Abstract
The aim of this study was to evaluate seven anti-TIMP-1 (tissue inhibitor of metalloproteinase-1) monoclonal antibodies by immunohistochemical (IHC) staining of formalin-fixed, paraffin-embedded (FFPE) tissue. Detection of the TIMP-1 protein was studied by IHC in FFPE human archival normal and neoplastic samples. Indirect IHC technique was used, and the seven antibodies (clones VT1, VT2, VT4, VT5, VT6, VT7, and VT8) were tested in various concentrations using different pretreatment protocols. All seven VT antibodies specifically immunostained the cytoplasm of islets of Langerhans cells in normal pancreas, epithelial cells of hyperplastic prostate, tumor cells of medullary thyroid carcinoma, and fibroblast-like cells of malignant melanoma. Specificity of the anti-TIMP-1 antibodies was confirmed by several controls, e.g., Western blotting on proteins extracted from FFPE tissue showed that the VT7 antibody reacted specifically with a protein band of ~28 kDa, corresponding to the molecular mass of TIMP-1. However, sensitivity varied with the different antibodies. Use of heat-induced epitope retrieval (HIER) and the VT7 clone applied at low concentrations demonstrated more intense immunoreactivity with the TIMP-1-positive cell types compared to the other six clones. Furthermore, when tested on a range of normal and neoplastic endocrine tissues, the VT7 clone demonstrated immunoreactivity with all neuroendocrine cell types. In conclusion, all seven antibodies detected TIMP-1 protein in various normal and neoplastic FFPE tissues, but one clone, VT7, was superior for IHC staining of TIMP-1 in FFPE tissue sections when using HIER.
Keywords: tissue inhibitor of metalloproteinase-1, VT7, immunohistochemistry, monoclonal antibodies, neuroendocrine tissue
Tissue inhibitor of metalloproteinase-1 (TIMP-1) is a 28.5-kDa glycoprotein consisting of 184 amino acids in the mature protein and is the most extensively studied of the four TIMP family proteins (TIMP-1, −2, −3, and −4) characterized so far (Welgus et al. 1979; Stricklin and Welgus 1983; Welgus and Stricklin 1983; Carmichael et al. 1986; Gomez et al. 1997). TIMP-1 was originally named for the ability to inhibit the activity of the matrix metalloproteinases (MMPs), which is a family of Zn2+- dependent endopeptidases (consisting so far of 24 members) (Mook et al. 2004) that are capable of degrading most, if not all, components of the extracellular matrix (Curran and Murray 1999). However, TIMP-1 also exhibits several other biological functions including stimulation of proliferation in various cell types (Brew et al. 2000; Fassina et al. 2000; Lafleur et al. 2003) and inhibition of apoptosis (Guedez et al. 1998; Li et al. 1999; Murphy et al. 2002; Lambert et al. 2003; Liu et al. 2003).
TIMP-1 is present in a wide range of normal and pathological tissues as well as in body fluids (Welgus and Stricklin 1983; Cawston et al. 1986; Tomita and Iwata 1996,1997,1999; Tomita 1997a,b; Isisag et al. 2003; Holten-Andersen et al. 2005; Liu et al. 2005). However, it is particularly the involvement of TIMP-1 in various cancers that has made this molecule the subject of many studies. Although early studies have shown TIMP-1 to have anti-tumor or anti-metastatic effects (Schultz et al. 1988; Khokha et al. 1989; Alexander and Werb 1992), more recent reports indicate a dual function with positive correlation between increased TIMP-1 tumor tissue levels and poor outcome in colorectal, breast, and non-small-cell lung cancer (Fong et al. 1996; Murashige et al. 1996; Ree et al. 1997; Wurtz et al. 2005). Our own studies have shown that plasma levels of TIMP-1 also carry the potential as a marker for colorectal cancer prognosis (Holten-Andersen et al. 2000). In addition, we have found that plasma TIMP-1 measurements can be used for the early detection of colorectal cancer (Holten-Andersen et al. 1999,2002). By immunohistochemistry (IHC), TIMP-1 localization studies in various neoplastic tissues have similarly demonstrated TIMP-1 as a potential tumor marker for different cancers, e.g., colorectal cancer (Holten-Andersen et al. 2005) and breast cancer (Jones et al. 1999). Common to these cancers is that TIMP-1 is primarily located in the stromal compartment of the tumors. In contrast, in various human neuroendocrine cancers, TIMP-1 protein is localized exclusively to the tumor cells, e.g., in islet cell tumors (Tomita and Iwata 1997), medullary thyroid carcinoma (MTC) (Tomita 1997b), parathyroid carcinoma (Tomita and Iwata 1999), and Merkel cell carcinoma (Massi et al. 2003).
IHC for detection of various tumor markers in neoplastic formalin-fixed, paraffin-embedded (FFPE) tissue sections have often demonstrated very conflicting interlaboratory results. Localization studies of TIMP-1 in FFPE colorectal cancer tissue have shown different results among research groups (Hewitt et al. 1991; Tomita and Iwata 1996; Holten-Andersen et al. 2005). The reason for these conflicting results may be due to differences in the IHC protocol used in the studies, e.g., variation in fixation time of the particular tissue, no or less-optimal antigen retrieval and, probably most importantly, differences in the specificity and sensitivity of the antibodies used. In order to validate antibodies for the detection of a protein in FFPE tissue sections, optimization of a range of parameters in the IHC protocol is required, e.g., methods for retrieval of antigenic determinants that are damaged or masked by fixation. Heating in different buffers and protease digestion of the tissue prior to the staining procedure have shown to be valuable tools for improving the IHC performance of antibodies (Shi et al. 1997,2001).
In the current study we characterized and optimized seven new TIMP-1 monoclonal antibodies (MAbs) for IHC staining. We used various normal and neoplastic human samples to compare and assess the seven antibodies with regard to sensitivity and specificity. The antibodies had previously been characterized biochemically with regard to affinities, specificities, linear epitopes, recognition, and their abilities to sandwich with each other (Moller Sorensen et al. 2005). Of the antibodies tested in the present study, one clone—VT7—exhibited the highest sensitivity and was chosen for further testing on a range of neuroendocrine tissue sections. Together the results demonstrate the VT7 antibody to be optimal for IHC detection of TIMP-1 in FFPE tissues.
Materials and Methods
Tissue Samples
Localization of the TIMP-1 protein was studied in human FFPE archival tissue samples obtained from the Department of Pathology, Odense University Hospital in accordance with approval by the Scientific Ethical Committee for the county of Funen (Fyns Amt), Denmark (VF 20050110). Tissues were routinely fixed in formalin prior to paraffin embedding.
For the comparative study of the seven anti-TIMP-1 MAbs, sections were cut from multi-tissue blocks (MTB) containing normal tissue from pancreas, colon, tonsil, liver, cerebellum, kidney, tissue from hyperplastic prostate, and neoplastic tissues from MTC and malignant melanoma.
For further characterization of the VT7 clone, a selection of normal and neoplastic archival human endocrine tissues was collected in five different MTBs. In addition, TIMP-1 immunoreactivity was tested on whole paraffin blocks of the following tissues: hyperplastic prostate, normal thyroid gland, normal parathyroid gland, MTC, and neuroblastoma.
