Abstract
Cellular stress leads to a change in distribution of RNA-binding proteins. HuR, a member of the ELAV/Hu family of RNA-binding proteins, is nuclear in distribution and following heat shock is found in large cytoplasmic stress granules where translation is inhibited. HuD, another ELAV/Hu RNA-binding protein, stabilizes the GAP-43 mRNA in response to nerve growth factor (NGF) stimulation in PC12 cells. We were interested in determining the nuclear distribution of HuD and if neurotrophic stimulation induced changes in the distribution of HuD. In PC12 cells, we found, as expected, that HuR translocates from the nucleus to the cytoplasm in response to heat shock. In response to heat shock, HuD forms large cytoplasmic stress granules, consistent with a role for HuD in the cessation of translation. In unstimulated cells, HuD is distributed in small granules in the cytoplasm and is consistently present at low levels in the nucleus. Stimulation of PC12 cells with NGF induces neuronal differentiation including outgrowth of neurites and increased levels of GAP-43 protein, whereas HuD remains localized in small cytoplasm granules and is still present in the nucleus. These results suggest that, following neurotrophic stimulation, the lack of changes in HuD distribution are due to continued steady state of HuD nuclear shuttling in PC12 cells, or that HuD is not normally shuttled from the nucleus in response to NGF. (J Histochem Cytochem 54:1129-1138, 2006)
Keywords: HuD, HuR, GAP-43, nuclear/cytoplasmic shuttling
HuR, a member of the ELAV/Hu family, is found in the nucleus of both non-neuronal and neuronal cells and has been shown to shuttle into the cytoplasm (Fan and Steitz 1998). The primary role for HuR appears to be shuttling mRNA out of the nucleus with rapid import back into the nucleus as the vast majority of steady-state HuR is found in the nucleus. Cytoplasmic HuR is involved in localization of mRNA that is protected from degradation (Peng et al. 1998). Differentiation-dependent movement of HuR into the cytoplasm is seen during myogenesis (Van Der Giessen et al. 2003), and HuR returns to a nuclear localization upon its completion (Figueroa et al. 2003). Thus, signaling of muscle cell differentiation is coincident with the movement of HuR from the nucleus. In the unstimulated state, it has been proposed that mRNA-binding proteins repress translation that is activated after a cellular stimulus (Barreau et al. 2005).
In contrast to HuR, which is found in numerous cell types, the rest of the ELAV/Hu family of RNA-binding proteins (HuB, HuC, and HuD) are found only in neuronal cells (Gao and Keene 1996; Akamatsu et al. 1999; Kasashima et al. 1999; Fornaro and Geuna 2001). Overexpression of HuB, HuD, and HuC in PC12 and P19 cells induces growth of neurites (Kasashima et al. 1999; Anderson et al. 2001), suggesting a role for these proteins in neuronal development. However, evidence to date for the neuronal members of the ELAV/Hu family indicates that these members bind to and stabilize mRNA species such as GAP-43 and localize to the cytoplasm. Interestingly, when PC12 cells are stimulated with nerve growth factor (NGF), transcription levels of GAP-43 do not change (Federoff et al. 1988; Perrone-Bizzozero et al. 1991,1993), but levels of GAP-43 protein increase 6-fold due to mRNA stabilization by HuD (Chung et al. 1997; Tsai et al. 1997). GAP-43 is a developmentally regulated neuronal protein that functions in axonal guidance (Shen et al. 2002). Our recent evidence indicates that HuD is present in axons and growth cones and is colocalized with GAP-43 mRNA and ribosomes (Smith et al. 2004). These observations suggest that HuD in neurites and growth cones could participate in stimulus-dependent protein translation. Thus, stimulation of axons may increase the local translation of mRNA in elongating axons and growth cones.
HuD is found at high levels in small cytoplasmic granules and at low levels in the nucleus (Gao and Keene 1996; Antic and Keene 1998; Toba et al. 2002; Anderson et al. 2003). However, cells transfected with HuD reporter constructs show primarily cytoplasmic localization of the reporter (Kasashima et al. 1999; Anderson et al. 2001). In adult hippocampus of mice overexpressing HuD, it is found only in the cytoplasm and not in the nucleus (Bolognani et al. 2006). If HuD is involved in shuttling between the nucleus and cytoplasm, it should have specific sequences. HuD has a nuclear export signal (Kasashima et al. 1999) and has been shown to bind TAP/NXF1, part of a nuclear export pathway (Saito et al. 2004). However, an effective nuclear localization signal (NLS) has not been reported for HuD (Kasashima et al. 1999). In addition, conditions under which HuD could be imported to or exported from the nucleus of neurons have not yet been elucidated.
In dendrites, another neuronal RNA-binding protein, Staufen, is associated with mRNA, localized to small granules in dendrites (Kiebler et al. 1999), and after electrical stimulation or treatment with growth factors is associated with increases in protein translation (Jiang and Schuman 2002). Many granules that contain mRNA and Staufen also contain ribosomes (Krichevsky and Kosik 2001). Staufen, like HuD, is localized almost exclusively to the cytoplasm in neurons (Marion et al. 1999; Miki and Yoneda 2004). One interpretation of the HuD and Staufen data is that some RNA-binding proteins are primarily involved in specific cytoplasmic localization or targeting of mRNA and may increase translation of the associated mRNA.
Our hypothesis is that the distribution of cytoplasmic RNA-binding proteins changes in response to trophic stimuli. We show that heat-shock of PC12 cells causes formation of large cytoplasmic HuD stress granules consistent with a decrease in translation induced by stress. Endogenous HuD with several microscopic techniques is found in unstimulated PC12 cells both in the cytoplasm as small granules and in the nucleus at low levels. Neurotrophic stimulation that causes neuronal differentiation does not lead to redistribution of HuD. Finally, we show that overexpressed HuD in PC12 cells maintains the same distribution as that of endogenous HuD. These results suggest that stimulation of PC12 cells to differentiate maintains the cytoplasmic distribution of HuD where, as previously suggested (Smith et al. 2004), it may be targeted to neurites for local translation of bound mRNA.
Materials and Methods
Cells and Culture
PC12 cells (PC12-N21 clone) were grown as indicated previously (Burry and Perrone-Bizzozero 1993). For experiments, cultures were rinsed twice in DMEM with 0.5% serum 6 hr prior to the beginning of incubations. Quantitation of neurite outgrowth was performed on phase optic micrographs of live cultures after 24 hr. The longest neurite of each of 100 cells was measured and expressed as a change from unstimulated cells on images.
