Abstract
Transport of several xenobiotics including pharmacological agents into or out of the central nervous system (CNS) involves the expression of ATP-dependent, membrane-bound efflux transport proteins such as P-glycoprotein (P-gp) at the blood-brain barrier (BBB). Previous studies have documented gene and protein expression of P-gp in brain microvessel endothelial cells. However, the exact localization of P-gp, particularly at the abluminal side of the BBB, remains controversial. In the present study we examined the cellular/subcellular distribution of P-gp in situ in rat and human brain tissues using immunogold cytochemistry at the electron microscope level. P-gp localizes to both the luminal and abluminal membranes of capillary endothelial cells as well as to adjacent pericytes and astrocytes. Subcellulary, P-gp is distributed along the nuclear envelope, in caveolae, cytoplasmic vesicles, Golgi complex, and rough endoplasmic reticulum (RER). These results provide evidence for the expression of P-gp in human and rodent brain capillary along their plasma membranes as well as at sites of protein synthesis, glycosylation, and membrane trafficking. In addition, its presence at the luminal and abluminal poles of the BBB, including pericytes and astrocyte plasma membranes, suggests that this glycoprotein may regulate drug transport processes in the entire CNS BBB at both the cellular and subcellular level.
Keywords: P-glycoprotein, brain capillary endothelial cell, immunogold cytochemistry, luminal plasma membrane, abluminal plasma membrane, blood-brain barrier
The primary interfaces between brain and peripheral circulation are the blood-brain barrier (BBB) and the blood-cerebrospinal fluid (CSF) barrier (Kusuhara and Sugiyama 2001; Fromm 2004). Because the surface area of the BBB is ~5000 times greater than that of the blood-CSF barrier, the BBB is considered to be the main route for the traffic of endogenous substances as well as xenobiotics into and out of the brain (Kusuhara and Sugiyama 2001). Physicochemical properties of these compounds (i.e., lipophilicity, molecular size) as well as BBB characteristics determine the extent of passive translocation across the capillary endothelial cells that form the barrier. However, permeation of several lipophilic drugs (i.e., doxorubicin, vincristine) across the BBB is much lower than expected (Kusuhara and Sugiyama 2001; Fromm 2004). One possible mechanism for this observation is the functional expression of ATP-dependent membrane-bound drug transporters such as P-glycoprotein (P-gp) at the BBB. P-gp, a 170-kDa membrane-associated glycoprotein and product of the multidrug resistance (MDR) genes, functions as an energy-dependent efflux pump that can transport a vast array of structurally unrelated compounds (DiDiodato and Sharom 1997). Numerous studies, including ours, have reported the molecular (i.e., gene and protein) expression of P-gp in brain microvessel endothelial cells (Tatsuta et al. 1992; Drion et al. 1997; Regina et al. 1998; Bendayan et al. 2002; Virgintino et al. 2002). Furthermore, in vivo studies have demonstrated that brain concentrations of several pharmacological agents are enhanced in mdr1 knockout mice, indicating that P-gp may restrict the brain entry of many xenobiotics (Schinkel et al. 1997; Kim et al. 1998).
Although the molecular expression of P-gp in brain microvessel endothelial cells is well documented, its exact localization at the BBB remains controversial. Several studies carried out in vitro (Tatsuta et al. 1992; Beaulieu et al. 1997; Seetharaman et al. 1998) have reported luminal localization of P-gp in cultured brain endothelial cells (i.e., mouse, rat, human) and isolated brain capillaries (i.e., rat, pig, human) (Biegel et al. 1995; Seetharaman et al. 1998; Miller et al. 2000). This was confirmed by immunoelectron microscopy in murine brain endothelial cells grown on porous membrane filters (Tatsuta et al. 1992) and in human brain endothelial cells (Biegel et al. 1995). Furthermore, using isolated luminal membranes from rat brain endothelial cells, Beaulieu et al. (1997) showed strong P-gp expression when compared with whole capillaries (17-fold) and whole cell membranes (400- to 500-fold); however, isolated abluminal membranes were not assessed in that study. On the other hand, some reports indicate that P-gp is also expressed at the abluminal membrane of the brain capillary endothelium. initial studies by Pardridge et al. reported that MRK16, a P-gp monoclonal antibody that recognizes an extracellular epitope, did not bind to the luminal membrane of brain endothelial cells but generated a signal at the abluminal side of the BBB on astrocyte foot processes (Pardridge et al. 1997). Subsequent findings obtained with unfixed brain capillaries from autopsy human brain specimens colocalized P-gp with glial fibrillary acidic protein (GFAP), a well-characterized astrocyte marker (Golden and Pardridge 1999). In addition, confocal microscopy studies on fresh-frozen brain tissue from healthy rhesus monkey demonstrated the colocalization of P-gp and GFAP on astrocytes (Schlachetzki and Pardridge 2003). Along this line, our previous work demonstrated the functional expression and cellular/subcellular localization of P-gp in primary cultures of rat astrocytes and in an immortalized rat astrocyte cell line (CTX TNA2) (Ronaldson et al. 2004).
