Abstract
The precursor membrane envelope (prME) proteins of all three tick-borne encephalitis virus (TBEV) subtypes were produced based on expression from Semliki Forest virus (SFV) replicons transcribed from recombinant plasmids. Vero E6 cells transfected by these plasmids showed specific reactivities in immunofluorescence and immunoblot assays by monoclonal antibodies against European and Far-Eastern subtype strains of TBEV, indicating proper folding of the expressed glycoproteins. The prME glycoproteins were secreted into the cell culture supernatant, forming TBEV subviral particles of 20 to 30 nm in diameter. IgM μ-capture and IgG monoclonal antibody (MAb)-capture enzyme immunoassays (EIAs) were developed based on prME Karelia-94 (Siberian subtype) particles. Altogether, 140 human serum samples were tested using these assays, and the results were compared to those obtained with a commercial IgM EIA, an in-house μ-capture IgM assay based on baculovirus-expressed antigen, a commercial IgG EIA, and a hemagglutination inhibition test. Compared to reference enzyme-linked immunosorbent assays (ELISAs), the sensitivities of the generated μ-capture IgM SFV-prME and IgG MAb-capture SFV-prME EIAs were 97.4 to 100% and 98.7%, respectively, and the specificities of the two assays were 100%. IgM and IgG immunofluorescence assays (IFAs) were created based on Vero E6 cells transfected with the recombinant plasmid carrying the TBEV Karelia-94 prME glycoproteins. The IgM IFA was 100% concordant with the μ-capture IgM bac-prME ELISA. The IgG IFA sensitivity and specificity were 98.7% and 100%, respectively, compared to those of the commercial ELISA. In conclusion, the tests developed based on SFV replicon-driven expression of TBEV glycoproteins provide safe and robust alternatives for conducting TBEV serology.
INTRODUCTION
Tick-borne encephalitis virus (TBEV) is the etiological agent of tick-borne encephalitis, a potentially fatal infection of the central nervous system occurring in a wide region throughout Europe and Asia, with thousands of cases occurring annually (1, 2). TBEV is the most important human pathogen of the mammalian tick-borne group of the genus Flavivirus within the family Flaviviridae (3).
Mature virions of TBEV are about 50 nm in diameter and are composed of a core surrounded by a lipid bilayer containing two envelope glycoproteins, E (envelope) and M (membrane). Intracellular (immature) virions contain a precursor prM protein, and the cleavage of prM to M occurs during the exit of virions from cells. The core is composed of a single capsid protein C and contains the viral genome, an unsegmented positive-stranded RNA of approximately 11 kb. The E protein is the major immunodominant surface protein of the viral particle. It binds with cell receptors and mediates virus-cell membrane fusion. It also induces virus-neutralizing antibodies that provide protective immune response (1, 4).
TBEV can be subdivided into three subtypes: European, Siberian, and Far-Eastern (1, 2). It has been shown that the Far-Eastern subtype causes severe clinical symptoms and shows a higher morbidity rate (5 to 20%) than the other two subtypes (5, 6). The European subtype induces a biphasic febrile illness and milder encephalitis, and its fatality rates are 0 to 2% (7, 8). The Siberian subtype causes less severe disease (case fatality rates, 2 to 3%) than the Far-Eastern subtype and is often associated with chronic disease (9). At present, little is known of the mechanisms of the differing clinical manifestations among the three subtypes.
After an incubation period of 7 to 14 days, the transmission of TBEV can cause febrile illness lasting for 4 to 10 days in the infected individual, followed by a symptomless interval of a few days, as well as meningitis or meningoencephalitis in about one-third of patients (1). Reverse transcription-PCR (RT-PCR) is sensitive only during the first mild phase of the illness when patients seek medical help only rarely, whereas TBEV antibodies are practically always present by the time central nervous system (CNS) symptoms occur. Therefore, the diagnosis of TBE is usually performed serologically. Several different enzyme immunoassays (EIAs) (IgM antibody-capture and IgG assays) have been developed over the last several years (10), including commercially available IgM and IgG EIAs, which are mainly based on purified and inactivated TBEV antigens. Although the use of commercially available EIAs in a diagnostic laboratory does not require any special safety precautions, the production and purification of TBEV antigens requires biosafety level 3 facilities and a specially trained staff. We have developed a specific and sensitive μ-capture IgM immunoassay (11) based on secreted recombinant TBEV precursor membrane envelope (prME) antigens produced in insect cells. Despite the advantages of this assay, the antigen titer in the cell culture supernatant was not high, and the presence of viable recombinant baculoviruses in the antigens entail the need for permits to work with genetically modified organisms (GMO) or special safety precautions to use GMO-contaminated antigen.
In order to resolve these problems and to further test the antigenic differences of the three TBEV subtypes, as well as to provide an optimally folded protein with a similar mammalian glycosylation pattern as in the viral glycoproteins expressed in the human body, we decided to construct Semliki Forest virus (SFV) prME recombinant replicons, providing expression of TBEV prME subviral particles of all the TBEV subtypes in mammalian cell culture. Furthermore, we report an evaluation of the diagnostic potential of such prME particles using IgM μ-capture and IgG monoclonal antibody (MAb)-capture enzyme immunoassays, as well as IgM and IgG immunofluorescence assays.
MATERIALS AND METHODS
Construction of the plasmids.
The prME genes of the three TBEV subtypes (strain Kumlinge A52, European subtype [12], strain Karelia-94, Siberian subtype [13], and strain Sofjin, Far-Eastern subtype [5]) were amplified by PCR from cDNA, using appropriate primers with incorporated initiation and termination codons and flanked by AclI and ApaI sites (Table 1). Phusion High-Fidelity DNA polymerase (Thermo Scientific) was used for PCR amplification. The amplified fragments were digested with AclI/ApaI and cloned into the SFV expression vector CMV-SFV-2SG to obtain the CMV-SFV-2SG-prME constructs (Fig. 1). The CMV-SFV-2SG plasmid was obtained by deleting the reporter gene enhanced green fluorescent protein (EGFP) with BamHI restriction enzyme from the vector CMV-SFV4-2SG-EGFP (14).
TABLE 1.
Primers used in this study
| Primer name | Sequencea |
|---|---|
| prM Kumlinge A52 fwd | 5′-TTTCTAACGTTCCTAATGTCAGCGACGGACTGGATG-3′ |
| prM Karelia-94 fwd | 5′-TTTCTAACGTTCCTAATGGCAACAACAGATTGGATG-3′ |
| prM Sofjin fwd | 5′-TTTCTAACGTTCCTAATGGCTGCAGTGGACTGGACA-3′ |
| E rev Kumlinge A52 | 5′-ACTGCGGGGCCCTTACGCCCCCACTC-3′ |
| E rev Karelia-94 | 5′-ACTGCAGGGCCCTTAAGCCCCCACTC-3′ |
| E rev Sofjin | 5′-ACTGCAGGGCCCTTAAGCTCCCACTC-3′ |
The recognition sequences for AclI and ApaI restriction enzymes are underlined.
FIG 1.
Recombinant plasmid is derived from SFV replicons encoding the SFV nonstructural proteins (Nsp1 to Nsp4) and either structural proteins of TBEV prM and E. Transcription from the CMV promoter generates the complete positive-strand RNA. TBEV structural proteins are under the transcriptional control of the native SFV subgenomic promoter. AclI and ApaI represent specific restriction sites used for cloning of the TBEV prME genes (nucleotide numbers refer to the position of these sites in recombinant plasmid).
