Abstract
This study aimed to determine the efficacy of human-simulated plasma exposures of 2 g ceftazidime plus 0.5 g avibactam every 8 h administered as a 2-h infusion or a ceftazidime regimen that produced a specific epithelial lining fluid (ELF) percentage of the dosing interval in which serum free drug concentrations remain above the MIC (fT>MIC) against 28 Pseudomonas aeruginosa isolates within a neutropenic murine pneumonia model and to assess the impact of host infection on pulmonary pharmacokinetics. The fT>MIC was calculated as the mean and upper end of the 95% confidence limit. Against the 28 P. aeruginosa strains used, the ceftazidime-avibactam MICs were 4 to 64 μg/ml, and those of ceftazidime were 8 to >128 μg/ml. The change in log10 CFU after 24 h of treatment was analyzed relative to that of 0-h controls. Pharmacokinetic studies in serum and ELF were conducted using ceftazidime-avibactam in infected and uninfected mice. Humanized ceftazidime-avibactam doses resulted in significant exposures in the lung, producing reductions of >1 log10 CFU against P. aeruginosa with ceftazidime-avibactam MICs of ≤32 μg/ml (ELF upper 95% confidence limit for fT>MIC [ELF fT>MIC] of ≥19%), except for one isolate with a ceftazidime-avibactam MIC of 16 μg/ml. No efficacy was observed against the isolate with a ceftazidime-avibactam MIC of 64 μg/ml (ELF fT>MIC of 0%). Bacterial reductions were observed with ceftazidime against isolates with ceftazidime MICs of 32 μg/ml (ELF fT>MIC of ≥12%), variable efficacy at ceftazidime MICs of 64 μg/ml (ELF fT>MIC of ≥0%), and no activity at a ceftazidime MIC of 128 μg/ml, where the ELF fT>MIC was 0%. ELF fT>MICs were similar between infected and uninfected mice. Ceftazidime-avibactam was effective against P. aeruginosa, with MICs of up to 32 μg/ml with an ELF fT>MIC of ≥19%. The data suggest the potential utility of ceftazidime-avibactam for treatment of lung infections caused by P. aeruginosa.
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative pathogen isolated in health care-associated and ventilator-associated pneumonia (1). These difficult to treat infections create challenges, because many antibiotics used against this organism have reduced potency and low concentrations in the lungs; thus, achieving optimal exposures is challenging (2, 3). Furthermore, more understanding is needed with emerging compounds about the pharmacodynamic targets needed at this site of infection.
Significant interest has been given to newer β-lactamase inhibitors paired with a β-lactam backbone. One of these compounds is avibactam, a novel non-β-lactam β-lactamase inhibitor that protects β-lactams from class A, class C, and some class D β-lactamases. The combination of ceftazidime-avibactam has been shown to provide enhanced potency compared with ceftazidime alone against a variety of Gram-negative pathogens. One study evaluated ceftazidime-avibactam against P. aeruginosa isolates from clinical specimens in France and found the MICs that inhibited 50% and 90% of the organisms (MIC50 and MIC90, respectively) to be 4 and 8 μg/ml, respectively (4). On the other hand, the MIC50 and MIC90 for ceftazidime alone against the same sample of isolates were 8 and 64 μg/ml, respectively (4). Similar distributions of activities, with a ceftazidime-avibactam MIC90 of 8 μg/ml and a ceftazidime MIC90 of 32 μg/ml, were found in a study of clinical isolates of P. aeruginosa in Canada (5). More recently, our group has described the activity of ceftazidime-avibactam compared with that of ceftazidime monotherapy within a P. aeruginosa murine thigh infection model (6). In that study, which evaluated human-simulated plasma exposures of ceftazidime-avibactam, predictable activity was observed against isolates with MICs of ≤16 μg/ml, where the pharmacodynamic target of interest, the percentage of the dosing interval in which serum free drug concentrations remain above the MIC (fT>MIC), was 62%.
In the clinic, ceftazidime-avibactam has been shown to be effective and well tolerated. In a phase II study evaluating ceftazidime-avibactam with metronidazole against meropenem in patients with complicated intra-abdominal infections, ceftazidime-avibactam produced similar results to meropenem, with 91.2 and 93.4% of patients, respectively, demonstrating a favorable clinical response at the test of cure visit (7). P. aeruginosa was isolated in 10 patients, 5 of whom received ceftazidime-avibactam and metronidazole and 5 of whom received meropenem. All had positive clinical responses. Similar results were observed in another phase II study evaluating ceftazidime-avibactam against imipenem-cilastatin in the treatment of complicated urinary tract infections (8). In this study, the favorable microbiologic response was 70.4% for patients receiving ceftazidime-avibactam versus 71.4% for patients receiving imipenem-cilastatin.
Ceftazidime itself is clinically effective against Gram-negative bacteria, including P. aeruginosa strains causing nosocomial pneumonia (1); more knowledge is needed to understand the potential role of ceftazidime-avibactam in this setting. This study therefore sought to evaluate the effect of the host's infection status on the pulmonary pharmacokinetics of ceftazidime-avibactam in the background of a murine model of simulated human pharmacokinetics (6) and to describe the activities of ceftazidime-avibactam and ceftazidime against highly resistant clinical isolates of P. aeruginosa in a lung infection model.