The goal of the present study was to examine the in situ cellular/subcellular localization of P-gp in rat and human whole BBB using the high-resolution quantitative immunogold cytochemical approach at the electron microscope level. These results demonstrate, for the first time on intact tissue, the distribution of P-gp not only at the level of the luminal and abluminal brain capillary endothelial plasma membranes but also the preferential association with caveolae and cytoplasmic vesicle as well as at the level of the rough endoplasmic reticulum (RER), nuclear envelope, and Golgi complex. Furthermore, this glycoprotein is present at the plasma membrane of pericytes and astrocytes, which indicates participation of the entire BBB in restricting brain permeability of several xenobiotics including pharmacological agents.
Materials and Methods
Materials
Murine monoclonal P-gp antibodies C219 and MRK16 were purchased from ID Labs (London, ON, Canada) and Kamiya Biomedical Co. (Seattle, WA), respectively. Rabbit polyclonal GFAP antibody was also obtained from Kamiya Biomedical Co. Colloidal gold-conjugated anti-mouse IgG (5 and 10 nm) and an anti-rabbit IgG-gold complex (10 nm) were purchased from Sigma-Aldrich (Oakville, ON, Canada) and British Biocell International (Cardiff, UK), respectively. Purified P-gp for control experiments was kindly provided by Dr. Frances Sharom (University of Guelph, Guelph, ON, Canada).
Tissue Preparation
All animal studies were carried out according to guidelines established by the Canadian Council on Animal Care. Male Sprague Dawley rats were anesthetized by an IP injection of urethane (1 g/kg). Small tissue fragments (~1 mm3) were sampled from the brain frontal cortex and immediately fixed by immersion with 1% glutaraldehyde in phosphate buffer (0.1 M, pH 7.2) for 1 hr at 4C. Tissues were then washed with phosphate buffer, dehydrated in graded methanol, and embedded in Lowicryl-K4M at −30C. Previous studies by our laboratory have shown that this tissue preparation protocol is appropriate for immunolabeling of P-gp (Lee et al. 2001; Bendayan et al. 2002; Ronaldson et al. 2004). Ultrathin sections of Lowicryl-embedded material were cut and mounted on Parlodion-carbon-coated nickel grids and processed for immunocytochemistry (Bendayan 1995).
Ethics approval for the collection of postsurgical human brain tissue was obtained from the Research Ethics Boards of the University Health Network and the University of Toronto. Human brain tissue was collected from the Departments of Neuropathology and Neurosurgery, Toronto Western Hospital, University Health Network, from consenting patients undergoing temporal lobectomies for intractable epilepsy. This procedure provides normal tissue at the resection margin and, in our study, all collected tissue samples were examined by a neuropathologist and confirmed to be normal. Immediately upon sampling, tissues were fixed in 1% glutaraldehyde and then processed for embedding in Lowicryl as described above.
Immunocytochemistry Studies
For ultrastructural detection of P-gp, thin tissue sections mounted on grids were first treated with a saturated solution of sodium metaperiodate for 10 min at room temperature. Upon rinsing with water, grids were then incubated with 1% ovalbumin in 0.01 M PBS for 30 min at room temperature to reduce nonspecific background labeling. The grids were then incubated with one of the specific monoclonal P-gp antibodies (C219 or MRK16) at 1:10 dilution overnight at 4C. The monoclonal C219 antibody recognizes a conserved intracellular epitope (VQEALD) on all human (i.e., MDR1, MDR2) and rodent (i.e., mdr1a, mdr1b, mdr2) isoforms of P-gp (Okochi et al. 1997). The monoclonal MRK16 antibody recognizes a conserved extracellular epitope on all drug-transporting isoforms (i.e., MDR1, mdr1a, mdr1b) of human and rat P-gp protein (Okochi et al. 1997). Grids were washed with PBS, treated again with 1% ovalbumin for 10 min, and incubated with the appropriate immunogold complex for 30 min at room temperature. Upon rinsing with PBS and water, grids were dried and stained with uranyl acetate and examined with a Philips 410 electron microscope (FEI System Canada; St-Laurent, Quebec, Canada). Specificity controls were carried out by incubating the grids with only the IgG-gold complex and omitting the specific antibody, as well as by incubating the grids with the antibody adsorbed with the purified antigen prior to incubation with the IgG-gold complex.
To firmly establish the identity of the astrocytes, double-labeling experiments (Bendayan 1995) were carried out with the anti-GFAP antibody (1/10 dilution) followed by the anti-rabbit IgG-gold complex (10 nm); a second series of incubations were then performed on the same grid with the MRK16 anti-P-gp antibody followed by the anti-mouse IgG complex (5 nm). Upon rinsing and staining, examination was carried out with the Philips electron microscope.
