Abstract
Here, we show that spiroindolone, an effective treatment for plasmodia, is also active against Toxoplasma gondii tachyzoites. In vitro, spiroindolone NITD609 is cidal for tachyzoites (50% inhibitory concentration [IC50], 1μM) and not toxic to human cells at ≥10μM. Two daily oral doses of 100 mg/kg of body weight reduced the parasite burden in mice by 90% (P = 0.002), measured 3 days after the last dose. This inhibition of T. gondii tachyzoites in vitro and in vivo indicates that spiroindolone is a promising lead candidate for further medicine development.
TEXT
Novel, improved medicines are needed to treat toxoplasmosis (1, 2, 3). One attractive approach to treat toxoplasmosis is to repurpose compounds effective against other, related apicomplexan parasites (4). Spirotetrahydro-β-carbolines (spiroindolones) inhibit Plasmodium falciparum and Plasmodium vivax blood stage parasites, with the most effective member of this family being NITD609 (5) (Fig. 1A). NITD609 is highly active, without cytotoxicity for brain, kidney, liver, or monocytes. A single dose of 100 mg/kg of body weight completely clears murine Plasmodium berghei infection, with pharmacokinetic properties amendable to once-daily oral treatment. It has a safety profile acceptable for medicine development and no adverse histopathologic findings in rodents (5). Spiroindolone blocks protein synthesis in P. falciparum in 1 h, with the cation-transporting P-type ATPase PFATP4 the proposed molecular target (5). Here, we report that spiroindolone is active against Toxoplasma gondii and that T. gondii likely shares an ATP4 target.
FIG 1.
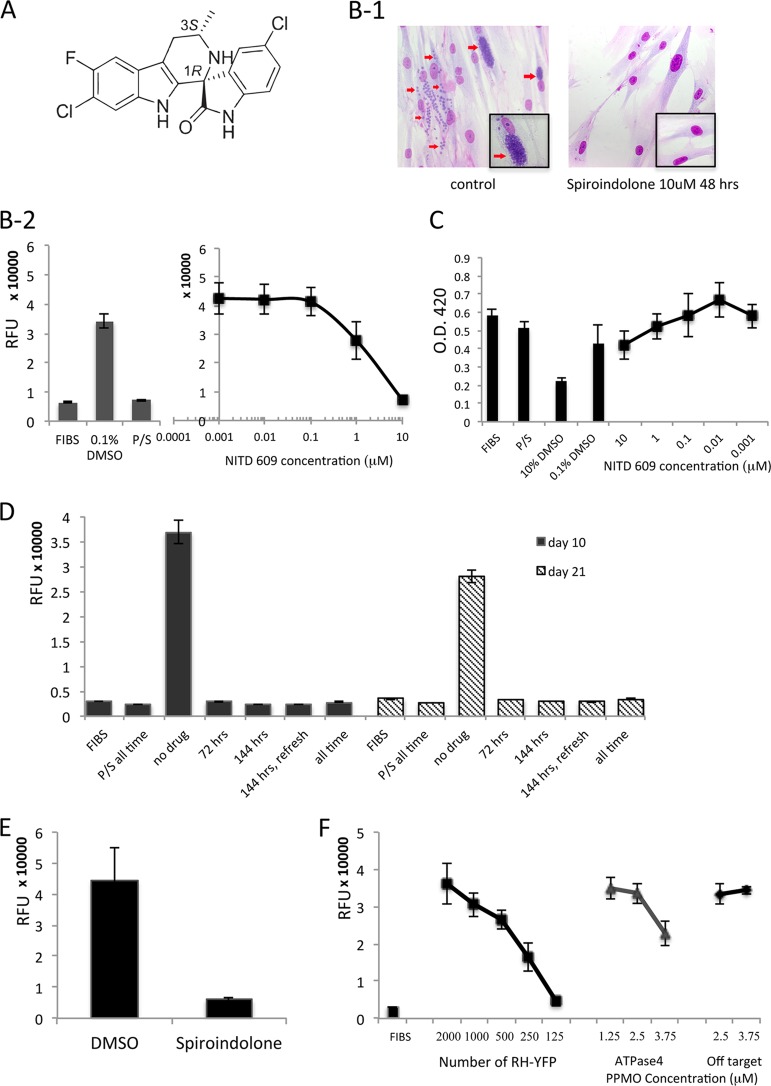
Effects of spiroindolone and PPMO directed against ATPase4 in tachyzoites of T. gondii. (A) Structure of lead spiroindolone compound NITD609, showing 1R,3S configuration. (B) Effect of NITD609 on T. gondii tachyzoites in vitro. (B-1) Giemsa-stained images 48 h after parasite infection of fibroblasts (FIBS) with and without spiroindolone (10 μM) treatment added 2 h after infection. Note replicating parasites in HFFs without spiroindolone (red arrows); representative single cells are shown in insets. The percentage of infected cells in the control cultures was 50%, with >8 parasites present in most vacuoles, whereas the spiroindolone cultures had almost no detectable recognizable parasites seen, making it infeasible to quantitate parasites per vacuole. (B-2) Fluorescence assays were performed to assess the effect of NITD609 on RH-YFP tachyzoites (96-well plates, 200 μl/well). (Left) Results for controls. RFU, relative fluorescence units; FIBS, unchallenged HFFs; P/S, pyrimethamine (40 ng/ml; Sigma) and sulfadiazine (10 μg/ml; Sigma) (standard T. gondii treatment and positive control); 0.1% DMSO, negative control. (Right) Parasite inhibition by spiroindolone at serial concentrations. At 10 μM and 1 μM, P < 0.001. (C) Effect of NITD609 on host