Abstract
The combination of efficacious treatment against bacterial infections and mitigation of antibiotic resistance amplification in gut microbiota is a major challenge for antimicrobial therapy in food-producing animals. In rats, we evaluated the impact of cefquinome, a fourth-generation cephalosporin, on both Klebsiella pneumoniae lung infection and intestinal flora harboring CTX-M-producing Enterobacteriaceae. Germfree rats received a fecal flora specimen from specific-pathogen-free pigs, to which a CTX-M-producing Escherichia coli strain had been added. K. pneumoniae cells were inoculated in the lungs of these gnotobiotic rats by using either a low (105 CFU) or a high (109 CFU) inoculum. Without treatment, all animals infected with the low or high K. pneumoniae inoculum developed pneumonia and died before 120 h postchallenge. In the treated groups, the low-inoculum rats received a 4-day treatment of 5 mg/kg of body weight cefquinome beginning at 24 h postchallenge (prepatent phase of the disease), and the high-inoculum rats received a 4-day treatment of 50 mg/kg cefquinome beginning when the animals expressed clinical signs of infection (patent phase of the disease). The dose of 50 mg/kg targeting the high K. pneumoniae inoculum cured all the treated rats and resulted in a massive amplification of CTX-M-producing Enterobacteriaceae. A dose of 5 mg/kg targeting the low K. pneumoniae inoculum cured all the rats and averted an outbreak of clinical disease, all without any amplification of CTX-M-producing Enterobacteriaceae. These findings might have implications for the development of new antimicrobial treatment strategies that ensure a cure for bacterial infections while avoiding the amplification of resistance genes of human concern in the gut microbiota of food-producing animals.
INTRODUCTION
Antimicrobial resistance is a major threat to human health, and the overuse of antibiotics in both human patients and animals is considered to be the main factor leading to the selection of resistant bacteria. It is also increasingly recognized that the gut microbiota constitutes one of the main reservoirs of resistance genes among commensal bacterial ecosystems (1–4), and that the antibiotic doses currently used in human and animal patients have not been optimized to prevent the collateral selection of antimicrobial resistance in the gut microbiota or its colonization by exogenous resistant strains (3, 5).
An examination of the interactions between antibiotics, pathogens, and the commensal flora, as well as an increased understanding of the key factors governing antimicrobial activity and resistance selection, might lead to the development of strategies combining maximal efficacy with minimal impact on the commensal bacterial ecosystems (2, 6, 7). For example, recent studies demonstrated that the degree of amplification of antimicrobial resistance in the gut microbiota was directly correlated with the magnitude of the antibiotic dose, regardless of the route of administration (8, 9).
Interestingly, some other studies have shown that the in vitro efficacy of antimicrobials can depend on the size of the bacterial inoculum, with drugs being more potent against low than against high inocula (10–12). It was subsequently shown in vivo that lower antibiotic doses given in the early prepatent phase of an infection, when the pathogen burden was still low, were as effective as higher doses administered during the patent phase of infection, as characterized by overt clinical symptoms and a high bacterial burden (13–17).
Therefore, we postulated that such prepatent phase adjusted doses might combine efficacy against the early infection and the mitigation (or absence) of resistance amplification in the gut microbiota.
To test this hypothesis, we evaluated the impact of two antibiotic dosage regimens selected to eradicate either high or low pulmonary pathogen burdens on the gut microbiota. For that purpose, we developed a model of pneumonia using Klebsiella pneumoniae in germfree rats previously colonized by fecal flora obtained from specific-pathogen-free (SPF) pigs, to which was added an Escherichia coli strain carrying an extended-spectrum beta-lactamase (ESBL) of the CTX-M group. We used cefquinome, a fourth-generation cephalosporin (possessing a molecular structure similar to that of cefpirome), which is marketed for veterinary use only for the treatment of pulmonary infections in food-producing animals.
MATERIALS AND METHODS
Microorganisms.
