Abstract
Enterococci are a major cause of health care-associated infections and account for approximately 10% of all bacteremias globally. The aim of this study was to determine the proportion of enterococcal bacteremia isolates in Australia that are antimicrobial resistant, with particular emphasis on susceptibility to ampicillin and the glycopeptides, and to characterize the molecular epidemiology of the Enterococcus faecalis and Enterococcus faecium isolates. From 1 January to 31 December 2011, 1,079 unique episodes of bacteremia were investigated, of which 95.8% were caused by either E. faecalis (61.0%) or E. faecium (34.8%). The majority of bacteremias were health care associated, and approximately one-third were polymicrobial. Ampicillin resistance was detected in 90.4% of E. faecium isolates but was not detected in E. faecalis isolates. Vancomycin nonsusceptibility was reported in 0.6% and 36.5% of E. faecalis and E. faecium isolates, respectively. Unlike Europe and the United States, where vancomycin resistance in E. faecium is predominately due to the acquisition of the vanA operon, 98.4% of E. faecium isolates harboring van genes carried the vanB operon, and 16.1% of the vanB E. faecium isolates had vancomycin MICs at or below the susceptible breakpoint of the CLSI. Although molecular typing identified 126 E. faecalis pulsed-field gel electrophoresis pulsotypes, >50% belonged to two pulsotypes that were isolated across Australia. E. faecium consisted of 73 pulsotypes from which 43 multilocus sequence types were identified. Almost 90% of the E. faecium isolates were identified as CC17 clones, of which approximately half were characterized as ST203, which was isolated Australia-wide. In conclusion, the Australian Enterococcal Sepsis Outcome Programme (AESOP) study has shown that although they are polyclonal, enterococcal bacteremias in Australia are frequently caused by ampicillin-resistant vanB E. faecium.
INTRODUCTION
Enterococci, which were initially believed to be harmless inhabitants of the gastrointestinal tract flora, have emerged as a major cause of health care-associated infections (1). Globally, they are now thought to account for approximately 10% of all bacteremias (2), and in North America and Europe are the fourth and fifth leading cause of sepsis, respectively (3).
In the 1970s, health care-associated enterococcal infections were associated with the introduction of third-generation cephalosporins and were primarily due to Enterococcus faecalis (4). However, following the increased use of vancomycin and broad-spectrum antibiotics, significant increases in the numbers of infections caused by the more frequently resistant E. faecium were reported in Europe and in North America (5, 6). Preceded by an increase in infections and outbreaks caused by ampicillin-resistant E. faecium, clinically significant isolates of vancomycin-resistant enterococci (VRE) were subsequently detected in the United Kingdom (7) and Europe (8) and shortly after in the United States (9). By the early 1990s, VRE had become the second most common nosocomial pathogen in the United States (9) and was endemic in many North American hospitals (10). Vancomycin resistance in E. faecium bacteremia isolates ranges from 5 to 35% in Europe (see www.earss.rivm.nl) to 60% in North America (11). The predominant VRE genotype in these two regions is vanA (12). Although the origins of vanA in these settings are unclear, the past use of the glycopeptide avoparcin as a growth promoter in animal husbandry has been proposed as a contributing factor in Europe (13).
In Australia, the first reported VRE was a vanA E. faecium from a liver transplant recipient in 1995 (14). Since this time, however, the vast majority of VRE have been E. faecium harboring the vanB operon (15). Although prevalence or incidence rates of VRE in Australian hospitals are not routinely collected, several reported studies have shown a significant increase in the number of patients infected or colonized with vanB E. faecium (16–18). In the 2010 Australian Group on Antimicrobial Resistance (AGAR) period prevalence study of key resistances in clinical isolates of Enterococcus species, vancomycin nonsusceptibility occurred in 36.5% of E. faecium isolates, of which 98% were vanB VRE (see http://www.agargroup.org/surveys).
In 2011, AGAR commenced the Australian Enterococcal Sepsis Outcome Programme (AESOP). In this study, we determined the proportion of bacteremia isolates of Enterococcus species demonstrating antimicrobial resistance, with particular emphasis on susceptibility to ampicillin and the glycopeptides and the molecular epidemiology of E. faecalis and E. faecium.
MATERIALS AND METHODS
The Australian Group on Antimicrobial Resistance (AGAR) is a network of laboratories located across Australia which provide microbiology services to over 80% of the country's tertiary acute care hospitals. AGAR has been conducting national resistance surveillance for more than 25 years (see http://www.agargroup.org), and for the last decade AGAR has been funded by the Australian Department of Health.
Twenty-nine laboratories from all six states, the Australian Capital Territory (ACT), and the Northern Territory (NT) participated in the inaugural 2011 AGAR Australian Enterococcal Sepsis Outcome Program (AESOP).
From 1 January to 31 December 2011, the 29 AGAR laboratories collected all enterococcal species isolated from blood cultures. Enterococci with the same species and antimicrobial susceptibility profiles isolated from a patient's blood culture within 14 days of the first positive blood culture were excluded. A new enterococcal sepsis episode in the same patient was recorded if it was confirmed by a further culture of blood taken more than 14 days after the initial positive culture.
A web-based data entry system was constructed to enable the collection of real-time data into a common database. To ensure patient anonymity, but to allow follow-up of discrepant results with each participating site, a record identifier unique to the participating laboratory was used.
Each episode of bacteremia was designated health care associated if any one of the following criteria applied (19, 20), (i) the first positive blood culture(s) in an episode of infection were collected >48 h after hospital admission, (ii) the patient resided in a nursing home or long-term-care facility within the year preceding the positive blood culture(s), (iii) the patient had a previous hospital admission for ≥2 days within the year preceding the positive blood culture(s), or (iv) the patient was receiving hemodialysis. If the patient did not meet the above criteria and the blood culture was taken before or within 48 h of hospital admission, then the episode was designated community associated (20).
Participating laboratories identified isolates to the species level by one of the following methods: API 20S (bioMérieux), API ID32Strep (bioMérieux), Vitek (bioMérieux), Phoenix (BD), Vitek-MS (bioMérieux), matrix-assisted laser desorption ionization (MALDI) Biotyper (Bruker Daltonics), PCR, or conventional biochemical tests.
