Abstract
This study characterizes the pharmacokinetics of ertapenem, a carbapenem antibiotic, in critically ill adult subjects receiving continuous renal replacement therapy (CRRT). Eight critically ill patients with suspected/known Gram-negative infections receiving continuous venovenous hemodialysis (CVVHD) or continuous venovenous hemodiafiltration (CVVHDF) and ertapenem were enrolled. One gram of ertapenem was infused over 30 min. Predialyzer blood samples were drawn with the first dose of ertapenem from the hemodialysis tubing at time zero, 30 min, and 1, 2, 4, 8, 12, 18, and 24 h after the start of the ertapenem infusion. Effluent was collected at the same time points. Ertapenem total serum, unbound serum, and effluent concentrations from all eight subjects were used simultaneously to perform a population compartmental pharmacokinetic modeling procedure using NONMEM. Monte Carlo simulations were performed to evaluate the ability of several ertapenem dosing regimens (500 mg once daily, 750 mg once daily, 500 mg twice daily, and 1,000 mg once daily) to obtain effective unbound serum concentrations above 0.5, 1, and 2 μg/ml. For our simulated patients, all regimens produced unbound ertapenem concentrations above 2 μg/ml for 40% of the dosing interval for at least 96% of simulated patients. (This study has been registered at ClinicalTrials.gov under registration no. NCT00877370.)
INTRODUCTION
Ertapenem is a carbapenem antibiotic with broad-spectrum antibacterial activity. It is indicated for many infections commonly seen in intensive care units, including complicated urinary tract infections, complicated intraabdominal infections, and complicated skin/skin structure infections (1). Sepsis is a common cause of acute kidney injury (AKI) in the intensive care unit, with severe cases often requiring renal replacement therapy (RRT) (2). Proper antibiotic dosing in critically ill septic patients is essential. However, currently there are no manufacturer's dosing recommendations for ertapenem in critically ill patients receiving continuous RRT (CRRT).
Ertapenem, unlike other carbapenems, is highly protein bound (85 to 95% in healthy volunteers), suggesting that it is unlikely to be removed substantially by CRRT (3, 4). However, an in vitro trial that employed dialysate/ultrafiltrate rates used commonly in practice suggested that ertapenem clearance during CRRT was approximately 30 to 40 ml/min, higher than would be predicted given ertapenem's protein binding values in healthy volunteers (5). Furthermore, critically ill patients receiving CRRT are likely to exhibit changes in volume of distribution, clearance (both renal and nonrenal), and protein binding (6–10). This study was designed to determine the pharmacokinetics of ertapenem in critically ill adults receiving continuous venovenous hemodialysis (CVVHD) or continuous venovenous hemodiafiltration (CVVHDF).
(The interim analysis of this study was presented in abstract form at the American Society of Nephrology “Kidney Week,” 8 to 13 November 2011, Philadelphia, PA [11].)
MATERIALS AND METHODS
This study was approved by the University of Michigan Investigational Review Board and was a prospective, open-label, first-dose pharmacokinetic study of 8 critically ill adults with suspected or confirmed Gram-negative infections receiving CVVHD or CVVHDF (CVVHD/F) and ertapenem. (This study has been registered at ClinicalTrials.gov under registration no. NCT00877370.)
Patients were considered eligible for the study if they were ≥18 years of age, receiving CVVHD/F, and prescribed ertapenem. Patients were excluded from the study if they were pregnant and/or breastfeeding, had an allergy to ertapenem or another carbapenem, or had a severe, life-threatening reaction to penicillins or cephalosporins. Patients with a history of a central nervous system (CNS) disorder or who were currently experiencing a CNS infection were excluded as well. Finally, patients who were not expected to complete 24 h of CVVHD/F or who were concurrently receiving other extracorporeal therapies, such as extracorporeal membrane oxygenation, plasmapheresis, or intermittent hemodialysis, were also excluded. Informed consent was obtained prior to study initiation for all subjects.
Patient age, weight, illness severity score, and albumin concentration were collected from medical records. CVVHD/F was performed with Prismaflex machines (Gambro, Lakewood, CO) using acrylonitrile Prismaflex M150 (surface area, 1.5 m2; Gambro) and polyarylethysulfone Prismaflex HF1400 hemodialyzers (surface area, 1.4 m2; Gambro). Ertapenem sodium (equivalent to 1 g ertapenem [Merck & Co., Whitehouse Station, NJ]) was administered as a half-hour intravenous infusion by an infusion pump. Two blood samples, one to measure total concentrations and one to measure unbound concentrations (5 ml each), were collected, one immediately following the other, from the CVVHD circuit at the sampling port just before the hemodialysis filter into nonheparinized evacuated red-top collection tubes (BD Diagnostic Systems, Franklin Lakes, NJ) at time zero (baseline), 30 min (end of infusion), and 1, 2, 4, 8, 12, 18, and 24 h after the start of the ertapenem infusion. At the same time points, effluent (5 ml) was also collected into polypropylene cryovials from the effluent port of the CVVHD/F circuit. The blood samples were allowed to clot and then were centrifuged, and the serum was harvested into polypropylene cryovials. Serum and effluent samples were stored at −80°C until analysis.
Assay.