Expression of recombinant prME.
Vero E6 cells (10th or 11th passage) were grown to 75 to 80% confluence in a 100-mm plate and transfected with 19 μg CMV-SFV-2SG-prME construct using FuGENE HD transfection reagent (Promega) in Opti-MEM (Invitrogen) without serum or antibiotics. The transfected cells were grown in Opti-MEM supplemented with 7.5% fetal calf serum and antibiotics. Thirty hours posttransfection, the supernatant and cells were harvested. The cells were detached from the plate by pipetting using phosphate-buffered saline (PBS). The harvested cells were pelleted at 400 × g for 5 min at 4°C and washed twice with PBS. The pellets were used as the antigen in immunofluorescence assays (IFA) and immunoblotting. The immunoblot assay was done as described earlier (11). Briefly, the samples were reduced with 2-mercaptoethanol, separated on sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. MAbs (3 μg/ml) in TEN-T (0.05% Tween 20 in 50 mM Tris-HCl, 5 mM EDTA, and 150 mM NaCl) with 3% skim milk powder were incubated on filters for 1 h at room temperature. Peroxidase-conjugated anti-mouse immunoglobulin (Dako, Glostrup, Denmark), diluted 1:1,000 in TEN-T with 3% skim milk, was incubated for 1 h. Excess MAbs and conjugate were washed with TEN-T. The enzyme reaction was detected with electrochemiluminescence. The supernatant (15 ml) was 75-fold concentrated using Amicon Ultra-15 30K columns (Millipore), according to the manufacturer's instructions. The concentrated supernatant was stored at 4°C and used for electron microscopy and as an antigen for the EIAs.
Electron microscopy.
The concentrated culture supernatant prepared as described above was processed for negative staining using Formvar-coated copper grids. The specimens were stained with sodium phosphotungstic acid and observed on an electron microscope, the Olympus Morada Soft imaging system (Japan), with a magnification of ×200,000.
Blood serum samples.
We studied a panel of 140 serum samples. The serum samples were sent to our diagnostic laboratory (Helsinki University Central Hospital Laboratory Diagnostics, Department of Virology, Zoonosis Unit) because of suspicion of TBE infection. Thirty-nine serum samples were TBEV IgM positive, and 101 controls were negative by μ-capture IgM immunoassay based on secreted recombinant TBEV prME antigens produced in insect cells (μ-capture IgM bac-prME assay) (11). This test has been used for 10 years in TBE diagnostics in the accredited (SFS-EN ISO/IEC 17025 and SFS-EN ISO 15189) diagnostic Helsinki University Hospital Laboratory (HUSLAB), Department of Virology and Immunology (a modification of the test was also commercialized recently by Reagena International, Ltd.). The same 140 serum samples were also tested using a commercial Immunozym FSME (TBE) IgM test (Progen Biotechnik GmbH, Heidelberg, Germany) as described below, as well as for TBEV-specific hemagglutinating antibodies by an in-house hemagglutination inhibition test and for IgG antibodies using commercial TBE virus (FSME) IgG ELISA (IBL International GmbH, Hamburg, Germany). According to the IgG ELISA, 75 samples were positive and 65 were negative. To detect possible cross-reactions with the antibodies and other flaviviruses, we also included eight serum samples positive for dengue virus IgM antibodies in a commercial dengue virus IgM test (Focus Technologies, Cypress, CA) and six serum samples positive for dengue virus IgG antibodies in a commercial dengue virus IgG test (IBL International GmbH, Hamburg, Germany).
Reference tests.
As an IgM reference test, we used the μ-capture IgM bac-prME assay, which was described earlier (11), and the Immunozym FSME (TBE) IgM test (Progen Biotechnik GmbH, Heidelberg, Germany), according to the manufacturer's instructions. The Immunozym FSME IgM test is a two-step EIA. The wells of the EIA strips are coated with purified inactivated TBEV. To exclude interferences caused by specific TBE IgG antibodies or rheumatoid factors, the serum samples were diluted in rheumatoid factor/IgG absorbent (anti-human IgG). After 15 min of incubation, the diluted serum samples and subsequently the anti-human IgM conjugate were incubated in the wells. The bound conjugate was detected by incubation with a substrate; the reaction was stopped with sulfuric acid, and the optical density was measured at a wavelength of 450 nm. The cutoff values were provided by the manufacturer in the test kits and had some variation (0.25 to 0.675), which was dependent on the lot used.
As an IgG reference test, we used a TBE virus (FSME) IgG ELISA (IBL International GmbH, Hamburg, Germany) according to the manufacturer's instructions. This is a two-step EIA and is based on EIA wells coated with inactivated TBEV. Briefly, diluted serum samples and subsequently the anti-human IgG conjugate were incubated in the wells, and the bound conjugate was detected by incubation with a substrate; the reaction was stopped with sulfuric acid, and the optical density was measured at a wavelength of 450 nm. The cutoff values were provided by the manufacturer in the test kits and had some variation (0.320 to 0.650), which was dependent on the lot used.
Total antibodies to TBEV from serum were also determined by a standard hemagglutination inhibition test (15). Briefly, serum samples were incubated with kaolin and goose erythrocytes to adsorb nonspecific agglutinating factors and tested subsequently at 2-fold dilutions, starting at 1:10. A dilution series of preadsorbed serum samples were incubated overnight at 4°C in microtiter wells with TBEV antigen (the inactivated TBEV-infected cell culture supernatant). On the next day, a 0.2% suspension of goose erythrocytes (pH 6.4) was incubated in each well at room temperature for 1 h, after which the result was read.
TBEV IgM μ-capture assay.
Human serum samples were analyzed for TBEV-specific IgM antibodies by a procedure described previously (11, 16). Goat anti-human IgM serum (Cappel, West Chester, PA), diluted 1:500 in 0.05 M carbonate buffer (pH 9.6) was incubated overnight in microtiter wells at room temperature. The patient and control serum samples that were diluted 1:200 in EIA buffer (PBS with 0.5% bovine serum albumin and 0.05% Tween 20) were incubated for 1 h at 37°C in duplicate wells. Recombinant SFV-expressed TBEV prME antigen diluted 1:8 in EIA buffer was incubated for 1 h at 37°C. Anti-TBEV MAb 1786 (17) was used at a concentration of 6 μg/ml and incubated for 1 h at 37°C. For the next step, peroxidase-conjugated anti-mouse antibody (Jackson ImmunoResearch, West Grove, PA) was added for 1 h at 37°C. Unbound excess antibodies, antigen, and conjugate were washed away after each incubation with PBS-0.05% Tween 20. Specific antibody binding was detected with tetramethylbenzidine substrate (Sigma). After incubation for 15 min at room temperature, the enzyme reaction was stopped with 0.5 M H2SO4, and the absorbance at 450 nm was measured. The mean values of the sample absorbances were adjusted to an average optical density (OD) value in this experiment (2,000) by dividing the measured absorbance of a sample by this average OD. To control the interplate variation, an acute-phase tick-borne encephalitis patient serum sample was used as a positive control and an internal standard in all plates. The positive-control values between the plates were adjusted as described above.
