Abstract
Hepatoblastoma is a pediatric liver tumor with epithelial components resembling embryonal and fetal liver cells. The existence of teratoid hepatoblastoma suggests the presence of stem cells in hepatoblastoma. The aim of this study was to analyze the expression of stem cell markers in hepatoblastomas. We studied specimens from 10 hepatoblastomas. Five of the hepatoblastomas were of epithelial and five of mixed type. Immunohistochemistry (IHC) for the stem cell markers CD34, Thy1, c-kit, and the hepatic or biliary lineage markers CK-18, OCH, CK-7, and CD56 was performed. Double IHC for stem cell and lineage markers was used to identify putative liver stem cells. The different markers showed distinct distributions on the tumor cells. Cells in atypical ducts were found to express simultaneously stem cell markers and hepatocytic or biliary lineage markers. Other cells in connective tissue showed c-kit expression, but not hepatic or biliary marker expression. The data show the presence of different cell populations bearing stem cell markers in human hepatoblastoma. Ductal cells co-expressing stem cell markers and hepatic lineage markers phenotypically resemble hepatic stem-like cells. These findings support the thesis that stem cells play a role in the histogenesis of hepatoblastoma.
Keywords: hepatoblastoma, liver stem cell, periatric liver tumors
Hepatoblastoma is the most common liver malignancy in children and shows various epithelial or mesenchymal lineages of differentiation (Abenoza et al. 1987). The epithelial components of hepatoblastomas exhibit features of embryonal and fetal liver differentiation (Ishak and Glunz 1967). Furthermore, cells resembling fetal or immature biliary lineages are also found in tissues of hepatoblastomas (Libbrecht et al. 2003). The presence of osteoid, cartilage, neural, or endocrine tissues in the mesenchymal components of mixed or teratoid hepatoblastomas suggested that stem cells play a role in the histogenesis of this tumor (Kim et al. 2001). Therefore, in the past several studies were aimed to find stem cell progenitors in the human liver by analyzing diseased pediatric livers with hepatoblastoma (Libbrecht and Roskams 2002). Ruck et al. (1996) were able to identify “small epithelial cells” with a characteristic phenotype in between hepatic and biliary cells by immunohistochemistry (IHC) and electron microscopic evaluation of hepatoblastomas. These cells expressed CK-7, albumin, and OV-6, as is known from stem cells (oval cells) present in the adult liver (Ruck et al. 1997). They were mainly found in anaplastic tumors, with a declining presence in embryonal or fetal differentiated hepatoblastomas. The existence of OV-6 positive cells in hepatoblastomas might point to the presence of oval cell progenitors; however, OV-6 was also expressed by terminally differentiated bile duct cells in normal livers (Roskams et al. 1996) or by regenerative hepatocytes (Crosby et al. 1998). Therefore, the existence and the role of a hepatic stem cell in the histogenesis of hepatoblastoma is still unclear. In the adult liver, hepatic stem cells (oval cells) with a clonogenic growth (Evarts et al. 1987, Lazaro et al. 1998) and a bipotential differentiation potential (either into hepatic or biliary cell lineages) were identified (Haruna et al. 1996). Oval cells show a characteristic co-expression of stem cell markers (e.g., CD34, Thy1, and others) simultaneously with hepatic lineage markers (e.g., CK-18, albumin, and others) (Thorgeirsson 1996). In this study, we aimed to identify stem cells in specimens of hepatoblastoma by IHC analysis for the expression of the stem cell markers CD34, c-kit, and Thy1. In addition, co-localization experiments by single and double IHC with differentiation markers of hepatic and biliary (CK-18, OCH, or CK-7) or neural cell types (N-CAM) were performed to further characterize cells bearing stem cell markers.
Materials and Methods
Harvest and Preparation of the Hepatoblastomas: H and E Staining
Ten hepatoblastomas (from patients aged 2 weeks to 12 years) resected after chemotherapy or directly as liver explantation after LTX were investigated. The tissue was snapfrozen in liquid nitrogen and stored at −80C for further processing. Cryostat sections were cut at 2 μm and were fixed with acetone at 4C for 90 sec. Slides were either stained directly or stored frozen at −20C until staining. Hematoxylin and eosin staining was performed, and the hepatoblastomas were classified histologically after the system of Ishak and Glunz (1967).
