Abstract
Among elastic system fibers, oxytalan fibers are known as a ubiquitous component of the periodontal ligament, but the localization and role of elastin-containing fibers, i.e., elastic and elaunin fibers, has yet to be clarified. In this study, we immunohistochemically investigated the localization of elastin and fibrillin, major proteins of elastin-containing fibers in the periodontal ligament of rat lower first molars. At the light microscope level, distribution of elastin-positive fibers was not uniform but often concentrated in the vicinity of blood vessels in the apical region of the ligament. In contrast, fibrillin-positive fibers were more widely distributed throughout the ligament, and the pattern of their distribution was comparable to the reported distribution of oxytalan fibers. At the ultrastructural level, assemblies or bundles of abundant fibrillin-containing microfibrils were intermingled with a small amount of elastin. This observation indicated that elastin-positive fibers observed under the light microscope were elaunin fibers. No mature elastic fibers, however, were found in the ligament. These results show that the major components of elastic system fibers in the periodontal ligament of the rat mandibular first molar were oxytalan and elaunin fibers, suggesting that the elastic system fibers play a role in the mechanical protection of the vascular system. (J Histochem Cytochem 54:1095-1103, 2006)
Keywords: elastic system fibers, elastin, fibrillin, immunohistochemistry, ultrastructure
Elastic system fibers are composed of elastic, elaunin, and oxytalan fibers (Kielty et al. 2002,2005). Oxytalan fibers are made up of bundles of 10- to 12-nm microfibrils with no associated elastin, whereas in elastic fibers a core of elastin is peripherally associated with small numbers of microfibrils. Elaunin fibers are believed to be an immature form of elastic fibers and are made up of bundles of microfibrils intermingled with small amounts of elastin. These fibers are widely distributed among the various types of connective tissue, particularly where elasticity is required such as in the tissues of the skin, lung, and blood vessels.
The periodontal ligament is a dense connective tissue localized between the tooth and the alveolar bone with the role of supporting the tooth. It is composed mainly of collagen fibers, and oxytalan fibers are a ubiquitous component of this ligament in a variety of species (Nanci and Somerman 2003). There have been a number of conflicting reports concerning the localization and distribution of elastin-containing fibers, i.e., elastic and elaunin fibers, in the periodontal ligament. For example, conventional elastin staining was reported to have revealed the presence of elastic fibers in the periodontal ligament in man (Hanazawa and Horie 1934; Higashi 1964; Sims 1976; Sampson 1979) and in a variety of animals including rat (Sakai 1968), dog (Hanazawa and Horie 1934; Shinohara 1996), cat (Sampson 1979), and sheep (Sampson 1979). Johnson and Pylypas (1992) demonstrated elaunin fibers in the mouse periodontal ligament stained with high-iron diamine after removal of the surrounding collagen fibers by treatment with sodium hydroxide. On the other hand, elastic fibers were not observed in the periodontal ligament of mouse (Sampson 1979), rat molars (Tashiro et al. 2002), rat incisor (Carmichael and Fullmer 1966), or monkey (Sampson 1979). Chantawiboonchai et al. (1998), using a confocal laser-scanning microscope following elastin staining, found no elastin-containing fibers in the mouse periodontal ligament.
In vitro studies have also shown conflicting results. Although some laboratories have demonstrated the presence of tropoelastin mRNA in vitro in the fibroblasts of human periodontal ligament (Palmon et al. 2001; Redlich et al. 2004), the opposite result was recently reported by Tsuruga et al. (2002a,b). These latter authors demonstrated neither tropoelastin mRNA expression in the culture of human periodontal ligament fibroblasts nor soluble tropoelastin in the cell matrix layer.
As described above, distribution and even the presence of elastin-containing fibers in the periodontal ligament are not yet clear. In this study, an attempt was made to observe the presence and distribution, in the rat molar periodontal ligament, of proteins of elastin-containing fibers, i.e., fibrillin (a major component of connective tissue microfibrils) and elastin by means of immunohistochemical (IHC) as well as immunoelectron microscopic examinations. The results obtained were analyzed in relation to the possible roles of elastic system fibers.
Materials and Methods
Animals
Twenty male Wistar rats weighing ~200 g each were used. Use and treatment of study animals were in accordance with the Guidelines for the Treatment of Experimental Animals of Tokyo Dental College, Tokyo, Japan.
