Abstract
Preventing relapses of Plasmodium vivax malaria through a radical cure depends on use of the 8-aminoquinoline primaquine, which is associated with safety and compliance issues. For future malaria eradication strategies, new, safer radical curative compounds that efficiently kill dormant liver stages (hypnozoites) will be essential. A new compound with potential radical cure activity was identified using a low-throughput assay of in vitro-cultured hypnozoite forms of Plasmodium cynomolgi (an excellent and accessible model for Plasmodium vivax). In this assay, primary rhesus hepatocytes are infected with P. cynomolgi sporozoites, and exoerythrocytic development is monitored in the presence of compounds. Liver stage cultures are fixed after 6 days and stained with anti-Hsp70 antibodies, and the relative proportions of small (hypnozoite) and large (schizont) forms relative to the untreated controls are determined. This assay was used to screen a series of 18 known antimalarials and 14 new non-8-aminoquinolines (preselected for blood and/or liver stage activity) in three-point 10-fold dilutions (0.1, 1, and 10 μM final concentrations). A novel compound, designated KAI407 showed an activity profile similar to that of primaquine (PQ), efficiently killing the earliest stages of the parasites that become either primary hepatic schizonts or hypnozoites (50% inhibitory concentration [IC50] for hypnozoites, KAI407, 0.69 μM, and PQ, 0.84 μM; for developing liver stages, KAI407, 0.64 μM, and PQ, 0.37 μM). When given as causal prophylaxis, a single oral dose of 100 mg/kg of body weight prevented blood stage parasitemia in mice. From these results, we conclude that KAI407 may represent a new compound class for P. vivax malaria prophylaxis and potentially a radical cure.
INTRODUCTION
Plasmodium vivax malaria causes between 70 million (1) and 390 million (2) clinical cases per year (for a review, see reference 3). Although vivax malaria is often referred to as “benign,” the disease is not mild, causing morbidity and mortality (4, 5). The parasite, which is dependent on the Duffy antigen for red blood cell (RBC) invasion, has been largely absent from West Africa, where the population is primarily Duffy negative. However, recent publications on P. vivax infections in Duffy-negative people (6–8) suggest that P. vivax is making its way into other areas of Africa, emphasizing the urgent need for new treatments.
One of the difficulties in fighting P. vivax stems from malaria relapses, caused by activation of dormant liver stage parasites, called hypnozoites (9). Currently, the only licensed drug for the radical cure of P. vivax malaria is primaquine (PQ), which is active against blood stage parasites (asexual and gametocyte stages) and liver stage parasites, including hypnozoites (10). However, PQ is contraindicated in glucose-6-phosphate dehydrogenase (G6PD)-deficient people, who can suffer from acute hemolytic anemia if treated with PQ (11). The CDC-recommended treatment schedule for PQ is 30 mg/day for 14 days (in non G6PD-deficient patients), limiting patient compliance, which could result in PQ resistance (12, 13).
Until now, the in vivo Plasmodium cynomolgi/rhesus monkey model has been the only model system exploited extensively to test new antimalarials for radical cure activity (14, 15, 53), as P. cynomolgi is one of the few parasite species that forms hypnozoites. Using this model, tafenoquine (16), elubaquine (also known as bulaquine or CDRI80/53) (17), and NPC1161 (18) were identified as antirelapse drugs, and these compounds are currently in preclinical development. However, all are 8-aminoquinolines, and all may have the same liabilities associated with PQ. Recently, Liu et al. (19) described a new non-8-aminoquinoline compound with radical cure properties in rhesus macaques, suggesting that new chemical structures may also act on hypnozoites.
The identification of new chemical entities with potential radical cure activity has been hampered by the lack of an in vitro assay to screen the parasite liver stages. We recently described the identification of imidazolopiperazines, which are active in vitro on both Plasmodium blood and liver stages (20). The in vitro liver stage assay used for screening is an image-based assay with HepG2-A16-CD81EGFP cells infected with Plasmodium yoelii sporozoites. The assay can identify compounds that that arrest or eliminate liver schizonts at various stages in development. Some of these have subsequently been shown to be effective causal prophylaxis agents in vivo. Although P. yoelii does not form hypnozoites, we surmise that compounds with activity on blood stages and liver schizonts are more likely to act on the dormant liver stages than randomly selected compounds or compounds with blood stage activity only. We used this assay to preselect liver stage-active compounds for evaluation on P. cynomolgi liver stages. The assay described in this paper assesses compound activity on liver stage cultures in vitro using P. cynomolgi parasites and is similar to assays previously described (21–24, 52) but is adapted to be more efficient for in vitro drug screening by reducing cultivation time and utilizing a fully automatic readout. In this adapted P. cynomolgi assay, the development of two distinct populations of parasites is monitored in the cultures: small forms that resemble hypnozoites both in appearance and in drug sensitivity profile and developing liver stage schizonts (21). Recently, Dembele et al. (25) have shown that the small forms can reactivate in vitro and can grow out to mature liver schizonts, confirming the hypothesis that these small forms are hypnozoites.
We evaluated the reproducibility of the P. cynomolgi in vitro liver stage assay and used it in a low-throughput screen of compounds with demonstrated antimalarial activity, identifying a new potent non-8-aminoquinoline compound that, similar to primaquine, efficiently kills the early developmental forms of all liver stages in vitro.
MATERIALS AND METHODS
Details of materials and methods are provided in the supplemental material.
Generation of P. cynomolgi sporozoites.
The Biomedical Primate Research Centre (BPRC) is an AAALAC-certified institute. All rhesus macaques (Macaca mulatta) used in this study were captive bred for research purposes and were housed at the BPRC facilities in compliance with the Dutch law on animal experiments, European directive 86/609/EEC, and with the Standard for Humane Care and Use of Laboratory Animals by Foreign institutions, identification number A5539-01, provided by the National Institutes of Health (NIH). Prior to the start of experiments, all protocols were approved by the local independent ethical committee, according to Dutch law. Rhesus macaques were infected with 1 × 106 P. cynomolgi M strain blood stage parasites and bled at peak parasitemia. Approximately 300 female Anopheles stephensi mosquitoes strain Sind-Kasur Nijmegen (Nijmegen University Medical Centre St. Radboud, Department of Medical Microbiology) were fed with this blood (26).
Isolation and maintenance of primary rhesus hepatocytes.
Rhesus monkey hepatocytes were isolated from liver lobes, as described by Guguen-Guillouzo et al. (27). Sporozoite infections were performed within 3 days after hepatocyte isolation.
Sporozoite infection of primary rhesus hepatocytes.
Sporozoite inoculation of primary rhesus hepatocytes was performed according to the methods of Mazier et al. (28) and Dembele et al. (21). Hepatocytes were washed with William's B medium before adding 50,000 (96-well plates) or 90,000 (LabTek chamber slides) sporozoites/well. Cultures were kept at 37°C in 5% CO2 with regular medium changes. To evaluate the development of P. cynomolgi liver stages, slides were fixed with cold methanol at the indicated time points.
Drug assays on liver stage parasites.
Compounds were diluted in William's B medium to 10, 1, and 0.1 μM. Atovaquone, primaquine, and medium only were used as controls. For 50% inhibitory concentration (IC50) determination 2-fold serial dilutions (10 to 0.04 μM final concentrations) were evaluated in duplicate. Statistical analyses were performed as described in the supplemental material.
Visualization of liver stages.
Intracellular P. cynomolgi malaria parasites were stained with the cross-reactive mouse polyclonal anti-Plasmodium falciparum Hsp70 antibody and secondarily stained with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (21). Alternatively, anti-P. cynomolgi Hsp70.1 (anti-PcyHsp70) and FITC-labeled goat anti-rabbit Ig antibodies were used. Anti-P. cynomolgi Hsp70 antisera were generated against part of the Hsp70 gene (amino acids 350 to 6860) (see the supplemental material) (29). The number of exoerythrocytic forms (EEFs) was determined for each well, using a Leica DMI6000 inverted microscope. In later assays, the number of intracellular parasites was determined using a high-content imaging system (Operetta; Perkin-Elmer). Validation of the Operetta analysis was performed by comparing manual-counting results of a number of plates with the outcome of Operetta analysis. The parasitophorous vacuolar membrane (PVM) was visualized using rabbit anti-P. cynomolgi Etramp serum generated against a mixture of two peptides [(C)-IISPNDELKKEGLD and (C)-IMKHRKKERKEMED, where "C" is cysteine, for coupling to column].
