Abstract
Increasing evidence suggests that colistin monotherapy is suboptimal at currently recommended doses. We hypothesized that front-loading provides an improved dosing strategy for polymyxin antibiotics to maximize killing and minimize total exposure. Here, we utilized an in vitro pharmacodynamic model to examine the impact of front-loaded colistin regimens against a high bacterial density (108 CFU/ml) of Pseudomonas aeruginosa. The pharmacokinetics were simulated for patients with hepatic (half-life [t1/2] of 3.2 h) or renal (t1/2 of 14.8 h) disease. Front-loaded regimens (n = 5) demonstrated improvement in bacterial killing, with reduced overall free drug areas under the concentration-time curve (fAUC) compared to those with traditional dosing regimens (n = 14) with various dosing frequencies (every 12 h [q12h] and q24h). In the renal failure simulations, front-loaded regimens at lower exposures (fAUC of 143 mg · h/liter) obtained killing activity similar to that of traditional regimens (fAUC of 268 mg · h/liter), with an ∼97% reduction in the area under the viable count curve over 48 h. In hepatic failure simulations, front-loaded regimens yielded rapid initial killing by up to 7 log10 within 2 h, but considerable regrowth occurred for both front-loaded and traditional regimens. No regimen eradicated the high bacterial inoculum of P. aeruginosa. The current study, which utilizes an in vitro pharmacodynamic infection model, demonstrates the potential benefits of front-loading strategies for polymyxins simulating differential pharmacokinetics in patients with hepatic and renal failure at a range of doses. Our findings may have important clinical implications, as front-loading polymyxins as a part of a combination regimen may be a viable strategy for aggressive treatment of high-bacterial-burden infections.
INTRODUCTION
Early efficacious antimicrobial treatment is the cornerstone in combating infections in critically ill patients (1). However, there is a dearth of new and effective antimicrobial agents available to combat extensively drug-resistant (XDR) Gram-negative strains, particularly for Pseudomonas aeruginosa (2–5). This has renewed interest in the once-abandoned polymyxin antibiotic colistin, also known as polymyxin E, which is often the only therapeutic option against XDR P. aeruginosa strains (6–9). However, a number of studies have highlighted potential shortcomings of colistin monotherapy when given following current dosing recommendations coupled with its high proclivity for nephrotoxicity, especially in critically ill patients (10–15).
Colistin is administered parenterally as colistin methanesulfonate (CMS), an inactive prodrug that undergoes hydrolysis in vivo to the active moiety colistin (16). Over the past 15 years with the resurgence of colistin, recent pharmacokinetic studies in critically ill patients demonstrated that the currently recommended dosage regimens of CMS result in suboptimal plasma concentrations of formed colistin (17, 18). Although these studies have provided significant insight into the disposition of CMS and formed colistin in the critically ill patient population, there has been little information to guide its clinical use in the setting of severe end-organ dysfunction. Haas et al. recently conducted the first pharmacokinetics study of CMS and formed colistin in defined groups of patients with stage 5 kidney disease or severe liver disease (Childs-Pugh class C) (19). We utilized this new information to evaluate pharmacokinetic/pharmacodynamic (PK/PD) relationships for colistin and propose optimal dosage regimens in these patient populations.
The objective of the current study was to investigate “front-loading” as a potential dosing strategy to optimize the pharmacodynamics of colistin by maximizing killing and minimizing total drug exposure. Our hypothesis was that polymyxins are suitable candidates for front-loading (20–22), as they demonstrate rapid bactericidal activity which has been shown to be active against high bacterial density (7, 22–24). An in vitro pharmacodynamic model was employed to examine the impact of front-loaded colistin regimens against P. aeruginosa by simulating the pharmacokinetics in patients with severe liver and renal diseases.
(Part of this study was presented at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 12 to 15 September 2010 [25].)
MATERIALS AND METHODS
Bacterial strains.
Two clinical isolates of P. aeruginosa, URMC1 and URMC2, which were obtained from the University of Rochester Medical Center, Rochester, NY, were studied as previous described (23). The MICs were determined by broth microdilution in four replicates using colistin (sulfate) according to Clinical and Laboratory Standards Institute guidelines in cation-adjusted Mueller-Hinton broth (CAMHB). The MIC was 1.0 mg/liter for URMC1 and URMC2.
Medium and antibiotic.
Colistin sulfate was purchased from Sigma-Aldrich, St. Louis, MO (lot number 011MZ062V). Fresh stock solutions of colistin (sulfate) were prepared and then sterilized by filtration with a 0.22-μm Millex-GP filter (Millipore, Bedford, MA, USA). Mueller-Hinton broth (Difco Laboratories, Detroit, MI) (CAMHB) supplemented with calcium (25 mg/liter) and magnesium (12.5 mg/liter) (SMHB) was utilized for susceptibility testing and in vitro models.
In vitro pharmacokinetic/pharmacodynamic model.
A one-compartment in vitro infection model (IVPM) was used to characterize the pharmacodynamics of colistin against clinical strains of P. aeruginosa as previously described (26). The IVPM experiments simulated the pharmacokinetics in critically ill patients with renal or hepatic impairment obtained from a recent pharmacokinetic study by Haas et al. (19). In brief, the experimental model consisted of sealed flasks representing compartments, each containing a working volume of 250 ml of CAMHB at 37°C with a magnetic stir bar to ensure adequate mixing. One of the flasks served as a control to characterize the growth dynamics of both clinical strains in the absence of colistin. Prior to each experiment, strains were subcultured onto Mueller-Hinton agar plates and incubated overnight at 37°C. Fresh bacterial colonies from this overnight growth were inoculated to SMHB and adjusted spectrophotometrically to a McFarland turbidity of 1.0. Each compartment was inoculated with 1.0 ml of this log-phase bacterial suspension to yield a starting inoculum of ∼108 CFU/ml.
Simulated colistin dosing regimens.