IHC
Animals were anesthetized with ketamine hydrochloride and fixed by perfusion with cold 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4, for 15 min. Mandibles were dissected out and placed in the same fresh fixative for 6 hr at 4C. They were then demineralized in 10% EDTA for 3 weeks at 4C, rinsed in 0.01 M PBS containing increasing amounts (10, 15, and 20%) of sucrose, and immersed in Tissue Tec OCT compound (Miles Laboratories; Naperville, IL) followed by quick freezing in liquid nitrogen. Fifteen-μm-thick sections were prepared with a cryostat (Leica; Nussloch, Germany) by cutting along either the sagittal or horizontal plane. The total number of sections prepared from the cervical to apical regions was 270.
Horizontal sections prepared from three regions—cervical, middle, and apical—and sagittal sections were first exposed to 5% normal sheep serum in PBS for 5 min and then incubated with primary antibodies for 16 hr at 4C. Primary antibodies used were rabbit anti-rat elastin polyclonal antibody (dilution 1:160, AB2039; Chemicon, Temecula, CA), and rabbit anti-bovine fibrillin antiserum (dilution 1:200, LB2297; LSL, Tokyo, Japan) in PBS. Negative control sections were incubated with either non-immunized rabbit serum or with PBS. After washing with PBS, they were incubated with peroxidase-linked F(abî)2 fragment of anti-rabbit IgG (from donkey) (dilution 1:100; Amersham, Buckinghamshire, UK) overnight at 4C. After washing with PBS, they were fixed with 0.5% glutaraldehyde in PBS for 5 min and incubated with 0.02% diaminobenzidine solution (Dojin Chemicals; Kumamoto, Japan) containing 0.003% hydrogen peroxide for 5 min at room temperature. Sections without counterstaining were washed with PBS, dehydrated in graded ethanol, and mounted on coverslips with a mounting medium for examination with a light microscope. Images were collected with a Zeiss Axiophot 2 microscope (Carl Zeiss; Hallbergmoos, Germany) equipped with a Zeiss Axio Cam HRC digital camera.
Immunoelectron Microscopy
Animals were fixed under anesthesia by perfusion with cold 4% paraformaldehyde-0.25% glutaraldehyde in 0.1 M sodium phosphate buffer, pH 7.4, for 15 min. Mandibles were dissected out and placed in the same fresh fixative for 6 hr at 4C. They were then demineralized in 10% EDTA for 3 weeks at 4C, rinsed in several changes of phosphate buffer, and left overnight at 4C. The tissue was dehydrated in a graded series of ethanol and embedded in Lowicryl K4M (Polysciences Inc.; Warrington, PA).
Thin sections were prepared with a Leica Ultracut microtome and placed on formvar-coated nickel grids. They were incubated with blocking solution (10% normal sheep serum in PBS) for 15 min and transferred onto the surface of a drop of primary antibody (dilution 1:50) in PBS in a humidified chamber for 2 hr at room temperature. After washing with PBS, grids were placed on the surface of a drop of goat anti-rabbit IgG (H + L) conjugated to 5-nm gold particles (dilution 1:50; Amersham) in PBS containing 1% BSA for 30 min. They were washed with PBS, postfixed with 2% glutaraldehyde in PBS for 10 min, and rinsed in distilled water. Immunolabeled sections were counterstained with uranyl acetate for 1 min followed by lead citrate for 30 sec and examined in an H-7100 electron microscope (Hitachi; Tokyo, Japan). Control sections were either incubated with non-immunized rabbit serum, or the incubation step with primary antibody was omitted.
Western Blotting
Rat periodontal ligament collected from isolated first molars was homogenized in lysis buffer consisting of 10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.5% Nonidet P-40, and complete protease inhibitor cocktail (Roche Diagnostics; Mannheim, Germany) and Phosphatase Inhibitor Cocktail 1 (Sigma-Aldrich; St Louis, MO) using a TissueLyser (Qiagen; Hilden, Germany) at 25 Hz for 3 min. Samples were centrifuged at 10,000 × g for 20 min and the supernatants were used for SDS-PAGE. Proteins were separated on 10% SDS-polyacrylamide gel and transferred to Immobilon P membrane (Millipore; Bedford, MA). Membrane was blocked with 5% skim milk and incubated with anti-elastin antibody (dilution 1:1000) or anti-fibrillin antiserum (dilution 1:1000), respectively, and detected with horseradish peroxidase-conjugated anti-rabbit IgG antibody (dilution 1:2000) using the ECL Plus system (GE Healthcare Bio-Science; Piscataway, NJ).