Density of immunocytochemical labeling was determined by morphometry using a Clemex Vision analysis unit (Montreal, Canada). Gold particles were considered associated with a particular site when they were within 10-15 nm of a cellular membrane (i.e., plasma membrane, nuclear envelope, RER, Golgi complex). Labeling was evaluated along the plasma membrane of capillary endothelial cells and associated caveolae. Length of membranes was measured, and numbers of gold particles aligned along these membranes were counted. We have also evaluated the percentage of total labeling present over the endothelial cells including plasma membrane, membrane-bound vesicles, mitochondria, nuclear envelope, RER, and Golgi apparatus as well as those particles free over the cytosol. In addition, numbers of caveolae lining the luminal and abluminal endothelial plasma membranes were counted. Images were recorded at X16,900 and enlarged to a final magnification of X40,000. Results are expressed in number of gold particles/μm. Morphometric evaluations were not carried out on human brain tissue due to the small number of available samples (i.e., n=2).
Statistical Analysis
Quantitative immunocytochemistry data are presented as mean ± SD from five individual animals. Fifty different fields originating from at least three tissue sections were analyzed for each animal. To determine the significance of these data, Student's t-test was used for unpaired experimental values; p<0.05 was considered statistically significant.
Results
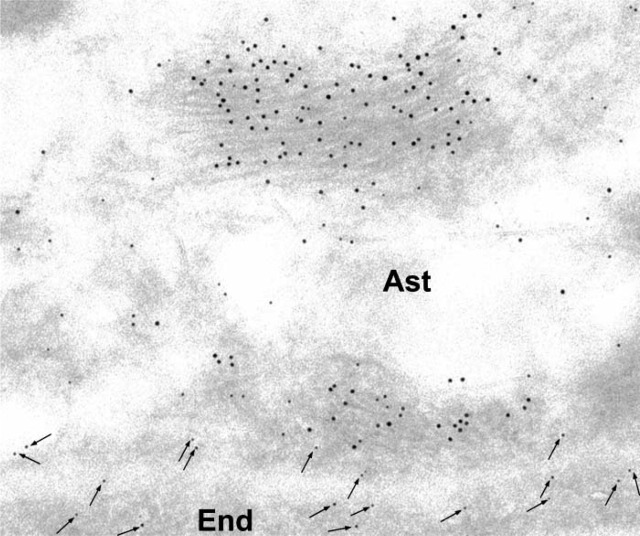
Electron micrographs (Figure 1) illustrate cross-sections of brain-blood capillaries surrounded by astrocytes and pericytes. Using the immunogold technique with the monoclonal anti-P-gp antibodies, gold particles are observed along the plasma membrane of the endothelial cells, pericytes, and astrocytes (Figure 1). Labeling was easily identified at both the luminal and abluminal plasma membranes of the capillary endothelial cells. Quantitative analysis demonstrates that labeling of the plasma membrane is 1.4-fold higher at the abluminal side of the endothelium than at the luminal surface (Table 1). Smooth membrane invaginations (i.e., caveolae) at the luminal and abluminal plasma membranes of the endothelial cells are also labeled (Figure 1B). The total number of caveolae is 1.7-fold higher (p<0.001) at the abluminal membrane than at the luminal one (Table 1) confirming previous reports (Bouchard et al. 2002). The number of caveolae displaying P-gp immunolabeling is almost 2-fold (p<0.001) higher at the abluminal membrane than at the luminal one (Table 1). P-gp antigenic sites are also observed in the RER, Golgi complex, and along the nuclear envelope of endothelial cells (Figure 2). Approximately 40% of P-gp immunolabeling is present along the plasma membrane, 20% along the nuclear envelope, 16% on vesicles (both cytoplasmic and membrane bound), 10% on the Golgi complex, and 9% on RER. Labeling over mitochondria and over cytosol, which could be considered as background labeling, represents <5% (Table 2). Immunocytochemical analysis using the MRK16 antibody resulted in a labeling pattern qualitatively identical to the results obtained with the C219 antibody. Control experiments carried out to assess labeling specificity show a negligible number of gold particles randomly distributed when the primary antibody was omitted or when it was adsorbed with purified P-gp (data not shown).
Figure 1.
