Abstract
Streptococcus pneumoniae is a significant bacterial pathogen that expresses >90 capsule serotypes. Conventional serotyping methods assume that each serotype is a genetically and antigenically distinct entity; however, recent investigations have revealed pneumococcal isolates that cannot be unambiguously serotyped because they share the properties of more than one serotype. Here, we employed a novel serotyping method and NMR spectroscopy to examine clinical isolates sharing properties of serotypes 11A and 11E. These ambiguous clinical isolates were provisionally named 11A variant (11Av) isolates. Serotype 11A pneumococci characteristically express capsule β-galactose-6-O-acetylation (βGal6OAc) mediated by the capsule synthesis gene wcjE, while 11E strains contain loss-of-function mutations in wcjE and completely lack the expression of βGal6OAc. Although 11Av isolates also contained mutated wcjE alleles, 11Av clinical isolates were composed of antigenically homogeneous bacteria expressing reduced amounts of 11A-specific capsule antigen. NMR data confirmed reduced but detectable amounts of βGal6OAc on 11Av capsule polysaccharide. Furthermore, the transformation of strains with wcjE alleles from 11Av strains was sufficient to restore partial βGal6OAc in an 11E background. We conclude that, instead of being distinct entities, serotypes 11A and 11E represent two extremes of an antigenic spectrum resulting from variable capsule O-acetylation secondary to heterologous wcjE mutations. These findings challenge whether all clinically relevant pneumococci can be definitively categorized into distinct serotypes.
INTRODUCTION
Nearly all clinical isolates of the major pathogen Streptococcus pneumoniae (pneumococcus) express a polysaccharide (PS) capsule that protects the bacterium against host immunity. Host exposure to capsule antigen, however, can induce adaptive immune responses that mediate effective, type-specific clearance of bacteria. Accordingly, pneumococci have evolved >90 capsule serotypes, each with a characteristic carbohydrate structure and capsule synthesis (cps) gene locus. Serotyping is an essential tool for understanding pneumococcal epidemiology and assessing the effects of pneumococcal vaccines. Although some molecular typing methods have emerged, commercial serological tests that employ polyclonal antisera (i.e., latex agglutination or Quellung test) are conventionally used to identify pneumococcal serotypes. The recent introduction of anticapsule monoclonal antibodies (MAbs) has improved the resolution of pneumococcal serotyping and has led to the discovery of multiple significant serotypes (1, 2).
For example, the recently discovered serotype 11E cannot be distinguished from serotype 11A using conventional serotyping methods (2). This is likely due to 11A and 11E capsule PSs being composed of nearly identical O-acetylated carbohydrate repeat units containing two glucose residues, two galactose residues, and a phospho-linked glycerol pendant (Fig. 1A). The 11A PS contains four O-acetylation sites, while the 11E PS has only three O-acetylation sites and lacks β-galactose-6-O-acetylation (βGal6OAc) (Fig. 1A, dotted box) (3). This βGal6OAc is the distinguishing difference between 11A and 11E PSs, although the initial NMR analysis of their capsule structures erroneously concluded that the site of 11A-specific O-acetylation was the phospho-linked glycerol (4). This error in the initial NMR analysis has been already reported as errata to the original report (4). The lack of βGal6OAc in serotype 11E strains is the result of inactivating mutations to the cps O-acetyltransferase gene wcjE (2, 3) (Fig. 1B, asterisk). Remarkably, each molecularly described serotype 11E isolate contains a unique wcjE mutation in its cps locus, ranging from single missense or nonsense mutations to insertion of transposable elements to gene deletions (2, 5).
FIG 1.
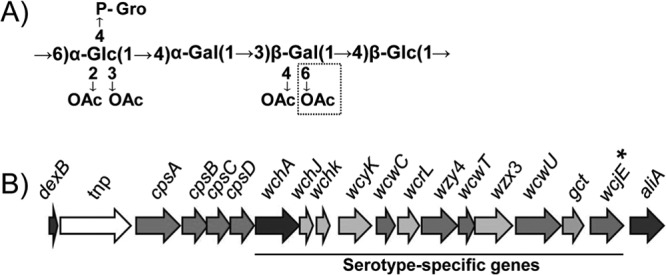
Serotype 11A polysaccharide repeat unit structure and cps locus. (A) Serotype 11A polysaccharide repeat unit structure. Dotted box, the O-acetyl substitution present in serotype 11A PS and absent in serotype 11E PS. Glc, glucose; Gal, galactose; P-Gro, 1-phosphoglycerol. (B) Serotype 11A cps locus. Horizontal line, the region of the serotype 11A cps locus containing serotype-specific genes examined in this study. cpsA to cpsD are genes conserved across the cps loci of almost all pneumococcal serotypes and are implicated in modulating capsule synthesis. All 11E cps loci contain mutations affecting wcjE (*). tnp, transposable element.
Despite identical polyclonal serum binding profiles, methods employing MAbs targeting the 11A-specific O-acetylation pattern and wcjE sequencing have been effective in identifying most serotype 11A and 11E isolates (2, 5). However, the MAb-based inhibition enzyme-linked immunosorbent assay (iELISA) originally developed to detect these serotypes identified a few clinical strains that appear to stably express small amounts of 11A-specific antigen despite harboring mutations in wcjE (5). Given the diversity of wcjE mutations observed in serotype 11E strains, we hypothesized that some mutations may inactivate gene activity only partially and result in pneumococci expressing antigenic properties intermediate between those of 11A and 11E. The existence of strains with intermediate phenotypes challenges the presumption that unambiguous distinctions can be always made between pneumococcal serotypes. Thus, we performed extensive serological, genetic, and structural analyses of these strains and their PS capsules.
MATERIALS AND METHODS
Pneumococcal clinical isolates.
All clinical isolates examined in this study and their tissues of isolation are listed in Table 1. Strain 3102-06 was obtained from the Centers for Disease Control and Prevention. All other clinical strains have been described previously, as noted in Table 1. All serogroup 11 isolates examined in this study were reactive to factor serum 11c but nonreactive to factor sera 11b and 11g (i.e., the serotype 11A/11E antigenic profile).
