Abstract
We fractionated leukocytes from three donors into >90% pure samples of granulocytes, lymphocytes, and monocytes and tested them for transcriptional and translational expression of three physiologically-proven lactate transporters, monocarboxylate transporter 1(MCT1), MCT2, and MCT4, using RT-PCR and affinity-purified rabbit antibody (Ab) to the C-terminal segment of each human MCT. Transcripts of all three MCTs were identified in each leukocyte fraction by RT-PCR and proven by sequencing of fragments extracted after isolation on agarose gels. Transporter protein of the appropriate size was demonstrated for each of the monocarboxylate transporters MCTs in lymphocytes and monocytes by Western blot, while lower-molecular-weight bands were found in granulocytes and are presumed to be degraded forms, because they were blocked by antibody-antigen (Ab-Ag) preincubation. IHC demonstrated all three MCTs in methanol-fixed droplets of all three leukocyte fractions; stain was abolished on omission of the primary Ab. Plasmalemmal staining occurred with all MCTs in all leukocyte fractions. Because the Km for lactate increases approximately fivefold at each step, with MCT2<1<4, leukocytes must use the full range of lactate binding to survive in acidic and hypoxic environments. Except for MCT4 in lymphocytes, all the MCTs also stained leukocyte cytoplasm, often with distinct granularity. Nuclear membrane staining was also seen with MCT1 and MCT2, while platelet plasmalemma stained only with MCT2.
Keywords: lactate transporters, monocarboxylate transporters, granulocytes, monocytes, lymphocytes, platelets, human leukocytes, immunohistochemistry, RT-PCR, Western blotting
Transport of lactate, pyruvate, and other mono-carboxylic acids across the plasma membrane is important for mammalian cells. Cells that employ glycolysis as a major source of ATP, such as red and white blood cells, skeletal muscle, and most tumor cells, must expel lactic acid to prevent acidosis. White muscle fibers may export accumulating lactate to adjacent red muscle fibers, which can convert it to pyruvate and oxidize it (Fishbein et al. 2002). This transport is controlled by hydrogen ion and metabolite gradients, and functions for a number of other monocarboxylic acids as well, so that their cell membrane transporters form a family of monocarboxylate transporters (MCTs) (for review, see Halestrap and Price 1999; Juel and Halestrap 1999; Juel 2001; Merezhinskaya and Fishbein 2001). Nine putative MCT family members have been identified on the basis of sequence similarity, and four of them, MCT1-4, have been proven physiologically to transport monocarboxylates (Kim-Garcia et al. 1994; Lin et al. 1998; Wilson et al. 1998; Grollman et al. 2000). To prevent confusion, we must point out that the gene and protein we refer to here as MCT4 was originally believed to be an MCT3 (Wilson et al. 1998) and was later renumbered with explanation (Pilegaard et al. 1999).
Whereas MCTs 1, 2, and 4 serve general lactate/pyruvate transport, other members have been tailored to more specific function or location. Thus, MCT3, with kinetics similar to MCT1, is present primarily in the retinal pigment epithelium in vertebrates (Philp et al. 2001), and MCT8 has recently been shown to exhibit remarkable substrate specificity for the transport of iodothyronines (Friesema et al. 2003). This latter finding, parenthetically, clouds the distinction between the MCT family and that of the α-amino acid transporters. Several MCTs can be expressed in the same cell or tissue, and the pattern of expression differs from one species to another. The dissociation constants (Km) of lactate and pyruvate increase roughly five-fold from MCT2 to 1 and again from MCT1 to 4, suggesting a simple kinetic reason why it may be useful to express all three in the same cell if it must function at times under moderately to extremely hypoxic conditions, as must leukocytes. We have recently evaluated the relative distribution of MCT1, MCT2, and MCT4 in a panel of human tissues, and with more detail in muscle fibers, using specific antibodies (Fishbein et al. 2002). Here we extend the investigation of MCT expression to fractionated white blood cells, about which there is very little information in the literature.
Materials and Methods
MCT1, MCT 2, and MCT4 Antiserum Preparation and Purification
The peptide sequences, of MCT1, MCT 2, and MCT4, each preceded by a cysteine, their apparent and theoretical molecular weight (MW), and their relationship to the C-terminus of the transporters are listed in Table 1. To immunize rabbits, the synthetic peptides were obtained for MCT1, MCT 2, and MCT4, [each verified by mass spectrometry (W.M. Keck Facility at Yale University; New Haven, CT)], conjugated to keyhole limpet hemocyanin via the cysteine, and emulsified with Freund's adjuvant. The sera were purified on protein A columns to obtain the IgG fraction, which was then affinity chromatographed on peptide-coupled Sulfolink columns (Pierce; Rockford, IL). After elution, dialysis, and concentration, they were assayed for specificity and sensitivity, then stored in 0.1-ml aliquots at −80C.
Table 1.
Peptide and protein antigen properties
|
| |||
|---|---|---|---|
| Transporter protein | Synthetic peptide used to immunize rabbits | C-terminus of protein | MW by Ab on SDS-PAGE MW by aa sequence |
|
| |||
| MCT1 | C-QKDTEGGPKEEESPV | 500 | 46 kD |
| aa = C + 486-500 | 54 kD | ||
| MCT2 | C-KVSNAQSVTSERETNI | 478 | 43 kD |
| aa = C + 463-478 | 52 kD | ||
| MCT4 | C-KAEPEKNGEVVHTPETSV | 575 | 45 kD |
| aa = C + 558-575 | 49.5 kD | ||
|
| |||
Preparation of Fractionated Leukocytes
Blood samples from three donors were collected (with written consent) in EDTA tubes and the cell layer first separated on Histopaque 1077 for isolation of granulocytes and red cells at the bottom after a 400 rpm × 35 mm spin. The red cells were lysed by a 10-min treatment with a two-to four-fold excess volume of 155 mM NH4Cl, 10 mM KHCO3, and 10 mM EDTA, pH 7.3 at 20C. After clearing, the solution was centrifuged at 300 × g for 10 min at 20C, and the pellet was washed to a barely pink color in 0.5% BSA and 2 mM EDTA in PBS, pH 7.3 (buffer A). After resuspension in PBS and preparation of droplet and smear microscope slides, the remainder was aliquotted and stored at −80C.
