Abstract
We reviewed our antifungal susceptibility data for micafungin, anidulafungin, fluconazole, and voriconazole against Candida species and compared resistance rates determined by the previous and recently revised CLSI antifungal breakpoints. With the new breakpoints, resistance was significantly increased for micafungin (from 0.8% to 7.6%), anidulafungin (from 0.9% to 7.3%), and voriconazole (from 6.1% to 18.4%) against Candida glabrata. Resistance was also increased for fluconazole against Candida albicans (from 2.1% to 5.7%).
TEXT
The Clinical and Laboratory Standards Institute (CLSI) recently revised the azole and echinocandin clinical breakpoints against Candida species (1). These new breakpoints are now both drug and species specific, whereas the previous breakpoints were not. For most species, with the exception of Candida parapsilosis and Candida guilliermondii against the echinocandins, the breakpoints have been lowered, such that previously susceptible MICs are now classified as resistant. In addition, Candida glabrata isolates are no longer considered susceptible to fluconazole but are rather classified only as either dose-dependent susceptible or resistant. The breakpoint revisions were made based on information from clinical studies, case reports describing clinical failure at MIC values below the previous breakpoints, results from pharmacokinetic/pharmacodynamic studies, and epidemiologic cutoff values (2, 3). Additionally, a goal of harmonizing the antifungal breakpoints with those set by EUCAST was sought. Epidemiologic cutoff values for individual species are used to optimize the detection of non-wild-type strains and, thus, the acquisition of resistance mechanisms and to prevent the breakpoints from dividing wild-type populations (2–4). The impact of the revised clinical breakpoints regarding categorical placement of Candida strains as resistant is unknown. Our objective was to evaluate what effect the new antifungal clinical breakpoints may have on azole and echinocandin resistance patterns in Candida species.
(This work was presented in part at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Denver, CO, 2013.)
The antifungal susceptibility database in the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio was reviewed. Susceptibility data for fluconazole, voriconazole, anidulafungin, and micafungin against Candida albicans, C. glabrata, Candida tropicalis, Candida krusei, and C. parapsilosis isolates sent to our laboratory for testing between 1 January 2008 and 31 December 2012 were reviewed. During this period, antifungal powders were obtained from the appropriate manufacturers (Pfizer and Astellas) and stock solutions were prepared in water for agents with aqueous solubility or in dimethyl sulfoxide (DMSO) as recommended (1, 5). Susceptibility testing was performed by broth microdilution methodology in RPMI according to the CLSI M27-A3 guidelines (5), and a 50% inhibition of growth compared to the growth control well was used as the endpoint for all agents. C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 served as the quality control isolates for each testing run, and the results were consistently within the range specified by the CLSI. Isolates were classified as resistant based on both the previous and the recently revised CLSI clinical breakpoints (Table 1) (1). For voriconazole against C. glabrata, the epidemiologic cutoff value of 0.5 μg/ml was used for the new threshold for resistance, as the CLSI has not set clinical breakpoints for this triazole against this species (1, 6, 7). Thus, C. glabrata isolates with a voriconazole MIC above this value (i.e., ≥1 μg/ml) were classified as resistant. Differences in resistance rates between the previous and revised breakpoints were assessed for significance by Fisher's exact test, and a P value of ≤0.05 was considered significant.
TABLE 1.
Previous and recently revised CLSI antifungal clinical breakpoints for resistance for fluconazole, voriconazole, anidulafungin, and micafungin against Candida speciesa
| Antifungal and CBP | Breakpoint (μg/ml) against: |
||||
|---|---|---|---|---|---|
| C. albicans | C. glabrata | C. tropicalis | C. krusei | C. parapsilosis | |
| Anidulafungin | |||||
| Previous | ≥4 | ≥4 | ≥4 | ≥4 | ≥4 |
| Revised | ≥1 | ≥0.5 | ≥1 | ≥1 | ≥8 |
| Micafungin | |||||
| Previous | ≥4 | ≥4 | ≥4 | ≥4 | ≥4 |
| Revised | ≥1 | ≥0.25 | ≥1 | ≥1 | ≥8 |
| Fluconazole | |||||
| Previous | ≥64 | ≥64 | ≥64 | ≥64 | ≥64 |
| Revised | ≥8 | ≥64 | ≥8 | ≥8 | ≥8 |
| Voriconazole | |||||
| Previous | ≥4 | ≥4 | ≥4 | ≥4 | ≥4 |
| Revised | ≥1 | ≥1 | ≥1 | ≥2 | ≥1 |
The epidemiologic cutoff value was used for voriconazole against C. glabrata. CBP, clinical breakpoints.
