Abstract
Identification of the strain of agent responsible for bovine spongiform encephalopathy (BSE) can be made histologically through the analysis of both distribution and intensity of brain vacuolar lesions after BSE transmission to mouse. Another useful way to distinguish the BSE agent from other prion strains is the study of the distribution of the abnormal prion protein (PrPres). For that purpose, paraffin-embedded tissue blot (PET-blot) method was applied on brains from C57Bl/6 mice infected with cattle BSE, experimental sheep BSE, or feline spongiform encephalopathy (FSE) from a cheetah. PrPres distribution was comparable, whichever of the three BSE agent sources was considered and was distinct from the PrPres distribution in C57Bl/6 mice inoculated with a French scrapie isolate or with a mouse-adapted scrapie strain (C506M3). These data confirm a common origin of infectious agent responsible for the British and French cattle BSE. They also indicate that PET-blot method appears as a precise complementary tool in prion strain studies because it offers easy and quick assessment of the PrPres mapping. Advantages and limits of the PET-blot method are discussed and compared with other established and validated methods of strain typing.
Keywords: bovine spongiform encephalopathy, mouse, paraffin-embedded tissue blot, prion, scrapie, strains
Prion diseases including scrapie in sheep and goats, bovine spongiform encephalopathy (BSE) in cattle, and Creutzfeldt-Jakob disease in human beings are fatal neurodegenerative disorders. Existence of distinct clinical forms of scrapie in sheep, including nervous and itching types, was first described in 1926 by Stockman (Pattison 1988). Transmission of this disease to goats also led to the observation of different clinical forms (Pattison and Millson 1961). A significant advance in scrapie research resulted from the successful transmission of the disease to mice by Chandler (1961,1963), who described different mouse susceptibilities to different scrapie isolates. Subsequent studies demonstrated many different phenotypic expressions of the disease in rodents. These observations led to the “strain” concept in the field of transmissible spongiform encephalopathies (TSEs), strains being defined by specific incubation periods, clinical signs, and neuropathological features such as distribution and intensity of spongiform changes (lesion profile) observed in different inbred mouse lines (Chandler 1963; Dickinson et al. 1968; Fraser and Dickinson 1968). Numerous experimental scrapie strains have been characterized using transmission to mice (Bruce 1991,1993) showing, at the same time, important interactions between the strain of TSE agent and genetic features of the host (Dickinson et al. 1968; Bruce et al. 1991). The use of mice for strain typing became particularly important with the appearance of BSE in cattle in 1986 (Wells et al. 1987), and this approach demonstrated that a number of different species including humans, cats, and some captive wild animals had probably been infected by the BSE agent (Fraser et al. 1994; Bruce et al. 1997; Hill et al. 1997).
The abnormal prion protein is derived from a normal host protein (PrPc) and accumulates in the brain mainly as an insoluble and partially proteaseresistant form of the protein (PrPres) during the course of the disease (McKinley et al. 1983). This abnormal protein is the only known specific marker for these diseases (Bolton et al. 1982). It contributes to the identification of the strain of TSE agent in the natural host as well as in mice experimentally infected (Jeffrey et al. 2001; Stack et al. 2002; Baron et al. 2004). In particular, in natural hosts, the histological strain-typing analyses are based on the fine study of differential PrP immunohistochemical (IHC) labeling of neurons and glial cells at the cellular level (Gonzalez et al. 2003), but in wild-type or transgenic mice this method has not yet been reported, and the neuroanatomical distribution of pathological PrP is used as a strain typing parameter (DeArmond et al. 1987,1993; Bruce et al. 1993,1994a; Green et al. 2004).
The present study investigated the distribution of PrPres accumulation in the brains of C57Bl/6 mice inoculated with three different origins of BSE agent and with two scrapie agents. Using an original method, PrPres distribution in mice was assessed with PET-blot that allowed easy, rapid, and precise identification of the neuro-anatomical distribution of PrPres in situ (Schulz-Schaeffer et al. 2000b). Limitations and advantages of this method are discussed using the example of transmission of BSE and scrapie to mice that show distinct BSE signature in terms of PrPres distribution.
Materials and Methods
Four- to 5-week-old female C57Bl/6 mice (Charles River; L'Arbresle, France) were intracerebrally challenged with 20 μl of an inoculum prepared from infected brain tissues from different affected species with TSEs as described in Table 1, diluted in physiological glucose at 1% for serial passages in wild-type mice, or at 10% for passage from ovine transgenic mice. Mice were inoculated after anesthesia and cared for and housed according to the guidelines of the French Ethical Committee (decree 87-848) and European Community Directive 68/609/EEC.
Table 1.