Antimicrobial susceptibility testing at the contributing site was performed according to each laboratory's routine standardized methodology (Clinical and Laboratory Standards [CLSI]-based disc diffusion, agar dilution, Vitek2, or Phoenix). Ampicillin and vancomycin susceptibilities were tested by all laboratories. In addition 810 (75.1%) isolates were tested against linezolid and 991 (91.8%) and 258 (23.9%) screened for high-level gentamicin and streptomycin resistance, respectively. For tests performed by the participating institution, CLSI breakpoints were utilized for interpretation (21). Isolates with an intermediate or resistant category were classified as nonsusceptible.
Quality control was conducted as part of the routine laboratory practice in all 29 ISO 15189-accredited laboratories.
Of the isolates obtained from the 1,033 Enterococcus faecium and Enterococcus faecalis sepsis episodes recorded by the participating laboratories, 963 (93.2%) isolates were referred to the Australian Centre for Enterococcus and Staphylococcus Species (ACCESS) Typing and Research for further susceptibility testing and molecular typing. Vancomycin and teicoplanin MICs were performed by Etest (bioMérieux) according to the manufacturer's instructions. Linezolid MICs were performed by Etest (bioMérieux) on linezolid-nonsusceptible isolates. Results were interpreted using both CLSI standards (21) and those of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (version 3.1 [see http://www.eucast.org/clinical_breakpoints/]). Pulsed-field gel electrophoresis (PFGE) of SmaI-digested DNA agarose plugs was performed on all referred isolates as previously described (22). Banding patterns were examined visually, scanned with a Quantity One device (Bio-Rad Laboratories Pty, Ltd.), and digitally analyzed using FPQuest (Bio-Rad Laboratories Pty., Ltd.). The Dice coefficient and the unweighted pair group method with arithmetic mean were used with settings for tolerance and optimization of 1.25% and 0.5%, respectively. Isolates with up to a six-band difference were considered the same pulsotype (23). Multilocus sequence typing (MLST) was performed as previously described on a representative isolate of each E. faecium PFGE pulsotype (24). If a PFGE pulsotype included vancomycin nonsusceptible and vancomycin susceptible E. faecium, MLST was performed on both phenotypes. The sequences were submitted to http://www.mlst.net/, where an allele profile was generated and a sequence type (ST) assigned. Bu use of the eBURST V3 algorithm at the same website, STs were classified into eBURST groups when a member of a group shared alleles at ≥5 of the 7 loci. Double-locus variants (dlvs) of an ST were included within an eBURST group only if the linking single-locus variant (slv) was present. The ancestral ST of an eBURST group was defined as the ST that differed from the largest number of other STs at only a single locus (i.e., the ST that has the greatest number of slvs). STs that diverged by no more than two of the seven MLST loci of the ancestral ST were considered to belong to the same clonal complex (CC).
vanA and vanB PCR was performed on the 963 referred E. faecium and E. faecalis isolates either by the participating laboratory or by ACCESS Typing and Research.
Regular audits for data discrepancies and potential duplicate entries were conducted and resolved with each of the participating sites during collection.
A chi-square test for comparison of two proportions was performed and 95% confidence intervals were determined using MedCalc for Windows, version 12.7 (MedCalc Software, Ostend, Belgium).
Approval to conduct the prospective data collection was given by the research ethics committee associated with each participating laboratory.
RESULTS
From 1 January to 31 December 2013, 1,079 unique episodes of enterococcal bacteremia were identified by the AGAR laboratories, of which 950 (88.0%) were health care associated (95% CI, 85.9 to 89.9%) and 359 (33.3%) were polymicrobial (95% CI, 30.5 to 36.2%). Twenty-eight patients had more than one episode.
Although eight species of Enterococcus were identified, 95.8% were either E. faecalis (658 isolates, 61.0%) or E. faecium (375, 34.8%). Forty-five enterococci were identified either as Enterococcus casseliflavus (15 isolates), E. gallinarum (14), E. avium (8), E. raffinosus (4), E. durans (2), or E. hirae (2). One isolate could not be identified to the species level.
Overall, 90.4% of E. faecium were ampicillin resistant (Table 1). Ampicillin resistance was not detected in E. faecalis. Vancomycin nonsusceptibility (CLSI) was reported by the participating laboratories in 0.6% and 36.5% of E. faecalis and E. faecium isolates, respectively. Of the 137 vancomycin-nonsusceptible E. faecium isolates, 133 (97.1%) were also ampicillin resistant. MICs were performed on 14 of the 15 isolates reported as linezolid nonsusceptible by the participating laboratory using CLSI interpretations (3 resistant and 11 intermediate). Eight isolates had MICs of 2 mg/liter (susceptible) and six isolates had MICs of 4 mg/liter (intermediate using CLSI interpretations and susceptible using EUCAST interpretations). High-level gentamicin resistance was reported by the participating laboratories in 65.0% of E. faecium and 34.8% of E. faecalis isolates. In contrast, 22.8% of E. faecium and 6.4% of E. faecalis isolates were high-level streptomycin resistant.
TABLE 1.
Enterococcus faecalis and Enterococcus faecium susceptibility results
| Antimicrobial agent |
E. faecalis |
E. faecium |
||
|---|---|---|---|---|
| No. tested | No. (%) resistant (95% CI)a | No. tested | No. (%) resistant (95% CI) | |
| Vancomycin | 658 | 3 (0.5) (0.1–1.4)b | 375 | 137 (36.5) (31.6–41.6)b |
| Ampicillin | 658 | 0 | 375 | 339 (90.4) (87.0–93.2) |
| Linezolid | 472 | 11 (2.3) (1.2–4.1)b | 300 | 4 (1.3) (0.4–3.3)b |
| High-level gentamicin | 607 | 211 (34.8) (31.0–38.7) | 340 | 221 (65.0) (59.7–70.1) |
| High-level streptomycin | 172 | 11 (6.4) (3.2–11.2) | 79 | 18 (22.8) (14.1–33.69) |
As described in CLSI M100-A23 (21).
Nonsusceptible.