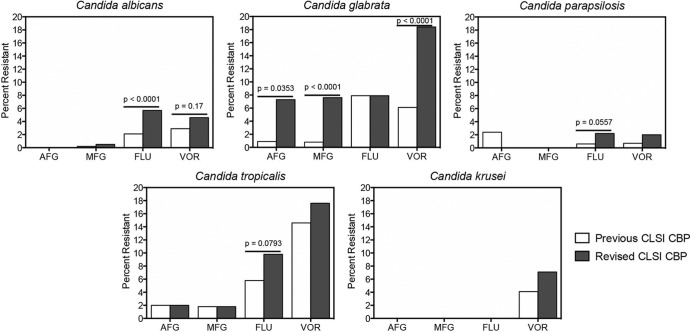
The number of each Candida species tested with each drug, the MIC range, the MIC50 and MIC90 values, and the percentage of isolates classified as resistant per the previous and revised breakpoints are shown in Table 2, and the MIC distributions are shown in Table 3. As shown in these tables and in Fig. 1, resistance did increase with the new breakpoints. For the echinocandins anidulafungin and micafungin, the most marked changes occurred against C. glabrata. For this species, the number of isolates classified as resistant increased from 1 (0.9%) to 8 (7.3%) of 110 isolates (P = 0.0353) for anidulafungin and from 3 (0.8%) to 27 (7.6%) of 354 isolates (P < 0.0001) for micafungin when the new CLSI clinical breakpoints were applied. Our rates of micafungin and anidulafungin resistance with the new CLSI clinical breakpoints are higher than those recently reported in the SENTRY study (1.3% and 1.6%, respectively) (6). However, others have found higher rates of echinocandin resistance for this species, with rates of micafungin and anidulafungin resistance reported to range from 9% to 12% between 2007 and 2010 at a large medical center in the United States (8). Against the other Candida species, the number of isolates considered to be resistant remained relatively low, and for anidulafungin against C. parapsilosis, the number actually decreased slightly from 2 to 0 isolates (2.4% to 0%), although this reduction was not statistically significant. This was not unexpected against this species, as the CLSI echinocandin breakpoints against C. parapsilosis were raised from ≥4 μg/ml as nonsusceptible to ≥8 μg/ml as resistant.
TABLE 2.
MIC ranges, MIC50 and MIC90 values, and percentages of isolates classified as resistant based on the previous and recently revised CLSI clinical breakpoints for antifungalsa
| Antifungal | Value for each species and drug |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Candida albicans |
Candida glabrata |
Candida tropicalis |
Candida krusei |
Candida parapsilosis |
||||||||||||||||
| AFG | MFG | FLU | VOR | AFG | MFG | FLU | VOR | AFG | MFG | FLU | VOR | AFG | MFG | FLU | VOR | AFG | MFG | FLU | VOR | |
| No. of strains | 118 | 433 | 1,196 | 593 | 110 | 354 | 882 | 522 | 49 | 170 | 327 | 205 | 28 | 65 | 98 | 83 | 269 | 496 | 298 | |
| MIC range | ≤0.015–0.25 | ≤0.015–8 | ≤0.125–>64 | ≤0.03–>16 | ≤0.015–4 | ≤0.015–4 | ≤0.125–>64 | ≤0.03–>16 | ≤0.015–8 | ≤0.015–8 | ≤0.125–>64 | ≤0.03–>16 | 0.03–0.25 | ≤0.015–0.25 | 0.03–4 | ≤0.015–4 | ≤0.015–2 | ≤0.125–>64 | ≤0.03–>16 | |
| MIC50 | 0.015 | 0.015 | 0.125 | 0.03 | 0.06 | 0.03 | 4 | 0.125 | 0.03 | 0.03 | 0.5 | 0.06 | 0.125 | 0.125 | 0.25 | 1 | 0.5 | 0.25 | 0.03 | |
| MIC90 | 0.06 | 0.03 | 0.5 | 0.125 | 0.25 | 0.06 | 32 | 2 | 0.125 | 0.06 | 8 | 16 | 0.25 | 0.25 | 1 | 2 | 1 | 0.5 | 0.06 | |
| % resistant to previous CBP | 0 | 0.2 | 2.1 | 2.9 | 0.9 | 0.8 | 7.9 | 6.1 | 2.0 | 1.8 | 5.8 | 14.6 | 0 | 0 | 4.1 | 2.4 | 0 | 0.6 | 0.7 | |
| % resistant to new CBP | 0 | 0.5 | 5.7 | 4.6 | 7.3 | 7.6 | 7.9 | 18.4 | 2.0 | 1.8 | 9.8 | 17.6 | 0 | 0 | 7.1 | 0 | 0 | 2.2 | 2.0 | |
CBP, clinical breakpoints; AFG, anidulafungin; MFG, micafungin; FLU, fluconazole; VOR, voriconazole.