The traditional and front-loaded regimens are described in Table 1. The simulated pharmacokinetic half-lives (t1/2) of colistin selected for the experimental setup were 14.8 h in renal failure patients and 3.2 h in hepatic failure patients), based on a pharmacokinetic study by Haas et al. in adult patients with renal failure (n = 10) and hepatic failure (n = 10) (19). Patients with stage 5 renal disease, maintained on intermittent hemodialysis and of any race or gender, were included in the renal failure group. Patients with severe hepatic disease or those currently being evaluated for liver transplant with a Child-Pugh score of C (27) were included in the hepatic failure group. Lactating or pregnant women were excluded from both the renal and hepatic failure groups. Patients with hepatic disease were excluded from the kidney renal failure group, and patients with stage 4 or 5 kidney disease were excluded from the hepatic failure group. The dosage regimens simulated in the in vitro model included a traditional CMS dose of 2.5 mg/kg/day, those that were recommended in the patient package insert (28), and higher-exposure dosage regimens administered in a front-loading fashion as shown in Table 1. Free drug areas under the concentration-time curves (fAUCs) from 0 to 24 h and from 24 to 48 h, were computed for each regimen using numeric integration of the functions of concentration versus time (ADAPT 5, Biomedical Simulations Resource [BMSR]).
TABLE 1.
Drug exposure by regimen simulating the pharmacokinetics in hepatic and renal failure patients
| Proposed dosing regimen | Total daily colistin dose (mg) |
fAUC (mg · h/liter) |
||
|---|---|---|---|---|
| 0–24 h | 24–48 h | 0–24 h | 24–48 h | |
| Hepatic | ||||
| Traditional | ||||
| 0.6 mg/liter every 24 h × 2 doses | 0.162 | 0.162 | 2.76 | 2.77 |
| 1.0 mg/liter every 24 h × 2 doses | 0.270 | 0.270 | 4.59 | 4.62 |
| 0.6 mg/liter every 12 h × 4 doses | 0.324 | 0.324 | 5.32 | 5.54 |
| 1.0 mg/liter every 12 h × 4 doses | 0.540 | 0.540 | 8.87 | 9.24 |
| 2.0 mg/liter every 24 h × 2 doses | 0.540 | 0.540 | 9.18 | 9.24 |
| 2.0 mg/liter every 12 h × 4 doses | 1.08 | 1.08 | 17.7 | 18.5 |
| High dose | ||||
| 4.0 mg/liter every 24 h × 2 doses | 1.08 | 1.08 | 18.4 | 18.5 |
| 4.0 mg/liter every 12 h × 4 doses | 2.16 | 2.16 | 35.5 | 36.9 |
| 8.0 mg/liter every 24 h × 2 doses | 2.16 | 2.16 | 36.7 | 36.9 |
| 8.0 mg/liter every 12h × 4 doses | 4.32 | 4.32 | 70.9 | 73.9 |
| Front loaded | ||||
| 6 mg/liter every 24 h × 1 dose followed by 2 mg/liter every 24 h × 1 dose | 1.62 | 0.540 | 27.6 | 9.33 |
| 6 mg/liter every 12 h × 1 dose followed by 2 mg/liter every 12 h × 3 doses | 2.16 | 0.540 | 36.1 | 18.6 |
| 10 mg/liter every 24 h × 1 dose followed by 2 mg/liter every 24 h × 1 dose | 2.70 | 0.540 | 45.9 | 9.44 |
| 10 mg/liter every 12 h × 1 dose followed by 2 mg/liter every 12 h × 3 doses | 3.24 | 0.540 | 54.5 | 18.7 |
| Renal | ||||
| Traditional | ||||
| 0.6 mg/liter every 24 h × 2 doses | 0.162 | 0.162 | 8.65 | 11.5 |
| 1.0 mg/liter every 24 h × 2 doses | 0.27 | 0.27 | 14.4 | 19.1 |
| 2.0 mg/liter every 24 h × 2 doses | 0.54 | 0.54 | 28.8 | 38.2 |
| High dose | ||||
| 4.0 mg/liter every 24 h × 2 doses | 1.08 | 1.08 | 57.7 | 76.4 |
| 8.0 mg/liter every 24 h × 2 doses | 2.16 | 2.16 | 115 | 153 |
| Front loaded | ||||
| 6 mg/liter every 24 h × 1 dose followed by 2 mg/liter every 24 h × 1 dose | 1.62 | 0.54 | 86.5 | 56.9 |
For both traditional and front-loaded regimens, serial samples were collected aseptically for determination of bacterial counts at 0, 0.5, 1, 2, 4, 6, 8, 24, 26, 28, 32, and 48 h for characterization of colistin pharmacodynamics. Serial dilutions with sterile saline were performed. Viable bacterial counts were determined by plating 50-μl samples of each diluted sample on drug-free Mueller-Hinton agar plates using an automatic WASP spiral plater (Microbiology International, Rockville, MD). Plates were incubated for 24 h at 37°C before colony counts were determined. Colony counts (log10 CFU/ml) were determined using an automated aCOLyte bacterial colony counter, (Symbiosis, Frederick, MD) with a limit of quantification of 2.0 CFU/ml (29). The colony count (log10 CFU/ml) data were plotted as a function of time for all tested drug regimens for each clinical isolate (26). Bactericidal activity at 24 h was defined as a ≥99.9% reduction (3 log10 reduction) in colony counts compared to the starting inoculum at 0 h (predose).
Samples collected from the in vitro PK/PD experiments were placed in microcentrifuge tubes and immediately stored at −80°C until analysis. Colistin concentrations were determined by a standard agar diffusion bioassay using antibiotic medium 9 as the base seed agar and antibiotic medium 10 agar which was inoculated with Escherichia coli ATCC 35218 as an indicator organism. Each standard and sample were tested in triplicate using a cork borer to establish 5-mm holes in the agar, which were filled with 25 μl of the sample. Concentrations which were used as standard curves for colistin were 0.25, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 mg/liter. The limit of detection for colistin was 0.25 mg/liter for control standards. The standard curves of the zone sizes versus the natural logarithm of the drug concentration were linear between 0.5 and 10 mg/liter when the standards were prepared in SMHB (r2 of ≥0.90, intraday coefficient of variation [CV] of ≤5.2%, and interday CV of ≤10.2%). Plates were incubated for 18 to 24 h at 37°C. The diameters of inhibition zones for samples and standards were measured to the nearest 0.1 mm with a vernier caliper. The observed colistin concentrations were within 10% of targeted concentrations.