K. pneumoniae strain ATCC 43816 was used to establish lung infections. E. coli strain 09F000898 was isolated from pig feces. It belongs to the phylogroup A and harbors a plasmid carrying a group 1 CTX-M beta-lactamase. This E. coli strain and a sample of feces from an SPF pig were simultaneously inoculated into the digestive tract of each germfree rat. The MIC of cefquinome was 0.125 μg/ml for K. pneumoniae and 64 μg/ml for the CTX-M-producing E. coli.
Animals.
Male OFA rats (Janvier, Saint Berthevin, France) were used for pharmacokinetic studies. Germfree male OFA rats (Charles River Laboratories, L'Arbresle, France) were used for all other studies. The protocol was approved by the French ethics committee (authorization no. 13/11/12-2).
Pharmacokinetic study.
A pharmacokinetic study was performed with six male OFA rats. The rats were subcutaneously injected with 10 mg/kg of body weight of cefquinome (Cobactan; Intervet), and blood samples were collected at several times, centrifuged, and stored at −80°C until the time of the assay. A sparse data analysis was performed with a nonlinear mixed effect model (one-compartment structural model with extravascular administration and constant error model) to fit the concentration-time profiles with Monolix 4.1.4 (Lixoft, Orsay, France).
Pneumonia model in gnotobiotic rats.
Germfree OFA male rats were inoculated intragastrically with 1 ml of a suspension containing a fecal sample from an SPF pig and 5 × 105 CFU/ml of the CTX-M-producing E. coli. The absence of cefotaxime-resistant (CTX-R) Enterobacteriaceae was checked in the SPF pig fecal samples after plating on MacConkey agar supplemented with cefotaxime (2 μg/ml). Pulmonary infection was induced in these rats as previously described (17–19). Briefly, the trachea of each rat was cannulated under general anesthesia, and the lungs were inoculated with 0.05 ml of a saline suspension of K. pneumoniae containing 2 × 106 CFU/ml (low inoculum; n = 8 rats) or 2 × 1010 CFU/ml (high inoculum; n = 8 rats). The control rats (n = 8) were inoculated with saline only. The clinical status of each infected rat was recorded twice daily.
In the high-inoculum group, 4 rats were treated and 4 rats were not treated. Treatment was launched when an animal expressed clinical signs of infection (coughing, close-set eyes, immobility, quilted coat, or hunched posture) and consisted of a subcutaneous injection of 50 mg/kg of cefquinome twice daily for 4 days (day 0 to day 3). In the low-inoculum group, 4 rats were not treated, and 4 rats received 5 mg/kg of cefquinome subcutaneously twice daily for 4 days beginning at day 0, starting at 24 h after challenge (day 0 to day 3). Stool samples were collected daily, starting 2 days before the antibiotic treatment (day −2) up to 23 days after the treatment (D23).
Bacterial counts.
The counts of total and resistant Enterobacteriaceae organisms were obtained from each stool sample (in duplicate) after plating serial dilutions of fecal samples on MacConkey agar only or supplemented with 2 μg/ml CTX. The colonies were counted after 24 h of incubation at 37°C. The lowest level of detection was 100 CFU/g feces.
At day 23, each surviving rat was euthanized and its lungs were aseptically removed and homogenized in 10 ml of 0.9% NaCl. The homogenates were centrifuged at 3,000 × g for 10 min, and the pellets were resuspended in 2.5 ml of 0.9% NaCl. The counts of K. pneumoniae organisms were obtained after plating serial dilutions of lung homogenates on Mueller-Hinton agar. The colonies were counted after 24 h of incubation at 37°C. The lowest level of detection was 100 CFU/lung, and the rats were considered to be microbiologically cured if they were found to have counts below this level.
RESULTS
Pharmacokinetics of cefquinome in OFA rats.