TBEV IgG MAb-capture assay.
The assay for TBEV-specific IgG was modified from a previously described assay (16). The optimal conditions for the assay were determined by box titrations of all the included reagents. Microtiter plates were coated with MAb 14D5 (5 μg/ml) against domain III of the glycoprotein E of TBEV (18), as described above. Concentrated TBEV prME antigen and negative-control antigen (concentrated supernatant from Vero E6 cells transfected with CMV-SFV-2SG) diluted 1:8 in EIA buffer were incubated alone for 1 h and then with duplicates of patient and control serum samples, diluted 1:200 in EIA buffer, for 1 h. Specific antibody binding was detected with 1:20,000 diluted peroxidase-conjugated goat anti-human IgG (Sigma) and tetramethylbenzidine substrate. The adjustment of measured absorbances to an average OD value in this experiment (1.4) was done as described above. To control the interplate variation, an acute-phase tick-borne encephalitis patient serum sample was used as an internal standard in all plates; the control values between the plates were adjusted as described above.
Precision.
The precision of the IgM μ-capture SFV-prME and IgG MAb-capture SFV-prME assays was measured as a coefficient of variation (%) from the mean value of the positive control (3 replicates on each plate). Both intra-assay precision (the reproducibility between wells within an assay) and interassay precision (the reproducibility between assays) were estimated.
Linearity of dilution.
To assess the dilution linearity of the standard curves of the developed EIAs, the positive human serum samples were initially diluted 1:20 and then serially titrated in EIA wells in the IgM μ-capture SFV-prME and IgG MAb-capture SFV-prME assays. The linear range of the curves was estimated following this step.
TBEV IgM and IgG IFA.
The Vero E6 cells transfected with the CMV-SFV4-2SG-prME construct and the cells transfected with the CMV-SFV4-2SG plasmid were fixed onto microscope slides with acetone for 7 min and stored at 4°C until use. Patient serum samples diluted 1:10, 1:40, 1:160, 1:640, and 1:2,560 in PBS were incubated for 3 h for the IgM IFA and for 1 h for the IgG IFA at 37°C. Fluorescein isothiocyanate-conjugated anti-human IgM or IgG (Jackson ImmunoResearch, West Grove, PA), diluted 1:100 and 1:30 in PBS, respectively, were incubated for 1 h at 37°C. Unbound antibodies and anti-human IgM and IgG were washed away with PBS and distilled water. The slides were covered with mounting medium and coverslips and read using a ×20 objective of fluorescence microscope Olympus IX71 (Japan).
RESULTS
Expression of TBEV prME genes by the recombinant CMV-SFV-2SG-prME replicon.
The full-length prME Karelia-94 (Siberian subtype) sequence, including the preceding signal sequence, was inserted under the native subgenomic promoter of the SFV in CMV-SFV-2SG vector plasmid. The resulting construct was designated CMV-SFV-2SG-prME Karelia-94. The same constructions were done for the Kumlinge A52 and Sofjin strains, which belong to the European and Far-Eastern subtypes of TBEV, respectively. These recombinant plasmids were designated CMV-SFV-2SG-prME Kumlinge A52 and CMV-SFV-2SG-prME Sofjin, respectively.
To confirm the expression of TBEV structural proteins from the recombinant plasmids, the CMV-SFV-2SG-prME constructs were transfected into Vero E6 cells. The transfected cells were stained for the expression of E protein with two panels of MAbs, which were originally prepared against European (17) and Far-Eastern (18) subtype strains of TBEV. Specific reactivities with all MAbs, except MAb 171 (Table 2), were detected in cells transfected with the CMV-SFV-2SG-prME constructs but not with the initial vector CMV-SFV-2SG lacking the prME genes. The transfection efficiencies for Vero E6 cells were 25%, 40%, and 35% for the recombinant plasmids, containing the prME genes of the Kumlinge A52, Karelia-94, and Sofjin strains of TBEV, respectively. All MAbs, except MAb 171 and MAb 13D6, recognized E protein (band of 50 kDa) in cells transfected with CMV-SFV-2SG-prME constructs in an immunoblot analysis (Table 2). The cells transfected with the empty parental vector were negative in the same test.
TABLE 2.
Reactivities of MAbs, prepared against European and Far-Eastern subtype strains of TBEV, with prME antigens in IFA and immunoblotting
| MAb | Reactivitiesa of the indicated MAb in: |
|||||
|---|---|---|---|---|---|---|
| IFA |
Immunoblotting assay |
|||||
| Kumlinge A52 | Karelia-94 | Sofjin | Kumlinge A52 | Karelia-94 | Sofjin | |
| European panel | ||||||
| 1493 | ++ | ++ | ++ | ++ | ++ | ++ |
| 1786 | ++ | + | + | ++ | + | + |
| 1418 | ++ | + | + | ++ | + | + |
| 1718 | ++ | (+) | (+) | ++ | (+) | (+) |
| 171 | −− | −− | −− | −− | −− | −− |
| Far-Eastern panel | ||||||
| 14D5 | + | ++ | ++ | + | ++ | ++ |
| 1B1 | + | ++ | ++ | + | ++ | ++ |
| 13D6 | + | + | + | −− | −− | −− |
| 10C2 | + | ++ | ++ | + | ++ | ++ |
| 7C2 | + | ++ | ++ | + | ++ | ++ |
| FVN-31b | + | ++ | ++ | + | ++ | ++ |
| FVN-32b | + | ++ | ++ | + | ++ | ++ |
Reactivity categories: ++, very strong; +, strong; (+), weak; −−, none.
Not published.
The culture supernatants from the transfected cells were collected and concentrated 75-fold. The concentrated supernatants were diluted and examined in pilot experiments for the presence of secreted prME proteins by EIAs (IgM μ-capture and IgG MAb-capture assays) with human TBEV-positive and -negative serum samples. The prME proteins were secreted from the cells transfected with the recombinant (but not control) plasmids. The expression level from the CMV-SFV-2SG-prME Karelia-94 construct, however, was considerably higher than the expression level from the other two vectors (data not shown). Based on these results and transfection efficiency data, the prME Karelia-94 construct was selected as a main tool for the production of TBEV antigen. It should also be noted that the nonconcentrated culture supernatants of cells transfected with the CMV-SFV-2SG-prME constructs gave only a weak signal in the pilot EIAs. The signals-to-noise ratios in this experiment were 3.2, 4.5, and 3.5 for the prME Kumlinge A52, prME Karelia-94, and prME Sofjin proteins, respectively.