fibroblast cells. Cell viability assay by water-soluble tetrazolium salt-1 (WST-1) incorporation and absorbance assay to assess NITD609 toxicity to HFFs. Graphs are representative of multiple experiments. O.D. 420, optical density at 420 nm. (D) NITD609 has a cidal effect on T. gondii tachyzoites in vitro. Fluorescence assays to determine parasitistatic/parasiticidal activity of NITD609 (10 μM) against RH-YFP tachyzoites were performed on days 3, 10, and 21. P/S all time, pyrimethamine and sulfadiazine were present for the duration of the experiment; 72 h, compound was present for 72 h and was then removed for the remaining duration of the experiment; 144 h, compound was present for 6 days continuously and was then removed; 144 h, refresh, compound was refreshed at day 3 and was then removed at day 6; all time, compound was present throughout the duration of the experiment. (E) Effect of NITD609 on T. gondii growth in vivo. Parasite burden in mouse peritoneal fluid was measured by fluorescence intensity. Protection was robust with a substantial dose of tachyzoites that resulted in an average parasite burden of 9.25 × 106 in untreated controls at the end of the experiment. (F) PPMO directed against ATPase4 inhibits tachyzoite replication. The left graph is the standard curve showing the fluorescence intensity directly reflecting RH-YFP growth. For all panels, error bars show standard deviations.
Spiroindolone NITD609 (Fig. 1A) was synthesized as described previously (5) by Reagents 4 Research. In vitro and in vivo assays were conducted as recently reported (1, 6, 7), with every experiment repeated at least twice (see the supplemental material). The significance of differences was determined using Student's t test, with a P value of <0.05 considered significant.
Spiroindolone NITD609 inhibited T. gondii (Fig. 1B-1) with a MIC90 for tachyzoites of 5 μM and a MIC50 of1μM (Fig. 1B-2), without toxicity to human foreskin fibroblasts (HFFs) at the highest concentration tested (10μM) (Fig. 1C). T. gondii tachyzoites were also treated with spiroindolone under four additional conditions: drug pressure (10 μM) was applied for a short time (72 h, as in a standard challenge assay) and replaced with fresh medium without drug, a longer time (6 days, as some medicines require a longer course of treatment to eliminate all parasites), or the entire duration of the experiment (21 days) (Fig. 1D). An additional 6-day group was included where, after 72 h of treatment, the growth medium was replaced with fresh medium with drug, in the event that the compound lost effectiveness by degradation over time. Parasite proliferation was assessed at several times over 21 days. Spiroindolone was cidal for T. gondii tachyzoites with 3, 10, or 21 days of treatment (Fig. 1D). It was not possible to create a viable insertional mutant parasite resistant to spiroindolone, suggesting that the drug's target is essential.
