Abstract
The microbial glycocalyx is composed of a variety of polyanionic exopolysac-charides and plays important roles in microbial attachment to different substrata and to other cells. Here we report the successful use of low-voltage scanning electron microscopy (LVSEM) to visualize the glycocalyx in two microbial models (Klebsiella pneumoniae and Enterococcus faecalis biofilms) at high resolution, and also the dependence on fixation containing polycationic dyes for its visualization. Fixation in a paraformaldehyde-glutaral-dehyde cocktail without cationic dyes was inadequate for visualizing the glycocalyx, whereas addition of various dyes (alcian blue, safranin, and ruthenium red) to the aldehyde cocktail appeared necessary for stabilization. The cationic dyes varied in size, shape, and charge density, and these factors appeared responsible for different phenotypic appearances of the glycocalyx with each dye. These results suggest that aldehyde fixation with cationic dyes for high-resolution LVSEM will be a useful tool for investigation of microbial biofilms as well as investigation of the extent and role of the glycocalyx in microbial attachment to surfaces.
Keywords: glycocalyx, polycatioic dyes, alcian blue, safranin O, low-voltage SEM, Enterococcus faecalis, Klebsiella pneumoniae, exopolysaccharides
The bacterial glycocalyx has been defined as polysaccharide components lying outside the outer membrane of gram-negative cells or the peptidoglycan layer of gram-positive cells (Costerton et al. 1981). This report uses the term glycocalyx to refer to the layer of exopolysaccharides (EPSs) surrounding the microbial cell, whether covalently bound or not. EPSs have recently been subdivided into three subtypes: linear, branched, and cyclic (Starkey et al. 2004). On the basis of cell association, the glycocalyx on the cell surface has been called capsular EPS, or “slime,” if unbound. In some cases, depending on the microbial species and environment, the glycocalyx has been described as forming polymeric strands that wrap around the surface, or strands that interact with one another to form helical duplexes (Mayer et al. 1999). The glycocalyx consists of a highly hydrated polyanionic matrix (>90% water) surrounding the bacterial cell and can be composed of hundreds to thousands of monomeric EPS units. The composition and degree of substitution of these EPS subunits may vary widely, and cells may make multiple forms (Schmitt and Flemming 1999; Starkey et al. 2004; Sutherland 2001). The glycocalyx may be involved in mediating bacterial adhesion, and the attachment of bacteria to form biofilms can actually increase EPS production in sessile cells compared with free-living planktonic cells (Vandevivere and Kirchman 1993).
Ultrastructural detection of the glycocalyx has been difficult because of its high polysaccharide content, which does not interact with the common postfixation stain osmium. Therefore, the glycocalyx scatters few electrons and is relatively indistinguishable in conventionally processed samples for transmission electron microscopy (TEM). Detection of the glycocalyx has been facilitated by development of stabilization methods using cationic probes such as ruthenium red (Luft 1971) and alcian blue (Behnke and Zelandeer 1970). Both probes facilitate deposition of osmium, which increases the electron density of the anionic polysaccharides. Detection of microbial glycocalyx with these methods at the TEM level has been reviewed (Erdos 1986; Fassel and Edmiston 1999). Several ultrastructural studies have used conventional scanning electron microscopy (SEM) to investigate the glycocalyx, but these studies (Marshall et al. 1971; Costerton et al. 1981; Fassel et al. 1991) were hampered by low resolution and also by the inability to use low voltages (<5 keV), which yield increased information from small topographical features (Pawley and Erlandsen 1989). The development of field emission SEM, together with the improvements in producing thin metal coatings of ~1 nm, has greatly improved SEM so that resolution in the range of 3–4 nm can be obtained on conventional specimens.
The aim of this study was to investigate the presence of the glycocalyx using cationic probes and high-resolution field emission SEM at low voltages. Two model microorganisms producing a prominent glycocalyx were selected for investigation: a gram-negative bacterium, Klebsiella pneumoniae, which produces a mucoid capsule, and a gram-positive bacterium, Enterococcus faecalis, which produces an extensive glycocalyx when forming biofilms on cellulose catheters (Erlandsen et al. 2004). Our results demonstrate that field emission SEM permits evaluation, previously unparalleled by TEM, of the extent and nature of the bacterial glycocalyx. In addition, the phenotypic visualization of the EPS was shown to vary with the type of cationic probe and the length of fixation.
Materials and Methods
Reagents
Standard laboratory chemicals and reagents were obtained from Sigma-Aldrich Chemical (St Louis, MO). Aldehyde solutions and osmium were obtained from Ted Pella (Redding, CA). Cationic probes were obtained from the following suppliers: alcian blue 8GX (C.I. no. 74240; Matheson Coleman and Bell Manufacturing Chemists, Norwood, OH), safranin O (C.I. no. 50240; Allied Chemical, New York, NY), L-lysine monohydrochloride (Sigma Chemical; St Louis, MO), and ruthenium red (K and K Laboratories; Plainview, NY).