Results
Immunoperoxidase Localization of Elastin
Following immunoperoxidase staining for elastin, the reaction product was unevenly distributed in the ligament. At the cervical region, no stain was observed in the ligament of the mesial, buccal, or lingual roots, and only a few dot-like stained structures were present at the distal side of the distal root (not shown). At the middle region, cellular cementum began to appear on the surface of the root dentin, and immunostaining occurred in limited areas close to blood vessels at the distal half, but not at the proximal side, of the ligament of the distal root. No significant staining was observed in the ligament of the mesial, lingual, or buccal roots (not shown).
From the middle to apical regions, the reaction product was abundant at the distal side of the ligament of the distal root (Figure 1A), and no staining was observed served at the proximal side. Distribution of dot-like stained structures was mainly limited to a band occupying the core of the layer of the ligament where small blood vessels were accumulated (Figure 1A). As compared with the abundance of dot-like structures stained for fibrillin in a comparable area (Figure 2C), elastin-stained structures were much less abundant. In sagittal sections, a small number of elastin-positive fibers were seen running in the apico-occlusal direction among small blood vessels (Figure 1B).
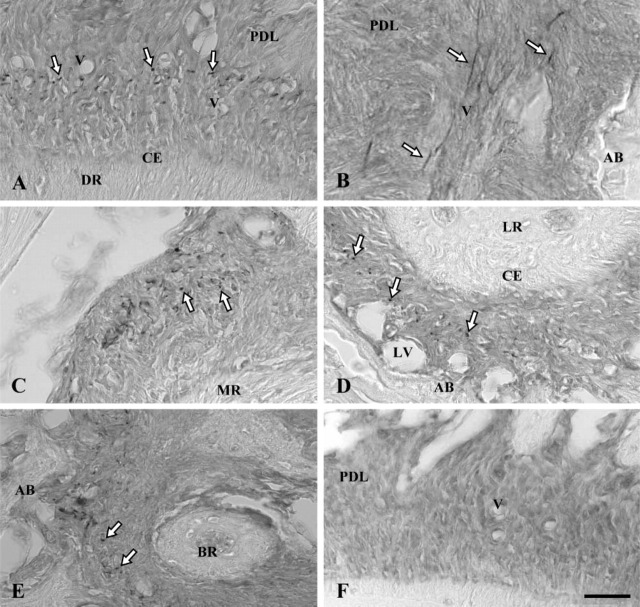
Figure 1.
Immunoperoxidase staining of rat molar periodontal ligament for elastin (A-E) with control section (F). AB, alveolar bone; CE, cellular cementum; PDL, periodontal ligament; V, small blood vessels; LV, large blood vessels. (A) Distal side of periodontal ligament of distal root (DR) sectioned horizontally. Dot-like stains (white arrows) are concentrated mainly in the central zone of the ligament where small blood vessels are localized. (B) Elastin-positive fibers (white arrows) are oriented in the apico-occulusal direction along blood vessels. (C) Stained dotlike structures (white arrows) are concentrated on the distal side of the periodontal ligament of the mesial root (MR). (D) On the proximal side of the ligament of the lingual root (LR), dot-like stains (white arrows) are distributed in the central zone of the periodontal ligament but not around large blood vessels localized in areas close to alveolar bone. (E) Immunoreaction products (white arrows) are present in the periodontal ligament around the apex of the buccal root (BR). (F) Control section in which no immunoreaction product is present. Bar = 80 μm.