All samples were shipped on dry ice to be analyzed by the Institute for Biomedical and Pharmaceutical Research (IBMP) in Nürnberg-Heroldsberg, Germany. Serum samples were analyzed for total ertapenem concentrations by high-performance liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS; Applied Biosystems/MDS Sciex), as previously described (5). Unbound ertapenem concentrations were assessed using equilibrium dialysis. Effluent ertapenem concentrations were determined using effluent samples (0.02 ml), to which 2 ml of 1 mM ammonium acetate buffer containing the internal standard had been added. After thorough mixing, an aliquot was past diluted with the mobile phase. Ten microliters of each sample was chromatographed. Under these conditions, ertapenem and the internal standard were eluted after approximately 0.9 min. The limit of quantification for the ertapenem assay was 0.5 μg/ml. Inter- and intraday precision values were all less than 12% for total and unbound serum and effluent ertapenem concentrations.
Descriptive statistics were used to summarize patient demographics, CRRT flow rates, and pharmacokinetic parameters. The sieving coefficient (SC) for ertapenem at each time point was calculated as the effluent concentration/predialyzer serum concentration. The effluent rate (Qef) was defined as the sum of dialysate flow rate and ultrafiltration rate, and hourly Qef values were averaged across the 24-h time period. At the University of Michigan, citrate (as an anticoagulant citrate dextrose solution A [ACD-A] solution) is infused directly after the CVVHD/F access catheter to prevent clotting (12). As a result, for the pharmacokinetic analysis, predialyzer ertapenem concentrations were adjusted for citrate dilution using the following equation:
| (1) |
where concentrations are in μg/ml, citrate infusion rate is in ml/min, Hct is hematocrit, and Qb is blood flow rate in ml/min (13).
Pharmacokinetic analysis.
Unbound ertapenem serum, total serum, and effluent concentrations from all eight subjects were used simultaneously to perform a population compartmental pharmacokinetic modeling procedure using NONMEM (version VII; Globomax LLC, MD, USA). A two-compartment structural pharmacokinetic model with first-order elimination from the central compartment was used to describe unbound ertapenem pharmacokinetics and dialytic clearance during CVVHD/F. Unbound ertapenem concentrations were assumed to be removed by dialysis from the central compartment. Ertapenem dialytic clearance was estimated using unbound serum and effluent concentrations through the developed compartmental model. Ertapenem total concentrations were linked to unbound concentrations using a nonlinear maximum binding model. The addition of this term was necessary due to an increase in unbound fraction at higher ertapenem concentrations. The equations describing the pharmacokinetic model are shown below.
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
Where X(1) and X(2) are the amounts of unbound drug in the central and peripheral compartments, VC and VP are the apparent volumes of distribution in the central and peripheral compartments, respectively, R0 is the zero-order infusion rate, and CLS and CLD are the systemic and distribution clearances, respectively. An indicator variable, DIAL, with a value of 1 or 0, was used to turn the effluent compartment on and off, respectively, if the CVVHD/F was turned off for any reason. CLdial refers to dialytic clearance. Transfer of drug between the compartments was assumed to follow first-order processes. Note that all pharmacokinetic parameters are based upon unbound concentrations. Cb is the bound ertapenem concentration, Cu is the unbound ertapenem concentration, Bmax is the maximum protein binding capacity, KD is the binding equilibrium dissociation constant, and Ct is the total ertapenem concentration. The best structural model to describe observed data was chosen based on goodness-of-fit plots, minimum value of objective function, as well as individual plots of observed and model-predicted concentrations versus time.
Interindividual variability of CLS, VC, VP, CLdial, and Bmax was modeled using an exponential interindividual variability model assuming log-normal distribution of the between-subject variability in population parameter estimates. Therefore, each subject's estimated CLS, VC, VP, CLdial, and Bmax were related to the corresponding population estimate using the following equation:
| (8) |
where Pj is the jth individual parameter estimate, PTV is the typical value (population estimate) of the parameter P, and eηP is the interindividual variability for this parameter.
Residual unexplained variability, including intraindividual variability, was modeled using a proportional error term (equation 9) with separate terms for unbound, total, and effluent concentrations.
| (9) |
Where yij is the ith observed concentration for the jth individual, is the ith model-predicted concentration for the jth individual, and εij is the residual error term for the ith observation of the jth individual.
Covariate testing.
The final structural model was used to test the effects of subject covariates on the model parameters. The effects of subject weight and age were examined on population parameter estimates for CLS, CLdial, and VC and on interindividual variability estimates for CLS. Covariates were kept in the model if their addition resulted in a statistically significant decrease in minimum value of object function (a decrease of 3.84 units is considered statistically significant at α = 0.05 using a chi-square test). The relationship between subject weight and each of CLS, CLdial, and VC was described using a power model after correcting each subject's weight for the median weight value according to the following equation:
| (10) |
where θ1 is the typical value (population estimate) of the parameter (CLS, CLdial, or VC) in a subject weighing 80 kg (median weight for the eight subjects), WT is the subject weight, and θ2 is the power term describing the effect of subject weight on the typical value of the parameter. The same formula was used to test the effect of age on the population estimate and interindividual variability for CLS.
Simulations.