Criteria for distinguishing large and small EEF parasites.
Based on the limited information available about in vivo hypnozoite morphology, we used the following definitions (30, 31): hypnozoite, a small (maximum 7-μm-diameter), round intracellular parasite with 1 nucleus; developing parasite, an intracellular parasite, larger than 7 μm and round or irregular in shape, with more than 1 nucleus.
Sporozoite infection and atovaquone treatment of rhesus macaques.
Sporozoites were isolated according to the method of Mazier et al. (28) and resuspended in phosphate-buffered saline (PBS); 6.37 × 106 sporozoites were injected into the vena saphena of two rhesus macaques while under ketamine sedation. One of the animals was given atovaquone (Mepron; 150 mg/ml; 10 mg/kg of body weight) via gavage immediately after the sporozoite injection, followed by five daily oral dosings. The other animal was treated with chloroquine (7.5 mg/kg) when blood stage positive. Parasitemia was monitored via thin-film slides, which were fixed with methanol and stained with Giemsa stain.
In vivo causal prophylaxis mouse model.
Female ICR mice (8 or 9 weeks old) were randomized into groups of four mice. The animals in each group were administered, via oral gavage, 20 or 100 mg/kg KAI407, 2.5 mg/kg atovaquone (in 0.5% [wt/vol] methylcellulose and 0.5% [vol/vol] Tween 80 in distilled water), or vehicle only. Within 2 h of oral dosing, all mice were inoculated intravenously via the lateral tail vein with 50 × 103 Plasmodium berghei ANKA luciferase sporozoites (32) in 5% PBS. Blood smears were obtained on days 5 to 9, 12, 16, 23, and 30 postinoculation. Mice were observed twice daily for clinical signs of illness, morbidity, and mortality. Animals were humanely euthanized when blood parasitemia exceeded 2% or mice survived to day 30 (completion of the prophylaxis study).
RESULTS
P. cynomolgi-infected rhesus monkeys provide a reliable source for sporozoite production.
To overcome the bottleneck of accessing sufficient numbers of sporozoites from P. vivax for in vitro hypnozoite drug assays, we set up a P. cynomolgi sporozoite production platform. Due to the very reproducible blood stage infection pattern of rhesus monkeys (33), this proved to be a reliable system for infecting A. stephensi mosquitoes, yielding an average of 50,000 sporozoites per mosquito over 53 infections. A detailed overview of blood stage parasitemia, transmission, oocyst count, and sporozoite yield is given in Table S1 in the supplemental material. Parasitemia ranged between 0.02 and 3.6% on the first day of mosquito feeding (feed 1, usually 11 days after infection of a rhesus monkey), and all but four transmissions were successful. The oocyst count was positively correlated with blood stage parasitemia at the time of feed 1 (Spearman's rho, 0.732; P < 10−8) (see Fig. S1A in the supplemental material). Interestingly, the four lowest parasitemia counts were found in rhesus monkeys of Burmese origin, resulting in low oocyst counts, as well. Monkeys of Indian or Chinese origin yielded better results. The number of sporozoites was weakly positively correlated with oocyst counts from feed 1 (Spearman's rho, 0.398; P = 0.0062) (see Fig. S1B in the supplemental material). The positive correlation between parasitemia and oocyst number is also found for feed 2 (at day 12 after rhesus infection), albeit weaker (Spearman's rho, 0.37; P = 0.0092) (see Fig. S1C in the supplemental material). The correlation coefficient between sporozoite yield and oocyst number (see Fig. S1D in the supplemental material) for feed 2 is 0.51 (P = 0.0017). A subset of sporozoites from the 53 mosquito feeds was used for the assays described below.
P. cynomolgi liver stage parasites develop to full maturity in vitro, with small liver stage parasites resembling hypnozoites remaining.
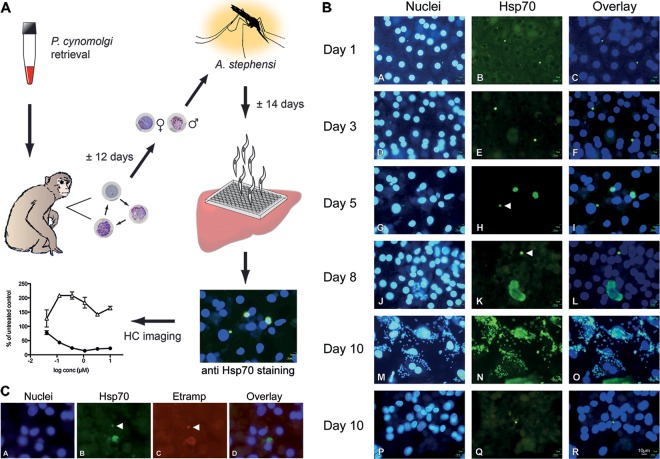
Freshly isolated primary rhesus hepatocytes from different monkeys were used for in vitro sporozoite infections (Fig. 1A). Hepatocytes were incubated with freshly dissected sporozoites, resulting in variable numbers of EEFs. Infected-cell numbers ranged from 0.09 to 3.0% (observations from 16 successful assays) (see Fig. S2 in the supplemental material). No clear correlation could be established between the backgrounds of hepatocyte donors and hepatocyte quality, but cell quality (visual evaluation involved cell density, granulation, and shape) played a role in sporozoite permissiveness, as EEF counts were reduced in visibly unhealthy cells. To follow EEF development over time, P. cynomolgi sporozoite-infected hepatocytes were fixed at several time points after infection and stained with anti-HSP70 antibodies (Fig. 1B). From day 5 postinfection (p.i.), two populations of parasites began to emerge, consisting of small forms that remained at the size of ∼3-day-old parasites (<7 μm) and a population of heterogeneously sized schizonts. At day 10 p.i., we could observe small forms that had remained small (Fig. 1B and P to R), as well as free merozoites (Fig. 1B and M to O). Incubation of RBCs with merosomes from the in vitro liver stage cultures did not result in P. cynomolgi-infected RBCs, probably because of the parasite's preference for reticulocytes. The small, uninucleate, persistent EEFs are likely to be hypnozoites, as was also noted by Hollingdale et al. (23), as well as Dembele et al. (21), who showed differential drug sensitivity of these forms. Further proof that the small forms we observed are indeed hypnozoites was recently provided by Dembele et al. (25), who showed that the small forms can reactivate in vitro. The small EEFs we therefore call hypnozoite forms can be positively stained for P. cynomolgi Etramp, a protein known to localize to the parasitophorous vacuole membrane (PVM) of EEFs (34) (Fig. 1C). This indicates that small EEFs are not the product of an aborted in vitro invasion but have successfully invaded a hepatocyte and formed a PVM.
FIG 1.