Immunohistochemistry
IHC analysis was performed using mouse monoclonal antibodies (MAbs) specific for CD34, Thy1, c-kit, CK-18, OCH, CK-7, and CD56 (specification and details in Table 1). Slides were rinsed with Tris buffer (Tris-buffered saline 0.05 M, pH 7.5) between the incubation steps.
Table 1.
Primary antibodies used for immunohistochemistry
|
| ||
|---|---|---|
| Marker | Antigen of | Antibody |
|
| ||
| CD 34 | Hematopoietic stem cells/ Hepatic oval cells | Mouse anti-human CD 34 (MCA 1578), Serotec, diluted 1:50 |
| Thy1 (CD 90) | Hematopoietic stem cells/ Hepatic oval cells | Mouse anti-human CDw90 (MCAP90), Serotec, diluted 1:10 |
| c-kit (CD 117) | Hematopoietic stem cells/ Hepatic oval cells | Mouse anti-human CD117 (clone 104 D2), DAKO, diluted 1:3000 |
| CD 56 (N-CAM) | Neuronal cells/ Reactive biliary epithelial cells | Mouse anti-human CD56 (clone T199), DAKO, diluted 1:40 |
| CK-18 | Liver and biliary epithelial cells | Mouse anti-human CK-18 (RGE53), Progen, diluted 1:10 |
| OCH | HCC antigen, hepatocyte marker | Mouse anti-human hepatocyte (clone OCH1E5), DAKO, diluted 1:40 |
| CK-7 | Biliary epithelial cells | Mouse anti-human CK-7 (clone OV-TL12/30), DAKO, diluted 1:100 |
|
| ||
Staining was performed using the alkaline phosphataseanti-alkaline phosphatase (APAAP) technique. Incubation with primary antibodies was 30 min. Antibodies were differently diluted (Table 1). Secondary marking was done with a rabbit anti-mouse IgG MAb diluted 1:50 for 30 min. Slides were then incubated with mouse-APAAP complex diluted 1:100 for 30 min. The alkaline phosphate substrate, New Fuchsin, was prepared as described elsewhere and the enzymatic reaction was allowed to proceed for 20 min. After rinsing in distilled water, slides were counterstained with hematoxylin.
Double Labeling of Thy1 and CK-18, Thy1 and CD56, or CK-18 and c-kit
For double staining cells were first stained with Thy1- or CK-18-APAAP as described above. After Thy1/CK-18 staining, slides were incubated with 20% horse serum (Vectastain; Vector Laboratories, Burlingame, CA) for 20 min. After rinsing in TRIS, the avidin and biotin blocking was performed for 15 min each. Slides were incubated with CK-18, CD56, or c-kit MAb for 30 min. A biotinylated antibody (Vectastain ABC kit) was then used as secondary antibody for 20 min. Avidin-biotin complex (Vectastain ABC kit) was prepared, and the enzymatic reaction was allowed to proceed for 20 min. The DAB staining reaction lasted for 10 min. Slides were counterstained with hematoxylin.
Results
Histological Classification of the Hepatoblastomas
Five of the 10 hepatoblastomas were classified as epithelial type and five as mixed type hepatoblastoma (Table 2). In two cases the epithelial type hepatoblastomas were pure fetal and in three cases mainly fetal. In the mixed type hepatoblastomas, the epithelial component was pure fetal in one case and mainly fetal in two cases (Table 2).
Table 2.
Human hepatoblastomas analyzed a
|
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|
| ||||||||||
| Age | 3 yr | 16 mo | 6 wk | 18 mo | 21 mo | 2.5 yr | 12 yr | 2 wk | 6 mo | 3 mo |
| Type | Mixed | Epithelial | Epithelial | Mixed | Mixed | Mixed | Epithelial | Epithelial | Epithelial | Mixed |
| Epithelial cells | Fetal | Mainly fetal | Mainly fetal | Fetal | Mainly fetal | Fetal | Fetal | Mainly fetal | Fetal | Embryonal |
| Pretreatment | No LTX | CTH | No | CTH | — | CTH | No | No | CTH | — |
|
| ||||||||||
aCTH, chemotherapy; LTX, allogenic liver transplantation; —, no data.