Of the 963 isolates referred to the Australian Centre for Enterococcus and Staphylococcus Species (ACCESS) Typing and Research, 622 (64.6%) were E. faecalis and 341 (35.4%) were E. faecium.
Enterococcus faecalis.
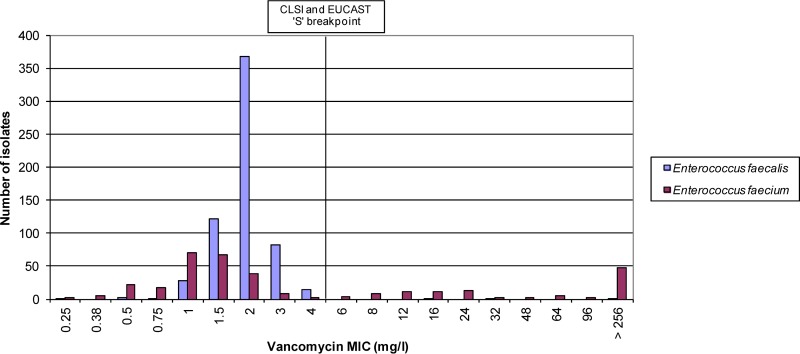
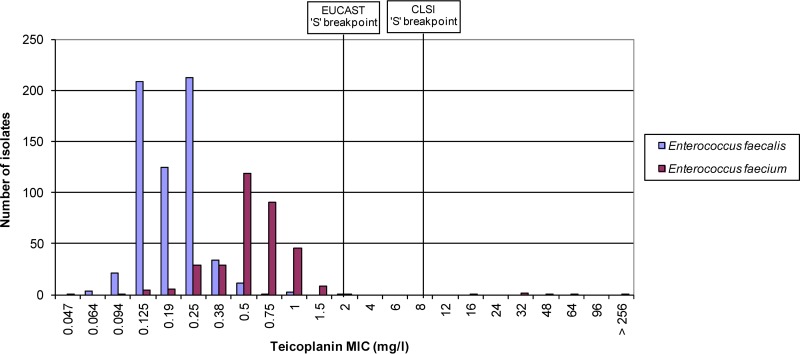
The vancomycin MICs for the 622 E. faecalis isolates ranged from 0.25 to >256 mg/liter, with a mode of 2 mg/liter (Fig. 1). The three vancomycin nonsusceptible isolates (>4 mg/liter, 0.6%) harbored the vanB gene and had vancomycin MICs of 16 mg/liter, 32 mg/liter, and >256 mg/liter. No vanA or vanB genes were detected in the vancomycin-susceptible isolates. The teicoplanin MICs ranged from 0.064 to 2 mg/liter, with a mode of 0.25 mg/liter (Fig. 2). The three vanB-containing isolates had teicoplanin MICs of 0.125 mg/liter (two isolates) and 0.25 mg/liter (one isolate), i.e., below the susceptible CLSI and EUCAST breakpoints.
FIG 1.
Enterococcus faecalis and Enterococcus faecium vancomycin MICs.
FIG 2.
Enterococcus faecalis and Enterococcus faecium teicoplanin MICs.
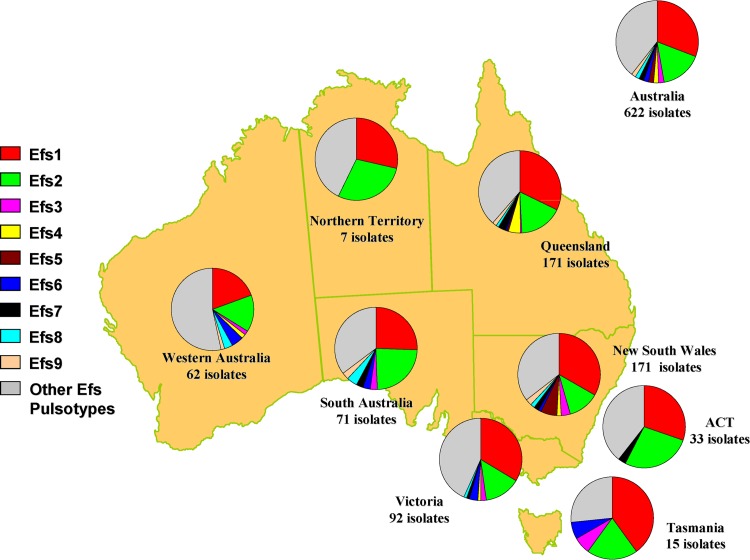
By PFGE, 618 of the 622 E. faecalis isolates were classified into 126 pulsotypes, of which nine pulsotypes (Efs1 to Efs9) had 10 or more isolates (Fig. 3). Four isolates could not be typed by PFGE. Of the 117 pulsotypes that had <10 isolates, 66 pulsotypes were represented by only one isolate. Geographically, the nine major pulsotypes were widely distributed, with the two predominant pulsotypes, Efs1 (191 isolates) and Efs2 (103 isolates), isolated across Australia. The three van B E. faecalis isolates were detected in pulsotype Efs2. Compared to non-Efs1/Efs2 bacteremia cases, a significantly higher percentage of Efs1 and Efs2 bacteremia cases were health care associated (80.8% versus 88.1%) (P = 0.0171).
FIG 3.
Distribution and proportion of Enterococcus faecalis (Efs) pulsotypes across Australia.
Enterococcus faecium.