Mice were infected intraperitoneally with 2,000 RH strain, yellow fluorescent protein-expressing (RH-YFP) tachyzoites per mouse. Spiroindolone was administered to mice by gavage on the day of and the day after infection (100 mg/kg/day), with control mice receiving the drug-suspending agent alone. Five days after infection, the peritoneal parasite burden was assessed by measuring the fluorescence intensity, which directly reflected the number of RH-YFP parasites present (6). Mice treated with spiroindolone had 90% fewer parasites than the control mice treated with dimethyl sulfoxide (DMSO) alone (P = 0.002) (Fig. 1E).
In plasmodia, ATPase4 has been identified as the target of spiroindolone. (5) The knockdown of T. gondii ATPase4 (TgATP4) with exon skipping or a translation-inhibiting peptide-conjugated phosphorodiamidate morpholino oligomer (PPMO; a molecular transporter-conjugated antisense morpholino) (7) but not off-target PPMO (7) showed that TgATP4 was also essential for replication (Fig. 1F).
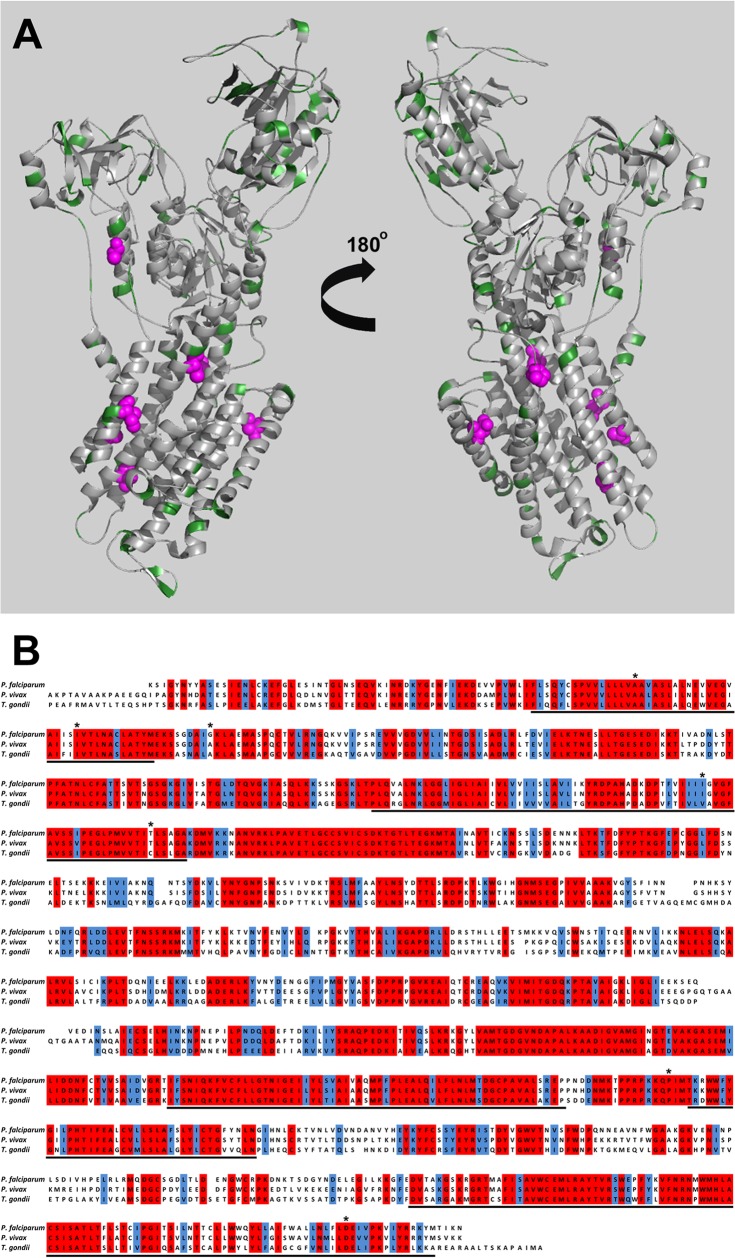
The structures of several ATPase4 homologues have been solved, revealing a conserved architecture that is likely to be similar within the apicomplexan parasites, as shown by Rottmann and coworkers (5). A model for TgATP4 was generated based against the Squalus acanthias crystal structure (PDB ID 2ZXE), using the Phyre2 server (8, 9). The TgATP4 model shares a large degree of sequence identity (Fig. 2A and B) with the ATPase4 of both P. falciparum (51%) and S. acanthias (25%). In particular, a large degree of sequence conservation is observed around the membrane-spanning region of the ATPase4 structure, which contains the proposed spiroindolone binding site at the protein-membrane interface (Fig. 2A). Multisequence alignments using ClustalW (10) (Fig. 2A) show strong conservation of the residues which confer resistance to spiroindolone in T. gondii and P. falciparum, with the majority of differences being in the solvent-exposed loops (Fig. 2B). Despite the high sequence identity and predicted structural homology in ATP4, a large difference in the efficacy of spiroindolone is seen. Subtle changes in structure and/or drug delivery, uptake, and efflux are likely to play a role in the differences in spiroindolone potency between different apicomplexan species. If the target of this spiroindolone in T. gondii is a sodium ATPase that regulates Na+ homeostasis (11), another possible reason for the difference in potency is that this ATPase could have a different localization than the PfATPase. This target could have been, for example, in the plant-like vacuole, where the parasite might require active extrusion of Na+ during extracellular life. However, two other groups studying these ATPases have found that the localization is the same in T. gondii and P. falciparum and is not in the plant-like vacuole (unpublished data). Nonetheless, differences in the sensitivity and regulation mechanism of the physiological process still might be the underlying reasons for the observed differences in the potency of the compound despite the strong conservation of the ATP4 target. Furthermore, we have not proven with detailed biochemical characterization that this is a sodium rather than a calcium ATPase. Also, we have not proven here that this TgATP4 definitely is the molecular target of the spiroindolone. These future characterizations will be important for understanding the mode of action of the spiroindolone on T. gondii, for further understanding of this ATP4 as a molecular target, and to determine definitively whether spiroindolone inhibits ATP4.