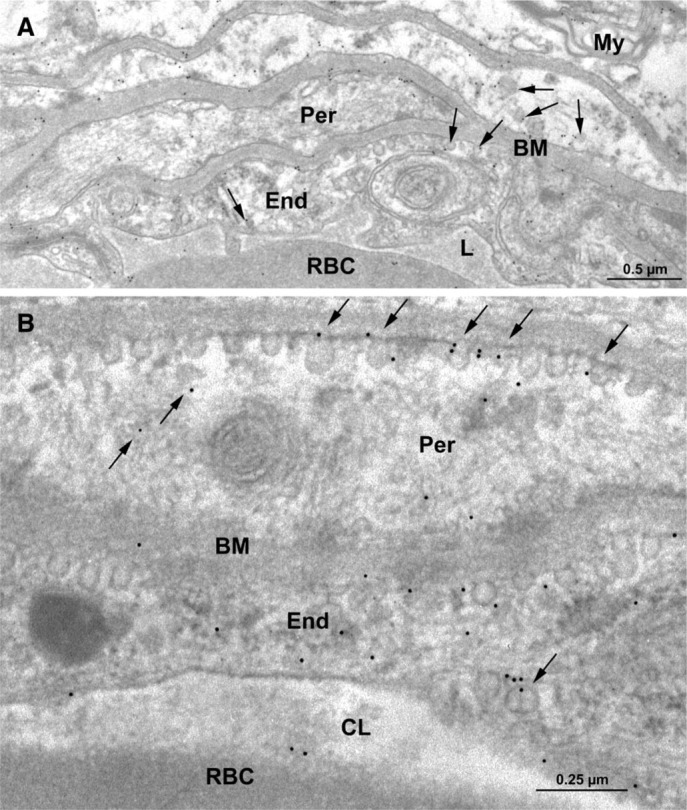
Electron microscopy of rat brain tissue. Immunocytochemical detection of P-gp using the monoclonal anti-P-gp antibody C219 (1:10 dilution). Labeling by gold particles is found to be associated with the plasma membrane and smooth membrane invaginations (arrows) of the capillary endothelial cells (End) (A). Labeling is also present along the plasma membrane of a pericyte (Per) and in some myelinated fibers (My) (A). At higher magnification, labeling is detected along the plasma membrane and clearly associated with smooth membrane invaginations (arrows) in an endothelial cell and pericyte (B). BM, basement membrane; CL, capillary lumen; RBC, red blood cell.
Table 1.
Quantitative immunocytochemical analysis of P-glycoprotein (P-gp) in capillary endothelial cells from rat brain tissue fixed in situ a
|
| |||
|---|---|---|---|
| Luminal membrane | Abluminal membrane | p value ∗ | |
|
| |||
| Total P-gp antigenic sites (gold particles/μm membrane) b 0.85 ± 0.03 | 1.17 ± 0.04 | p<0.001 | |
| Total smooth membrane invaginations, i.e., caveolae (caveolae/μm membrane) b | 1.42 ± 0.29 | 2.46 ± 0.35 | p<0.001 |
| Caveolae containing P-gp antigenic sites | 8.3% (330 caveolae examined) | 16% (300 caveolae examined) | N/A c |
|
| |||
n = five animals.
For quantitation, 1500-μm plasma membrane was evaluated.
N/A, not applicable.
Statistical significance evaluated by Student's t-test.
Figure 2.
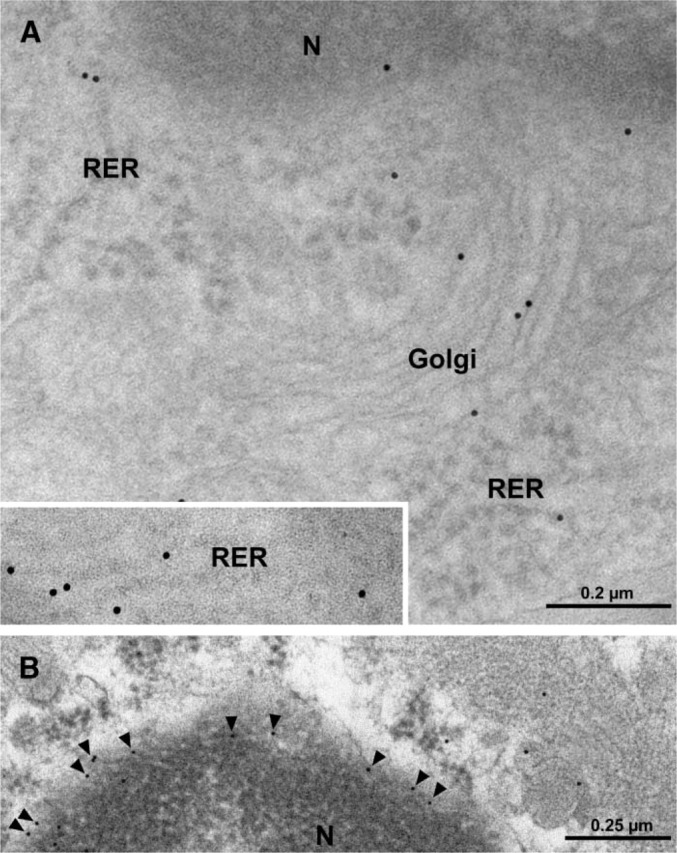
Electron micrographs of capillary endothelial cells from rat brain tissue. Immunolabeling for P-gp by gold particles is observed in the rough endoplasmic reticulum (RER; inset and A) and Golgi complex (A) as well as along the nuclear envelope (arrowheads in B). N, nucleus.