In vitro liver stage assay, intracellular development of P. cynomolgi, and PVM staining of small EEFs. (A) Schematic representation of the P. cynomolgi in vitro liver stage assay workflow. The experiment starts with the infection of a rhesus monkey with blood stage parasites from thawed stock. Mosquitoes are fed on the parasitized rhesus blood around peak parasitemia. Salivary gland sporozoites are harvested ∼14 days after the infected blood meal to infect primary rhesus hepatocytes. After sporozoite invasion, compounds are added in a concentration series, and the assay mixtures are incubated for 6 days. Parasites are visualized by staining Hsp70 with fluorescent antibodies. Small and large liver stage parasites are differentially counted using a high-content imager, resulting in a dose-response curve. (B) Sporozoite-infected primary rhesus hepatocytes were fixed 1, 3, 5, 8, or 10 days after inoculation with P. cynomolgi sporozoites. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (left column). Parasites were stained with anti-Hsp70 antibodies and fluorescein isothiocyanate (FITC)-labeled secondary antibodies (middle column). An overlay is shown in the right column. Within 24 h after infection, intracellular parasites are visible (images A to C), containing one nucleus (image A). The growth rates of all intracellular parasites appear to be similar until day 3 (images D to F). After 5 (images G to I) and 8 (images J to L) days of intracellular development, schizonts and hypnozoite forms (arrowhead) can be distinguished. Approximately 53% of the EEFs develop into mature schizonts, and 47% of the parasites remain small for the duration of the assay. Ten days after sporozoite inoculation, in the same well, free merozoites were observed (images M to O), as well as persistent small forms with a single nucleus (P to R). The developing liver stage parasites grow out to full maturity in a similar time frame, as reported in vivo (51). (C) Etramp staining of P. cynomolgi liver stage parasites. A liver schizont and a hypnozoite form (arrowheads) are seen in this field. Images: A, nuclei of P. cynomolgi-infected primary rhesus hepatocytes stained with DAPI; B, mouse anti-Hsp70/anti-mouse FITC staining of P. cynomolgi liver stage parasites; C, PVM stained with rabbit-anti-P. cynomolgi Etramp/anti-rabbit-tetramethyl rhodamine isocyanate (TRITC), showing the presence of a PVM in both parasites; D, merge of images A, B, and C. The presence of a PVM indicates that small EEFs are not the product of an aborted in vitro invasion but, rather, that the parasites have successfully invaded the hepatocyte.
A reproducible drug assay platform allows testing of antihypnozoite activity.
With a view to developing a higher-throughput drug assay than previously reported (21, 22), we amended the assay described by Dembele et al. (21) by exposing EEFs to compounds immediately after sporozoite invasion and incubating them until day 6, rather than from days 5 to 8. In addition, we implemented an automatic high-content imaging readout. To validate the new assay, we infected monolayers of primary rhesus hepatocytes with 50,000 P. cynomolgi sporozoites to which test compounds were added approximately 3 h after sporozoite inoculation. Six days after sporozoite infection, the plates were fixed and stained with anti-P. cynomolgi Hsp70 antibodies. Small and large EEFs were separately counted, based on size and number of parasite nuclei; 53% ± 12.7% (range, 28 to 74%) of the EEFs consisted of developing liver stage parasites, independent of the infection level of the cells, while 47% ± 12.7% (range, 26 to 72%) of the parasites remained small. Essentially, we observed drug sensitivity profiles identical to those with the day 5 to 8 treatment schedule described by Dembele et al. (21). More specifically, the small, uninucleate EEFs were found to be susceptible only to PQ and not to atovaquone (Fig. 2A) or pyrimethamine, similar to hypnozoites, and are different from developing EEFs, which are killed by all 3 drugs.
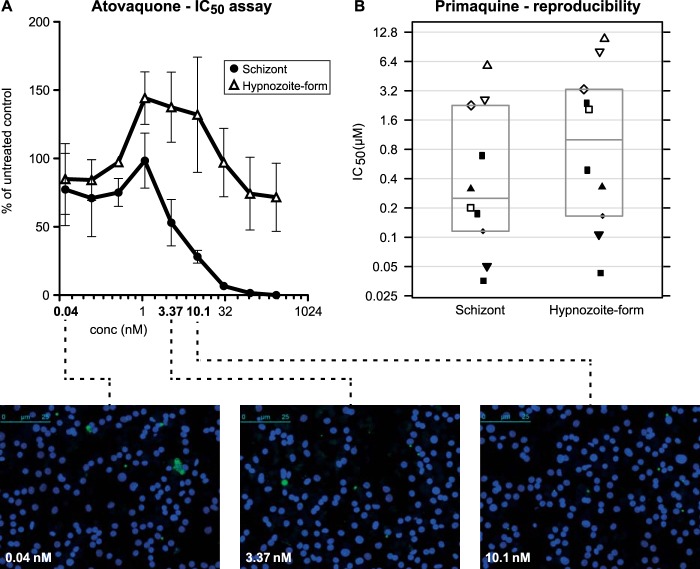
FIG 2.
P. cynomolgi liver stage drug assay validation. (A) Atovaquone sensitivity profile of large EEFs and hypnozoite forms. Three independent atovaquone IC50 assays were averaged and are shown here (± standard deviations [SD]) with representative images of assay wells. Developing EEFs are more sensitive to the compound than hypnozoite forms. At higher atovaquone concentrations, only small parasites are visible. (B) Reproducibility of the primaquine IC50 assay. Ten independent P. cynomolgi primaquine in vitro liver stage drug assays were performed. IC50s were determined for individual assays and plotted. Indicated are the mean values and the 95% confidence intervals. IC50s from the same experiment for schizonts and hypnozoite forms are indicated with identical symbols. Note that the IC50 for hypnozoite forms is always higher than for schizonts within a single assay.
Collectively, these data show the presence of hypnozoite forms, both by appearance and in the drug sensitivity profile. Further support for the presence of hypnozoites in culture comes from comparison to an in vivo drug test in a P. cynomolgi sporozoite-infected rhesus monkey. The monkey was treated with atovaquone for 6 days, starting immediately after sporozoite injection, similar to the in vitro drug assay. While an untreated control monkey developed primary blood stage parasitemia on day 9, the atovaquone-treated monkey developed blood stage parasitemia only on day 19, at the time of the first relapse of the untreated animal. Thus, in vitro, only hypnozoite forms remain after atovaquone treatment, and in vivo, such parasites reactivate and cause malaria relapse. Recently, these observations were further substantiated by Dembele et al., who showed in vitro reactivation of hypnozoite forms in atovaquone-treated P. cynomolgi cultures (25).
To assess the robustness of the in vitro drug assay, we performed 10 independent PQ IC50 determinations. Assays were performed with independent hepatocyte and sporozoite batches using independent PQ dilutions. The mean IC50 for PQ was 0.374 μM (range, 0.036 to 5.780 μM) for developing liver stage parasites and 0.839 μM (range, 0.043 to 10.924 μM) for hypnozoite forms (Fig. 2B). We attributed the significant assay-to-assay variation of PQ IC50s to variability of the primary hepatocytes used in the assay. PQ is converted to an active metabolite and thus may be affected by hepatocyte viability and the presence of active cytochrome P450.
Evaluators blinded to reference compounds with known anti-blood stage and/or anti-liver stage activity evaluated them in a three-point, 10-fold-dilution fingerprinting series. The data are largely in agreement with what is known of their antimalarial activities (Table 1). Anti-blood stage compounds, like chloroquine and artesunate, did not show any significant anti-EEF activity. On the other hand, 8-aminoquinolines, known to prevent relapses in vivo, were all active against hypnozoite forms and schizonts (Table 1). They included PQ; NPC1161B (35); tafenoquine, currently in clinical trials (36); and bulaquine, also currently in clinical trials (37–41). Atovaquone was the only compound tested that specifically inhibited developing EEFs to a high degree without affecting hypnozoite forms. Cycloheximide, a protein biosynthesis inhibitor, showed dose-dependent inhibition of developing EEFs, as well as a limited effect on hypnozoite forms, but only at the highest dose tested. This suggests that low-level protein biosynthesis may be ongoing in the small, persistent liver forms. However, at the highest cycloheximide dose, hepatocyte viability may have been compromised due to its general toxicity, even though we did not observe significant hepatocyte loss based on nucleus counts. This may also have contributed to reduced numbers of hypnozoite forms. All reference drugs displayed the expected activity pattern on liver stages, suggesting a strong predictive value of this in vitro liver stage/hypnozoite form drug assay.
TABLE 1.