Monte Carlo simulations were performed in NONMEM VII to evaluate the ability of several ertapenem dosing regimens to obtain effective unbound serum concentrations above the FDA breakpoint of 0.5 μg/ml (the MIC breakpoint of Enterobacteriaceae and Haemophilus spp.), 1 μg/ml (breakpoint of Streptococcus spp.), and 2 μg/ml (breakpoint of Staphylococcus aureus) (1). Ertapenem concentrations were simulated over a 3-day period. Each population simulation consisted of 1,000 simulations obtained using the model-estimated population parameters (VC, VP, CLS, CLD, CLdial, Bmax, and KD) and interindividual variability terms. The serum concentration-time profiles were simulated according to the following regimens: (i) 500 mg every 12 h (q12h); (ii) 500 mg q24h; (iii) 750 mg q24h; and (iv) 1,000 mg q24h.
The simulations were used to generate serum concentration-time curves (means ± standard deviations [SD]) over the time period. For each of the simulated dosing regimens, we calculated the percentage of time within a single dosing interval where unbound serum concentrations were equal to or above the 0.5, 1, and 2 μg/ml breakpoints. This was calculated for each of the 1,000 simulations within the same dosing regimen, and then the percentage of subjects achieving a time above MIC (T > MIC) of more than or equal 40% of the dosing interval (termed ≥40% T > MIC; a pharmacodynamic target for carbapenems suggested by animal models) was calculated (14). The suitability of the simulated dosing regimens was judged by comparing the percentage of simulated subjects achieving the pharmacodynamic outcome (≥40% T > MIC) under each regimen.
RESULTS
Eight subjects meeting inclusion criteria provided consent and were enrolled between April 2009 and March 2011. Subject demographics are presented in Table 1. Urine output was minimal (<50 ml per 24 h) for all subjects. Mean serum albumin concentrations were 3.0 ± 0.5 g/dl. Patients 1 to 4 received CVVHD with low ultrafiltration rates to maintain fluid balance, and patients 5 to 8 received CVVHDF. Dialysate flow rates ranged from 8 to 38 ml/kg/h (1,000 to 3,000 ml/h), and ultrafiltration rates ranged from 5 to 26 ml/kg/h (380 to 1,800 ml/h). Subjects 1 and 2 received CRRT utilizing the Prisma M150 filter, and subjects 3 to 8 received CRRT utilizing the Prismaflex HF1400 (Gambro). Table 1 also outlines the CRRT blood, dialysate, ultrafiltration, and effluent (dialysate plus ultrafiltrate) rates used for each subject. During the pharmacokinetic sampling period, subject 3 received a portion (∼20%) of another dose 16 h after the first dose was hung, and subject 8 received the next ertapenem dose before the 24-h blood draw, so sampling was limited to 18 h for that subject. Subjects 1, 2, and 6 had CRRT stopped (and then restarted) for 135, 142, and 276 min during the sampling interval due to filter changes (2 subjects) and an off-floor procedure (1 subject). Each of these alterations was handled within the pharmacokinetic modeling procedure. No adverse reactions to ertapenem were observed during the study interval.
TABLE 1.
Subject demographics and continuous renal replacement therapy parametersa
| Subject no. | Sex | Age (yr) | Wt (kg) | APACHE III score | Albumin (g/dl) | Qb (ml/min) | Qd (ml/h/kg) | Quf (ml/h/kg) | Qef (ml/h/kg) | SC |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 74 | 79.1 | 123 | 3.6 | 150 | 38 | 5 | 43 | 0.19 |
| 2 | M | 71 | 82.6 | 77 | 4.0 | 200 | 24 | 8 | 33 | 0.14 |
| 3 | F | 31 | 82.0 | 80 | 2.6 | 200 | 24 | 8 | 33 | 0.22 |
| 4 | F | 58 | 58.7 | 84 | 3.0 | 200 | 34 | 12 | 46 | 0.15 |
| 5 | F | 71 | 68.0 | 85 | 2.4 | 150 | 29 | 21 | 51 | 0.33 |
| 6 | M | 46 | 56.0 | 63 | 2.7 | 200 | 18 | 26 | 44 | 0.26 |
| 7 | F | 69 | 119.2 | NA | 2.9 | 150 | 8 | 12 | 21 | 0.18 |
| 8 | M | 78 | 85.2 | 68 | 2.7 | 200 | 15 | 21 | 36 | 0.24 |
| Total (means ± SD) | 62 ± 16 | 78.9 ± 19.8 | 83 ± 19 | 3.0 ± 0.5 | 181 ± 26 | 24 ± 10 | 14 ± 8 | 38 ± 10 | 0.21 ± 0.06 |
M, male; F, female; NA, not available; Qb, blood flow rate; Qd, dialysate flow rate; Quf, ultrafiltration rate; Qef, effluent flow; SC, sieving coefficient. APACHE III, Acute Physiology Chronic Health Evaluation III score.