Antibodies
Seven mouse MAbs raised against recombinant human TIMP-1 (clones VT1, VT2, VT4, VT5, VT6, VT7, VT8) were included (SSI; Copenhagen, Denmark). All clones are IgG1 subtype. TIMP-1 antibodies were used at concentrations ranging from 0.1 to 10 μg/ml as specified in the Results section. In addition, MAbs against chromogranin A (1:100, clone DAK-A3, cat. #M0869) and Aspergillus niger glucose oxidase (negative control antibody, IgG1, cat. #X0931) were obtained from Dako A/S (Glostrup, Denmark).
IHC
Reagents used for IHC staining were obtained from Dako A/S and were used according to the manufacturer's instructions.
Paraffin sections (5 μm) were dewaxed with xylene and rehydrated through ethanol/water dilutions. Each antibody was tested with six different protocols: (1) without antigen retrieval of the sections; (2) proteolytic treatment by proteinase K (cat. #S3020) digestion for 5 min at room temperature or with heat-induced epitope retrieval (HIER) in one of four different buffers; (3) 10 mM citrate buffer, pH 6; (4) target retrieval solution, pH 6.1 (cat. #S1700); (5) target retrieval solution, pH 9 (cat. #S2368); (6) target retrieval solution, pH 9.9 (cat. #S3308). HIER procedure was performed by placing the sections in preheated retrieval solution for 40 min at 95-99C (not boiling) followed by 20 min at room temperature. Endogenous peroxidase activity in the sections was quenched by immersion in 3% hydrogen peroxide (cat. #S2023) for 5 min. Sections were incubated with primary antibody and diluted in antibody diluent (cat. #S3022) for 30 min at room temperature. MAbs were detected with mouse/rabbit Envision+ (cat. #K5007), and the reactions were visualized by incubating the sections with DAB+ (cat. #K5007) for two periods of 3 min. Washes between incubations were carried out with TBS containing 0.05% Tween 20, pH 7.6 (cat. #S3006). Sections were counterstained with Mayer's hematoxylin, and all staining procedures were performed in a Dako Autostainer.
For each experiment the following specificity control was included: one section was incubated with an irrelevant MAb against Aspergillus niger glucose oxidase diluted to the same IgG concentration as the primary antibody. In addition, one section was incubated with VT7 antibody pre-absorbed with a 10-fold molar excess of purified recombinant soluble TIMP-1 protein (R&D Systems; Minneapolis, MN).
IHC Analysis
Immunostaining of tissue sections was assessed semi-quantitatively using + and − symbols as a measure of the intensity in particular cell/tissue types: + indicates pale staining, ++ indicates marked staining, and +++ signifies intense immunostaining. A score of +/- means very weak staining in~1% of the cells, whereas a score of − means that there is no positive staining in any cell.
Protein Extraction
Proteins were extracted from FFPE tissue sections essentially as described previously (Ikeda et al. 1998). In brief, paraffin sections (5-μm thick) were cut for IHC staining and 20-μm-thick parallel sections were cut for protein extraction and mounted on Superfrost glass slides. Twelve 20-μm-thick sections for protein extraction were dewaxed in xylene, rehydrated in graded ethanol, immersed in distilled water, and air dried. Tissues were transferred to 400 μl of radioimmunoprecipitation (RIPA) buffer (50 mM Tris-HCl, pH 7.4, 1% NP40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 2% SDS, 10 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin A, 100 μg/mL Pefa-block). Samples were incubated at 100C for 20 min followed by incubation at 60C for 2 hr. After incubation, tissue lysates were centrifuged at 15,000 × g for 20 min at 4C. Supernatants were collected and proteins were precipitated by adding 1:9 ice-cold 96% ethanol and incubating the mixture overnight. Samples were centrifuged at 15,000 × g for 30 min at 4C. Supernatants were discarded and pellets were resuspended in RIPA buffer and stored at −20C until use for protein assay and Western blot analysis.
Protein Assay
Protein concentration of the lysates was determined with the BCA Protein assay kit (Pierce Biotechnology; Rockford, IL). Reagents were added to the lysates, and the absorbance was read at 570 nm. Protein concentration was determined based on the standard curve using BSA.
Western Blot Analysis
Proteins (~45 μg) were separated on a 12% polyacrylamide gel and blotted on nitrocellulose paper. The blot was blocked in PBS containing 0.1% Tween 20 and 5% dry milk for 1 hr and then incubated overnight with the primary antibody (anti-TIMP-1 mouse MAb, clone VT7, 3 μg/ml) in PBS containing 0.1% Tween 20 and 1% dry milk. Subsequently, the blot was washed 3X for 10 min in PBS containing 0.1% Tween 20, incubated with horseradish peroxidase-conjugated secondary antibody (1:1000, goat anti-mouse antibody, code #P0447; Dako A/S) in PBS containing 0.1% Tween 20 1% dry milk for 1 hr followed by washing 3X for 10 min in PBS containing 0.1% Tween 20. The blot was developed by ECL+ detection system (Amersham; Buckinghamshire, UK) according to the manufacturer's instructions.
Results
Comparison of Seven MAbs for Detection of TIMP-1 Protein in Various Normal and Neoplastic Human Tissues
All seven anti-TIMP-1 antibodies detected the TIMP-1 protein in the cytoplasm of cells from normal and neoplastic tissues. These cell types/tissues included islets of Langerhans cells in normal pancreas, tumor cells of the MTC (Figure 1Bi), epithelial cells of the hyperplastic prostate (Figure 1Biii), and fibroblast-like cells of malignant melanoma. Furthermore, in three types of tissues, some of the antibodies showed immunoreactivity. In normal colon, single cells scattered among the epithelial cells at the bottom of the colon crypts were detected by all the TIMP-1 antibodies with exception of VT1 (Table 1; Figures 2D and 2F). Localization and morphology (bottle shape) of these cells suggested a neuroendocrine origin, which was confirmed by IHC staining with a monoclonal anti-chromogranin A antibody, a marker of neuroendocrine cells, and VT7 on parallel sections. The TIMP-1-positive cells colocalized with a subpopulation of chromogranin A-positive cells (not shown). Furthermore, a fraction of lymphocytes in the normal tonsil immunoreacted with the VT2, VT5, VT6, VT7, and VT8 MAbs and the VT7 detected the Purkinje cells of the normal cerebellum.
Figure 1.