Reference compounds evaluated in three-point 10-fold dilution series on P. cynomolgi liver stages
| Compound name | Activity againsta: |
|
|---|---|---|
| Hypnozoite forms | Schizonts | |
| Amodiaquineb | − | − |
| Artesunateb | − | − |
| Dihydroartimisinb | − | − |
| Lumefantrineb | − | − |
| Piperaquineb | − | − |
| Chloroquineb | − | ± |
| Doxycyclineb | ± | ± |
| Mefloquineb | ± | + |
| Proguanilb | ± | + |
| Atovaquonec | − | +++ |
| Cycloheximided | + | ++ |
| Primaquinee | +++ | +++ |
| NPC-1161Bf | +++ | +++ |
| Tafenoquinef | ++ | +++ |
| Bulaquinef | +++ | +++ |
Activities of compounds (reduction of parasite counts at 10 μM) are represented as follows: −, 0 to 10%; ±, 10 to 20%; +, 20 to 70%; ++, 70 to 90%; +++, >90%. Of the nonaminoquinoline antimalarials, only atovaquone reduced the number of developing liver stage parasites by more than 90%. The nonspecific protein synthesis inhibitor cycloheximide had a minor effect on the number of small and large liver stage parasites at the highest concentration tested. All 8-aminoquinolines tested were active in the P. cynomolgi in vitro assay, efficiently killing both schizonts and hypnozoite forms.
Reference compound had known blood stage activity.
Reference compound had known blood and liver stage activity.
Reference compound is a known protein synthesis inhibitor.
Reference compound is an 8-aminoquinoline, used as a radical cure, active against gametocytes and liver stages (including hypnozoites).
Reference compound is an 8-aminoquinoline.
Small-scale screening of malaria box compounds (preselected compounds with bloodstage activity) identified a new potent anti-liver stage compound class.
We screened 14 selected compounds that previously showed activity against the malaria blood and/or liver stage (42). These compounds were primary hits or had been subject to some preliminary lead optimization. The selection was based on diversity of chemical structure and opportunities to further modify the compounds. Compounds were initially screened in three-point 10-fold dilutions (see Table S2 in the supplemental material). Interestingly, one of the compounds active against developing liver stages, QN254, is a known dihydrofolate reductase (DHFR) inhibitor (11). The fact that it was not active against hypnozoite forms highlights the assay's expected ability to distinguish compounds that are active only against large EEFs.
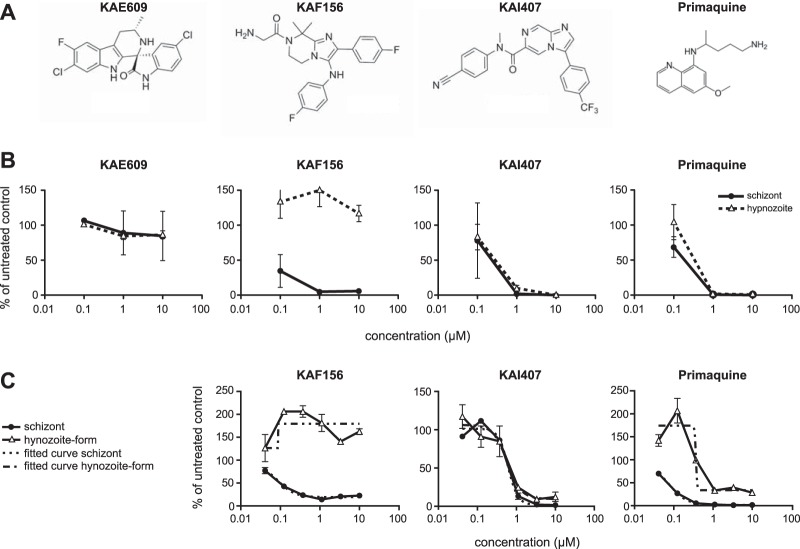
We focused on three different compounds, evaluating their activities against P. cynomolgi hypnozoite forms. These compounds were the spiroindolone KAE609, which displays subnanomolar activity against P. falciparum and P. vivax blood stages (43); the imidazolopiperazine KAF156, an optimized clinical candidate that has low nanomolar activity against blood and liver stages (44); and the imidazopyrazine KAI407 (Fig. 3A). KAI407 was derived from a lead compound (imidazolopyridine, compound 1 in Table 2) identified in a high-throughput blood stage screen (42), which also showed weak activity in an in vitro assay using P. yoelii sporozoites and HepG2 cells (20). Introduction of a methyl group to the amide nitrogen produced compound 2, which improved blood stage activity nearly 100-fold but lost liver stage activity. By modification of both aryl appendages, we obtained compound 3, with blood and liver stage activity. Chemical modifications of the 6,5-heterocyclic core provided an activity breakthrough. Introducing a nitrogen to the 5 position of the bicyclic core generated the imidazopyrazine KAI407 (compound 4), with good potency (IC50 < 100 nM) on both blood and liver stage parasites (Table 2).
FIG 3.
P. cynomolgi drug assays with selected malaria box compounds. (A) Structure formulas of KAE609, KAF156, KAI407, and primaquine. (B) Three-point 10-fold dilution series (0.1, 1, and 10 μM) on P. cynomolgi liver stage cultures. The percentage of untreated control is shown as a function of the compound concentration. Differential counting of schizonts and hypnozoite forms was performed based on size and number of parasite nuclei. The results of one representative assay are shown; assays were performed at least twice. KAE609 was not active against liver stage parasites and was therefore not taken forward for IC50 determination. (C) IC50 determination for KAF156, KAI407, and primaquine. Drugs were tested in a 2-fold dilution series. The percentage of untreated control is shown as a function of the compound concentration. Differential counting of schizonts and hypnozoite forms was performed based on size and number of parasite nuclei. IC50s were calculated based on fitted curves (nonlinear fit). The results of one representative assay are shown, and at least two independent assays were performed. The activity of KAF156 is limited to schizonts, while KAI407 and primaquine also kill hypnozoite forms. The error bars indicate standard deviations.
TABLE 2.
Lead optimization toward KAI407a
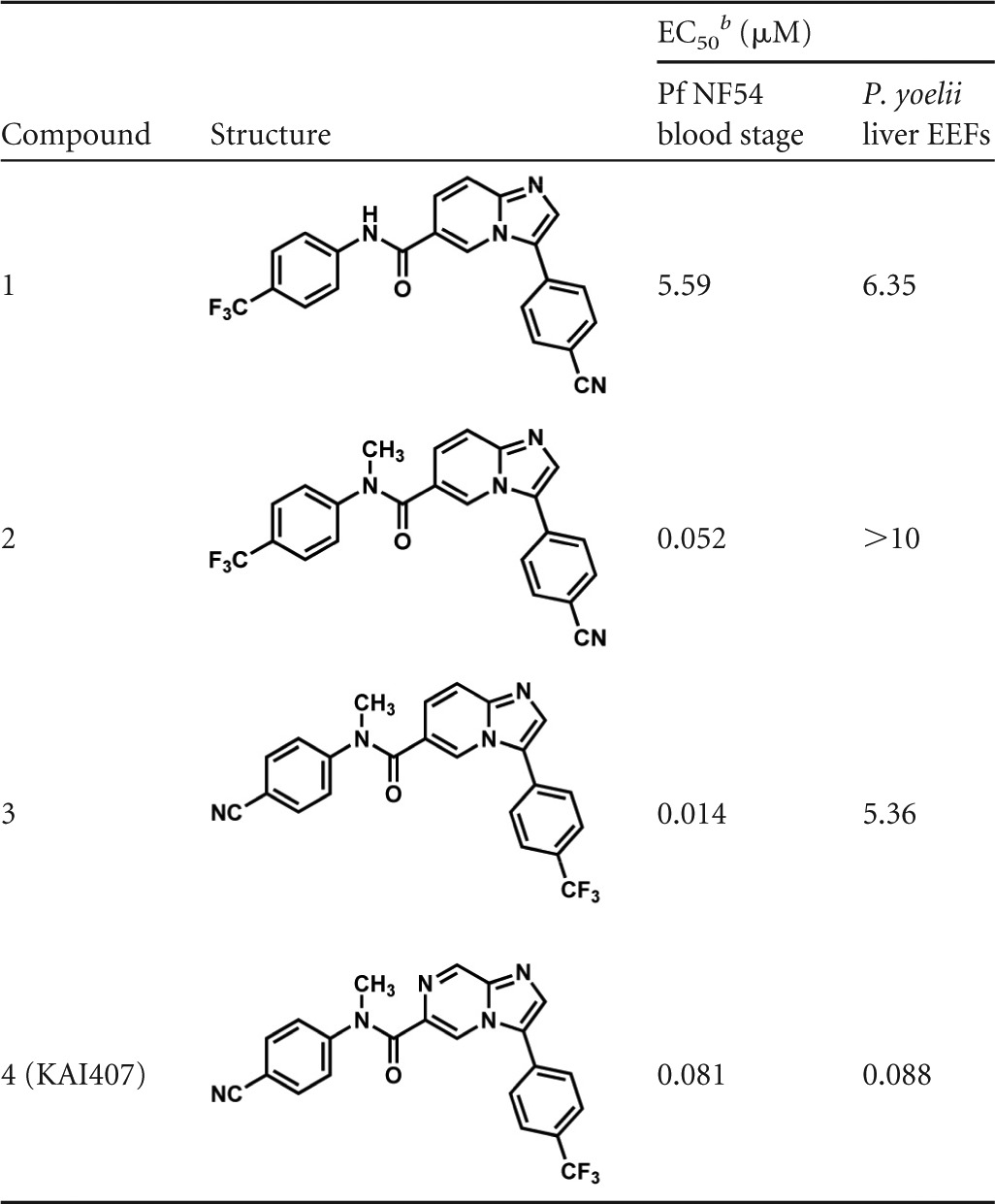
KAI407 was derived from lead compound 1, identified in a high-throughput blood stage screen, which also showed weak activity in an in vitro assay using P. yoelii sporozoites and HepG2 cells. Introduction of a methyl group to the amide nitrogen provided compound 2 and improved blood stage activity nearly 100-fold, but lost liver stage activity. By modification of both aryl appendages, we obtained compound 3, with blood and liver stage activity. Chemical modifications of the 6,5-heterocyclic core by introducing a nitrogen to the 5 position of the bicyclic core generated the imidazolopyrazine KAI407 (compound 4) with good potency (IC50 < 100 nM) on both blood and liver stage parasites.