PK/PD analysis.
The initial rate of kill was quantified as the initial slope of the log CFU/ml versus time as well as the initial and maximum extents of killing relative to those for the growth control for both isolates and all regimens. The colistin overall killing activity was quantified as the percent reduction in integrated bacterial burden. This was computed by first integrating the area under the CFU/ml curves (AUC_CFUtreatment and AUC_CFUgrowth control were determined using the linear up, log down trapezoidal rule), for 24 h and 48 h, for all experiments. The percent reduction in the bacterial burden was then computed using equation 1:
| (1) |
RESULTS
Regimens simulating the pharmacokinetics of colistin in hepatic failure.
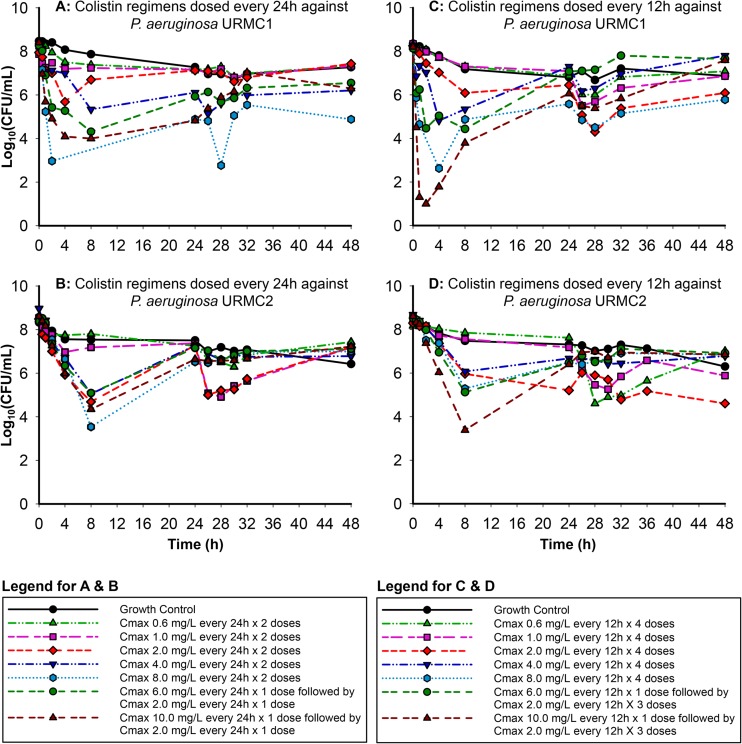
Colistin exhibited rapid concentration-dependent bactericidal initial activity against both clinical isolates (URMC1 and URMC2) (Fig. 1 and 2) for the higher-dosage regimens. The traditional regimens simulating hepatic failure against URMC1 dosed every 24 h (Fig. 1A) resulted in a reduction of up to 2.26 log10 by 4 h. The high-dosage regimens resulted in a reduction of up to 3.32 log10 by 8 h, while the front-loaded regimens resulted in a reduction of up to 4.40 log10 in the same time period (Fig. 1A). All regimens resulted in regrowth starting at 24 h. The traditional regimens simulating hepatic failure against URMC2 dosed every 24 h (Fig. 1B) resulted in a reduction of up to 3.80 log10 by 8 h which was not sustained beyond 24 h, with almost complete regrowth similar to that for the growth control by 48 h. The high-dosage regimens showed the greatest decrease in bacterial burden, with reductions of up to 5.05 log10 by 8 h. The front-loaded regimens resulted in a reduction of up to 4.30 log10 by 8 h.
FIG 1.
In vitro killing of two clinical P. aeruginosa isolates (URMC1 and URMC2) by colistin using pharmacokinetic profiles simulated for hepatic failure patients.
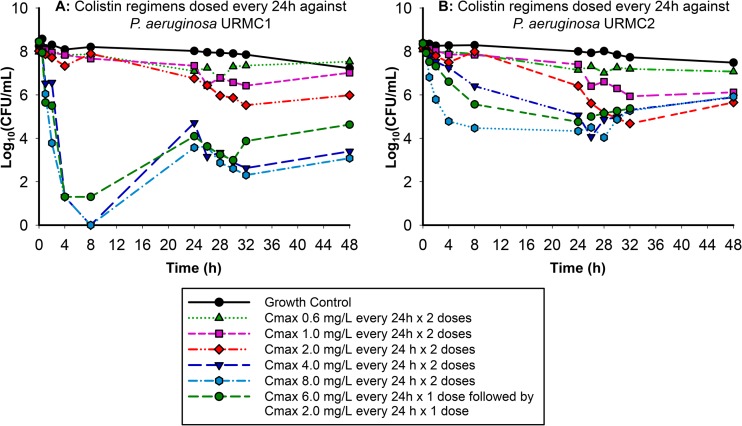
FIG 2.
In vitro killing of two clinical P. aeruginosa isolates (URMC1 and URMC2) by colistin using pharmacokinetic profiles simulated for renal failure patients.
Traditional regimens simulating hepatic failure dosed every 12 h against URMC1 resulted in minimal reductions by 4 h (Fig. 1C). While the high-dosage regimens resulted in a reduction of up to 5.59 log10 by 4 h, the reduction attained by the front-loaded regimens ranged from 3.67 to 7.19 log10. The every-12-h (q12h) dosing regimens, with fAUCs similar to or higher than those of the q24h regimens, exhibited the most aggressive regrowth pattern. The regimens simulating hepatic failure dosed every 12 h against URMC2 (Fig. 1D) demonstrated bactericidal activity for the high-dosage and front-loaded regimens. The high-dosage regimens displayed aggressive regrowth compared to the traditional regimens, while the front-loaded regimens resulted in a reduction of up to 5.22 log10 by 8 h, with considerable regrowth of ∼6.84 log10 by 48 h.