TABLE 1.
Strains and their mean fluorescence intensity values according to the flow cytometric serotyping assay
| Strain | Serotypea | FCSA MFIb |
FCSA serotype | Isolation tissue | cps locus genetics | |
|---|---|---|---|---|---|---|
| Hyp11AM1 | Hyp11AG2 | |||||
| Clinical isolates | ||||||
| MNZ264 | 11E | 44.2 (+) | 2.8 (−) | 11E | Blood | |
| MNZ268 | 11E | 40.0 (+) | 2.6 (−) | 11E | Blood | |
| MNZ741 | 11E | 16.6 (+) | 2.7 (−) | 11E | Blood | |
| MNZ267 | 11E | 71.6 (+) | 2.7 (−) | 11E | Blood | |
| MNZ265 | 11E | 69.7 (+) | 5.5 (low) | 11Av | Blood | |
| MNZ2322 | 11E | 117.9 (+) | 6.2 (low) | 11Av | Conjunctiva | |
| MNZ2293-C | 11E | 160.0 (+) | 12.7 (low) | 11Av | Blood | |
| 3102-06 | − | 101.8 (+) | 19.7 (low) | 11Av | Blood | |
| MNZ2292 | 11E | 172.0 (+) | 44.0 (low) | 11Av | Blood | |
| MNZ2293-A | 11A | 320.7 (+) | 1,076.2 (high) | 11A | Blood | |
| MNZ272 | 11A | 170.1 (+) | 1,460.9 (high) | 11A | Blood | |
| MNZ273 | 11A | 170.7 (+) | 1,601.6 (high) | 11A | Blood | |
| MNZ271 | 11A | 150.3 (+) | 1,789.0 (high) | 11A | Blood | |
| MNZ274 | 11A | 155.1 (+) | 2,201.0 (high) | 11A | Blood | |
| MNZ2321 | 11A | 132.5 (+) | 2,452.0 (high) | 11A | Conjunctiva | |
| Recombinant strains | ||||||
| JC01 | 9V | 3.3 (−) | 2.8 (−) | NTc | ||
| JC03 | − | 450.5 (+) | 570.4 (high) | 11A | MNZ270 rpsL::rpsL TIGRd | |
| JC04 | − | 397.2 (+) | 2.6 (−) | 11E | JC03 wcjE::Januse | |
| JC12 | − | 150.1 (+) | 11.3 (low) | 11Av | JC04 Janus::wcjE 3102-06 | |
| JC13 | − | 154.2 (+) | 2.6 (−) | 11E | JC04 Janus::wcjE MNZ741 | |
| AMB01 | − | 309.1 (+) | 743.6 (high) | 11A | JC04 Janus::wcjE MNZ2321 | |
| AMB02 | − | 153.6 (+) | 7.6 (low) | 11Av | JC04 Janus::wcjE MNZ2322 | |
| AMB03 | − | 3.1 (−) | 2.6 (−) | NTc | JC03 wchA-wcrL::Janus | |
Previously reported serotype for each strain (2, 5, 6). −, strains were first analyzed in this study. All examined clinical isolates were typed as 11A by conventional polyclonal serum-based serotyping methods.
In FCSA experiments, bacteria were stained with Hyp11AM1 or Hyp11AG2 murine monoclonal antibodies (MAbs) and anti-murine IgM or anti-murine IgG secondary antibodies, respectively. MFI values determined after staining with each MAb in a representative experiment are shown. MFI values were considered negative (−) if they were indistinguishable from negative-control values (i.e., MFI values of <4.0). Hyp11AM1 MFI values were considered positive (+) if they were greater than 4. Hyp11AG2 MFI values were considered high if they were at least 100-fold greater than the negative-control values (i.e., MFI of >400.0) and low if they were greater than negative-control values but less than 400.
NT, nontypeable by 11A/11E FCSA. AMB03 was determined to be nonencapsulated according to colony morphology and loss of reactivity with MAbs and serotyping factor sera.
The rpsL TIGR allele mediates streptomycin resistance (7).
The Janus cassette encodes kanamycin resistance (see Materials and Methods).
Recombinant strains.
We employed a previously described allelic replacement method (2, 6) to construct serogroup 11 isogenic recombinant strains containing different wcjE alleles. Briefly, the streptomycin-resistant serotype 11A strain JC03 was transformed to strain JC04 by recombinatorial replacement of wcjE with a Janus cassette, which conferred resistance to kanamycin and sensitivity to streptomycin (7). Streptomycin-resistant strains JC11, JC12, JC13, AMB01, and AMB02 were created by recombinatorial replacement of the Janus cassette in JC04 with amplicons of the wcjE alleles from clinical isolates MNZ265, 3102-06, MNZ741, MNZ2322, and MNZ2321, respectively. Successful recombination was verified by sequencing of the wcjE locus in all recombinant strains. The capsule-null variant AMB03 was created by recombinatorial replacement of the JC03 cps region spanning from wchA to wcrL with a Janus cassette containing the respective flanking regions, as described previously (8). Loss of capsule expression was verified by colony morphology and the Quellung reaction using factor serum 11c, which contains antibodies that bind to serotype 11A, 11C, 11D, and 11E capsule PSs (3). The serotype 9V strain JC01 is described elsewhere (6).
Monoclonal antibodies and flow cytometric serotyping assay.
Murine hybridomas Hyp11AM1 and Hyp11AG2 were produced using a previously described method (9) and selected for binding to serotype 11A capsule PS. Hyp11AM1 produces IgM monoclonal antibody (MAb) and Hyp11AG2 produces IgG MAb. Hybridoma supernatants containing MAbs were used in a flow cytometric serotyping assay (FCSA), as described previously (3, 6). Briefly, bacteria (1 × 107 CFU/ml) were incubated with 1:10 and 1:100 dilutions of Hyp11AM1 and Hyp11AG2 hybridoma supernatants, respectively, for 10 min at 4°C. After washing, antibodies bound to the surface of bacteria were detected using either anti-murine IgM or anti-murine IgG fluorescently labeled antibodies. As a negative control, bacteria were incubated with secondary antibodies only. Data were acquired using a FACSCalibur system (BD Biosciences, San Jose, CA) and analyzed with FCS Express (De Novo Software, Los Angeles, CA).