Different TSE sources
|
|
|---|
| Origin of inocula |
|
|
| Scrapie 1 (Scr1): From a natural scrapie case from a Charollais × Texel (VRQ/VRQ) (Ardennes, France) |
| Year of death: 1999 (Baron et al. 2004) |
| Second passage |
| C506M3: mouse-adapted scrapie strain |
| From a natural case of scrapie in sheep (US) (Maignien et al. 1999) |
| Multiple passages |
| C57Bl mouse-adapted scrapie strain |
| From a cow with BSE (UK) (Lasmézas et al. 2001) |
| Multiple passages |
| Experimental transmission of a French BSE case to a Lacaune sheep (ARQ/ARQ) |
| (SB1) (Baron et al. 2000) |
| Second passage |
| Cheetah born in 1987, reared in Whipsnade Zoo (UK) for 6 years, then sent to Peaugres Zoo (France) |
| Year of death: 1997 (Baron et al. 1997) |
| Second passage |
| French cattle BSE transmitted to a Lacaune sheep (ARQ/ARQ) (SB1), then inoculated to TgOvPrP4 ovine transgenic mice (Crozet et al. 2001a) |
| First passage |
|
|
TSE, transmissible spongiform encelphalopathy; BSE, bovine spongiform encephalopathy.
Once inoculated, mice were regularly observed twice weekly. When the first clinical signs occurred, evolution of the disease was followed daily as recommended by others (Dickinson et al. 1968; Bruce et al. 1991; Thackray et al. 2002). When the intensity of clinical symptoms appeared life threatening, mice were sacrificed, and brains were quickly collected and fixed in 4% buffered paraformaldehyde (n=6 to 13 per group). Four coronal brain sections were prepared as originally described by Fraser and Dickinson (1968). Most of the brains were cut using a dedicated matrix allowing the standardization of the level of sections as well as assuring symmetrical sections. Brain pieces were then placed in a cassette, routinely dehydrated, and embedded in paraffin.
PET-blot Analysis
PET-blot method was used as previously described with few changes, in order to visualize in situ the resistant form of abnormal PrP, PrPres, after digestion with a high concentration of proteinase K (PK) (Schulz-Schaeffer et al. 2000b). Five-μm paraffin sections were cut and collected onto 0.45-μm pore nitrocellulose membranes (Bio-Rad Laboratories; Marnes-la-Coquette, France) and then dried for 1 hr at 55C. Membranes were dewaxed by immersion in xylene (45C, 20 min) and rinsed in isopropanol 100 (2 × 10 min) followed by stepwise rehydration. Membranes were dried at room temperature. After wetting with TBST (10 mM Tris HCl, pH 7.8, 100 mM NaCl, 0.05% Tween 20; Euromedex, Mundolsheim, France), enzymatic digestion was performed using 250 μg/ml PK (Roche Diagnostics; Meylan, France) in a buffer consisting of 10 mM Tris HCl, pH 7.8, 100 mM NaCl, 0.1% Brij 35 for 8 hr at 55C, so that only PK PrPres will be detectable. After washing with TBST, sections were treated for 10 min with guanidine isothiocyanate (3 M). Guanidine was washed out five times in TBST. Immunodetection was performed after preincubation in blocking solution (skimmed milk diluted at 0.2% in TBST). Monoclonal antibody used was SAF84 (1/2000) overnight at room temperature. After three washes in TBST, a phosphatase alkaline-coupled anti-mouse antibody (Clini-Sciences; Montrouge, France) was used (1/500 in TBST, 37C, 45 min). Membranes were washed three times in TBST and pH was adjusted to 9 by incubating in NTM buffer (100 mM Tris-HCl, pH 9.5,100 mM NaCl, 50 mM MgCl2). Finally, 5-bromo 4-chloro 3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT; CliniSciences) was used to visualize the reaction product (dark blue deposits). PET-blot membranes were assessed using a stereomicroscope (Olympus; Rungis, France) coupled to an image analysis workstation (Biocom; Les Ulis, France). To better define the different structural levels actually targeted by PrPres deposition that was not possible to confidently assign using PET-blot, we used PrP IHC or hematoxylin/eosin-stained serial sections as well as mouse brain atlas (Sidman et al. 1971).
Brain sections of uninfected C57Bl/6 mice and PrP0/0 mice were also used to check that no specific staining was observable on such tissue control.
Results
Specific Detection of PrP res Using PET-blot
Specificity of PET-blot was confirmed by the results obtained on brain stem and cerebellum samples of uninfected mice that did not present any staining (Figure 1C) in comparison to the PrPres labeling visualized in the same structures in mice infected with scrapie or BSE agents (Figures 1A and 1B). Detail of the cerebellum in the stratum moleculare (sm) of mice affected with these two agents shows the ability of this method to detect different types of PrPres accumulation that remain remarkably accessible despite being quite unrefined as compared with IHC data.
Figure 1.