Bacterial Strains
The OG1RF strain of E. faecalis was cultivated in tryptic soy broth in 24-well microtiter plates as described (Kristich et al. 2004). Hollow cellulose tubing (Spectra/Por; Spectrum, Houston, TX), 200 μm in diameter and 3–5 mm in length, was immersed in ~0.6 ml tryptic soy broth, and the medium was inoculated with E. faecalis. Medium was changed daily and fixation of biofilms on cellulose tubing was performed after 4 days of culture at 37C (Erlandsen et al. 2004). K. pneumoniae (ATCC 13,883) was cultured overnight at 37C in tryptic soy broth, and stationary phase cells were concentrated and washed with sterile PBS. Cells were attached for ~20–30 min to glass chips pretreated with a thin film of 0.1% poly-L-lysine to increase adhesion, and then were immersed in the desired fixative.
Fixation Protocol
Multiple experiments (four to six) were performed to investigate the role of cationic probes in visualizing the bacterial glycocalyx. Either cellulose tubing with biofilms of E. faecalis or glass chips with adherent K. pneumoniae were immersed for 4–22 hr in a mixture of 2% paraformaldehyde and 2% glutaraldehyde in 0.15 M sodium cacodylate buffer, pH 7.4, containing no cationic additives. Probes used to stabilize the anionic glycocalyx included the cationic dyes alcian blue, safranin O, and ruthenium red, and also the diamine L-lysine hydrochloride. The properties of these probes are listed in Table 1. To test the effect of different cationic probes, the same aldehyde fixative was modified by adding one of the following probes: 0.15% alcian blue 8GX, 0.15% safranin O, 0.15% ruthenium red, 0.15% lysine monohydrochloride, a mixture of 0.15% alcian blue and 0.15% lysine, or a mixture of 0.15% alcian blue and 0.15% ruthenium red. Lysine added to the aldehyde cocktail at a concentration of 0.15% tended to polymerize, producing loss of sample, so the concentration was reduced to 0.0075%.
Table 1.
Common cationic probes for detection of anionic polysaccharides in the microbial glycocalyx
|
| ||||
|---|---|---|---|---|
| Alcian blue | Safranin O a | Ruthenium red | L-lysine | |
|
| ||||
| MW(Daltons) | 1299 | 351 | 858 | 182.6 |
| Charge | +4 | +2-+3 | +6 | +2 |
| Shape | Planar | Planar | Spherical | Planar |
| Size | ~4 × 4 nm | ~3 × 2 nm | 1.1 nm | ~1 × 2 nm |
|
| ||||
aMW (molecular weight) for safranin O may vary because the dye is a mixture of dimethyl and trimethyl isomers (Luft 1971; Erdos 1986; Fassel and Edmiston 1999; Horobin and Kiernan 2002).
Tissue Processing and Low-voltage SEM
After primary fixation in aldehyde, with or without cationic dye additives, the samples were washed in 0.15 M cacodylate buffer and postfixed for 90–120 min in 1% OsO4 in 0.15 M cacodylate buffer containing 1.5% potassium ferrocyanide. Samples were then rinsed in cacodylate buffer and dehydrated in an ascending ethanol series [50, 70, 80, 95, and 100% (twice)] before critical point drying with CO2. Specimens were mounted on adhesive carbon films and then coated with ~1 nm of platinum using an Ion Tech argon ion beam coater. Examination of samples was performed in a Hitachi S-4700 field emission SEM operated at 2 keV, and secondary electron images were collected as TIFF files.
Results
To investigate the preservation of the bacterial glycocalyx by low-voltage SEM, an aldehyde fixation containing 2% paraformaldeyde and 2% glutaraldehyde in cacodylate buffer, which has been successfully used for TEM studies of microbial glycocalyx, was chosen as the vehicle for dissolving different cationic probes (Springer and Roth 1973; Fassel et al. 1997,1998). All probes, except lysine, were used at a concentration of 0.15%. Lysine added to glutaraldehyde can form crosslinks through its two amino groups, and prolonged fixation usually leads to solidification, resulting in sample loss (Fassel and Edmiston 1999). To reduce solidification, lysine was used at a concentration of 0.0075%. All probes were dissolved in the aldehyde cocktail, and buffer rinses and postfixation steps were performed without added probe.
Immersion fixation of K. pneumoniae in the paraformaldehyde-glutaraldehyde cocktail without added cationic probes revealed a complete lack of detectable glycocalyx on the cell surface (Figures 1A and 1B). Addition of alcian blue to the aldehyde fixation and 4 hr of fixation produced an entirely different appearance. Thin spike-like processes extended from the cell circumference to the substratum (Figures 1C and 1D).