The observed and predicted plasma concentrations of cefquinome in rats (10 mg/kg dose, subcutaneous administration) are shown in Fig. 1, and the pharmacokinetic parameters are presented in Table 1. From these results, and assuming dose proportionality, we simulated different cefquinome plasma concentration profiles to determine the pivotal curative dose giving a time (h) concentrations are above the MIC (T>MIC) equal to 50% of the dosing interval (20). The dose was 50 mg/kg for a twice-daily administration schedule (Table 2).
FIG 1.
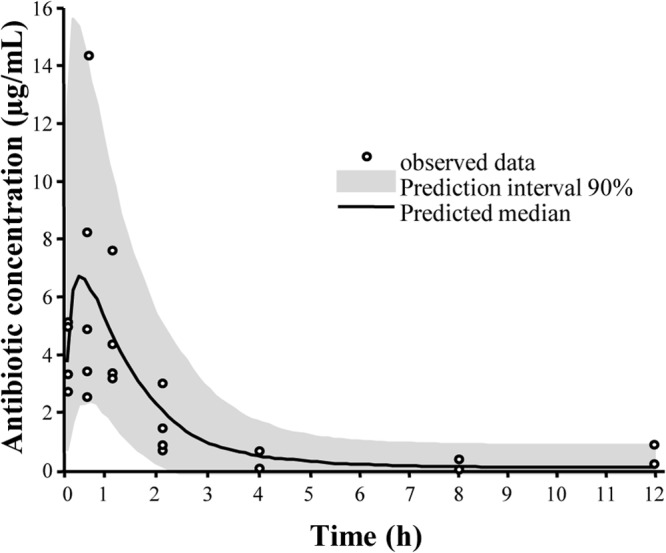
Observed and predicted median cefquinome plasma concentrations versus time in rats after a single subcutaneous administration.
TABLE 1.
Population pharmacokinetic parameters of cefquinome in rats after subcutaneous injection
| Parametera | Population value | Interindividual variability (%) |
|---|---|---|
| Dose (mg/kg) | 10 | |
| Ka (1/h) | 5.53 | 115 |
| V/F (ml/kg) | 900 | 49.6 |
| CL/F (ml/h/kg) | 912 | 26.8 |
| AUC[0–∞] (μg × h/ml) | 10.9 | |
| T1/2elim (h) | 0.68 |
Ka, absorption rate constant; V/F, apparent volume of distribution; CL/F, apparent plasma clearance; AUC, area under the plasma concentration curve; T1/2elim, terminal half-life.
TABLE 2.
Values of the PK/PD index T>MIC for two cefquinome doses
| T>MIC parameter | Dose (mg/kg of body weight) |
|
|---|---|---|
| 50 | 5 | |
| Time (h) | 6 | 3.9 |
| % of dosing interval | 50 | 32.5 |
Pneumonia.
In the low-inoculum group, the 4 untreated rats presented clinical signs of infection between 48 and 72 h postchallenge and died between 72 and 120 h postchallenge. The animals receiving the 5-mg/kg cefquinome dose presented no signs of infection before and after treatment, and they were microbiologically cured (defined as absence of lung K. pneumoniae) at day 23. In the high-inoculum group, the 8 rats presented clinical signs of infection between 12 and 48 h postchallenge. Four rats were treated when clinical signs occurred, and the 4 untreated rats died between 24 and 72 h postchallenge. All the rats receiving the 50-mg/kg cefquinome dose were clinically cured, and they were microbiologically cured at day 23 (absence of lung K. pneumoniae).
Fecal flora.