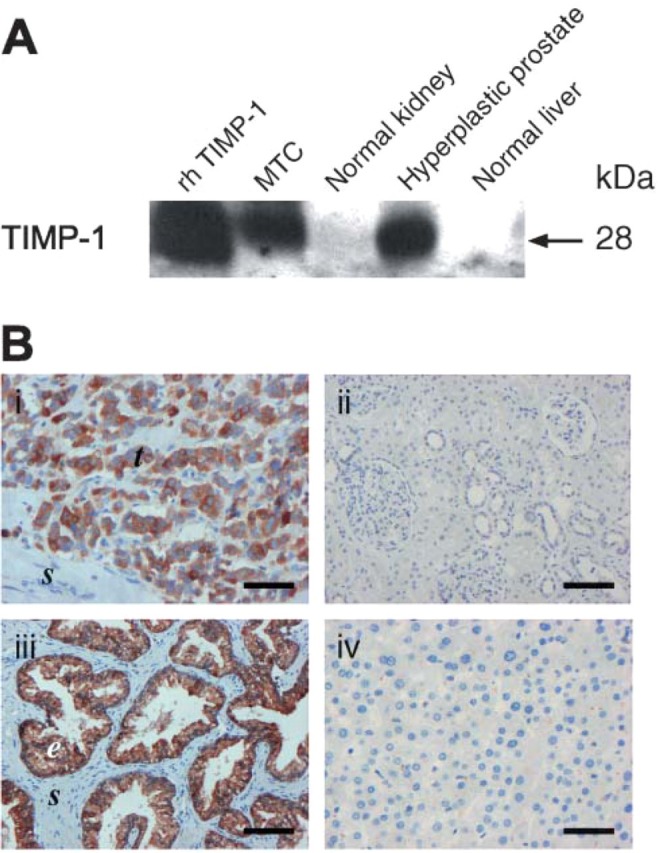
(A) Western blot analysis with VT7 of proteins extracted from FFPE tissue sections from medullary thyroid carcinoma (MTC), normal kidney, hyperplastic prostate, and normal liver. For each sample, ~45 μg of extracted protein was loaded on gels. As a positive control, recombinant human TIMP-1 (rhTIMP-1) was included (5 ng). The TIMP-1 protein was detected in the MTC and in the hyperplastic prostate, whereas normal kidney and normal liver were negative. (B) Immunostaining of parallel FFPE tissue sections from the paraffin blocks used for Western blotting. TIMP-1 immunoreactivity [VT7 monoclonal antibody (MAb), 0.4 μg/mL] was demonstrated in the tumor cells (t) of MTC (i) and in the epithelial cells (e) of hyperplastic prostate (iii). Normal kidney (ii) and normal liver (iv) showed no TIMP-1 immunostaining. Bars: B (i,iv) = 20 μm; (ii,iii) = 10 μm. s, stromal cells.
Table 1.
Normal and neoplastic tissues immunoreactive with VT1, VT2, VT4, VT5, VT6, VT7, and VT
|
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No antigen retrieval (concentration in μg/mL) | Proteolytic treatment (proteinase K) (concentration in μg/mL) | Target retrieval solution, pH 6.1 (concentration in μg/mL) | ||||||||||
|
|
|
|
||||||||||
| Cell type | Clone | 0.1 | 10 | 0.1 | 1 | 10 | 0.1 | 1 | 10 | |||
|
| ||||||||||||
| Langerhans cells of pancreas islets | VT-1 | − | − | + | − | − | +/− | − | − | − | ||
| VT-2 | − | + | ++ | − | − | +++∗ | − | − | + | |||
| VT-4 | − | ++ | +++ | − | + | ++ | +/-+ | + | ||||
| VT-5 | − | +/− | +++ | − | − | +/− | − | +/− | + | |||
| VT-6 | − | +/− | − | − | − | − | +/− | +++∗ | ||||
| VT-7 | − | +++ | +++∗ | − | − | − | + | +++ | +++∗∗ | |||
| VT-8 | − | + | +++ | − | − | +/− | − | − | + | |||
| Epithelial cells of hyperplastic prostate | VT-1 | − | + | +/− | + | − | +/− | + | +/− | ++ | ++ | |
| VT-2 | − | + | +++∗ | − | +/− | ++∗ | − | +/− | ++∗ | |||
| VT-4 | − | + | ++∗ | − | + | +++∗ | +/− | +++ | +++∗ | |||
| VT-5 | − | +/− | + | − | − | +∗ | +/− | ++ | +++∗ | |||
| VT-6 | − | − | − | − | − | − | + | +++ | ||||
| VT-7 | − | ++ | +++∗ | − | − | − | + | +++∗ | +++∗∗ | |||
| VT-8 | − | + | ++∗ | − | +/− | ++ | +/− | ++ | +++∗ | |||
| Tumor cells of medullary thyroid carcinoma | VT-1 | − | − | − | − | − | − | − | + | + | ||
| VT-2 | − | +/− | ++∗ | − | +/− | ++ | − | + | ++ | |||
| VT-4 | − | NA | NA | − | NA | NA | +/− | NA | NA | |||
| VT-5 | − | − | +/− | − | − | +/− | +/− | ++ | +++∗ | |||
| VT-6 | − | − | − | − | − | − | − | + | ++ | |||
| VT-7 | − | + | +++∗ | − | − | +/− | + | +++ | +++∗∗ | |||
| VT-8 | − | − | +/− | − | − | +/− | +/− | ++ | +++∗ | |||
| Fibroblast-like cells of malignant melanoma | VT-1 | − | − | ++ | − | − | − | − | − | − | ||
| VT-2 | − | + | + | − | + | + | − | + | +++ | |||
| VT-4 | − | +/− | − | − | − | − | +/− | − | +/− | |||
| VT-5 | − | − | − | − | − | − | − | ++ | +++ | |||
| VT-6 | − | − | − | − | − | − | +/− | +/− | +/− | |||
| VT-7 | − | + | +++∗ | − | − | − | + | +++ | +++∗∗ | |||
| VT-8 | − | − | +/− | − | − | − | +/− | +/− | ++∗ | |||
| Neuroendocrine cells of normal colon | VT-1 | − | − | − | − | − | − | − | − | − | ||
| VT-2 | − | +++ | +++ | − | +/− | +++ | − | − | +++ | |||
| VT-4 | − | − | +/− | − | − | +/− | − | ++ | ++∗ | |||
| VT-5 | − | − | +/− | − | − | +/− | − | ++ | +++∗ | |||
| VT-6 | − | − | − | − | − | − | − | − | ++∗ | |||
| VT-7 | − | +/− | +++∗ | − | − | − | +/− | +++ | +++∗∗ | |||
| VT-8 | − | − | +/− | − | − | − | − | − | +/− | |||
|
| ||||||||||||
+, Pale staining in this cell type. ++, Marked staining. +++, Intense immunoreactivity. A score of +/- means very weak staining in approximately 1% of the cell type, whereas a score of - means that there is no positive staining in any cell of this type. ∗Some nonspecific staining and ∗∗strong nonspecific staining. Antibodies are tested at three concentrations: 0.1 μg/mL, 1 μg/mL, and 10 μg/mL, each in three different immunohistochemical (IHC) protocols: no antigen retrieval, proteolytic treatment (proteinase K), and heat-induced epitope retrieval (HIER) in a target retrieval solution, pH 6.1.
N/A, not applicable. Boldface type indicates optimal staining with the VT7 antibody.
Figure 2.