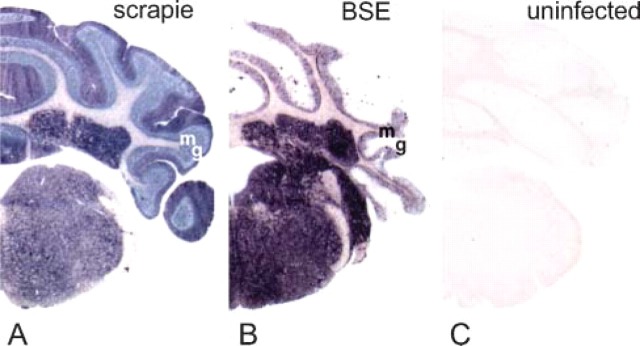
Paraffin-embedded tissue (PET)-blot analysis of the proteinase K (PK)-resistant form of the prion protein (PrPres) (dark blue deposits) in the brain stem and cerebellum of mice infected with scrapie (A) and bovine spongiform encephalopathy (BSE) (B). Control section from a non-infected mouse shows absence of PrPres labeling (C). g, granular layer; m, molecular layer.
PrP res Profiles in Brain of Infected Mice with Scrapie Agents and BSE
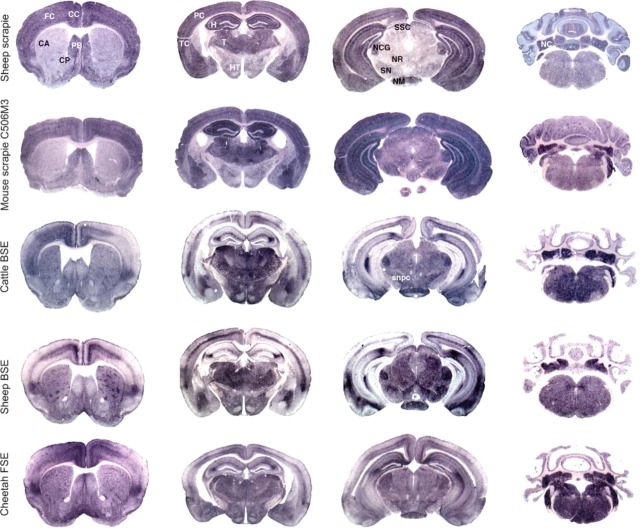
Figure 2 shows comparative views of PET-blot analysis of brain sections from C57Bl/6 mice inoculated with different TSE sources such as a French natural scrapie case Scr1 (three mice, analyzed on two sections) (row 1), a murinized scrapie strain, C506M3 (two mice, analyzed on two to four sections) (row 2), a British cow naturally affected with BSE (two mice analyzed on two sections) (row 3), an experimentally infected sheep with French BSE (row 4) (seven mice analyzed on one to three sections), and a cheetah diagnosed with FSE in France (one mouse analyzed on two sections) (row 5), as indicated in Table 1.
Figure 2.
Neuro-anatomical distribution of PrPres (PET-blot) in the brain of C57Bl/6 mice infected with natural scrapie case (row 1), C506M3 mouse-adapted scrapie strain (row 2), BSE agent from a cow (row 3), ovine BSE (row 4), and FSE agent from a cheetah (row 5). The different sources of BSE agent induced a uniform abnormal PrP distribution distinct from those seen in the case of scrapie sources. FC, frontal cortex; CC, cingulated cortex; CA, caudate putamen; PB, paraterminal body; CP, caudate putamen; PC, parietal cortex; TC, temporal cortex; H, hippocampus; T, thalamus; HT, hypothalamus; SSC, stratum moleculare of the cerebellum; NCG, nucleus corporis geniculati; NR, nucleus rubber; SN, substantia nigra; NM, nucleus mammilaris; NC, deep nuclei of the cerebellum; snpc, substantia nigra pars compacta.
Results are presented to compare PrPres distribution for each anatomical level (four sections) of the different experimentations. The description was performed on the brain of mice infected with scrapie compared with mice infected with BSE sources, in order to identify which brain areas could better allow BSE and scrapie discrimination. The main distinct areas identified between BSE and scrapie sources are summarized in Table 2.
First Level of Brain Section
In the cortex of mice infected with cattle BSE, cheetah FSE, and experimental ovine BSE, dense PrPres deposits were observed in some specific layers, whereas others were spared except in the insular area located in the lateroventral region of the cortex that shows a dense zone of PrPres accumulation (Figure 2, column 1, rows 3-5). The limiting glia also accumulated significant amounts of PrPres for these three experiments. Comparatively, in mice infected with the two scrapie agents, PrPres accumulation was dense and homogeneous, affecting all cortical layers except the limiting glia (Figure 2, column 1, rows 1 and 2).
In the caudate putamen nuclei, dense clusters of PrPres deposits were always identified with more or less intensity (rows 3-5) in mice infected with BSE agent. In comparison, this type of labeling was never observed with scrapie agents in this site (rows 1 and 2).