Figure 1.
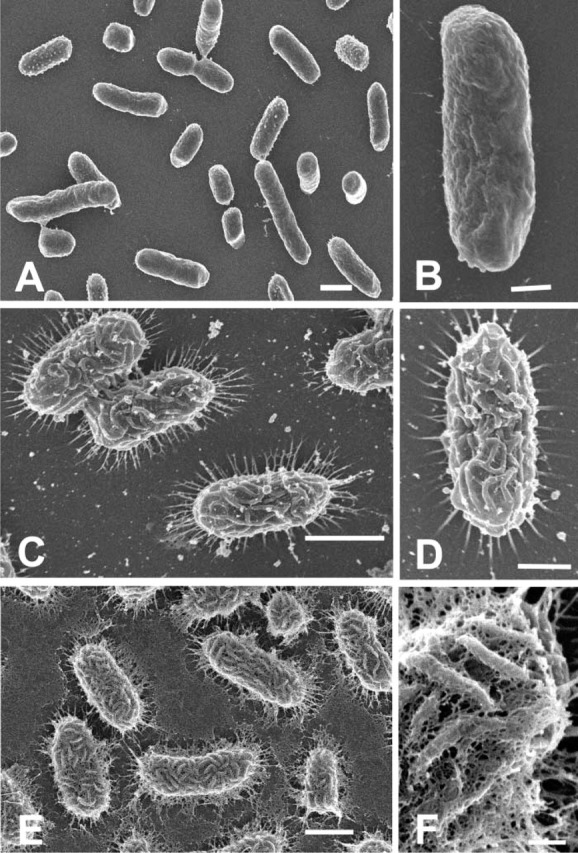
(A) Low magnification of K. pneumoniae fixed for 22 hr in a mixture of 2% paraformaldehyde and 2% glutaraldehyde in cacodylate buffer without any added cationic probes. Individual cells are rod-shaped bacilli and do not possess any detectable surface structure. Bar = 1 μm. (B) Higher magnification of an individual cell of K. pneumoniae showing the absence of any discernible features resembling the glycocalyx. Bar = 0.5 μm. (C) SEM of K. pneumoniae fixed for 4 hr in identical aldehydes fixative as in A, but containing added 0.15% alcian blue. Long spikes can be seen extending outwards for 0.2–0.3 μm from the circumference of the cell. Bar = 1 μm. (D) Higher magnification reveals surface arranged in rugae and circumferential spikes (EPS) extending to substratum. Bar = 0.5 μm. (E) Fixation for 22 hr in aldehyde fixation containing 0.15% alcian blue produces increased retention of EPS. Substratum is covered with thin mat-like covering interpreted as extended slime layer. Bar = 1 μm. (F) Higher magnification of E showing an extensive network of filaments (capsular EPS) bridging between individual rugae on the cell surface. Bar = 150 nm.
The surface of the cells exhibited a rugose-like appearance of anastamosing folds. Extending the fixation time in alcian blue from 4 to 22 hr also produced a different appearance (Figures 1E and 1F). The attached cells still possessed spike-like processes, but the surface of the substratum was covered with a web of thin filaments originating from the surface of the K. pneumoniae. Close examination of the rugae on the cell surface (Figure 1F) also revealed a fine meshwork of filaments extending between the rugae. Aldehyde fixation in the presence of 0.0075% lysine resulted in a surface topography that was distinct from that shown using alcian blue. Fixation for 4 hr in the presence of lysine resulted in retention of the spike-like processes around the cell circumference, but the cell surface now possessed many short, fine fibrils and small globule-like particles (Figures 2A-2C). Extending the fixation time from 4 to 22 hr did not appear to produce any changes in the surface topography (Figure 2C). Fixation in the presence of ruthenium red (Figure 2D) had an effect similar to that seen in the 4-hr fixation in the presence of alcian blue (see Figures 1C and 1D) and lysine (Figure 2C), in that spike-like fibrils were seen extending between the cell margin and the substratum. These spikes appeared thicker than those seen when alcian blue or lysine was added, and the surface topography appeared rugose, with a few fibrils present between adjacent folds (Figure 2D).
Figure 2.