The mononuclear cells at the interface were harvested, washed, and spun for 10 min at 200 × g at 24C to separate them from most of the platelets. Monocytes were separated from lymphocytes by selective adsorption onto anti-CD14-coupled magnetic microbeads (Miltenyi Biotech; Auburn, CA). Cells were resuspended in 500 μl of ice-cold buffer A, and anti-CD14 microbeads were added to the cells (100 μl beads per 5 × 107 mononuclear cells). After incubation for 15 min at 40C, the cells were washed in buffer A and spun down at 300 × g for 10 min at 20C. An MS(+) positive selection column was placed into the magnetic field of a MACS separator attached to a metal stand and washed with 500 μl of degassed buffer A. Then 500 μl of cell suspension was applied to the column and the unbound flow-through fraction containing lymphocytes was collected. The column was rinsed with 500 μl of degassed buffer A four times and then removed from the magnetic field. One ml of buffer A was added to the column, and monocytes were eluted from the column with the aid of a plunger. The separated lymphocytes and monocytes were each washed with buffer A, resuspended in 500 μl PBS, used to prepare droplet and smear slides, then aliquotted and stored at −80C.
RNA Isolation and RT-PCR for Evaluation of Transcripts
Total RNA from the separated granulocytes, lymphocytes, and monocytes was obtained using RNAzolB (TEL-TEST; Friendswood, TX). Briefly, cells were lysed in RNAzolB and chloroform was added. After vigorous shaking, incubation on ice, and centrifugation, the colorless upper aqueous phase containing RNA was precipitated by treatment with isopropanol, washed, air dried, and dissolved in 1 mM EDTA, pH 7.0. The mRNA was isolated from 50 μl of total RNA using the QuickPrep Micro mRNA Purification Kit (Pharmacia; Piscataway, NJ) as recommended by the manufacturer. RTPCR of MCT1, −2, and −4 was carried out using First Strand cDNA Synthesis Kit (Pharmacia) followed by PCR. The first strand synthesis reaction and PCR amplification were performed using 8 μl of mRNA and the primers listed in Table 2, synthesized by Midland Certified Reagents (Midland, TX). AmpliTaq polymerase (PE Applied Biosystems; Foster City, CA) with 2 μM primers and 2 mM MgCl2 was used for the PCR reaction. The reaction mixture was overlaid with mineral oil and heat denatured for 3 min at 95C, followed by 40 cycles of 15-sec denaturation (95C), 30 sec of annealing (60C), and 36+6 sec/cycle of elongation (72C). After a final 10-min polishing at 72C, the PCR products were electrophoresed in agarose gels. PCR product bands of expected size were excised from agarose gels and extracted using Gene Clean (BIO101; Carlsbad, CA) as recommended by the manufacturer. The PCR amplimers were sequenced using a 3730 DNA Analyzer (Applied Biosystems) and compared with the nucleotide NCBI database.
Table 2.
Primer sets used for RT-PCR of MCT1, MCT2, and MCT4 a
|
| ||||
|---|---|---|---|---|
| MCT | Primers | Sequence | Total bp | Coding bases |
|
| ||||
| MCT1 | P1 | GGAGGTCTTGGGCTTGCCTTCAACT | 799 | 358-382 |
| P2 | GGACTCTTTGCACCTTTGGTGT | 331 | 826-847 | |
| P3 | GGATTCTGTGTCTATGCGGGATTC | 94 | 1063-1086 | |
| RP1 | CAACAAGGTCCATCAATGTTTCAA | 1157-1134 | ||
| MCT2 | P1 | CGGGCGCCCACCCTGCGCCAGAGACCAG | 650 | (−161)-(−134) |
| P2 | TAATTGGCAAATACTTCTATAGGAAGCG | 636 | 401-428 | |
| RP1 | AGGAGCCAATGAACTTAAGAAAACAGGA | 489-462 | ||
| RP2 | AATACAGCATATAATACCAGGCTTGT | 1037-1012 | ||
| MCT4 | P1 | GGCGAGAGGCGGGCTGAGGCGGCCCAG | 582 | (−62)-(−36) |
| P2 | CCCTGTCTTCCTGTGTGCCCTGAG | 601 | 468-492 | |
| P3 | CCATCGTGGGCACCCACAAGTTCT | 419 | 1034-1057 | |
| RP1 | AGCGGTCCTGCAGCAGCTGCCCCAGCG | 520-594 | ||
| RP2 | CAATGGCACTGGAGAACTTGTGGG | 1069-1046 | ||
| RP3 | AAGCGTTGCCGGCTTCTGTACCTC | 1453-1430 | ||
|
| ||||
aThe correspondingly numbered P and RP were used as primer pairs for each amplification except for MCT1, where one RP was used for all. P is a forward primer (5′ × 3′) and RP is a reverse primer (3′ × 5′). The coding bases count from the start codon's first nucleotide. Nucleotides in parentheses precede the coding region counting 5′(−) backward before the first coding base. Total bp listed in column 4.
Cell Membrane Preparation and Western Blotting Analysis
Fractionated white blood cells were thawed, added to lysis buffer (20 mM Tris-HCl, pH 7.5), with Complete Protease Inhibitor Cocktail with EDTA (Boehringer; Indianapolis, IN) and freeze thawed (dry ice and 37C water bath) three times for 10 min each cycle. The suspension was then sonicated at 2.5 output control, 40% duty cycle for 30 sec on ice with the microprobe (Heat Systems/Ultrasonics; Plainview, NY). Membrane and cytosolic fractions were separated by centrifugation at 20,000 × g for 30 min at 4C, and the membrane fraction was dissolved in 2.5% SDS in PBS. A 40-60-μg aliquot of membrane protein was heated at 100C in sample buffer (62.5 mM Tris-HCl, pH 6.8, 50 mM dithiothreitol, 10% glycerol, 0.1% bromphenol blue, 2.5% SDS) and loaded on 12% polyacrylamide Tris-glycine gels (NOVEX; San Diego, CA). After electrophoresis at 120 V for 1 hr, the proteins were electroblotted in an ice bath at 100 V for 1 hr onto Immobilon-PSQ polyvinyldifluoride membranes (Millipore; Bedford, MA). The blots were blocked with 5% nonfat milk and 0.1% Tween-20 in PBS for 1 hr, then incubated for 2 hr with affinity-purified anti-MCT1, −2, or −4 antibodies diluted in 3% BSA plus 0.1% Tween-20 in PBS. Antibody dilutions were: MCT1, 1:1000; MCT2, 1:2500; and MCT4, 1:500. After three washes in 2.5% milk plus 0.1% Tween-20 in PBS, the blots were incubated with goat anti-rabbit IgG coupled to horseradish peroxidase (Sigma; St Louis, MO). After three more washes in 2.5% milk plus 0.1% Tween-20 in PBS, SuperSignal West Dura Extended Duration chemiluminescent substrate (Pierce) was diluted 1:2 with water and added to the blots for 5 min. The blots were drained and exposed to Biomax MR film (Eastman Kodak; Rochester, NY) for various periods of time (minutes).