FIG 2.
(A) Modeling T. gondii ATPase4. The modeled structure of TgATPase4 is shown from the front and back in cartoon format. Residues which are significantly different in T. gondii, P. falciparum, and P. vivax are shown in green, and residues which have been shown by mutational analysis to confer spiroindolone resistance are shown as magenta spheres. The figure was produced using Pymol. (B) Multiple sequence alignment of P. falciparum, P. vivax, and T. gondii ATPase4. Residues which are fully conserved and similar are highlighted in red and blue, respectively; residues which are proposed to be associated with the membrane are underlined; and residues which confer resistance to the spiroindolone derivatives NITD678-R and NITD609-R are highlighted by asterisks.
Future studies may use transductive peptides, which can significantly improve the delivery of molecular cargo across membranes and, importantly, into bradyzoites (12). Future studies of bradyzoites will be of special interest. Once the most effective spiroindolone is identified, the mode of solubilization and administration can be optimized. A variety of models are available for such future studies (1, 4, 7, 12, 13).
Even before such studies to try to improve spiroindolone's selectivity and bioavailability for effectiveness against T. gondii, the work described here has immediate practical value for care of patients. Second-line medicines for T. gondii are greatly needed when hypersensitivity or other unacceptable toxicity issues with currently used medicines are present. Since spiroindolone is being developed as a medicine effective against malaria, it may soon be available for clinical use (5, 14, 15). The data presented here suggest that if this medicine clears all needed safety and efficacy hurdles to be a potent antimalarial, it could be a potential medicine effective against T. gondii (5, 14, 15). Future studies of bioavailability in eye, brain, and placenta, as well as toxicity and teratogenicity studies for humans, will be key in whether and when this compound will be clinically useful.
The work described here identifies spiroindolones, a family of compounds already defined as active against Plasmodium and with promising pharmacokinetic properties, as perorally effective against T. gondii. This work opens the way for the development of spiroindolones as medicines effective against toxoplasmosis.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful for the financial support provided by NIAID grant U01 AI082180, The Cornwell-Mann Family Foundation, The Pritzker Family Foundation, and The Rooney Alden, Engel, and Mussillami Families. S.M. is supported by an MRC Career Development fellowship (no. G1000567).
Footnotes
Published ahead of print 23 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02225-13.