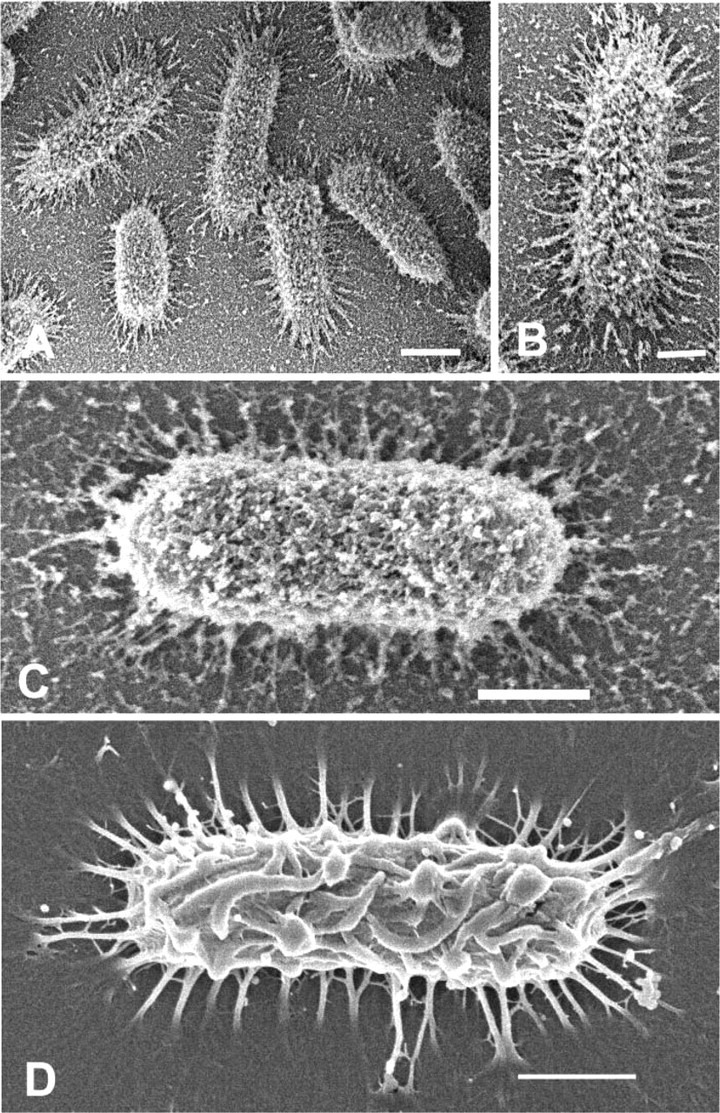
(A) Low magnification of K. pneumoniae fixed for 4 hr in the presence of 0.15% lysine. Fine spikes extend from the cell margin to the substratum, and the surface of bacterium appears covered by fine filaments with bulbous tips. Bar = 1 μm. (B) Higher magnification of cell from A. Fine spikes at the margin of cell and network of filaments are now discernible on the cell surface. Bar = 0.5 μm. (C) High magnification of K. pneumoniae fixed for 22 hr in an aldehyde mixture containing both 0.15% alcian blue and 0.15% l-lysine. Long spikes extend from cell margin to substratum; however, spikes are decorated with short branches. EPS consists of short fibrils and globule-like particles seen on cell the surface and on the substratum. Bar = 0.5 μm. (D) K. pneumoniae cell fixed in 0.15% ruthenium red in paraformaldehyde-glutaraldehyde fixative for 22 hr. Long spikes of the glycocalyx are seen projecting from the circumference of the cell to the substratum. Note similarity of the cell surface to that of cells in shown in Figures 1C and 1D fixed in alcian blue for 4 hr. Bar = 0.5 μm.
A model system using biofilms of the gram-positive bacterium E. faecalis, formed on cellulose catheters, has been shown to produce an extensive glycocalyx associated with sessile bacterial colonies (Erlandsen et al. 2004). Fixation of biofilms in paraformaldehyde-glutaraldehyde cocktail without probes allowed visualization of a few fibrils that appeared to anchor the cocci to the surface of the substratum (Figures 3A and 3B). Cross-walls related to cell division were obvious, and small fibrils could be seen on the cell surface. In the same experiment, biofilms fixed in an aldehyde cocktail containing the cationic probe alcian blue for 22 hr resulted in a different appearance of glycocalyx (Figures 3C and 3D). Fine fibrils formed a branching anastomotic network on the coccal surface and also formed a thick mat of fibrils on the substratum. When a different cationic probe, safranin O, was used for 22 hr in place of alcian blue, a totally different appearance was obtained for the glycocalyx (Figures 3E and 3F). In the presence of safranin O, far fewer filaments were present and there was considerable variation in filament diameter. Filaments were directly attached to the cell surface and formed a meshwork above cells attached to the cellulose substratum. Addition of ruthenium red to the aldehyde cocktail (Figure 4A) permitted visualization of finger-like projections from the glycocalyx, but the density of these projections was less than that seen with addition of alcian blue or safranin. Likewise, addition of 0.0075% lysine had little effect in enhancing projections from the glycocalyx (Figure 4B). Both ruthenium red and lysine allowed visualization of fibrils connecting the microbial surface to the substratum, but other surface projections were minimal in number. Using a mixture of both alcian blue and safranin O in the aldehyde cocktail preserved a fine meshwork of filaments that appeared polar in distribution, and large fibers, possibly aggregates of smaller fibrils, were seen to originate directly from the cell surface (Figures 4C and 4D).