In the central area of the para-terminal body (septal nucleus), a weaker PrPres accumulation was observed in comparison to the periphery of this structure in mice infected with BSE sources (rows 3-5). In comparison, in mice infected with scrapie agents (rows 1 and 2), a homogeneous PrPres accumulation was visualized.
In the ventral limit of the diagonal band of Broca (glia limitans), dense amounts of PrPres were identified in mice infected with BSE (rows 3-5), which was not observed in mice infected with scrapie agents (rows 1 and 2).
Second Level of Brain Section
In the cortex, identical criteria as presented for the first section are maintained allowing the differentiation of BSE from scrapie agents.
In the hippocampus, specific PrPres accumulation occurred with all three BSE sources. The stratum lacunosum moleculare of the hippocampus (CA2 region) was specifically stained for PrPres as well as the limiting zone of the sm of the dentate gyrus where a dense PrPres accumulation was observed. In the hippocampus of mice infected with scrapie, all molecular layers accumulate significant amounts of PrPres delimitating the dentate gyrus that appear unlabeled (Figure 2, column 2, rows 1 and 2).
In the thalamus and hypothalamus of mice infected with the three BSE sources, PrPres was detected as well in both structures (Figure 2, column 2, rows 3-5). In comparison, in mice infected with scrapie sources, PrPres accumulation was significantly more important in the thalamus.
Third Level of Brain Section
In the cerebral cortex and in the hippocampus (Figure 2, column 3), the previously described particularities of PrPres profiles in either BSE or scrapie sources were also maintained in this third brain section, both in mice infected with BSE and in mice infected with scrapie.
In the mesencephalon, PrPres accumulation was relatively homogeneous in this section with the BSE agent. However, the nucleus corporis geniculati, the nucleus mammilari, the stratum superficiale colliculi, and the pars compacta of substantia nigra accumulate large amounts of PrPres.
In comparison, in mice infected with the French scrapie agent, PrPres accumulation was particularly intense in these four nuclei compared with other adjacent structures that accumulate lesser amounts of PrPres. With C506M3 scrapie strain, PrPres accumulation in this structure was nearly identical to that induced by the BSE agent, but the red nucleus also accumulated high amounts of PrPres.
Fourth Level of Brain Section
In the cerebellum (Figure 2, column 4) of mice infected with BSE, a homogeneous PrPres accumulation was observed in the stratum granulosum (sg), and numerous dense aggregates occurred in the sm (Figure 1 and Figure 2). Comparatively, in mice infected with scrapie agents, similar PrPres distribution was observed in the sg, but PrPres accumulation appears as homogeneous stripes in the sm perpendicular to the cerebellar surface (Figure 1 and Figure 2).
In mice infected with BSE, more abundant PrPres accumulation was observed at the level of the pons when compared with posterior and anterior sections. This was not easily illustrated here because levels of sections were not strictly identical.
Features of BSE Strain From a Cow Successively Passaged in Sheep, TgOvPrP4, and C57Bl/6 Mice
PET-blot method was also performed to study PrPres distribution in C57Bl/6 mice (n=2) infected with the BSE agent transmitted to ovine transgenic mice (TgOvPrP4 mouse line expressing the A136 R154 Q171 ovine PrP protein) after a first experimental passage from cattle to sheep (A136 R154 Q171 homozygous genotype of the prnp gene).
Table 2.
Summary of the main distinctive criteria (anatomical site and intensity of PrPres) resulting from a PET-blot analysis that allows differentiation of BSE from scrapie agent a
|
| |||
|---|---|---|---|
| Intensity of PrPres accumulation revealed by PET-blot | |||
|
|
|||
| Neuro-anatomical sites | BSE agent | Scrapie agent | |
|
| |||
| Cerebral cortex (rows 1-3) | Dense in 1 or 2 layers | Dense, affecting all cortical structures | |
| Caudate putamen (row 1) | Dense clusters | Homogeneous | |
| Paraterminal body (row 1) | Moderate and peripheral | Homogeneous and central | |
| Hippocampus (rows 2 and 3) | Dense in CA2 region | Dense in all molecular layers | |
| Thalamus/hypothalamus (row 2) | Dense in the thalamus | Dense in the thalamus | |
| Dense in the hypothalamus | Moderate in the hypothalamus | ||
| Cerebellar cortex, molecular layer (row 4) | Moderate, dense depositions (plaques as revealed by immunohistochemistry | Dense and homogeneous, with stripes in the molecular layer | |
|
| |||
Rows from Figure 2.
PrPres, protein-resistant prion; PET-blot, paraffin-embedded tissue blot.