PS purification.
We purified capsule PS from the following strains: MNZ2293-A, MNZ2293-C, 3102-06, MNZ264, JC03, JC04, JC12, and JC13. Capsule PS purification was performed as described previously (10). Briefly, PS was obtained by ethanol precipitation from autolyzed bacterial cultures and purified by ion exchange and size exclusion chromatography. To de-O-acetylate samples for NMR analysis, PS was incubated in 0.2 M sodium hydroxide for 3 h at room temperature.
NMR spectroscopy.
NMR samples were prepared by dissolving 1 to 2 mg of lyophilized PS in 0.6 ml of D2O. The one-dimensional (1D) 1H NMR data for purified PS were collected with a Bruker DRX (600-MHz) spectrometer equipped with a cryoprobe at 25°C. Data were analyzed with ACD/NMR Processor Academic Edition (Advanced Chemistry Development, Inc., Toronto, Canada). Two-dimensional (2D) 1H-13C heteronuclear multiple quantum coherence (HMQC) data were collected at 45°C with a Bruker Avance II (700-MHz 1H) spectrometer equipped with a cryoprobe. Data were processed with NMRPipe software (11) and analyzed with NMRView software (12). Limited chemical shift assignments were made by comparison to recent NMR analyses of serogroup 11 capsule PS structures, which used similar PS purification and data acquisition methods (3, 8).
Sequencing analysis.
Sequences of the cps locus spanning from the last 48 bp of cpsD to bp 26 of aliB (Fig. 1B, horizontal line) from strains 3102-06, MNZ2293-A, MNZ2293-C, and MNZ2292 were obtained using previously described primer sets (2). Sequences of the downstream portion of the cps locus containing wcjE from MNZ2293-A, MNZ2293-C, and MNZ2292 were described previously (5).
Nucleotide sequence accession numbers.
Sequences for the cps locus from strains 3102-06, MNZ2293-A, MNZ2293-C, and MNZ2292 were uploaded to GenBank and assigned accession numbers KF676986, KF676984, KF676983, and KF676985, respectively.
RESULTS
Flow cytometry confirms serological intermediates between the 11A and 11E phenotypes.
To explain the ambiguous phenotype of some serogroup 11 strains, it was necessary to distinguish between a sample composed of a phenotypically homogeneous culture and a sample composed of two subpopulations (2, 13). Although iELISA effectively assesses the antigenic properties of a bacterial culture as a whole, it cannot directly examine surface antigens on individual bacteria. Therefore, we employed a novel MAb-based flow cytometric serotyping assay (FCSA) to investigate the O-acetylation state of individual pneumococci in isolates previously serotyped by iELISA and wcjE sequencing.
FCSA histograms of strains that were positively stained with Hyp11AM1 and Hyp11AG2 MAbs contain single peaks (Fig. 2), confirming that these strains are composed of bacteria stably expressing a single capsule phenotype. Table 1 lists the mean fluorescent intensity (MFI) values of strains stained with Hyp11AM1 and Hyp11AG2 MAbs in a representative experiment. Hyp11AM1 MAb bound to both serotype 11A and 11E strains but did not bind to the serotype 9V strain JC01. In contrast, Hyp11AG2 MAb is 11A specific; it strongly bound to serotype 11A strains (i.e., MFI greater than 500), but its binding to most serotype 11E strains was indistinguishable from its binding to strain JC01 (i.e., MFI less than 3). Thus, the FCSA could clearly identify nearly all serotype 11A and 11E strains based on Hyp11AG2 MAb binding, with a few notable exceptions.
FIG 2.
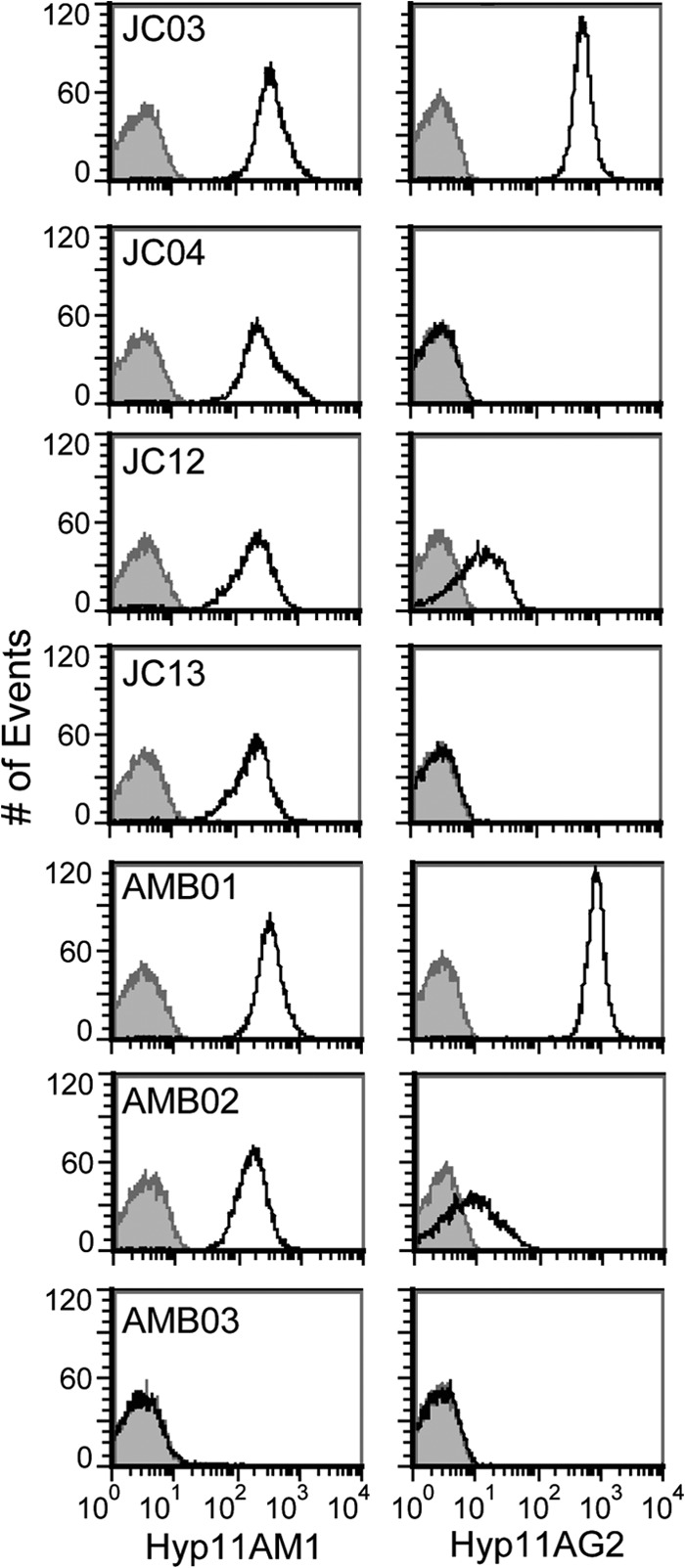