Immunohistochemistry and Comparison of Fixatives
To evaluate fixatives, 6-μm sequential cryostat sections of unfixed frozen human skeletal muscle were air dried on microscope slides and immersed in 4C solutions of methanol, ethanol, acetone, or 10% formalin (3.7% formaldehyde) in PBS for 30 min. They were rinsed in PBS, air dried, and treated as described below, using anti-MCT1 as primary antibody, along with a sequential slide that remained unfixed, and compared at X100-200 magnification.
Freshly separated white blood cells that had been drop dried or smeared on glass slides and fixed with 100% methanol for 30 min at 4C were used for antibody (Ab) staining. This was performed using Elite Rabbit IgG Vectastain ABC kit (Vector; Burlingame, CA) with 3,3′-diaminobenzidine substrate (DAB), after quenching with 0.3% H2O2 and blocking with normal goat serum. After 30 min with primary MCT Ab and washing, the biotinylated secondary Ab was added for 30 min, washed, then followed by preformed avidin DH-biotinylated horseradish peroxidase H complex for 30 min. Slides were then overlaid with DAB for 9 min, rinsed, dried, mounted, and coverslipped.
Protein concentrations were measured using the Lowry procedure (Lowry et al. 1951) modified to 0.8 ml final volume to triple the sensitivity; BSA provided the reference standard. For rabbit antisera purification, UV absorption was measured and calculated using the known specific absorption of human IgG. Standard Wright-Giemsa staining (Raber and Buckner 1994) was used to evaluate the purity of the three white cell fractions, and the reverse ATPase reaction was used to evaluate the three muscle fiber types in frozen sections (Gregory and Griffin 1994). Preparation of frozen sections for fluorescence microscopy and instrumentation used were the same as previously described (Fishbein et al. 2002), and statistical analysis employed the GraphPad Prism 4 software (San Diego, CA).
Results
Preparation of Fractionated White Blood Cells
The purity of the three fractionated white blood cell groups, granulocytes, lymphocytes, and monocytes, was assessed by microscopy at ×400-1000 (oil) of Wright-Giemsa-stained slides. Each of the mononuclear cell fractions had >90% purity from the other, and both of them versus the granulocyte isolates shared <2% contamination. Residual red cells in the granulocyte isolate and platelets in the mononuclear cell isolates were not considered in this evaluation.
RT-PCR of MCT1, MCT2, and MCT4
RT-PCR was performed to evaluate transcription of MCTs, using two or three primer pairs for each of three MCTs and the mRNA isolated from each of the three cell types. The primer pairs used are listed in Table 2. The PCR product amplimers were run on agarose gels and visualized by ethidium bromide staining. An example is shown in Figure 1, in which three amplimers of MCT1 were obtained for granulocytes, lymphocytes, and monocytes, although the granulocyte bands are quite weak for two of the three PCR products. Figure 2 shows another agarose gel in which an amplimer for each of the three MCTs is present, in this instance from a monocyte isolate. At least two different bands of expected size for each MCT were cut out from the various gels, extracted, and sequenced. All of the sequences provided perfect matches to the database sequences of the corresponding MCTs, thus confirming the transcription of all three MCTs in each of the three white cell isolates. The number of base pairs sequenced for each amplimer are listed in Table 3.
Figure 1.
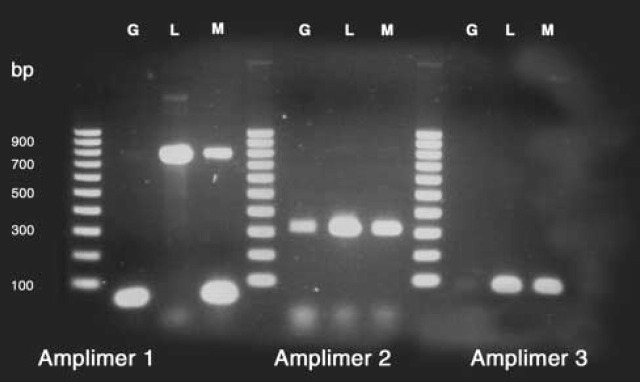
Three amplimers identifying mRNA of MCT1 in granulocytes (G), lymphocytes (L), and monocytes (M). The oligonucleotide standards in lanes 1, 5, and 9 are 100-bp increments from 100 to 1000 bp. The blurry bands near the bottom are excess primers, while the strong bands just above that level in lanes 2 and 4 are primer-dimers of 90 bp. The expected amplimer sizes were, respectively, 799, 331, and 94, and presented strong bands for L and M. The corresponding bands for G are quite weak, but upon extraction and sequencing, gave exact matches to the database sequence, as did the strong bands. See Table 3 for further amplimer data.
Figure 2.
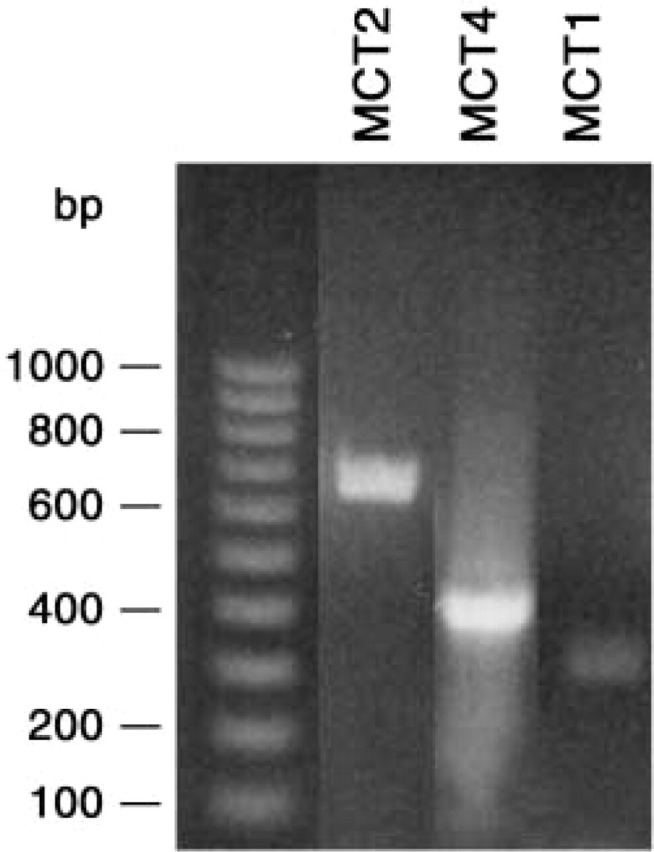
Examples of amplimers of MCT1, −2, and −4 in the same acrylamide gel before excision for extraction and sequencing. Extraneous lanes have been removed and the oligonucleotide 100-bp stepladder is the same as in Figure 1. The amplimers here were obtained from a monocyte fraction, and the total base pairs sequenced (see Table 3) were, respectively, 331 for MCT1, 636 for MCT2, and 419 for MCT4.