PC12 cells were transfected with c-myc-HuD (Anderson et al. 2001) using Lipofectamine 2000 (Invitrogen; Carlsbad, CA) and examined 3 days after transfection.
Light Microscopic Immunocytochemistry (ICC)
Cells grown in four-well Permanox Lab-Tek chamber slides (Nalge Nunc; Naperville, IL) were fixed with 2% paraformaldehyde in PBS, pH 7.3, with sucrose for 30 min and rinsed in PBS. Incubation and rinse buffer was 5% normal goat serum, 1% BSA, and 0.02% sodium azide (PBS+). Cells were incubated for 2 hr in 0.2% Triton X-100 in PBS at room temperature. Primary antibodies were incubated for 3 hr and secondary antibodies for 1 hr, all at room temperature with agitation. Antibodies used were monoclonal anti-HuD (16C12, Clonegene; Hartford, CT) (Smith et al. 2004), monoclonal anti-p97 (Importin-β, MA3-070; Affinity Bio-Reagents, Golden, CO), monoclonal c-myc (ascites, 9E10; Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA), goat anti-mouse IgG Alexa Fluor-488 (A-11029; Molecular Probes, Eugene, OR), goat anti-mouse Alexa Fluor-546 (A-11030; Molecular Probes). We have previously reported both the generation and characterization of the 16C12 monoclonal anti-HuD antibody (Smith et al. 2004). For nuclear labeling experiments, DRAQ5 (Biostatus Limited; Leicestershire, UK) was used at 0.5 μl/ml. For double-labeling experiments, samples were first incubated with anti-HuD and Alexa Fluor-547, blocked with a 1 hr incubation of normal mouse serum (015-000-120, 1:20 dilution; Jackson Immuno Research, West Grove, PA), rinsed four times with buffer, incubated 1 hr with goat anti-mouse IgG Fab at 20 μg/ml (015-007-003; Jackson ImmunoResearch), and then incubated in Importin-β and Alexa Fluor-488. For wide-field microscopy, images were collected on a Zeiss Axioskop (Carl Zeiss; Jena, Germany) with an Olympus MagniFire camera system (Olympus; Tokyo, Japan). Images were also collected on a Zeiss 510 META LSM confocal on an Axioplan 2 microscope (Carl Zeiss). Microscopes are located in the Campus Microscopy and Imaging Facility at The Ohio State University.
To show specificity for each of the monoclonal antibodies used in the double-labeling ICC, several sets of controls were performed. First, the double labeling was compared with the individual labeling for each primary antibody and found to be the same. Second, to show that the blocking step removed the possible binding of the second secondary antibody (GAM 546) to the first primary antibody (HuD), the secondary primary antibody (p97) was eliminated and showed only HuD labeling with 488. Third, to show that the second secondary antibody (GAM 546) did not bind to the first primary antibody (HuD), the first secondary antibody (GAM 488) was eliminated and showed only p97 labeling with 546. Fourth, to show that the blocking step eliminated all binding of a secondary antibody (GAM 546) to a first primary antibody (HuD), the first secondary antibody (GAM 488) and the secondary primary antibody (p97) were eliminated and showed no labeling. These controls showed that the blocking steps eliminated the binding of antibodies between the first and second antibody incubations, and that the labeling for each primary antibody was specific.
Nuclear/cytoplasmic ratios of fluorescence intensity were determined with the LSM 510 software. Images from cultures labeled with Alexa Fluor-546 for HuD were captured on the Zeiss 510 META LSM at 12 bits for both the 546 and DIC channels and analyzed with Zeiss software. Each sample was an average from 50 cells, and p values were calculated with the t-test.
Cryo-ultramicrotomy
PC12 cultures in 35-mm dishes were fixed as described above, scraped, and pelleted in 2% gelatin in PBS. After equilibration in 20% sucrose, samples were frozen in liquid nitrogen on stubs. Samples were sectioned at 400 nm on a Leica EM UC6 ultramicrotome (Leica; Wetzlar, Germany) with an FC6 cryo attachment at −100C on glass knives. Sections were picked up in a loop and placed on coverslips to thaw. Subsequent ICC and microscopy were performed as described above.
Electron Microscopic ICC
Cultures were prepared for pre-embedding electron microscopic ICC with 1.4-nm Nanogold (Nanoprobes; Yaphank, NY) and silver enhancement as previously described (Burry 1995; Gilerovitch et al. 1995) with modifications indicated below. Following incubation in 1:500 monoclonal anti-HuD and 1:50 goat anti-mouse Nanogold, cells were silver enhanced with a modified silver-enhancement solution. NPG concentration was reduced from 0.36 mg/ml to 0.09 mg/ml, which allowed for more reproducible silver-enhancement times. Final concentrations for the silver-enhancement solution were gum arabic 0.25 g/ml, 0.1 M 2-(N-morpholino)ethanesulfonic acid (MES), 0.09 mg/ml NPG, and 1.1 mg/ml silver lactate. Cells were embedded and sectioned as previously described (Gilerovitch et al. 1995). Control samples with no primary antibody showed no particles.
Immunoblots
Twenty five μg of total protein was loaded per well in a 10% polyacrylamide gel and transferred to a polyvinylidene fluoride membrane. Immunobloting was performed with blocking in Tris-buffered saline with 5% milk and 0.05% Tween 20. The membrane was incubated in monoclonal anti-HuD at 1:1000 for 3 hr. After four rinses, the membrane was incubated in goat anti-mouse IgG-HRP (1:1000 dilution; Cappel, Durham, NC) for 1 hr. After exposure to ECL Western Blotting Reagent (Amersham Biosciences; Piscataway, NJ), the membrane was exposed to ECL Hyper-film (Amersham Biosciences). Some membranes were stripped in 25 mM glycine, 1% SDS, pH 2.0, for 30 min. Incubations with sheep anti-GAP-43 (Benowitz et al. 1988) at a 1:1000 dilution, rabbit anti-sheep horseradish peroxidase (HRP; Cappel) at a 1:5000 dilution and ERK 1 (sc-093; Santa Cruz Biotechnology, Santa Cruz, CA) plus ERK 2 (sc-154; Santa Cruz Biotechnology) at a 1:66 dilution and goat anti-rabbit HRP (1:1000 dilution; Cappel) were done in the same buffers
Results
Heat Shock Causes Formation of Large Stress Granules of HuD
Because HuR is shown to form stress granules and to change its nuclear/cytoplasmic distribution in response to stress (Gallouzi et al. 2000), we tested the hypothesis that in PC12 cells HuD would also change its distribution in response to stress. In unstimulated PC12 cells, HuR is nuclear in its distribution (Figure 1A). When cells were heat shocked at 45C for 1 hr formation of large perinuclear stress granules was observed (Figure 1B). Note that red-labeled nuclear HuR in control cells combined with the blue label for DNA results in a pink nuclear label. In the heat-shocked cells, HuR labeling in the nucleus (Figure 1B) is decreased. Quantitative analysis indicated that HuR levels in heat-shock-treated cells dropped significantly (p>0.001; Figure 1G).