Regimens simulating the pharmacokinetics of colistin in renal failure.
The regimens simulating renal failure against URMC1 dosed every 24 h (Fig. 2A) displayed a clear separation in the rate and extent of kill achieved by the higher-dose regimens and the front-loaded regimen (the ratio of initial rate of kill up to 2 h of these higher-dosage regimens compared to the lowest-dosage regimen, with a maximum concentration [Cmax] of 0.6 mg/liter, ranged from 38.7 to 98.1). The regimens with a Cmax of ≥4 mg/liter resulted in bacterial counts below the limit of quantification (i.e., 2 log10 CFU/ml) by 4 h, and the bactericidal activity for these high-dosage regimens was sustained up to 48 h with minimal regrowth. The regimens simulating renal failure against URMC2 dosed every 24 h (Fig. 2B) demonstrated bactericidal killing activity for the higher-dosage regimens and the front-loaded regimen by 24 h. The antimicrobial pharmacodynamic activity observed against URMC2 was attenuated compared to the activity of these regimens against URMC1 (the ratio of the initial slope of the kill curves up to 2 h for these higher-dosage regimens compared to the lowest-dosage regimen, with a Cmax of 0.6 mg/liter, ranged from 6.47 to 19.7) (Fig. 2A).
Pharmacodynamics of colistin exposure against P. aeruginosa.
The regimens simulating renal and hepatic failure are described in Table 1 with the corresponding fAUCs at the two integrated endpoints of 24 h and 48 h. The analysis of these fAUCs based on equation 1 is presented in Table 2. The fAUCs (0 to 48 h) for the regimens simulating renal failure ranged from 20 to 268 mg · h/liter, while the fAUCs for the regimens simulating hepatic failure ranged between 6 and 73 mg · h/liter. Against URMC1, among regimens simulating hepatic failure, the front-loaded regimen with a Cmax of 6 mg/liter × 1 dose followed by a Cmax of 2 mg/liter × 1 dose outperformed the traditional regimens with a Cmax of 2.0 mg/liter every 12 h × 4 doses and a Cmax of 4.0 mg/liter every 24 h × 2 doses. Regimens with a Cmax of 10 mg/liter every 12 h × 1 dose followed by a Cmax of 2.0 mg/liter every 12 h × 3 doses, 4.0 mg/liter every 12 h × 4 doses, and 8.0 mg/liter every 24 h × 2 doses, with similar 0- to 48-h fAUCs, had very comparable percent reductions in bacterial burden during the first 24 h, but the performance of these regimens significantly diverged during the second 24 h. Regimens with a Cmax of 8.0 mg/liter every 24 h × 2 doses, with identical fAUCs during 0 to 24 h and 24 to 48 h, had the highest reduction in bacterial burden among all the hepatic regimens. In contrast, even though the front-loaded regimen with a Cmax of 10 mg/liter every 12 h × 1 dose followed by a Cmax of 2.0 mg/liter every 12 h × 3 doses had the same 0- to 24-h and 24- to 48-h fAUCs, the increased dosing frequency of q12h did not perform as well as regimens with a q24h dosing frequency and similar fAUCs. The front-loaded regimen with a Cmax of 10.0 mg/liter every 12 h × 1 dose followed by 2 mg/liter every 12 h × 3 doses had the highest fAUC of 54.5 during 0 to 24 h in this group, with an fAUC of 73.1 during 0 to 48 h, resulting in a 98.6% reduction during the first 24 h and only a 77.1% reduction over 48 h.
TABLE 2.
Pharmacodynamic response against P. aeruginosa URMC1 and URMC2 for regimens simulating those for hepatic and renal failure patients
| Dosage regimen |
fAUC (mg · h/liter) |
URMC1 |
URMC2 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log10 AUCCFUa (CFU · h/ml) |
Reduction in bacterial burden (%)b |
Log10 AUCCFU (CFU · h/ml) |
Reduction in bacterial burden (%) |
|||||||
| 0–24 h | 0–48 h | 0–24 h | 0–48 h | 0–24 h | 0–48 h | 0–24 h | 0–48 h | 0–24 h | 0–48 h | |
| Hepatic | ||||||||||
| Traditional | ||||||||||
| 0.6 mg/liter every 24 h × 2 doses | 2.76 | 5.53 | 8.93 | 9.08 | 36.9 | 25.4 | 9.08 | 9.18 | -8.07 | 12.0 |
| 1.0 mg/liter every 24 h × 2 doses | 4.59 | 9.21 | 8.63 | 8.88 | 68.2 | 53.4 | 8.91 | 9.09 | 28.4 | 33.3 |
| 0.6 mg/liter every 12 h × 4 doses | 5.32 | 10.9 | 8.89 | 8.97 | 42.5 | 41.6 | 9.11 | 9.17 | -13.4 | -3.24 |
| 1.0 mg/liter every 12 h × 4 doses | 8.87 | 18.1 | 8.89 | 8.94 | 41.9 | 46.5 | 9.07 | 9.09 | 4.61 | 20.9 |
| 2.0 mg/liter every 24 h × 2 doses | 9.18 | 18.4 | 8.36 | 8.76 | 82.7 | 64.7 | 8.50 | 8.66 | 72.2 | 68.1 |
| 2.0 mg/liter every 12 h × 4 doses | 17.7 | 36.2 | 8.41 | 8.43 | 81.0 | 83.4 | 8.74 | 8.75 | 55.8 | 63.5 |
| High dose | ||||||||||
| 4.0 mg/liter every 24 h × 2 doses | 18.4 | 36.8 | 8.26 | 8.32 | 86.4 | 87.1 | 8.82 | 8.91 | 40.3 | 40.1 |
| 4.0 mg/liter every 12 h × 4 doses | 35.5 | 72.4 | 8.32 | 8.92 | 84.4 | 49.0 | 8.88 | 8.94 | 36.6 | 40.9 |
| 8.0 mg/liter every 24 h × 2 doses | 36.7 | 73.7 | 7.68 | 7.72 | 96.4 | 96.7 | 8.65 | 8.81 | 59.9 | 53.1 |