Neuro-anatomical PrPres distribution in infected C57Bl/6 mice is illustrated in Figure 3 at only three levels of brain sections compared with Figure 2. Apparent differences are observed at the level of the thalamus compared with that shown in Figure 2 (column 2, row 3), and these are linked to a less-intense accumulation and to a level of the tissue sections slightly more rostral in Figure 3 compared with sections of Figure 2 (column 2). However, PrPres distribution is quite comparable to that previously described in C57Bl/6 mice directly infected with cattle BSE or with experimental ovine BSE. Hallmarks of BSE infection that have previously been described distinct from mice infected with scrapie sources were also present in the cortex, hippocampus, and brain stem, as well as in the cerebellum.
Figure 3.
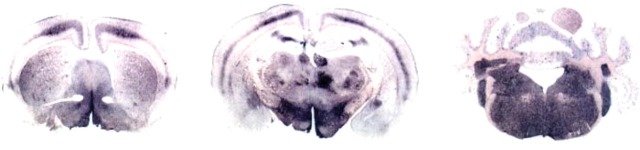
Neuro-anatomical distribution of PrPres (PET-blot) in three sections of brain of C57Bl/6 mice infected with a brain homogenate from TgOvPrP4 infected with ovine BSE (successive transmission of cattle BSE to a sheep, then to TgOvPrP4 mice, and finally to C57Bl/6) revealed a PrPres distribution reminiscent of the one described for the other BSE sources illustrated here.
Discussion
Among the different approaches for studying TSE strains after transmission of the disease to mice, topographical distribution of the abnormal form of the prion protein has a significant place, in particular because of its specificity compared with the study of vacuolar lesions. It is more and more studied using PrP IHC but does not appear easily and precisely transcriptable onto a map of the mouse brain. PET-blot method seems to offer the most adapted strategy to study PrPres brain distribution rapidly and precisely. Here we assessed this strategy successfully on C57Bl/6 mice infected with different TSE sources.
Sensitivity of this method was previously demonstrated for diagnosis of BSE in cows (Schulz-Schaeffer et al. 2000a). In addition to its high sensitivity, this method allows greater anatomical resolution when compared with histoblots (Telling et al. 1994; Scott et al. 1999). Indeed, the use of fixed and paraffin-embedded tissues before PK digestion offered better preservation of the samples compared with histoblot method performed on unfixed frozen samples. As for histoblot, PET-blot approach is similar to the Western blot method because of the extensive pretreatment with PK that ensures complete digestion of all proteins including PrPc, whereas abnormal PrP is only partially degraded to PrPres (Schulz-Schaeffer et al. 2000a,2000b; Ritchie et al. 2004). Even more precisely, PK conditions used in the PET-blot method are more severe than those used in the Western blot method (Baron et al. 2004).
Remarkably, even if this method lacks high cellular precision, it is possible to distinguish different types of PrPres deposition. However, these criteria cannot be used instead of a precise cellular analysis that only IHC offers. Advantageously, PET-blot method also offers the possibility to analyze old tissue block collections of paraffin-embedded samples that represent an important source of archival materials. Above all and without doubt, the main advantage of the PET-blot is a rapid and acute approach to examine PrPres distribution in the infected brain.
Nevertheless, PET-blot data cannot be considered alone to establish the identification of a given strain because, in some cases, PrPres is present at a minimal or undetectable level (Lasmézas et al. 1997). PrPres is also not correlated with the presence of vacuolar lesions (Manson et al. 2000). In any case and even though the distribution of vacuolar degeneration is most often closely correlated with the distribution of abnormal PrP accumulation in infected mice (Bruce et al. 1989,1994b), a precise comparison of both approaches with different prion strains is needed to validate this method, as the lesion profile method benefits from several years of experience.
Identification of Scrapie Agents
Although all three groups of mice infected with BSE or FSE showed a consistent pattern of PrP distribution, this pattern was easily differentiated from that found with two different scrapie sources. This included an experimental mouse-adapted scrapie strain (C506M3) and transmission in C57Bl/6 mice from a French natural scrapie isolate (Scr1). Molecular studies following transmission in wild-type and ovine transgenic mice of this Scr1 isolate have recently been described (Baron et al. 2004). Our study now extends these observations to the characterization of PrPres distribution in the brain of C57Bl/6 mice.
Differences observed between scrapie and BSE sources in PrPres brain targeting argues for a selective spread of the infectious agent. Specific neural cells appear to accumulate abnormal PrP, which could be the result of differential cellular susceptibilities, PrPres processing, and tropism following infection with different agents. Other preliminary results showed that with mouse-adapted scrapie strains 79A and 22A inoculated into C57Bl/6 mice, abnormal PrP brain distribution is clearly different not only from BSE but also from Scr1 and C506M3 scrapie sources studied here (data not shown). Thus, the present data suggest that this approach may also be useful for scrapie strain typing.