Figure 1.
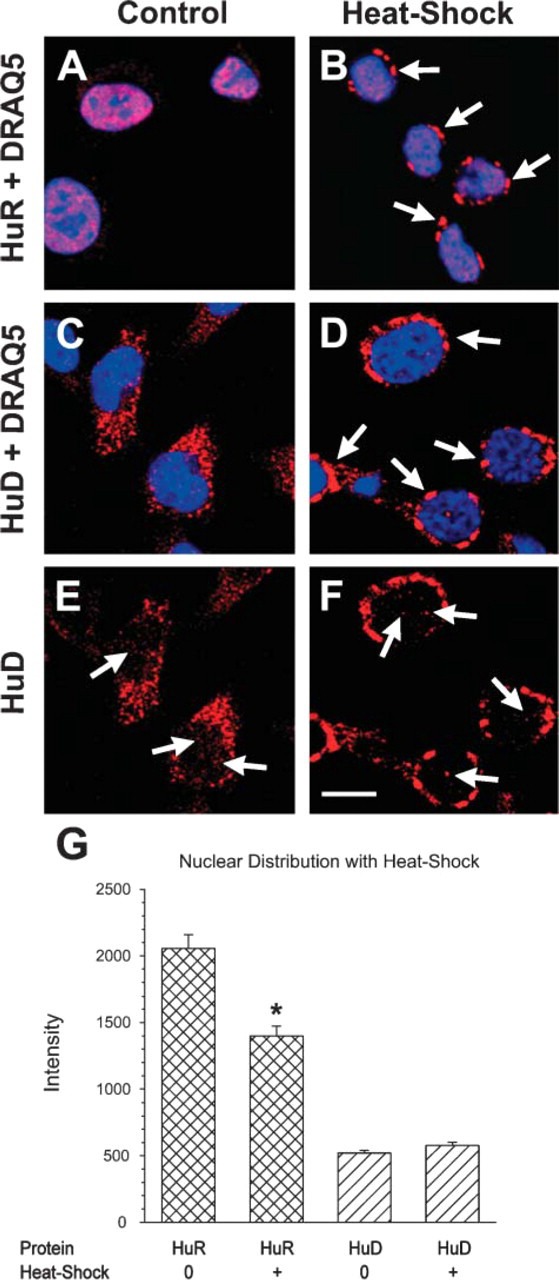
Redistribution of HuR and HuD following 1 hr heat-shock treatment of PC12 cells at 45C. (A,B) HuR (red) is seen exclusively in the nucleus (blue) in untreated PC12 cells resulting in a pink-colored nucleus (A). Following heat shock, large cytoplasmic HuR granules are seen (arrows) and HuR in the nucleus is reduced (B). (C-F) HuD is mainly cytoplasmic in small granules (C), and following heat shock HuD is found in large cytoplasmic granules (arrows in D). In micrographs without the nuclear stain, HuD is seen in the nucleus of control and heat-shocked cells (arrows in E,F). (G) Quantitation of the intensity of HuR and HuD labeling shows a statistically significant reduction in nuclear intensity of HuR following heat shock but not in nuclear intensity of HuD. (∗ = p>0.001). Bar = 10 μm.
HuD distribution in untreated control cells was a mix of cytoplasmic and nuclear (Figure 1C) with few pink nuclear granules. In response to heat shock, HuD formed perinuclear stress granules (Figure 1D) similar to those formed by HuR. In addition, nuclear HuD was found in both control and heat-shocked cells (Figures 1E-1G). This is the first report of HuD forming heat-shock granules and is surprising because there was no change in the already-low HuD levels in the nucleus. Thus, both HuR and HuD can form stress granules in heat-shocked cells.
HuD Is Found in the Nucleus of Unstimulated PC12 Cells
With the above indication of both cytoplasmic and nuclear localization of HuD we first examined the distribution of endogenous HuD with antibodies that only recognize HuD. To determine if endogenous HuD was present in the nucleus of unstimulated PC12 cells, we examined cells labeled for HuD using ICC. Shuttling of many proteins into the nucleus is mediated by an NLS-containing protein such as importin-β that binds to proteins in the cytoplasm (Chi et al. 1995,1997). Thus, importin-β is found in low levels in the nucleus because the protein is rapidly returned to the cytoplasm. In unstimulated control cultures, HuD labeling was found in small cytoplasmic granules and frequently concentrated at one side of the nuclear envelope (Figure 2A). Importin-β labeling was concentrated at the nuclear envelope and was localized more broadly in the cytoplasm, but not in the nucleus (Figure 2B). Although the level of HuD labeling in the nucleus is less than that in the cytoplasm, HuD was consistently found in cell nuclei (Figure 2C).
Figure 2.