| 8.0 mg/liter every 12 h × 4 doses | 70.9 | 144 | 7.65 | 7.71 | 94.5 | 95.2 | 8.75 | 8.85 | 54.9 | 53.7 |
| Front loaded | ||||||||||
| 6 mg/liter every 24 h × 1 dose followed by 2 mg/liter every 24 h × 1 dose | 27.6 | 36.9 | 8.05 | 8.22 | 91.6 | 89.7 | 8.72 | 8.89 | 52.6 | 43.0 |
| 6 mg/liter × 1 dose followed by 2mg/liter × 3 doses | 36.1 | 54.7 | 8.04 | 9.07 | 91.8 | 28.0 | 8.77 | 8.90 | 47.8 | 45.7 |
| 10 mg/liter every 24 h × 1 dose followed by 2mg/liter every 24 h × 1 dose | 45.9 | 55.4 | 7.83 | 8.27 | 94.9 | 88.5 | 8.56 | 8.75 | 67.3 | 58.6 |
| 10 mg/liter every 12 h × 1 dose followed by 2mg/liter every 12 h × 3 doses | 54.5 | 73.1 | 7.27 | 8.53 | 98.6 | 77.1 | 8.60 | 8.77 | 68.2 | 60.5 |
| Renal | ||||||||||
| Traditional | ||||||||||
| 0.6 mg/liter every 24 h × 2 doses | 8.65 | 20.1 | 9.13 | 9.28 | 61.0 | 60.4 | 9.17 | 9.26 | 62.2 | 65.3 |
| 1.0 mg/liter every 24 h × 2 doses | 14.4 | 33.5 | 9.06 | 9.11 | 66.9 | 73.2 | 9.17 | 9.18 | 62.2 | 70.8 |
| 2.0 mg/liter every 24 h × 2 doses | 28.8 | 67.0 | 9.04 | 9.05 | 68.1 | 76.7 | 9.13 | 9.13 | 65.4 | 74.2 |
| High dose | ||||||||||
| 4.0 mg/liter every 24 h × 2 doses | 57.7 | 134 | 8.10 | 8.10 | 96.3 | 97.4 | 8.47 | 8.49 | 92.3 | 94.2 |
| 8.0 mg/liter every 24 h × 2 doses | 115 | 268 | 8.06 | 8.06 | 96.7 | 97.6 | 8.24 | 8.27 | 95.6 | 96.5 |
| Front loaded | ||||||||||
| 6 mg/liter every 24 h × 1 dose followed by 2 mg/liter every 24 h × 1 dose | 86.5 | 143 | 8.07 | 8.07 | 96.6 | 97.6 | 8.06 | 8.10 | 97.0 | 97.6 |
Log10 AUCCFU, log of the area under the CFU/ml-versus-time curve.
Reduction in bacterial burden was computed as per equation 1.
Against URMC2, the higher-dosage regimens simulating renal failure with the favorable pharmacokinetics and a longer t1/2 (14.8 h) performed well against URMC1 compared to the regimens simulating hepatic failure. The substantial increase in fAUC associated with the regimen of a Cmax of 8.0 mg/liter every 24 h × 2 doses compared to those with a Cmax of 4.0 mg/liter every 24 h × 2 doses and a Cmax of 6 mg/liter every 24 h for 1 dose followed by 2 mg/liter every 24 h × 1 dose against both strains did not result in an increased reduction in the bacterial burden. Interestingly, against URMC2, even though the two Pseudomonas aeruginosa clinical isolates had the same MICs, the pharmacodynamic activity was markedly different. The regimens with a q12h dosing frequency did not perform as well as the regimens with q24h dosing despite the increase in drug exposure (fAUC). Overall, none of the regimens were able to achieve good pharmacodynamic activity against URMC2.
DISCUSSION
Colistin is a last-resort antibiotic increasingly used in critically ill patients infected with P. aeruginosa strains which are resistant to nearly all other commercially available antibiotics (6, 8, 30, 31). However, there is a paucity of clinical studies focused on the critically ill patient population to guide its optimal use (17, 18). As a result, dosing recommendations for CMS have substantial deficiencies, and resultant plasma concentrations in critically ill patients are suboptimal (32). In the present study, we investigated new front-loaded dosing schemes simulating concentration-time profiles of formed colistin in patients with severe liver and renal disease against two clinical P. aeruginosa isolates (19) from a recent study. This pharmacokinetic study (19) demonstrated that the disposition of formed colistin was significantly different for the hepatic failure (t1/2 of 3.2 h) and the renal failure (t1/2 of 14.8 h) patients. Interestingly, the terminal half-life in renal failure patients from this study was similar to that in critically ill patients as determined by Plachorous et al. (t1/2 of 14.4 h) and Garonzik et al. (t1/2 of 13.0 h) (17, 18). Mohamed et al., using a previously developed PK/PD model based on in vitro time-kill studies describing the bactericidal activity of colistin against a resistant P. aeruginosa strain (33), found that a loading dose of 480 to 720 mg of CMS (6 to 9 million units [MU]) administered to critically ill patients was very effective in decreasing the bacterial load by 3 log10 CFU/ml during the first 5 to 6 h of therapy. Colistin concentrations measured in these patients were, on average, 1.34 mg/liter at 8 h following the loading dose of 480 mg (34). Taken together, these new pharmacokinetic findings have vastly improved our understanding of the disposition of CMS and formed colistin in critically ill patients. Therefore, we hypothesized that front-loaded colistin regimens will result in a rapid reduction of a high bacterial density of P. aeruginosa and reduce cumulative drug exposure.