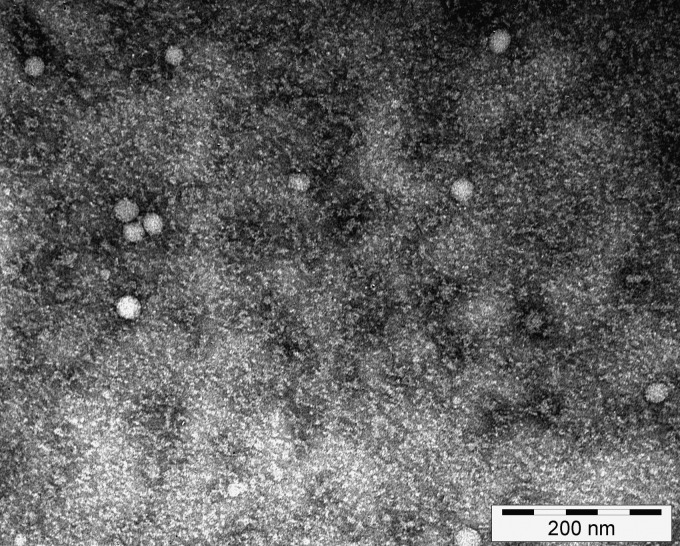
To characterize the prME Karelia-94 antigen, we examined concentrated culture supernatant with electron microscopy. The analysis showed small round structures with a diameter of around 20 to 30 nm, indicating that the secreted glycoproteins form subviral particles (Fig. 2). As expected, prME particles were not observed in culture supernatant of the cells transfected with the control plasmid CMV-SFV-2SG.
FIG 2.
Electron micrograph of TBEV prME Karelia-94 subviral particles. The concentrated culture supernatant of the cells transfected with CMV-SFV-2SG-prME Karelia-94 was processed for negative staining and observed under an electron microscope (×200,000 magnification).
In order to use the subviral particles as an antigen in the IgM and IgG assays, the dilution linearity of the standard curves, the intra- and interassay precision, the titer of the prME particles, and optimal dilutions of the expressed particles and serum samples were determined in pilot experiments by box titration. It was shown that the linear range of the curves extended from 0.17 to at least 2.3 optical densities for the IgM μ-capture SFV-prME assay and from 0.2 to at least 2.2 optical densities for the IgG MAb-capture SFV-prME assay (data not shown). Both assays had a coefficient of variation of <10% for intra- and interassay precision. The titer of subviral particles for both tests was 1:64, the optimal dilution of the concentrated prME antigen in both assays was 1:8, and the optimal dilution of human serum samples was 1:200. After this evaluation and optimization, 140 human serum samples were tested in IgM μ-capture and IgG MAb-capture assays.
IgM μ-capture assay.
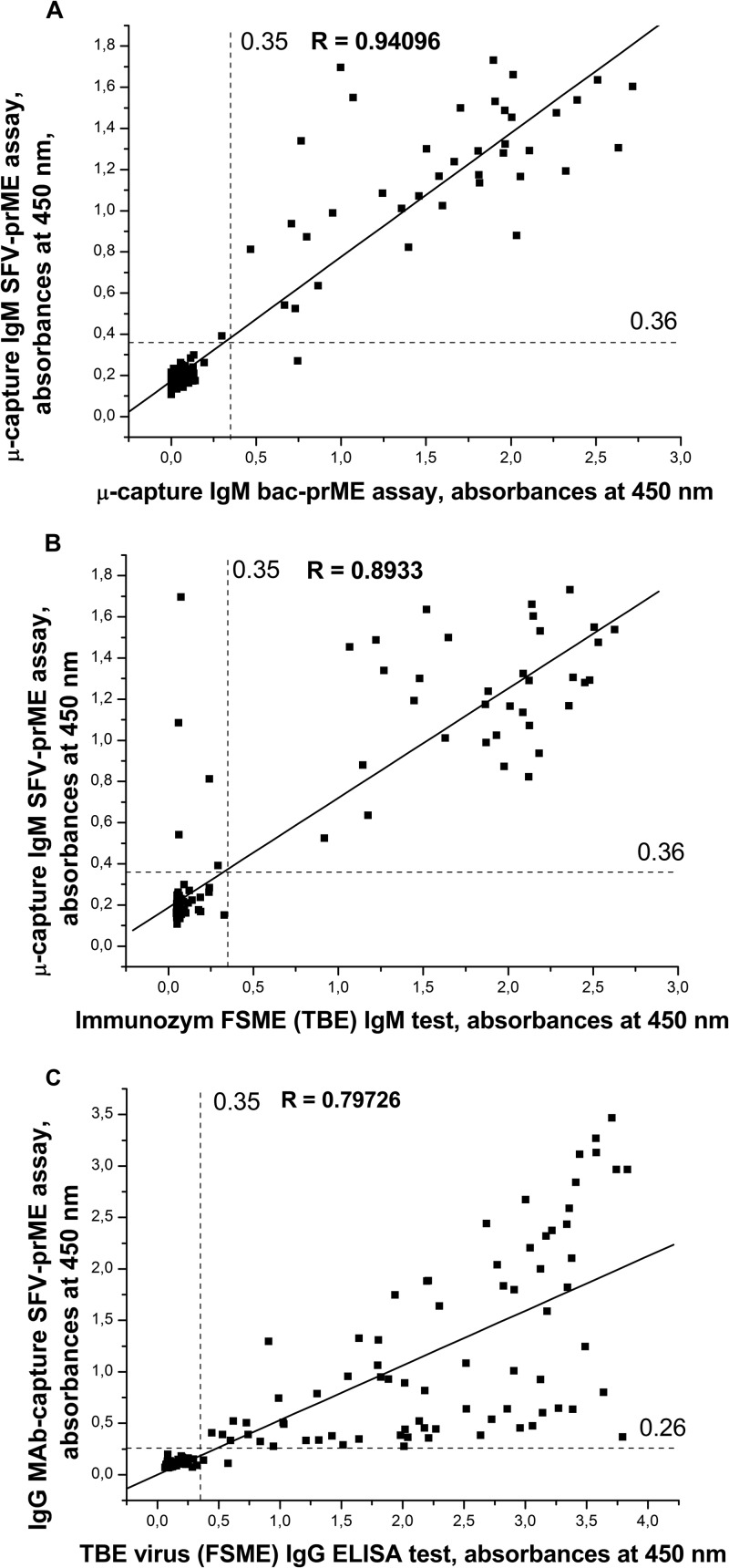
A μ-capture IgM assay was performed based on SFV-expressed TBEV prME Karelia-94 subviral particles (μ-capture IgM SFV-prME assay) and anti-TBEV E MAb 1786 (17) for the detection of IgM antibodies in human serum samples. It has been shown that MAb 1786 binds with the European and Far-Eastern subtypes of TBEV in ELISA and immunoblotting (17). This MAb was also used in a similar μ-capture IgM assay (11) and demonstrated efficient immunochemical properties in this test. The panel of 140 serum samples was studied, and the results were compared to those obtained with the reference μ-capture IgM bac-prME assay and the commercial IgM test (Fig. 3A and B). One hundred negative-control serum samples were first used to adjust the cutoff value of the assay, and the mean absorbance plus three standard deviations was 0.182 + 3 × 0.0363 = ∼0.29. The cutoff was adjusted to a 25% higher value to 0.36 in order to show the gray zone and to increase the specificity of the assay after initial evaluations. In the μ-capture IgM bac-prME assay, 39 of the 140 serum samples were positive and 101 were negative. Of these 39 positive serum samples, 38 were positive and one (2d) was negative in the μ-capture IgM SFV-prME test, and 34 were positive and 5 (1a, 2a, 2b, 2c, and 2d) were negative in the commercial IgM test (Table 3). Of the 101 serum samples that were negative in a μ-capture IgM bac-prME assay, all were negative in the μ-capture IgM SFV-prME test and in the commercial TBEV IgM test (Table 4).
FIG 3.