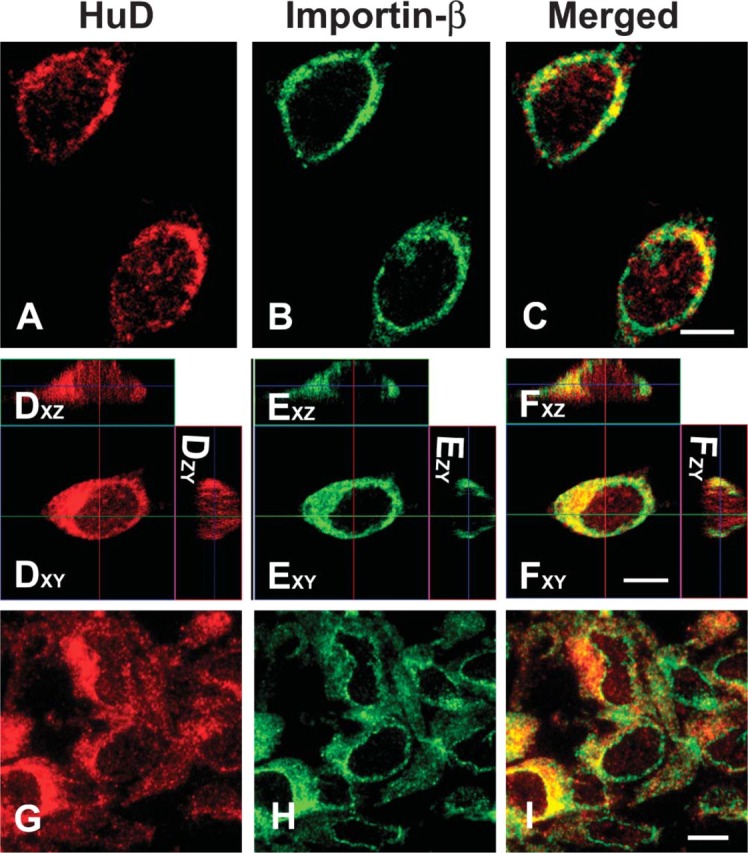
Immunocytochemistry (ICC) labeling of HuD (red) and Importin-β (green) in PC12 cells grown on a coverslip and examined with confocal microscopy (A-F) or cryo-ultramicrotome sections examined with wide-field fluorescence (G-I). (A-C) PC12 cells in a single optical section labeled with HuD (red) and Importin-β (green) and shown as a merged image. (D-F) Stack of optical sections of PC12 cells shown in an orthogonal reconstruction. Sections in the xz and zy planes are perpendicular to the xy plane. Image labeled xy is in the plane of the section and xz image is reconstructed from a plane indicated by the horizontal line in xy image. Likewise, the zy image is reconstructed from a plane indicated by the vertical line in xy image. (G-I) PC12 cells fixed, scraped, and frozen. Four-hundred-nm cryo-ultramicrotome sections were thawed on coverslips, labeled for ICC as cultured cells, and micrographs taken from a wide-field fluorescence microscope. Bar = 5 μm.
To further demonstrate nuclear HuD, we chose different methods of viewing PC12 cells to show that HuD is indeed in the nucleus. First, we examined reconstructed Z-series of cells labeled with HuD. From a Z-series of optical sections in the xy plane, we examined orthogonally reconstructed sections in the xz and zy planes perpendicular to the xy plane (Figures 2D-2F). The image labeled xy is in the plane of the optical section and the image xz is reconstructed from a plane indicated by the horizontal line in the xy image. Likewise, the image yz is reconstructed from a plane indicated by the vertical line in the xy image. Three-dimensional sections of PC12 cells show in the xz and zy plane that HuD individual granules were seen in the nucleus (Figure 2D). These are not due to bleed-through from adjacent sections because the importin-β images do not show nuclear granules even though there is perinuclear labeling (Figure 2E). These results show, with confocal microscopy, that HuD granules are present in the nucleus of unstimulated PC12 cells.
A second method to examine nuclei for HuD-labeled granules is to cut thin sections of PC12 cells at a thickness of <5% of the diameter of the cell. We used 400-nm-thick frozen cryo-ultramicrotome sections labeled with the same antibodies as the intact cultures above. These sections were thin enough that sections through the center of these 15-μm-diameter cells would not contain any HuD labeling from cytoplasm above or below the section. In wide-field fluorescent microscopy, these sections showed nuclear HuD-labeled granules consistent with that seen in the optical sections from confocal microscopy of unstimulated cells (Figures 2G-2I). HuD-labeled granules are seen within an importin-β negative nucleus (Figure 2I). Distribution of cytoplasmic HuD and Importin-β in these cells was similar to that seen in the optical sections.
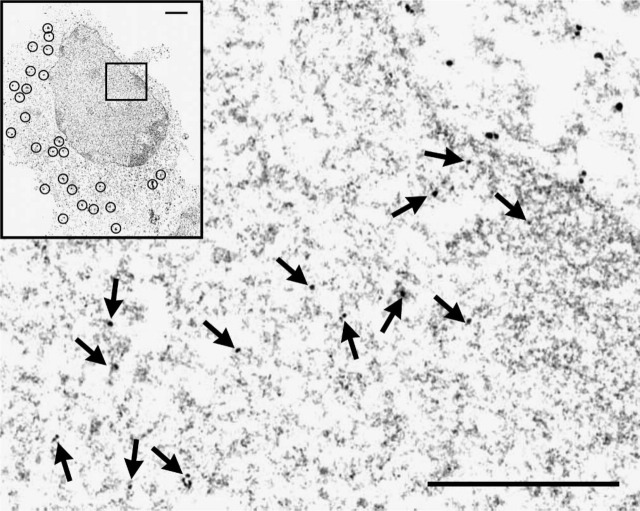
Finally, to confirm that HuD was present within the nucleus, we used electron microscopic ICC. HuD antibody was localized with silver-enhanced small gold-labeled secondary antibody. Low magnification micrographs show many small granules in the cytoplasm (Figure 3; inset). In the nucleus at higher magnification (Figure 3), there are numerous HuD-labeled granules within the nucleus (Figure 3). The silver-enhanced gold particles form a large cluster or granule with no other silver-enhanced gold particles nearby. Thus, three different microscopic approaches, confocal microscopy of whole mounts, wide-field microscopy of ultrathin sections, and electron microscopy, have demonstrated that endogenous HuD is within the nucleus of unstimulated PC12 cells.
Figure 3.
Pre-embedding electron microscopic ICC of cultured PC12 cells for HuD. Inset is an electron micrograph of the entire cell at low magnification, and cytoplasmic HuD-positive granules are labeled with silver-enhanced gold particles (shown in circles). The small box within the inset indicates the field shown in the rest of the figure. In the larger image the cytoplasm is oriented in the upper right, and the HuD-labeled particles in the nucleus are indicated by arrows. Bar = 500 nm; Inset = 1 μm.
HuD Distribution Following Stimulation of PC12 Cells
In PC12 cells, the HuD response to heat shock was the formation of cytoplasmic stress granules, with little change in nuclear/cytoplasmic distribution (Figure 1).
We next investigated the hypothesis that NGF stimulates PC12 cells to change the levels and distribution of HuD. PC12 cells were stimulated with NGF for 24 hr, and the nuclear/cytoplasmic ratio of HuD was analyzed quantitatively. Level of nuclear HuD labeling in stimulated cells was similar to that of untreated cells (Figure 4A). To show that these PC12 cells were sufficiently stimulated, we measured the length of neurites and found that after 24 hr NGF resulted in a 2-fold increase in length of neurites (Figure 4B) and an increase in the levels of GAP-43 protein. However, NGF treatment did not influence levels of HuD protein (Figure 4C).