Total Enterobacteriaceae fecal counts remained stable at around 108 CFU/g in all rats from day −2 to day 23 (not shown). The CTX-R Enterobacteriaceae fecal counts are shown in Fig. 2. In the three groups of rats (patent phase dose, prepatent phase dose, and control groups), the basal CTX-R Enterobacteriaceae fecal counts were around 104 CFU/g between day −2 and day 0. For the patent phase dose group, the CTX-R Enterobacteriaceae counts increased to >108 CFU/g between day 1 and day 4, remained stable between day 4 and day 16, and slightly decreased to <108 CFU/g at day 23. For the prepatent phase dose and control groups, the CTX-R Enterobacteriaceae counts remained stable at 104 CFU/g between day 1 and day 23 (Fig. 2). The mean percentage of CTX-R Enterobacteriaceae mirrored the absolute counts, because the total counts were unchanged (Fig. 3). In the three groups, the basal CTX-R percentage was around 0.01% between day −2 and day 0. For the patent phase dose group, the basal CTX-R percentage increased from 0.01% to 100% between day 1 and day 4, remained at 100% between day 4 and day 16, and decreased to <100% at day 23 (Fig. 3). For the prepatent phase dose and control groups, the CTX-R percentage remained stable at around 0.01% between day 1 and day 23.
FIG 2.
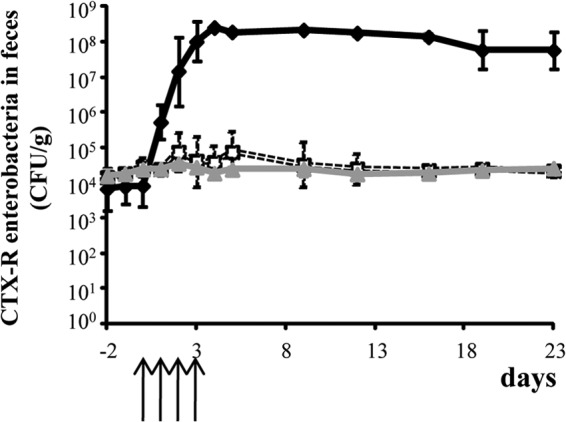
Impact of the different cefquinome dosage regimens on cefotaxime-resistant Enterobacteriaceae in the fecal flora of rats before, during, and after treatment. ⧫, Patent-phase-adjusted dose (50 mg/kg of body weight); □, prepatent-phase-adjusted dose (5 mg/kg);  , control untreated group. Data are means ± standard deviations (SDs). The arrows indicate the days of antibiotic administration.
, control untreated group. Data are means ± standard deviations (SDs). The arrows indicate the days of antibiotic administration.
FIG 3.
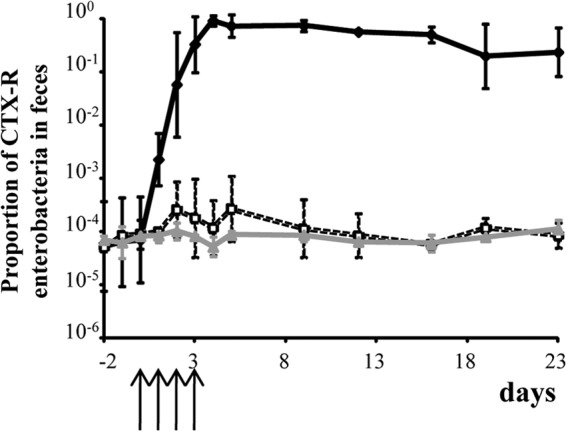
Impact of the different cefquinome dosage regimens on the proportion of cefotaxime-resistant Enterobacteriaceae in the fecal flora of rats before, during, and after treatment. ⧫, Patent-phase-adjusted dose (50 mg/kg); □, prepatent-phase-adjusted dose (5 mg/kg);  , control untreated group. Data are means ± SDs. The arrows indicate the days of antibiotic administration.
, control untreated group. Data are means ± SDs. The arrows indicate the days of antibiotic administration.
DISCUSSION
In the present study, we developed an original model allowing a concomitant assessment of the impact of antibiotics on lung pathogens and intestinal microbiota. Our main result was to demonstrate that a cefquinome treatment targeting a low pathogenic inoculum was able to fully cure a lung infection caused by K. pneumoniae, all without any measurable amplification of intestinal CTX-R Enterobacteriaceae.