(A) Comparison of μ-capture IgM SFV-prME test and μ-capture IgM bac-prME assay adjusted absorbance values for 140 blood serum samples that were analyzed with the same IgM bac-prME test and thus are comparable to each other. The correlation coefficient between the tests was 0.94. The cutoff value of μ-capture IgM SFV-prME test was adjusted to 0.36, and to 0.35 for the IgM bac-prME test. (B) Comparison of μ-capture IgM SFV-prME test and commercial IgM test adjusted absorbance values for 140 serum samples that were analyzed with the same commercial test. The correlation coefficient between the tests was 0.89. The cutoff value of μ-capture IgM SFV-prME test was adjusted to 0.36, and for commercial test in this lot, it was 0.35. (C) Comparison of IgG MAb-capture SFV-prME test and commercial IgG ELISA adjusted absorbance values for 140 serum samples analyzed with the same commercial test. The correlation coefficient between the tests was 0.79. The cutoff value of IgG MAb-capture SFV-prME test was adjusted to 0.26, and for commercial test in this lot, it was 0.35.
TABLE 3.
Comparison of the results obtained in commercial EIAs and EIAs using recombinant antigens with the results obtained in IgM and IgG IFAs and hemagglutination inhibition test for inconclusive samples
| Patient no. | Result for IgM EIA (OD) |
IgM IFA result | Result for IgG EIA (OD) |
IgG IFA result | HI titer | |||
|---|---|---|---|---|---|---|---|---|
| Commercial test | SFV-prME test | bac-prME test | IgG commercial test | IgG SFV-prME test | ||||
| 1a | 0.062 | 0.541a | 0.667a | +a | 0.109 | 0.121 | − | <20 |
| 2a | 0.060 | 1.084a | 1.244a | +a | 0.129 | 0.116 | − | <20 |
| 2b | 0.074a | 1.696 | 0.998 | + | 0.741 | 0.389 | + | 20 |
| 2c | 0.242a | 0.812 | 0.468 | + | 0.196 | 0.180 | − | 20 |
| 2db | 0.123 | 0.270 | 0.744 | + | 0.287 | 0.074 | − | 80 |
| 1b | 0.052 | 0.107 | 0.103 | − | 0.577a | 0.110 | − | <20 |
Likely false-positive (1a, 2a, and 1b) and false-negative (2b and 2c) results.
Sample for patient no. 2d was inconclusive.
TABLE 4.
Comparison of the results obtained in μ-capture IgM immunoassay based on secreted recombinant TBEV prME antigen produced in insect cells (bac-prME) and ELISA using recombinant SFV-prME Karelia-94 particles and two ELISAs
| Results by IgM ELISA type | μ-capture IgM SFV- prME test results (no.) |
Immunozym FSME (TBE) IgM test results (no.) |
||||
|---|---|---|---|---|---|---|
| Positive | Negative | Total | Positive | Negative | Total | |
| μ-capture IgM bac-prME test | ||||||
| Positive | 38 | 1 | 39 | 34 | 5 | 39 |
| Negative | 0 | 101 | 101 | 0 | 101 | 101 |
| Total | 38 | 102 | 140 | 34 | 106 | 140 |
| Immunozym FSME (TBE) IgM test | ||||||
| Positive | 34 | 0 | 34 | |||
| Negative | 4 | 102 | 106 | |||
| Total | 38 | 102 | 140 | |||
The sensitivity of the μ-capture IgM SFV-prME test was 97.4% (38/39) compared to the reference μ-capture IgM bac-prME test. Compared to the commercial IgM test, the sensitivity of the μ-capture IgM SFV-prME test was 100% (34/34). Using the μ-capture IgM bac-prME assay as a reference test, the sensitivity of the commercial IgM test was 87.2% (34/39).
Eight acute-phase serum samples from patients infected with dengue virus were included to evaluate the heterologous flavivirus reactivity of the μ-capture IgM SFV-prME test. Seven samples (87.5%) were negative in the μ-capture IgM SFV-prME test.
IgG MAb-capture assay.
To study the applicability of the concentrated SFV prME particles for the detection of human IgG antibodies against TBEV, the same 140 patient serum samples were studied in an MAb-capture assay. The results were compared to those obtained with a commercially available IgG ELISA (Fig. 3C). Sixty-five negative-control serum samples were first used to adjust the cutoff value of the assay, and the mean absorbance plus three standard deviations was 0.104 + 3 × 0.0356 = ∼0.21. In order to show the gray zone and to increase the specificity of the assay after the initial evaluations, the cutoff was adjusted to a 25% higher value, to 0.26. Of the 75 serum samples shown to be positive by the commercial IgG ELISA, 74 were positive and one sample (1b) was negative in the IgG MAb-capture SFV-prME assay, for a calculated sensitivity of 98.7%. All 65 serum samples that were negative by the commercial IgG ELISA were also negative in the IgG MAb-capture SFV-prME assay, for a calculated specificity of 100% (Table 5).
TABLE 5.
Comparison of the results obtained in commercial IgG ELISA and ELISA using recombinant SFV-prME Karelia-94 particles
| TBE virus (FSME) IgG ELISA test results | IgG MAb-capture SFV-prME test results (no.) |
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 74 | 1 | 75 |
| Negative | 0 | 65 | 65 |
| Total | 74 | 66 | 140 |
Six dengue virus IgG-positive serum samples were evaluated by the IgG MAb-capture SFV prME ELISA; all were negative.
IgM and IgG IFAs.
The IgM and IgG IFAs were done using the cells transfected with the plasmid CMV-SFV-2SG-prME Karelia-94 and human serum samples (Fig. 4). The results were compared to those obtained with IgM and IgG EIAs as well as with the hemagglutination inhibition test. All 101 serum samples that were negative in the μ-capture IgM bac-prME test were negative in the IgM IFA. Of the 39 serum samples that were positive in the μ-capture IgM bac-prME test, 39 were positive in the IgM IFA. Of these 39 serum samples, 37 samples had a hemagglutination inhibition (HI) titer of ≥20. These 37 serum samples (except samples 2c and 2d) were also positive in IgG EIAs and IgG IFA. The other two samples (1a and 2a) that were negative in the hemagglutination inhibition test were also negative in a commercial IgM test, the IgG EIAs, and the IgG IFA (Table 3). Based on these results, these two samples were probably false positive by the IgM IFA. According to these data, the IgM IFA probably identified all true IgM-positive samples. Therefore, the resolved sensitivity of the developed IgM IFA test was 100%. To determine the heterologous flavivirus reactivity of the IgM IFA, eight serum samples positive for dengue virus IgM antibodies were included in our assay. Out of these eight samples, seven (87.5%) were negative by the IgM IFA.
FIG 4.
Immunofluorescence labeling of Vero E6 cells. Vero E6 cells were transfected with the recombinant plasmid CMV-SFV-2SG-prME Karelia-94 and used in the IgM and IgG IFA tests. Shown are IgM-positive (top panel) and IgM-negative (bottom panel) serum samples (A), IgG-positive (top panel) and IgG-negative (bottom panel) serum samples (B), and MAb 14D5 against glycoprotein E of TBEV (18) (top panel) and MAb 4G2 against glycoprotein G2 of Puumala virus (32) (bottom panel) (C).