Figure 4.
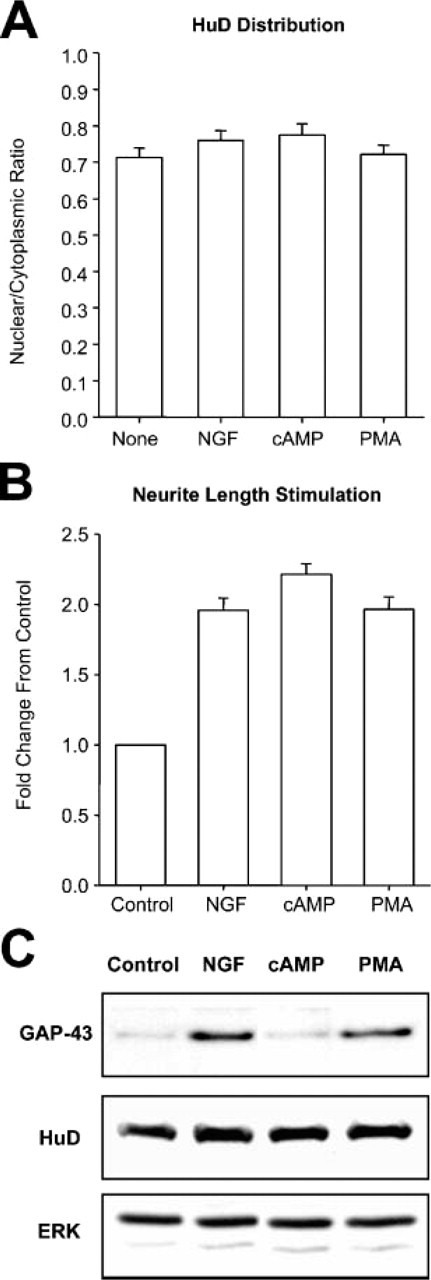
Evaluation of PC12 cells stimulated to differentiate. (A) Relative intensity of nuclear HuD labeling in PC12 cells unstimulated (None) or stimulated with nerve growth factor (NGF), dibutyryl AMP (1 mM), and phorbol ester (PMA) 1 μM. There was no significant change in the mean values with these treatments as compared with unstimulated cells. (B) To show the differentiation response of PC12 cells, neurite length was measured. All stimulations caused an increase of 1-fold in neurite length at 24 hr. (C) To measure steady-state levels of GAP-43, cultures incubated for 24 hr were analyzed by immunoblot. NGF and PMA treatments resulted in increased levels of GAP-43, but HuD levels were similar to controls. Levels of ERK 1 (top dark band) were used as internal loading controls.
It is known that elevated levels of cytoplasmic cAMP stimulate neuronal differentiation of PC12 cells. We used dibutyryl AMP and phorbol ester (PMA) to stimulate neurite outgrowth and assayed for altered HuD nuclear labeling. Dibutyryl AMP stimulated neurite outgrowth (Figure 4B) but did not increase levels of GAP-43 (Figure 4C). PMA stimulated both neurite outgrowth (Figure 4B) and increased levels of GAP-43 (Figure 4C). Nuclear levels of HuD in cells stimulated for 24 hr with dibutyryl AMP (1 mM) or PMA (1 μM) were not changed significantly from those of untreated cells (Figure 4A). These results are consistent with the previous findings that stimulation of PC12 cells with NGF or PMA, but not dibutyryl AMP, increases GAP-43 mRNA stability and expression (Perrone-Bizzozero et al. 1993) via HuD-dependent mechanisms (Mobarak et al. 2000) mediated by the increased binding of this protein to mRNA (Tsai et al. 1997). We conclude that stimulation of PC12 cells with compounds that activated different signaling pathways did not change the levels of nuclear HuD.
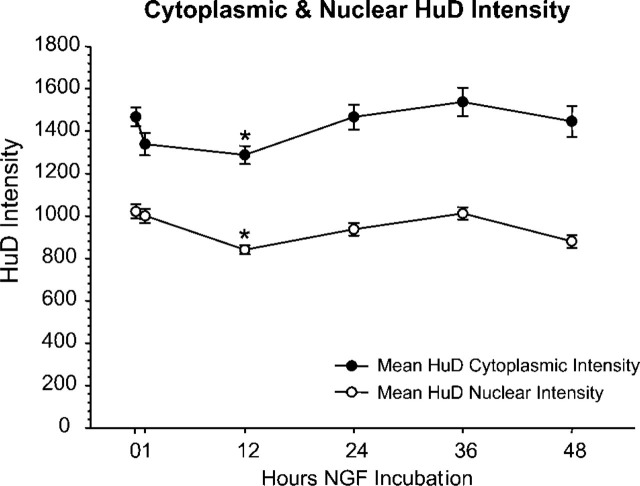
Finally, to determine if changes in HuD distribution occurred earlier than 24 hr, we examined the time course of HuD distribution in PC12 cells stimulated with NGF. Examining the average intensity of HuD labeling in the cytoplasm or the nucleus showed that there is a small but significant drop in both compartments at 12 hr (Figure 5), but the nuclear/cytoplasmic ratio at 12 hr was not significantly different from the control value (data not shown). This result is consistent with the the previously reported decline in levels of whole cell HuD at 6 hr after NGF stimulation (Smith et al. 2004). As a control for data collection, levels of the nuclear stain DRAQ5 were determined, and levels of nuclear label showed that HuD labeling was similar at all time points (data not shown).
Figure 5.
Time course of HuD levels following NGF stimulation. Both the cytoplasmic and nuclear HuD intensity showed little change from untreated values. At 12 hr there was a significant decrease in intensity of both cytoplasmic and nuclear compartments (asterisks); however, nuclear/cytoplasmic ratios showed no significant changes during the 48 hr examined.
Overexpression of HuD Does Not Change HuD Distribution
Overexpression of HuD causes an increase in length of neurites and growth of neurites in cells not stimulated with NGF (Kasashima et al. 1999; Mobarak et al. 2000). It is not known if overexpression of HuD causes changes in HuD distribution. To evaluate this possibility, we examined PC12 cells transfected and overexpressing HuD-c-myc. With ICC for c-myc, there were high levels of diffuse cytoplasmic HuD and low but consistent levels of nuclear HuD in the transfected cells (Figures 6A-6C). Thus, overexpressed HuD exhibited the same nuclear/cytoplasmic distribution as endogenous HuD, even though it stimulated growth of neurites, supporting data obtained examining endogenous HuD with ICC.