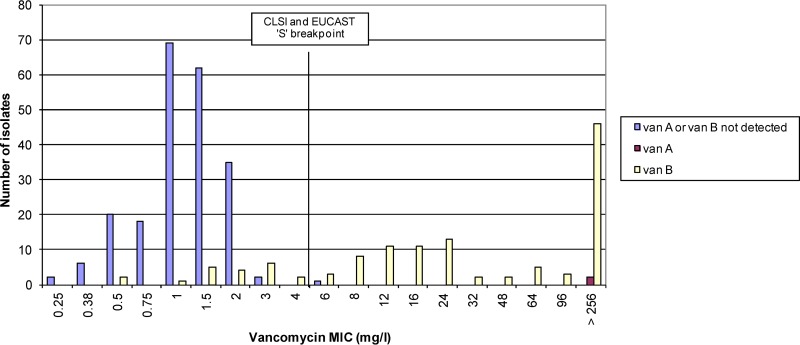
The vancomycin MICs for the 341 E. faecium isolates ranged from 0.25 to >256 mg/liter, with a mode of 1.0 mg/liter (Fig. 1). Of the 234 vancomycin-susceptible isolates (MIC ≤4 mg/liter), 20 (8.5%) harbored the vanB gene and had vancomycin MICs that ranged from 0.5 mg/liter to 4.0 mg/liter (Fig. 4). Of the 107 vancomycin-nonsusceptible isolates (MIC >4 mg/liter), two isolates harbored the vanA gene (vancomycin MIC >256 mg/liter) and 104 harbored the vanB gene (vancomycin MICs 6 to >256 mg/liter) (Fig. 4). One isolate with a vancomycin MIC of 6 mg/liter did not harbor vanA or vanB genes. The teicoplanin MICs ranged from 0.047 to >256 mg/liter, with a mode of 0.5 mg/liter (Fig. 2). The two vanA isolates had teicoplanin MICs of 32 and 64 mg/liter. Of the 124 vanB isolates, one was teicoplanin intermediate (MIC 16 mg/liter) and three were resistant (MIC >16 mg/liter) by CLSI criteria, and five were resistant by EUCAST criteria (MIC >2 mg/liter).
FIG 4.
MICs of vanA-, vanB-, and vanA/B-negative Enterococcus faecium.
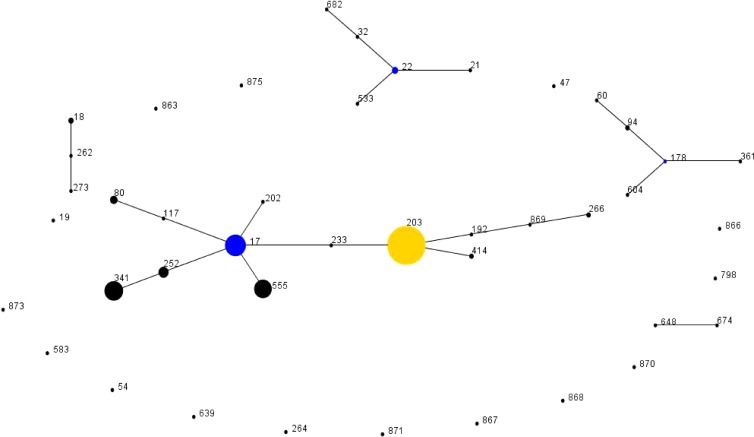
By PFGE, the 341 E. faecium isolates were classified into 73 pulsotypes from which 43 multilocus STs were identified (Table 2). Three of the seven housekeeping genes in PFGE pulsotype Efm56 could not be amplified using the recommended MLST primers and therefore a ST could not be assigned. By eBURST, 28 of the 43 STs were grouped into five eBURST groups (Fig. 5). The remaining 15 STs were classified as singletons. eBURST group 1 consisted of 13 STs of which 9 STs formed clonal complex (CC) 17. The ancestral ST within CC17 was identified as ST17, from which seven single-locus variants (slvs) and the double locus variant (dlv) ST203 were identified. ST203 was considered a cofounder within eBURST group 1 from which an additional four STs were linked. Overall, 38 pulsotypes were identified in eBURST group 1, of which 12 and 11 pulsotypes were characterized as ST203 and ST17, respectively. A further two CCs were characterized, including CC22 in eBURST group 2 and CC178 in eBURST group 3. An ancestral ST was not identified in eBURST groups 4 and 5.
TABLE 2.
Molecular epidemiology of health care- and community-associated Enterococcus faecium isolates
| Group | CC | ST | MLST allelic profile | Pulsotypes identified by PFGE (no. of isolates) | Total no. (%) of isolates | No. of isolates with vanA gene | No. of isolates with vanB gene | No. of health care-associated isolates | No. of community-associated isolates |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | 203 | 15-1-1-1-1-20-1 | Efm1 (77), Efm2 (48), Efm6 (15), Efm12 (4), Efm14 (3), Efm15 (3), Efm16 (3), Efm17 (2), Efm35 (1), Efm41 (1), Efm50 (1), Efm69 (1) | 159 (46.6) | 79 | 153 | 6 | |
| 17 | 1-1-1-1-1-1-1 | Efm5 (26), Efm9 (4), Efm10 (4), Efm11 (4), Efm18 (2), Efm19 (2), Efm36 (1), Efm46 (1), Efm49 (1), Efm54 (1), Efm57 (1) | 47 (13.8) | 4 | 47 | ||||
| 341 | 15-5-1-1-1-1-1 | Efm3 (37), Efm32 (1) | 38 (11.1) | 1 | 33 | 38 | |||
| 555 | 4-1-1-1-1-1-1 | Efm4 (34) | 34 (10.0) | 2 | 30 | 4 | |||
| 252 | 1-5-1-1-1-1-1 | Efm7 (10), Efm38 (1) | 11 (3.2) | 1 | 11 | ||||
| 80 | 9-1-1-1-12-1-1 | Efm8 (5), Efm28 (1) | 6 (1.8) | 1 | 2 | 6 | |||
| 202 | 1-1-1-1-1-7-1 | Efm48 (1) | 1 (0.3) | 1 | |||||