Table 3.
Matching sequences by RT-PCR of MCT1, −2, and −4 in all three white blood cell subtypes
|
| ||||||
|---|---|---|---|---|---|---|
| MCT1 | MCT2 | MCT4 | ||||
|
|
|
|
||||
| PCR products | Coding bases | Amplimer total bp | Coding bases | Amplimer total bp | Coding bases | Amplimer total bp |
|
| ||||||
| Amplimer 1 | 358-1157 | 799 | (−161)-489 | 650 | (−62)-520 | 582 |
| Amplimer 2 | 826-1157 | 331 | 401-1037 | 636 | 468-1069 | 601 |
| Amplimer 3 | 1063-1157 | 94 | 1034-1453 | 419 | ||
|
| ||||||
Western Blotting Analysis of White Blood Cell Membranes
Because the presence of mRNA transcripts does not guarantee their translation into proteins, expression of the MCT antigens was also assessed by Western blotting analysis. The bulk of the cellular membranes were concentrated by removing the 25,000 × g supernatant fractions before SDS extraction of the precipitates. After SDS-PAGE and blot transfer, we tested for the presence of MCT1, MCT2, and MCT4 using our specific antibodies. SDS-solubilized human heart membranes were also loaded to serve as a positive control, because they have ample levels of all three MCTs (Fishbein et al, 2002). Both lymphocytes and monocytes were positive for all three MCTs, with apparent MWs corresponding to those present in other tissues, including our positive control (Figure 3; MW data listed in Table 1). Granulocytes showed faint lower-MW bands for MCT1 and MCT2 (~28–32 kD) and an extremely faint 45-kD band (or no band at all) for MCT4. It is probable that this is a result of proteolysis by these highly degradative cells, despite the presence of protease inhibitors during preparation. On occasion, as shown in Figure 3, one patient sample would contain the 45-kD band while another patient sample prepared simultaneously would exhibit a low-MW band. However, all of the bands observed for the three MCTs, including those with lower MW, were blocked by preincubation of MCT Ab with specific peptide before application to the blot.
Figure 3.
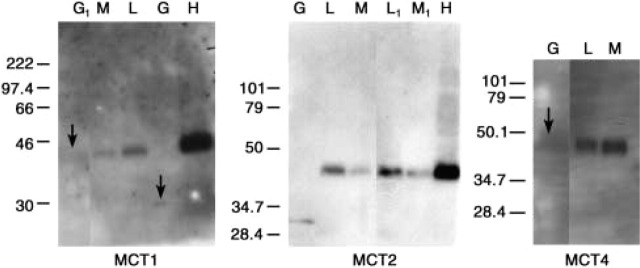
Western blots of the three MCT transporter proteins in granulocytes (G), lymphocytes (L), monocytes (M), and heart (H) as the positive control. Each of the blots is from a single gel, with extraneous lanes removed. The numbers at left show the location of globular protein standards relevant to the bands of interest. For the MCT1 blot, G1 indicates that the granulocyte preparation is from a different blood sample than G, and the same distinction follows for L1 and M1 vs L and M in the MCT2 blot. Arrows point to the very weak granulocyte bands.
Optimal Fixative Selection
Leukocytes could not be studied satisfactorily without fixation because of autolysis on storage, and fluorescence microscopy was not suitable because the purified cell fractions displayed prominent autofluorescence even after treatment with reducing agents. We therefore employed a standard dichroic light immunochemical procedure for this study (which would also satisfy routine clinical pathology IHC requirements) and selected four fixatives to evaluate.
The results are shown in Figure 4. In the four upper segments (Figures 4A-4D), the best fixatives, methanol (Figure 4C) and acetone (Figure 4D), are compared with the unfixed stained section (Figure 4A), and a companion unfixed (and non-serial) section stained with omission of the first Ab (Figure 4B), which yields a barely detectable image. Although the photographic fields of Figures 4A, 4C, and 4D are not identical, the asterisks mark three fibers that are identical, so that the overlapping areas are easily compared. The methanol fixation mimics faithfully the unfixed section, emphasizing the sarcolemma and located in the same fibers, which were differentiated with the reverse ATPase stain (not shown). The fibers lining the vascular channel are primarily type 2b, which show minimal or no staining with MCT1 (Fishbein et al. 2002). Although acetone fixation also gives good preservation and staining, it is not as accurate for identification, because the cytoplasm is stained quite strongly and is not infrequently discordant with the sarcolemmal staining.
Figure 4.
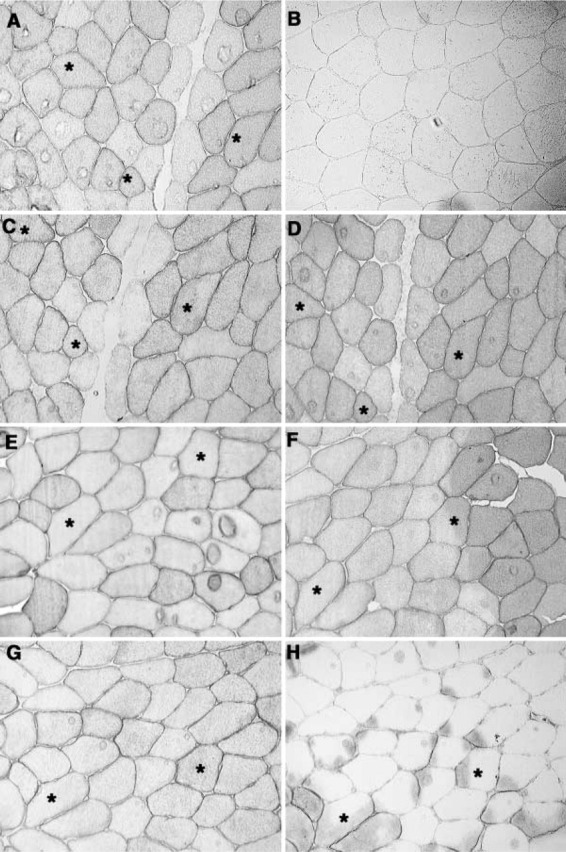
IHC of MCT1 in serial 6-μm sections of frozen human muscle after immersion in various fixatives for 30 min at 4C. (A) Unfixed section; (C) methanol, same field; (D) acetone, same field; (B) unfixed section, primary antibody omitted, different field, light decreased to increase refractive effects. Asterisks identify the same fibers in A, C, D. (E-H) A different field; again, the asterisks identify the same fibers in all four sections. (E) unfixed section; (F) formalin; (G) methanol; (H) ethanol. The essential feature for comparison is the plasmalemmal staining, not the cytoplasmic staining.