TABLE 3.
Comparison of in vitro susceptibilities of anidulafungin, micafungin, fluconazole, and voriconazole against Candida isolates
| Species and agenta | No. of isolates tested | % of isolates at an MIC (μg/ml) of: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | ||
| C. albicans | ||||||||||||||
| AFG | 118 | 60.2 | 29.7 | 4.2 | 3.4 | 2.5 | ||||||||
| MFG | 433 | 75.5 | 21.0 | 1.4 | 0.5 | 0.5 | 0.7 | 0.2 | 0.2 | |||||
| FLU | 1,196 | 69.6 | 17.4 | 3.2 | 1.3 | 1.0 | 1.8 | 1.5 | 1.3 | 0.8 | 2.1 | |||
| VOR | 593 | 84 | 4.9 | 3.2 | 2.4 | 1.0 | 0.8 | 0.8 | 0.5 | 0.5 | 1.9 | |||
| C. glabrata | ||||||||||||||
| AFG | 110 | 2.7 | 26.4 | 29.1 | 27.3 | 7.3 | 2.7 | 1.8 | 1.8 | 0.9 | ||||
| MFG | 354 | 48.0 | 39.0 | 3.4 | 2.0 | 2.3 | 2.0 | 1.1 | 1.4 | 0.8 | ||||
| FLU | 882 | 0.2 | 1.8 | 7.0 | 12.6 | 27.2 | 27.3 | 6.7 | 4.1 | 5.1 | 7.9 | |||
| VOR | 522 | 14.6 | 24.5 | 22.2 | 11.9 | 8.4 | 7.5 | 4.8 | 4.8 | 1.1 | 0.2 | |||
| C. tropicalis | ||||||||||||||
| AFG | 49 | 26.5 | 30.6 | 24.5 | 12.2 | 4.1 | 2.0 | |||||||
| MFG | 170 | 21.2 | 64.1 | 8.8 | 2.9 | 1.2 | 1.8 | |||||||
| FLU | 327 | 10.1 | 37.3 | 26.6 | 8.5 | 5.5 | 2.4 | 1.2 | 2.1 | 0.6 | 5.8 | |||
| VOR | 205 | 39.0 | 21.0 | 12.7 | 6.3 | 3.4 | 1.5 | 1.5 | 1.5 | 0.5 | 12.6 | |||
| C. krusei | ||||||||||||||
| AFG | 28 | 21.4 | 14.3 | 57.1 | 7.1 | |||||||||
| MFG | 65 | 3.1 | 6.1 | 13.8 | 58.5 | 18.5 | ||||||||
| VOR | 98 | 4.1 | 3.1 | 25.5 | 41.8 | 11.2 | 7.1 | 3.1 | 4.1 | |||||
| C. parapsilosis | ||||||||||||||
| AFG | 83 | 1.2 | 2.4 | 10.8 | 28.9 | 38.6 | 15.7 | 2.4 | ||||||
| MFG | 269 | 0.7 | 1.5 | 0.7 | 3.3 | 21.6 | 43.1 | 27.9 | 1.1 | |||||
| FLU | 496 | 8.9 | 56.1 | 25.8 | 2.8 | 3.0 | 1.2 | 1.2 | 0.4 | 0.6 | ||||
| VOR | 298 | 88.9 | 4.0 | 3.4 | 1.0 | 0.7 | 0.7 | 0.7 | 0.3 | 0.3 | ||||
AFG, anidulafungin; MFG, micafungin; FLU, fluconazole; VOR, voriconazole.