Reduced expression of 11A-specific epitopes on 11Av pneumococci. FCSA histograms depict Hyp11AM1 and Hyp11AG2 MAb binding to strains JC03 (11A), JC04 (11E), JC12 (11Av), JC13 (11E), AMB01 (11A), AMB02 (11Av), and ABM03 (isogenic, nonencapsulated control). Black curves, bacteria incubated with the corresponding MAb and secondary antibody; gray curves, negative-control preparations incubated with secondary antibody only.
Strains MNZ2293-A and MNZ2293-C were coisolated from the blood of a single bacteremic patient and were previously serotyped as 11A and 11E, respectively, by iELISA and wcjE sequencing (5). Unlike most 11E strains, however, MNZ2293-C partially interacts with 11A-specific MAbs in the iELISA. In the FCSA, Hyp11AG2 MAb strongly bound to MNZ2293-A pneumococci (MFI of 1076.2) and displayed detectable, though markedly reduced, levels of binding to MNZ2293-C pneumococci (MFI of 12.7). Reduced levels of Hyp11AG2 binding (MFI of 44.0) were also observed in the FCSA for strain MNZ2292, a blood isolate that behaves like MNZ2293-C in the iELISA (5). A FCSA screen of isolates in our collection identified three additional strains, i.e., MNZ265, MNZ2322, and 3102-06, that displayed marginal levels of Hyp11AG2 MAb binding (MFI values of 5.5, 6.2, and 19.7, respectively). The intermediate-Hyp11AG2 phenotype is stable, as clonal cultures of these isolates expressed levels of Hyp11AG2 antigen comparable to those of their parent strains (data not shown) and all MAb-binding histograms revealed single distinct peaks.
In summary, we identified five clinical isolates, i.e., MNZ265, MNZ2292, MNZ2293-C, MNZ2322, and 3102-06, that express Hyp11AG2 epitopes at higher levels than most serotype 11E strains but lower levels than 11A strains. As Hyp11AG2 binding defined serotype 11A in this system, the intermediate phenotype was provisionally named 11A variant (11Av).
The cps loci of 11A and 11Av strains differ only in wcjE-associated mutations.
Since integrity of the cps O-acetyltransferase gene wcjE determines expression of serotype 11A or 11E, we analyzed the cps locus sequences of 11Av strains. Serotype 11A and 11E strains can contain two highly similar but distinct cps loci, which for simplicity are named here the 11A-1 allele (exemplified by GenBank accession no. GU074952) (2) and the 11A-2 allele (exemplified by GenBank accession no. CR931653) (14). On alignment analysis, almost all sequence mismatches are located in the 3′ region of the locus, including 20-bp substitutions in wcjE. Figure 3 depicts the alignment of the gene products encoded by wcjE alleles in the 11A-1 and 11A-2 cps loci. Since strains MNZ265, MNZ2322, and 3102-06 contain the 11A-1 allele but strains MNZ2292 and MNZ2293-C contain the 11A-2 allele (2, 5), 11Av expression is not linked to either allele of the serotype 11A cps locus.
FIG 3.

Alignment of protein sequences encoded by various wcjE alleles. The complete protein sequence encoded by the wcjE allele of the 11A-1 cps locus is bold, and asterisks indicate every tenth amino acid. Amino acid substitutions encoded by the wcjE alleles of strain 3102-06 and the 11A-2 cps locus are shown.
Compared with the sequences of their 11A counterparts, each 11Av cps locus contains a unique downstream mutation associated with wcjE. Noticeably, 11Av mutations maintain the wcjE gene largely intact, in comparison with serotype 11E strains. For example, the strain 3102-06 wcjE allele (GenBank accession no. KF676986) contains only a T323G substitution, encoding Arg108Ile in the predicted amino acid sequence (Fig. 3), while the 11E strain MNZ264 contains a deletion of the start codon and the upstream portion of wcjE (2). Remarkably, the wcjE gene of the 11Av strain MNZ2292 is intact, though the transposable element IS1167A is inserted immediately upstream of the gene (5).