Table 2.
Distribution of P-gp antigenic sites in rat brain capillary endothelial cells fixed in situ ∗
|
| |
|---|---|
| Cellular structure | P-gp antigenic sites (%) |
|
| |
| Plasma membrane | 38.75 ± 1.57 ∗∗ |
| Nuclear envelope | 19.16 ± 2.45 ∗∗ |
| Vesicles (cytoplasmic/membrane) | 16.33 ± 1.16 ∗∗ |
| Golgi complex | 9.5 ± 1.80 ∗∗ |
| Rough endoplasmic reticulum | 8.5 ± 0.83 ∗∗ |
| Cytosol | 4.42 ± 0.45 |
| Mitochondria | 3.28 ± 0.50 |
|
| |
1895 gold particles evaluated over 75 photographic fields.
Labeling significantly greater than background (i.e., cytosol and mitochondrial labeling).
p<0.001.
To identify the astrocytic nature of the cells, double-labeling experiments were carried out on rat brain sections using an anti-GFAP and anti-P-gp antibodies. GFAP is selectively present in astrocytes, and its expression is widely used as a cellular marker. Results from the double labeling allowed us to establish that pericapillary astrocytes do express P-gp. Indeed, labeling for GFAP is intense over the bundles of glial intermediate filaments (Figure 3) identifying the cells as astrocytes. The same cells display specific labeling for P-gp at their plasma membrane (Figure 3).
Figure 3.
Rat brain tissue. Double-labeling experiment. Glial fibrillary acidic protein (GFAP) and P-gp were simultaneously revealed on the same tissue section using specific corresponding antibodies (anti-GFAP and MRK-16) and immunogold complexes of 5 and 10 nm. GFAP labeling (10 nm) is concentrated over the bundles of neurofilaments. P-gp labeling (5-nm arrows) is associated with the plasma membrane of the astrocyte (Ast). Labeling for P-gp (arrows) is also present in endothelial cells (End).
The capillary endothelium in human brain tissue lined by pericytes and astrocytes displays immunocytochemical labelings similar to rat tissue (i.e., along the luminal and abluminal plasma membranes as well as in plasmalemmal vesicles). In addition, discrete immunogold labeling was observed along the plasma membranes of the surrounding pericytes and astrocytes (Figure 4).
Figure 4.
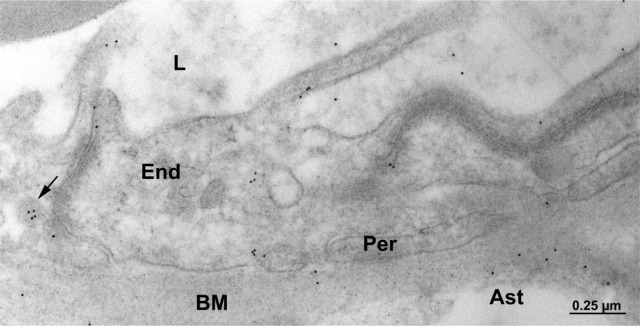
Electron micrograph of human brain tissue. Immunocytochemical detection of P-gp. Labeling by gold particles is present along the plasma membrane of the brain capillary endothelial (End) cells as well as the pericyte (Per) and astrocyte (Ast). Labeling was also detected in a cytoplasmic vesicle of an endothelial cell (arrow). BM, basement membrane; L, capillary lumen.
Discussion
Previous studies have reported that mdr1/MDR1 mRNA and corresponding protein product, P-gp, are expressed in rat and human brain capillary endothelium (Beaulieu et al. 1997; Regina et al. 1998; Bendayan et al. 2002). However, the polarized localization of the protein along the plasma membranes of the endothelial cells remains controversial. Although studies on isolated brain microvessels (Seetharaman et al. 1998; Miller et al. 2000) and in human brain tissue (Virgintino et al. 2002) have localized P-gp primarily to the endothelial luminal surface, a few reports have suggested that P-gp is present at the endothelial abluminal membrane and at the end-foot astrocyte processes lining the capillary endothelium (Pardridge et al. 1997; Golden and Pardridge 1999; Schlachetzki and Pardridge 2003). Applying confocal microscopy and using antibodies to P-gp and to the facilitated glucose transporter type-1 (GLUT1), Schlachetzki and Pardridge (2003) observed an almost identical distribution pattern of P-gp and GLUT1 at the capillary endothelium. Because the expression of GLUT1 is 4-fold greater at the abluminal membrane than at the luminal one (Farrell and Pardridge 1991), these data indirectly imply greater proportions of P-gp localized at the abluminal surface of brain capillary endothelial cells.