EC50, 50% effective concentration.
To determine if liver stage activity on the rodent parasite would predict activity against P. cynomolgi liver stages, a three-point 10-fold dilution series was performed with KAE609, KAF156, and KAI407 (Fig. 3B). Similar to what was previously reported for rodent malaria liver stages (20), KAE609 showed no activity against either P. cynomolgi developing liver stages or hypnozoite forms. KAF156 was active only against developing liver stages, in agreement with the known causal prophylactic activity of members of these series, such as GNF179 (16). In contrast, KAI407 displayed activity against both P. cynomolgi developing liver stages and hypnozoite forms, similar to PQ. Subsequent IC50 determinations for the two liver stage-active compounds showed IC50s of 0.09 μM for KAF156 and 0.64 μM (0.86 to 0.42 μM) for KAI407 on developing liver stages and >10 μM (no dose response) for KAF156 and 0.69 μM (0.84 to 0.55 μM) for KAI407 on hypnozoite forms (80 nM against P. yoelii in vitro EEFs). This is slightly better than PQ, for which IC50s in the same assays were 1.3 μM (0.29 to 2.31 μM) for developing liver stages and 1.8 μM (3.27 to 0.33 μM) for hypnozoite forms (Fig. 3C) (7). Overall, based on activity testing of this limited series, there seems to be concordance between the rodent malaria and P. cynomolgi liver stage activities, although activity on hypnozoite forms cannot be predicted.
In vivo causal prophylaxis mouse model.
KAI407 as a lead compound needs further optimization before in vivo efficacy evaluation can be done in the P. cynomolgi rhesus relapse model. We thus could not yet evaluate the prophylactic and radical curative activities of this compound in the rhesus model. Based on its ability to eliminate developing liver stages, analogous to atovaquone and KAF156, we therefore further tested whether KAI407 could prevent the establishment of blood stage parasitemia after sporozoite inoculation in a causal prophylactic rodent model. Four groups of four mice each were treated by oral gavage with 20 or 100 mg/kg KAI407, 2.5 mg/kg atovaquone, or vehicle only and then infected with P. berghei ANKA luciferase sporozoites (32) within 2 h of dosing. The vehicle-only group became blood stage positive 5.75 ± 0.46 days p.i. The low-dose KAI407-treated group (20 mg/kg) became blood stage positive at day 6.5 ± 0.53 p.i., and neither the atovaquone-treated mice nor the 100-mg/kg KAI407-treated mice became blood stage positive over 30 days of monitoring, achieving 100% protection in the two groups (Table 3). Although these data do not speak to its potential radical cure activity, they demonstrate that KAI407 is active against malaria tissue stages in vivo.
TABLE 3.
Causal prophylaxis activity of KAI407 in an in vivo rodent malaria model using P. berghei
| Compound | Dosea (mg/kg) | Survival (%) | Prepatent periodb (days ± SD) |
|---|---|---|---|
| Vehicle (untreated) | 0 | 5.75 ± 0.46 | |
| Atovaquone | 2.5 | 100 | NAd |
| KAI407c | 20 | 0 | 6.5 ± 0.53 |
| KAI407c | 100 | 100 | NAd |
A single dose was administered via oral gavage 1–2 h prior to P. berghei sporozoite infection.
Average number of days before blood-stage parasitemia was detected by microscopy.
Formulated in 0.5% (wt/vol) methylcellulose, 0.1% (vol/vol) Tween 80.
NA, not applicable; 100% prophylaxis achieved.
DISCUSSION
The quiescent nature of malaria hypnozoites in the livers of P. vivax- or Plasmodium ovale-infected individuals represents a significant challenge for malaria eradication. Hypnozoites are found only in these two human malaria parasites and in a few nonhuman primate malaria parasites, one of which is P. cynomolgi. Primaquine remains the only treatment option for P. vivax patients but has potentially severe side effects and contraindications (for a recent review, see reference 45). Bolstering the malaria pipeline with new chemical entities that overcome the liabilities associated with primaquine is now a top priority. Historically, P. cynomolgi-infected monkeys were used for screening compounds to detect hypnozoitocidal activity. A large screening effort (14) in which hundreds of monkeys were treated with experimental compounds only confirmed the potency of 8-aminoquinolines as radical cure compounds. More recently, a compound from a different chemical class, a 2-guanidino-4-oxoimidazoline derivative (19), was found to cure relapsing P. cynomolgi infections in rhesus monkeys. In vivo large-scale screens are currently ethically and financially restricted. Moreover, they are extremely low throughput and time-consuming, which explains the dearth of new chemical entities with proven hypnozoitocidal activity. The development and application of an in vitro assay that can assess activity against hypnozoite forms can bridge the current gap between blood stage (P. falciparum) and liver schizont (P. yoelii) active compounds and the P. cynomolgi-infected rhesus monkey model. We chose P. cynomolgi rather than P. vivax as a way to reliably access sufficient numbers of sporozoites from a single parasite strain, which is currently not possible for P. vivax. Our data show that mosquito feeding on P. cynomolgi-infected rhesus monkey blood provides a reliable source of sufficient numbers of sporozoites for in vitro assays.
Time course immunofluorescence assay images of P. cynomolgi-infected hepatocytes show uniform EEF development (based on size and shape) until day 3 and two EEF populations emerging after that, with ∼53% of schizonts progressing to merozoites by day 10 and ∼47% of EEFs remaining as small uninucleate forms, essentially as previously described for in vivo-derived EEFs (46). The persisting small EEFs share characteristics of hypnozoites, including the morphologies derived from the limited description of in vivo-derived hypnozoites (30, 31) and their atovaquone and primaquine drug sensitivity profiles (21). Further evidence that these in vitro-cultured small forms could indeed be hypnozoites comes from our comparative in vivo drug treatment experiment, in which we observed no primary blood stages in an atovaquone-treated animal, which relapsed simultaneously with an untreated animal. In vitro, liver schizonts were completely absent and only small hypnozoite forms remained after atovaquone treatment. Dembele (25) showed in long-term in vitro cultures that these hypnozoite forms spontaneously started to develop into liver stage schizonts after the atovaquone treatment was stopped. Collectively, these data link the in vitro-cultured hypnozoite forms to the appearance of relapse in vivo following initial atovaquone treatment of infection and thus strengthen the case for in vitro-cultured hypnozoites. Additionally, despite the small sample size of in vivo prophylactic atovaquone-treated animals, the outcome corroborates occasional clinical observations of P. vivax and P. ovale infections in travelers, despite atovaquone/proguanil prophylaxis (47). When taken together with our in vitro data, this suggests that atovaquone is effective against the early developing liver stage forms destined to become primary hepatic schizonts but ineffective against the forms destined to become latent hypnozoites. Thus, when prophylaxis is discontinued, relapses are likely to occur.