REFERENCES
- 1.Fomovska A, Huang QQ, El Bissati K, Mui EJ, Witola WH, Cheng G, Zhou Y, Sommerville C, Roberts CW, Bettis S, Prigge ST, Afanador GA, Hickman MR, Lee PJ, Leed SE, Auschwitz JM, Pieroni M, Stec J, Muench SP, Rice DW, Kozikowski AP, McLeod R. 2012. Novel N-benzoyl-2-hydroxybenzamide disrupts unique parasite secretory pathway. Antimicrob. Agents Chemother. 56:2666–2682. 10.1128/AAC.06450-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLeod R, Khan AR, Noble GA, Latkany P, Jalbrzikowski J, Boyer K, Toxoplasmosis Study Group 2006. Severe sulfadiazine hypersensitivity in a child with reactivated congenital toxoplasmic chorioretinitis. Pediatric Infect. Dis. J. 25:270–272. 10.1097/01.inf.0000202070.59190.9a [DOI] [PubMed] [Google Scholar]
- 3.McLeod R, Boyer KM, Lee D, Mui E, Wroblewski K, Karrison T, Noble AG, Withers S, Swisher CN, Heydemann PT, Sautter M, Babiarz J, Rabiah P, Meier P, Grigg ME, Toxoplasmosis Study Group 2012. Prematurity and severity are associated with parasite alleles (NCCTS, 1981–2009). Clin. Infect. Dis. 54:1595–1605. 10.1093/cid/cis258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mui EJ, Milhous WK, Hsu H, Roberts CW, Kirisits M, Muench S, Rice D, Dubey JP, Fowble JW, Rathod PK, Queener SF, Liu SR, Jacobus DP, McLeod R. 2008. Novel triazine JPC-2067-B inhibits Toxoplasma gondii in vitro and in vivo. PLoS Negl. Trop. Dis. 2:e190. 10.1371/journal.pntd.0000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, González-Páez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. 2010. Spiroindolones, a potent compound class for the treatment of malaria. Science 329:1175–1180. 10.1126/science.1193225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubbels MJ, Li C, Striepen B. 2003. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob. Agents Chemother. 47:309–316. 10.1128/AAC.47.1.309-316.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai B-S, Witola W, El Bissati K, Zhou Y, Mui E, Fomovska A, McLeod R. 2012. Molecular target validation, antimicrobial delivery, and potential treatment of Toxoplasma gondii infections. Proc. Natl. Acad. Sci. U. S. A. 109:14182–14187. 10.1073/pnas.1208775109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley LA, Sternberg MJE. 2009. Protein structure prediction on the web: a case study using the phyre server. Nat. Protoc. 4:363–371. 10.1038/nprot.2009.2 [DOI] [PubMed] [Google Scholar]
- 9.Shinoda T, Ogawa H, Cornelius F, Toyoshima C. 2009. Crystal structure of the sodium-potassium pump at 2.4Å resolution. Nature 459:446–450 [DOI] [PubMed] [Google Scholar]
- 10.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acid Res. 22:4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spillman NJ, Allen RJ, McNamara CW, Yeung BK, Winzeler EA, Diagana TT, Kirk K. 2013. Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe 13:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel BU, Hearn B, Mack D, Wender P, Rothbard J, Kirisits MJ, Mui E, Wernimont S, Roberts CW, Muench SP, Rice DW, Prigge ST, Law AB, McLeod R. 2003. Delivery of antimicrobials into parasites. Proc. Natl. Acad. Sci. U. S. A. 100:14281–14286. 10.1073/pnas.2436169100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLeod R, Muench SP, Rafferty JB, Kyle DE, Mui EJ, Kirisits MJ, Mack DG, Roberts CW, Samuel BU, Lyons RE, Dorris M, Milhous WK, Rice DW. 2001. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab 1. Int. J. Parasitol. 31:109–113. 10.1016/S0020-7519(01)00111-4 [DOI] [PubMed] [Google Scholar]
- 14.Mäser P, Wittlin S, Rottmann M, Wenzler T, Kaiser M, Brun R. 2012. Antiparasitic agents: new drugs on the horizon. Curr. Opin. Pharmacol. 12:562–566. 10.1016/j.coph.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 15.van Pelt-Koops JC, Pett HE, Graumans W, van der Vegte-Bolmer M, van Gemert GJ, Rottmann M, Yeung BK, Diagana TT, Sauerwein RW. 2012. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to Anopheles mosquito vector. Antimicrob. Agents Chemother. 56:3544–3548. 10.1128/AAC.06377-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.