A number of observations emerged from the present investigation. First, the current achievable plasma concentrations and dosing recommendations for colistin point to the fact that there is a need for a loading dose and a change in the current suggested CMS dosing strategy (17, 35). Clearly, CMS doses at the upper end of the product label recommendation have resulted in suboptimal concentrations. For example, the median of the average concentration at steady state (CSSavg) determined by Garonzik et al. (18) was 2.36 mg/liter by 24 h, which barely exceeds the breakpoint of 2 mg/liter. Second, colistin-induced nephrotoxicity has been shown to be dose limiting and reversible upon discontinuation of treatment (36, 37). Pogue et al. reported an 8-fold increase in the propensity for developing nephrotoxicity in patients receiving CMS 4.0 mg/kg/day (colistin base activity [CBA]) or greater versus patients receiving 2.0 mg/kg/day (CBA) or less (38). A prospective study by Paul et al. (39) compared 200 patients treated with colistin (average colistin dose of 6 MU/day, equivalent to 180 mg of colistin base activity) with 295 patients treated with comparator antibiotics (imipenem, meropenem, or ampicillin-sulbactam). Although one of the confounding factors of this study was that the patient population was comprised of those already at increased risk for developing nephrotoxicity and the colistin arm was comprised of patients with worse prognostic factors, a major conclusion of the study was that there was an increased incidence of nephrotoxicity and a lower survival rate associated with the colistin treatment arm (the 30-day mortality for the colistin treatment arm was 39%, compared to the 28.8% for the comparator arm) (39). With increased and improved supportive care of critically ill patients, close monitoring of their renal function and avoidance of coadministration of nephrotoxic agents has led to colistin-related nephrotoxicity being less prominent (6). Recent reports support these claims, with lower rates of nephrotoxicity (10 to 30%) (40) compared to those found in older studies (50%) (41). Therefore, increasing the daily dose for colistin for a short duration seems a promising and clinically viable strategy to decrease the potential for nephrotoxicity while enhancing the antimicrobial killing activity. This strategy has been applied for other antimicrobials (42–44) and may be particularly promising for colistin, which can achieve rapid and concentration-dependent bactericidal activity (7). However, these findings should also be balanced with toxicodynamic evaluations of these new regimens, as it has been shown that the fAUC is the driver for nephrotoxicity for colistin (41, 45).
Third, in the current study, we determined that front-loaded regimens, with a high Cmax for a short duration (for front-loaded regimens simulating both renal and hepatic failure), resulted in different killing activity than traditional regimens with similar fAUC values. The longer half-life seen in the renal failure population combined with a colistin front-loaded regimen provided a highly beneficial strategy to achieve high initial peak concentrations followed by de-escalation of therapy, resulting in lower exposures and at least ≥3-log10 reductions in the bacterial burden against both isolates. These IVPM experiments have illustrated that both front-loaded and higher-than-current traditional regimens selected to have maximal colistin drug exposure were unable to successfully eradicate the two clinical isolates. Although the front-loaded regimens showed initial promising bactericidal killing against both clinical isolates, when maximal levels of drug were administered, extensive regrowth occurred beyond 8 h.
Collectively, front-loading CMS/colistin combination regimens in the clinical setting may be considered as a strategy to treat high-bacterial-density infections (46). At even higher bacterial inocula, as seen in bacterial ventilator-associated pneumonia (VAP) (47), achieving high colistin concentrations against more-difficult-to-treat polymyxin-heteroresistant strains may pose a formidable challenge. Bergen et al. investigated the combination of colistin plus doripenem against P. aeruginosa in vitro, simulating colistin pharmacokinetics obtained from critically ill patients (35). Their results suggested that the addition of doripenem to clinically achievable colistin regimens (e.g., Cmax of 0.5 and 2.0 mg/liter) substantially improved antibacterial activity compared to colistin monotherapy. Hence, simply increasing the Cmax of colistin monotherapy is not likely to provide complete bacterial reduction in immunocompromised patients. Therefore, the addition of a synergistic second antimicrobial agent may be a promising strategy to serve as a backbone of a combination regimen by sustaining the initial killing achieved by a colistin front-loaded regimen.
We acknowledge potential limitations of the current study, as bacterial eradication is multifactorial and depends on the choice of the antibiotic, the dose selected, the type of offending agent, the severity of the infection, and the immune status of the host. Our study utilized only two clinical isolates which may not be representative of all P. aeruginosa strains. Further in vivo studies are warranted to strengthen the translation of these in vitro findings before these results can guide the selection of optimal dosage regimens for critically ill patients. Overall, the results from the current study are promising, as they herald a new dosing strategy for polymyxins.