MATERIALS AND METHODS
Antimicrobial test agents.
Analytical-grade ceftazidime (Sigma-Aldrich, St. Louis, MO) and avibactam (provided by AstraZeneca Pharmaceuticals, Waltham, MA) were corrected for potency and utilized for all in vitro analyses. Analytical-grade ceftazidime and avibactam powders were weighed in a quantity sufficient to achieve the required concentrations and reconstituted immediately prior to use. For in vivo experimentation, commercially available ceftazidime (Fortaz, lot no. L716; GlaxoSmithKline, Philadelphia, PA) was obtained from the Hartford Hospital Pharmacy Department, Hartford, CT, and analytical-grade avibactam was utilized. Clinical vials of ceftazidime were reconstituted as described in the package insert. Drug dosing solutions of ceftazidime and ceftazidime-avibactam were diluted in 0.9% normal saline to achieve the desired concentrations.
Bacterial isolates and susceptibility testing.
A total of 28 clinical isolates of P. aeruginosa, provided by AstraZeneca Pharmaceuticals and the Center for Anti-Infective Research and Development (CAIRD), were utilized for these studies. MICs of ceftazidime and ceftazidime-avibactam were determined for each isolate by the broth microdilution methodology as outlined by the Clinical and Laboratory Standards Institute, and a minimum of five replicates and modal MICs were determined (9). For ceftazidime-avibactam, doubling dilutions of ceftazidime were utilized in combination with a fixed concentration of 4 μg/ml of avibactam.
Neutropenic lung infection model.
Pathogen-free female ICR mice weighing approximately 25 g were acquired from Harlan Laboratories (Indianapolis, IN). This study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee. Animals were maintained and used in accordance with National Research Council recommendations and were provided food and water ad libitum. Mice were rendered neutropenic by administering intraperitoneal injections of cyclophosphamide (Baxter, Deerfield, IL) at doses of 250 and 100 mg/kg body weight 4 days and 1 day, respectively, prior to inoculation. Uranyl nitrate at a dose of 5 mg/kg was also administered 3 days prior to inoculation to induce renal impairment. Mice were anesthetized with isoflurane and inoculated with 0.05 ml of a 107-CFU/ml suspension of the infecting P. aeruginosa isolate in 3% porcine stomach mucin (Sigma-Aldrich, St. Louis, MO). The inoculum was administered into the mouths of the mice while blocking their nares to induce aspiration (10).
Confirmatory pharmacokinetic studies.
A dosing regimen previously described by our group was used for the ceftazidime-avibactam and ceftazidime human simulated regimens (6). Confirmatory pharmacokinetic studies were done to verify concentrations in a murine lung infection model similar to those seen in a murine thigh model. Briefly, these regimens were derived from a human pharmacokinetic model based on phase I and phase II clinical data (11). The time course of free plasma concentrations was an average of those predicted to occur in humans following the administration of 2 g ceftazidime plus 0.5 g avibactam every 8 h as a 2-h infusion. For free drug calculations, protein binding values of 26% and 15% were used for ceftazidime in mice and humans, respectively (6, 11). These values for avibactam were 10% and 8%, respectively (6, 11). The ceftazidime-avibactam murine free drug serum concentration-time profile was further defined over the entire 24-h experiment by sampling at specific time points. Studies were conducted as described above, and blood was collected via cardiac puncture. The fT>MIC was calculated over the three dosing intervals. Serum samples were separated by centrifugation and stored at −80°C until analysis. The mean fT>MIC was then calculated over the entire experiment.
During confirmatory pharmacokinetic studies, concentrations in the epithelial lining fluid (ELF) were measured over the third interval, and the mean ELF fT>MIC was calculated using time points over the third interval of the experiment (i.e., 16 to 24 h). Protein binding was assumed to be negligible in the ELF based on previous reports (12). Given that the ELF fT>MIC is calculated using a composite of data derived from multiple animals and given the pharmacokinetic variability between animals, the upper 95% confidence limit of the ELF fT>MIC was also calculated to describe the upper end of the population range. ELF was collected via bronchoalveolar lavage (BAL), which was performed by inserting a catheter into the trachea and instilling four aliquots of 0.4 ml normal saline followed by immediate removal of the fluid. Blood samples were also obtained as described above. Serum and BAL samples were separated by centrifugation and stored at −80°C until analysis. In order to calculate the ELF concentrations, serum and BAL fluid samples were also utilized for urea determination (Teco Diagnostics blood urea nitrogen [BUN] reagent set), as previously described (13). The ceftazidime and avibactam concentrations in ELF were calculated using the equation CAZBAL × (ureaserum/ureaBAL). The term “CAZBAL” represents the ceftazidime or avibactam concentration within the BAL fluid, and “ureaserum” and “ureaBAL” represent the concentrations of urea in the serum and BAL fluid, respectively.
Directed ceftazidime pharmacokinetic studies.