IHC for Hepatic or Biliary Epithelial Markers CK-18, OCH, CK-7, and Neural Cell Adhesion Molecule N-CAM (CD56)
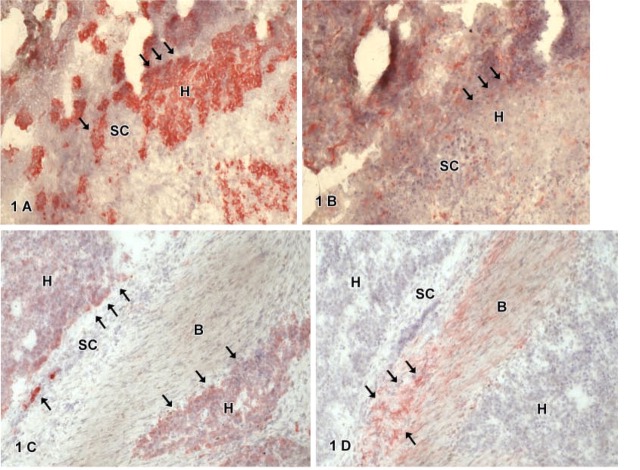
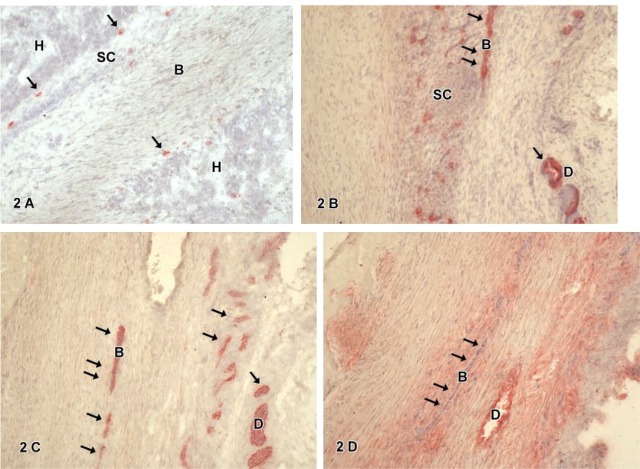
Cytokeratin-18-positive cells were found in all tumors. These cells were epithelial cells with a regular-shaped round nucleus, a small cytoplasm:nucleus ratio, and a polygonal cell shape resembling embryonal or fetal hepatocytic cells (H). Such cells were arranged either in sheets or groups surrounded by smaller cells (SC) (Figure 1A) or embedded in connective tissue (Figure 1C) not expressing CK-18. Furthermore, CK-18-positive cells were also found in atypical ducts (D) of the tumor (Figures 3A, 4C, and 4D). By CK-7 IHC, atypical bile ducts embedded in connective tissue (Figure 3C) or cell layers resembling fetal biliary cells (B) could be distinguished from hepatocytic cells. These CK-7-positive fetal biliary cells were arranged as streaks (Figures 1D and 2C) or as rosettes (Figure 2C). Fetal or embryonal hepatocytic cells, small cells, and connective tissue did not show any staining reaction for CK-7. Staining for the hepatocyte marker OCH confirmed the different staining reaction of hepatocytic cells (H) and biliary cells (B) in the hepatoblastomas (Table 3). CD 56-positive cells were found in some tumors. These cells were spindle shaped with a small nucleus, and formed small oval or round groups resembling ganglionic cells embedded in connective tissue (Figure 4A). In addition, some atypical ducts also showed CD56-expressing cells (not shown).
Figure 1.