| 117 | 9-1-1-1-1-1-1 | Efm72 (1) | 1 (0.3) | 1 | |||||
| 233 | 1-1-1-1-1-20-1 | Efm37 (1) | 1 (0.3) | 1 | |||||
| UD | 414 | 15-5-1-1-1-20-1 | Efm23 (1), Efm64 (1) | 2 (0.6) | 2 | 1 | 1 | ||
| UD | 192 | 15-1-1-1-1-7-1 | Efm24 (1) | 1 (0.3) | 1 | ||||
| UD | 266 | 15-1-6-6-1-7-1 | Efm20 (2) | 2 (0.6) | 2 | ||||
| UD | 869 | 15-1-1-6-1-7-1 | Efm55 (1) | 1 (0.3) | 1 | ||||
| 2 | 22 | 22 | 2-3-1-2-1-1-1 | Efm13 (3), Efm40 (1) | 4 (1.2) | 4 | |||
| 682 | 3-3-2-2-1-1-1 | Efm29 (1) | 1 (0.3) | 1 | |||||
| 533 | 2-3-1-14-1-1-1 | Efm31 (1) | 1 (0.3) | 1 | |||||
| 32 | 3-3-1-2-1-1-1 | Efm63 (1) | 1 (0.3) | 1 | |||||
| 21 | 9-3-1-2-1-1-1 | Efm71 (1) | 1 (0.3) | 1 | |||||
| 3 | 178 | 361 | 13-8-8-8-6-51-6 | Efm26 (1) | 1 (0.3) | 1 | |||
| 94 | 13-8-8-8-6-10-6 | Efm30 (1), Efm61 (1) | 2 (0.6) | 1 | 1 | ||||
| 604 | 13-8-8-23-6-27-6 | Efm60 (1) | 1 (0.3) | 1 | |||||
| 60 | 13-8-8-8-11-10-6 | Efm62 (1) | 1 (0.3) | 1 | |||||
| 178 | 13-8-8-8-6-27-6 | Efm67 (1) | 1 (0.3) | 1 | |||||
| 4 | UDb | 18 | 7-1-1-1-5-1-1 | Efm21 (1), Efm22 (1), Efm45 (1) | 3 (0.9) | 3 | |||
| UD | 273 | 7-3-1-1-5-7-1 | Efm53 (1) | 1 (0.3) | 1 | ||||
| UD | 262 | 7-1-1-1-5-7-1 | Efm51 (1) | 1 (0.3) | 1 | ||||
| 5 | UD | 674 | 25-8-14-22-10-73-6 | Efm47 (1) | 1 (0.3) | 1 | |||
| UD | 648 | 25-8-14-22-10-71-6 | Efm68 (1) | 1 (0.3) | 1 | ||||
| Sa | S | 19 | 7-1-1-1-1-11-1 | Efm27 (1) | 1 (0.3) | 1 | |||
| S | S | 54 | 2-9-6-6-1-11-1 | Efm25 (1) | 1 (0.3) | 1 | |||
| S | S | 264 | 5-4-6-6-2-1-8 | Efm33 (1) | 1 (0.3) | 1 | |||
| S | S | 870 | 25-8-14-22-10-19-11 | Efm34 (1) | 1 (0.3) | 1 | |||
| S | S | 583 | 5-18-14-22-8-26-6 | Efm39 (1) | 1 (0.3) | 1 | |||
| S | S | 867 | 3-7-3-73-1-1-1 | Efm42 (1) | 1 (0.3) | 1 | |||
| S | S | 639 | 2-40-12-3-1-1-1 | Efm43 (1) | 1 (0.3) | 1 | |||
| S | S | 798 | 5-13-18-17-8-19-6 | Efm44 (1) | 1 (0.3) | 1 | |||
| S | S | 868 | 4-5-1-6-1-92-1 | Efm52 (1) | 1 (0.3) | 1 | |||
| S | S | 863 | 4-59-1-1-1-20-1 | Efm58 (1) | 1 (0.3) | 1 | 1 | ||
| S | S | 47 | 9-2-1-11-1-14-5 | Efm59 (1) | 1 (0.3) | 1 | |||
| S | S | 873 | 5-4-1-3-1-1-5 | Efm65 (1) | 1 (0.3) | 1 | |||
| S | S | 871 | 36-21-9-17-10-59-6 | Efm66 (1) | 1 (0.3) | 1 | |||
| S | S | 875 | 15-1-1-22-1-20-6 | Efm70 (1) | 1 (0.3) | 1 | |||
| S | S | 866 | 13-8-58-72-6-27-6 | Efm73 (1) | 1 (0.3) | 1 | |||
| NTc | Efm56 (1) | 1 (0.3) | 1 |
UD, undefined.
S, singleton.
NT, nontypeable using recommended Enterococcus faecium MLST primers.
FIG 5.
eBURST-generated population snapshot of Enterococcus faecium sequence types (STs) isolated in the 2011 Australian Enterococcus Sepsis Outcome Program (AESOP). Each ST is represented by a black dot. The numbers refer to a particular sequence type (ST). The size of each dot reflects the number of isolates within a ST. The ancestral ST of a clonal complex is represented by a blue dot. The yellow-colored dot (ST203) is considered a subgroup cofounder.
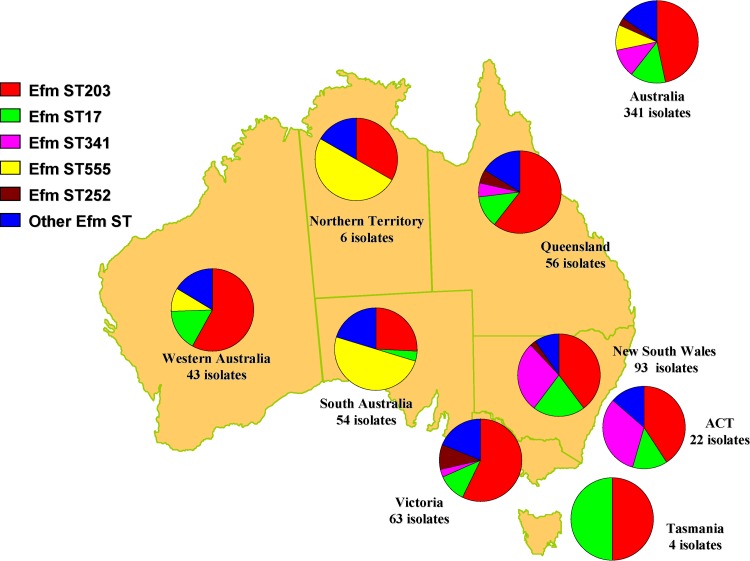
Overall, 304 (89.1%) E. faecium isolates were grouped into eBURST group 1. Five STs, all found in CC17, had more than 10 isolates (Fig. 6). The two major STs, ST203 (159 isolates) and ST17 (47 isolates), were isolated across Australia. ST341 (38 isolates) and ST252 (11 isolates) were isolated only in the eastern regions of Australia, and ST555 (34 isolates) was isolated in the western and central regions.