Adaptations to the original in vitro model (21) were necessary in order to allow larger-scale in vitro compound screening. The implemented adaptations were (i) culture times reduced from 8 to 6 days to increase throughput; (ii) exposure to compounds continuously from day 0 to day 6 rather than from days 5 to 8 (here, we found that shorter exposure to primaquine reduced assay sensitivity, so we did not decrease the assay time any further); (iii) replacement of manual readout by automated high-content imaging, which dramatically increased assay throughput. We used the adapted assay to screen (blinded) a number of reference compounds and identified a number of active compounds, which were all 8-aminoquinolines known to prevent in vivo relapses, suggesting good predictive value of the assay for activity against day 0 to 6 hypnozoites.
In an initial (blinded) small-scale screen of selected antimalarial compounds (some of which had already been subjected to lead optimization), we confirmed the reported activities on rodent malaria liver stages in P. cynomolgi liver stages: the spiroindolone KAE609 showed no activity against P. cynomolgi liver stages, while the imidazolopiperazine KAF156 was active against P. cynomolgi liver stages (20, 48). Like atovaquone, KAF156 affected only developing liver stages in a dose-dependent manner and had no activity on hypnozoite forms.
With the modified drug treatment schedule, starting immediately after invasion, it became possible to identify different drug sensitivities of the liver stage parasites. We believe this difference reflects commitment of the sporozoites to become either a hypnozoite or a proliferative liver stage schizont at a very early time point. The very early hypnozoite is relatively insensitive to all drugs tested, showing reduced sensitivity to primaquine in comparison to developing parasites. This includes resistance to atovaquone, a licensed causal prophylaxis against P. falciparum (49). In contrast, the developing parasite population is sensitive, at similar concentrations, to anti-liver stage antimalarials (atovaquone and KAF156) that block P. yoelii from growing in hepatocytes. This suggests that commitment to the hypnozoite developmental pathway occurs early on, before, during, or immediately after infection of the hepatocyte. Despite this early commitment, parasites destined for the two pathways have indistinguishable rates of growth up to day 3. After 3 days of development, clear morphological differentiation of hypnozoites and developing liver stage parasites becomes evident. Thus, parasites committed to a period of dormancy require a period of growth subsequent to invasion before entering latency.
Whether, immediately subsequent to day 3, parasites have already entered a dormant state that can be reactivated is as yet unclear. In our hands and with our P. cynomolgi strain, the earliest relapse observed in vivo (in an animal that was treated with atovaquone from days 0 to 6 p.i. to kill all the primary liver stages) with this P. cynomolgi strain occurred 19 days p.i. In addition, another animal treated with chloroquine from days 14 to 16 to cure the primary blood stage parasitemia relapsed by day 21 p.i. Chloroquine treatment may have delayed the appearance of blood stage parasites in this animal. Assuming that (i) blood stage parasites detected at day 19 are caused by merozoites directly emerging from the liver (a reasonable assumption, because the animals were infected with over 6 million sporozoites, leading to a large liver load) and (ii) reactivated hypnozoites need 6 further days of development before merozoites are released, reactivation of hypnozoites in vivo could start around day 13 p.i. This suggests the possibility that, for a period between days 3 and 13 post-hepatocyte infection, further hypnozoite development may be required until a reactivation-competent parasite is established. Under our current assay format, both of the early stages (days 0 to 3 of treatment) that are committed to hypnozoite development but are indistinguishable from nonlatent forms and those that have engaged in a morphologically distinguishable hypnozoite-specific developmental pathway (days 4 to 6 of treatment) are analyzed for drug sensitivity. However, further research is needed to better understand hypnozoite development and to determine whether there is a period of development during which the hypnozoite-committed lineage cannot be activated.
In a focused hit-to-lead effort, we identified a series of blood stage-active compounds that were optimized for activity against rodent liver stages. For one compound, KAI407, the liver stage activity against rodent parasites translated into activity on both P. cynomolgi developing EEFs and hypnozoite forms. This compound displayed an activity profile identical to that of primaquine in the same assay. Interestingly KAI407 activity was less variable between assays than primaquine, suggesting a different metabolic pathway or mode of action, as conversion to an active metabolite does not seem to be required for activity (KAI407 is stable in the presence of liver microsomes). The 100% protection of mice by a single oral dose of 100 mg/kg KAI407 indicates that the compound is active as causal prophylaxis against P. berghei,and is an interesting candidate for causal prophylaxis against P. vivax-type parasites.
The exciting potential of the in vitro P. cynomolgi liver stage drug assay, as well as the newly discovered compound, is clear. Like primaquine, the newly discovered compound eliminated the early hepatic stages destined to become the latent hypnozoites. However, further optimization of the P. cynomolgi liver stage assay and miniaturization to increase the throughput are required before screens of larger, more chemically diverse libraries can be achieved. Improved hypnozoite cultures will permit more detailed study of hypnozoite biology, including work to identify reactivation triggers (50). More detailed investigation of the antirelapse properties of the KAI407 series of compounds as a critical step to determining their clinical potential will require assessment in vivo. KAI407 is currently the focus of a lead optimization effort to provide compounds suitable for testing in P. cynomolgi-infected rhesus monkeys for prophylactic and antirelapse activities, the gold standard for identifying antirelapse compounds. The outcome of such an in vivo experiment will be an important next step in the further validation of the in vitro P. cynomolgi liver stage drug assay and the discovery and further development of new antirelapse drugs for clinical use.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dominique Mazier and Jean-François Franetich of the UPMC, Paris, France, for their help with setting up P. cynomolgi in vitro liver stage cultures. We thank Henk van Westbroek for help with figures and Bart Faber and Marjolein van der Eijk for generating recombinant P. cynomolgi Hsp70 protein. We thank the staff of the BPRC Animal Science Department for skillful technical assistance with P. cynomolgi infections of rhesus macaques. We are grateful to the mosquito-breeding facilities in Nijmegen, The Netherlands, for provision of numerous batches of A. stephensi mosquitoes.
C.W.M., K.K., R.J.G., A.N., B.K.S.Y., T.T.D., E.A.W., and J.R. own Novartis stock and declare a competing financial interest.
This work was supported by a grant from the Medicines for Malaria Venture, a translational research grant (WT078285) from the Wellcome Trust, and funding from the European Community's Seventh Framework Programme (FP7/2007-2013), the EVIMALAR Network of Excellence under grant agreement 242095, and the EC-FP7 project MALSIG (contract number 223044).
Footnotes
Published ahead of print 23 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01927-13.