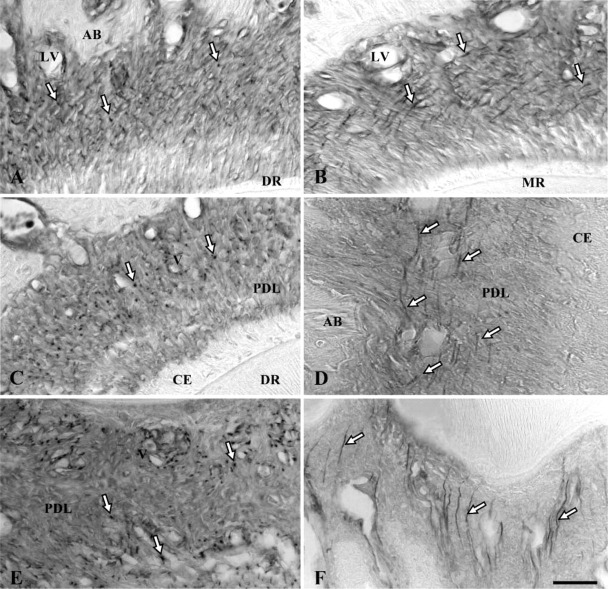
Figure 2.
Immunoperoxidase staining of rat molar periodontal ligament for fibrillin. Dot-like or fibrous stained structures (white arrows) are evenly distributed throughout the central zone of the ligament mainly around blood vessels. AB, alveolar bone; CE, cellular cementum; PDL, periodontal ligament; V, small blood vessels; LV, large blood vessels. (A) Horizontal section of the cervical region of the distal root (DR). (B) Cervical region of the mesial root (MR) with obliquely sectioned stained fibrilous structures. (C) Apical region of the distal root (DR). (D) Longitudinal section of the apical region of the distal root. (E) Horizontal section of the periodontal ligament of interradicular bone. (F) Longitudinal section of the periodontal ligament of interradicular bone. Bar = 80 μm.
At the apical region of the mesial (Figure 1C) and lingual (Figure 1D) roots, the reaction product was present at both the proximal and distal sides of the ligament. They were not found, however, in areas close to alveolar bone where larger vessels were localized. At the most apical region of the buccal root, reaction product was evenly distributed throughout the ligament (Figure 1E). No reaction product was seen in control sections (Figure 1F).
Immunoperoxidase Localization of Fibrillin
Horizontal sections of the cervical, middle, and apical regions were observed following immunoperoxidase staining for fibrillin. At the cervical (Figures 2A and 2B), middle, and apical (Figure 2C) regions, abundant fibrillin-positive, small dot-like, or short (obliquely sectioned) fibrillar structures were localized at the central zone of the layer of the ligament. They were mainly concentrated in an area where small blood vessels were accumulated. The number of dot-like (Figures 2A and 2C) or obliquely sectioned fibrillar structures (Figure 2B) stained for fibrillin in these areas was much larger than that of the dot-like structures stained for elastin (Figure 1A) as described above. In sagittal sections, a number of fibrillin-positive fibers were observed running parallel to one another along with blood vessels in the apico-occlusal direction (Figure 2D). Some fibers were bent in the direction of the root surface and inserted into the cementum at the cervical region (not shown). In the periodontal ligament of interradicular bone, a large number of fibrillin-positive dot-like structures (Figure 2E) and fibers (Figure 2F) were observed.
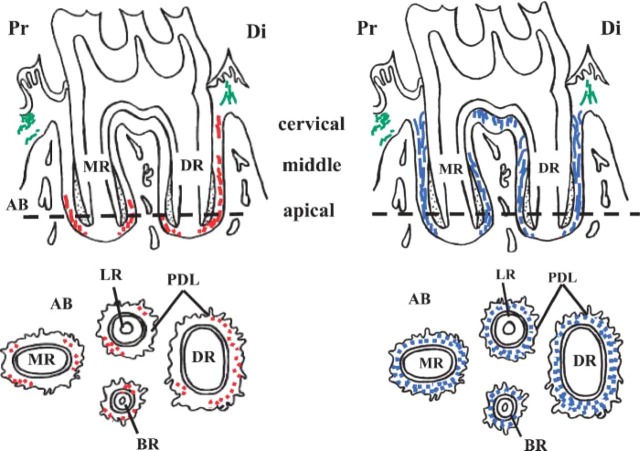
In summary of the results of immunoperoxidase staining at the light microscope level, localization of elastin and fibrillin are shown schematically in Figure 3. In the sagittal section of the roots of the first molar (top illustration), elastin (red) is localized mainly at the apical region of the periodontal ligament. On the other hand, fibrillin (blue) is ubiquitously distributed throughout the periodontal ligament. Elastin in the gingival connective tissue (green) is also shown for comparison. View of the horizontal section along the plane indicated by the dotted line in the upper half of Figure 3 is shown in the lower half.