Figure 3.
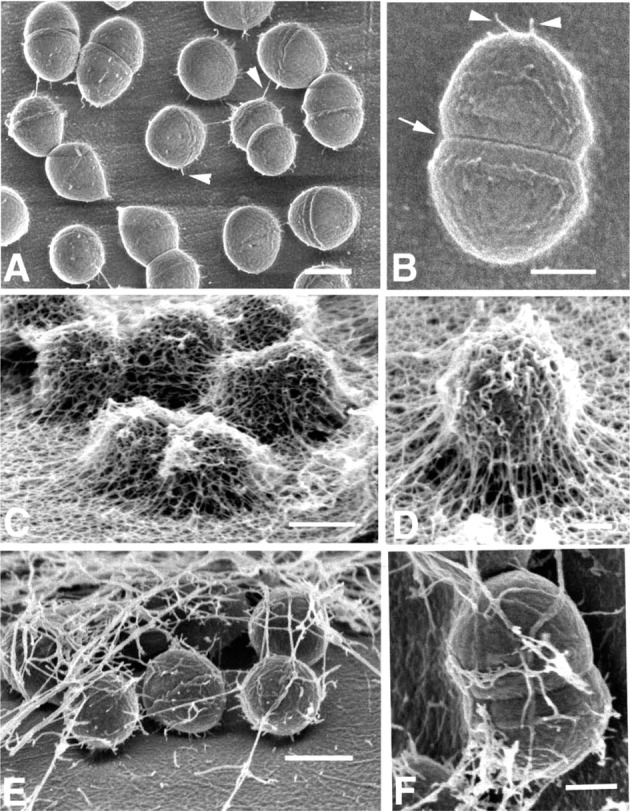
Low-voltage SEM of E. faecalis biofilm. (A) Low magnification of enterococci adhering to the surface of cellulose tubing fixed for 22 hr in paraformaldehyde and glutaraldehyde. Short fibrils extend from the surface of diplococci to substratum (arrowheads). Bar = 0.4 μm. (B) High magnification of a single diplococcus showing adherent fibrils (arrowhead), cross-wall (arrow), and sparse fibril-like material on the cell surface. Bar = 300 nm. (C) E. faecalis biofilm fixed for 22 hr in same aldehyde fixative as A but containing 0.15% alcian blue. Glycocalyx or capsular EPS exists as extensive network of filaments covering diplococci and substratum. Bar = 0.5 μm. (D) Higher magnification of E. faecalis cell fixed in alcian blue as in C. Note cross-branching of fibrillar network forming glycocalyx. Bar = 300 nm. (E) E. faecalis cells in biofilm fixed in aldehyde containing 0.15% safranin O for 22 hr. EPS forming glycocalyx consists of fibrils of various diameters that appear less numerous than seen with alcian blue in C and D. Bar = 0.5 μm. (F) Higher magnification of a diplococcus fixed in 0.15% safranin O showing the origin of fibrils at the cell surface. Variation in fibril diameter is evident. Bar = 200 nm.
Figure 4.
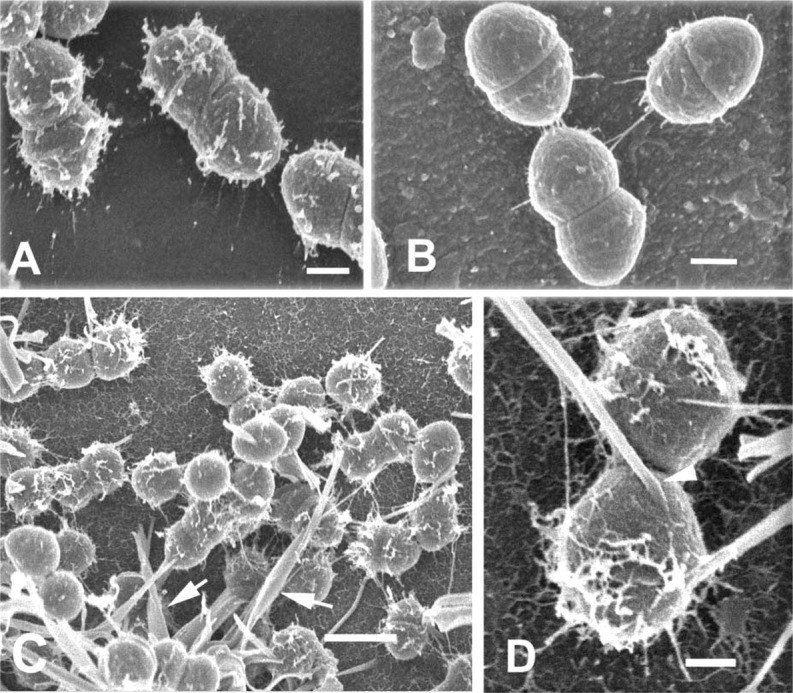
(A) Low-voltage SEM of E. faecalis biofilm. E. faecalis cell fixed in aldehyde cocktail containing 0.15% ruthenium red. Finger-like projections extend outward from the glycocalyx. Bar = 200 nm. (B) Fixation of E. faecalis in aldehyde containing 0.0075% lysine. Short fibrils anchor cells to the substratum, but only sparse fibrils can be seen on the glycocalyx. Bar = 200 nm. (C) Low magnification of E. faecalis biofilm fixed in aldehyde containing 0.15% alcian blue and 0.15% safranin O. Glycocalyx consists of short filaments protruding from the cell surface and large, thick, curved rods (arrows). Bar = 1 μm. (D) Higher magnification of glycocalyx from cells fixed as in C, showing the origin of large, curved rods directly at the cell surface (arrowhead). Short fibrils can be seen on both glycocalyx and substratum. Bar = 200 nm.