(A-D, and 2A) Immunohistochemistry for hepatic marker CK-18 (A, C), biliary marker CK-7 (D), and stem cell markers CD34 (B) or c-kit (2A) of non-pretreated epithelial hepatoblastomas with mainly fetal cells. CK-18 staining shows positive hepatocytic cells (H, arrows) arranged as sheets (A) or groups (C) surrounded by small cells (SC) not positive for CK-18. Hepatocytic or small cells show no expression for CD 34 (B, arrows; CD34-negative hepatocytic cells). Immature biliary cells (B) expressing CK-7 (D) show no expression of CK-18 (C) or c-kit (2A). C-kit positive cells (arrows) are single cells embedded within the connective tissue; hepatocytic (H) or small cells (SC) show no expression of c-kit (2A).
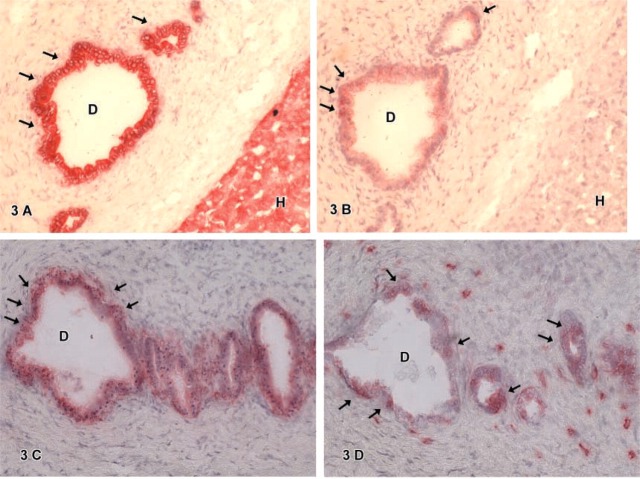
Figure 3.
(A-D) Immunohistochemistry for hepatic marker CK-18 (3A) showed positive cells located in atypical bile ducts (D, arrows) and CK-18 positive hepatocytic cells (H). CD 34 expression (3B) was observed by some cells in atypical bile duct cells (D, arrows). Hepatocytic cells (H) showed no staining reaction with CD 34 IHC. Cells in atypical bile ducts also showed expression of biliary marker CK-7 (3C) or stem cell marker c-kit (3D). Note the presence of single c-kit-positive cells in the surrounding connective tissue not positive for CK-7 (3C, 3D).
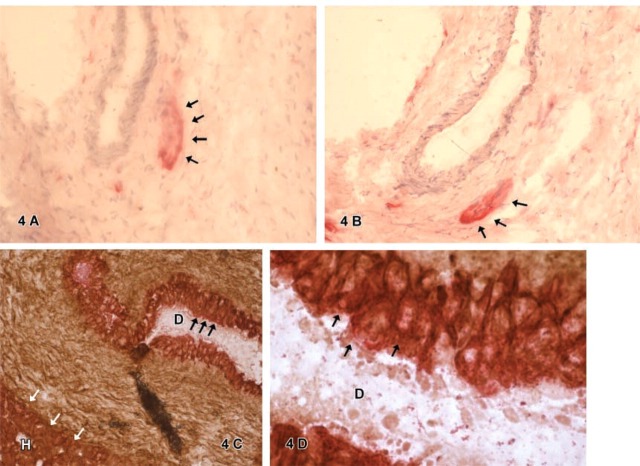
Figure 4.
(A-D) Round or oval groups of spindle shaped cells resembling ganglionic cells were found to express neural cell adhesion molecule (N-CAM, CD56) (4A) and stem cell marker Thy1 (4B). Double IHC for hepatic marker CK-18 (APAAP, red) and stem cell marker Thy1 (DAB, brown) showed cells simultaneously positive for both markers (black arrows) in atypical bile ducts (D) (4C; closer view 4D). Hepatocytic cells (H) stained only for CK-18 positive (white arrows, 4C).
Figure 2.