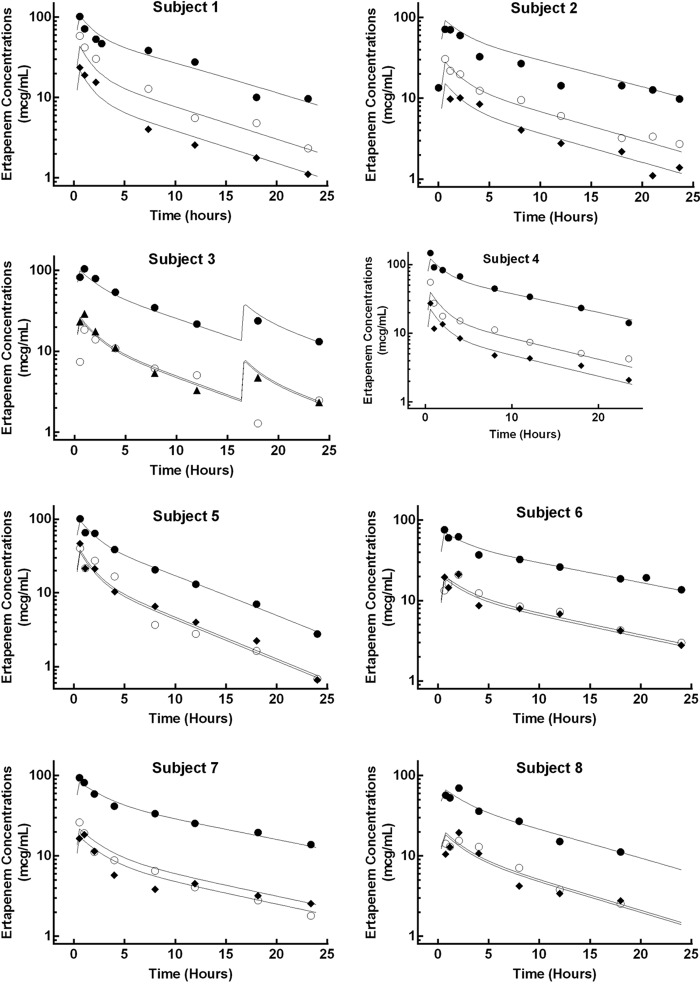
The mean ± SD sieving coefficient was 0.21 ± 0.06 (Table 1). The average total and unbound ertapenem concentrations are presented in Fig. 1. Table 2 describes the ertapenem population pharmacokinetic parameters derived from the simultaneous fitting of unbound, total, and dialysate concentrations. Figure 2 illustrates the individual model fits in all eight subjects for the total, unbound, and effluent concentrations. Ertapenem protein binding was affected by concentration, with the unbound fraction increasing at higher ertapenem total concentrations (Fig. 3).
FIG 1.
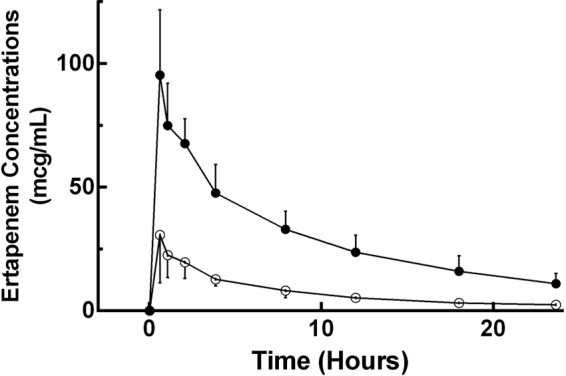
Average total and unbound ertapenem concentrations over a 24-h dosing interval. Closed circles represent total ertapenem serum concentrations, and open circles represent unbound concentrations. Lines connecting the data points are for clarity. Vertical bars represent the SD of the mean ertapenem concentration at the respective time point.
TABLE 2.
Ertapenem population pharmacokinetic parameters and error estimates from final pharmacokinetic model
| Parametera | Population estimate | Relative SE (%) | Interindividual variability (%) |
|---|---|---|---|
| CLS, unbound (ml/min) | 48 | 10 | 23 |
| VC, unbound (liter) | 32 | 167 | 33 |
| VP, unbound (liter) | 21 | 23 | 20 |
| CLD, unbound (ml/min) | 115 | 41 | NA |
| CLdial, unbound (ml/min) | 36 | 13 | 32 |
| Bmax (μg/ml) | 144 | 26 | 17 |
| KD (μg/ml) | 38 | 25 | NA |
CLS, unbound, the systemic unbound clearance; VC, unbound, the apparent volume of distribution in the central compartment; VP, unbound, apparent volume of distribution in the peripheral compartments; CLD, unbound, unbound distribution clearances; CLdial, dialytic clearance of unbound ertapenem; Bmax, maximum protein binding capacity; KD, binding equilibrium dissociation constant.
FIG 2.
Observed and modeled ertapenem concentration-time profile for the eight subjects. The closed circles (total), open circles (unbound), and diamonds (effluent) depict the observed ertapenem concentrations. The solid lines depict the model-derived concentrations.
FIG 3.
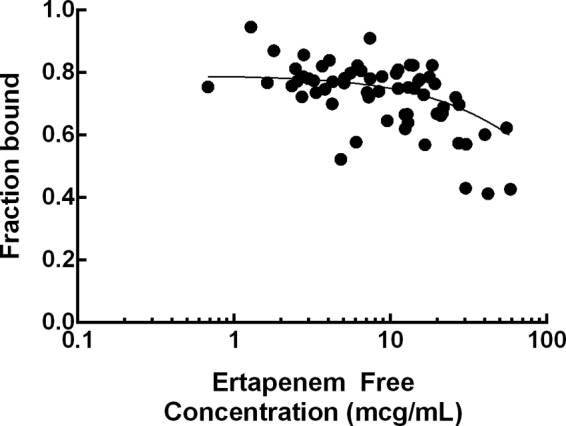
Relationship between free ertapenem serum concentrations and protein binding.