Figure 6.
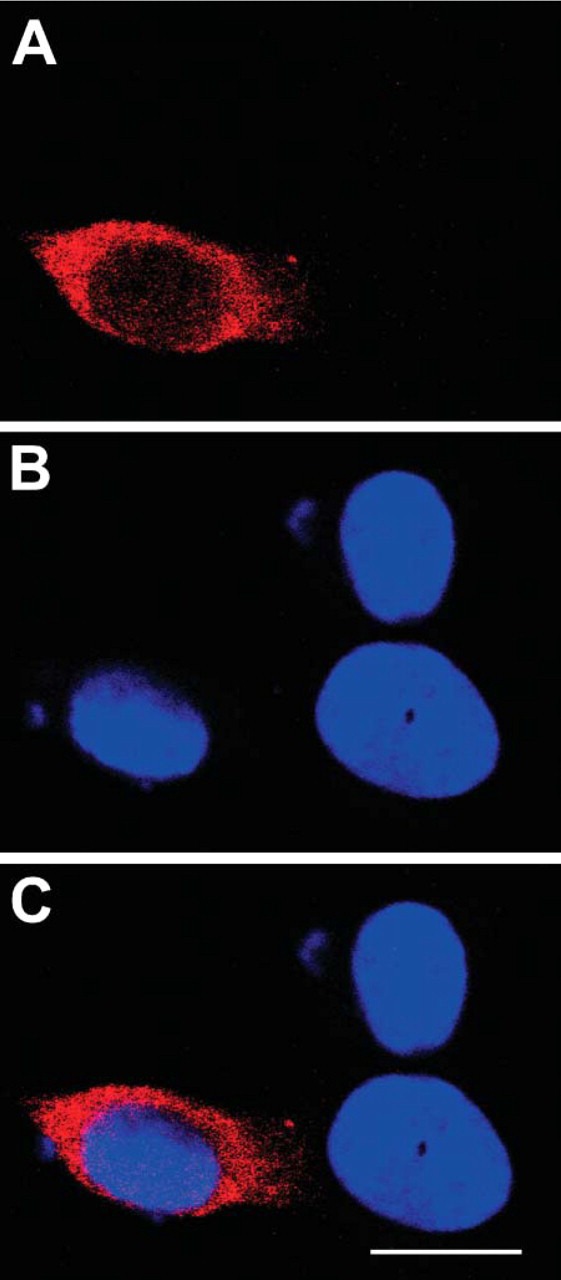
PC12 cells transfected with c-myc-HuD, fixed, and labeled for c-myc (A) and the nuclear marker DRAQ5 (B). Transfected cells had high levels of c-myc in the cytoplasm and little in the nucleus as seen in the merged image (C). In these overexpressing cells, HuD-c-myc levels are higher than would be expected for endogenous HuD. The blue of the nuclear marker indicates adjacent untransfected PC12 cells. Bar = 10 μm.
Discussion
HuD Nuclear/Cytoplasmic Shuttling
The RNA-binding protein, HuD, is mainly distributed to the cytoplasm with a small but consistent localization in the nucleus. To evaluate nuclear localization of HuD, we used three different microscopic techniques. With confocal microscopy we reconstructed optically sectioned cells to show that HuD granules were clearly within the nucleus. With cryo-ultramicrotome sections and wide-field fluorescence microscopy, HuD granules were found in nuclei of cells sectioned at 400 nm. Finally, with electron microscopic pre-embedding ICC, silver-enhanced gold particles were found within nuclei, indicating a small but consistent presence of HuD in the nucleus.
Another member of the ELAV/Hu family, HuR, is localized to the nucleus, shuttles to the cytoplasm, and forms large granules when stimulated with stress. In this study we have shown that, with heat shock, HuD accumulates in large granules, and that the HuD in these large granules is derived from HuD already in the cytoplasm. This redistribution of the ELAV/Hu family members in response to stress leads to the hypothesis that RNA-binding proteins in neuronal cells may respond to neurotrophic stimulation.
Previous results have shown that when the NLS of HuD is replaced with a strong NLS, HuD is found in both the nucleus and cytoplasm of unstimulated PC12 cells (Kasashima et al. 1999). This result suggests that the endogenous HuD NLS is very weak or does not function well. One possibility is that the HuD NLS requires other unidentified proteins to be shuttled into the nucleus, and that this may be dependent on NGF stimulation of PC12 cells to differentiate. In PC12 cells differentiation is associated with an increase in cytoplasmic GAP-43 mRNA, which binds to and is stabilized by HuD. With these observation of PC12 cells following stimulation with NGF, we hypothesized that HuD may shuttle into the nucleus.
It has been suggested that shuttled proteins that accumulate in the cytoplasm do so because the export is more efficient than the import (Kaffman and O'Shea 1999). We tested this idea with leptomycin B, an inhibitor of CRM-dependent export, and did not find an increase in levels of HuD in the nucleus (data not shown). However, it is possible that other non-CRM1 export pathways, like the transportin 2 pathway (Gallouzi and Steitz 2001), are involved. A recent report showed that HuD binds TAP/NXF1, a primary export protein for mRNA, suggesting that HuD uses the bulk mRNA export pathway (Saito et al. 2004) and not a regulated pathway. Evidence that HuD is involved in nuclear pre-mRNA splicing (Hua Lou, personal communication) and thus has an active role in the nucleus would support the need for HuD shuttling. Combined with our evidence that HuD levels in the nucleus do not change following neurotrophic stimulation, the possibility of a nuclear function for HuD suggests that shuttling occurs. Our evidence does not show a change in HuD distribution in response to NGF incubation. This result is supported by the HuD-dependent increases in GAP-43 mRNA stability following NGF stimulation (Chung et al. 1997; Tsai et al. 1997), an increase in cytoplasmic GAP-43 (Van Hooff et al. 1986; Burry and Perrone-Bizzozero 1993), and only a small change in GAP-43 transcription following NGF stimulation (Federoff et al. 1988; Perrone-Bizzozero et al. 1991,1993). Thus, HuD steady-state levels in the nucleus do not change following NGF stimulation, whereas an increase in cytoplasmic GAP-43 protein is seen.