Figure 3.
Schematic drawings of distribution of elastin and fibrillin in the periodontal ligament of rat mandibular first molars. Top: Sagittal section of roots of first molar. Bottom: Section along the horizontal plane at a level indicated by the broken line in the top drawing. Elastin (left, indicated in red) is mainly concentrated in the apical region of the periodontal ligament. Fibrillin (right, indicated in blue) is more widely distributed throughout the ligament. Elastin of gingival connective tissue is indicated in green. AB, alveolar bone; PDL, periodontal ligament; DR, distal root; MR, mesial root; LR, lingual root; BR, buccal root; Di, distal side; Pr, proximal side.
Immunogold Localization of Elastin and Fibrillin
For further examination of detailed ultrastructural distribution of elastin and fibrillin, observations with immunogold labeling were done at the ultrastructural level mainly in areas of the ligament where positive immunostaining was observed at the light microscope level. Particularly, areas of the distal side of the distal root at the middle to apical regions were examined in detail.
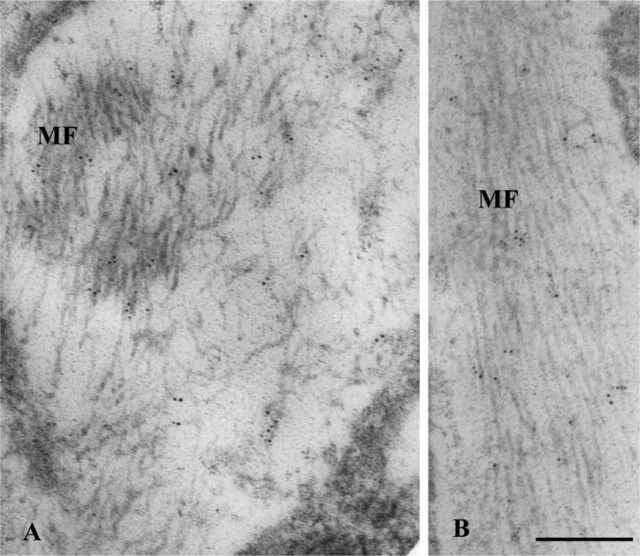
To examine possible specific distribution of elastin within elastin-positive dot-like stains seen at the light microscope level, tissue immunogold labeled for elastin was observed at the ultrastructural level. Gold particles were mainly localized over assemblies of microfibrils in areas in the vicinity of fibroblasts (Figure 4A). In addition, those gold particles had a tendency to localize in the form of discrete small groups over the area of assemblies of microfibrils as observed in longitudinal (Figure 4B) as well as perpendicular-oblique sections of the ligament (Figure 4C).
Figure 4.
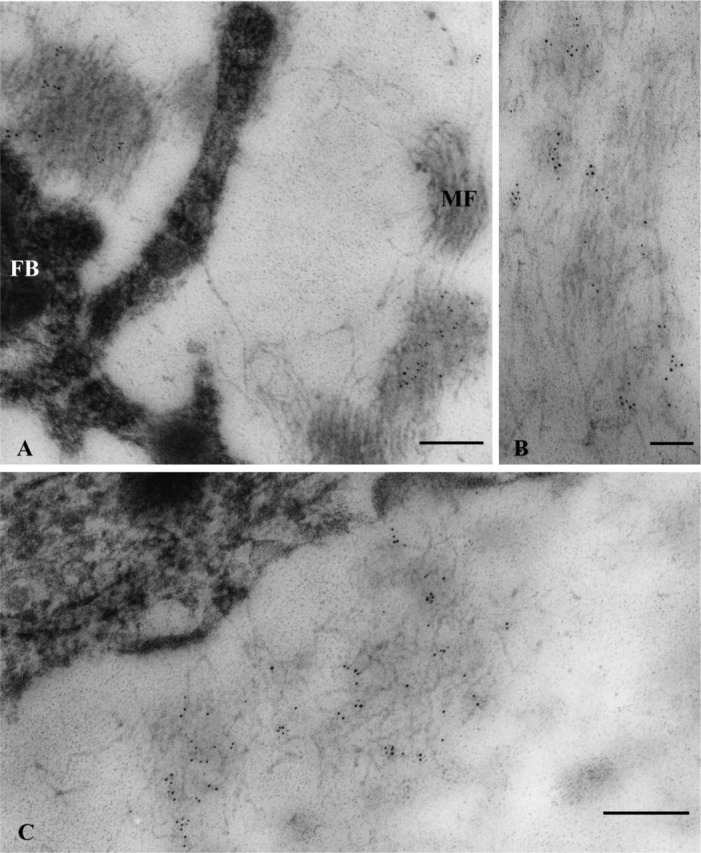
Immunogold localization of elastin in the distal region of the periodontal ligament of the distal root. (A) Five-nm gold particles are scattered within assemblies of microfibrils (MF) localized very close to a fibroblast (FB) in the vicinity of blood vessels. (B) Longitudinal section of assemblies of microfibrils onto which clusters of gold particles are localized. (C) Clusters of gold particles are more clearly seen within assemblies of oblique-transversely sectioned microfibrils. Bars: A,C = 200 nm; B = 100 nm.