Discussion
In this study we have demonstrated that low-voltage SEM can be used to directly visualize the glycocalyx in both a gram-negative and a gram-positive bacterium treated with cationic probes. For nearly three decades, cationic probes such as alcian blue and ruthenium red have been used to increase the electron density of the glycocalyx for TEM analysis of a wide variety of microbes (Pate and Ordal 1967; Springer and Roth 1973; Costerton et al. 1981; Erdos 1986; Fassel and Edmiston 1999) and, more recently, lysine has been used to improve preservation of the glycocalyx (Boyles et al. 1985; Jacques and Graham 1989; Fassel et al. 1997; Fassel and Edmiston 1999). Although providing information on the existence of the glycocalyx, these TEM studies were limited by section thickness and the necessity of the cationic probe to increase the electron scattering capability for visualization of the glycocalyx. Our approach, using cationic dyes to protect the glycocalyx from collapse during dehydration/critical point drying and using low-voltage SEM, has several distinct advantages over TEM. First, SEM with secondary electron imaging does not depend on increasing density with osmication but instead relies on a fine heavy-metal film (~1 nm) produced by ion beam sputtering. The thin metal coating deposited on the stabilized glycocalyx will generate the secondary electron signal used for image formation. In addition, owing to the directionality of the secondary electron detector, the microbial image appears 3D-like, facilitating the interpretation of surface topography of the entire cell. This approach permits visualization of the arrangement of filaments over the entire microbial surface and permits examination of the interaction of these filaments with the substratum and other bacteria.
Our results confirm and extend what others have done using cationic dyes at the TEM level, and supports the finding that aldehyde fixation alone is inadequate for visualization of the bacterial glycocalyx (Fassel et al. 1993). Our study is unique because we have demonstrated that visualization of different components of the glycocalyx is dependent on the duration of fixation and also on the choice of cationic probe. As shown in Table 1, the cationic probes we employed (alcian blue, safranin O, ruthenium red, and lysine) vary considerably in both charge and shape. The biochemical composition of the microbial glycocalyx is known to contain many anionic mono- and polysaccharides together with proteins, nucleic acids, and lipids (Cooksey 1992; Nielsen et al. 1997; Flemming and Wingender 2001; Starkey et al. 2004). It has been proposed that preservation of the different elements in the glycocalyx might be related to a charge-coupling effect (Fassel and Edmiston 1999). Thus, cationic probes such as ruthenium red and lysine are quite small (~1 nm) compared with the larger planar molecules (~4 nm) of alcian blue and safranin O, and charge density varies considerably in these probes.