Given the potency of ceftazidime-avibactam and that the MIC90 against P. aeruginosa is ≤8 μg/ml, there were limited isolates available to test at the high end of the MIC distribution (i.e., ≥64 μg/ml). Therefore, we used ceftazidime as a surrogate to describe efficacy, as this had been shown in a previous study to correlate well with fT>MIC efficacy targets derived for ceftazidime-avibactam against P. aeruginosa (6). These studies were conducted to design a regimen in mice that resulted in a sufficiently low ELF fT>MIC so that we were able to observe a loss of efficacy within the lung infection model. Mice were prepared as described above, and serum and ELF were collected to measure ceftazidime concentrations. These pharmacokinetic studies were conducted over the third interval of the experiment (i.e., 16 to 24 h).
In vivo samples.
Ceftazidime concentrations in serum were analyzed at CAIRD (Hartford, CT) using a previously described high-performance liquid chromatography assay (14). Avibactam concentrations were determined by Eurofins Medinet, Inc. (Chantilly, VA), using a previously described liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay (15). Urea concentration determination was assayed at CAIRD (Hartford, CT).
In vivo efficacy.
Twenty-eight P. aeruginosa isolates were used in the experiments. Two hours after inoculation, groups of six mice received predetermined ceftazidime-avibactam or ceftazidime doses administered as 0.2-ml subcutaneous injections. Control animals were administered normal saline at the same volume, route, and frequency as the treatment regimens. A group of six mice were sacrificed just prior to the initiation of therapy (0-h control animals) to serve as a baseline measurement of lung bacterial density. All other control and treatment mice were euthanized 24 h after initiation of therapy. Mice that did not survive to the end of the experiment (24 h) had their lungs removed at the time of expiration. Following sacrifice, lungs were aseptically harvested, rinsed with normal saline, and then homogenized in 1.0 ml of normal saline. Serial dilutions of lung homogenate were plated onto 5% sheep blood agar and Trypticase soy agar and incubated at 37°C for 24 h. The change in bacterial density was calculated as the difference in log10 CFU from ceftazidime-avibactam- or ceftazidime-treated mice after 24 h from the level in the 0-h control animals.
For nine isolates (ceftazidime-avibactam MICs of 4 to 16 μg/ml), a phenotypic assessment of resistance was conducted by plating the lung homogenate on blood agar plates containing 32 μg/ml of ceftazidime and 4 μg/ml of avibactam and incubating the plates overnight.
Pharmacokinetic studies of host infection status.
Pharmacokinetic studies were performed with ceftazidime-avibactam, using the human simulated dosing regimen (6), administered to both infected and uninfected mice to determine if the host's infection status has an impact on the pulmonary pharmacokinetic profile. Utilizing the BAL concentration-time profile of the mean data, fT>MICs were also compared for infected and uninfected mice.
RESULTS
Bacterial isolates.
The phenotypic profiles for the P. aeruginosa isolates used in this study are listed in Table 1. There was little variability between the five individually prepared replicates of the MIC test for each isolate; thus, the modal MIC has been utilized as the best estimate of in vitro potency using conventional MIC testing methodology.
TABLE 1.
Phenotypic data for the 28 P. aeruginosa isolates utilized during in vivo efficacy studies of ceftazidime-avibactam and ceftazidime
| P. aeruginosa isolatea | MIC (μg/ml)b: |
|
|---|---|---|
| CAZ-AVI | CAZ | |
| PSA 22 | 4 | 64 |
| PSA 971 | 4 | 16 |
| PSA 1383* | 4 | 64 |
| PSA 1384* | 4 | 64 |
| PSA 37-8 | 4 | 64 |
| PSA 856 | 8 | 8 |
| PSA 1382* | 8 | 128 |
| PSA 1386* | 8 | 128 |
| PSA 1387* | 8 | 32 |
| PSA 1388* | 8 | 128 |
| PSA 1389* | 8 | 64 |
| PSA 4-32 | 8 | 32 |
| PSA 4-39 | 8 | 32 |
| PSA 2-69 | 8 | 64 |
| PSA 8-16 | 8 | 64 |
| PSA 24-2 | 8 | 32 |
| PSA 28-19 | 8 | 128 |
| PSA 968 | 16 | 32 |
| PSA 1391* | 16 | 128 |
| PSA 1394* | 16 | 64 |
| PSA 4-36 | 16 | 64 |
| PSA 4-84 | 16 | 64 |
| PSA 1-25 | 16 | 128 |
| PSA 1-29 | 16 | 32 |
| PSA 3-9 | 16 | >128 |
| PSA 11-54 | 32 | 32 |
| PSA 7-6 | 32 | 128 |
| PSA 14-28 | 64 | 128 |
Asterisks indicate isolates used to assess development of resistance.
CAZ, ceftazidime; AVI, avibactam.
Ceftazidime-avibactam confirmatory pharmacokinetic studies.