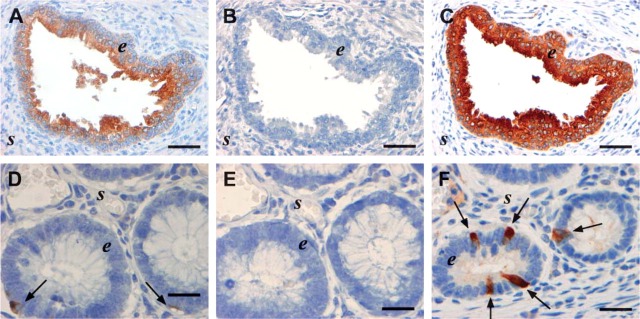
Immunohistochemistry (IHC) for TIMP-1 performed on parallel tissue sections of hyperplastic prostate (A-C) and normal colon (D-F) with the VT7 MAb (1 μg/mL) using different IHC protocols concerning antigen retrieval. (A,D) No antigen retrieval. (B,E) Proteolytic pretreatment (proteinase K). (C) Heat-induced epitope retrieval (HIER) in 10 mM citrate buffer, pH 6. (F) HIER in target retrieval solution, pH 6.1. Strong immunoreactivity is obtained in both tissues with HIER pretreatment (C,F), and weak staining is also seen without pretreatment (A,D). In prostate, staining is observed in the epithelial cells (e) of A,C with no staining in the stroma (s). In normal colon mucosa, immunoreactivity is seen in neuroendocrine cells (arrows in D,F). Bars: A-C = 50 μm; D-F = 20 μm.
Normal kidney (Figure 1Bii) and hepatocytes of the normal liver (Figure 1Biv) displayed no TIMP-1 staining with any of the antibodies used.
Specificity of the Seven VT Clones
The negative control antibody (mouse IgG1) diluted to the same IgG concentration as the anti-TIMP-1 antibody was used on all tissues and displayed no staining (not shown). Similarly, the pre-absorption control test in which the sections were incubated with VT7 antibody pre-absorbed with recombinant TIMP-1 was negative.
Furthermore, a Basic Local Alignment Search Tool (BLAST) analysis was performed in order to test if the specific sequences (linear epitopes) of the TIMP-1 protein that are bound by VT2, VT6, and VT7 (Moller Sorensen et al. 2005) also could be found in other known proteins. The result was that no known sequences in other human proteins matched the sequences of the TIMP-1 protein that react with the VT2, VT6, and VT7 antibodies. Finally, extraction of proteins from two tissues positive for TIMP-1 by IHC (MTC and hyperplastic prostate) and two tissues negative for TIMP-1 (normal kidney and normal liver) followed by Western blotting and VT7 incubation demonstrated an ~28-kDa band in the TIMP-1 IHC-positive tissues, whereas the TIMP-1 IHC-negative tissues were also negative by Western blotting (Figure 1A).
Sensitivity of the Seven VT Clones
Sensitivity of the seven TIMP-1 MAbs depended strongly on three parameters: the antigen retrieval protocol, the concentration of the antibodies, and the cell type/tissue tested.
Optimal IHC results were, for most of the tissues and antibodies, obtained using MAbs in combination with a citrate buffer for antigen retrieval, either the “classic” 10-mM citrate buffer, pH 6, or target retrieval solution, pH 6.1 (modified commercial citrate buffer). However, using the target retrieval solution, pH 9, in the HIER protocol resulted in a less-optimal staining (low signal/noise ratio). Furthermore, when using the target retrieval solution, pH 9.9, as a pretreatment buffer, the procedure resulted in an impaired morphology. In order to improve the performance of these buffers, HIER protocol was changed using a more gentle procedure (20-min heating in the buffer instead of 40 min). This did not, however, improve the staining or the morphology.
Performance of the MAbs in combination with an antigen retrieval protocol using proteolytic treatment (proteinase K) resulted in a weak or negative result for all MAbs (Table 1).
Finally, absence of antigen retrieval resulted likewise in a less-optimal performance of the antibodies except for the staining of the islets of Langerhans cells in normal pancreas. The antibodies performed equally well or slightly better with this tissue without antigen retrieval as when using HIER in combination with a citrate buffer.
IHC performance of the seven antibodies diluted to a very low concentration (0.1 μg/mL), a relatively low concentration (1 μg/mL), or a very high concentration (10 μg/mL) is listed in Table 1. Furthermore, IHC results are shown for three IHC protocols: proteolytic treatment (proteinase K), HIER in a citrate buffer (target retrieval solution, pH 6.1), or without antigen retrieval. The antibodies were also tested in concentrations between 1 μg/mL and 10 μg/mL, but these are omitted from the table because the highest concentration (10 μg/mL) is generally needed for all the clones, with the exception of VT7, to give a strong signal in the positive tissues. For the VT7 antibody, a concentration of 10 μg/mL results in pronounced background staining, regardless of the pretreatment used.
For detection of TIMP-1 in islets of Langerhans cells in normal pancreas, all antibodies showed immunoreactivity to the protein. However, VT7 was the only antibody to demonstrate an intense immunoreactivity (+++) when used at the low concentration (1 μg/mL) (with or without antigen retrieval), and even at the very low concentration of 0.1 μg/mL, this antibody demonstrated a pale (+) immunostaining. The intense specific VT7 staining was accompanied by some nonspecific staining indicating that the optimal concentration lies somewhere between 0.1 and 1.0 μg/mL. The high-intensity staining (+++) could also be achieved with the other VT clones, even without pretreatment, but only when applied at the highest concentration (10 μg/mL).
TIMP-1 in the epithelial cells of hyperplastic prostate was detected by all MAbs even at the relatively low concentration (1 μg/mL). However, VT4 and VT7 were the only clones to demonstrate an intense immunoreactivity (+++) at the low concentration (1 μg/mL) and a pale immunoreactivity (+) when further diluted 10 times. Again, this performance was achieved only when the tissue had been treated with HIER in a citrate buffer. The other antibodies also showed optimal performance in combination with antigen retrieval. However, they were capable of demonstrating only a pale (+) or moderate signal (++). Comparison of the performance of the VT7 clone in combination with three different antigen retrieval protocols is shown for the hyperplastic prostate in Figures 2A-2C.
Equally, tumor cells of the MTC were detected by all the MAbs. VT7 and VT4 were again the only antibodies to demonstrate an intense immunoreactivity (+++) at the concentration of 1 μg/mL (Figure 3) and a pale immunostaining in the 10-fold dilution. These results were again obtained only when the tissue was treated with HIER. The other antibodies demonstrated only very weak staining of pale (+) or moderate intensity (++), even at the highest concentration (10 μg/mL).
Figure 3.
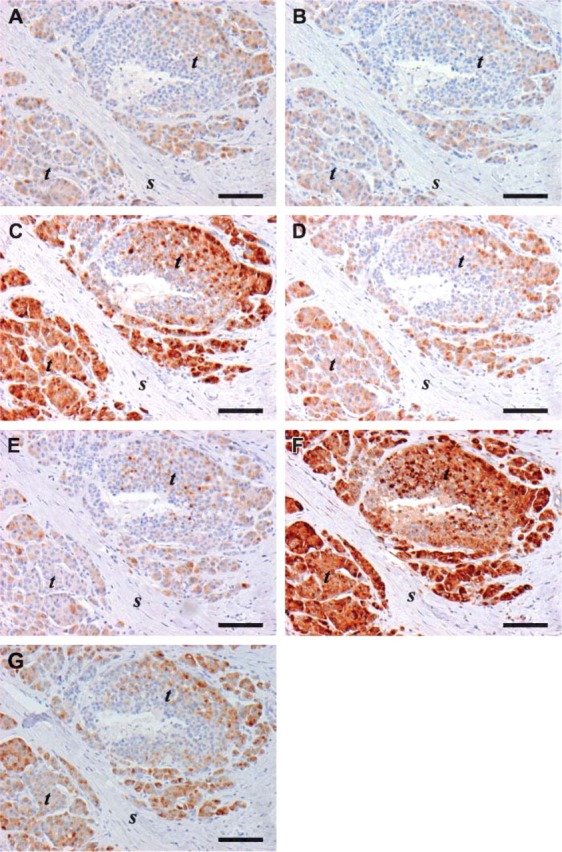
IHC performed on parallel tissue sections of human MTC with the seven anti-TIMP-1 MAbs (A-G): (A) VT1. (B) VT2. (C) VT4. (D) VT5. (E) VT6. (F) VT7. (G) VT8 used at the same concentration (1 μg/mL). Immunostaining is seen in tumor cells (t) with all seven antibodies with little or no staining observed in the stroma (s). The strongest immunoreactivity is seen with clone VT4 (C) and VT7 (F). The target retrieval solution, pH 6.1, is used in the HIER protocol for all the stainings. Bar = 100 μm.