FIG 6.
Distribution and proportion of Enterococcus faecium (Efm) multilocus sequence types (ST) across Australia.
Apart from ST863 (pulsotype Efm58), isolates harboring a vanA or vanB gene were located in eBURST group 1. However, ST863, a ST203dlv, was not included in eBURST group 1 because no linking slv was identified. van genes were identified in the five major STs and in ST80 and ST414. Although only 8.5% (4/47) of ST17 isolates harbored vanB, vanB genes were identified in 50.6% (79/153) of ST203 isolates, including seven of the 12 ST203 pulsotypes. All 48 of the ST203 Efm2 isolates were vanB positive.
Overall, 94.4% of E. faecium isolates were health care associated. Compared to non-group 1 E. faecium bacteremia, a significantly higher percentage of cases of group 1 E. faecium bacteremia were health care associated, 78.4% versus 96.4% (P < 0.0001).
DISCUSSION
Similar to the situation in the United States (25) and in Europe (26), in Australia enterococcal bacteremia, and notably bacteremia caused by multidrug-resistant E. faecium, has become a significant problem. In the AESOP 2011 study, approximately one in three cases of enterococcal bacteremia was due to E. faecium, of which 90.4% were ampicillin resistant and 36.5% were vancomycin nonsusceptible. However, unlike Europe and the United States, where vancomycin resistance in E. faecium is predominately due to the acquisition of the vanA operon, almost all E. faecium blood culture isolates in Australia harboring van genes carried the vanB operon (98.4%). Twenty (16.1%) of the 124 vanB E. faecium isolates had vancomycin MICs at or below the CLSI susceptible breakpoint (≤4 mg/liter) and would not have been identified using routine phenotypic antimicrobial susceptibility methods.
Similar to the study performed by Pinholt and colleagues in Denmark (2), in our study the majority of bacteremia cases were health care associated, and approximately one-third of episodes were polymicrobial.
With the use of PFGE, both enterococcal species were shown to be very polyclonal, confirming the enormous plasticity of the enterococcal genome, which has been demonstrated to acquire genetic elements that can account for up to 25% of the genome (27). Although 126 E. faecalis PFGE pulsotypes were identified, 47.6% of the isolates belonged to either Efs1 or Efs2. Isolated across Australia, these two strains have had success in extending their geographical range, which may be due to the acquisition of mobile genetic elements and single nucleotide polymorphisms that favor spread and persistence. However, this hypothesis will need to be confirmed by whole-genome sequencing. Both ampicillin and vancomycin resistance in E. faecalis were uncommon. Only three Efs2 isolates harbored van genes, and all were vanB.
The relative clinical importance of E. faecium bacteremia has increased with the emergence of resistance to antimicrobials such as ampicillin and vancomycin (28, 29). In at least one Australian institution, VRE bacteremia has reportedly surpassed methicillin-resistant Staphylococcus aureus (MRSA) bacteremia (18). Globally, hospital-derived E. faecium isolates have been shown to be part of a single clonal lineage, designated CC17 after its presumed founder ST17, that has successfully adapted to hospital environments (30). CC17 is characteristically ampicillin and quinolone resistant, and subsequent acquisition of vanA- or vanB-containing transposons by horizontal gene transfer in CC17 clones has resulted in VRE with pandemic potential. In the AESOP 2011 study, the five major blood culture E faecium clones were all grouped into CC17. In addition, seven of the eight clones harboring the vanA or vanB genes were also CC17 clones. The eighth clone (ST863), although not a CC17 clone, was an ST203dlv.
ST203, a dlv of ST17, was first described in Australia in 2010 as the cause of a sustained outbreak of vanB E. faecium bacteremia in a hospital located on the eastern seaboard of Australia (18). Subsequent to this report, ST203 has been identified as a major vanB E. faecium clone in other Australian regions (31), Outside of Australia, ST203 has also been reported in many Asian and European countries (http://efaecium.mlst.net/), although isolates from these countries almost always possess the vanA gene. In our study, 46.6% of E. faecium isolates were characterized as ST203, and these were isolated Australia wide. Approximately 50% of ST203 isolates carried the vanB operon, while none harbored vanA genes. Although 12 PFGE ST203 pulsotypes were identified, almost 80% of isolates were classified as either pulsotype Efm1 or pulsotype Efm2. Why some clones within CC17 dominate over other clones, and why within a clone some pulsotypes dominate over other pulsotypes, requires further genetic analysis.
ST17, the presumed founder of CC17, is thought to have successfully adapted to the hospital environment by the cumulative acquisition of resistance (ampicillin and vancomycin) and genes (espEfm, hylEfm, and fms) that result in a putative selective advantage (32). The acquisition of these genes has been followed by the genetic diversification of ST17, resulting in many slvs and dlvs that presumably have acquired or lost genetic elements. This appears to have facilitated clones in gaining further selective advantages in hospital environments.
Importantly, the successful transmission of a genetically adapted vancomycin-resistant E. faecium clone in a hospital may not be attributable solely to suboptimal infection control practices. Recently, de novo generation of vancomycin-resistant vanB E. faecium has been demonstrated to occur within patients, presumably occurring in the normal colonic flora by acquisition of the vanB operon from anaerobic flora (33). The acquisition of a selective advantage by a VRE clone not necessarily lead to the long-term survival of that clone. In 2004, we described a large single-strain outbreak of vanB E. faecium across several hospitals in Western Australia (17). Characterized as CC17 ST173 (34), once eradicated from the hospital environment this strain has not subsequently been isolated in Western Australia, nor was it detected in the AESOP 2011 bacteremia study. Furthermore, failure to isolate ST173 during long-term follow-up screening of previously colonized patients suggests the strain no longer had a selective advantage once outside the hospital environment. Whole-genome sequencing of isolates from the AESOP 2011 study will be an important resource in our efforts to understand this species and to develop novel infection control strategies to prevent the emergence of this hospital superbug.
Our study had a number of limitations. Although achieving national coverage, the participating laboratories service only a minority of the Australian hospitalized population. Further, MIC assays for vancomycin, teicoplanin, and linezolid were performed by a commercial gradient diffusion method, and not the standard reference broth microdilution method.