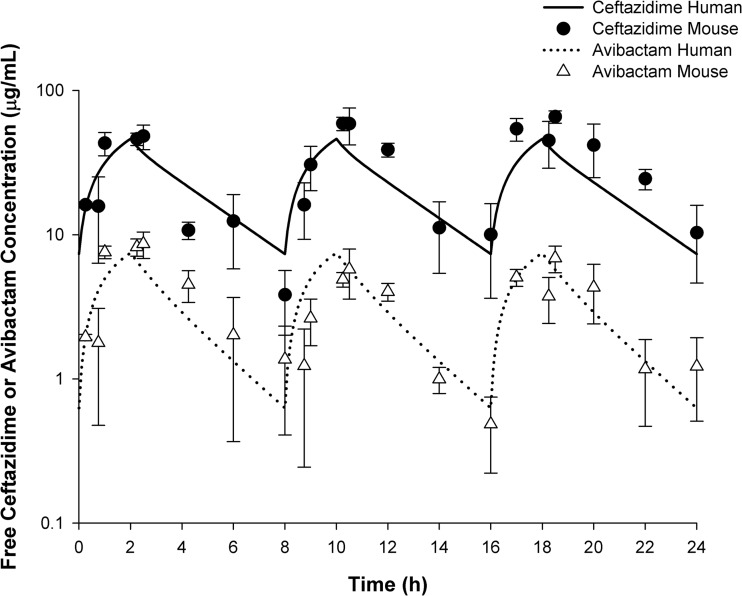
The free drug plasma concentration-time profile for 2 g ceftazidime–0.5 g avibactam administered as a 2-h infusion every 8 h in humans and free drug serum concentration-time profile in mice are shown in Fig. 1. The ceftazidime-avibactam ELF concentration-time profile in mice is shown in Fig. 2. The associated ELF pharmacodynamic parameters fT>MIC and the upper 95% confidence limit of fT>MIC are described in Table 2. The mean serum fT>MIC is also described in Table 2 (6).
FIG 1.
Serum concentration-time profile after human simulated plasma doses of 2 g ceftazidime–0.5 g avibactam every 8 h as a 2-h infusion in humans compared with that observed in infected female ICR mice.
FIG 2.
Epithelial lining fluid concentration-time profile after human simulated plasma doses of 2 g ceftazidime–0.5 g avibactam every 8 h as a 2-h infusion in humans compared with that observed in infected female ICR mice.
TABLE 2.
Pharmacodynamic parameters for the ceftazidime-avibactam and ceftazidime regimens used in the in vivo efficacy studies
| Regimen and fT>MIC typea | % with MIC (μg/ml): |
||||
|---|---|---|---|---|---|
| 4 | 8 | 16 | 32 | 64 | |
| Ceftazidime-avibactam | |||||
| Serum, mean (6) | 99 | 88 | 62 | 34 | 0 |
| ELF | |||||
| Mean | 100 | 88 | 63 | 0 | 0 |
| Upper 95% CL | 100 | 100 | 63 | 19 | 0 |
| Ceftazidime (directed) | |||||
| Serum, mean | 100 | 100 | 91 | 60 | 6 |
| ELF | |||||
| Mean | 100 | 69 | 28 | 6 | 0 |
| Upper 95% CL | 100 | 100 | 34 | 12 | 0 |
For each regimen, the mean ELF fT>MIC is reported, as well as the upper 95% confidence limit (CL) and the mean serum fT>MIC for ceftazidime from the ceftazidime-avibactam and ceftazidime (directed) regimens.
Directed ceftazidime pharmacokinetic determination.
The drug concentrations in ELF (mean ± standard deviation [SD]) after administration of the ceftazidime regimen at 17, 18.5, and 24 h were 19.13 ± 11.91, 33.74 ± 3.97, and 5.45 ± 3.35 μg/ml, respectively. The ELF fT>MIC was calculated and is described in Table 2.
In vivo efficacy of ceftazidime-avibactam.
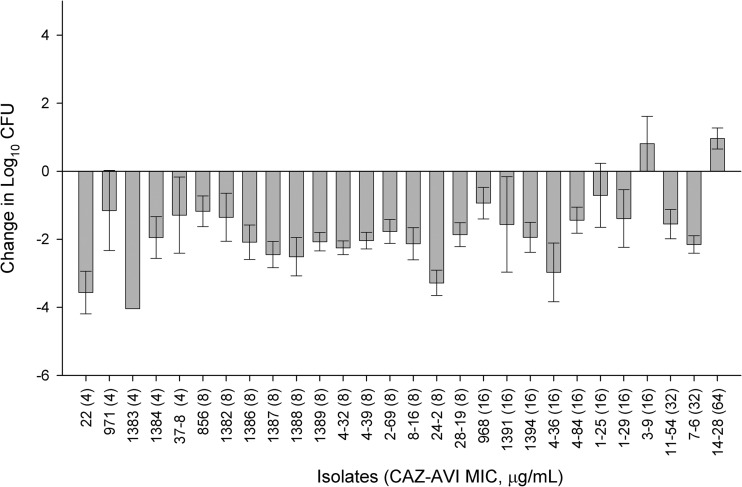
The mean ± SD bacterial density for the 0-h neutropenic control mice at the start of dosing was 5.98 ± 0.44 log10 CFU. The mean bacterial density of the 24-h control mice increased to 9.13 ± 0.80 log10 CFU. Seventy-four percent of control mice did not survive the entire 24 h; hence, their lungs were harvested at the time of expiration. Bacterial densities from these animals were found to be similar to those in mice that did survive 24 h and were included in the data analysis. The efficacy of ceftazidime-avibactam observed against the P. aeruginosa isolates is depicted in Fig. 3. Activity was observed with ceftazidime-avibactam against the P. aeruginosa isolates with MICs of 32 μg/ml and less, with the exception of one isolate at a MIC of 16 μg/ml. Activity was not observed against the lone isolate with a MIC of 64 μg/ml. After the direct homogenate had been plated onto drug-containing plates, no growth was observed, signifying no development of resistance was present.