First, we showed that early treatment targeting a low K. pneumoniae inoculum in the lungs was efficacious with a much lower dose (the prepatent phase adjusted dose) than that required against a high K. pneumoniae inoculum in sick animals (the patent phase adjusted dose). We observed in another rodent model of pneumonia that the prepatent phase adjusted dose of cefquinome was not active (100% mortality) in sick animals harboring a high pathogen load (M. V. Vasseur, A. A. Ferran, M. Z. Lacroix, P. L. Toutain, and A. Bousquet-Mélou, submitted for publication). This finding is supported by those of previous studies using various classes of antibiotics or bacterial species (13–17, 19) and reinforces the generic relevance of this so-called inoculum effect. A combination of several mechanisms might explain this effect, such as (i) the inoculum size dependency of antimicrobial activity as demonstrated in vitro for several antibiotics (11, 12) and (ii) the impact of pathogen burden on the saturable granulocytic clearance of bacteria (21, 22). For the early interventions, we decided to use a cefquinome dosage regimen that was 10-fold lower than the curative one used in sick rats. In previous in vitro experiments, we determined that the antimicrobial activity of cefquinome was higher against low than against high bacterial inocula (M. V. Vasseur, A. A. Ferran, M. Z. Lacroix, P. L. Toutain, and A. Bousquet-Mélou, submitted for publication). In addition, a set of published works on the influence of inoculum size on the in vivo efficacy of fluoroquinolones or beta-lactams against various Gram-positive or Gram-negative pathogens (11, 15, 17, 19, 23) supports the hypothesis that efficacious doses can be lower in early than in later interventions. In particular, Mizunaga et al. (11) determined that the 50% effective doses (ED50s) of 3 carbapenems were 12 to 50 times lower in Pseudomonas aeruginosa-infected mice when the initial bacterial inoculum was 100-fold lower.
The ability of prompt antibiotic treatments to eliminate growing but still small pathogen loads might be taken into consideration and have practical consequences for both human therapy and control of infectious outbreaks in food-producing animals. Indeed, one current strategy of infectious disease control at the herd level, called metaphylaxis or control, consists of very early administration of antibiotics during a collective infectious episode while most animals in the group are still in their prepatent phase of infection. However, two main characteristics of this strategy support the opinion that it probably generates an excessive consumption of antibiotics: (i) all animals in the group are treated, even when a proportion of animals does not become ill, meaning that antibiotics are not indicated at that time for those animals, and (ii) the doses used in this situation are those cleared by the marketing authority for treating animal patients with established bacterial infections, characterized by overt clinical signs and a high bacterial burden. Therefore, in the context of limiting antibiotic use in food animal production, our objective was to test if antibiotic dosage regimens adjusted to the early prepatent phase of an infection contribute to a reduction in the amount of antibiotics used.
Moreover, given the key role played by the gut microbiota in the amplification and spread of antimicrobial resistance from food-producing animals to humans, it was necessary to evaluate if the prepatent phase adjusted dosage regimen produced a differential impact on such commensal flora compared to a classical (patent phase adjusted) dosage regimen. As cefquinome is mainly used in pigs, and because the intestinal flora is very different between rats and pigs, we used a model of gnotobiotic rats harboring porcine fecal flora samples enriched with a CTX-R E. coli strain. Indeed, it has been shown that the implanted flora were stable and comparable to natural flora from the sampled individuals (24, 25). No measurable enrichment of CTX-R Enterobacteriaceae was observed with the prepatent phase adjusted dose, whereas rapid and massive amplification of CTX-R Enterobacteriaceae was observed with the patent phase adjusted dose. Cavaco et al. (26) demonstrated the emergence of CTX-R Enterobacteriaceae after cefquinome administration in healthy pigs by using the dose recommended for the treatment of respiratory infections. Here, we confirmed in sick animals that the cefquinome dose used for treating a fully established infection led to a rapid amplification of indigenous CTX-R Enterobacteriaceae. These results support the hypothesis that the gut microbiota is likely to be an important site for the amplification of antibiotic resistance, when a selective pressure is exerted by classical antibiotic treatments (1–4). We also showed in our experiments that this reservoir might release high levels of CTX-R Enterobacteriaceae into the environment for a long period (≥20 days) after the antibiotic selective pressure ended. Woerther et al. (27) recently pointed out that exposure to antibiotics might impact the carriage of ESBL-producing Enterobacteriaceae at the community level, in association with other factors, such as the exchanges of resistant strains or horizontal transfer of plasmids. Such observations support the objective of reducing global antibiotic exposure at the herd level. Moreover, in the context of human health protection, the most realistic objective is probably not to reduce the proportion of animals carrying ESBL-producing Enterobacteriaceae but rather to suppress antibiotic-driven resistance amplification that might result in massive excretion of CTX-R Enterobacteriaceae from the gastrointestinal (GI) tracts of animals into the environment or at slaughter.