In sections immunolabeled for fibrillin, gold particles were mainly localized along and over microfibrils in sections cut obliquely (Figure 5A) and longitudinally (Figure 5B) against the microfibril axis.
Figure 5.
Immunogold localization of fibrillin in the distal region of the ligament of the distal root. Gold particles are associated with microfibrils (MF) sectioned obliquely (A) and longitudinally (B). Bar = 200 nm.
Western Blotting of Elastin and Fibrillin
To confirm the result of IHC detection of elastin and fibrillin in the rat molar periodontal ligament, Western blotting for these components was done. However, no positive immunoreaction for both elastin and fibrillin was detected in the extract of the ligament (data not shown).
Discussion
In this study, localization and distribution of two major components of elastin-containing fibers, i.e., elastin and fibrillin, were examined by IHC in the periodontal ligament of rat mandibular first molars at the light and electron microscope levels. To date, IHC detection of these components in the periodontal ligament was not reported. A possible reason might have been poor availability of antigens for immunoreactions due to improper orientations of the tissue for sectioning. Therefore, cross-sections of elastin-containing fibers were chosen in this study, and such sections were prepared by cutting the ligament transversely in the cervical, middle, and apical regions. In those sections the extent of antigen exposure was found to be adequate for immunoreactions. Ultrastructural observations showed that the elastin-positive fibers seen under the light microscope (cross-section of fibers appeared as stained dot-like structures) were in fact composed of dense bundles of fibrillin-positive microfibrils within which small patches of elastin were deposited as evidenced by observation of clusters of gold particles after labeling for elastin. This type of ultrastructure clearly demonstrated that these fibers were elaunin fibers.
The presence of abundant elaunin fibers was previously reported following histochemical staining in the mouse periodontal ligament (Johnson and Pylypas 1992) and were anchored to a variety of structures including cementum, alveolar bone, and others. In contrast, in this study with the rat periodontal ligament the presence of elaunin fibers was limited to the vicinity of blood vessels in the apical region of the ligament. The role of elaunin fibers has not been clear. A basket-like formation of blood vessels was reported in the apical region of the rat molar ligament (Selliseth and Selvig 1994; Tsukada et al. 2000), and it was suggested that the function of such a vascular network was to cushion against occlusal forces. If this is the case, the role of elaunin fibers present close to blood vessels may be for the restoration of the vascular system and recuperation of blood vessels from deformations.
Oxytalan fibers localized among elaunin fibers may also protect the integrity of the vascular system along with elaunin fibers. The pattern of distribution of fibrillin-positive fibers was basically the same as that of oxytalan fibers (Tashiro et al. 2002), and various roles of oxytalan fibers have been proposed including anchoring, maintenance of elasticity, guidance of cell migration, and stabilization of blood vessels (Beertsen et al. 1974; Fullmer et al. 1974; Chantawiboonchai et al. 1998; Everts et al. 1998; Tashiro et al. 2002). In the periodontal ligament of developing rat molars, the size and number of oxytalan fibers were reported to increase concurrently with the development of the vascular system, and it indicated the possibility that a role of these fibers was in the maintenance of the integrity of the vascular system (Tashiro et al. 2002). For such a protective function, biomechanical properties of different types of elastic system fibers would certainly play a significant role and will be the subject of future studies. Also, the state of the elastin (fibrillated vs. amorphous precursor) affects the function of elastic system fibers.