In the present study, identification of the precise scrapie agent isolated from the French sheep scrapie case is still not possible, as only partial results have been published previously about abnormal PrP distribution in the brain of mice infected with experimental scrapie. However, similar abnormal PrP accumulation has been reported in C57Bl/6 inoculated with the scrapie strain ME7, which presented identical prion accumulation in the hippocampus and cerebellum (Bruce et al. 1994b). A characteristic zonal pattern of abnormal PrP in the molecular layer of the cerebellum was also described, labeling appearing as stripes perpendicular to the cerebellar surface that was considered to be associated with the dendrites of Purkinje cells (Bruce et al. 1994b). Furthermore, intense abnormal PrP accumulation in the hippocampal area was also described with ME7 (Bruce et al. 1994b). These data suggest that the isolated strain of the present scrapie case may be a ME7-like strain. PET-blot data obtained by another group with ME7 in C57Bl mice in sagittal brain sections also showed comparable results (Schulz-Schaeffer et al. 2000b). Actually, PrPres distribution differences observed with our isolate Scr1 and C506M3 experimental scrapie strain are faint and both agents present similarities with ME7 (hippocampal and cerebellar PrPres signature). Furthermore, ME7 strain is one of the most commonly isolated strains by passage in mice from different cases of natural scrapie, at least in the UK (Bruce and Dickinson 1987; Bruce et al. 2002).
To the best of our knowledge, characterization of French scrapie by transmission in wild-type mice has only been described for a single scrapie case (Lasmézas et al. 2001). In this case, vacuolar lesion profiles found following transmission in C57Bl/6 mice were clearly different from that observed in mice infected with C506M3 strain. However, comparisons of these data could support the hypothesis that different strains of scrapie affect French sheep flocks, which is also observed in the UK.
Identification of the BSE Agent Among Species
The infectious agent responsible for BSE has been previously isolated from different cattle sources, and transmission studies in mice have revealed the existence of a single major strain of agent (Bruce et al. 1994a,2002; Green et al. 2005). These studies also showed that the BSE agent was unchanged when passaged through a range of different species (Foster et al. 1996; Bruce et al. 1997), and our PET-blot study now provides additional data sustaining the BSE strain stability among species. First, PrPres distribution substantiates further that ARQ/ARQ sheep experimentally infected with a brain homogenate from a French BSE cow developed a TSE due to the BSE agent inoculated with similar properties to the BSE agent isolated from British cattle also studied here (Baron et al. 2000; Lezmi et al. 2004). PET-blot data are also sustaining the hypothesis of a common origin of cattle BSE epidemic in France and the UK, as previously demonstrated for the Swiss cases by the study of vacuolar lesion profiles (Bruce 1996).
Here we showed that PrPres study allows the identification of the BSE agent, especially by analyzing the cortex, hippocampus, thalamus/hypothalamus, and cerebellum areas that appeared to be the best BSE discriminative regions. In another recent study, specific amounts of abnormal PrP were detected by IHC in other brain areas such as the locus coeruleus, facial nucleus, and cochlear nucleus (Green et al. 2005). Accumulation of PrPres was marked only in the cochlear nucleus in this study, but this staining was also observed in the two scrapie agent transmission studies.
With regard to the agent isolated from the cheetah affected with FSE, analysis of PrPres distribution suggested a possible link with the BSE agent originating from cattle. Even if lesion profile studies are needed to confirm this hypothesis, this observation is consistent with data previously reported using the lesion profile method in mice infected with FSE from three domestic cats (Fraser et al. 1994). Biochemical analyses also showed high levels of di-glycosylated form of PrPres in cheetah and in mice infected with FSE, as generally reported with the BSE agent. These similarities again suggest that BSE and FSE in different feline species may be due to the same infectious agent.
Finally, analysis of PrPres brain distribution in C57Bl/6 mice infected with a brain homogenate of transgenic mice expressing the sheep prion protein (TgOvPrP4) (Crozet et al. 2001b) infected with ovine BSE also revealed the PET-blot typical BSE signature. This result argues for the BSE strain stability even after passage in a murine transgenic model, which apparently did not induce changes of the BSE agent strain property. The BSE agent was indeed easily recognized by PET-blot approach following transmission to C57Bl/6 mice, as well as by its molecular features (Baron et al. 2004).
In conclusion, although PET-blot may have some limitations, the advantage is that this method identifies the PK-resistant form of the prion protein, PrPres, in situ and allows the rapid and accurate identification of the distribution of PrPres in brain, thus describing the type of strain for the agent responsible for the different TSEs. PET-blot approach should be considered as a complementary method in strain typing studies in murine models.
Acknowledgments
This work was supported in part by grants from Programme National de Recherche sur les ESST et les Prions. S.L. was financially supported by a grant from Agence Française de Securite Sanitaire des Aliments (AFSSA).