(B-D) Immunohistochemistry for hepatic marker CK-18 (B), biliary marker CK-7 (C), and stem cell marker Thy1 (D) of mixed type hepatoblastoma with mainly fetal cells. CK-18 staining (B) shows single positive biliary cells (arrows) embedded by small cells (SC) not positive for CK-18. CK-7 expression (C) is seen in aberrant bile ducts (B, arrows) or atypical biliary ducts (D). Thy1-positive cells (D) are found in atypical ducts (D); note that fetal biliary cells (B) do not express Thy1 (arrows). The connective tissue showed a diffuse staining in the Thy1 immunocytochemistry.
Table 3.
Marker expression in human hepatoblastoma tissue a
|
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Age | 3 yr | 16 mo | 6 wk | 18 mo | 21 mo | 2.5 yr | 12 yr | 2 wk | 6 mo | 3 mo |
| HE | Mixed | Epith. | Epith. | Mixed | Mixed | Mixed | Epith. | Epith. | Epith. | Mixed |
| CK-18 | H+ D+ | H+ D+ | SC- H+ | H+ B+ D+ | SC- H+ B(+) | SC- H+ | H+ | H+ | (+) | Neg |
| CK-7 | D+ | D+ | — | H- B+ D+ | SC- H- B+ | — | (+) | (+) | (+) | — |
| OCH | H+ D- | H+ | SC- | (+) | — | H+ | H+ | H+ | (+) | — |
| CD34 | D+ | D+ | SC(+) | — | — | — | — | — | — | |
| Thy1 | D+ CT+ GG+ | D+ CT+ GG+ | CT+ | CT+ D+ | CT+ | CT+ | CT+ | CT+ | CT(+) | |
| c-kit | D+ CT+ | D+ CT+ | SC(+) | CT+ | SC(+) | — | CT(+) | + | + | — |
| CD56 | D+ GG+ | D+ GG+ | — | — | — | — | — | (+) | (+) | — |
|
| ||||||||||
aB, biliary cells; CT, connective tissue; D, duct cells; H, hepatocytic cells; GG, ganglionic groups; SC, small (undifferentiated) cells; —, no data.
IHC for Hematopoietic Stem Cell Markers CD34, Thy1, and c-kit
CD34-positive cells were found in some hepatoblastomas. Cells expressing CD34 were mainly located in atypical ducts (D) (Figure 3B). These ductal cells (D) also expressed CK-18 (Figure 3A) or CK-7 (Figure 3C). In one case, rare single CD34-positive cells were diffusely found in the small cell component (SC) of the tumor (Figure 1B). Hepatocytic cells (H) positive for CK-18 did not express CD34 in this case (Figure 1A).
C-kit-positive cells showed a different distribution. Cells in atypical ducts (D) showed expression of c-kit (Figure 3D) as well as CD34 expression. In contrast, single cells expressing c-kit were found in the connective tissue (Figure 3D). These cells showed no CD34 expression.
Thy1-positive cells were either in atypical ducts (D) (Figures 4C-4D) or in ganglionic cell groups (Figure 4B). Thy1-positive ganglionic cells did not express CD34 or c-kit, whereas atypical ducts showed an expression of both markers, as mentioned above. Furthermore, a Thy1-positive staining reaction was found diffusely within the connective tissue of all tumors, which was not specifically a cellular reaction. This unspecific reaction could be clearly distinguished from the observed defined cellular reaction of Thy1-positive stained cells.
Double IHC for CK-18 and Thy1 or CK-18 and c-kit
Double IHC for CK-18 and Thy1 showed a positive reaction for CK-18 of hepatocytic cells (H) and in atypical ducts (D) (Figure 4D). Whereas cells also positive for Thy1 were found in atypical ducts and in some ganglionic groups, hepatocytic cells were not found to express Thy1 in the double immunostaining. CK-18 and c-kit double IHC confirmed the staining reaction found for both markers in the single staining. Atypical ducts contained cells positive for both CK-18 and c-kit. Single cells spreading out into the connective tissue were positive for c-kit but not for CK-18. Hepatocytic cells showed CK-18 expression but no c-kit expression.