FIG 3.
Efficacy of human simulated serum doses of 2 g ceftazidime–0.5 g avibactam every 8 h as a 2-h infusion against P. aeruginosa in the neutropenic lung infection model. Error bars represent means ± SD.
In vivo efficacy of directed ceftazidime.
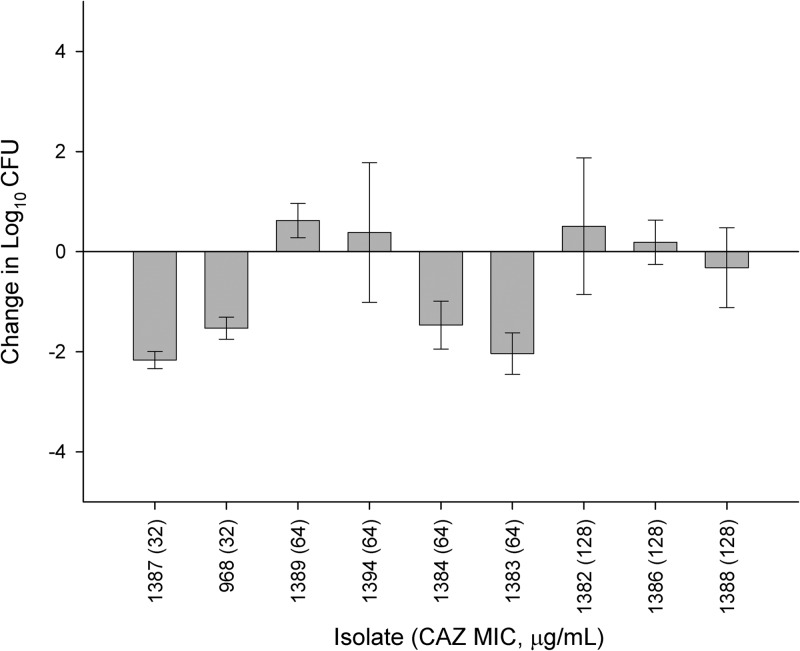
Respective bacterial densities in control mice at the initiation of dosing were 5.89 ± 0.30 log10 CFU, increasing to 8.75 ± 0.93 log10 CFU after 24 h. Isolates tested against ceftazidime had MICs between the range of 32 and 128 μg/ml. As illustrated in Fig. 4, activity was observed against those isolates with MICs of 32 μg/ml. Efficacy was variable against isolates with MICs of 64 μg/ml; three isolates for which the ceftazidime MIC was 128 μg/ml displayed variable activity (a log10-CFU change from −0.32 to +0.51) when the ceftazidime monotherapy regimen was used.
FIG 4.
Efficacy of ceftazidime as a directed epithelial lining fluid (ELF) fT>MIC regimen against P. aeruginosa in the neutropenic lung infection model. Error bars represent means ± SD.
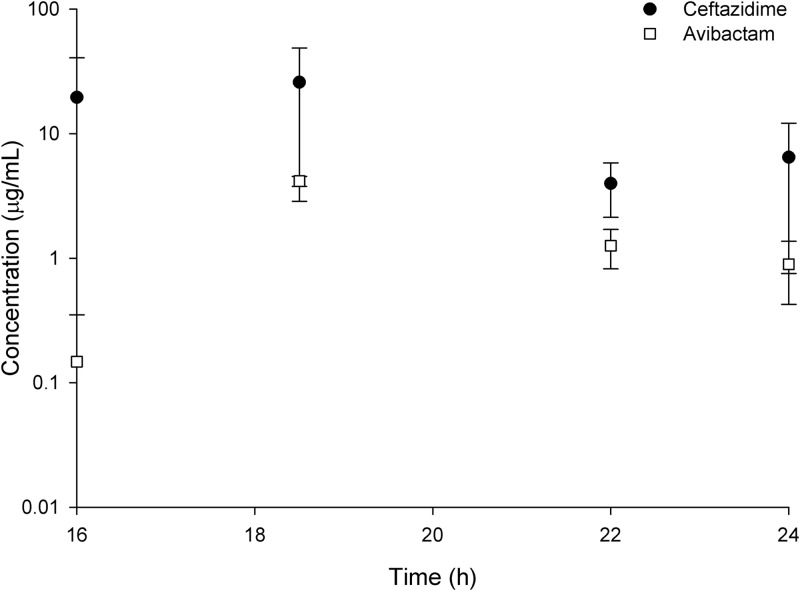
Pharmacokinetic determination and host infection status.