FIG 1.
Percentage of Candida isolates per species classified as resistant to anidulafungin (AFG), micafungin (MFG), fluconazole (FLU), and voriconazole (VOR) per the previous and recently revised CLSI antifungal clinical breakpoints (CBP).
The azoles fluconazole and voriconazole were also affected by the revised breakpoints. Against C. albicans, the number of isolates classified as resistant to fluconazole by the new breakpoints significantly increased from 25 (2.1%) to 68 (5.7%) of a total of 1,196 strains tested (P < 0.0001) (Table 2 and Fig. 1) compared to the previous threshold for resistance. This rate of fluconazole resistance in C. albicans is higher than what was recently reported in isolates from North American institutions in the SENTRY study (0.6%) (6). There were also trends for increased resistance to fluconazole with the new breakpoints against C. tropicalis (19 to 32 of 327 isolates [6% to 9.9%]; P = 0.0557) and C. parapsilosis (3 to 11 of 497 isolates [0.6% to 2.2%]; P = 0.0793). A similar observation was found for voriconazole against C. albicans (17 to 27 of 593 isolates [2.9% to 4.6%]; P = 0.17), although this difference was not significant. In contrast, only minor increases in voriconazole resistance against C. tropicalis (30 to 36 of 205 isolates [14.6% to 17.6%]) and C. krusei (4 to 7 of 98 isolates [4.1% to 12.2%]) were observed when the new breakpoints were applied. A significant increase in the number of C. glabrata isolates that were classified as resistant to voriconazole (32 to 96 of 522 isolates [6.1% to 18.4%]; P < 0.0001) was observed when the epidemiologic cutoff value was used. The revised CLSI breakpoints for the azoles are supported by epidemiologic cutoff values and the results of various pharmacodynamic analyses (9–12), as well as by the cross-resistance observed among different members of this class in Candida species (13–16). For most species, our rates of fluconazole and voriconazole resistance were higher than those reported in the SENTRY study (6). This may be a reflection of the nature of our reference laboratory in that we may often be sent isolates from patients exposed to antifungals who are failing therapy. Thus, some of the isolates we receive for susceptibility testing may be more likely to be non-wild-type strains. However, the overall MIC distributions from our study shown in Table 3 are similar to those reported by others, including those from large surveillance studies (6, 7, 17).
The results from our laboratory demonstrate that the rates of resistance for the echinocandins and the azoles may be increased with the use of the new CLSI antifungal breakpoints. These changes were especially marked for micafungin, anidulafungin, and voriconazole against C. glabrata and for fluconazole against C. albicans. One limitation of this study is that we did not determine if the isolates harbored mechanisms of acquired antifungal resistance. Although this information is important, it would not change how an isolate is classified as susceptible or resistant by the CLSI breakpoints, something which is determined by the phenotypic MIC. The clinical relevance of our findings is unknown. Studies have indicated that in vitro resistance may be indicative of clinical failure (8, 18, 19). The results from other laboratories and institutions are needed to confirm our observations.
ACKNOWLEDGMENTS
We thank Carmita Sanders for her assistance with this work.
N.P.W. has received research support from Pfizer, Merck, Basilea, Astellas, Viamet, bioMérieux, and Medicis and has served on advisory boards for Merck, Astellas, Toyama, and Viamet.