Since it is possible that serotype-specific cps genes other than wcjE mediate the 11Av phenotypes, we sequenced over 11 kbp of the serotype-specific genes (Fig. 1B) of strains MNZ2292, MNZ2293-A, MNZ2293-C, and 3102-06 (GenBank accession numbers KF676985, KF676984, KF676983, and KF676986, respectively). Other than the substitution noted above, the 3102-06 cps locus sequence is 100% identical to the published 11A-1 cps sequence. The cps locus sequence of strain MNZ2293-A, a serotype 11A strain, is identical to the 11A-2 cps sequence with only three differences, i.e., a putatively noncoding G to T substitution 28 bp downstream of the GCT stop codon, an insertion of the transposable element IS1167A in the putatively noncoding region 59 bp downstream of the wcjE stop codon, resulting in duplication of the sequence TGTCTTCT, and a 224-bp deletion in the putatively noncoding region from 81 bp to 304 bp downstream of wcjE. Consistent with prior reports (5), the cps loci of MNZ2293-C and MNZ2292 are identical to the MNZ2293-A sequence except for a nonsense mutation at codon 88 of MNZ2293-C wcjE and the transposable element insertion in the MNZ2292 cps locus, as described above. These findings strongly support the 11Av phenotype being linked to mutations affecting wcjE.
Allelic replacement of wcjE from 11Av donors into an 11E strain partially restores 11A-specific epitope expression.
To examine the hypothesis that wcjE-associated mutations alone can determine whether strains express the 11E or 11Av serological phenotype, we constructed and performed FCSA analysis of recombinant strains isogenic to the 11A strain JC03 (Table 1 and Fig. 2). All encapsulated strains expressed comparable levels of Hyp11AM1 antigen. Hyp11AG2 MAb displayed negative binding to the wcjE-null strain JC04 and the recombinant strain JC13, which contains the wcjE allele from the 11E clinical isolate MNZ741. In contrast, the Hyp11AG2 MFI values for strain JCO3 and strain AMB01, which contains the wcjE allele obtained from the 11A strain MNZ2321, were comparably high. The recombinant strains JC12 and AMB02 contain wcjE alleles obtained from the 11Av strains 3102-06 and MNZ2322, respectively. In FCSA analyses, their Hyp11AG2 MFI values were low positive (Table 1), indicating that introduction of specific wcjE mutations is sufficient for the partial expression of 11A-specific epitopes characteristic of 11Av strains.
The 11Av capsule PS contains reduced molar amounts of β-Gal-4,6-OAc, in comparison with serotype 11A capsule PS.
To explore the structural basis of the 11Av phenotype, we conducted 1D and 2D NMR experiments with capsular PS purified from various clinical and recombinant strains expressing serotypes 11A, 11E, and 11Av. The 1D 1H NMR spectra of the de-O-acetylated capsule PSs from all strains were indistinguishable (Fig. 4). This indicated that 11Av, 11A, and 11E capsules contain identical carbohydrate backbones (Fig. 1B) and that 11Av antigenic properties likely arise from a unique O-acetylation pattern.
FIG 4.
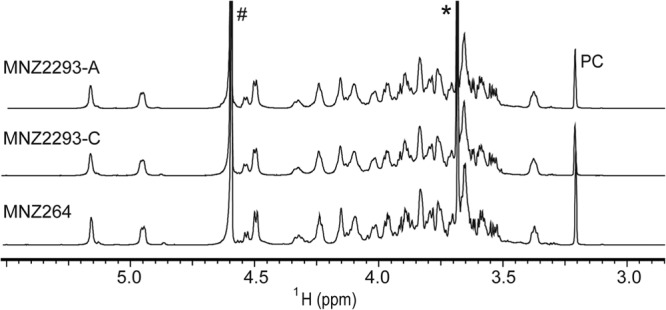
1H NMR spectra of de-O-acetylated serotype 11A (MNZ2293-A), 11Av (MNZ2293-C), and 11E (MNZ264) capsule PSs, which are indistinguishable. #, NMR signals arising from residual water; *, NMR signals arising from residual Tris-HCl from buffer; PC, NMR signals arising from phosphocholine residues on contaminating teichoic acid.
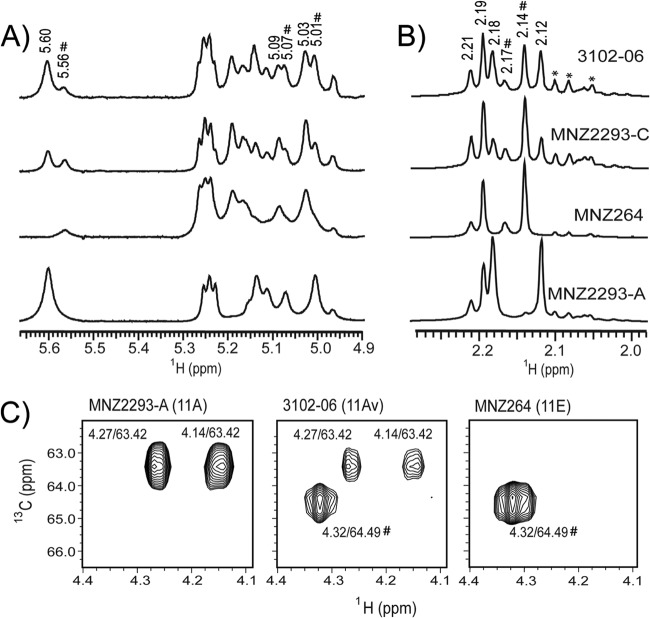
Indeed, NMR analysis of native O-acetylated capsule PS revealed that 11Av PS contains both 11A- and 11E-specific features. As shown in Fig. 5A, the 1D 1H NMR spectra of native 3102-06 and MNZ2293-C (11Av) PSs clearly contain signals observed in the native MNZ2293-A (11A) PS spectrum, e.g., signals at 5.60, 5.07, and 5.01 ppm, and signals observed in the native MNZ264 (11E) PS spectrum, e.g., signals at 5.56, 5.09, and 5.03 ppm. Figure 5B displays the hydrolysis-sensitive 1H signals between 2.10 and 2.25 ppm that arise from methyl protons on O-acetyl substitutions. All 1H NMR spectra contain signals at 2.19 and 2.21 ppm, assigned to the acetyl groups of αGlc-2-OAc and αGlc-3-OAc, respectively (3). As expected, well-resolved 1H signals at 2.12 and 2.18 ppm, arising from βGal-4,6-OAc acetyl groups (3), are observed in the MNZ2293-A spectrum but absent from the MNZ264 spectrum. These signals are clearly observed in 3102-06 and MNZ2293-C 1H NMR spectra at lesser intensities than in the MNZ2293-A spectrum.