Finally, fibroblast-like cells of malignant melanoma were detected by all the VT antibodies. However, only VT7 antibody was capable of demonstrating an intense (+++) immunoreactivity, and this was obtained only with HIER and an antibody concentration of 1 μg/mL.
Further optimization of the VT7 MAb using concentrations in the range of 0.1-1 μg/mL on sections of the MTB showed that a concentration of 0.4 μg/mL was optimal with strong staining intensity and negligible background staining (data not shown).
These IHC results, together with previous published biochemical analyses (Moller Sorensen et al. 2005), indicate that the seven MAbs (VT1, VT2, VT4, VT5, VT6, VT7, VT8) are highly specific for TIMP-1 detection in archival human FFPE tissue sections. However, regarding sensitivity, results demonstrate that the VT7 clone is superior to the other clones.
IHC Staining of Normal Endocrine Tissue Sections with VT7
In order to further test the specificity and sensitivity of the VT7 antibody, a range of various normal and neoplastic human endocrine FFPE tissue sections was stained for TIMP-1 protein immunoreactivity using the VT7 MAb (0.4 μg/mL) in combination with a HIER protocol (target retrieval solution, pH 6.1). Tissues were selected in order to cover an array of tissues from various endocrine organs, thereby gaining an overview of the IHC specificity of the VT7 clone.
For the normal tissues, TIMP-1 stainings were strongly (+++) positive in scattered cells (“neuroendocrine cells”, see above) of the normal colon as shown previously for the higher concentration (1 μg/mL) of the antibody (Table 1; Figure 2). All other cell types in this tissue were negative, including the epithelial cells (Figures 4A and 4B). The same staining pattern was also seen in the rest of the tissues of the alimentary tract including esophagus, stomach, small intestine, and rectum (Table 2). In pancreas, the VT7 clone detected TIMP-1 protein in islets of Langerhans cells in normal pancreas with high intensity (+++), whereas the acinar cells were negative (Table 2; Figures 4C and 4D). VT7 detected with equally high intensity TIMP-1 protein in chief cells of normal parathyroid gland (Table 2; Figures 4E and 4F) and with moderate intensity chromaffin cells of the adrenal medulla (Table 2; Figures 4G and 4H), both belonging to the neuroendocrine cell population of the human body. In all cells mentioned, subcellular localization of the TIMP-1 protein was the cytoplasmic compartment. Identity of TIMP-1-positive cells as neuroendocrine cells was confirmed by a chromogranin A (neuroendocrine marker) staining of all endocrine tissues (data not shown).
Figure 4.
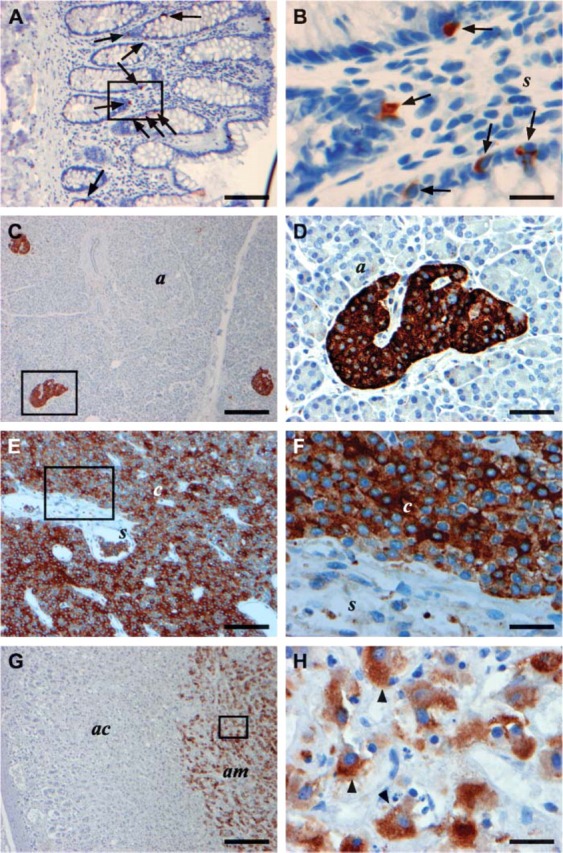
TIMP-1 immunoreactivity in various human normal endocrine archival FFPE tissue sections stained with the VT7 MAb (A-H). Framed areas in A,C,E,G are shown at higher magnifications in B,D,F,H, respectively. Immunostaining for TIMP-1 is seen in neuroendocrine cells of normal colon mucosa (arrows in A,B) with no staining of the epithelial cells (e) or the stromal cells (s), in islets of Langerhans cells (l) in pancreas with no staining in the acinar cells (a) (C,D), in chief cells (c) of the normal parathyroid (E,F), and in chromaffin cells of normal adrenal medulla (am) with no staining seen in the adrenal cortex (ac) (G) and arrowheads in H. Bars: A,E,G = 100 μm; C = 200 μm; B,F,H = 20 μm; = 50 μm.
Table 2.
Normal and neoplastic endocrine tissues immunoreactive with VT7
|
| ||||
|---|---|---|---|---|
| Tissue | Cell type | n | IHC score | |
|
| ||||
| Normal endocrine tissues | ||||
| Parathyroid glands | Chief cells | 3 | +++ | |
| Adrenal gland | Chromaffin cells | 1 | ++ | |
| Esophagus | Neuroendocrine cells | 1 | ++ | |
| Stomach | Neuroendocrine cells | 1 | +++ | |
| Small intestine | Neuroendocrine cells | 2 | +++ | |
| Colon | Neuroendocrine cells | 4 | +++ | |
| Rectum | Neuroendocrine cells | 1 | +++ | |
| Pancreas | Cells of the Langerhans islets | 4 | +++ | |
| Neoplastic endocrine tissues | ||||
| Parathyroid adenoma | Chief cells | 3 | +++ | |
| Lung carcinoid | Tumor cells | 4 | +++ | |
| Appendiceal adenocarcinoid | Tumor cells | 3 | +++ | |
| Rectal carcinoid | Tumor cells | 2 | +++ | |
| Small intestinal carcinoid | Tumor cells | 4 | +++ | |
| Pheochromocytoma | Tumor cells | 6 | +++ | |
| Medullary thyroid carcinoma | Tumor cells | 4 | +++ | |
| Merkel cell carcinoma | Tumor cells | 1 | + | |
| Neuroblastoma | Tumor cells | 3 | +++ | |
|
| ||||
+, Pale staining in this cell type. ++, Marked staining. +++, Intense immunoreactivity. ∗Nonspecific staining. n, number of different samples stained for each tissue.