We are grateful to Dr. Norman Barlow (Sanofi-Aventis) for critical evaluation of the English and to Dr. Michel Solsona (AFSSA-Lyon) for technical suggestions
Literature Cited
- Baron T, Belli P, Madec JY, Moutou F, Vitaud C, Savey M. (1997) Spongiform encephalopathy in an imported cheetah in France. Vet Rec 141:270–271 [DOI] [PubMed] [Google Scholar]
- Baron T, Crozet C, Biacabe A-G, Philippe S, Verchere J, Bencsik A, Madec J-Y, et al. (2004) Molecular analysis of the proteaseresistant prion protein in scrapie and bovine spongiform encephalopathy transmitted to ovine transgenic and wild-type mice. J Virol 78:6243–6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron T, Madec J-Y, Calavas D, Richard Y, Barillet F. (2000) Comparison of French natural scrapie isolates with bovine spongiform encephalopathy and experimental scrapie infected sheep. Neurosci Lett 284:175–178 [DOI] [PubMed] [Google Scholar]
- Bolton DC, McKinley MP, Prusiner SB. (1982) Identification of a protein that purifies with the scrapie prion. Science 218:1309–1311 [DOI] [PubMed] [Google Scholar]
- Bruce M. (1996) Strain typing studies of scrapie and BSE. In Baker H, Ridley RM, eds. Prion Diseases. Totowa, NJ, Humana Press, 223–236 [Google Scholar]
- Bruce M, Chree A, McConnell I, Foster J, Pearson G, Fraser H. (1994a) Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos Trans R Soc London B Biol Sci 343:405–411 [DOI] [PubMed] [Google Scholar]
- Bruce ME. (1993) Scrapie strain variation and mutation. Br Med Bull 49:822–838 [DOI] [PubMed] [Google Scholar]
- Bruce ME, Boyle A, Cousens S, McConnell I, Foster J, Goldmann W, Fraser H. (2002) Strain characterization of natural sheep scrapie and comparison with BSE. J Gen Virol 83:695–704 [DOI] [PubMed] [Google Scholar]
- Bruce ME, Dickinson AG. (1987) Biological evidence that scrapie agent has an independent genome. J Gen Virol 68:79–89 [DOI] [PubMed] [Google Scholar]
- Bruce ME, McBride PA, Farquhar CF. (1989) Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci Lett 102:1–6 [DOI] [PubMed] [Google Scholar]
- Bruce ME, McBride PA, Jeffrey M, Scott JR. (1994b) PrP in pathology and pathogenesis in scrapie-infected mice. Mol Neurobiol 8:105–112 [DOI] [PubMed] [Google Scholar]
- Bruce ME, McConnell I, Fraser H, Dickinson AG. (1991) The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol 72:595–603 [DOI] [PubMed] [Google Scholar]
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, et al. (1997) Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389:498–501 [DOI] [PubMed] [Google Scholar]
- Chandler RL. (1961) Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet 1:1378–1379 [DOI] [PubMed] [Google Scholar]
- Chandler RL. (1963) Experimental scrapie in the mouse. Res Vet Sci 4:276–285 [Google Scholar]
- Crozet C, Bencsik A, Flamant F, Lezmi S, Samarut J, Baron T. (2001a) Florid plaques in ovine PrP transgenic mice infected with an experimental ovine BSE. EMBO reports 2:952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozet C, Flamant F, Bencsik A, Aubert D, Samarut J, Baron T. (2001b) Efficient transmission of two different sheep scrapie isolates in transgenic mice expressing the ovine PrP gene. J Virol 75:5328–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeArmond SJ, Mobley WC, DeMott DL, Barry RA, Beckstead JH, Prusiner SB. (1987) Changes in the localization of brain prion proteins during scrapie infection. Neurology 50:1271–1280 [DOI] [PubMed] [Google Scholar]
- DeArmond S, Yang S-L, Lee A, Bowler R, Taraboulos A, Groth D, Prusiner SB. (1993) Three scrapie prion isolates exhibit different accumulation patterns of the prion protein scrapie isoform. Proc Natl Acad Sci USA 90:6449–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson AG, Meikle VMH, Fraser H. (1968) Identification of a gene which controls the incubation period of some strains of scrapie agent in mice. J Comp Pathol 78:293–299 [DOI] [PubMed] [Google Scholar]
- Foster JD, Bruce M, Mc Connell I, Chree A, Fraser H. (1996) Detection of BSE infectivity in brain and spleen of experimentally infected sheep. Vet Rec 138:546–548 [DOI] [PubMed] [Google Scholar]
- Fraser H, Dickinson AG. (1968) The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol 78:301–311 [DOI] [PubMed] [Google Scholar]
- Fraser H, Pearson GR, McConnell I, Bruce ME, Wyatt JM, Gruffydd-Jones TJ. (1994) Transmission of feline spongiform encephalopathy to mice. Vet Rec 134, 449. [DOI] [PubMed] [Google Scholar]