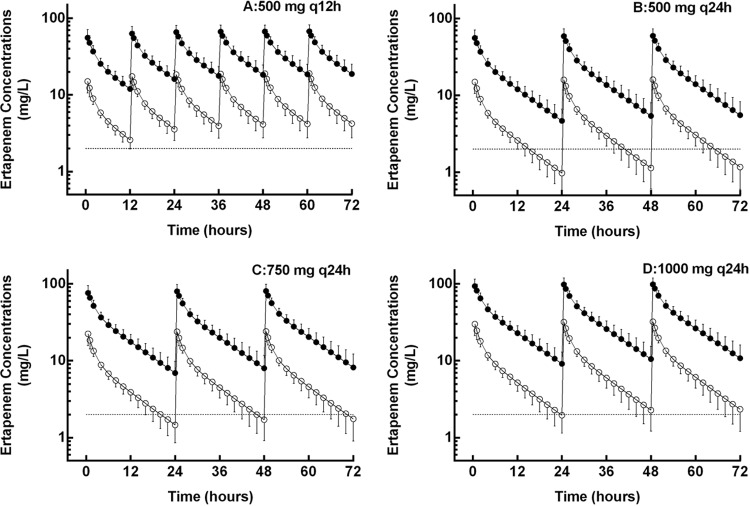
Ertapenem exposure was simulated for four dosing regimens. Figure 4 shows the simulated unbound serum concentration-time profiles (means ± SD, 1,000 simulations) of the four regimens for 72 h. Table 3 reports the probability of the simulated unbound concentrations to achieve the pharmacodynamic endpoint (≥40% T > MIC), as well as median time above MIC for each regimen. Only the first-dose pharmacodynamics are illustrated in the table. As accumulation is expected to occur after the first dose, the percentage of simulated subjects achieving the pharmacodynamics outcome will be at or above those shown for the first dose.
FIG 4.
Simulated mean ± SD concentration-time profiles (means of 1,000 simulations) for four ertapenem regimens: 500 mg q12h (A), 500 mg q24h (B), 750 mg q24h (C), and 1,000 mg q24h (D). The closed circles represent total ertapenem serum concentrations, and open circles represent unbound concentrations. Lines connecting the data points are for clarity. The dashed line at 2 μg/liter depicts the sensitivity breakpoint for methicillin-sensitive Staphylococcus aureus.
TABLE 3.
Probability of simulated subjects achieving unbound concentrations of ≥40% T > MIC following the first dose for each regimen and fraction of the dosage interval spent above the MIC for each regimen
| Regimen | Result for MIC (mg/liter) of: |
|||||
|---|---|---|---|---|---|---|
| 0.5 |
1 |
2 |
||||
| Probability of target attainment (≥40% T> MIC) | Fraction of dosing interval with unbound concn > MIC median (range) | Probability of target attainment (≥40% T > MIC) | Fraction of dosing interval with unbound concn > MIC median (range) | Probability of target attainment (≥40% T > MIC) | Fraction of dosing interval with unbound concn > MIC median (range) | |
| 500 mg q12h | 1.000 | 0.999 (0.999–0.999) | 1.000 | 0.999 (0.833–0.999) | 1.000 | 0.992 (0.50–0.999) |
| 500 mg q24h | 1.000 | 0.999 (0.583–0.999) | 0.999 | 0.916 (0.333–0.999) | 0.962 | 0.583 (0.167–1.000) |
| 750 mg q24h | 1.000 | 0.999 (0.667–0.999) | 1.000 | 0.999 (0.5–0.999) | 0.999 | 0.750 (0.333–1.000) |
| 1,000 mg q24h | 1.000 | 0.999 (0.75–0.999) | 1.000 | 0.999 (0.583–0.999) | 0.999 | 0.917 (0.330–1.000) |
DISCUSSION
Optimal antibiotic dosing in critically ill patients requiring CRRT can be challenging, as both critical illness and CRRT can alter drug pharmacokinetics. Capillary leak and aggressive fluid resuscitation contribute to an increased volume of distribution in septic individuals (6, 15). Furthermore, protein binding is altered in the severely ill, resulting in altered unbound drug concentrations (16).
These alterations in pharmacokinetics and unbound drug concentrations can lead to changes in dosing requirements. Although large-scale pharmacokinetic data on critically ill patients is often lacking, several Monte Carlo simulations suggest that we are underdosing antibiotics in the critically ill population, even when they are prescribed at manufacturer-recommended dosages (17, 18). It has been suggested that this is sometimes the case for ertapenem. Unbound (free) ertapenem concentrations are best associated with antimicrobial activity, and animal models have suggested that maintaining carbapenem concentrations above an organism's MIC for at least 40% of the dosing interval (≥40% T > MIC) maximizes bactericidal effect (14, 19).