ACKNOWLEDGMENTS
This study was funded in part by the Joseph F. Dasta Critical Care Research Grant from the Society of Critical Care Medicine and grant R01AI079330 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. J.B.B. is an Australian Research Council DECRA fellow (DE120103084). N.S.L was partially supported by an AFPE predoctoral fellowship.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Published ahead of print 16 December 2013
REFERENCES
- 1.Paterson DL, Lipman J. 2007. Returning to the pre-antibiotic era in the critically ill: the XDR problem. Crit. Care Med. 35:1789–1791. 10.1097/01.CCM.0000269352.39174.A4 [DOI] [PubMed] [Google Scholar]
- 2.Lim T-P, Lee W, Tan T-Y, Sasikala S, Teo J, Hsu L-Y, Tan T-T, Syahidah N, Kwa AL. 2011. Effective antibiotics in combination against extreme drug-resistant Pseudomonas aeruginosa with decreased susceptibility to polymyxin B. PLoS One 6:e28177. 10.1371/journal.pone.0028177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, Bartlett JG. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657–668. 10.1086/499819 [DOI] [PubMed] [Google Scholar]
- 4.Souli M, Galani I, Giamarellou H. 2008. Emergence of extensively drug-resistant and pandrug-resistant Gram-negative bacilli in Europe. Euro Surveill. 13(47):pii=19045 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19045 [PubMed] [Google Scholar]
- 5.Rossolini GM, Mantengoli E. 2005. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosa. Clin. Microbiol. Infect. 11(Suppl 4):17–32. 10.1111/j.1469-0691.2005.01161.x [DOI] [PubMed] [Google Scholar]
- 6.Falagas ME, Kasiakou SK, Saravolatz LD. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin. Infect. Dis. 40:1333–1341. 10.1086/429323 [DOI] [PubMed] [Google Scholar]
- 7.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601. 10.1016/S1473-3099(06)70580-1 [DOI] [PubMed] [Google Scholar]
- 8.Kallel H, Bahloul M, Hergafi L, Akrout M, Ketata W, Chelly H, Hamida CB, Rekik N, Hammami A, Bouaziz M, Pavleas J, Skiada A, Daikos GL, Rigas K, Mega A, Archondoulis N, Vernikos P, Salatas K, Thomopoulos G. 2006. Colistin as a salvage therapy for nosocomial infections caused by multidrug-resistant bacteria in the ICU Int. J. Antimicrob. Agents 28:366–369. 10.1016/j.ijantimicag.2006.07.008 [DOI] [PubMed] [Google Scholar]
- 9.Landman D, Georgescu C, Martin DA, Quale J. 2008. Polymyxins revisited. Clin. Microbiol. Rev. 21:449–465. 10.1128/CMR.00006-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M. 2010. Steady-state pharmacokinetics and BAL concentration of colistin in critically ill patients after IV colistin methanesulfonate administration. Chest 138:1333–1339. 10.1378/chest.10-0463 [DOI] [PubMed] [Google Scholar]
- 11.De Pascale G, Sandroni C, Antonelli M. 2011. Colistin use in critically ill patients. Chest 139:234. 10.1378/chest.10-2031 [DOI] [PubMed] [Google Scholar]
- 12.Roberts J, Roberts M, Semark A, Udy A, Kirkpatrick C, Paterson D, Roberts M, Kruger P, Lipman J. 2011. Antibiotic dosing in the ‘at risk' critically ill patient: linking pathophysiology with pharmacokinetics/pharmacodynamics in sepsis and trauma patients. BMC Anesthesiol. 11:3. 10.1186/1471-2253-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varghese JM, Roberts JA, Lipman J. 2010. Pharmacokinetics and pharmacodynamics in critically ill patients. Curr. Opin. Anesthesiol. 23:472–478. 10.1097/ACO.0b013e328339ef0a [DOI] [PubMed] [Google Scholar]
- 14.Michalopoulos A, Falagas ME. 2008. Colistin and polymyxin B in critical care. Crit. Care Clin. 24:377–391. 10.1016/j.ccc.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 15.Lim LM, Ly N, Anderson D, Yang JC, Macander L, Jarkowski A, Forrest A, Bulitta JB, Tsuji BT. 2010. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 30:1279–1291. 10.1592/phco.30.12.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, Etherington C, Turnidge J. 2003. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J. Antimicrob. Chemother. 52:987–992. 10.1093/jac/dkg468 [DOI] [PubMed] [Google Scholar]
- 17.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by Gram-negative bacteria. Antimicrob. Agents Chemother. 53:3430–3436. 10.1128/AAC.01361-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories Of Patients. Antimicrob. Agents Chemother. 55:3284–3294. 10.1128/AAC.01733-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas C, Kaufman D, Yohonn L, Forrest A, Tsuji B, Li J, He H, Nation RL. 2009. Colistin pharmacokinetics (PK) in the presence of renal and liver disease, poster 822. 38th Critical Care Congress, Nashville, TN. Crit. Care Med. 37(Suppl):A402 [Google Scholar]
- 20.Akins RL, Rybak MJ. 2001. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 45:454–459. 10.1128/AAC.45.2.454-459.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pankuch GA, Jacobs MR, Appelbaum PC. 2003. Bactericidal activity of daptomycin against Streptococcus pneumoniae compared with eight other antimicrobials. J. Antimicrob. Chemother. 51:443–446. 10.1093/jac/dkg091 [DOI] [PubMed] [Google Scholar]
- 22.Poudyal A, Howden BP, Bell JM, Gao W, Owen RJ, Turnidge JD, Nation RL, Li J. 2008. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J. Antimicrob. Chemother. 62:1311–1318. 10.1093/jac/dkn425 [DOI] [PubMed] [Google Scholar]
- 23.Bulitta JB, Yang JC, Yohonn L, Ly NS, Brown SV, D'Hondt RE, Jusko WJ, Forrest A, Tsuji BT. 2010. Attenuation of colistin bactericidal activity by high inoculum of Pseudomonas aeruginosa characterized by a new mechanism-based population pharmacodynamic model. Antimicrob. Agents Chemother. 54:2051–2062. 10.1128/AAC.00881-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen RJ, Li J, Nation RL, Spelman D. 2007. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J. Antimicrob. Chemother. 59:473–477. 10.1093/jac/dkl512 [DOI] [PubMed] [Google Scholar]