REFERENCES
- 1.Mendis K, Sina BJ, Marchesini P, Carter R. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97–106 [DOI] [PubMed] [Google Scholar]
- 2.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. 2004. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect. Dis. 4:327–336. 10.1016/S1473-3099(04)01043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77:79–87 [PMC free article] [PubMed] [Google Scholar]
- 4.Baird JK. 2013. Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin. Microbiol. Rev. 26:36–57. 10.1128/CMR.00074-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. 2012. Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv. Parasitol. 80:151–201. 10.1016/B978-0-12-397900-1.00003-7 [DOI] [PubMed] [Google Scholar]
- 6.Menard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, Thonier V, Carod JF, Domarle O, Colin Y, Bertrand O, Picot J, King CL, Grimberg BT, Mercereau-Puijalon O, Zimmerman PA. 2010. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc. Natl. Acad. Sci. U. S. A. 107:5967–5971. 10.1073/pnas.0912496107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendes C, Dias F, Figueiredo J, Mora VG, Cano J, de Sousa B, do Rosario VE, Benito A, Berzosa P, Arez AP. 2011. Duffy negative antigen is no longer a barrier to Plasmodium vivax: molecular evidences from the African west coast (Angola and Equatorial Guinea). PLoS Negl. Trop. Dis. 5:e1192. 10.1371/journal.pntd.0001192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woldearegai TG, Kremsner PG, Kun JF, Mordmuller B. 2013. Plasmodium vivax malaria in Duffy-negative individuals from Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 107:328–331. 10.1093/trstmh/trt016 [DOI] [PubMed] [Google Scholar]
- 9.Cogswell FB. 1992. The hypnozoite and relapse in primate malaria. Clin. Microbiol. Rev. 5:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edgcomb JH, Arnold J, Yount EH, Jr, Alving AS, Eichelberger L, Jeffery GM, Eyles D, Young MD. 1950. Primaquine, SN 13272, a new curative agent in vivax malaria; a preliminary report. J. Natl. Malar. Soc. 9:285–292 [PubMed] [Google Scholar]
- 11.Alving AS, Carson PE, Flanagan CL, Ickes CE. 1956. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science 124:484–485 [DOI] [PubMed] [Google Scholar]
- 12.Collins WE, Jeffery GM. 1996. Primaquine resistance in Plasmodium vivax. Am. J. Trop. Med. Hyg. 55:243–249 [DOI] [PubMed] [Google Scholar]
- 13.Baird JK. 2009. Resistance to therapies for infection by Plasmodium vivax. Clin. Microbiol. Rev. 22:508–534. 10.1128/CMR.00008-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt LH. 1983. Appraisals of compounds of diverse chemical classes for capacities to cure infections with sporozoites of Plasmodium cynomolgi. Am. J. Trop. Med. Hyg. 32:231–257 [DOI] [PubMed] [Google Scholar]
- 15.Sodeman TM, Contacos PG, Collins WE, Smith CS, Jumper JR. 1972. Studies on the prophylactic and radical curative activity of RC-12 against Plasmodium cynomolgi in Macaca mulatta. Bull. World Health Organ. 47:425–428 [PMC free article] [PubMed] [Google Scholar]
- 16.Eyles DE, Coatney GR. 1962. Effect of certain drugs on exoerythrocytic parasites of Plasmodium cynomolgi. Am. J. Trop. Med. Hyg. 11:175–185 [DOI] [PubMed] [Google Scholar]
- 17.Dutta GP, Puri SK, Bhaduri AP, Seth M. 1989. Radical curative activity of a new 8-aminoquinoline derivative (CDRI 80/53) against Plasmodium cynomolgi B in monkeys. Am. J. Trop. Med. Hyg. 41:635–637 [DOI] [PubMed] [Google Scholar]
- 18.LaMontagne MP, Blumbergs P, Strube RE. 1982. Antimalarials. 14.5-(Aryloxy)-4-methylprimaquine analogues. A highly effective series of blood and tissue schizonticidal agents. J. Med. Chem. 25:1094–1097 [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Wang X, Li Q, Kozar MP, Melendez V, O'Neil MT, Lin AJ. 2011. Synthesis and antimalarial activity of 2-guanidino-4-oxoimidazoline derivatives. J. Med. Chem. 54:4523–4535. 10.1021/jm200111g [DOI] [PubMed] [Google Scholar]
- 20.Meister S, Plouffe DM, Kuhen KL, Bonamy GM, Wu T, Barnes SW, Bopp SE, Borboa R, Bright AT, Che J, Cohen S, Dharia NV, Gagaring K, Gettayacamin M, Gordon P, Groessl T, Kato N, Lee MC, McNamara CW, Fidock DA, Nagle A, Nam TG, Richmond W, Roland J, Rottmann M, Zhou B, Froissard P, Glynne RJ, Mazier D, Sattabongkot J, Schultz PG, Tuntland T, Walker JR, Zhou Y, Chatterjee A, Diagana TT, Winzeler EA. 2011. Imaging of Plasmodium liver stages to drive next-generation antimalarial drug discovery. Science 334:1372–1377. 10.1126/science.1211936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dembele L, Gego A, Zeeman AM, Franetich JF, Silvie O, Rametti A, Le Grand R, Dereuddre-Bosquet N, Sauerwein R, van Gemert GJ, Vaillant JC, Thomas AW, Snounou G, Kocken CH, Mazier D. 2011. Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS One 6:e18162. 10.1371/journal.pone.0018162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisk TL, Millet P, Collins WE, Nguyen-Dinh P. 1989. In vitro activity of antimalarial compounds on the exoerythrocytic stages of Plasmodium cynomolgi and P. knowlesi. Am. J. Trop. Med. Hyg. 40:235–239 [DOI] [PubMed] [Google Scholar]
- 23.Hollingdale MR, Collins WE, Campbell CC, Schwartz AL. 1985. In vitro culture of two populations (dividing and nondividing) of exoerythrocytic parasites of Plasmodium vivax. Am. J. Trop. Med. Hyg. 34:216–222 [DOI] [PubMed] [Google Scholar]
- 24.Millet P, Collins WE, Monken CE, Brown BG. 1990. In vitro maturation of the exoerythrocytic stage of Plasmodium knowlesi observed under phase contrast microscopy. J. Parasitol. 76:923–925. 10.2307/3282816 [DOI] [PubMed] [Google Scholar]
- 25.Dembele L, Franetich JF, Lorthiois A, Gego A, Zeeman AM, Kocken CHM, LeGrand R, Dereuddre-Bosquet N, Gemert GJ, von Sauerwein R, Vaillant JC, Hannoun L, Fuchter MJ, Diagana T, Malmquist NA, Scherf A, Snounou G, Mazier D. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. A potential role for the epigenetic control of quiescence. Nat. Med., in press [DOI] [PubMed] [Google Scholar]
- 26.Ponnudurai T, Lensen AH, Van Gemert GJ, Bensink MP, Bolmer M, Meuwissen JH. 1989. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98:165–173. 10.1017/S0031182000062065 [DOI] [PubMed] [Google Scholar]
- 27.Guguen-Guillouzo C, Campion JP, Brissot P, Glaise D, Launois B, Bourel M, Guillouzo A. 1982. High yield preparation of isolated human adult hepatocytes by enzymatic perfusion of the liver. Cell Biol. Int. Rep. 6:625–628. 10.1016/0309-1651(82)90187-4 [DOI] [PubMed] [Google Scholar]
- 28.Mazier D, Beaudoin RL, Mellouk S, Druilhe P, Texier B, Trosper J, Miltgen F, Landau I, Paul C, Brandicourt O, et al. 1985. Complete development of hepatic stages of Plasmodium falciparum in vitro. Science 227:440-442. 10.1126/science.3880923 [DOI] [PubMed] [Google Scholar]
- 29.Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. 1994. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol. Res. 80:16–21. 10.1007/BF00932618 [DOI] [PubMed] [Google Scholar]
- 30.Krotoski WA, Krotoski DM, Garnham PCC, Bray RS, Killick-Kendrick R, Draper CC, Targett GAT, Guy MW. 1980. Relapses in primate malaria: discovery of two popualtions of exoerythrocytic stages. Br. Med. J. 280:153–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krotoski WA, Garnham PC, Bray RS, Krotoski DM, Killick-Kendrick R, Draper CC, Targett GA, Guy MW. 1982. Observations on early and late post-sporozoite tissue stages in primate malaria. I. Discovery of a new latent form of Plasmodium cynomolgi (the hypnozoite), and failure to detect hepatic forms within the first 24 hours after infection. Am. J. Trop. Med. Hyg. 31:24–35 [PubMed] [Google Scholar]