We have shown that a small amount of HuD is found in the nucleus of PC12 cells, and that levels of nuclear HuD do not change in response to stimulation with signals that induce neuronal differentiation. Although we were able to detect nuclear loss of HuR in response to stress, we did not detect changes in nuclear HuD in stress- or neurotrophin-treated cells. The specific steady-state rates of HuD movement in and out of the nucleus cannot be determined by these studies, and our results suggest that localization of HuD in the nucleus is not regulated by the same stimuli that induce PC12 cells to differentiate. These results are consistent with the idea that following NGF or PMA stimulation the increase in GAP-43 mRNA stability is not regulated by shuttling.
Shuttling of mRNA involves distinct nuclear and cytoplasmic events in the localization pathway and suggests that the binding of specific RNA-binding proteins in the nucleus can direct RNA to its final destination in the cytoplasm (Kress et al. 2004). RNA-binding proteins and mRNA form ribonucleoprotein complexes (RNPs) that in the nucleus include proteins that bind to pre-mRNA as well as to mRNA (Dreyfuss et al. 2002). Identity of the proteins bound to mRNA is a topic of active investigation, and the distinctions between proteins involved in splicing and shuttling have become blurred. In fact, recent evidence shows that ELAV in flies (Soller and White 2005) and HuD in PC12 cells (Hua Lou, unpublished data) are involved in splicing pre-mRNA species, supporting a role for HuD in shuttling.
Model of HuD Distribution Following NGF Stimulation
Our results, along with previous observations, allow us to propose a model for HuD in neuronal cells. The model is based on the idea that HuD has functions in the nucleus and the cytoplasm in regulating mRNA. An important part of this model is that HuD is found at consistent levels seen in the nucleus and the cytoplasm.
Nuclear HuD is likely involved in splicing of pre-mRNA (Hua Lou, personal communication) and is exported to the cytoplasm by TAP/NXF1 binding to the hinge region of HuD (Saito et al. 2004). Little is known about the mechanisms of HuD shuttling, but our demonstration of consistent steady-state levels of HuD in the nucleus suggests that NGF stimulation does not change the normal rate of HuD nuclear shuttling. This is in contrast to HuR, which loses its mainly nuclear distribution during myogenesis (Van Der Giessen et al. 2003). Following completion of differentiation, HuR is again mainly nuclear.
Our previous study showed that HuD is associated with GAP-43 mRNA and ribosomes in growth cones (Smith et al. 2004), where it may regulate translation. This association completes our model by suggesting that the major role of HuD in the cytoplasm is targeting mRNA and potentially regulating translation in growth cones.
Acknowledgments
This research was supported by DiMarco Foundation for Spinal Cord Research (to RWB) and a Hunt-Curtis Visiting Scholar Award from The Ohio State University (to CLS).
The authors thank Nora Perrone-Bizzozero for the HuD-c-myc plasmid, Kathy Wolken for the cryo-ultramicrotomy, and Brian Kemmenoe for the image analysis. The authors are indebted to The Ohio State University Campus Microscopy and Imaging Facility for microscope services and to Andy Fischer for help with the manuscript.
Literature Cited
- Akamatsu W, Okano HJ, Osumi N, Inoue T, Nakamura S, Sakakibara SI, Miura M, et al. (1999) Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems. Proc Natl Acad Sci USA 96:9885–9890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KD, Merhege MA, Morin M, Bolognani F, Perrone-Bizzozero NI. (2003) Increased expression and localization of the RNA-binding protein HuD and GAP-43 mRNA to cytoplasmic granules in DRG neurons during nerve regeneration. Exp Neurol 183:100–108 [DOI] [PubMed] [Google Scholar]
- Anderson KD, Sengupta J, Morin M, Neve RL, Valenzuela CF, Perrone-Bizzozero NI. (2001) Overexpression of HuD accelerates neurite outgrowth and increases GAP-43 mRNA expression in cortical neurons and retinoic acid-induced embryonic stem cells in vitro. Exp Neurol 168:250–258 [DOI] [PubMed] [Google Scholar]
- Antic D, Keene JD. (1998) Messenger ribonucleoprotein complexes containing human ELAV proteins: interactions with cytoskeleton and translational apparatus. J Cell Sci 111:183–197 [DOI] [PubMed] [Google Scholar]
- Barreau C, Paillard L, Osborne HB. (2005) AU-rich elements and associated factors: are there unifying principles?. Nucleic Acids Res 33:7138–7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Apostolides PJ, Perrone-Bizzozero NI, Finklestein SP, Zwiers H. (1988) Anatomical distribution of the growth-associated protein GAP-43/B-50 in the adult rat brain. J Neurosci 8:339–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognani F, Tanner DC, Merhege M, Deschenes-Furry J, Jasmin B, Perrone-Bizzozero NI. (2006) In vivo post-transcriptional regulation of GAP-43 mRNA by overexpression of the RNA-binding protein HuD. J Neurochem 96:790–801 [DOI] [PubMed] [Google Scholar]
- Burry RW. (1995) Pre-embedding immunocytochemistry with silver-enhanced small gold particles. In Hayat MA, ed. Immunogold-Silver Staining: Principles, Methods, and Applications. Boca Raton, FL, CRC Press, 217–230 [Google Scholar]
- Burry RW, Perrone-Bizzozero NI. (1993) Nerve growth factor stimulates GAP-43 expression in PC12 cell clones independently of neurite outgrowth. J Neurosci Res 36:241–251 [DOI] [PubMed] [Google Scholar]
- Chi NC, Adam EJ, Adam SA. (1995) Sequence and characterization of cytoplasmic nuclear protein import factor p97. J Cell Biol 130:265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Adam EJ, Adam SA. (1997) Different binding domains for Ran-GTP and Ran-GDP/RanBP1 on nuclear import factor p97. J Biol Chem 272:6818–6822 [DOI] [PubMed] [Google Scholar]
- Chung S, Eckrich M, Perrone-Bizzozero NI, Kohn DT, Furneaux HM. (1997) The Elav-like proteins bind to a conserved regulatory element in the 3î-untranslated region of GAP-43 mRNA. J Biol Chem 272:6593–6598 [DOI] [PubMed] [Google Scholar]