In the present study we have examined the BBB localization of P-gp using high-resolution quantitative immunogold cytochemistry at the electron microscope level in conjunction with monoclonal P-gp antibodies that recognize highly conserved intra- and extracellular epitopes on all P-gp isoforms (Georges et al. 1990). The results have unambiguously demonstrated the presence of P-gp at both the luminal and abluminal plasma membranes of brain capillary endothelial cells from rat and human brain tissue. Quantitative analysis of the immunolabeling shows ~1.4-fold greater density of P-gp antigenic sites at the abluminal membrane (Table 1) when compared with the luminal one, indicating asymmetrical distribution of P-gp along the endothelial cell plasma membranes. Expression of the glycoprotein at both surfaces of the capillary endothelium implies that drug efflux processes may be involved in the export of substrates not only toward the blood capillary interface but also into the brain interstitial fluid at the abluminal side. Interestingly, the multidrug resistance-associated protein 4 (MRP4), which has been shown to export cyclic nucleotides (i.e., cAMP, cGMP), has also been localized to the abluminal plasma membrane in bovine brain microvessel endothelial cells (Zhang et al. 2004). In addition, our data also reveal the presence of P-gp along the plasma membrane of both astrocytes and pericytes in rat and human brain tissues. Identification of astrocytes on rat brain tissue was further established by revealing the GFAP-positive intermediate filaments. This is supported by previous studies from our group (Ronaldson et al. 2004) and others (Golden and Pardridge 1999; Ballerini et al. 2002), which have reported the in vitro expression of P-gp in astrocytes isolated from mammalian brain tissue. Recently, MDR1 mRNA expression was detected in primary cultures of pericytes isolated from bovine brain cortex (Berezowski et al. 2004). Using confocal laser-scanning microscopy in conjunction with the monoclonal P-gp antibody JSB-1, Virgintino et al. (2002) also observed the presence of P-gp in microvascular pericytes from human brain microvessels. crovessels. Our electron microscopy observation that P-gp is present along the plasma membrane of pericytes and astrocytes strengthens the hypothesis that P-gp is expressed in all cells that constitute the BBB.
Our studies show P-gp localization at several subcellular sites (i.e., caveolae, nuclear envelope, cytoplasmic vesicles, RER, and Golgi complex) in the capillary endothelial cells. In particular, we demonstrate the presence of caveolae at both the luminal and abluminal membranes with a higher number at the abluminal one. Similar asymmetric distribution of caveolae at the BBB has been reported by Bouchard et al. (2002) and Virgintino et al. (2002). In parallel, our study demonstrates that P-gp distribution to caveolae is ~2.0-fold greater at the abluminal membrane of the endothelial cells when compared with the luminal one. Previous studies, including our own, performed with cultured cells have demonstrated the association of P-gp with caveolae in cultured brain microvessel endothelial cells (Jodoin et al. 2003; Schlachetzki and Pardridge 2003), human cerebral cortex microvessels (Virgintino et al. 2002), and astrocytes (Ronaldson et al. 2004). Caveolae are invaginated plasma membrane microdomains involved in a wide variety of cellular processes including signal transduction, cholesterol transport, endocytosis, and potocytosis. Several P-gp substrates are large molecules, and expression of this protein within caveolae may act to restrict their uptake by endocytosis processes (Jodoin et al. 2003). Further studies are needed to fully assess the functional significance of P-gp localization within this subcellular compartment.
We previously reported localization of P-gp at the nuclear envelope and cytoplasmic vesicles in cultured rat brain microvessel endothelial cells (Bendayan et al. 2002) and astrocytes (Ronaldson et al. 2004), indicating that P-gp may play a role in the intracellular distribution/kinetics of several pharmacological agents. In the present study we confirm such subcellular localization in rat and human brain tissue. It has been proposed that P-gp localization along the nuclear envelope may serve as a protective mechanism that could restrict the permeation of xenobiotics into the nuclear matrix, a subcellular site of action of several xenobiotics including chemotherapeutic agents (Molinari et al. 2002). Similarly, it has been proposed that P-gp localization in cytoplasmic vesicles serves to sequester drugs into those vesicles (Shapiro et al. 1998). Together this subcellular localization implies that the transport protein could participate in the overall MDR phenotype in the CNS by promoting drug efflux from nuclei, which ultimately prevents drug-DNA interactions and drug sequestration in vesicles, which will result in low cytoplasmic cellular concentrations.
The high sensitivity of our immunocytochemical approach has further enabled the detection of P-gp at sites of protein synthesis (i.e., RER) as well as glycosylation and protein trafficking (i.e., Golgi complex). Recent immunocytochemistry and fluorescent confocal microscopy studies on a human uterine carcinoma cell line (i.e., HeLa) transfected with P-gp indicated that the protein is first localized to the RER before moving to the Golgi, presumably to undergo posttranslational modification (i.e., glycosylation) (Fu et al. 2004). This same study also reported that cellular treatment with inhibitors of protein traffic (i.e., brefeldin A, monensin) decreased cell surface expression of P-gp but increased localization in the RER and Golgi (Fu et al. 2004), indicating that intracellular P-gp trafficking via the RER and Golgi complex does occur before the protein is expressed at the cell surface.