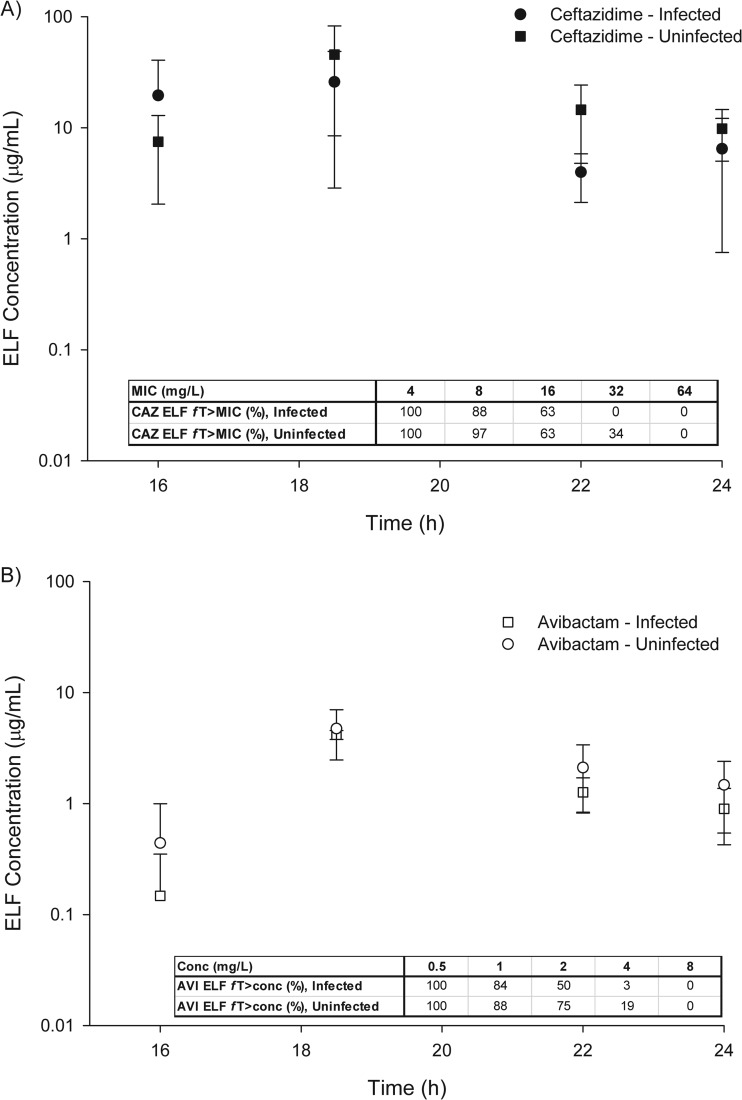
The comparison of ELF drug concentrations between infected and uninfected mice is shown in Fig. 5A for ceftazidime and B for avibactam. In ELF, the pharmacodynamic drivers of efficacy, fT>MIC for ceftazidime or fT>CT (threshold concentration) for avibactam, were similar between infected and uninfected mice.
FIG 5.
Epithelial lining fluid (ELF) concentration-time profile after human simulated plasma doses of 2 g ceftazidime–0.5 g avibactam every 8 h as a 2-h infusion observed in infected and uninfected mice monitored during the third and final 8-h period.
DISCUSSION
Ceftazidime-avibactam is a novel β-lactam β-lactamase inhibitor currently being investigated for the treatment of serious infections caused by Gram-negative pathogens, including P. aeruginosa. Given its potent in vitro activity, we evaluated the efficacy of ceftazidime-avibactam within a murine lung infection model against P. aeruginosa, a likely pathogen isolated within the lung.
Efficacy with ceftazidime-avibactam against P. aeruginosa was observed in the murine lung infection model up to ceftazidime-avibactam MICs of 32 μg/ml, where the serum fT>MIC was 32% and the ELF fT>MIC was as high as 19%. No activity was observed against a single isolate against which the ceftazidime-avibactam MIC was 64 μg/ml. It is important to note that with conventional doubling dilution MIC techniques, a single modal MIC may be overly precise. The break in activity observed at an MIC of 64 μg/ml is likely to be somewhere between 32 and 64 μg/ml. Given the limited isolates with ceftazidime-avibactam MICs higher than 32 μg/ml, ceftazidime was used to help predict the break in efficacy, as this had been shown previously to be a good predictor of ceftazidime-avibactam efficacy (6). With the ceftazidime directed fT>MIC regimen, efficacy was observed against those isolates for which the ELF fT>MIC was ≥12% and the serum fT>MIC was ≥60%. At ceftazidime MICs of 64 and 128 μg/ml, variable to no activity was observed where there was no appreciable fT>MIC in the ELF. The serum fT>MIC at 64 μg/ml was 6%, suggesting the necessary fT>MIC in the blood needed for activity is between 6 and 60%. Based on these observations, efficacy would be expected when the ELF fT>MIC is less than the standard pharmacodynamic target of 60% for β-lactams in the blood (16).