Discussion
Stem Cell Markers Are Found on Different Hepatoblastoma Cells
Hematopoietic stem cell markers were used to identify possible candidates for stem cells in human hepatoblastomas. The results showed that the different stem cell markers studied were present in distinct varying cell types. CD34-positive cells were rarely observed in hepatoblastoma. We observed CD34 positive cells mainly located in atypical ducts (Figures 3 and 4). Furthermore, some small cells in one tumor were also found to be CD34-positive. Ruck et al. (1995) investigated the expression of the stem cell marker CD34 and additional markers of endothelial cells (CD31, von Willebrand factor) by IHC in hepatoblastoma. In contrast to our study, they found CD34-positive staining of liver sinusoids and capillaries in one of the studied specimens. This might be due to the different type of the tumors investigated. In the study of Ruck et al. the tumors were mainly anaplastic, whereas we investigated mainly fetal type tumors. IHC for the stem cell marker Thy1 showed a cell-specific reaction for Thy1 in atypical biliary ducts as well as in some ganglionic cells. Furthermore, a diffuse reaction of the connective tissue was observed. The positive staining reaction of the connective tissue for Thy1 was also found by others and was explained by an unspecific binding by the fibers of the connective tissue or by extracellular Thy1 accumulation (Morris and Ritter 1980; Morris and Beech 1984). C-kit-positive cells were found in atypical biliary ducts, as observed with CD34 or Thy1 positive cells. Additionally c-kit positive cells were found within the connective tissue: These were small cells located as isolated cells within the connective tissue fibers (overview Table 4). Baumann et al. (1999) identified c-kit positive cells in tissue specimens of biliary atresia in a previous study. Double staining showed that biliary CK-19 positive ductal cells occasionally expressed the stem cell marker c-kit. Some cells located around the portal tract also expressed both markers. From these observations, we concluded that cells co-expressing both markers might be progenitor cells of the human liver. Interestingly, these stem cells were found in ductal structures, but not in periportal areas or within hepatocytic cells. This was also observed in our study. Stem cell marker-positive cells for CD34, Thy1, and c-kit were located mainly in atypical ducts. On rare occasions, c-kit-positive cells were found as single cells within the connective tissue, or Thy1-positive cells were found in ganglionic groups. No such cells were observed in hepatocytic tumor cells, and only in one specimen were small cells positive for CD34 or c-kit found.
Table 4.
Stem cell marker-bearing cells represented in hepatoblastoma
|
| ||
|---|---|---|
| Cell type | Phenotypical marker expression | Location |
|
| ||
| Hepatocytic stem-like cells | CD 34 +, Thy 1 +, c-kit + and CK-18 +, CK-7 + | Atypical biliary ducts (D) |
| Neuronal progenitor cells | Thy 1 + (CD 34 neg, c-kit neg) and CD 56 + (CK-18/CK-7 neg) | Ganglionic groups located periductally in connective tissue |
| Primitive stem cells | c-kit + (CD 34/Thy 1 neg) | Single cells spreading in CK-18/CK-7/CD 56 neg connective tissue, small cells (SC) |
|
| ||
Stem-like cells Resembling Oval Cells Are Located in Atypical Ducts of Human Hepatoblastomas
Double IHC for the hepatic lineage marker CK-18 and the stem cell markers c-kit or Thy1 showed cells staining positive for both markers located in atypical bile ducts. Single staining furthermore characterized these duct cells as CD34-, CK-7-, and CD56-positive (Figures 3 and 4). The phenotypical marker expression of these cells identified in human hepatoblastoma equals the pattern of oval cells observed in adult human liver, which typically show the co-expression of stem cell markers (e.g., CD34, Thy1, c-kit) and hepatic lineage markers (Alison et al. 1996; Thorgeirsson 1996; Sell 2000; Vessey and Hall 2001). Other groups investigated the presence of the oval cell-associated antigen OV-6 to identify possible liver stem cells in hepatoblastoma by IHC studies (Crosby et al. 1998). Ruck et al. (1997) found small epithelial cells expressing CK-7, albumin, and OV-6. On this basis, it was suggested that the small epithelial cells described may be tumorous equivalents of adult oval cells in hepatoblastomas. Our study shows that small cells in hepatoblastoma do not regularly bear stem cell markers; therefore, we assume that these cells are not tumorous equivalents of oval cells. Cells with a similar pattern of marker expression, such as oval cells, were found to be distinct cells located in atypical ducts. This is consistent with findings published recently by Badve et al. (2003), who analyzed CD34 and bcl-2 expression in hepatoblastomas. They found no stem cell marker expression in small epithelial cells or in HepPar-1 (as hepatocytic marker) or CK-19 (as biliary marker)-positive small epithelial cells. From these observations it was speculated that small epithelial cells might be more primitive stem cells of hepatoblastoma than oval cell-like cells. Our data may strengthen this hypothesis. Well-characterized oval-cell-like cells co-expressing stem cell and hepatic lineage markers were located in atypical ducts. Small cells observed in our study were found in different locations. In rare instances they expressed stem cell markers (CD34 or c-kit) but never liver-like differentiation markers.