In the four lower segments (Figures 4E-4H) from another field, we compare methanol fixation (Figure 4G), which again mimics that of the unfixed section (Figure 4E), with formalin (Figure 4F) and ethanol (Figure 4H). Formalin leads to blotchy staining and, more importantly, the correspondence of sarcolemmal staining with that of the unfixed section often cannot be established with confidence. Formalin also produced a noticeable and progressive loss in reactivity with time in the fixative, which was quantified by automated exposure times in fluorescent microscopy in comparison to serial unfixed sections. Those results are listed in Table 4 and show negligible loss after 10 min but then progressive loss reaching 82% at 24 hr fixation. In contrast, methanol and acetone treatments did not degrade the MCT antigens, and gave fluorescence results closely matching those of the unfixed frozen sections. Ethanol fixation (Figure 4H) was extremely deleterious, with large areas failing to stain, while the stained areas are anatomically irrelevant and show virtually no correspondence with the unfixed specimen. Because methanol fixation was innocuous and has long been the procedure of choice for blood specimens, we chose it for this study of the lactic acid transporters.
Table 4.
Inactivation of MCT1 Ag by formalin fixation a
|
| ||||
|---|---|---|---|---|
| % Loss of fluorescence intensity | ||||
|
|
||||
| Time in formaldehyde (3.7%) at 4C | Trials | Mean | SD | SE |
|
| ||||
| 10 min | 4 | 3.5 | 12.5 | 6.2 |
| 2 hr | 3 | 49.7 | 9.1 | 5.2 |
| 3 hr | 3 | 72.0 | 17.8 | 10.3 |
| 24 hr | 3 | 82.0 | 10.6 | 6.1 |
|
| ||||
aLoss of fluorescence intensity was measured by automated exposure time required to produce the same photographic image of the same field of view as an unfixed frozen section, after various immersion times of sequential 6-μm frozen human muscle sections in 10% formalin (3.7% formaldehyde) in PBS at 4C Analysis of variance (1-way) yielded an F-ratio of 26.38 and p<0.01%. Post hoc testing gave significant linear trend increase at the 0.01% p level. The Tukey test showed that the 10-min mean differed at the 1% level or beyond from all later means, and the Newman-Keuls test added a significant difference between the 2-hr and 24-hr means at p<5%.
Immunohistochemistry and Immunocytochemistry
In our preliminary studies, MCTs could not be demonstrated effectively by fluorescent microscopy because of marked autofluorescence of the leukocyte isolates even after sodium borohydride treatment. We therefore turned to dichroic light methods, which would be the procedure of choice, in any event, for clinical diagnostic pathology. Blood samples from three male donors, two of them Caucasian, ages 70 and 52, and one Afro-American, age 22, were fractionated and methanol-fixed for this study.
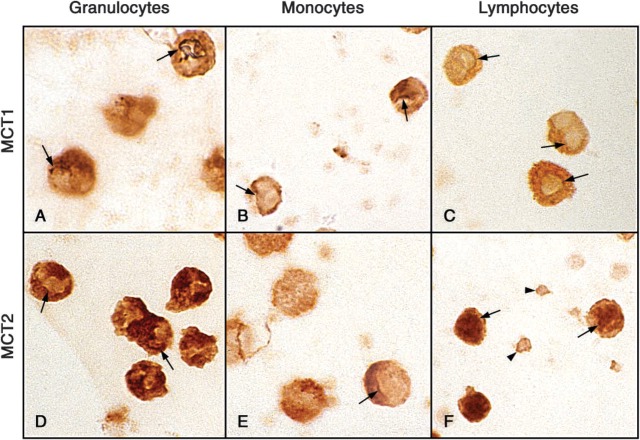
Figure 5 shows, in composite, the full range of ICC performed. Figure 5M is at ×700; all others are at ×415. Although the Wright-Giemsa-stained samples show that the smears (Figures 5A, 5F, and 5K) detail the structure of the mononuclear cells more distinctly than do the droplets (Figures 5B, 5G, and 5L), it was very hard to find enough cells to evaluate the frequency of each feature, so the droplet method was used for ICC. The granulocytes stained strongly, with MCT2 > MCT1 ≫ MCT4. A feature of MCT1 staining was a dark nuclear membrane, based on our criterion of a line, following part of the nuclear envelope (or a fold in it), which was darker than the nucleoplasm and cytoplasm on either side. Arrows in Figure 5C show two examples. This feature was found in only a subset of cells, whereas cytoplasmic and plasma membrane stainings were almost universal and were occasionally distinctly granular. MCT2 stained the cytoplasm so darkly that our criteria for nuclear membrane staining were difficult to satisfy, but Figure 6, which shows selected cells from each cell fraction at ×1615, presents examples we consider convincing. In general, MCT2 stained the plasmalemma while sparing the nucleoplasm. The arrowheads in Figure 5D point to unstained red cells as a negative control, because these cells express only MCT1. MCT4 staining was weaker but evident in the plasma membrane and cytosol, which again were sometimes granular. We could not find convincing examples of nuclear envelope staining by MCT4 in any of the three cell types.
Figure 5.