Footnotes
Published ahead of print 8 January 2014
REFERENCES
- 1.CLSI 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; 4th informational supplement. CLSI document M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 2.Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D, CLSI Subcommittee for Antifungal Susceptibility Testing 2010. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist. Updat. 13:180–195. 10.1016/j.drup.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS, CLSI Subcommittee for Antifungal Susceptibility Testing 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 14:164–176. 10.1016/j.drup.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 125:S3–S13. 10.1016/j.amjmed.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 5.CLSI 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—3rd ed. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 2013. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J. Clin. Microbiol. 51:2571–2581. 10.1128/JCM.00308-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfaller MA, Boyken LB, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, Diekema DJ. 2011. Validation of 24-hour posaconazole and voriconazole MIC readings versus the CLSI 48-hour broth microdilution reference method: application of epidemiological cutoff values to results from a global Candida antifungal surveillance program. J. Clin. Microbiol. 49:1274–1279. 10.1128/JCM.02437-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 56:1724–1732. 10.1093/cid/cit136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller MA, Diekema DJ, Sheehan DJ. 2006. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol. Rev. 19:435–447. 10.1128/CMR.19.2.435-447.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuesta I, Bielza C, Cuenca-Estrella M, Larranaga P, Rodriguez-Tudela JL. 2010. Evaluation by data mining techniques of fluconazole breakpoints established by the Clinical and Laboratory Standards Institute (CLSI) and comparison with those of the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrob. Agents Chemother. 54:1541–1546. 10.1128/AAC.01688-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clancy CJ, Yu VL, Morris AJ, Snydman DR, Nguyen MH. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49:3171–3177. 10.1128/AAC.49.8.3171-3177.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Tudela JL, Almirante B, Rodriguez-Pardo D, Laguna F, Donnelly JP, Mouton JW, Pahissa A, Cuenca-Estrella M. 2007. Correlation of the MIC and dose/MIC ratio of fluconazole to the therapeutic response of patients with mucosal candidiasis and candidemia. Antimicrob. Agents Chemother. 51:3599–3604. 10.1128/AAC.00296-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panackal AA, Gribskov JL, Staab JF, Kirby KA, Rinaldi M, Marr KA. 2006. Clinical significance of azole antifungal drug cross-resistance in Candida glabrata. J. Clin. Microbiol. 44:1740–1743. 10.1128/JCM.44.5.1740-1743.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Messer SA, Boyken L, Rice C, Tendolkar S, Hollis RJ, Diekema DJ. 2004. Cross-resistance between fluconazole and ravuconazole and the use of fluconazole as a surrogate marker to predict susceptibility and resistance to ravuconazole among 12,796 clinical isolates of Candida spp. J. Clin. Microbiol. 42:3137–3141. 10.1128/JCM.42.7.3137-3141.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller MA, Messer SA, Boyken L, Rice C, Tendolkar S, Hollis RJ, Diekema DJ. 2007. Use of fluconazole as a surrogate marker to predict susceptibility and resistance to voriconazole among 13,338 clinical isolates of Candida spp. Tested by clinical and laboratory standards institute-recommended broth microdilution methods. J. Clin. Microbiol. 45:70–75. 10.1128/JCM.01551-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller MA, Messer SA, Boyken L, Tendolkar S, Hollis RJ, Diekema DJ. 2008. Selection of a surrogate agent (fluconazole or voriconazole) for initial susceptibility testing of posaconazole against Candida spp.: results from a global antifungal surveillance program. J. Clin. Microbiol. 46:551–559. 10.1128/JCM.01952-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. 2013. In vitro activities of isavuconazole and comparator antifungal agents tested against a global collection of opportunistic yeasts and molds. J. Clin. Microbiol. 51:2608–2616. 10.1128/JCM.00863-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeiffer CD, Garcia-Effron G, Zaas AK, Perfect JR, Perlin DS, Alexander BD. 2010. Breakthrough invasive candidiasis in patients on micafungin. J. Clin. Microbiol. 48:2373–2380. 10.1128/JCM.02390-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baddley JW, Patel M, Bhavnani SM, Moser SA, Andes DR. 2008. Association of fluconazole pharmacodynamics with mortality in patients with candidemia. Antimicrob. Agents Chemother. 52:3022–3028. 10.1128/AAC.00116-08 [DOI] [PMC free article] [PubMed] [Google Scholar]