In conclusion, the AESOP 2011 study has shown that the majority of Australian enterococcal bacteremias are health care associated, and though predominantly caused by E. faecalis, they are frequently caused by ampicillin-resistant vanB E. faecium. Molecular typing characterized over 50% of E. faecalis isolates as two PFGE pulsotypes, and almost 90% of E. faecium isolates as CC17 clones, of which approximately half were ST203. Further studies of the enterococcal genome will contribute to our understanding of the evolution of enterococci in the hospital environment and assist in preventing their nosocomial transmission.
ACKNOWLEDGMENTS
We gratefully acknowledge Hui-leen Tan, Yung Ching Lee, and Lynne Wilson from the Department of Microbiology and Infectious Diseases, PathWest Laboratory Medicine-WA, Royal Perth Hospital; Frances O'Brien and Ka Yan Wong from the Australian Collaborating Centre for Enterococcus and Staphylococcus Species (ACCESS) Typing and Research, School of Biomedical Sciences, Curtin University; and the WA Genome Resource Centre, Department of Clinical Immunology and Biochemical Genetics, Royal Perth Hospital, for the molecular typing of enterococci.
This study was primarily funded by a grant from the Australian Government Department of Health and Ageing.
Members of the AGAR in 2011 were, in the Australian Capital Territory, Peter Collignon and Susan Bradbury (The Canberra Hospital); in New South Wales, Tom Gottlieb and Graham Robertson (Concord Hospital), Miriam Paul and Richard Jones (Douglass Hanly Moir Pathology), James Branley and Donna Barbaro (Nepean Hospital), George Kotsiou and Peter Huntington (Royal North Shore Hospital), Colin MacLeod and Bradley Watson (Royal Prince Alfred Hospital), Iain Gosbell and Annabelle LeCordier (Liverpool Hospital), David Mitchell and Lee Thomas (Westmead Hospital); in the Northern Territory, Jann Hennessy and Rob Baird (Royal Darwin Hospital); in Queensland, Enzo Binotto and Bronwyn Thomsett (Pathology Queensland Cairns Base Hospital), Graeme Nimmo and Narelle George (Pathology Queensland Central Laboratory), Petra Derrington and Sharon Dal-Cin (Pathology Queensland Gold Coast Hospital), Chris Coulter and Sonali Coulter (Pathology Queensland Prince Charles Hospital), Joan Faoagali and Joel Douglas (Pathology Queensland Princess Alexandra Hospital), Jenny Robson and Georgia Peachey (Sullivan Nicolaides Pathology); in South Australia, Kelly Papanoum and Nicholas Wells (SA Pathology [Flinders Medical Centre]), Morgyn Warner and Fleur Manno (SA Pathology [Royal Adelaide Hospital]), John Turnidge and Jan Bell (SA Pathology [Women's and Children's Hospital]); in Tasmania, Mhisti Rele and Kathy Wilcox (Launceston General Hospital), Louise Cooley and Rob Peterson (Royal Hobart Hospital); in Victoria, Denis Spelman and Michael Huysmans (The Alfred Hospital), Benjamin Howden and Peter Ward (Austin Hospital), Tony Korman and Despina Kotsanas (Monash Medical Centre), Suzanne Garland and Gena Gonis (Royal Women's Hospital), Mary Jo Waters and Linda Joyce (St Vincent's Hospital); in Western Australia, David McGechie and Rebecca Wake (PathWest Laboratory Medicine–WA Fremantle Hospital), Barbara Henderson and Ronan Murray (PathWest Laboratory Medicine-WA Queen Elizabeth II Hospital), Keryn Christiansen, Denise Daley, and Geoffrey Coombs (PathWest Laboratory Medicine-WA Royal Perth Hospital), Victoria D'Abrera and Sindy Budalich (St John of God Pathology).
Footnotes
Published ahead of print 3 January 2014
REFERENCES
- 1.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK, National Healthcare Safety Network Team Participating National Healthcare Safety Network Facilities 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011. 10.1086/591861 [DOI] [PubMed] [Google Scholar]
- 2.Pinholt M, Ostergaard C, Arpi M, Bruun NE, Schonheyder HC, Gradel KO, Sogaard M, Knudsen JD, for the Danish Collaborative Bacteraemia Network 2013. Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006–2009: A population-based cohort study. Clin. Microbiol. Infect. 10.1111/1469-0691.12236 [DOI] [PubMed] [Google Scholar]
- 3.Deshpande LM, Fritsche TR, Moet GJ, Biedenbach DJ, Jones RN. 2007. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 58:163–170. 10.1016/j.diagmicrobio.2006.12.022 [DOI] [PubMed] [Google Scholar]
- 4.Murray BE. 1990. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3:46–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonsen GS, Smabrekke L, Monnet DL, Sorensen TL, Moller JK, Kristinsson KG, Lagerqvist-Widh A, Torell E, Digranes A, Harthug S, Sundsfjord A. 2003. Prevalence of resistance to ampicillin, gentamicin and vancomycin in Enterococcus faecalis and Enterococcus faecium isolates from clinical specimens and use of antimicrobials in five Nordic hospitals. J. Antimicrob. Chemother. 51:323–331. 10.1093/jac/dkg052 [DOI] [PubMed] [Google Scholar]
- 6.Treitman AN, Yarnold PR, Warren J, Noskin GA. 2005. Emerging incidence of Enterococcus faecium among hospital isolates (1993 to 2002). J. Clin. Microbiol. 43:462–463. 10.1128/JCM.43.1.462-463.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uttley AH, Collins CH, Naidoo J, George RC. 1988. Vancomycin-resistant enterococci. Lancet i:57–58 [DOI] [PubMed] [Google Scholar]
- 8.Leclercq R, Derlot E, Duval J, Courvalin P. 1988. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 319:157–161. 10.1056/NEJM198807213190307 [DOI] [PubMed] [Google Scholar]
- 9.Frieden TR, Munsiff SS, Low DE, Willey BM, Williams G, Faur Y, Eisner W, Warren S, Kreiswirth B. 1993. Emergence of vancomycin-resistant enterococci in New York City. Lancet 342:76–79. 10.1016/0140-6736(93)91285-T [DOI] [PubMed] [Google Scholar]