Recently, several studies have suggested that the localization and functional expression of P-gp may be altered due to disease and/or exposure to pharmacological agents. For example, P-gp expression in brain capillary endothelial cells and astrocytes may be modulated during epilepsy (Volk et al. 2005), HIV-1 encephalitis (Langford et al. 2004), and Alzheimer's disease (Vogelgesang et al. 2004). Many of these conditions involve the production of cytokines (i.e., tumor necrosis factor-α, interleukin-1β, interleukin-6), which have been shown to modulate the molecular expression of P-gp in vitro in rat brain endothelial (i.e., GPNT) and human glioblastoma (i.e., U373MG) cell lines (Fernandez et al. 2004). Previous studies have also reported that xenobiotics, including pharmacological agents, can alter the molecular expression and functional activity of P-gp (Chandler et al. 2003) through the activation of nuclear orphan receptors (Bauer et al. 2004). With the demonstration of P-gp throughout the BBB, further studies are required to evaluate the effect of pathological conditions and pharmacotherapy on the functional expression of P-gp in all cellular compartments of the BBB (i.e., brain capillary endothelial cells, astrocytes, pericytes).
In summary, the present study is the first to demonstrate P-gp localization at both the luminal and abluminal plasma membranes in brain capillary endothelial cells as well as in pericytes and astrocytes from human and rodent brain tissue. We also observed the subcellular localization of P-gp along the nuclear envelope and in caveolae, cytoplasmic vesicles, Golgi complex, and RER, reflecting synthesis, posttranslational changes, and membrane trafficking. Together our data suggest an important role for P-gp throughout all cellular components of the BBB, restricting brain permeability of several pharmacological agents at both the cellular surface and subcellular sites.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (MOP-56976 to RB; MOP-90027 to MB) and the Ontario HIV Treatment Network. P.T.R. is a recipient of an Ontario HIV Treatment Network Studentship Award.
The authors thank Dr. Taufik Valiante and Dr. Patrick Shannon, Toronto Western Hospital, University Health Network for the providing human brain tissue.
Literature Cited
- Ballerini P, Di Iorio P, Ciccarelli R, Nargi E, D'Alimonte I, Traversa U, Rathbone MP, et al. (2002) Glial cells express multiple ATP binding cassette proteins which are involved in ATP release. Neuroreport 13:1789–1792 [DOI] [PubMed] [Google Scholar]
- Bauer B, Hartz AM, Fricker G, Miller DS. (2004) Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 66:413–419 [DOI] [PubMed] [Google Scholar]
- Beaulieu E, Demeule M, Ghitescu L, Beliveau R. (1997) P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J 326:539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M. (1995) Colloidal gold post-embedding immunocytochemistry. Prog Histochem Cytochem 29:1–159 [DOI] [PubMed] [Google Scholar]
- Bendayan R, Lee G, Bendayan M. (2002) Functional expression and localization of P-glycoprotein at the blood brain barrier. Microsc Res Tech 57:365–380 [DOI] [PubMed] [Google Scholar]
- Berezowski V, Landry C, Dehouck MP, Cecchelli R, Fenart L. (2004) Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res 1018:1–9 [DOI] [PubMed] [Google Scholar]
- Biegel D, Spencer DD, Pachter JS. (1995) Isolation and culture of human brain microvessel endothelial cells for the study of blood-brain barrier properties in vitro. Brain Res 692:183–189 [DOI] [PubMed] [Google Scholar]
- Bouchard P, Ghitescu LD, Bendayan M. (2002) Morpho-functional studies of the blood-brain barrier in streptozotocin-induced diabetic rats. Diabetologia 45:1017–1025 [DOI] [PubMed] [Google Scholar]
- Chandler B, Almond L, Ford J, Owen A, Hoggard P, Khoo S, Back D. (2003) The effects of protease inhibitors and nonnucleoside reverse transcriptase inhibitors on p-glycoprotein expression in peripheral blood mononuclear cells in vitro. J Acquir Immune Defic Syndr 33:551–556 [DOI] [PubMed] [Google Scholar]
- DiDiodato G, Sharom FJ. (1997) Interaction of combinations of drugs, chemosensitizers, and peptides with the P-glycoprotein multidrug transporter. Biochem Pharmacol 53:1789–1797 [DOI] [PubMed] [Google Scholar]
- Drion N, Risede P, Cholet N, Chanez C, Scherrmann JM. (1997) Role of P-170 glycoprotein in colchicine brain uptake. J Neurosci Res 49:80–88 [PubMed] [Google Scholar]
- Farrell CL, Pardridge WM. (1991) Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci USA 88:5779–5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C, Buyse M, German-Fattal M, Gimenez F. (2004) Influence of the pro-inflammatory cytokines on P-glycoprotein expression and functionality. J Pharm Pharm Sci 7:359–371 [PubMed] [Google Scholar]