With the limited information available for ceftazidime-avibactam pharmacodynamics in the lung at this time, the pharmacodynamic targets of ceftazidime from other studies were utilized to understand the potential efficacy of ceftazidime-avibactam in humans. Muller and colleagues recently published evidence to define the necessary pharmacodynamic serum targets for ceftazidime in patients with nosocomial pneumonia (17). This study utilized data from a multicenter phase III study to evaluate the pharmacodynamic indices via a population pharmacokinetic model and correlated those data with microbiological outcome. The authors concluded that an fT>MIC of >45% in serum would likely predict the microbiological and positive clinical outcome in nosocomial pneumonia. However, no information on pulmonary distribution was provided. Boselli et al. described the pulmonary penetration of ceftazidime following a 30-min infusion of 2 g and then a continuous infusion of 4 g over 24 h for at least 2 days (3). A total of 15 patients with nosocomial pneumonia were enrolled and equally distributed into three different time points. Samples from plasma and BAL fluid were obtained simultaneously. Penetration was calculated as the ratio of the concentration in ELF to the free drug concentration in plasma and resulted in a penetration of 20.6% ± 8.9%. Coupling of the data derived from Muller and Boselli, it is likely that the ceftazidime-avibactam ELF fT>MIC required for activity within the lung is <45%. A recent study by our group reported that an fT>MIC in serum of 53% is needed for microbiologic success in patients being treated with ceftazidime or cefepime for confirmed Gram-negative ventilator-associated pneumonia (18). This is concordant with our observations in the present study of the activity of ceftazidime-avibactam in the lung against P. aeruginosa, when the ELF fT>MIC was approximately 20% or higher and the serum fT>MIC was 32%.
One unpublished study reported that the penetration of ceftazidime-avibactam into the lungs of healthy human volunteers was approximately 30%, with a maximum concentration of drug in serum (Cmax) in the ELF of 23.2 μg/ml after administration of 2 g ceftazidime–0.5 g avibactam every 8 h (19). Accepting that ceftazidime-avibactam penetrates into the ELF of humans, future studies will be necessary to confirm pharmacodynamic breakpoints in patients.
Finally, the impact of infection status upon the pharmacokinetics of antibiotics is important. A previous assessment in an animal model compared the penetration into the ELF of four fluoroquinolones in healthy and infected mice and found no differences between the groups (20). Additional work with ceftriaxone demonstrated no difference in penetration (defined as the area under the concentration-time curve [AUC] in the lung tissue or serum) between infected and uninfected mice (21). Of note, infected mice did display higher overall concentrations of ceftriaxone in serum and ELF than did uninfected mice (21). More recently, enhanced ELF penetration has been described for tigecycline in infected tissue compared to that in healthy mice (22). In our observations with ceftazidime-avibactam, we found no difference in ELF concentrations between healthy and infected female ICR mice. Similarly, little difference was observed in either ceftazidime or avibactam ELF/plasma exposure ratios between infected and uninfected mice when renal clearance was unimpaired (23). When calculating the pharmacodynamic driver for β-lactams, fT>MIC, there were slight numerical differences between the infected and uninfected groups, but these were not deemed to be significant as the differences occurred at MICs or concentrations viewed as not pharmacodynamically relevant. While this provides important information in the context of murine lung pharmacokinetics, additional data are needed in patients to link these animal data to the clinic.
Ceftazidime-avibactam demonstrated substantial activity in a murine lung infection model against P. aeruginosa, with ceftazidime-avibactam MICs up to 32 μg/ml, where the ELF fT>MIC was ≥19% suggesting the required fT>MIC in the lung is less than previously expected. Observations in the present study, a recent murine dose fractionation study (24), and an ongoing clinical efficacy study will aid in the prediction of the efficacies of ceftazidime and avibactam against P. aeruginosa in human lung infections.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicius, Henry Christensen, Mao Hagihara, Jennifer Hull, Rebecca Keel, Debora Santini, Christina Sutherland, Pam Tessier, Lindsay Tuttle, and Dora Wiskirchen from the Center for Anti-Infective Research and Development for assistance with the in vivo experimentation. James Li is acknowledged for the modeled human pharmacokinetic profiles.
This study was funded by AstraZeneca Pharmaceuticals, Inc. (Waltham, MA).
D. P. Nicolau has received research grants and an honorarium and participates in the advisory board for AstraZeneca. W. W. Nichols is an employee of AstraZeneca.