Burkhardt et al. collected pharmacokinetic data from 17 critically ill patients without renal failure who suffered from ventilator-associated pneumonia, and they found that estimated mean unbound ertapenem concentrations exceeded 2 μg/ml (the MIC90 of penicillin-resistant S. pneumoniae in an in vitro report) for only 25% of the dosing interval (20, 21). Similarly, Brink et al. measured unbound ertapenem concentrations in eight critically ill patients with severe sepsis and normal renal function and found pharmacokinetic parameters to be highly variable, with only half of patients achieving unbound concentrations above 2 μg/ml for 40% of the dosing interval (22).
Although critically ill patients with AKI requiring RRT exhibit decreased renal clearance, decreased protein binding in this population means more unbound drug available for clearance by RRTs. In some instances, critically ill patients requiring RRTs require higher doses of antibiotic than recommended for individuals receiving intermittent hemodialysis for end-stage renal disease (13, 23, 24). A pharmacokinetic study of ertapenem in critically ill subjects requiring extended daily dialysis (blood and dialysate flow rates of 160 ml/min, treatment duration of 480 min) suggested that a dose of 1 g every 24 h, substantially higher than the manufacturer's suggested dosing for patients with creatinine clearance of <30 ml/min/1.73 m2 (500 mg q24h) or receiving maintenance hemodialysis (500 mg within 6 h prior to hemodialysis, supplementary 150 mg following the hemodialysis session), is more appropriate to achieve bactericidal targets (1, 25).
Our study, which also utilized a dose of 1 g every 24 h, is the first to examine ertapenem pharmacokinetics in CVVHD/F. Our model was able to describe the changes in pharmacokinetics that occur in critically ill individuals requiring CRRT, as well as the change in ertapenem protein binding as a function of unbound concentrations. In our study of critically ill patients with AKI, the unbound fraction (fu; 20 to 40%) was markedly increased compared to those reported for healthy volunteers (fu, 5 to 15%) (3, 4). There was an observed increase in unbound fraction at higher ertapenem concentrations, which indicates saturation of albumin binding sites. Our population estimate of unbound CLdial was 36 ml/min, which is in the 30- to 40-ml/min range predicted by an in vitro study (5). CLdial contributed substantially to total body clearance (84 ml/min).
During the study sampling period, the dose of 1 g every 24 h produced unbound ertapenem concentrations that remained above 2 μg/ml for an average of 90% of the dosing interval, achieving the pharmacodynamic targets in all eight patients. Monte Carlo simulations revealed that 99.9% of simulated subjects would achieve unbound ertapenem concentrations above 2 μg/ml for at least 40% of the interval, with concentrations remaining above 2 μg/ml for a median of 92% (range, 33 to 100%) of the dosing interval.
All of the simulated regimens produced unbound ertapenem concentrations above 2 μg/ml for 40% of the dosing interval for at least 96% of the simulated patients. However, although animal models suggest a T > MIC of 40% as a target for carbapenems, it appears prudent to aim for a longer time above the MIC in critically ill humans. For example, in 101 adult patients with lower-respiratory-tract infections treated with meropenem, unbound meropenem concentrations above the MIC for >54% of the dosing interval were associated with microbiological eradication (26). In another study of 60 febrile neutropenic patients with bacteremia treated with meropenem, clinical responders achieved unbound meropenem concentrations above the MIC for a mean time of 83% of the dosing interval, while nonresponders exhibited an average time above the MIC of 59% of the dosing interval (27). Because it is crucial to optimize pharmacokinetic parameters in critically ill patients to promote clinical responses and prevent the development of resistance, the time above the MIC for beta-lactams should be maximized (28, 29). Particularly in critically ill patients where organisms with high MICs are suspected, it may be necessary to give doses higher than the 500-mg q24h ertapenem dose recommended by the manufacturer for patients with a creatinine clearance of less than 30 ml/min, as our data suggest that concentrations remained above 2 μg/ml for an interquartile range of only 50 to 67% of the dosing interval.
As with all time-dependent antibiotics, the benefits of maximizing time above the MIC of ertapenem should be weighed against the risk of developing adverse effects associated with doses that are too high. Ertapenem is generally well tolerated, with most side effects reported to be mild to moderate in severity (30). The most common clinical adverse events of ertapenem in clinical trials were diarrhea (5%), infusion vein complications (4%), nausea (3%), and headache (2%), while the risk of seizures with ertapenem is low (0.2%) (30). However, risk factors associated with carbapenem neurotoxicity do include renal insufficiency, as well as a history of central nervous system abnormalities, low body weight, and advanced age (31). In critically ill patients, achieving pharmacodynamic targets often outweighs the risks of adverse effects, and ertapenem is a well-tolerated antibiotic; however, caution may be warranted in patients presenting with multiple risk factors for seizure development.
Monte Carlo simulations in the current study indicated that at least 96% of simulated subjects achieved concentrations above the higher MIC of 2 μg/ml for at least 40% of the dosing interval for all dosing regimens tested. However, because the effluent rate is the most important CRRT variable contributing to drug clearance, it is possible that units that utilize lower or higher CRRT effluent flow rates will require different ertapenem doses to achieve pharmacodynamic goals (32–34). This was also a single-dose pharmacokinetic study, so it is likely that some ertapenem will accumulate with repeated dosing (Fig. 3). Regimens should be assessed in multiple-dose trials and safety and efficacy evaluated in larger, outcome-oriented studies.