As to the origin of elastin, in vitro studies with fibroblasts of the human periodontal ligament reported expression of a tropoelastin gene (Redlich et al. 2004) and secretion of elastin (Howard et al. 1998). On the other hand, no expression of tropoelastin mRNA was reported in other studies (Tsuruga et al. 2002a,b). Fibroblastic cells were likely to be the main source of elastin in human gingival connective tissue (Chavrier et al. 1988; Chavrier 1990). Although examining the origin of elastin was beyond the scope of this study, ultrastructural observation of the preferential close localization of elaunin fibers to fibroblasts may indicate the possibility that fibroblasts are involved in the production of elastin in the periodontal ligament.
No mature elastic fibers were detected in the rat molar periodontal ligament in this study. Remodeling of the rodent periodontal ligament is known to be rapid, and degradation of maturing elastic fibers may be occurring. If this is the case, there is a possibility that fibroblast-derived elastase is participating in such a degradation (Garant 2003). Alternatively, rapid remodeling of the components of the ligament may not allow sufficient time for the full maturation of elastic fibers (Bradamante and Svajger 1977).
Finally, a further attempt was made to reconfirm the presence of elastin and fibrillin in the rat periodontal ligament with the Western blot method. However, it was not successful because of the extreme difficulty in collecting a sufficient amount of isolated periodontal ligament for this technique.
In summary, IHC study of the periodontal ligament of rat molars at the light microscope and ultrastructural levels showed that the elastic system fibers of the ligament were composed of oxytalan and elaunin fibers with no elastic fibers. Oxytalan fibers, dense bundles of fibrillin-containing microfibrils, were widely distributed in the ligament. On the other hand, the presence of elaunin fibers was limited mainly to the apical region of the ligament in close association with blood vessels. Elaunin fibers were particularly rich in the distal half of the ligament of the distal root and also concentrated at the apical region of the ligament in other roots. These specific areas rich in elaunin fibers in the periodontal ligament are probably more exposed to mechanical stress as compared with other areas. Results of this study suggest that the role of the elastic system fibers is in the protection and maintenance of the integrity of the vascular system in the ligament during mastication.
Literature Cited
- Beertsen W, Everts V, van den Hooff A. (1974) Fine structure of fibroblasts in the periodontal ligament of the rat incisor and their possible role in tooth eruption. Arch Oral Biol 19:1087–1098 [DOI] [PubMed] [Google Scholar]
- Bradamante Z, Svajger A. (1977) Pre-elastic (oxytalan) fibres in the developing elastic cartilage of the external ear of the rat. J Anat 123:735–743 [PMC free article] [PubMed] [Google Scholar]
- Carmichael GG, Fullmer HM. (1966) The fine structure of the oxytalan fiber. J Cell Biol 28:33–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantawiboonchai P, Warita H, Ohya K, Soma K. (1998) Confocal laser scanning-microscopic observations on the three-dimensional distribution of oxytalan fibres in mouse periodontal ligament. Arch Oral Biol 43:811–817 [DOI] [PubMed] [Google Scholar]
- Chavrier C. (1990) The elastic system fibres in healthy human gingiva. Arch Oral Biol 35:223–225s [DOI] [PubMed] [Google Scholar]
- Chavrier C, Hartmann DJ, Couble ML, Herbage D. (1988) Distribution and organization of the elastic system fibres in healthy human gingiva. Ultrastructural and immunohistochemical study. Histochemistry 89:47–52 [DOI] [PubMed] [Google Scholar]