Footnotes
Published ahead of print 16 December 2013
REFERENCES
- 1.American Thoracic Society, Infectious Diseases Society of America 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388–416. 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 2.Lodise TP, Sorgel F, Melnick D, Mason B, Kinzig M, Drusano GL. 2011. Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia. Antimicrob. Agents Chemother. 55:1606–1610. 10.1128/AAC.01330-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boselli E, Breilh D, Rimmele T, Poupelin JC, Saux MC, Chassard D, Allaouchiche B. 2004. Plasma and lung concentration of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med. 30:989–991. 10.1007/s00134-004-2171-2 [DOI] [PubMed] [Google Scholar]
- 4.Levasseur P, Girard AM, Claudon M, Goossens H, Black MT, Coleman K, Miossec C. 2012. In vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 56:1606–1608. 10.1128/AAC.06064-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walkty A, DeCorby M, Lagacé-Wiens PRS, Karlowsky JA, Hoban DJ, Zhanel GG. 2011. In vitro activity of ceftazidime combined with NXL104 versus Pseudomonas aeruginosa isolates obtained from patients in Canadian hospitals (CANWARD 2009 Study). Antimicrob. Agents Chemother. 55:2992–2994. 10.1128/AAC.01696-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crandon JL, Schuck VJ, Banevicius MA, Beaudoin ME, Nichols WW, Tanudra MA, Nicolau DP. 2012. Comparative in vitro and in vivo efficacies of human simulated doses of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 56:6137-6146. 10.1128/AAC.00851-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. 2013. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double blind, phase II trial. J. Antimicrob. Chemother. 68:1183–1192. 10.1093/jac/dks523 [DOI] [PubMed] [Google Scholar]
- 8.Vazquez JA, Gonzalez Patzan LD, Stricklin D, Duttaroy DD, Kreidly Z, Lipka J, Sable C. 2012. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr. Med. Res. Opin. 28:1921–1931. 10.1185/03007995.2012.748653 [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute 2011. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, 8th ed. CLSI publication M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10.Tessier PR, Keel RA, Hagihara M, Crandon JL, Nicolau DP. 2012. Comparative in vivo efficacies of epithelial lining fluid exposures of tedizolid, linezolid, and vancomycin for methicillin-resistant Staphylococcus aureus in a mouse pneumonia model. Antimicrob. Agents Chemother. 56:2342–2346. 10.1128/AAC.06427-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Knebel W, Riggs M, Zhou D, Nichols W, Das S. 2012. Population pharmacokinetic modeling of ceftazidime (CAZ) and avibactam (AVI) in healthy volunteers and patients with complicated intra-abdominal infection (cIAI), abstr A634. Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 12.Kiem S, Schentag JJ. 2008. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob. Agents Chemother. 52:24–36. 10.1128/AAC.00133-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laohavaleeson S, Tessier PR, Nicolau DP. 2008. Pharmacodynamic characterization of ceftobiprole in experimental pneumonia caused by phenotypically diverse Staphylococcus aureus strains. Antimicrob. Agents Chemother. 52:2389–2394. 10.1128/AAC.01422-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolau DP, Nightingale CH, Banevicius MA, Fu Q, Quintiliani R. 1996. Serum bactericidal activity of ceftazidime: continuous infusion versus intermittent injections. Antimicrob. Agents Chemother. 40:61–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiskirchen DE, Crandon JL, Furtado GH, Williams G, Nicolau DP. 2011. In vivo efficacy of a human-simulated regimen of ceftaroline combined with NXL104 against extended-spectrum-beta-lactamase (ESBL)-producing and non-ESBL-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 55:3220–3225. 10.1128/AAC.00024-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10. 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 17.Muller AE, Punt N, Mouton JW. 2013. Optimal exposures of ceftazidime predict the probability of microbiological and clinical outcome in the treatment of nosocomial pneumonia. J. Antimicrob. Chemother. 68:900–906. 10.1093/jac/dks468 [DOI] [PubMed] [Google Scholar]
- 18.MacVane SH, Kuti JL, Nicolau DP. 2013. Clinical pharmacodynamics of antipseudomonal cephalosporins in patients with ventilator-associated pneumonia, abstr A-297. 53rd Intersci. Conf. Antimicrob. Agents Chemother [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolau DP, Siew L, Armstrong J, Edeki T, Bouw R. 2013. Concentration of avibactam (AVI) and ceftazidime (CAZ) in plasma and epithelial lining fluid (ELF) in healthy volunteers, abstr A-1027. Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 20.Vallee E, Azoulay-Dupuis E, Pocidalo JJ, Bergogne-Berezin E. 1991. Pharmacokinetics of four fluoroquinolones in an animal model of infected lung. J. Antimicrob. Chemother. 28(Suppl C):39–44. 10.1093/jac/28.suppl_C.39 [DOI] [PubMed] [Google Scholar]
- 21.Wang E, Bergeron Y, Bergeron MG. 2005. Ceftriaxone pharmacokinetics in interleukin-10-treated murine pneumococcal pneumonia. J. Antimicrob. Chemother. 55:721–726. 10.1093/jac/dki085 [DOI] [PubMed] [Google Scholar]
- 22.Crandon JL, Kim A, Nicolau DP. 2009. Comparison of tigecycline penetration into the epithelial lining fluid of infected and uninfected murine lungs. J. Antimicrob. Chemother. 64:837–839. 10.1093/jac/dkp301 [DOI] [PubMed] [Google Scholar]
- 23.Berkhout J, Melchers MJ, van Mil CH, Seyedmousavi S, Lagarde CMC, Nichols WW, Mouton JW. 2013. Penetration of ceftazidime and avibactam into epithelial lining fluid (ELF) in thigh- and lung-infected mice, abstr A-1025. Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkhout J, Melchers MJ, van Mil CH, Seyedmousavi S, Lagarde CMC, Schuck V, Nichols WW, Mouton JW. 2013. Pharmacodynamics of ceftazidime and avibactam in a neutropenic mouse lung model, abstr A-1022. Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother [DOI] [PMC free article] [PubMed] [Google Scholar]