The variability seen in the phenotypic retention of the glycocalyx for both K. pneumoniae and E. faecalis is probably a result of the selectivity of each probe interacting with different anionic species as a function of probe charge density, and the ability of each probe to penetrate into this highly charged hydrophilic layer. Lysine also has the ability to form polymers with glutaraldehyde, which can be diminished by adding paraformaldehyde to the fixative, but high concentrations of lysine should be avoided to minimize the potential for polymerization of the fixative (Fassel et al. 1997; Fassel and Edmiston 1999). Fine branches on surface fibrils were seen in Figures 2A–2C, and therefore short fibrils on filaments may be a “decorative artifact” resulting from lysine polymerization. Because of the cytochemical variation in the visualization of glycocalyx phenotype with different cationic probes, it was difficult to determine which one represented the correct topography or whether the actual appearance would be a composite of all methods. The spike-like extensions surrounding the cell circumference were first detected by Springer and Roth (1973), but the increased presence of small fibrils linking adjacent rugae (Figures 1E and 1F), or the fine fibrillar material visualized with lysine (Figures 2A–2C), has not been described. Likewise, the dramatic visualization of glycocalyx in E. faecalis biofilms seen with alcian blue is quite distinct from that seen with safranin O, ruthenium red, or lysine (cfompare Figures 3C and 3D to Figures 3E and 3F and Figures 4A and 4B). Direct comparisons of visualization of the glycocalyx by alcian blue or ruthenium red have suggested that alcian blue stabilizes a more extensive glycocalyx than does ruthenium red (Fassel et al. 1992). Attempts to improve visualization of microbial glycocalyx by constructing cocktails of different dyes, including alcian blue and ruthenium red, alcian blue and safranin O, as well as alcian blue and lysine, were less successful than the use of individual cationic probes (data not shown). Complex mechanisms related to charge coupling and diffusion in highly charged hydrophilic films, together with other components of the glycocalyx, may be involved in protecting the dye-EPS interaction against forces (surface tension) that may collapse the glycocalyx during dehydration or critical point drying.
Our results suggest that the design of fixations containing cationic dyes may require a trial-and-error approach. Because very little information exists about the type(s) and number of polyanionic subunits in the glycocalyx (Schmitt and Flemming 1999; Starkey et al. 2004; Sutherland 2001), it will be necessary to vary time and dye concentrations for application to other microorganisms. On the basis of our experience with cationic dyes to visualize polyanionic substances in the glycocalyx, we predict that anionic dyes (ponceau 2R, orange G, or biebrick scarlet) may be more useful in detecting and enhancing polycationic cell surfaces as found in some strains of Staphlylococcus epidermidis (Starkey et al. 2004).
In summary, our study shows that low-voltage SEM can provide high-resolution imaging of the bacterial glycocalyx and allow visualization of the filaments that compose it. We also observed that cationic dyes can dramatically increase the visualization of glycocalyx structure, and found that different cationic dyes may selectively permit visualization of different components in the glycocalyx. The actual determination of the “native” structure of the glycocalyx may require the application of cryo-immobilization methods. Instead of using cationic probes to preserve components of the glycocalyx, the cryo-immobilization and cryo-sublimation would avoid typical processing artifacts (such as fixation or dehydration), and water would be sublimated at cryo-temperature and vacuum conditions designed to prevent molecular collapse (Erlandsen et al. 2001). This approach has been successfully used to discern the glycocalyx of the bacterium Proteus mirabilus, and this study revealed that the glycocalyx consisted of a fine meshwork of anastomotic fibrils (Erlandsen et al. 2003). Our current knowledge of the bacterial glycocalyx has been based on experiments with cationic dyes in TEM. This study demonstrates the potential of field emission SEM for enhancing and visualizing the bacterial glycocalyx and, together with cryo-SEM approaches, may help to clarify the role of the glycocalyx in bacterial adhesion and biofilm formation.
Acknowledgments
Supported in part by NIH Grant HL-51987 to GMD, NIH Grants RO1 GM-066751 and GM-059221 to CLW, and NIH Training Grant HD-07381-12 and NIH Fellowship AI-56684-01 to CJK.
We wish to thank Rob Garni and Lisa Erickson for their excellent technical assistance. We also thank Chris Frethem (Electron Microscopy Laboratory in the Characterization Facility at the University of Minnesota) for excellent technical assistance.