- Everts V, Niehof A, Jansen D, Beertsen W. (1998) Type VI collagen is associated with microfibrils and oxytalan fibers in the extracellular matrix of periodontium, mesenterium and periosteum. J Periodontal Res 33:118–125 [DOI] [PubMed] [Google Scholar]
- Fullmer HM, Sheetz JH, Narkates AJ. (1974) Oxytalan connective tissue fibers: a review. J Oral Pathol 3:291–316 [DOI] [PubMed] [Google Scholar]
- Garant PR. (2003) Oral cells and tissues. Chicago, Quintessence Publishing, 153–177 [Google Scholar]
- Hanazawa K, Horie K. (1934) Über die elastischen fasern im zahne, besunders im parodontium (in Japanese). Shikwa Gakuho 39, 117–138, 231–263 [Google Scholar]
- Higashi S. (1964) The elastic fibers in the periodontal membrane and cementum of human teeth (in Japanese). Shikwa Gakuho 64:453–477 [Google Scholar]
- Howard PS, Kucich U, Taliwal R, Korostoff JM. (1998) Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J Periodontal Res 33:500–508 [DOI] [PubMed] [Google Scholar]
- Johnson RB, Pylypas SP. (1992) A re-evaluation of the distribution of the elastic meschwork within the periodontal ligament of the mouse. J Periodontal Res 27:239–249 [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Marson A, Baldoc C. (2005) Fibrillin microfibrils. Adv Protein Chem 70:405–436 [DOI] [PubMed] [Google Scholar]
- Kielty CM, Sherratt MJ, Shuttleworth CA. (2002) Elastic fibres. J Cell Sci 115:2817–2828 [DOI] [PubMed] [Google Scholar]
- Nanci A, Somerman MJ. (2003) The Periodontium. In Nanci A, ed. Ten Cate's Oral Histology, 6th ed. Development, structure, and function. St Louis, Mosby, 240–274 [Google Scholar]
- Palmon A, Roos H, Reichenberg E, Grosskop A, Bar Kana I, Pitaru S, Redlich M. (2001) Basic fibroblast growth factor suppresses tropoelastin gene expression in cultured human periodontal fibroblasts. J Periodontal Res 36:65–70 [DOI] [PubMed] [Google Scholar]
- Redlich M, Asher Roos H, Reichenberg E, Zaks B, Mussig D, Baumert U, Golan I, et al. (2004) Expression of tropoelastin in human periodontal ligament fibroblasts after simulation of orthodontic force. Arch Oral Biol 49:119–124 [DOI] [PubMed] [Google Scholar]
- Sakai N. (1968) Histochemical investigations on the age changes of fibrous components in the rat molar periodontium (in Japanese). Shigaku 55:488–512 [PubMed] [Google Scholar]
- Sampson WJ. (1979) A comparative light microscopic evaluation of oxytalan fiber staining with a variety of dye substances. Stain Technol 54:181–191 [DOI] [PubMed] [Google Scholar]
- Selliseth NJ, Selvig KA. (1994) The vasculature of the periodontal ligament: a scanning electron microscopic study using corrosion casts in the rat. J Periodontol 65:1079–1087 [DOI] [PubMed] [Google Scholar]
- Shinohara C. (1996) Changes in elastic fibers in the periodontal ligament during orthodontic tooth movement. Viscoelasticity and three-dimensional ultrastructure (in Japanese). J Jpn Orthodontal Soc 55:287–299 [Google Scholar]
- Sims MR. (1976) Reconstitution of the human oxytalan system during orthodontic tooth movement. Am J Orthod 70:38–58 [DOI] [PubMed] [Google Scholar]
- Tashiro K, Sawada T, Inoue S, Yanagisaw T. (2002) Development of oxytalan fibers in the rat molar periodontal ligament. J Periodontal Res 37:345–352 [DOI] [PubMed] [Google Scholar]
- Tsukada H, Ishikawa H, Nakamura S, Yashida S. (2000) Developmental changes of the vasculature in the periodontal ligament of rat molars: a scanning electron microscopic study of microcorrosion casts. J Periodontal Res 35:201–207 [DOI] [PubMed] [Google Scholar]
- Tsuruga E, Irie K, Sakakura Y, Yajima T. (2002a) Tropoelastin expression by periodontal fibroblasts. J Dent Res 81:198–202 [PubMed] [Google Scholar]
- Tsuruga E, Irie K, Sakakura Y, Yajima T. (2002b) Expression of fibrillins and tropoelastin by human gingival and periodontal ligament fibroblasts in vitro. J Periodontal Res 37:23–28 [DOI] [PubMed] [Google Scholar]