Conclusions.
In our study of critically ill patients with acute kidney injury, ertapenem pharmacokinetic parameters were much different than those seen in healthy individuals, with much lower protein binding values. In our 8 patients, 1 g of ertapenem resulted in unbound concentrations at or above the 2 μg/ml breakpoint for a mean of 90% of the dosing interval. At the effluent rates employed in our CRRT system, ertapenem was cleared to a substantial degree. It appears that doses higher than the manufacturer-recommended dose for creatinine clearance of <30 ml/min may be necessary for critically ill patients receiving CRRT, particularly in those infected with organisms with elevated MICs.
ACKNOWLEDGMENTS
Rachel F. Eyler and Daryl D. DePestel were employees of the University of Michigan College of Pharmacy when this study was conducted. Ahmed M. Nader was an employee of Purdue University College of Pharmacy when this study was conducted.
Merck & Co. provided funding for this investigator-initiated study.
Footnotes
Published ahead of print 9 December 2013
REFERENCES
- 1.Merck & Co., Inc 2010. Ertapenem package insert. Merck & Co., Inc., Whitehouse Station, NJ. http://www.merck.com/product/usa/pi_circulars/i/invanz/invanz_pi.pdf
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BESTKidney) Investigators 2005. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813-818. 10.1001/jama.294.7.813 [DOI] [PubMed] [Google Scholar]
- 3.Majumdar AK, Musson DG, Birk KL, Kitchen CJ, Holland S, McCrea J, Mistry G, Hesney M, Xi L, Li SX, Haesen R, Blum RA, Lins RL, Greenberg H, Waldman S, Deutsch P, Rogers JD. 2002. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob. Agents Chemother. 46:3506–3511. 10.1128/AAC.46.11.3506-3511.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musson DG, Majumdar A, Holland S, Birk K, Xi L, Mistry G, Sciberras D, Muckow J, Deutsch P, Rogers JD. 2004. Pharmacokinetics of total and unbound ertapenem in healthy elderly subjects. Antimicrob. Agents Chemother. 48:521–524. 10.1128/AAC.48.2.521-524.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevenson JM, Patel JH, Churchwell MD, Vilay AM, Depestel DD, Sörgel F, Kinzig M, Jakob V, Mueller BA. 2008. Ertapenem clearance during modeled continuous renal replacement therapy. Int. J. Artif. Organs 31:1027–1034 [DOI] [PubMed] [Google Scholar]
- 6.Boucher BA, Wood GC, Swanson JM. 2006. Pharmacokinetic changes in critical illness. Crit. Care Clin. 22:255–271. 10.1016/j.ccc.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 7.Sánchez M, Jiménez-Lendínez M, Cidoncha M, Asensio MJ, Herrerot E, Collado A, Santacruz M. 2011. Comparison of fluid compartments and fluid responsiveness in septic and non-septic patients. Anaesth. Intensive Care 39:1022–1029 [DOI] [PubMed] [Google Scholar]
- 8.Van Dalen R, Vree TB, Baars IM. 1987. Influence of protein binding and severity of illness on renal elimination of four cephalosporin drugs in intensive-care patients. Pharm. Weekbl. Sci. 9:98–103. 10.1007/BF01960743 [DOI] [PubMed] [Google Scholar]
- 9.Zielmann S, Mielck F, Kahl R, Kazmaier S, Sydow M, Kolk J, Burchardi H. 1994. A rational basis for the measurement of free phenytoin concentration in critically ill trauma patients. Ther. Drug Monit. 16:139–144. 10.1097/00007691-199404000-00005 [DOI] [PubMed] [Google Scholar]
- 10.Zamacona MK, Suárez E, Aguilera L, Rodríguez-Sasiaín Aguirre C, Calvo R. 1997. Serum protein binding of propofol in critically ill patients. Acta Anaesthesiol. 41:1267–1272. 10.1111/j.1399-6576.1997.tb04643.x [DOI] [PubMed] [Google Scholar]
- 11.Eyler RF, Vilay AM, Heung M, Pleva M, Sowinski KM, DePestel DD, Mueller BA. 2011. Pharmacokinetics of ertapenem in critically ill patients receiving continuous venovenous hemodialysis (CVVHD) or hemodiafiltration (CVVHDF). J. Am. Soc. Nephrol. 22:314A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swartz R, Pasko D, O'Toole J, Starmann B. 2004. Improving the delivery of continuous renal replacement therapy using regional citrate anticoagulation. Clin. Nephrol. 61:134–143. 10.5414/CNP61134 [DOI] [PubMed] [Google Scholar]
- 13.Vilay AM, Grio M, Depestel DD, Sowinski KM, Gao L, Heung M, Salama NN, Mueller BA. 2011. Daptomycin pharmacokinetics in critically ill patients receiving continuous venovenous hemodialysis. Crit. Care Med. 39:19–25. 10.1097/CCM.0b013e3181fa36fb [DOI] [PubMed] [Google Scholar]