Other recent studies have investigated the impact of manipulating therapeutic regimens on the amplification of antimicrobial resistance in the gut microbiota. For example, Zhang et al. (8) recently demonstrated that both the doses and routes of administration of tetracycline and ampicillin influenced the levels of antibiotic resistance found in the gut microbiota. On the other hand, when testing six different dosing regimens covering a 3-fold range of ciprofloxacin doses in humans, Fantin et al. (5) observed no measurable differences in the probability of the emergence of resistance in the gut microbiota. In their subjects, they measured antibiotic stool concentrations high enough to exceed the mutant preventive concentrations (MPC) for the dominant Enterobacteriaceae flora, associated with a transient disappearance of the total Enterobacteriaceae flora during the treatment and an appearance of resistant strains several days after the end of the treatment, when ciprofloxacin concentrations dropped to below the MPC (3, 5). However, it might be speculated that much lower ciprofloxacin doses produce different impacts on resistance amplification in gut microbiota, as was shown in piglets by Nguyen et al. (9), using a 10-fold range of ciprofloxacin doses. Obviously, such low doses do not produce any advantage if they cannot cure infected patients. Second, the picture of resistance development in the gut microbiota is different for classical doses of beta-lactams, such as ampicillin or cefquinome, for which resistance amplification in gut microbiota occurs as soon as the first day of treatment, as seen in the present study and elsewhere (8, 26, 28, 29). These results are probably explained by the pharmacokinetic and pharmacodynamic characteristics of beta-lactam drugs, which are excreted in the GI tract to a lesser extent than fluoroquinolones, leading to lower intestinal concentrations that are unable to kill highly resistant strains, such as those harboring ESBL genes.
All together, our results suggest that prepatent phase adjusted doses might constitute a promising strategy for the optimization of antibiotic dosage regimens by providing a way to ensure the control of infectious diseases in food-producing animals while minimizing the animal reservoirs of resistance genes of human concern.
ACKNOWLEDGMENTS
We thank Marlène Lacroix and Sylvie Puel for performing analytical assays.