Of the 75 serum samples that were positive in a commercial IgG ELISA, 74 (98.7%) samples were positive and one sample (1b) was negative in the IgG IFA (Table 3). This negative sample had an HI of <20, and it was also negative in the IgG MAb-capture SFV-prME test, the IgM EIAs, and the IgM IFA. According to these data, the IgG IFA probably identified all true IgG-positive samples. Of the 74 serum samples that were positive in the IgG EIAs and the IgG IFA, 35 samples were positive in the IgM EIAs and the IgM IFA, and 39 samples were negative in the IgM EIAs and the IgM IFA. All 65 samples that were negative in a commercial IgG ELISA were negative in the IgG IFA, as well as in the IgM EIAs and IgM IFA.
To estimate the heterologous flavivirus reactivity of the IgG IFA, six serum samples positive for dengue virus IgG antibodies were tested, and five (83.4%) were negative by IgG IFA.
DISCUSSION
At present, commercial IgM and IgG ELISAs used to diagnose TBE are mostly based on inactivated TBE virus. The production and inactivation of live TBEV for use as a diagnostic antigen require special safety precautions, as TBEV is classified as a biosafety level 3 agent, and furthermore, this does not allow sensitive capture assays with antigen in solution. To resolve these problems, several EIAs based on the recombinant TBEV antigens have been developed (11, 19, 20). In 2001, Marx et al. (19) described an IgG ELISA based on truncated E protein expressed in insect cells. The expressed protein was found in the insoluble fraction, and after the protein purification under denaturing conditions, it demonstrated activity in the IgG ELISA with serum samples from vaccinated patients. However, the sensitivity and specificity of this test were not high. Out of 48 serum samples from patients vaccinated against TBEV, only 35 were positive, whereas all six serum samples from patients vaccinated against yellow fever virus were positive in this TBEV IgG ELISA (19). A more specific and sensitive μ-capture IgM immunoassay based on secreted recombinant TBEV prME antigen produced in insect cells was developed in our laboratory (11). Despite its high sensitivity and specificity, this test system has some disadvantages, such as low antigen titer in the cell culture supernatant and the presence of viable recombinant baculoviruses. It should also be noted that both antigens described above were produced in Sf9 cells, which provide insect–cell-type glycosylation of the expressed proteins. In 2003, Yoshii et al. (20) described IgM and IgG EIAs for the detection of TBEV antibodies, based on the prME particles that were secreted from mammalian cells, transfected with the recombinant plasmid pCAGprME. Despite the high level of expression and secretion of particles, the sensitivities of the IgM and IgG assays using this antigen were only 80.7% and 87.7%, respectively (20).
In this study, we describe the production of TBEV subviral prME particles using SFV replicons and their evaluation as antigens in the IgM and IgG EIAs and the IgM and IgG IFAs. The SFV system was selected for expression in our study because of the high level of cytoplasmic expression of heterologous proteins with mammalian-cell-type glycosylation, rapid construction of recombinant DNA molecules, and the absence of infectious SFV viral contaminants in the culture supernatant of the transfected cells (21). In addition, the vector CMV-SFV-2SG does not require in vitro manipulation of RNAs because its expression is RNA polymerase II dependent. It should also be noted that the alphavirus replicon vectors have already been successfully used for high-level expression of prME particles of different flaviviruses (21–23), but to the best of our knowledge, they have never been used for the expression of TBEV proteins.
It has been shown in a number of studies that expression of the flavivirus prME sequence from either a DNA expression vector or from alphavirus replicons resulted in the secretion of subviral particles containing correctly processed E and M proteins (20–22). The expression of both full-length prM and E, including the hydrophobic domains (signal and anchor sequences), was required for proper maturation and secretion of subviral particles (20, 21). To achieve this, we inserted the full-length prME sequence together with the preceding signal sequence under the subgenomic promoter of the CMV-SFV-2SG vector. Using this system, we constructed three recombinant plasmids based on the three subtypes of TBEV. The transfected cells expressing each prME antigen were successfully stained in an IFA by monoclonal antibodies, which were prepared against the European and Far-Eastern subtype strains of TBEV. Only MAb 171 could not recognize expressed proteins in the IFA, similar to what was found earlier by Jääskeläinen et al. (11), for the prME antigen of the TBEV European subtype expressed in insect cells. This might be due to the low affinity of MAb 171, conformational changes occurring posttranslationally, or during fixation. The transfected cells were also used for detection of the prME antigen in the immunoblot assay. All monoclonal antibodies, except MAb 171 and MAb 13D6, recognized the E proteins of the European, Siberian, and Far-Eastern subtypes in this assay. Using the Far-Eastern panel of MAbs, it was shown earlier that only epitopes of MAb 13D6 seemed to be sensitive to SDS-denaturing conditions (18). To summarize this part of our study, both the IFA and immunoblot assay clearly showed evidence of proper folding of the expressed prME proteins and the low subtype variability of the glycoprotein E of TBEV; these findings are in agreement with previous results (24, 25).
To investigate whether the prME antigen is secreted from the cells, the nonconcentrated culture supernatants of transfected cells were tested by the IgM and IgG EIAs. The results of these tests indicated that the prME antigen was indeed secreted into the culture supernatant. However, the signals in both assays were weak. We assume that the moderate level of expression and modest secretion of the prME proteins were linked to the wild-type nature of the SFV replicon vector. It is known that nonattenuated SFV vectors lead to high protein expression in mammalian cells, but expression is transient due to cytopathic effects caused by the vector (26). In our case, we were able to collect culture supernatant during a short period only, 30 h after transfection. After this period, a massive apoptotic effect was observed.
The concentrated culture supernatant was analyzed by electron microscopy. The results indicated that TBEV subviral particles of 20 to 30 nm in diameter were secreted into the culture medium of Vero E6 cells transfected by prME Karelia-94 construct. Yoshii et al. (20) and Ferlenghi et al. (27) obtained recombinant TBEV prME particles of the same size, secreted from transfected mammalian cells. Similar results for recombinant West Nile virus prME particles have been reported by Takahashi et al. (28). In all these reports, the difference in size of the prME particles was probably linked to the absence of capsid protein, which plays an essential role in the formation of viral particles.
IgM EIAs have been used extensively in the diagnosis of flavivirus infections due to the high specific activity of the antigen and the ability to detect IgM antibodies during the early phase of infection. The concentrated culture supernatant of the Vero E6 cells transfected with the recombinant plasmid CMV-SFV-2SG-prME-Karelia-94 was subsequently used for the development of a μ-capture IgM assay to detect TBE virus-specific IgM antibodies in human serum samples. The μ-capture IgM SFV-prME assay using subviral prME particles identified 97.4% of the samples that were positive by the reference μ-capture IgM bac-prME assay. However, a commercial IgM test identified only 87.2% of the samples as positive by the reference assay. To obtain more information about these five inconclusive serum samples, the data from the IgM assay, IgG assay, and the hemagglutination inhibition tests were compared (Table 3). In addition, the available clinical and epidemiological data of these patients were taken into account. These data suggest that samples 1a and 2a were likely true negatives. Samples 1a and 2a were collected in November and in May, respectively. It would seem highly unusual according to the seasonal pattern of ticks and TBE in Finland that these patients were bitten and infected by ticks 1 to 3 weeks earlier in their localities. Samples 2b and 2c were likely true positives and therefore false negatives by the commercial IgM test. Sample 2d was inconclusive; it was positive in the μ-capture IgM bac-prME assay and the IgM IFA but negative in the commercial IgM assay and in the μ-capture IgM SFV-prME test, all while demonstrating HI activity. All of the 101 serum samples that had a negative result with the commercial test and the IgM bac-prME assay were also negative in the μ-capture IgM SFV-prME test. If we take these data into account and discard the inconclusive sample 2d, the resolved sensitivity of the μ-capture IgM SFV-prME test was 100%. The μ-capture IgM SFV-prME test also demonstrated high specificity (100%) compared to the reference μ-capture IgM bac-prME ELISA. The heterologous flavivirus reactivity of the IgM μ-capture SFV-prME assay was 87.5%.