- Fromm MF. (2004) Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci 25:423–429 [DOI] [PubMed] [Google Scholar]
- Fu D, Bebawy M, Kable EP, Roufogalis BD. (2004) Dynamic and intracellular trafficking of P-glycoprotein-EGFP fusion protein: implications in multidrug resistance in cancer. Int J Cancer 109: 174–181 [DOI] [PubMed] [Google Scholar]
- Georges E, Bradley G, Gariepy J, Ling V. (1990) Detection of P- glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci USA 87:152–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden PL, Pardridge WM. (1999) P-glycoprotein on astrocyte foot processes of unfixed isolated human brain capillaries. Brain Res 819:143–146 [DOI] [PubMed] [Google Scholar]
- Jodoin J, Demeule M, Fenart L, Cecchelli R, Farmer S, Linton KJ, Higgins CF, et al. (2003) P-glycoprotein in blood-brain barrier endothelial cells: interaction and oligomerization with caveolins. J Neurochem 87:1010–1023 [DOI] [PubMed] [Google Scholar]
- Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. (1998) The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest 101:289–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuhara H, Sugiyama Y. (2001) Efflux transport systems for drugs at the blood-brain barrier and blood-cerebrospinal fluid barrier (Part 1). Drug Discov Today 6:150–156 [DOI] [PubMed] [Google Scholar]
- Langford D, Grigorian A, Hurford R, Adame A, Ellis RJ, Hansen L, Masliah E. (2004) Altered P-glycoprotein expression in AIDS patients with HIV encephalitis. J Neuropathol Exp Neurol 63: 1038–1047 [DOI] [PubMed] [Google Scholar]
- Lee G, Schlichter L, Bendayan M, Bendayan R. (2001) Functional expression of P-glycoprotein in rat brain microglia. J Pharmacol Exp Ther 299:204–212 [PubMed] [Google Scholar]
- Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. (2000) Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol 58:1357–1367 [DOI] [PubMed] [Google Scholar]
- Molinari A, Calcabrini A, Meschini S, Stringaro A, Crateri P, Toccacieli L, Marra M, et al. (2002) Subcellular detection and localization of the drug transporter P-glycoprotein in cultured tumor cells. Curr Protein Pept Sci 3:653–670 [DOI] [PubMed] [Google Scholar]
- Okochi E, Iwahashi T, Tsuruo T. (1997) Monoclonal antibodies specific for P-glycoprotein. Leukemia 11:1119–1123 [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Golden PL, Kang YS, Bickel U. (1997) Brain microvascular and astrocyte localization of P-glycoprotein. J Neurochem 68:1278–1285 [DOI] [PubMed] [Google Scholar]
- Regina A, Koman A, Piciotti M, El Hafny B, Center MS, Bergmann R, Couraud PO, et al. (1998) Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. J Neurochem 71:705–715 [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan M, Gingras D, Piquette-Miller M, Bendayan R. (2004) Cellular localization and functional expression of P-glycoprotein in rat astrocyte cultures. J Neurochem 89:788–800 [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, van der Valk MA, et al. (1997) Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci USA 94:4028–4033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlachetzki F, Pardridge WM. (2003) P-glycoprotein and caveolin-1alpha in endothelium and astrocytes of primate brain. Neuroreport 14:2041–2046 [DOI] [PubMed] [Google Scholar]
- Seetharaman S, Barrand MA, Maskell L, Scheper RJ. (1998) Multidrug resistance-related transport proteins in isolated human brain microvessels and in cells cultured from these isolates. J Neurochem 70:1151–1159 [DOI] [PubMed] [Google Scholar]
- Shapiro AB, Fox K, Lee P, Yang YD, Ling V. (1998) Functional intracellular P-glycoprotein. Int J Cancer 76:857–864 [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Naito M, Oh-hara T, Sugawara I, Tsuruo T. (1992) Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem 267:20383–20391 [PubMed] [Google Scholar]
- Virgintino D, Robertson D, Errede M, Benagiano V, Girolamo F, Maiorano E, Roncali L, et al. (2002) Expression of P-glycoprotein in human cerebral cortex microvessels. J Histochem Cytochem 50: 1671–1676 [DOI] [PubMed] [Google Scholar]
- Vogelgesang S, Warzok RW, Cascorbi I, Kunert-Keil C, Schroeder E, Kroemer HK, Siegmund W, et al. (2004) The role of P-glycoprotein in cerebral amyloid angiopathy; implications for the early pathogenesis of Alzheimer's disease. Curr Alzheimer Res 1: 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk H, Potschka H, Loscher W. (2005) Immunohistochemical localization of P-glycoprotein in rat brain and detection of its increased expression by seizures are sensitive to fixation and staining variables. J Histochem Cytochem 53:517–531 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuetz JD, Elmquist WF, Miller DW. (2004) Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exp Ther 311:449–455 [DOI] [PubMed] [Google Scholar]