- 10.Murray BE. 2000. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 342:710–721. 10.1056/NEJM200003093421007 [DOI] [PubMed] [Google Scholar]
- 11.Bearman GM, Wenzel RP. 2005. Bacteremias: a leading cause of death. Arch. Med. Res. 36:646–659. 10.1016/j.arcmed.2005.02.005 [DOI] [PubMed] [Google Scholar]
- 12.Cetinkaya Y, Falk P, Mayhall CG. 2000. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 13:686–707. 10.1128/CMR.13.4.686-707.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klare I, Heier H, Claus H, Reissbrodt R, Witte W. 1995. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol. Lett. 125:165–171. 10.1111/j.1574-6968.1995.tb07353.x [DOI] [PubMed] [Google Scholar]
- 14.Kamarulzaman A, Tosolini FA, Boquest AL, Geddes JE, Richards MJ. 1995. Vancomycin resistant Enterococcus faecium in a liver transplant recipient. Aust. N. Z. J. Med. 25:560 [Google Scholar]
- 15.Bell J, Turnidge J, Coombs G, O'Brien F. 1998. Emergence and epidemiology of vancomycin-resistant enterococci in Australia. Commun. Dis. Intell. 22:249–252 [PubMed] [Google Scholar]
- 16.Cooper E, Paull A, O'Reilly M. 2002. Characteristics of a large cluster of vancomycin-resistant enterococci in an Australian hospital. Infect. Control Hosp. Epidemiol. 23:151–153. 10.1086/502027 [DOI] [PubMed] [Google Scholar]
- 17.Christiansen KJ, Tibbett PA, Beresford W, Pearman JW, Lee RC, Coombs GW, Kay ID, O'Brien FG, Palladino S, Douglas CR, Montgomery PD, Orrell T, Peterson AM, Kosaras FP, Flexman JP, Heath CH, McCullough CA. 2004. Eradication of a large outbreak of a single strain of vanB vancomycin-resistant Enterococcus faecium at a major Australian teaching hospital. Infect. Control Hosp. Epidemiol. 25:384–390. 10.1086/502410 [DOI] [PubMed] [Google Scholar]
- 18.Johnson PD, Ballard SA, Grabsch EA, Stinear TP, Seemann T, Young HL, Grayson ML, Howden BP. 2010. A sustained hospital outbreak of vancomycin-resistant Enterococcus faecium bacteremia due to emergence of vanB E. faecium sequence type 203. J. Infect. Dis. 202:1278–1286. 10.1086/656319 [DOI] [PubMed] [Google Scholar]
- 19.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance (ABCs) Investigators MRSA 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 20.Klevens RM, Morrison MA, Fridkin SK, Reingold A, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Fosheim G, McDougal LK, Tenover FC. 2006. Community-associated methicillin-resistant Staphylococcus aureus and healthcare risk factors. Emerg. Infect. Dis. 12:1991–1993. 10.3201/eid1212.060505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. CLSI M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 22.Kulski JK, Wilson RD, Bending R, Grubb W. 1998. Antibiotic resistance and genomic analysis of enterococci in an intensive care unit and general wards. Pathol. 30:68–72. 10.1080/00313029800169705 [DOI] [PubMed] [Google Scholar]
- 23.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homan WL, Tribe D, Poznanski S, Li M, Hogg G, Spalburg E, Van Embden JD, Willems RJ. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963–1971. 10.1128/JCM.40.6.1963-1971.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309–317. 10.1086/421946 [DOI] [PubMed] [Google Scholar]
- 26.de Kraker ME, Jarlier V, Monen JC, Heuer OE, van de Sande N, Grundmann H. 2013. The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clin. Microbiol. Infect. 19:860–868. 10.1111/1469-0691.12028 [DOI] [PubMed] [Google Scholar]
- 27.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 10:266–278. 10.1038/nrmicro2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwen PC, Kelly DM, Linder J, Hinrichs SH, Dominguez EA, Rupp ME, Patil KD. 1997. Change in prevalence and antibiotic resistance of Enterococcus species isolated from blood cultures over an 8-year period. Antimicrob. Agents Chemother. 41:494–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheah AL, Spelman T, Liew D, Peel T, Howden BP, Spelman D, Grayson ML, Nation RL, Kong DC. 2013. Enterococcal bacteraemia: factors influencing mortality, length of stay and costs of hospitalization. Clin. Microbiol. Infect. 19:E181–E189. 10.1111/1469-0691.12132 [DOI] [PubMed] [Google Scholar]
- 30.Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, Grundmann H, Bonten MJ. 2005. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg. Infect. Dis. 11:821–828. 10.3201/1106.041204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamolvit W, Sidjabat HE, Nimmo GR, Anuj SN, Bergh H, Richardson LJ, Paterson DL. 2013. Predominance of VREfm ST203 subgroup in Queensland. Pathol. 45:99. 10.1097/PAT.0b013e32835b68d2 [DOI] [PubMed] [Google Scholar]
- 32.Top J, Willems R, Bonten M. 2008. Emergence of CC17 Enterococcus faecium: from commensal to hospital-adapted pathogen. FEMS immunol Med. Microbiol. 52:297–308. 10.1111/j.1574-695X.2008.00383.x [DOI] [PubMed] [Google Scholar]
- 33.Howden BP, Holt KE, Lam MM, Seemann T, Ballard S, Coombs GW, Tong SY, Grayson ML, Johnson PD, Stinear TP. 2013. Genomic insights to control the emergence of vancomycin-resistant enterococci. mBio 4:eoo412–13. 10.1128/mBio.00412-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freitas AR, Tedim AP, Novais C, Ruiz-Garbajosa P, Werner G, Laverde-Gomez JA, Canton R, Peixe L, Baquero F, Coque TM. 2010. Global spread of the hyl(Efm) colonization-virulence gene in megaplasmids of the Enterococcus faecium CC17 polyclonal subcluster. Antimicrob. Agents Chemother. 54:2660–2665. 10.1128/AAC.00134-10 [DOI] [PMC free article] [PubMed] [Google Scholar]