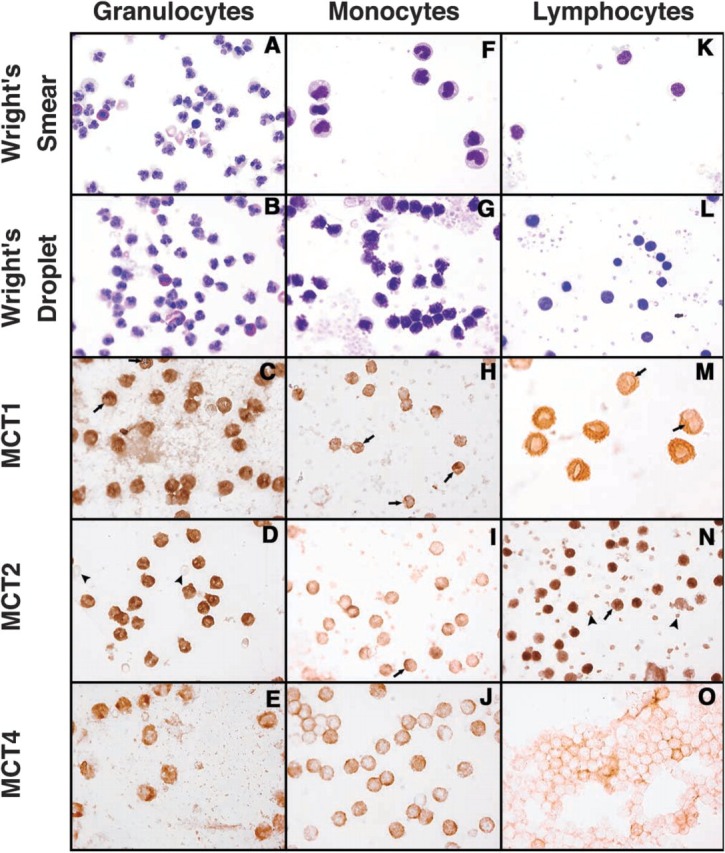
Wright's stain and ICC stain of the three transporters in the three human leukocyte fractions. All panels are ×415 save for M, which is ×700. The smears in F and K give better morphological distinction for the mononuclear cells than do the droplets in G and L, but the cells are much more widely spaced and difficult to quantify for specific features. ICC was therefore confined to droplet preparations. Arrows in C, H, I, M, and N show regions of suspected nuclear membrane staining. Arrowheads in D show unstained erythrocytes as a negative control, while those in N show convincing staining of platelet plasmalemma by MCT2.
Figure 6.
Evidence for nuclear envelope staining by MCT1 and MCT2 antibodies in each of the three leukocyte fractions at high magnification (×1615). In each panel, the arrows touch a curvy line at nuclear folds or perimeter, which is darker than the nucleoplasm or cytoplasm on either side; this is our requirement for a convincing indicator. In F, the arrowheads point to two platelets with plasmalemmal staining.
Monocytes had a high level of expression with all three lactate transporters. MCT1 was expressed on plasma and nuclear membranes (arrows in Figure 5H), while the nucleoplasm in these cells was usually light but occasionally dark. Cytoplasmic staining was variable as well, and sometimes had a distinctly granular pattern. MCT2 stained the cytosol and plasmalemma, and occasionally showed a hint of nuclear membrane (arrow in Figure 5I; seen better in Figure 6E) but did not stain the nucleoplasm. MCT4 was expressed only in plasma membrane and cytoplasm, which often appeared distinctly granular (see Figure 5J).
Lymphocytes expressed staining patterns similar to those of granulocytes, with MCT2 > MCT1 ⋙ MCT4. Uniquely in this cell type, MCT2 stained nucleoplasm darker than cytosol and plasmalemma; yet in a number of cells, evidence of nuclear envelope staining could still be identified (see arrows in Figures 5N and 6F). MCT2 also stained the residual platelets in the isolate, emphasizing their plasma membrane (arrowheads in the same figure panels). MCT1 stained both plasma membrane and cytoplasm but not the nucleoplasm. Convincing staining of the nuclear envelope could often be detected, as shown by the arrows in Figures 5M and 6C (these panels are, respectively, ×700 and ×1615). MCT4 expression was very weak in lymphocytes and was limited to the plasma membrane.
Platelets present in the preparation of lymphocytes showed membrane staining only with MCT2, as noted above, and in all experiments, no staining was present if the primary Ab was omitted from the staining regimen. In all preparations from all three donors, virtually all of the leukocytes in each fraction showed some staining with each of the MCT antibodies, but the intensity of staining in various locations, i.e., nucleoplasm vs cytoplasm vs nuclear envelope vs plasma-lemma varied considerably, so that only approximate and semiquantitative estimates are appropriate for these ICC studies. In addition, the ICC data indicate that attempts to reliably quantify Western blots are compromised by the many cell compartments that may be variably contributing MCTs to the SDS extracts (as well as to the cells themselves), unlike the situation encountered in most other tissues (Fishbein et al. 2002).
Discussion
Although concerns are periodically raised about the unresolved problems of damage to and alteration of the structure and reactivity of cells and tissues after fixation (Montero 2003), no simple solutions have been promulgated and most studies ignore the problem. Here we introduce a simple test, although it may be restricted in application. Because of its turgor, resistance to autolysis, and great axial ratio, frozen muscle in cross section provides an ideal medium for comparing stains on fixed and unfixed tissue. The cells (i.e., fibers) can easily be tracked through multiple sections and the staining compared, provided that the antigen is discretely located and in a bound state that is not readily reversible. Such is the case for the MCTs in muscle. Our presumptions are that unfixed frozen tissue gives minimal artifactual staining, that localization will be consistent within a single cell or fiber, and that fixation is not tissue specific, so that effects in muscle will be representative of other tissues. Therefore, fixed specimens should reproduce the major result found in the unfixed frozen muscle, which for MCT1 in fluorescence microscopy, was the staining of the sarco-lemma of type 1 fibers (Fishbein et al. 2002). Although additional staining may occur after fixation (since that may increase resolution and detail), it should not alter or confuse the evaluation of sarcolemmal staining. In this study, we stained individual 6-μm sections, which is reasonable for comparison with leukocyte smears or droplets.
Four fixatives were evaluated in comparison to the unfixed frozen section, selecting the same microscopic fields. In summary, the staining pattern of the unfixed specimen = methanol fix ≥ acetone fix > formalin fix ⋙ ethanol fix. This satisfied our requirements, because methanol was so effective, but more fixatives and reactivation techniques could be added. In addition, we were able to quantify the decay of antigen activity during formalin fixation by automated photomicrography exposure times using the same field of view.