- 14.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions “bug and drug.” Nat. Rev. Microbiol. 2:289–300. 10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- 15.Varghese JM, Roberts JA, Lipman J. 2011. Antimicrobial pharmacokinetic and pharmacodynamic issues in the critically ill with severe sepsis and septic shock. Crit. Care Clin. 27:19–34. 10.1016/j.ccc.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 16.Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. 2011. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 50:99–110. 10.2165/11539220-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 17.Ulldemolins M, Roberts JA, Wallis SC, Rello J, Lipman J. 2010. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: special emphasis on unbound pharmacokinetics. J. Antimicrob. Chemother. 65:1771–1778. 10.1093/jac/dkq184 [DOI] [PubMed] [Google Scholar]
- 18.Zelenitsky SA, Ariano RE, Zhanel GG. 2011. Pharmacodynamics of empirical antibiotic monotherapies for an intensive care unit (ICU) population based on Canadian surveillance data. J. Antimicrob. Chemother. 66:343–349. 10.1093/jac/dkq348 [DOI] [PubMed] [Google Scholar]
- 19.Nix DE, Matthias KR, Ferguson EC. 2004. Effect of ertapenem protein binding on killing of bacteria. Antimicrob. Agents Chemother. 48:3419–3424. 10.1128/AAC.48.9.3419-3424.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wexler HM. 2004. In vitro activity of ertapenem: review of recent studies. J. Antimicrob. Chemother. 53(Suppl 2):ii11–ii21. 10.1093/jac/dkh204 [DOI] [PubMed] [Google Scholar]
- 21.Burkhardt O, Kumar V, Katterwe D, Majcher-Peszynska J, Drewelow B, Derendorf H, Welte T. 2007. Ertapenem in critically ill patients with early-onset ventilator-associated pneumonia: pharmacokinetics with special consideration of free-drug concentration. J. Antimicrob. Chemother. 59:277–284. 10.1093/jac/dkl485 [DOI] [PubMed] [Google Scholar]
- 22.Brink AJ, Richards GA, Schillack V, Kiem S, Schentag J. 2009. Pharmacokinetics of once-daily dosing of ertapenem in critically ill patients with severe sepsis. Int. J. Antimicrob. Agents 33:432–436. 10.1016/j.ijantimicag.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 23.Seyler L, Cotton F, Taccone FS, De Backer D, Macours P, Vincent JL, Jacobs F. 2011. Recommended β-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit. Care. 15:R137. 10.1186/cc10257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel K, Roberts JA, Lipman J, Tett SE, Deldot ME, Kirkpatrick CM. 2011. Population pharmacokinetics of fluconazole in critically ill patients receiving continuous venovenous hemodiafiltration: using Monte Carlo simulations to predict doses for specified pharmacodynamic targets. Antimicrob. Agents Chemother. 55:5868–5873. 10.1128/AAC.00424-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkhardt O, Hafer C, Langhoff A, Kaever V, Kumar V, Welte T, Haller H, Fliser D, Kielstein JT. 2009. Pharmacokinetics of ertapenem in critically ill patients with acute renal failure undergoing extended daily dialysis. Nephrol. Dial. Transplant. 24:267–271. 10.1093/ndt/gfn472 [DOI] [PubMed] [Google Scholar]
- 26.Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 51:1725–1730. 10.1128/AAC.00294-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ariano RE, Nyhlén A, Donnelly JP, Sitar DS, Harding GK, Zelenitsky SA. 2005. Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann. Pharmacother. 39:32–38. 10.1345/aph.1E271 [DOI] [PubMed] [Google Scholar]
- 28.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 31:345–351. 10.1016/j.ijantimicag.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 29.Roberts JA, Paratz J, Paratz E, Krueger WA, Lipman J. 2007. Continuous infusion of beta-lactam antibiotics in severe infections: a review of its role. Int. J. Antimicrob. Agents 30:11–18. 10.1016/j.ijantimicag.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 30.Burkhardt O, Derendorf H, Welte T. 2007. Ertapenem: the new carbapenem 5 years after first FDA licensing for clinical practice. Expert Opin. Pharmacother. 8:237–256. 10.1517/14656566.8.2.237 [DOI] [PubMed] [Google Scholar]
- 31.Seto AH, Song JC, Guest SS. 2005. Ertapenem-associated seizures in a peritoneal dialysis patient. Ann. Pharmacother. 39:352–356. 10.1345/aph.1E421 [DOI] [PubMed] [Google Scholar]
- 32.Choi G, Gomersall CD, Tian Q, Joynt GM, Li AM, Lipman J. 2009. Principles of antibacterial dosing in continuous renal replacement therapy. Crit. Care Med. 37:2268–2282. 10.1097/CCM.0b013e3181aab3d0 [DOI] [PubMed] [Google Scholar]
- 33.Kielstein JT, Burkhardt O. 2011. Dosing of antibiotics in critically ill patients undergoing renal replacement therapy. Curr. Pharm. Biotechnol. 12:2015–2019. 10.2174/138920111798808275 [DOI] [PubMed] [Google Scholar]
- 34.Kielstein JT. 2009. Dosage of renal replacement therapy in acute renal failure. Dtsch. Med. Wochenschr. 134:1697–1699. 10.1055/s-0029-1234005 [DOI] [PubMed] [Google Scholar]