FIG 5.
NMR data of 11Av PS, revealing biochemical signatures of both 11A and 11E PSs. (A and B) The anomeric (A) and methyl (B) regions of the 1H NMR spectra of native capsule PSs purified from strains 3102-06 (11Av), MNZ2293-C (11Av), MNZ264 (11E), and MNZ2293-A (11A). O-Acetylation-associated signals discussed in the text are labeled with their respective chemical shift values (in ppm). *, hydrolysis-resistant signals arising from contaminating teichoic acid. (C) Select regions of the 2D 1H-13C HMQC NMR spectra of native capsule PSs purified from strains MNZ2293-A, 3102-06, and MNZ264. Signals are labeled with their corresponding shift values (1H/13C, in ppm). In A to C, all spectra are normalized according to the signal intensity arising from the H2 proton of βGlc at 3.36 ppm (see Fig. 4). #, signals present in 11E and 11Av spectra that are grossly absent in 11A spectra.
Confirmatory 1H-13C HMQC experiments using native capsule PS were consistent with 1D NMR findings. The 1H-13C cross-peaks characteristic of the C6 group of βGal-4,6-OAc (3) are observed at 4.14/63.42 (1H/13C) and 4.27/63.42 ppm in the native MNZ2293-A (Fig. 5C, left), 3102-06 (Fig. 5C, middle), and MNZ2293-C PS spectra but absent from the spectra of native MNZ264 PS (Fig. 5C, right) and de-O-acetylated MNZ2293-A PS (data not shown).
No signals identified in the 1D and 2D NMR spectra were unique to native 11Av PS, confirming that the 11Av capsule is composed exclusively of a combination of 11A and 11E carbohydrate repeat units. Consistent with this model is the observation of multiple signals in the MNZ2293-C, 3102-06, and MNZ264 PS NMR spectra that are absent from the MNZ2293-A PS spectrum (Fig. 5, pound signs). The proportions of repeat units containing βGal-6-O-acetylation can be estimated by integrating the area under the curve at 2.12 ppm and normalizing the result to the integral of the hydrolysis-resistant signal at 3.36 ppm arising from the H2 proton of βGlc (Fig. 4) (3, 15). By this method, the βGal-6-O-acetylation in MNZ2293-C and 3102-06 PSs was estimated to be 55% and 36% of the amount in MNZ2293-A PS, respectively.
Capsule PS purified from the recombinant strains JC04 (11E), JC03 (11A), JC12 (11Av), and JC13 (11E) revealed similar findings in NMR experiments. Signals at 2.12 and 2.18 ppm are observed in the JC03 PS 1H NMR spectrum (Fig. 6, asterisks) and, at a lesser intensity, in the JC12 PS spectrum but are absent in the JC04 PS and JC13 PS spectra. Altogether, these NMR data confirm that 11Av pneumococci express capsule PS repeat units containing βGal-4,6-OAc (Fig. 1), albeit in reduced amounts, compared with 11A pneumococci.
FIG 6.
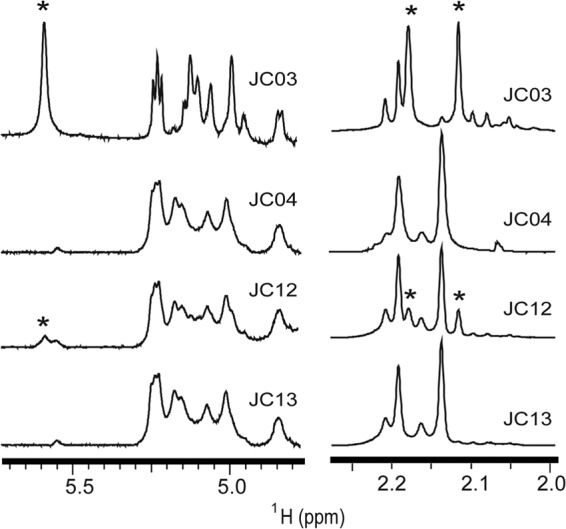
1H NMR spectra of native PSs purified from serogroup 11 recombinant strains. The anomeric (left) and methyl (right) regions of the 1H NMR spectra of native capsule polysaccharide expressed by strains JC03 (11A), JC04 (11E), JC12 (11Av), and JC13 (11E) are shown. *, serotype 11A-specific peaks that are present in the JC03 and JC12 spectra but absent in the JC04 and JC13 spectra. Spectra are normalized according to the signal intensity arising from the H2 proton of βGlc at 3.36 ppm (see Fig. 4).
DISCUSSION
Due to the lack of a widely available method to distinguish serotype 11A from serotype 11E, both serotypes are reported as serotype 11A in almost all studies. Following the widespread use of pneumococcal conjugate vaccines, serotype 11A has become one of the most prevalent serotypes among pneumococci isolated from the nasopharynx (16–18) and, in one U.S. multicenter study, isolated from invasive disease (19). It can be postulated that pneumococci expressing serotypes 11A and 11E have contributed equally to the increase in “serotype 11A” prevalence. However, the two serotypes have strikingly contrasting ecologies; less than 1% of carriage isolates and approximately 30 to 50% of invasive disease isolates originally typed as serotype 11A actually express 11E (5). Coupled with the lack of clonality among serotype 11E strains (see above), it appears that 11A pneumococci can readily colonize the nasopharynx and are clonally transmitted between hosts, while 11E pneumococci are nontransmissible and subsist almost exclusively in disease sites (e.g., middle ear effusions, conjunctiva, and blood). Understanding why these highly similar pneumococci display differing clinical behaviors is important in light of the increasing epidemiological relevance of serogroup 11 pneumococci. Thus, we sought to clearly define the distinctions between serotypes 11A and 11E and to develop methods capable of detecting and distinguishing the two serotypes.