The VT7 antibody is diluted to a concentration of 0.4 μg/mL, and the target retrieval solution, pH 6.1, is used in the HIER IHC protocol.
IHC Staining of Neoplastic Endocrine Tissues with VT7
For the neoplastic endocrine tissues, neuroendocrine cells also showed intense TIMP-1 staining. All carcinoids included in the study showed an intense TIMP-1 staining of the tumor cells including lung carcinoid that originates from Kulchitsky cells (K-cells) of the bronchi (Table 2), appendiceal carcinoid (Table 2), rectal carcinoid (Table 2), and small intestinal carcinoid (Table 2; Figures 5A and 5B). Furthermore, insulinoma of the pancreas that originates from the β-cells of the islets of Langerhans (Table 2; Figures 5C and 5D), MTC that originates from the C-cells of the thyroid gland (Table 2; Figures 5E and 5F), and pheochromocytoma that originates from the chromaffin cells of the adrenal medulla showed a strong staining of the tumor cells (Table 2; Figures 5G and 5H). In addition, tumor cells of a neuroblastoma (Table 2) likewise demonstrated an intense immunoreactivity (+++). Finally, tumor cells of a Merkel cell tumor showed a pale immunoreactivity (+) with the VT7 MAb (Table 2). Similar to the normal endocrine tissues, subcellular localization of the TIMP-1 protein in the cells was the cytoplasmic compartment of all tumor cells tested, and the identity of the TIMP-1-positive cells as belonging to the neuroendocrine cell population was confirmed by a chomogranin A (neuroendocrine marker) staining (not shown).
Figure 5.
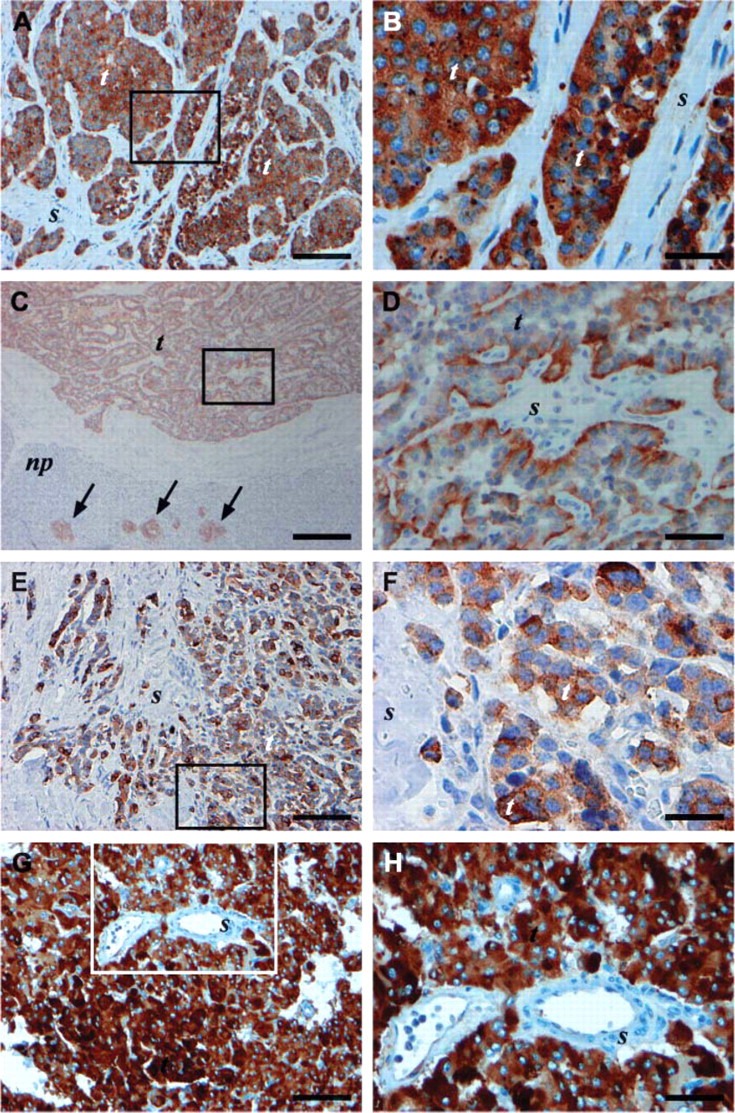
TIMP-1 immunoreactivity in various human neoplastic endocrine archival FFPE tissue sections with the VT7 MAb (A-H). Framed areas in A,C,E,G are shown at higher magnifications in B,D,F,H, respectively. Immunoreactivity for TIMP-1 was seen in tumor cells (t) of small intestine carcinoid (A,B), insulinoma (C,D), MTC (E,F), and pheochromocytoma (G,H) with little or no immunostaining in the stroma (s) of the four tumors. The islets of Langerhans cells of normal pancreas adjacent to the insulinoma are positive for TIMP-1 (arrows in C). Bars: A,E,G = 100 μm; B,F = 20 μm; C = 200 μm; D,H = 50 μm. np, normal pancreas tissue.
Stability of IHC Staining with VT7
All tissues used for optimization of IHC staining with the anti-TIMP-1 antibodies in this study were obtained from archival samples with no registration of fixation time. In order to test whether the performance of the VT7 MAb varied according to the fixation time, the VT7 antibody was tested on normal colon tissue sections fixed in 4% neutral-buffered formalin for 6 h, 24 h, 96 h, or 6 days. No difference in the staining intensity of the TIMP-1-positive cells in the colon tissue was observed (data not shown).
Furthermore, to test if prolonged storage of paraffin sections affects immunostaining, tissue sections were cut from the MTB and stored at 4C for 30, 21, 7, or 1 day(s), respectively, before staining with the VT7 antibody. There was no difference in staining intensity of TIMP-1-positive cells (data not shown).
Discussion
Seven TIMP-1 MAbs that had previously been biochemically characterized (Moller Sorensen et al. 2005) were tested in this study in order to determine the sensitivity and specificity for IHC detection of TIMP-1 in FFPE tissue sections. Results of this study show that when a specified IHC protocol was followed, all seven antibodies resulted in a similar staining pattern in the cytoplasm of cells from normal and neoplastic tissues, which previously had been demonstrated by other research groups to be immunoreactive for TIMP-1. These cell types/tissues included islets of Langerhans cells in normal pancreas (Tomita and Iwata 1997), epithelial cells of the hyperplastic prostate (Isisag et al. 2003; Liu et al. 2005), tumor cells of the MTC (Tomita 1997b), and fibroblast-like cells of malignant melanoma (Airola et al. 1999). Furthermore, antibodies showed positive immunoreactivity of neuroendocrine cells of normal colon mucosa, Purkinje cells of cerebellum, and a fraction of cells in the germinal centers of normal tonsil. To our knowledge, TIMP-1 protein has not previously been demonstrated by IHC in these cell types in human tissue, but our results are supported by findings from other research groups. Although TIMP-1 has not been shown previously in neuroendocrine cells of normal colon mucosa, it has been demonstrated in several other human neuroendocrine cell types (Tomita 1997b; Tomita and Iwata 1997,1999). Furthermore, similar to our finding in human cerebellum, Vaillant et al. (1999) demonstrated TIMP-1 by IHC in Purkinje cells of rat cerebellum. Finally, it has not been clarified which cells are responsible for immunoreactivity with the VT antibodies in the germinal centers of normal tonsil. However, one possibility is B cells because TIMP-1 mRNA has been previously demonstrated by Northern blot analysis in isolated tonsillar B cells (Stetler-Stevenson et al. 1997). In order to validate IHC results for the three cell types/tissues, further studies and a more extensive sample material are needed.