- Gonzalez L, Martin S, Jeffrey M. (2003) Distinct profiles of PrP(D) immunoreactivity in the brain of scrapie- and BSE-infected sheep: implications for differential cell targeting and PrP processing. J Gen Virol 84:1339–1350 [DOI] [PubMed] [Google Scholar]
- Green R, Horrocks C, Wilkinson A, Hawkins SAC, Ryder SJ. (2005) Primary isolation of the bovine spongiform encephalopathy agent in mice: agent definition based on a review of 150 transmissions. J Comp Pathol 132:117–131 [DOI] [PubMed] [Google Scholar]
- Hill A, Desbruslais M, Joiner S, Sidle KCL, Gowland I, Collinge J. (1997) The same prion strain causes vCJD and BSE. Nature 389:448–450 [DOI] [PubMed] [Google Scholar]
- Jeffrey M, Martin S, Gonzalez L, Ryder SJ, Bellworthy SJ, Jackman R. (2001) Differential diagnosis of infections with the bovine spongiform encephalopathy (BSE) and scrapie agents in sheep. J Comp Pathol 125:271–284 [DOI] [PubMed] [Google Scholar]
- Lasmézas CI, Deslys J-P, Robain O, Jaegly A, Beringue V, Peyrin J-M, Fournier J-G, et al. (1997) Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 275:402–405 [DOI] [PubMed] [Google Scholar]
- Lasmézas CI, Fournier J-G, Nouvel V, Boe H. (2001) Adaptation of the bovine spongiform encephalopathy agent to primates and comparison with Creutzfeldt-Jakob disease: implications for human health. Proc Natl Acad Sci USA 98:4142–4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezmi S, Martin S, Simon S, Comoy E, Bencsik A, Deslys J-P, Grassi J, et al. (2004) Comparative molecular analysis of the abnormal prion protein in field scrapie cases and experimental bovine spongiform encephalopathy in sheep by use of western blotting and immunohistochemical methods. J Virol 78:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maignien T, Lasmezas CI, Beringue V, Dormont D, Deslys JP. (1999) Pathogenesis of the oral route of infection of mice with scrapie and bovine spongiform encephalopathy agents. J Gen Virol 80:3035–3042 [DOI] [PubMed] [Google Scholar]
- Manson JC, Barron R, Jamieson E, Baybutt H, Tuzi N, McConnell I, Melton D, et al. (2000) A single amino acid alteration in murine PrP dramatically alters TSE incubation time. Arch Virol 16:95–102 [DOI] [PubMed] [Google Scholar]
- McKinley MP, Bolton DC, Prusiner SB. (1983) A protease-resistant protein is a structural component of the scrapie prion. Cell 35:57–62 [DOI] [PubMed] [Google Scholar]
- Pattison IH. (1988) Fifty years with scrapie: a personal reminiscence. Vet Rec 123:661–666 [PubMed] [Google Scholar]
- Pattison IH, Millson GC. (1961) Experimental transmission of scrapie to goats and sheep by the oral route. J Comp Pathol 71:171–176 [Google Scholar]
- Ritchie DL, Head MW, Ironside JW. (2004) Advances in the detection of prion protein in peripheral tissues of variant Creutzfeldt-Jakob disease patients using paraffin-embedded tissue blotting. Neuropathol Appl Neurobiol 30:360–368 [DOI] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ, Fatzer R, Vandevelde M, Kretzschmar HA. (2000a) Detection of PrPsc in subclinical BSE with the paraffinembedded tissue (PET) blot. Arch Virol 16:173–180 [DOI] [PubMed] [Google Scholar]
- Schulz-Schaeffer WJ, Tschoke S, Kranefuss N, Drose W, Hause-Reitner D, Giese A, Groschup MH, et al. (2000b) The paraffinembedded tissue blot detects PrPsc early in the incubation time in prion disease. Am J Pathol 156:51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MR, Will R, Ironside J, Nguyen H-O, Tremblay P, DeArmond SJ, Prusiner SB. (1999) Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci USA 96:15137–15142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Angevine JB, Taberpiece E. (1971) Atlas of the Mouse Brain and Spinal Cord. Cambridge, MA, Harvard University Press [Google Scholar]
- Stack J, Chaplin MJ, Clark J. (2002) Differentiation of prion protein glycoforms from naturally occurring sheep scrapie sheep-passaged scrapie strains (CH1641 and SSBP1) bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol (Berl) 104:279–286 [DOI] [PubMed] [Google Scholar]
- Telling GC, Scott M, Hsiao KK, Foster D, Yang SL, Torchia M, Sidle KC, et al. (1994) Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc Natl Acad Sci USA 91:9936–9940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray AM, Klein MA, Aguzzi A, Bujdoso R. (2002) Chronic subclinical prion disease induced by low-dose inoculum. J Virol 76:2510–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GAH, Scott AC, Johnson CT, Gunning RF, Hancock RD, Jeffrey M, Dawson M, et al. (1987) A novel progressive spongiform encephalopathy in cattle. Vet Rec 121:419–420 [DOI] [PubMed] [Google Scholar]