- 25.Rao GG, Ly NS, Haas CE, Garonzik SM, Forrest AF, Bulitta JB, Kelchlin P, Li J, Nation RL, Tsuji BT. 2010. Abstr. 50th Intersci. Conf. Antimicrob. Agents Chemother., abstr A1-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harigaya Y, Bulitta JB, Forrest A, Sakoulas G, Lesse AJ, Mylotte JM, Tsuji BT. 2009. Pharmacodynamics of vancomycin at simulated epithelial lining fluid concentrations against methicillin-resistant Staphylococcus aureus (MRSA): implications for dosing in MRSA pneumonia. Antimicrob. Agents Chemother. 53:3894–3901. 10.1128/AAC.01585-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lucey MR, Brown KA, Everson GT, Fung JJ, Gish R, Keeffe EB, Kneteman NM, Lake JR, Martin P, McDiarmid SV, Rakela J, Shiffman ML, So SK, Wiesner RH. 1997. Minimal criteria for placement of adults on the liver transplant waiting list: a report of a national conference organized by the American Society of Transplant Physicians and the American Association for the Study of Liver Diseases. Liver Transplant. Surg. 3:628–637. 10.1002/lt.500030613 [DOI] [PubMed] [Google Scholar]
- 28.Monarch Pharmaceuticals 2005. Coly-Mycin M parenteral package insert. Monarch Pharmaceuticals, Bristol, TN [Google Scholar]
- 29.Begic D, von Eiff C, Tsuji BT. 2009. Daptomycin pharmacodynamics against Staphylococcus aureus hemB mutants displaying the small colony variant phenotype. J. Antimicrob. Chemother. 63:977–981. 10.1093/jac/dkp069 [DOI] [PubMed] [Google Scholar]
- 30.Giamarellou H, Poulakou G. 2009. Multidrug-resistant Gram-negative infections: what are the treatment options? Drugs 69:1879–1901. 10.2165/11315690-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 31.Michalopoulos AS, Karatza DC. 2010. Multidrug-resistant Gram-negative infections: the use of colistin. Expert Rev. Anti Infect. Ther. 8:1009–1017. 10.1586/eri.10.88 [DOI] [PubMed] [Google Scholar]
- 32.Bergen PJ, Li J, Nation RL. 2011. Dosing of colistin-back to basic PK/PD. Curr. Opin. Pharmacol. 11:464–469. 10.1016/j.coph.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohamed A, Carrs O, Friberg LE. 2011. Pharmacokinetic-pharmacodynamic modeling of pre-existing and emerging resistance of Pseudomonas aeruginosa to colistin, abstr 2223. Abstr. Annu. Meet. Popul Approach Group, Europe, Athens, Greece [Google Scholar]
- 34.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob. Agents Chemother. 56:4241–4249. 10.1128/AAC.06426-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergen PJ, Tsuji BT, Bulitta JB, Forrest A, Jacob J, Sidjabat HE, Paterson DL, Nation RL, Li J. 2011. Synergistic killing of multidrug-resistant Pseudomonas aeruginosa at multiple inocula by colistin combined with doripenem in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob. Agents Chemother. 55:5685–5695. 10.1128/AAC.05298-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch-Weser JAN, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. 1970. Adverse effects of sodium colistimethate manifestations and specific reaction rates during 317 courses of therapy. Ann. Intern. Med. 72:857–868. 10.7326/0003-4819-72-6-857 [DOI] [PubMed] [Google Scholar]
- 37.Levin AS, Barone AA, Penco J, Santos MV, Marinho IS, Arruda EA, Manrique EI, Costa SF. 1999. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin. Infect. Dis. 28:1008–1011. 10.1086/514732 [DOI] [PubMed] [Google Scholar]
- 38.Pogue JM, Marchaim D, Kaye D, Kaye KS. 2011. Revisiting “older” antimicrobials in the era of multidrug resistance. Pharmacotherapy 31:912–921. 10.1592/phco.31.9.912 [DOI] [PubMed] [Google Scholar]
- 39.Paul M, Bishara J, Levcovich A, Chowers M, Goldberg E, Singer P, Lev S, Leon P, Raskin M, Yahav D, Leibovici L. 2010. Effectiveness and safety of colistin: prospective comparative cohort study. J. Antimicrob. Chemother. 65:1019–1027. 10.1093/jac/dkq069 [DOI] [PubMed] [Google Scholar]
- 40.Falagas M, Kasiakou S. 2006. Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit. Care 10:R27. 10.1186/cc3995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JM, Dorman DC, Roy LP. 1970. Acute renal failure due to overdosage of colistin. Med. J. Aust. 2:923–924 [DOI] [PubMed] [Google Scholar]
- 42.Tsuji BT, Brown T, Parasrampuria R, Brazeau D, Forrest A, Kelchlin PA, Holden PN, Peloquin CA, Hanna D, Bulitta JB. 2012. Front-loaded linezolid regimens result in increased killing and suppression of the accessory gene regulator system of Staphylococcus aureus. Antimicrob. Agents Chemother. 56:3712–3719. 10.1128/AAC.05453-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuji BT, Okusanya OO, Bulitta JB, Forrest A, Bhavnani SM, Fernandez PB, Ambrose PG. 2011. Application of pharmacokinetic-pharmacodynamic modeling and the justification of a novel fusidic acid dosing regimen: raising Lazarus from the dead. Clin. Infect. Dis. 52:S513–S519. 10.1093/cid/cir166 [DOI] [PubMed] [Google Scholar]
- 44.Drusano GL, Liu W, Brown DL, Rice LB, Louie A. 2009. Impact of short-course quinolone therapy on susceptible and resistant populations of Staphylococcus taureus. J. Infect. Dis. 199:219–226. 10.1086/595739 [DOI] [PubMed] [Google Scholar]
- 45.Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. 2008. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 61:636–642. 10.1093/jac/dkm511 [DOI] [PubMed] [Google Scholar]
- 46.Petrosillo N, Ioannidou E, Falagas ME. 2008. Colistin monotherapy vs. combination therapy: evidence from microbiological, animal and clinical studies. Clin. Microbiol. Infect. 14:816–827. 10.1111/j.1469-0691.2008.02061.x [DOI] [PubMed] [Google Scholar]
- 47.Zavascki A, Barth A, Fernandes J, Moro A, Goncalves A, Goldani L. 2006. Reappraisal of Pseudomonas aeruginosa hospital-acquired pneumonia mortality in the era of metallo-beta-lactamase-mediated multidrug resistance: a prospective observational study. Crit Care 10:R114. 10.1186/cc5006 [DOI] [PMC free article] [PubMed] [Google Scholar]