Specificity of the VT antibodies for TIMP-1 was confirmed by various controls: MTB sections incubated with an irrelevant MAb against Aspergillus niger glucose oxidase, IgG1, diluted to the same IgG concentration as the primary antibody, as well as MTB sections incubated with VT7 antibody pre-absorbed with a 10-fold molar excess of purified recombinant soluble TIMP-1 protein displayed no staining. Furthermore, BLAST analysis of the specific sequences (linear epitopes) of the TIMP-1 protein that react with VT2, VT6, and VT7 (Moller Sorensen et al. 2005) demonstrated that no known sequences in other human proteins matched the sequences of the TIMP-1 protein that react with these antibodies.
The fact that some of the seven antibodies bind to different epitopes on the TIMP-1 protein but at the same time show the same IHC staining pattern further supports the specificity of these antibodies. Finally, extraction of proteins from FFPE tissue sections followed by Western blotting with the VT7 antibody demonstrated an ~28-kDa band in the tissues that had proven positive for TIMP-1 by IHC (hyperplastic prostate and MTC), whereas no band was detected in the tissues negative by IHC (normal kidney and normal liver).
Sensitivity of the seven TIMP-1 MAbs depended strongly on three parameters: the antigen retrieval protocol, the concentration of the antibodies, and the tested cell type/tissue. For all VT antibodies, HIER in citrate buffer, either the “classic” 10 mM citrate buffer, pH 6, or target retrieval solution, pH 6.1 (modified commercial citrate buffer), generally resulted in an increase in intensity of immunoreactivity. However, the VT7 clone was found superior because this antibody demonstrated an optimal staining intensity (+++) even at very low concentrations (0.1-1 μg/mL) for all TIMP-1-positive tissues tested when using a citrate buffer for antigen retrieval.
A potential difficulty with FFPE tissue is formalin cross-linking of tissue proteins. In addition, the process of tissue embedding can mask or destroy antibody epitopes. This is particularly problematic for MAbs if the single epitope recognized is sensitive to formalin fixation. A way to circumvent this problem is to unmask cross-linked epitopes by pretreatment of the tissue sections with an antigen retrieval method such as digestion with proteases or by HIER in pretreatment buffers (Shi et al. 1997,2001). As demonstrated in the comparison of the seven VT antibodies in this study, different antibodies to TIMP-1 exhibit different sensitivities to antigen retrieval procedures. Therefore, it is unlikely that a single optimized method for IHC staining of TIMP-1 in FFPE tissue sections will be achievable for all TIMP-1 antibodies. However, for the VT7 antibody, optimal staining intensity was accomplished by using HIER in combination with a citrate buffer for detection of TIMP-1 protein in various tissues.
In order to further test the performance of the VT7 antibody, we used the optimized protocol (including HIER in a citrate buffer) on a range of neuroendocrine tissues previously described as immunoreactive with TIMP-1 antibodies (Tomita 1997a,b; Tomita and Iwata 1997,1999) and on three other endocrine tissues (pheochromocytomas, various carcinoids, and adrenal glands). Results of this study confirmed that the VT7 antibody at a very low concentration (0.4 μg/mL) reacts specifically and with high-staining intensity with various normal, benign, and malignant neuroendocrine tissues (see Table 2; Figure 4 and Figure 5). Therefore, these data support the work of Tomita and Iwata (1999) and their suggestion that IHC staining for TIMP-1 could be used as a new marker for neuroendocrine cells. Little is known regarding the function of TIMP-1 in endocrine tissues, but it has been suggested that the balance between MMPs and TIMPs may contribute to the structure-function relationships of the endocrine organs (Tomita and Iwata 1999).
In summary, a direct comparison of seven different TIMP-1 antibodies revealed that all the antibodies detected the TIMP-1 protein in various normal and neoplastic FFPE tissues and in clone VT7 was superior for IHC detection of TIMP-1 in FFPE tissue sections when using HIER.
Acknowledgments
We thank the Ministry of Science, Technology and Innovation, Copenhagen, Denmark for financial support.
We express our gratitude to Peter Staben, Dako A/S, for excellent technical assistance regarding the graphic implementation of the figures.
Literature Cited
- Airola K, Karonen T, Vaalamo M, Lehti K, Lohi J, Kariniemi AL, Keski-Oja J, et al. (1999) Expression of collagenases-1 and −3 and their inhibitors TIMP-1 and −3 correlates with the level of invasion in malignant melanomas. Br J Cancer 80:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CM, Werb Z. (1992) Targeted disruption of the tissue inhibitor of metalloproteinases gene increases the invasive behavior of primitive mesenchymal cells derived from embryonic stem cells in vitro. J Cell Biol 118:727–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477:267–283 [DOI] [PubMed] [Google Scholar]
- Carmichael DF, Sommer A, Thompson RC, Anderson DC, Smith CG, Welgus HG, Stricklin GP. (1986) Primary structure and cDNA cloning of human fibroblast collagenase inhibitor. Proc Natl Acad Sci USA 83:2407–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston TE, Noble DN, Murphy G, Smith AJ, Woodley C, Hazleman B. (1986) Rapid purification of tissue inhibitor of metalloproteinases from human plasma and identification as a gamma-serum protein. Biochem J 238:677–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran S, Murray GI. (1999) Matrix metalloproteinases in tumour invasion and metastasis. J Pathol 189:300–308 [DOI] [PubMed] [Google Scholar]
- Fassina G, Ferrari N, Brigati C, Benelli R, Santi L, Noonan DM, Albini A. (2000) Tissue inhibitors of metalloproteases: regulation and biological activities. Clin Exp Metastasis 18:111–120 [DOI] [PubMed] [Google Scholar]
- Fong KM, Kida Y, Zimmerman PV, Smith PJ. (1996) TIMP1 and adverse prognosis in non-small cell lung cancer. Clin Cancer Res 2: 1369–1372 [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. (1997) Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 74:111–122 [PubMed] [Google Scholar]
- Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Mansoor A, Stetler-Stevenson M. (1998) In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest 102:2002–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt RE, Leach IH, Powe DG, Clark IM, Cawston TE, Turner DR. (1991) Distribution of collagenase and tissue inhibitor of metalloproteinases (TIMP) in colorectal tumours. Int J Cancer 49:666–672 [DOI] [PubMed] [Google Scholar]