A novel serotyping approach that allows quantification of capsule epitopes expressed on individual bacteria, i.e., FCSA, led us to discover that some pneumococcal isolates have serological and capsule properties intermediate between those of 11A and 11E. We named these strains serotype 11A variant (11Av) strains. Although we did not directly measure WcjE function, structural and molecular evidence strongly indicates that 11Av wcjE-encoded enzymes partially conserve their βGal-6-O-acetylation function. Discovery of 11Av strains reveals that pneumococcal serotypes 11A and 11E are not two distinct antigenic entities but instead are two extremes of an antigenic spectrum putatively mediated by differential activities of the O-acetyltransferase gene wcjE.
The molecular mechanisms responsible for reducing wcjE-mediated O-acetylation in 11Av strains remain unclear. The missense mutations contained in the wcjE alleles of strains 3102-06 and MZN265 may reduce without fully neutralizing O-acetylation activity. It can be argued that stop codon readthrough (20) and ribosomal frameshifting (21) could mediate low-level correction of the MNZ2292 wcjE nonsense mutation and the MNZ2232 wcjE point deletion, respectively, resulting in marginal gene expression. Lastly, insertion of the IS1167 element upstream of MNZ2292 wcjE may either interrupt gene expression control by upstream transcriptional or translational elements or mediate an inhibitory interaction with the IS1167 element located downstream of wcjE.
As these possibilities are investigated, it should be noted that FCSA and NMR data can provide discrepant results for wcjE-mediated capsule features. The βGal-6-O-acetylation rates of 11Av strains according to NMR data are greater than predicted rates according to FCSA MFI values, i.e., 30 to 60% versus 1 to 10% of 11A βGal-6-O-acetylation. However, FCSA measures cell surface availability of the epitope for Hyp11AG2 MAb, and epitope availability may not linearly correlate with βGal-6-O-acetylation. Therefore, NMR data may reflect the chemical composition of the 11Av PS better than FCSA.
wcjE-mediated capsule features have been described for pneumococcal serotype 9V. The 9V strains evolve serotype 9A expression following mutation of wcjE and resulting loss of capsule βManNAc-6-O-acetylation (6, 15). Akin to 11Av isolates, 9Vv (previously 9Aα) isolates express reduced amounts of serotype 9V-specific epitopes according to FCSA, in a wcjE allele-dependent fashion (6). Based on our studies with 11A, 11E, and 11Av, we propose that pneumococcal serotypes 9V and 9A also form two extremes of an antigenic spectrum with intermediate serovariants. Indeed, intermediate serovariants may exist in multiple serogroups, considering that wcjE and wcjE-like O-acetyltransferase genes (i.e., wciG, wciZ, wciX, wcyO, and wcyH) are encoded in the cps loci of multiple serogroups (10, 14). These include a few cases in which gene inactivation putatively results in loss of capsule O-acetylation and expression of a new serotype, e.g., 33A/33F and 15B/15C (22–24). We are developing methods to detect capsule O-acetylation in other serogroups and investigating the role these capsule features play in pneumococcal ecology.
Similar to intermediate serovariants, we recently characterized pneumococci that express capsule hybrids of two serotypes. For instance, serotype 6F PS contains a combination of serotype 6A and 6C repeat units (25). Serotype 6F expression results from point mutations in the cps gene wciN, putatively encoding a bispecific glycosyltransferase capable of producing both 6A and 6C PS repeat units. Conventional serotyping methods assume that serotypes are discrete entities and that each bacterial isolate belongs to a single discrete serotype. Thus, the incorporation of hybrid serotypes and intermediate serovariants in pneumococcal epidemiological studies may necessitate modification of current serotyping concepts.
These findings also pose a practical challenge to molecular serotyping techniques. The 11A-1 and 11A-2 cps loci present in JC03 and MNZ2293-A, respectively, contain multiple coding and noncoding nucleotide polymorphisms, but FCSA and NMR data reveal that both strains express 11A PS repeat units. In contrast, wcjE sequences of the 11Av strains 3102-06, MZN265, and MNZ2293-C differ from the wcjE sequences of their 11A counterparts by only one nucleotide substitution. Similarly, sequences of the serotype 11D and 11A-1 cps loci differ by only a single nucleotide substitution (8) and, as discussed above, nucleotide polymorphisms resulting in notable antigenic changes are reported for serogroups 6 and 9 (6, 25). In light of this evidence, genetic profiling of cps loci cannot unambiguously predict capsule identity, and the ability to serologically characterize pneumococcal strains remains crucial.
ACKNOWLEDGMENTS
This work was supported by NIH grants (R56 AI 31473 [to M.H.N.], F31-A1093103 [to J.J.C.], and T32-AI007051 [to A.M.B.]).
The University of Alabama at Birmingham (UAB) has intellectual property rights for some of the reagents used in this study, and all the authors are UAB employees.
M.H.N. serves as a consultant to Merck Inc. on S. pneumoniae diagnostic assays.