Footnotes
Published ahead of print 6 January 2014
REFERENCES
- 1.Andremont A. 2003. Commensal flora may play key role in spreading antibiotic resistance. ASM News 69:601–607 [Google Scholar]
- 2.Baquero F, Coque TM, de la Cruz F. 2011. Ecology and evolution as targets: the need for novel eco-evo drugs and strategies to fight antibiotic resistance. Antimicrob. Agents Chemother. 55:3649–3660. 10.1128/AAC.00013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lastours V, Cambau E, Guillard T, Marcade G, Chau F, Fantin B. 2012. Diversity of individual dynamic patterns of emergence of resistance to quinolones in Escherichia coli from the fecal flora of healthy volunteers exposed to ciprofloxacin. J. Infect. Dis. 206:1399–1406. 10.1093/infdis/jis511 [DOI] [PubMed] [Google Scholar]
- 4.Phillips I, Casewell M, Cox T, De Groot B, Friis C, Jones R, Nightingale C, Preston R, Waddell J. 2004. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 53:28–52. 10.1093/jac/dkg483 [DOI] [PubMed] [Google Scholar]
- 5.Fantin B, Duval X, Massias L, Alavoine L, Chau F, Retout S, Andremont A, Mentré F. 2009. Ciprofloxacin dosage and emergence of resistance in human commensal bacteria. J. Infect. Dis. 200:390–398. 10.1086/600122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez MN, Papich MG, Drusano GL. 2012. Dosing regimen matters: the importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 56:2795–2805. 10.1128/AAC.05360-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantón R, Morosini MI. 2011. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 35:977–991. 10.1111/j.1574-6976.2011.00295.x [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Huang Y, Zhou Y, Buckley T, Wang HH. 2013. Antibiotic administration routes significantly influence the levels of antibiotic resistance in gut microbiota. Antimicrob. Agents Chemother. 57:3659–3666. 10.1128/AAC.00670-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen TT, Chachaty E, Huy C, Cambier C, de Gunzburg J, Mentr F, Andremont A. 2012. Correlation between fecal concentrations of ciprofloxacin and fecal counts of resistant Enterobacteriaceae in piglets treated with ciprofloxacin: toward new means to control the spread of resistance? Antimicrob. Agents Chemother. 56:4973–4975. 10.1128/AAC.06402-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferran A, Dupouy V, Toutain PL, Bousquet-Mélou A. 2007. Influence of inoculum size on the selection of resistant mutants of Escherichia coli in relation to mutant prevention concentrations of marbofloxacin. Antimicrob. Agents Chemother. 51:4163–4166. 10.1128/AAC.00156-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizunaga S, Kamiyama T, Fukuda Y, Takahata M, Mitsuyama J. 2005. Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenems. J. Antimicrob. Chemother. 56:91–96. 10.1093/jac/dki163 [DOI] [PubMed] [Google Scholar]
- 12.Udekwu KI, Parrish N, Ankomah P, Baquero F, Levin BR. 2009. Functional relationship between bacterial cell density and the efficacy of antibiotics. J. Antimicrob. Chemother. 63:745–757. 10.1093/jac/dkn554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eagle H. 1949. The effect of the size of the inoculum and the age of the infection on the curative dose of penicillin in experimental infections with streptococci, pneumococci, and Treponema pallidum. J. Exp. Med. 90:595–607. 10.1084/jem.90.6.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eagle H, Magnuson HJ, Fleischman R. 1947. Relation of the size of the inoculum and the age of the infection to the curative dose of penicillin in experimental syphilis, with particular reference to the feasibility of its prophylactic use. J. Exp. Med. 85:423–440. 10.1084/jem.85.4.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferran AA, Kesteman AS, Toutain PL, Bousquet-Mélou A. 2009. Pharmacokinetic/pharmacodynamic analysis of the influence of inoculum size on the selection of resistance in Escherichia coli by a quinolone in a mouse thigh bacterial infection model. Antimicrob. Agents Chemother. 53:3384–3390. 