- Holten-Andersen MN, Christensen IJ, Nielsen HJ, Stephens RW, Jensen V, Nielsen OH, Sorensen S, et al. (2002) Total levels of tissue inhibitor of metalloproteinases 1 in plasma yield high diagnostic sensitivity and specificity in patients with colon cancer. Clin Cancer Res 8:156–164 [PubMed] [Google Scholar]
- Holten-Andersen MN, Hansen U, Brunner N, Nielsen HJ, Illemann M, Nielsen BS. (2005) Localization of tissue inhibitor of metalloproteinases 1 (TIMP-1) in human colorectal adenoma and adenocarcinoma. Int J Cancer 113:198–206 [DOI] [PubMed] [Google Scholar]
- Holten-Andersen MN, Murphy G, Nielsen HJ, Pedersen AN, Christensen IJ, Hoyer-Hansen G, Brunner N, et al. (1999) Quantitation of TIMP-1 in plasma of healthy blood donors and patients with advanced cancer. Br J Cancer 80:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten-Andersen MN, Stephens RW, Nielsen HJ, Murphy G, Christensen IJ, Stetler-Stevenson W, Brunner N. (2000) High pre-operative plasma tissue inhibitor of metalloproteinase-1 levels are associated with short survival of patients with colorectal cancer. Clin Cancer Res 6:4292–4299 [PubMed] [Google Scholar]
- Ikeda K, Monden T, Kanoh T, Tsujie M, Izawa H, Haba A, Ohnishi T, et al. (1998) Extraction and analysis of diagnostically useful proteins from formalin-fixed, paraffin-embedded tissue sections. J Histochem Cytochem 46:397–403 [DOI] [PubMed] [Google Scholar]
- Isisag A, Nese N, Ermete M, Lekili M, Ayhan S, Kandiloglu AR. (2003) Col IV and Fn distribution in prostatic adenocarcinoma and correlation of 67LR, MMP-9 and TIMP-1 expression with Gleason score. Anal Quant Cytol Histol 25:263–272 [PubMed] [Google Scholar]
- Jones JL, Glynn P, Walker RA. (1999) Expression of MMP-2 and MMP-9, their inhibitors, and the activator MT1-MMP in primary breast carcinomas. J Pathol 189:161–168 [DOI] [PubMed] [Google Scholar]
- Khokha R, Waterhouse P, Yagel S, Lala PK, Overall CM, Norton G, Denhardt DT. (1989) Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science 243:947–950 [DOI] [PubMed] [Google Scholar]
- Lafleur MA, Handsley MM, Edwards DR. (2003) Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med 2003:1–39 [DOI] [PubMed] [Google Scholar]
- Lambert E, Boudot C, Kadri Z, Soula-Rothhut M, Sowa ML, Mayeux P, Hornebeck W, et al. (2003) Tissue inhibitor of metalloproteinases-1 signalling pathway leading to erythroid cell survival. Biochem J 372:767–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Fridman R, Kim HR. (1999) Tissue inhibitor of metalloproteinase-1 inhibits apoptosis of human breast epithelial cells. Cancer Res 59:6267–6275 [PubMed] [Google Scholar]
- Liu AY, Zhang H, Sorensen CM, Diamond DL. (2005) Analysis of prostate cancer by proteomics using tissue specimens. J Urol 173:73–78 [DOI] [PubMed] [Google Scholar]
- Liu XW, Bernardo MM, Fridman R, Kim HR. (2003) Tissue inhibitor of metalloproteinase-1 protects human breast epithelial cells against intrinsic apoptotic cell death via the focal adhesion kinase/phosphatidylinositol 3-kinase and MAPK signaling pathway. J Biol Chem 278:40364–40372 [DOI] [PubMed] [Google Scholar]
- Massi D, Franchi A, Ketabchi S, Paglierani M, Pimpinelli N, Santucci M. (2003) Expression and prognostic significance of matrix metalloproteinases and their tissue inhibitors in primary neuroendocrine carcinoma of the skin. Hum Pathol 34:80–88 [DOI] [PubMed] [Google Scholar]
- Moller Sorensen N, Dowell BL, Stewart KD, Jensen V, Larsen L, Lademann U, Murphy G, et al. (2005) Establishment and characterization of 7 new monoclonal antibodies to tissue inhibitor of metalloproteinases-1. Tumour Biol 26:71–80 [DOI] [PubMed] [Google Scholar]
- Mook OR, Frederiks WM, Van Noorden CJ. (2004) The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta 1705:69–89 [DOI] [PubMed] [Google Scholar]
- Murashige M, Miyahara M, Shiraishi N, Saito T, Kohno K, Kobayashi M. (1996) Enhanced expression of tissue inhibitors of metalloproteinases in human colorectal tumors. Jpn J Clin Oncol 26:303–309 [DOI] [PubMed] [Google Scholar]
- Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, et al. (2002) Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem 277:11069–11076 [DOI] [PubMed] [Google Scholar]
- Ree AH, Florenes VA, Berg JP, Maelandsmo GM, Nesland JM, Fodstad O. (1997) High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin Cancer Res 3:1623–1628 [PubMed] [Google Scholar]
- Schultz RM, Silberman S, Persky B, Bajkowski AS, Carmichael DF. (1988) Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res 48: 5539–5545 [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Taylor CR. (2001) Antigen retrieval techniques: current perspectives. J Histochem Cytochem 49:931–937 [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Taylor CR. (1997) Antigen retrieval immunohistochemistry: past, present, and future. J Histochem Cytochem 45:327–343 [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson M, Mansoor A, Lim M, Fukushima P, Kehrl J, Marti G, Ptaszynski K, et al. (1997) Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in reactive and neoplastic lymphoid cells. Blood 89:1708–1715 [PubMed] [Google Scholar]
- Stricklin GP, Welgus HG. (1983) Human skin fibroblast collagenase inhibitor. Purification and biochemical characterization. J Biol Chem 258:12252–12258 [PubMed] [Google Scholar]
- Tomita T. (1997a) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in pituitary adenomas: possible markers of neuroendocrine cells. Endocr Pathol 8:305–313 [DOI] [PubMed] [Google Scholar]
- Tomita T. (1997b) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in thyroid C-cells and medullary thyroid carcinomas. Histopathology 31:150–156 [DOI] [PubMed] [Google Scholar]
- Tomita T, Iwata K. (1996) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in colonic adenomas-adenocarcinomas. Dis Colon Rectum 39:1255–1264 [DOI] [PubMed] [Google Scholar]
- Tomita T, Iwata K. (1997) Gelatinases and inhibitors of gelatinases in pancreatic islets and islet cell tumors. Mod Pathol 10:47–54 [PubMed] [Google Scholar]
- Tomita T, Iwata K. (1999) Matrix metalloproteinases in some endocrine organs and their tumors. Endocr Pathol 10:15–26 [Google Scholar]
- Vaillant C, Didier-Bazes M, Hutter A, Belin MF, Thomasset N. (1999) Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J Neurosci 19:4994–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus HG, Stricklin GP. (1983) Human skin fibroblast collagenase inhibitor. Comparative studies in human connective tissues, serum, and amniotic fluid. J Biol Chem 258:12259–12264 [PubMed] [Google Scholar]
- Welgus HG, Stricklin GP, Eisen AZ, Bauer EA, Cooney RV, Jeffrey JJ. (1979) A specific inhibitor of vertebrate collagenase produced by human skin fibroblasts. J Biol Chem 254:1938–1943 [PubMed] [Google Scholar]
- Wurtz SO, Schrohl AS, Sorensen NM, Lademann U, Christensen IJ, Mouridsen H, Brunner N. (2005) Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr Relat Cancer 12:215–227 [DOI] [PubMed] [Google Scholar]