Footnotes
Published ahead of print 18 December 2013
REFERENCES
- 1.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. 2007. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J. Clin. Microbiol. 45:1225–1233. 10.1128/JCM.02199-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calix JJ, Nahm MH. 2010. A new pneumococcal serotype, 11E, has variably inactivated wcjE gene. J. Infect. Dis. 202:29–38. 10.1086/653123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calix JJ, Nahm MH, Zartler ER. 2011. Elucidation of structural and antigenic properties of pneumococcal serotype 11A, 11B, 11C, and 11F polysaccharide capsules. J. Bacteriol. 193:5271–5278. 10.1128/JB.05034-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zartler ER, Porambo RJ, Anderson CL, Chen LH, Yu J, Nahm MH. 2009. Structure of the capsular polysaccharide of pneumococcal serotype 11A reveals a novel acetylglycerol that is the structural basis for 11A subtypes. J. Biol. Chem. 284:7318–7329 (Erratum, 286:43588, 2011; erratum, 286:8707, 2011.) 10.1074/jbc.M807952200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calix JJ, Dagan R, Pelton SI, Porat N, Nahm MH. 2012. Differential occurrence of Streptococcus pneumoniae serotype 11E between asymptomatic carriage and invasive pneumococcal disease isolates reflects a unique model of pathogen microevolution. Clin. Infect. Dis. 54:794–799. 10.1093/cid/cir953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calix JJ, Oliver MB, Sherwood LK, Beall BW, Hollingshead SK, Nahm MH. 2011. Streptococcus pneumoniae serotype 9A isolates contain diverse mutations to wcjE that result in variable expression of serotype 9V-specific epitope. J. Infect. Dis. 204:1585–1595. 10.1093/infdis/jir593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung CK, Li H, Claverys JP, Morrison DA. 2001. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl. Environ. Microbiol. 67:5190–5196. 10.1128/AEM.67.11.5190-5196.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliver MB, Jones C, Larson TR, Calix JJ, Zartler ER, Yother J, Nahm MH. 2013. Streptococcus pneumoniae serotype 11D has a bispecific glycosyltransferase and expresses two different capsular polysaccharide repeating units. J. Biol. Chem. 288:21945–21954. 10.1074/jbc.M113.488528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Lin J, Benjamin WH, Jr, Waites KB, Lee CH, Nahm MH. 2005. Rapid multiplex assay for serotyping pneumococci with monoclonal and polyclonal antibodies. J. Clin. Microbiol. 43:156–162. 10.1128/JCM.43.1.156-162.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calix JJ, Porambo RJ, Brady AM, Larson TR, Yother J, Abeygunwardana C, Nahm MH. 2012. Biochemical, genetic and serological characterization of two capsule subtypes among Streptococcus pneumoniae serotype 20 strains: discovery of a new pneumococcal serotype. J. Biol. Chem. 287:27885–27894. 10.1074/jbc.M112.380451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. 1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6:277–293 [DOI] [PubMed] [Google Scholar]
- 12.Johnson BA, Blevins RA. 1994. NMR View: a computer-program for the visualization and analysis of NMR data. J. Biomol. NMR 4:603–614. 10.1007/BF00404272 [DOI] [PubMed] [Google Scholar]
- 13.Venkateswaran PS, Stanton N, Austrian R. 1983. Type variation of strains of Streptococcus pneumoniae in capsular serogroup 15. J. Infect. Dis. 147:1041–1054. 10.1093/infdis/147.6.1041 [DOI] [PubMed] [Google Scholar]
- 14.Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, Samuel G, Skovsted IC, Kaltoft MS, Barrell B, Reeves PR, Parkhill J, Spratt BG. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:e31. 10.1371/journal.pgen.0020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calix JJ, Saad JS, Brady AM, Nahm MH. 2012. Structural characterization of Streptococcus pneumoniae serotype 9A capsule polysaccharide reveals role of glycosyl 6-O-acetyltransferase wcjE in serotype 9V capsule biosynthesis and immunogenicity. J. Biol. Chem. 287:13996–14003. 10.1074/jbc.M112.346924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. 2005. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 116:e408–e413. 10.1542/peds.2004-2338 [DOI] [PubMed] [Google Scholar]
- 17.Kellner JD, Scheifele D, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ. 2008. Effects of routine infant vaccination with the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization with Streptococcus pneumoniae in children in Calgary, Canada. Pediatr. Infect. Dis. J. 27:526–532. 10.1097/INF.0b013e3181658c5c [DOI] [PubMed] [Google Scholar]
- 18.Spijkerman J, van Gils EJ, Veenhoven RH, Hak E, Yzerman EP, van der Ende A, Wijmenga-Monsuur AJ, van den Dobbelsteen GP, Sanders EA. 2011. Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, the Netherlands. Emerg. Infect. Dis. 17:584–591. 10.3201/eid1704.101115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Beekmann SE, Doern GV. 2009. Changing epidemiology of antimicrobial-resistant Streptococcus pneumoniae in the United States, 2004–2005. Clin. Infect. Dis. 48:e23–e33. 10.1086/595857 [DOI] [PubMed] [Google Scholar]
- 20.Tate WP, Mannering SA. 1996. Three, four or more: the translational stop signal at length. Mol. Microbiol. 21:213–219. 10.1046/j.1365-2958.1996.6391352.x [DOI] [PubMed] [Google Scholar]
- 21.Atkins JF, Bjork GR. 2009. A gripping tale of ribosomal frameshifting: extragenic suppressors of frameshift mutations spotlight P-site realignment. Microbiol. Mol. Biol. Rev. 73:178–210. 10.1128/MMBR.00010-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin FL, Vinogradov E, Deng C, Zeller S, Green BA, Jansen KU, Pavliak V. 2013. Identification of the common antigenic determinant shared by Streptococcus pneumoniae serotypes 33A, 35A, and 20 capsular polysaccharides. Carbohydr. Res. 380:101–107. 10.1016/j.carres.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 23.van Selm S, van Cann LM, Kolkman MA, van der Zeijst BA, van Putten JP. 2003. Genetic basis for the structural difference between Streptococcus pneumoniae serotype 15B and 15C capsular polysaccharides. Infect. Immun. 71:6192–6198. 10.1128/IAI.71.11.6192-6198.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones C, Lemercinier X. 2005. Full NMR assignment and revised structure for the capsular polysaccharide from Streptococcus pneumoniae type 15B. Carbohydr. Res. 340:403–409. 10.1016/j.carres.2004.12.009 [DOI] [PubMed] [Google Scholar]
- 25.Oliver MB, van der Linden MP, Kuntzel SA, Saad JS, Nahm MH. 2013. Discovery of Streptococcus pneumoniae serotype 6 variants with glycosyltransferases synthesizing two differing repeating units. J. Biol. Chem. 288:25976–25985. 10.1074/jbc.M113.480152 [DOI] [PMC free article] [PubMed] [Google Scholar]