Other Stem Cell Marker-bearing Tumor Cells in Hepatoblastoma
Staining for stem cell marker Thy1 identified positive periductular clusters of cells resembling ganglionic groups (Figure 4). These cells also expressed the neuronal cell marker N-CAM (CD56). The presence of neuronal cells in close relationship to developing bile ducts was observed by Libbrecht et al. (2001) and Fabris et al (2000). These findings indicate that these groups might be neuronal progenitor cells. Furthermore, we observed distinct CD56-positve cells in atypical ducts. N-CAM (CD56) was first established as a marker for reactive biliary epithelial cells by Van den Heuvel et al. (2001). By immunocytochemical analysis of normal and diseased human liver, CD56 was found to be expressed by reactive bile ducts in most liver diseases. Normal biliary cells did not express CD56. Therefore, the CD56 expression of biliary cells might be explained by a reactive state of the tumorous bile ducts.
In short, the IHC analysis for the hematopoietic stem cell markers Thy1, c-kit, and CD34 and the hepatic or biliary lineage markers CK-18, CK-7, and CD56 revealed the presence of different types of stemcell marker positive cells in human hepatoblastoma (see Table 4). We observed cells positive for CK-18 and Thy1/c-kit resembling liver stem-like cells. These cells also expressed the stem cell marker CD34, the biliary marker CK-7, and CD56. Furthermore, we found cells positive for CD56 and the stem cell marker Thy1, which might be progenitors of neural cell lineages, and c-kit positive cells within the connective tissue negative for all other markers. The data indicate the presence of different types of stem cells during the histogenesis of hepatoblastoma.
Acknowledgments
We wish to thank Mrs B. Teichmann, Dept. of Pediatric Surgery, Medizinische Hochschule Hannover, for technical assistance.
Literature Cited
- Abenoza P, Manivel JC, Wick MR, Hagen K, Dehner LP. (1987) Hepatoblastoma: an immunohistochemical and ultrastructural study. Hum Pathol 18:1025–1035 [DOI] [PubMed] [Google Scholar]
- Alison MR, Golding MHC, Sarraf CE. (1996) Pluripotential liver stem cells: facultative stem cells located in the biliary tree. Cell Prolif 29:373–402 [DOI] [PubMed] [Google Scholar]
- Badve S, Logdberg L, Lal A, de Davila MT, Greco MA, Mitsudo S, Saxena R. (2003) Small cells in hepatoblastoma lack “oval” cell phenotype. Mod Pathol 16:930–936 [DOI] [PubMed] [Google Scholar]
- Baumann U, Crosby HA, Ramani P, Kelly DA, Strain AJ. (1999) Expression of the stem cell factor c-kit in normal and diseased pediatric livers: identification of a human hepatic progenitor cell? Hepatology 30:112–117 [DOI] [PubMed] [Google Scholar]
- Crosby HA, Hubscher SG, Joplin RE, Kelly DA, Strain AJ. (1998) Immunolocalization of OV-6, a putative progenitor cell marker in human fetal and diseased pediatric liver. Hepatology 28:980–985 [DOI] [PubMed] [Google Scholar]
- Evarts RP, Nagy P, Marsden E, Thorgeirsson SS. (1987) A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis 8:1737–1740 [DOI] [PubMed] [Google Scholar]