Literature Cited
- Behnke O, Zelandeer T. (1970) Preservation of intercellular substances by the cationic dye alcian blue in preparative procedures for electron microscopy. J Ultrastruct Res 31:424–438 [DOI] [PubMed] [Google Scholar]
- Boyles JK, Anderson L, Hutchinson P. (1985) A new fixative for the preservation of actin filaments: fixation of pure actin filament pellets. J Histochem Cytochem 33:1116–1128 [DOI] [PubMed] [Google Scholar]
- Cooksey KE. (1992) Extracellular polymers in biofilms. In Melo LF, Fletcher M, Bott TR, eds. Biofilms: Science and Technology. Dordrecht, Kluwer, 137–147 [Google Scholar]
- Costerton JW, Irvin RT, Cheng K-J. (1981) The bacterial glycocalyx in nature and disease. Annu Rev Microbiol 35:299–324 [DOI] [PubMed] [Google Scholar]
- Erdos GE. (1986) Localization of carbohydrate-containing molecules. In Aldrich HC, Todd WJ, eds. Ultrastructural Techniques for Microorganisms. New York, Plenum Press, 399–420 [Google Scholar]
- Erlandsen SL, Kristich SJ, Dunny GM. (2004) Ultrastructure of Enterococcus faecalis biofilms. Biofilms (in press)
- Erlandsen SL, Lei M, Martine-Lacave I, Dunny G, Wells C. (2003) High resolution cryoFESEM of microbial surfaces. Microsc Microanal 9:273–278 [DOI] [PubMed] [Google Scholar]
- Erlandsen SL, Ottenwaelter C, Frethem C, Chen Y. (2001) Cryo field emission SEM (FESEM). BioTechniques 31:300–305 [DOI] [PubMed] [Google Scholar]
- Fassel TA, Edmiston CE., Jr (1999) Bacterial biofilms: strategies for preparing glycocalyx for electron microscopy. In Doyle R, ed. Methods in Enzymology. Vol 310 New York, Academic Press, 194–205 [DOI] [PubMed] [Google Scholar]
- Fassel TA, Sanger JR, Edmiston CE. (1993) Lysine effect on ruthenium red and alcian blue preservation and staining of the staphy-lococcal glycocalyx. Cells Mater 3:327–336 [Google Scholar]
- Fassel TA, Schaller MJ, Remsen CC. (1992) Comparison of alcian blue and ruthenium red effects on preservation of outer envelope ultrastructure in methanotrophic bacteria. Microsc Res Tech 20:87–94 [DOI] [PubMed] [Google Scholar]
- Fassel TA, Van Over JE, Hauser CC, Edmiston CE, Sanger JR. (1991) Adhesion of staphylococci to breast prosthesis biomaterials: an electron microscopic evaluation. Cells Mater 1:199–208 [Google Scholar]
- Fassel TAA, Mozdziak PE, Sanger JR, Edmiston CE. (1997) Paraformaldehyde effect on ruthenium red and lysine preservation and staining of the staphylococcal glycocalyx. Microsc Res Tech 36:422–427 [DOI] [PubMed] [Google Scholar]
- Fassel TA, Mozdziak PE, Sanger JR, Edmiston CE. (1998) Superior preservation of the staphylococcal glycocalyx with aldehyde-rutheium red and select lysine salts using extended fixation times. Micro Res Tech 4:291–297 [DOI] [PubMed] [Google Scholar]
- Flemming H-C, Wingender J. (2001) Relevance of microbial polyumeric substances (EPSs)—part 1: structural and ecological aspects. Water Sci Technol 43:1–8 [PubMed] [Google Scholar]
- Horobin RW, Kiernan JA. (2002) Conn's Biological Stains. 10th ed. Oxford, BIOS Scientific; [Google Scholar]
- Jacques M, Graham L. (1989) Improved preservation of bacterial capsule for electron microscopy. J Electron Microsc Tech 11:167–169 [DOI] [PubMed] [Google Scholar]
- Kristich CJ, Li Y-H, Cvitkovitch DG, Dunny GM. (2004) Esp-independent biofilm formation by Enterococcus faecalis. J Bacteriol 186:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft JH. (1971) Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanisms of action. Anat Rec 171:347–368 [DOI] [PubMed] [Google Scholar]
- Marshall KC, Stout R, Mitchell R. (1971) Mechanism of the initial events in the sorption of marine bacteria to surfaces. J Gen Microbiol 68:337–348 [Google Scholar]
- Mayer C, Morita R, Kirschner C, Borchard W, Maivbaum R, Wingender J, Flemming HC. (1999) The role of intermolecular interactions: studies on a model systems for bacterial biofilms. Int J Biol Macromol 26:3–16 [DOI] [PubMed] [Google Scholar]
- Nielsen PH, Jahn A, Palmgren R. (1997) Conceptual model for production and composition of exopolymers in biofilms. Water Sci Technol 36:11–19 [Google Scholar]
- Pate J, Ordal EJ. (1967) The fine structure of Chondrococcus columnaris. J Cell Biol 35:37–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawley JB, Erlandsen SL. (1989) The case for low voltage high resolution scanning electron microscopy of biological samples. Scanning Microsc 3(suppl):16–173 [PubMed] [Google Scholar]
- Schmitt J, Flemming H-C. (1999) Water binding in biofilms. Water Sci Technol 39:77–82 [Google Scholar]
- Springer EL, Roth IL. (1973) The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol 74:21–31 [DOI] [PubMed] [Google Scholar]
- Starkey M, Gray KA, Chang S, Parsek MR. (2004) A sticky business: the extracellular polymeric substance matrix of bacterial biofilms. In Ghannoum M, O'Toole GA, eds. Microbial Biofilms. Washington, DC, ASM Press, 174–191 [Google Scholar]
- Sutherland IW. (2001) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9 [DOI] [PubMed] [Google Scholar]
- Vandevivere P, Kirchman DL. (1993) Attachment stimulates exo-polysaccharide synthesis by a bacterium. Appl Environ Microbiol 59:3280–3286 [DOI] [PMC free article] [PubMed] [Google Scholar]