There has been little study of MCTs in leukocytes and none in separated cell types. This is somewhat surprising when one considers that these cells are among the most dependent on glycolysis of all the cells in the body. They must function in moderately to markedly hypoxic environments, such as abscesses, and can undertake such elaborate functions as phagocytosis under such stress, fueled purely by glycolysis. This has been demonstrated for monocytes and macrophages (Cline and Lehrer 1968; Cohn 1968), neutrophils (Karnovsky 1962), and eosinophils (Cline et al. 1968). We would therefore presume that, as in contracting skeletal muscle, leukocytes would also express lactic acid transporters to facilitate its efflux in such states, so as to decrease intracellular acidosis.
Although studies are limited, MCT4 expression in mixed white blood cell populations has been observed at both the mRNA and the protein level. Price et al. (1998) found MCT4 mRNA in leukocytes by Northern blotting analysis, and the protein antigen was identified by Western blotting analysis by Wilson et al. (1998). Data on MCT1 and MCT2 expression have not been consistent. Hahn et al. (2000) observed MCT1 mRNA and protein expression in peritoneal macrophages, whereas both Price et al. (1998) and Lin et al. (1998) failed to find MCT1 mRNA in mixed leukocytes by Northern blot, although the latter investigators did find it expressed in lymph nodes. MCT2 mRNA was identified in leukocytes by Lin et al. (1998), whereas Price et al. (1998) reported it to be absent. The inconsistency may be explained by the lower sensitivity of the Northern blotting analysis compared with RT-PCR. Hahn et al. (2000) could not detect any MCT1 mRNA in a macrophage cell line by Northern blotting, yet the protein antigen was expressed in the same cells, as identified by specific antibodies.
Using RT-PCR, we were able to verify two or three different sequences of each MCT cDNA in each of the three cell types, thus confirming transcriptional expression. Because each isolate contained <10% contamination by the other white cell types, it is unlikely that any of the Western blots are confounded by a false-positive, and this judgment is supported by the direct staining of the individual cells by ICC. Lymphocyte and monocyte extracts gave discrete bands with specific antibodies to each of the three MCTs on Western blotting analysis, with the same mobility in the leukocytes as in the tissue used as positive control. Granulocyte extracts, save for a rare weak band of 45 kD for MCT4, gave weak bands of greater mobility, which were in the 28–32-kD range for both MCT1 and MCT2. However, these lower-MW bands were competed out by the corresponding specific peptide antigen just as effectively as were the full-size bands of the mononuclear cell extracts, and are therefore considered to be proteolytic fragments of the MCT1 and MCT2 protein antigens. Proteolysis is very pronounced in granulocytes because of their large stock of enzymes committed to degradation of foreign agents and damaged host cells and macromolecules.
High expression of all three MCTs was observed in monocytes, but they might have been in a stimulated state, because they were isolated by Ab binding to their CD14 receptor site. The entire separation procedure, through slide preparation and fixation, was completed within a matter of hours, so it is quite unlikely that a true activated state could have been attained. It would be necessary to compare positive with negative (i.e., depletion) selection to decipher the possible contribution of stimulation to MCT expression.
Brooks et al. (1999) presented strong evidence for the presence of MCT1 in mitochondria of cardiac and skeletal muscle; so it would seem reasonable to expect it to be expressed in the mitochondria of other cells and tissues as well. However, we see cytoplasmic staining with all three MCTs in all three leukocyte isolates (save for MCT4 in lymphocytes). Whether this represents mitochondria, leukocyte granules (i.e., lysosomes), or other intracellular compartments will require subcellular fractionation and/or electron microscopy to answer. Similarly, nuclear envelope staining seems convincing to us for MCT1 and MCT2 in all three isolates, although this is not definitive. The isolation of intact nuclei would permit a much improved test specimen that could still be evaluated by light microscopy and combined with subcellular compartment enzymology. We point out that Hanu et al. (2000) observed strong punctate cytosolic labeling of cultured rat astrocytes with both MCT1 and MCT2. In addition, MCT1 labeled aster-like structures near the nuclei, and MCT2 labeled both the nuclei and the trans-Golgi network in their study.
It appears that for certain nuclear functions, even aggregated mitochondria at the nuclear envelope are insufficiently proximate to provide timely levels of ATP via oxidative phosphorylation, unless macromolecular shuttling by creatine kinase or adenylate kinase is also operating in a bucket-brigade fashion (Bessman and Carpenter 1985; Dzeja et al. 1996,2002). Indeed, this is further evidence of the inadequacy of simple micromolecular diffusion to satisfy cell requirements. Note that MCT Ab staining of mitochondrial aggregates on or at the nuclear membrane is also another possible explanation for the apparent nuclear membrane staining, which often appears granular or stippled. Otherwise, the presence of MCTs in the nuclear membrane indicates that facilitated transport of some carboxylic acid must be important and that it may not be lactic acid, because it is unlikely that glycolysis is more active in nucleoplasm than in cytoplasm.
The expression of three lactate transporters (with approximate respective Km values of 1 mM, 5 mM, and 25 mM for lactate) in all three major leukocyte fractions means that the export of lactic acid will be at maximal efficiency throughout the range of expected physiological accumulation, i.e., ~40 mM to <1 mM. In addition, because the three MCTs are coded by separate genes on separate chromosomes (Garcia et al. 1994; Lin et al. 1998; Halestrap and Price 1999), marked redundancy is present. The loss of one gene would not eliminate facilitated diffusion, although it may be slowed, and the loss of two genes would be extremely improbable.
On the basis of this work and our previous report (Fishbein et al. 2002), the presence of an inactivating mutation in any one of these MCTs should cause loss of expression of that MCT in muscle and in all blood cells. One might not expect the patient to have notable muscle symptoms except under conditions of extreme exercise or comparable stress, such as activity at high environmental temperatures, or in the presence of anemia, or anoxemia from working in a low-O2 environment. Under such conditions, the otherwise healthy person might develop rhabdomyolysis or, less threatening, muscle cramping and elevated creatine kinase, as was found in a military drill sergeant with defective erythrocyte lactate efflux (Fishbein 1986a). Later, two other military personnel with repeated bouts of muscle cramping and elevated creatine kinase levels were also found to have erythrocyte lactate transport less than half the normal rate. On genetic analysis, all three were found to be heterozygous for a missense mutation in MCT1 that was rare in our normal population (Merezhinskaya et al. 2000). Whether this was the cause of their symptoms or simply a surprising coincidence is still uncertain, and there has been no further investigation of this question to date.