10.1128/AAC.01347-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferran AA, Toutain PL, Bousquet-Mélou A. 2011. Impact of early versus later fluoroquinolone treatment on the clinical; microbiological and resistance outcomes in a mouse-lung model of Pasteurella multocida infection. Vet. Microbiol. 148:292–297. 10.1016/j.vetmic.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kesteman AS, Ferran AA, Perrin-Guyomard A, Laurentie M, Sanders P, Toutain PL, Bousquet-Mélou A. 2009. Influence of inoculum size and marbofloxacin plasma exposure on the amplification of resistant subpopulations of Klebsiella pneumoniae in a rat lung infection model. Antimicrob. Agents Chemother. 53:4740–4748. 10.1128/AAC.00608-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakker-Woudenberg IA, de Jong-Hoenderop JY, Michel MF. 1979. Efficacy of antimicrobial therapy in experimental rat pneumonia: effects of impaired phagocytosis. Infect. Immun. 25:366–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesteman AS, Perrin-Guyomard A, Laurentie M, Sanders P, Toutain PL, Bousquet-Mélou A. 2010. Emergence of resistant Klebsiella pneumoniae in the intestinal tract during successful treatment of Klebsiella pneumoniae lung infection in rats. Antimicrob. Agents Chemother. 54:2960–2964. 10.1128/AAC.01612-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig WA. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. North Am. 17:479–501. 10.1016/S0891-5520(03)00065-5 [DOI] [PubMed] [Google Scholar]
- 21.Drusano GL, Fregeau C, Liu W, Brown DL, Louie A. 2010. Impact of burden on granulocyte clearance of bacteria in a mouse thigh infection model. Antimicrob. Agents Chemother. 54:4368–4372. 10.1128/AAC.00133-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drusano GL, Vanscoy B, Liu W, Fikes S, Brown D, Louie A. 2011. Saturability of granulocyte kill of Pseudomonas aeruginosa in a murine model of pneumonia. Antimicrob. Agents Chemother. 55:2693–2695. 10.1128/AAC.01687-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jumbe N, Louie A, Leary R, Liu W, Deziel MR, Tam VH, Bachhawat R, Freeman C, Kahn JB, Bush K, Dudley MN, Miller MH, Drusano GL. 2003. Application of a mathematical model to prevent in vivo amplification of antibiotic-resistant bacterial populations during therapy. J. Clin. Invest. 112:275–285. 10.1172/JCI200316814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazenberg MP, Bakker M, Verschoor-Burggraaf A. 1981. Effects of the human intestinal flora on germ-free mice. J. Appl. Bacteriol. 50:95–106. 10.1111/j.1365-2672.1981.tb00874.x [DOI] [PubMed] [Google Scholar]
- 25.Mallett AK, Bearne CA, Rowland IR, Farthing MJ, Cole CB, Fuller R. 1987. The use of rats associated with a human faecal flora as a model for studying the effects of diet on the human gut microflora. J. Appl. Bacteriol. 63:39–45. 10.1111/j.1365-2672.1987.tb02415.x [DOI] [PubMed] [Google Scholar]
- 26.Cavaco LM, Abatih E, Aarestrup FM, Guardabassi L. 2008. Selection and persistence of CTX-M-producing Escherichia coli in the intestinal flora of pigs treated with amoxicillin, ceftiofur, or cefquinome. Antimicrob. Agents Chemother. 52:3612–3616. 10.1128/AAC.00354-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woerther PL, Angebault C, Jacquier H, Clermont O, El Mniai A, Moreau B, Djossou F, Peroz G, Catzeflis F, Denamur E, Andremont A. 2013. Characterization of fecal extended-spectrum-beta-lactamase-producing Escherichia coli in a remote community during a long time period. Antimicrob. Agents Chemother. 57:5060–5066. 10.1128/AAC.00848-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bibbal D, Dupouy V, Ferré JP, Toutain PL, Fayet O, Prère MF, Bousquet-Mélou A. 2007. Impact of three ampicillin dosage regimens on selection of ampicillin resistance in Enterobacteriaceae and excretion of blaTEM genes in swine feces. Appl. Environ. Microbiol. 73:4785–4790. 10.1128/AEM.00252-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bibbal D, Dupouy V, Prère MF, Toutain PL, Bousquet-Mélou A. 2009. Relatedness of Escherichia coli strains with different susceptibility phenotypes isolated from swine feces during ampicillin treatment. Appl. Environ. Microbiol. 75:2999–3006. 10.1128/AEM.02143-08 [DOI] [PMC free article] [PubMed] [Google Scholar]