- 32.Ploemen IH, Prudencio M, Douradinha BG, Ramesar J, Fonager J, van Gemert GJ, Luty AJ, Hermsen CC, Sauerwein RW, Baptista FG, Mota MM, Waters AP, Que I, Lowik CW, Khan SM, Janse CJ, Franke-Fayard BM. 2009. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One 4:e7881. 10.1371/journal.pone.0007881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocken CH, Remarque EJ, Dubbeld MA, Wein S, van der Wel A, Verburgh RJ, Vial HJ, Thomas AW. 2009. Statistical model to evaluate in vivo activities of antimalarial drugs in a Plasmodium cynomolgi-macaque model for Plasmodium vivax malaria. Antimicrob. Agents Chemother. 53:421–427. 10.1128/AAC.00576-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackellar DC, O'Neill MT, Aly AS, Sacci JB, Jr, Cowman AF, Kappe SH. 2010. Plasmodium falciparum PF10_0164 (ETRAMP10.3) is an essential parasitophorous vacuole and exported protein in blood stages. Eukaryot. Cell 9:784–794. 10.1128/EC.00336-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nanayakkara NP, Ager AL, Jr, Bartlett MS, Yardley V, Croft SL, Khan IA, McChesney JD, Walker LA. 2008. Antiparasitic activities and toxicities of individual enantiomers of the 8-aminoquinoline 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline succinate. Antimicrob. Agents Chemother. 52:2130–2137. 10.1128/AAC.00645-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nasveld PE, Edstein MD, Reid M, Brennan L, Harris IE, Kitchener SJ, Leggat PA, Pickford P, Kerr C, Ohrt C, Prescott W. 2010. Randomized, double-blind study of the safety, tolerability, and efficacy of tafenoquine versus mefloquine for malaria prophylaxis in nonimmune subjects. Antimicrob. Agents Chemother. 54:792–798. 10.1128/AAC.00354-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajgor DD, Gogtay NJ, Kadam VS, Kamtekar KD, Dalvi SS, Chogle AR, Aigal U, Bichile LS, Kain KC, Kshirsagar NA. 2003. Efficacy of a 14-day primaquine regimen in preventing relapses in patients with Plasmodium vivax malaria in Mumbai, India. Trans. R. Soc. Trop. Med. Hyg. 97:438–440. 10.1016/S0035-9203(03)90082-4 [DOI] [PubMed] [Google Scholar]
- 38.Gogtay NJ, Kamtekar KD, Dalvi SS, Chogle AR, Aigal U, Kshirsagar NA. 2004. Preliminary report of the evaluation of the gametocytocidal action of bulaquine, in adult patients with acute, Plasmodium falciparum malaria. Ann. Trop. Med. Parasitol. 98:525–528. 10.1179/000349804225003541 [DOI] [PubMed] [Google Scholar]
- 39.Adak T, Valecha N, Sharma VP. 2001. Plasmodium vivax polymorphism in a clinical drug trial. Clin. Diagn. Lab. Immunol. 8:891–894. 10.1128/CDLI.8.5.891-894.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gogtay NJ, Kamtekar KD, Dalvi SS, Mehta SS, Chogle AR, Aigal U, Kshirsagar NA. 2006. A randomized, parallel study of the safety and efficacy of 45 mg primaquine versus 75 mg bulaquine as gametocytocidal agents in adults with blood schizonticide-responsive uncomplicated falciparum malaria [ISCRTN50134587]. BMC Infect. Dis. 6:16. 10.1186/1471-2334-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krudsood S, Wilairatana P, Tangpukdee N, Chalermrut K, Srivilairit S, Thanachartwet V, Muangnoicharoen S, Luplertlop N, Brittenham GM, Looareesuwan S. 2006. Safety and tolerability of elubaquine (bulaquine, CDRI 80/53) for treatment of Plasmodium vivax malaria in Thailand. Korean J. Parasitol. 44:221–228. 10.3347/kjp.2006.44.3.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F, Matzen JT, Anderson P, Nam TG, Gray NS, Chatterjee A, Janes J, Yan SF, Trager R, Caldwell JS, Schultz PG, Zhou Y, Winzeler EA. 2008. In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. Proc. Natl. Acad. Sci. U. S. A. 105:9059–9064. 10.1073/pnas.0802982105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, Gonzalez-Paez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. 2010. Spiroindolones, a potent compound class for the treatment of malaria. Science 329:1175–1180. 10.1126/science.1193225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagle A, Wu T, Kuhen K, Gagaring K, Borboa R, Francek C, Chen Z, Plouffe D, Lin X, Caldwell C, Ek J, Skolnik S, Liu F, Wang J, Chang J, Li C, Liu B, Hollenbeck T, Tuntland T, Isbell J, Chuan T, Alper PB, Fischli C, Brun R, Lakshminarayana SB, Rottmann M, Diagana TT, Winzeler EA, Glynne R, Tully DC, Chatterjee AK. 2012. Imidazolopiperazines: lead optimization of the second-generation antimalarial agents. J. Med. Chem. 55:4244–4273. 10.1021/jm300041e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myint HY, Berman J, Walker L, Pybus B, Melendez V, Baird JK, Ohrt C. 2011. Review: improving the therapeutic index of 8-aminoquinolines by the use of drug combinations: review of the literature and proposal for future investigations. Am. J. Trop. Med. Hyg. 85:1010–1014. 10.4269/ajtmh.2011.11-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krotoski WA, Collins WE, Broderson JR, Warren M, Krotoski DM. 1981. The 48-hour exoerythrocytic stage of Plasmodium cynomolgi bastianellii. Am. J. Trop. Med. Hyg. 30:31–37 [DOI] [PubMed] [Google Scholar]
- 47.Jimenez BC, Navarro M, Huerga H, Lopez-Roman E, Mendoza A, Lopez-Velez R. 2006. Tertian malaria (Plasmodium vivax and Plasmodium ovale) in two travelers despite atovaquone-proguanil prophylaxis. J. Travel Med. 13:373–375. 10.1111/j.1708-8305.2006.00073.x [DOI] [PubMed] [Google Scholar]
- 48.Wu T, Nagle A, Kuhen K, Gagaring K, Borboa R, Francek C, Chen Z, Plouffe D, Goh A, Lakshminarayana SB, Wu J, Ang HQ, Zeng P, Kang ML, Tan W, Tan M, Ye N, Lin X, Caldwell C, Ek J, Skolnik S, Liu F, Wang J, Chang J, Li C, Hollenbeck T, Tuntland T, Isbell J, Fischli C, Brun R, Rottmann M, Dartois V, Keller T, Diagana T, Winzeler E, Glynne R, Tully DC, Chatterjee AK. 2011. Imidazolopiperazines: hit to lead optimization of new antimalarial agents. J. Med. Chem. 54:5116–5130. 10.1021/jm2003359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shanks GD, Kremsner PG, Sukwa TY, van der Berg JD, Shapiro TA, Scott TR, Chulay JD, Malarone Clinical Trials Study Group 1999. Atovaquone and proguanil hydrochloride for prophylaxis of malaria. J. Travel Med. 6(Suppl. 1):S21–S27 [PubMed] [Google Scholar]
- 50.Hulden L. 2011. Activation of the hypnozoite: a part of Plasmodium vivax life cycle and survival. Malar. J. 10:90. 10.1186/1475-2875-10-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eyles DE. 1963. The species of simian malaria: taxonomy, Morphology, life cycle, and geographical distribution of the monkey species. J. Parasitol. 49:866–887. 10.2307/3275712 [DOI] [PubMed] [Google Scholar]
- 52.Millet P, Collins WE, Aikawa M, Cochrane AH, Nguyen-Dinh P. 1990. Use of non-human primate hepatocytes for in vitro study of the pre-erythrocytic stages of malaria parasites. Bull. World Health Organ. 68(Suppl):60–65 [PMC free article] [PubMed] [Google Scholar]
- 53.Dow GS, Gettayacamin M, Hansukjariya P, Imerbsin R, Komcharoen S, Sattabongkot J, Kyle D, Milhous W, Cozens S, Kenworthy D, Miller A, Veazey J, Ohrt C. 2011. Radical curative efficacy of tafenoquine combination regimens in Plasmodium cynomolgi-infected Rhesus monkeys (Macaca mulatta). Malar. J. 10:212. 10.1186/1475-2875-10-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.