We know now that these patients should also have the mutation in their white blood cells as well, although there was no clinical information to arouse suspicion. Perhaps homozygosity for the mutation is necessary here, and even then these patients might only manifest a slower than normal recovery from bacillary infections and poor athletic stamina, and thus be cursed with the label of being constitutionally inadequate. The lactic acid transporters therefore, like myo-adenylate deaminase, may be “perquisitory” catalysts, providing perquisites for maximal performance, rather than essential functions, and mutations would cause “diseases of healthy people” (Fishbein 1986b). Only further studies can settle this question, but it would be a grave mistake to disregard “perquisitory catalysts” and “diseases of healthy people.” Imagine the number of “constitutional inadequates” there would be in a world without eyeglasses and contact lenses.
Literature Cited
- Bessman SP, Carpenter CL. (1985) The creatine-creatine phosphate energy shuttle. Annu Rev Biochem 54:831–862 [DOI] [PubMed] [Google Scholar]
- Brooks GH, Brown MA, Butz CE, Sicurello JP, Dubochaud H. (1999) Cardiac and skeletal muscle mitochondria have a mono-carboxylate transporter MCT1. J Appl Physiol 87:1713–1718 [DOI] [PubMed] [Google Scholar]
- Cline MJ, Hanifin J, Lehrer RI. (1968) Phagocytosis by human eosinophils. Blood 32:922–934 [PubMed] [Google Scholar]
- Cline MJ, Lehrer RI. (1968) Phagocytosis by human monocytes. Blood 32:423–435 [PubMed] [Google Scholar]
- Cohn ZA. (1968) The structure and function of monocytes and macrophages. Adv Immunol 9:163–214 [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Bortolon R, Perez-Terzic C, Holmuhamedov EL, Terzic A. (2002) Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer. Proc Natl Acad Sci USA 99:10156–10161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja PP, Zeleznikar RJ, Goldberg ND. (1996) Suppression of creatine kinase-catalyzed phosphotransfer results in increased phosphoryl transfer by adenylate kinase in intact skeletal muscle. J Biol Chem 271:12847–12851 [DOI] [PubMed] [Google Scholar]
- Fishbein WN. (1986a) Lactate transporter defect: a new disease of muscle. Science 234:1254–1256 [DOI] [PubMed] [Google Scholar]
- Fishbein WN. (1986b) Myoadenylate deaminase deficiency. In Engel AG, Banker BQ, eds. Myology. Vol 2 New York, McGraw-Hill, 1745–1762 [Google Scholar]
- Fishbein WN, Merezhinskaya N, Foellmer J. (2002) Relative distribution of three major lactate transporters in frozen human tissues and their localization in unfixed skeletal muscle. Muscle Nerve 26:101–112 [DOI] [PubMed] [Google Scholar]
- Friesema ECH, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. (2003) Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135 [DOI] [PubMed] [Google Scholar]
- Garcia CK, Li X, Luna J, Francke U. (1994) cDNA cloning of the human monocarboxylate transporter 1 and chromosomal localization of the SLC16A1 locus to 1p13.2-p12. Genomics 23:500–503 [DOI] [PubMed] [Google Scholar]
- Gregory CE, Griffin JL. (1994) Enzyme histochemistry of skeletal muscle. In Mikel UV, ed. Advanced Laboratory Methods in Histology and Pathology. Washington, DC, Armed Forces Institute of Pathology, American Registry of Pathology, 161–207 [Google Scholar]
- Grollman EF, Philp NJ, McPhie P, Ward RD, Sauer B. (2000) Determination of transport kinetics of chick MCT3 monocarboxylate transporter from retinal pigment epithelium by expression in genetically modified yeast. Biochemistry 39:9351–9357 [DOI] [PubMed] [Google Scholar]
- Hahn EL, Halestrap AP, Gamelli R. (2000) Expression of the lactate transporter MCT1 in macrophages. Shock 13:253–260 [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Price NT. (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 343:281–299 [PMC free article] [PubMed] [Google Scholar]
- Hanu R, McKenna M, O'Heill A, Resneck WG, Bloch R. (2000) Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am J Physiol 278:C921–930 [DOI] [PubMed] [Google Scholar]
- Juel C. (2001) Current aspects of lactate exchange: lactate/H+ transport in human skeletal muscle. Eur J Appl Physiol 86:12–16 [DOI] [PubMed] [Google Scholar]
- Juel C, Halestrap AP. (1999) Lactate transport in skeletal muscle - role and regulation of the monocarboxylate transporter. J Physiol 517:633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky ML. (1962) Metabolic basis of phagocytic activity. Physiol Rev 42:143–168 [DOI] [PubMed] [Google Scholar]
- Kim-Garcia C, Goldstein JL, Pathak RK, Anderson RGW, Brown MS. (1994) Molecular characterization of a membrane transporter for lactate, pyruvate and other monocarboxylates: implications for the Cori cycle. Cell 76:865–873 [DOI] [PubMed] [Google Scholar]
- Lin RY, Vera JC, Chaganti RS, Golde DW. (1998) Human monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate transporter. J Biol Chem 273:28959–28965 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Merezhinskaya N, Fishbein WN. (2001) Monocarboxylate transporters. In Wiley Encyclopedia of Molecular Medicine. Vol 2 New York, John Wiley, 2119–2123 [Google Scholar]
- Merezhinskaya N, Fishbein WN, Davis JI, Foellmer JW. (2000) Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle Nerve 23:90–97 [DOI] [PubMed] [Google Scholar]
- Montero C. (2003) The antigen-antibody reaction in immunohistochemistry. J Histochem Cytochem 51:1–4 [DOI] [PubMed] [Google Scholar]
- Philp NJ, Yoon H, Lombardi L. (2001) Mouse MCT3 gene is expressed preferentially in retinal pigment and choroid plexus epithelia. Am J Physiol 280:C1319–1326 [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Terzis G, Halestrap A, Juel C. (1999) Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. Am J Physiol 276:E843–E848 [DOI] [PubMed] [Google Scholar]
- Price NT, Jackson VN, Halestrap AP. (1998) Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J 329:321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber TL, Buckner L., III (1994) Cytopathology techniques. In Mikel UV, ed. Advanced Laboratory Methods in Histology and Pathology. Washington, DC, Armed Forces Institute of Pathology, American Registry of Pathology, 230–231 [Google Scholar]
- Wilson MC, Jackson VN, Neddle C, Price NT, Pilegaard H, Juel C, Bonen A, et al. (1998) Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J Biol Chem. 273:15920–15926 [DOI] [PubMed] [Google Scholar]