At present, different IgG EIA kits are often used for the detection of IgG antibodies in order to confirm successful vaccination against TBEV or previous infection by TBEV, in order to study seroprevalence or endemicity level. However, the close antigenic relationship between different Flavivirus species can lead to false-positive serological results with serum samples from individuals after, e.g., infection with dengue virus, West Nile virus, or vaccination against yellow fever (10). In this study, prME Karelia-94 particles produced in Vero E6 cells by SFV replicons were used also for the generation of a highly sensitive and specific IgG MAb-capture SFV-prME assay for the detection of IgG antibodies in human serum samples. The IgG MAb-capture SFV-prME assay identified 74 positive serum samples out of 75 samples indicated to be positive by the commercial IgG ELISA. It was shown later that the one inconclusive serum sample was probably a false positive in a commercial IgG ELISA, because it was also negative by the hemagglutination inhibition test and the IgG IFA (Table 3). Therefore, the resolved sensitivity and specificity of the IgG MAb-capture SFV prME test were 100% compared to the commercial IgG test. Six dengue virus IgG-positive serum samples were evaluated by the IgG MAb-capture SFV prME ELISA, and all were negative. However, more serum samples from individuals with known flavivirus vaccination or exposure history are needed to assess the level of cross-reactivity of the developed tests with other flavivirus infections. In particular, specimens from documented Omsk hemorrhagic fever (OHF) cases should be tested, as OHF virus is very closely related to TBEV. It should also be noted that a considerable number of serum samples demonstrated low positive signals in the IgG MAb-capture SFV-prME assay (Fig. 3C). This might reflect differences in the epitopes present in the “sandwich” in the IgG MAb-capture assay compared to the inactivated directly coated antigen in the commercial assay.
Immunofluorescence assays are widely used today in the serodiagnosis of flavivirus infections (29–31). In this work, we further described the design of IgM and IgG IFAs based on transfected Vero E6 cells producing prME proteins of the Siberian subtype of TBEV. The IgM IFA test was 100% concordant with the reference μ-capture IgM bac-prME test. The sensitivity and specificity of the IgG IFA test were 98.7% and 100%, respectively, compared to a commercial IgG ELISA. As noted above, the one sample negative by the IgG IFA and positive by the commercial ELISA was likely a true negative. These data demonstrate that the Vero E6 cells transfected with the plasmid CMV-SFV-2SG-prME Karelia-94 might be a useful tool for the detection of IgM and IgG antibodies against TBEV in an IFA. However, additional heterologous flavivirus reactivity studies using more specimens and a variety of flavivirus infections are warranted.
In conclusion, the μ-capture IgM SFV-prME and IgG MAb-capture SFV-prME assays were designed based on subviral prME particles expressed in Vero E6 cells from a CMV-SFV-2SG-prME Karelia-94 construct. Using these transfected cells, IgM and IgG IFAs were also developed. The constructed plasmid CMV-SFV-2SG-prME Karelia-94 can also serve as a vector, providing TBEV glycoproteins for pseudotyping purposes. The assays developed in this work demonstrated high sensitivity and specificity compared to reference ELISAs. Therefore, they can be applied for the detection of anti-TBEV IgM and IgG antibodies, both of which should be positive in acute TBE patients in their second phase of disease for a TBE diagnosis to be made.
ACKNOWLEDGMENTS
This study was supported by CIMO, the Finnish Cultural Foundation, the Academy of Finland, and The Finnish Funding Agency for Technology and Innovation (TEKES).
We appreciate the skillful technical assistance of Irina Suomalainen, Kirsti Räihä, and Kirsi Aaltonen. We thank Irja Luoto for technical assistance with electron microscopy experiments. The CMV-SFV4-2SG-EGFP vector was obtained from Margus Varjak, University of Tartu, Estonia. We also thank Andres Merits from the University of Tartu and Ph.D. student Rommel Iheozor-Ejiofor from the Haartman Institute, University of Helsinki, for reading the manuscript and providing useful comments.
Footnotes
Published ahead of print 26 December 2013
REFERENCES
- 1.Gritsun TS, Lashkevich VA, Gould EA. 2003. Tick-borne encephalitis. Antiviral Res. 57:129–146 [DOI] [PubMed] [Google Scholar]
- 2.Lindquist L, Vapalahti O. 2008. Tick-borne encephalitis. Lancet 371:1861–1871. 10.1016/S0140-6736(08)60800-4 [DOI] [PubMed] [Google Scholar]
- 3.Gubler DJ, Kuno G, Markoff L. 2007. Flaviviruses, p 1153–1252 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA. (ed), Fields virology, 5th ed. Lippincott, Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 4.Gehrke R, Ecker M, Aberle SW, Allison SL, Heinz FX, Mandl CW. 2003. Incorporation of tick-borne encephalitis virus replicons into virus-like particles by a packaging cell line. J. Virol. 77:8924–8933. 10.1128/JVI.77.16.8924-8933.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zilber LA, Soloviev VD. 1946. Far-Eastern tick-borne spring-summer (spring) encephalitis. Am. Rev. Sov. Med. 5:1–80 [PubMed] [Google Scholar]
- 6.Levkovich EN, Pogodina VV. 1967. The problem of tick-borne encephalitis. Vestn. Acad. Med. Nauk. SSSR 22:53–59 (Article in Russian.) [PubMed] [Google Scholar]
- 7.Blaskovic D. 1970. Tick-borne encephalitis in Czechoslovakia. Arch. Environ. Health 21:453–461. 10.1080/00039896.1970.10667263 [DOI] [PubMed] [Google Scholar]
- 8.Gustafson R. 1994. Epidemiological studies of Lyme borreliosis and tick-borne encephalitis. Scand. J. Infect. Dis. Suppl. 92:1–63 [PubMed] [Google Scholar]
- 9.Bakhvalova VN, Rar VA, Tkachev SE, Matveev VA, Matveev LE, Karavanov AS, Dobrotvorsky AK, Morozova OV. 2000. Tick-borne encephalitis virus strains of Western Siberia. Virus Res. 70:1–12. 10.1016/S0168-1702(00)00174-X [DOI] [PubMed] [Google Scholar]
- 10.Niedrig M, Vaisviliene D, Teichmann A, Klockmann U, Biel SS. 2001. Comparison of six different commercial IgG-ELISA kits for the detection of TBEV-antibodies. J. Clin. Virol. 20:179–182. 10.1016/S1386-6532(00)00178-5 [DOI] [PubMed] [Google Scholar]
