Helden PD. 1991. Improved method for the routine identification of toxigenic Escherichia coli by DNA amplification of a conserved region of the heat-labile toxin A subunit. J. Clin. Microbiol. 29:158–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beaudry M, Zhu C, Fairbrother JM, Harel J. 1996. Genotypic and phenotypic characterization of Escherichia coli isolates from dogs manifesting attaching and effacing lesions. J. Clin. Microbiol. 34:144–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gunzburg ST, Tornieporth NG, Riley LW. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sethabutr O, Venkatesan M, Yam S, Pang LW, Smoak BL, Sang WK, Echeverria P, Taylor DN, Isenbarger DW. 2000. Detection of PCR products of the ipaH gene from Shigella and enteroinvasive Escherichia coli by enzyme linked immunosorbent assay. Diagn. Microbiol. Infect. Dis. 37:11–16. 10.1016/S0732-8893(00)00122-X [DOI] [PubMed] [Google Scholar]
- 32.Haque R, Roy S, Siddique A, Mondal U, Rahman SMM, Mondal D, Houpt E, Petri WA. 2007. Multiplex real-time PCR assay for detection of Entamoeba histolytica, Giardia intestinalis, and Cryptosporidium spp. Am. J. Trop. Med. Hyg. 76:713–717 [PubMed] [Google Scholar]
- 33.Ibrahim A, Liesack W, Griffiths MW, Robins-Browne RM. 1997. Development of a highly specific assay for rapid identification of pathogenic strains of Yersinia enterocolitica based on PCR amplification of the Yersinia heat-stable enterotoxin gene (yst). J. Clin. Microbiol. 35:1636–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirai H, Nishibuchi M, Ramamurthy T, Bhattacharya SK, Pal SC, Takeda Y. 1991. Polymerase chain reaction for detection of the cholera enterotoxin operon of Vibrio cholerae. J. Clin. Microbiol. 29:2517–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez J, Sota M, Vivanco AB, Perales I, Cisterna R, Rementeria A, Garaizar J. 2004. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. J. Clin. Microbiol. 42:1734–1738. 10.1128/JCM.42.4.1734-1738.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogunsanya TI, Rotimi VO, Adenuga A. 1994. A study of the aetiological agents of childhood diarrhoea in Lagos, Nigeria. J. Med. Microbiol. 40:10–14. 10.1099/00222615-40-1-10 [DOI] [PubMed] [Google Scholar]
- 37.Reither K, Ignatius R, Weitzel T, Seidu-Korkor A, Anyidoho L, Saad E, Djie-Maletz A, Ziniel P, Amoo-Sakyi F, Danikuu F, Danour S, Otchwemah RN, Schreier E, Bienzle U, Stark K, Mockenhaupt FP. 2007. Acute childhood diarrhoea in northern Ghana: epidemiological, clinical and microbiological characteristics. BMC Infect. Dis. 7:104. 10.1186/1471-2334-7-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randremanana R, Randrianirina F, Gousseff M, Dubois N, Razafindratsimandresy R, Hariniana ER, Garin B, Randriamanantena A, Rakotonirina HC, Ramparany L, Ramarokoto CE, Rakotomanana F, Ratsitorahina M, Rajatonirina S, Talarmin A, Richard V. 2012. Case-control study of the etiology of infant diarrheal disease in 14 districts in Madagascar. PLoS One 7:e44533. 10.1371/journal.pone.0044533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy K, Hubbard AE, Eisenberg JN. 2009. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int. J. Epidemiol. 38:1487–1496. 10.1093/ije/dyn260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qadri F, Svennerholm A-M, Faruque ASG, Sack RB. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465–483. 10.1128/CMR.18.3.465-483.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen TV, Le Van P, Le Huy C, Gia KN, Weintraub A. 2005. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J. Clin. Microbiol. 43:755–760. 10.1128/JCM.43.2.755-760.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vilchez S, Reyes D, Paniagua M, Bucardo F, Mollby R, Weintraub A. 2009. Prevalence of diarrhoeagenic Escherichia coli in children from Leon, Nicaragua. J. Med. Microbiol. 58:630–637. 10.1099/jmm.0.007369-0 [DOI] [PubMed] [Google Scholar]
- 43.Hien BTT, Scheutz F, Cam PD, Serichantalergs O, Huong TT, Thu TM, Dalsgaard A. 2008. Diarrheagenic Escherichia coli and Shigella strains isolated from children in a hospital case-control study in Hanoi, Vietnam. J. Clin. Microbiol. 46:996–1004. 10.1128/JCM.01219-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng J, Yang J, Jin Q. 2009. The molecular evolutionary history of Shigella spp. and enteroinvasive Escherichia coli. Infect. Genet. Evol. 9:147–152. 10.1016/j.meegid.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 45.Ajjampur SSR, Sarkar R, Sankaran P, Kannan A, Menon VK, Muliyil J, Ward H, Kang G. 2010. Symptomatic and asymptomatic Cryptosporidium infections in children in a semi-urban slum community in southern India. Am. J. Trop. Med. Hyg. 83:1110–1115. 10.4269/ajtmh.2010.09-0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatei W, Wamae CN, Mbae C, Waruru A, Mulinge E, Waithera T, Gatika SM, Kamwati SK, Revathi G, Hart CA. 2006. Cryptosporidiosis: prevalence, genotype analysis, and symptoms associated with infections in children in Kenya. Am. J. Trop. Med. Hyg. 75:78–82 [PubMed] [Google Scholar]
- 47.Khan WA, Rogers KA, Karim MM, Ahmed S, Hibberd PL, Calderwood SB, Ryan ET, Ward HD. 2004. Cryptosporidiosis among Bangladeshi children with diarrhea: a prospective, matched, case-control study of clinical features, epidemiology and systemic antibody responses. Am. J. Trop. Med. Hyg. 71:412–419 [PubMed] [Google Scholar]
- 48.Semenza JC, Nichols G. 2007. Cryptosporidiosis surveillance and water-borne outbreaks in Europe. Euro Surveill 12:pii=711 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=711 [DOI] [PubMed] [Google Scholar]
- 49.Vargas M, Gascon J, Casals C, Schellenberg D, Urassa H, Kahigwa E, Ruiz J, Vila J. 2004. Etiology of diarrhea in children less than five years of age in Ifakara, Tanzania. Am. J. Trop. Med. Hyg. 70:536–539 [PubMed] [Google Scholar]
- 50.My PV, Thompson C, Phuc HL, Tuyet PT, Vinh H, Hoang NV, Minh PV, Vinh NT, Thuy CT, Nga TT, Hau NT, Campbell J, Chinh NT, Thuong TC, Tuan HM, Farrar J, Baker S. 2013. Endemic norovirus infections in children, Ho Chi Minh City, Vietnam, 2009–2010. Emerg. Infect. Dis. 19:977–980. 10.3201/eid1906.111862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lerner A, Romano J, Chmelnitsky I, Navon-Venezia S, Edgar R, Carmeli Y. 2013. Rectal swabs are suitable for quantifying the carriage load of KPC-producing carbapenem-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 57:1474–1479. 10.1128/AAC.01275-12 [DOI] [PMC free article] [PubMed] [Google Scholar]