- Fabris L, Strazzabosco M, Crosby HA, Ballardini G, Hubscher SG, Kelly DA, Neuberger JM, et al. (2000) Characterization and isolation of ductular cells coexpressing neural cell adhesion molecule and bcl-2 from primary cholangiopathies and ductal plate malformations. Am J Pathol 156:1599–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. (1996) Identification of bipotential progenitor cells in normal and injured livers. Hepatology 23:476–481 [DOI] [PubMed] [Google Scholar]
- Ishak KG, Glunz PR. (1967) Hepatoblastoma and hepatocarcinoma in infancy and childhood: report of 47 cases. Cancer 20:396–422 [DOI] [PubMed] [Google Scholar]
- Kim L, Park YN, Kim SE, Noh TW, Park C. (2001) Teratoid hepatoblastoma: multidirectional differentiation of stem cell of the liver. Yonsei Med J 42:431–435 [DOI] [PubMed] [Google Scholar]
- Lazaro CA, Rhim JA, Yamada Y, Fausto N. (1998) Generation of hepatocytes from oval cell precursors in culture. Cancer Res 58:5514–5522 [PubMed] [Google Scholar]
- Libbrecht L, Cassiman D, Desmet V, Roskams T. (2001) Expression of neural cell adhesion molecule in human liver development and in congenital and acquired liver diseases. Histochem Cell Biol 116:233–239 [DOI] [PubMed] [Google Scholar]
- Libbrecht L, Desmet V, Roskams T. (2003) Stages of normal and aberrant intrahepatic bile duct development in a mixed hepatoblastoma. Histopathology 42:618–620 [DOI] [PubMed] [Google Scholar]
- Libbrecht L, Roskams T. (2002) Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol 13:389–396 [DOI] [PubMed] [Google Scholar]
- Morris RJ, Beech JN. (1984) Differential expression of Thy1 on various components of connective tissue of rat nerve during postnatal development. Dev Biol 102:32–42 [DOI] [PubMed] [Google Scholar]
- Morris RJ, Ritter MA. (1980) Association of Thy1 cell surface differentiation antigen with certain connective tissues in vivo. Cell Tissue Res 206:459–475 [DOI] [PubMed] [Google Scholar]
- Roskams T, DeVos R, Desmet V. (1996) Undifferentiated progenitor cells in focal nodular hyperplasia of the liver. Histopathology 28:292–296 [DOI] [PubMed] [Google Scholar]
- Ruck P, Xiao JC, Kaiserling E. (1995) Immunoreactivity of sinusoids in hepatoblastoma: an immunohistochemical study using lectin UEA-1, and antibodies against endothelium-associated antigens, including CD34. Histopathology 26:451–455 [DOI] [PubMed] [Google Scholar]
- Ruck P, Xiao JC, Kaiserling E. (1996) Small epithelial cells and the histogenesis of hepatoblastoma: electron microscopic, immunoelectron microscopic, and immunohistochemical findings. Am J Pathol 148:321–329 [PMC free article] [PubMed] [Google Scholar]
- Ruck P, Xiao JC, Pietsch T, Von Schweinitz D, Kaiserling E. (1997) Hepatic stem-like cells in hepatoblastoma: expression of cytokeratin 7, albumin and oval cell associated antigens detected by OV-1 and OV-6. Histopathology 31:324–329 [DOI] [PubMed] [Google Scholar]
- Sell S. (2000) Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology 33:738–750 [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS. (1996) Liver regeneration 9: hepatic stem cells in liver regeneration. FASEB J 10:1249–1256 [PubMed] [Google Scholar]
- Van den Heuvel MC, Sloof MJH, Visser L, Muller M, De Jong KP, Poppema S, Gouw AH. (2001) Expression of anti-OV6 antibody and anti-N-CAM antibody along the biliary line of normal and diseased human livers. Hepatology 33:1387–1393 [DOI] [PubMed] [Google Scholar]
- Vessey CJ, Hall PM. (2001) Hepatic stem cells: a review. Pathology 33:130–141 [PubMed] [Google Scholar]