- 11.Jääskeläinen A, Han X, Niedrig M, Vaheri A, Vapalahti O. 2003. Diagnosis of tick-borne encephalitis by a mu-capture immunoglobulin M-enzyme immunoassay based on secreted recombinant antigen produced in insect cells. J. Clin. Microbiol. 41:4336–4342. 10.1128/JCM.41.9.4336-4342.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brummer-Korvenkontio M, Saikku P, Korhonen P, Oker-Blom N. 1973. Arboviruses in Finland. I. Isolation of tick-borne encephalitis (TBE) virus from arthropods, vertebrates, and patients. Am. J. Trop. Med. Hyg. 22:382–389 [PubMed] [Google Scholar]
- 13.Jääskeläinen AE, Sironen T, Murueva GB, Subbotina N, Alekseev AN, Castrén J, Alitalo I, Vaheri A, Vapalahti O. 2010. Tick-borne encephalitis virus in ticks in Finland, Russian Karelia and Buryatia. J. Gen. Virol. 91:2706–2712. 10.1099/vir.0.023663-0 [DOI] [PubMed] [Google Scholar]
- 14.Rausalu K, Iofik A, Ulper L, Karo-Astover L, Lulla V, Merits A. 2009. Properties and use of novel replication-competent vectors based on Semliki Forest virus. Virol. J. 6:33. 10.1186/1743-422X-6-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vene S, Haglund M, Vapalahti O, Lundkvist A. 1998. A rapid fluorescent focus inhibition test for detection of neutralizing antibodies to tick-borne encephalitis virus. J. Virol. Methods 73:71–75 [DOI] [PubMed] [Google Scholar]
- 16.Vapalahti O, Lundkvist A, Kallio-Kokko H, Paukku K, Julkunen I, Lankinen H, Vaheri A. 1996. Antigenic properties and diagnostic potential of puumala virus nucleocapsid protein expressed in insect cells. J. Clin. Microbiol. 34:119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niedrig M, Klockmann U, Lang W, Roeder J, Burk S, Modrow S, Pauli G. 1994. Monoclonal antibodies directed against tick-borne encephalitis virus with neutralizing activity in vivo. Acta Virol. 38:141–149 [PubMed] [Google Scholar]
- 18.Tsekhanovskaya NA, Matveev LE, Rubin SG, Karavanov AS, Pressman EK. 1993. Epitope analysis of tick-borne encephalitis (TBE) complex viruses using monoclonal antibodies to envelope glycoprotein of TBE virus (persulcatus subtype). Virus Res. 30:1–16 [DOI] [PubMed] [Google Scholar]
- 19.Marx F, Gritsun TS, Grubeck-Loebenstein B, Gould EA. 2001. Diagnostic immunoassays for tick-borne encephalitis virus based on recombinant baculovirus protein expression. J. Virol. Methods 91:75–84. 10.1016/S0166-0934(00)00251-2 [DOI] [PubMed] [Google Scholar]
- 20.Yoshii K, Hayasaka D, Goto A, Obara M, Araki K, Yoshimatsu K, Arikawa J, Ivanov L, Mizutani T, Kariwa H, Takashima I. 2003. Enzyme-linked immunosorbent assay using recombinant antigens expressed in mammalian cells for serodiagnosis of tick-borne encephalitis. J. Virol. Methods 108:171–179. 10.1016/S0166-0934(02)00283-5 [DOI] [PubMed] [Google Scholar]
- 21.Khromykh AA, Varnavski AN, Westaway EG. 1998. Encapsidation of the flavivirus kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J. Virol. 72:5967–5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholle F, Girard YA, Zhao Q, Higgs S, Mason PW. 2004. trans-packaged West Nile virus-like particles: infectious properties in vitro and in infected mosquito vectors. J. Virol. 78:11605–11614. 10.1128/JVI.78.21.11605-11614.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fayzulin R, Scholle F, Petrakova O, Frolov I, Mason PW. 2006. Evaluation of replicative capacity and genetic stability of West Nile virus replicons using highly efficient packaging cell lines. Virology 351:196–209. 10.1016/j.virol.2006.02.036 [DOI] [PubMed] [Google Scholar]
- 24.Klockmann U, Krivanec K, Stephenson JR, Hilfenhaus J. 1991. Protection against European isolates of tick-borne encephalitis virus after vaccination with a new tick-borne encephalitis vaccine. Vaccine 9:210–212 [DOI] [PubMed] [Google Scholar]
- 25.Holzmann H, Vorobyova MS, Ladyzhenskaya IP, Ferenczi E, Kundi M, Kunz C, Heinz FX. 1992. Molecular epidemiology of tick-borne encephalitis virus: cross-protection between European and Far-Eastern subtypes. Vaccine 10:345–349 [DOI] [PubMed] [Google Scholar]
- 26.Casales E, Aranda A, Quetglas JI, Ruiz-Guillen M, Rodriguez-Madoz JR, Prieto J, Smerdou C. 2010. A novel system for the production of high levels of functional human therapeutic proteins in stable cells with a Semliki Forest virus noncytopathic vector. New Biotechnol. 27:138–148. 10.1016/j.nbt.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 27.Ferlenghi I, Clarke M, Ruttan T, Allison SL, Schalich J, Heinz FX, Harrison SC, Rey FA, Fuller SD. 2001. Molecular organization of a recombinant subviral particle from tick-borne encephalitis virus. Mol. Cell 7:593–602. 10.1016/S1097-2765(01)00206-4 [DOI] [PubMed] [Google Scholar]
- 28.Takahashi H, Ohtaki N, Maeda-Sato M, Tanaka M, Tanaka K, Sawa H, Ishikawa T, Takamizawa A, Takasaki T, Hasegawa H, Sata T, Hall WW, Kurata T, Kojima A. 2009. Effects of the number of amino acid residues in the signal segment upstream or downstream of the NS2B-3 cleavage site on production and secretion of prM/M-E virus-like particles of West Nile virus. Microbes Infect. 11:1019–1028. 10.1016/j.micinf.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 29.Malan AK, Stipanovich PJ, Martins TB, Hill HR, Litwin CM. 2003. Detection of IgG and IgM to West Nile virus. Development of an immunofluorescence assay. Am. J. Clin. Pathol. 119:508–515. 10.1309/WJJ7UE42DFHTTF1X [DOI] [PubMed] [Google Scholar]
- 30.Niedrig M, Sonnenberg K, Steinhagen K, Paweska JT. 2007. Comparison of ELISA and immunoassays for measurement of IgG and IgM antibody to West Nile virus in human sera against virus neutralization. J. Virol. Methods 139:103–105. 10.1016/j.jviromet.2006.09.009 [DOI] [PubMed] [Google Scholar]
- 31.Niedrig M, Kürsteiner O, Herzog C, Sonnenberg K. 2008. Evaluation of an indirect immunofluorescence assay for detection of immunoglobulin M (IgM) and IgG antibodies against yellow fever virus. Clin. Vaccine Immunol. 15:177–181. 10.1128/CVI.00078-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundkvist A, Niklasson B. 1992. Bank vole monoclonal antibodies against Puumala virus envelope glycoproteins: identification of epitopes involved in neutralization. Arch. Virol. 126:93–105 [DOI] [PubMed] [Google Scholar]