- Dreyfuss G, Kim VN, Kataoka N. (2002) Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol 3:195–205 [DOI] [PubMed] [Google Scholar]
- Fan XC, Steitz JA. (1998) HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc Natl Acad Sci USA 95:15293–15298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federoff HJ, Grabczyk E, Fishman MC. (1988) Dual regulation of GAP-43 gene expression by nerve growth factor and glucocorticoids. J Biol Chem 263:19290–19295 [PubMed] [Google Scholar]
- Figueroa A, Cuadrado A, Fan J, Atasoy U, Muscat GE, Munoz-Canoves P, Gorospe M, et al. (2003) Role of HuR in skeletal myogenesis through coordinate regulation of muscle differentiation genes. Mol Cell Biol 23:4991–5004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M, Geuna S. (2001) Confocal imaging of HuC/D RNA-binding proteins in adult rat primary sensory neurons. Ann Anat 183:471–473 [DOI] [PubMed] [Google Scholar]
- Gallouzi IE, Brennan CM, Stenberg MG, Swanson MS, Eversole A, Maizels N, Steitz JA. (2000) HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc Natl Acad Sci USA 97:3073–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallouzi IE, Steitz JA. (2001) Delineation of mRNA export pathways by the use of cell-permeable peptides. Science 294:1895–1901 [DOI] [PubMed] [Google Scholar]
- Gao FB, Keene JD. (1996) Hel-N1/Hel-N2 proteins are bound to poly(A)+ mRNA in granular RNP structures and are implicated in neuronal differentiation. J Cell Sci 109:579–589 [DOI] [PubMed] [Google Scholar]
- Gilerovitch HG, Bishop GA, King JS, Burry RW. (1995) The use of electron microscopic immunocytochemistry with silver-enhanced 1.4-nm gold particles to localize GAD in the cerebellar nuclei. J Histochem Cytochem 43:337–343 [DOI] [PubMed] [Google Scholar]
- Jiang C, Schuman EM. (2002) Regulation and function of local protein synthesis in neuronal dendrites. Trends Biochem Sci 27:506–513 [DOI] [PubMed] [Google Scholar]
- Kaffman A, O'Shea EK. (1999) Regulation of nuclear localization: a key to a door. Annu Rev Cell Dev Biol 15:291–339 [DOI] [PubMed] [Google Scholar]
- Kasashima K, Terashima K, Yamamoto K, Sakashita E, Sakamoto H. (1999) Cytoplasmic localization is required for the mammalian ELAV-like protein HuD to induce neuronal differentiation. Genes Cells 4:667–683 [DOI] [PubMed] [Google Scholar]
- Kiebler MA, Hemraj I, Verkade P, Kohrmann M, Fortes P, Marion RM, Ortin J, et al. (1999) The mammalian staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: implications for its involvement in mRNA transport. J Neurosci 19:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress TL, Yoon YJ, Mowry KL. (2004) Nuclear RNP complex assembly initiates cytoplasmic RNA localization. J Cell Biol 165:203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky AM, Kosik KS. (2001) Neuronal RNA granules: a link between RNA localization and stimulation-dependent translation. Neuron 32:683–696 [DOI] [PubMed] [Google Scholar]
- Marion RM, Fortes P, Beloso A, Dotti C, Ortin J. (1999) A human sequence homologue of Staufen is an RNA-binding protein that is associated with polysomes and localizes to the rough endoplasmic reticulum. Mol Cell Biol 19:2212–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Yoneda Y. (2004) Alternative splicing of Staufen2 creates the nuclear export signal for CRM1 (Exportin 1). J Biol Chem 279:47473–47479 [DOI] [PubMed] [Google Scholar]
- Mobarak CD, Anderson KD, Morin M, Beckel-Mitchener A, Rogers SL, Furneaux HM, King P, et al. (2000) The RNA-binding protein HuD is required for GAP-43 mRNA stability, GAP-43 gene expression, and PKC-dependent neurite outgrowth in PC12 cells. Mol Biol Cell 11:3191–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng SS, Chen CY, Xu N, Shyu AB. (1998) RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J 17:3461–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone-Bizzozero NI, Cansino VV, Kohn DT. (1993) Posttranscriptional regulation of GAP-43 gene expression in PC12 cells through protein kinase C-dependent stabilization of mRNA. J Cell Biol 120:1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone-Bizzozero NI, Neve RL, Irwin N, Lewis S, Fischer I, Benowitz LI. (1991) Post-transcriptional regulation of GAP-43 mRNA levels during neuronal differentiation and nerve regeneration. Mol Cell Neurosci 2:402–409 [DOI] [PubMed] [Google Scholar]
- Saito K, Fujiwara T, Katahira J, Inoue K, Sakamoto H. (2004) TAP/NXF1, the primary mRNA export receptor, specifically interacts with a neuronal RNA-binding protein HuD. Biochem Biophys Res Commun 321:291–297 [DOI] [PubMed] [Google Scholar]
- Shen Y, Mani S, Donovan SL, Schwob JE, Meiri KF. (2002) Growth-associated protein-43 is required for commissural axon guidance in the developing vertebrate nervous system. J Neurosci 22:239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Afroz R, Bassell GJ, Furneaux HM, Perrone-Bizzozero NI, Burry RW. (2004) GAP-43 mRNA in growth cones is associated with HuD and ribosomes. J Neurobiol 61:222–235 [DOI] [PubMed] [Google Scholar]
- Soller M, White K. (2005) ELAV multimerizes on conserved AU4–6 motifs important for ewg splicing regulation. Mol Cell Biol 25:7580–7591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba G, Qui J, Koushika SP, White K. (2002) Ectopic expression of Drosophila ELAV and human HuD in Drosophila wing disc cells reveals functional distinctions and similarities. J Cell Sci 115:2413–2421 [DOI] [PubMed] [Google Scholar]
- Tsai KC, Cansino VV, Kohn DT, Neve RL, Perrone-Bizzozero NI. (1997) Post-transcriptional regulation of the GAP-43 gene by specific sequences in the 3î untranslated region of the mRNA. J Neurosci 17:1950–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Giessen K, DiMarco S, Clair E, Gallouzi IE. (2003) RNAimediated HuR depletion leads to the inhibition of muscle cell differentiation 9. J Biol Chem 278:47119–47128 [DOI] [PubMed] [Google Scholar]
- Van Hooff COM, de Graan PNE, Boostra J, Oestreicher AB, Schmidt-Michels MH, Gispen WH. (1986) Nerve growth factor enhances the level of the protein kinase C substrate B-50 in pheochromocytoma PC12 cells. Biochem Biophys Res Commun 139:644–651 [DOI] [PubMed] [Google Scholar]